Abstract
Aim
To assess prospectively which patients with left breast cancer have the dosimetric benefit from the use of deep-inspiration breath-hold radiotherapy (DIBH-RT).
Background
Improvement in selection of patients for DIBH-RT would spare time of radiotherapy staff by removing the need for preparation of two comparative treatment plans.
Materials and methods
Thirty-one patients qualified for whole left breast irradiation underwent breath-hold training and their free-breathing (FB) and DIBH planning-CT were included in the analysis of dosimetric outcome. Mean heart dose (MHD), heart V20 Gy (V20Heart), maximum dose to LAD (LADmax), V20 for left lung (V20L.lung) were recorded from FB and DIBH plans and the differences (Δ) of these values were calculated. If relative improvement of at least 20% for any evaluated dosimetric parameter was found for the DIBH-RT, this plan was selected for treatment. Correlations of Δ of dosimetric parameters with patient-related parameters (BMI, age, PTV, cardiac contact distance [CCD], lung volume at FB) were sought.
Results
In 30 of 31 patients, a predefined reduction in evaluated parameters was achieved. CCD, BMI correlated positively with ΔMHD, ΔV20Heart, ΔLADmax; PTV with ΔMHD and ΔV20Heart (p < 0.05; AUC > 0.6); Lung volume correlated negatively with ΔLADmax, ΔMHD and ΔV20Heart. No specific thresholds for the lack of predefined improvement of any dosimetric parameters was identified in ROC analysis. 19/30 (63%) patients with dosimetric benefit completed their RT with DIBH.
Conclusions
Despite a strong correlation between patient-related and dosimetric parameters, we cannot use the anatomical characteristics’ thresholds to select patients for whom DIBH-RT will not be considered.
Keywords: Breast cancer, Deep inspiration breath hold, Heart-sparing, Whole breast irradiation
1. Background
Postoperative radiotherapy (RT) following breast conserving surgery (BCS) in patients with early breast cancer reduces a risk of local relapse by about 16%, which is related to the reduction of the risk of death by 5% within 15 years following diagnosis.1 Epidemiological and clinical data show the increased risk of cardiac death in women who received RT until the 1980s for left-sided breast cancer compared with patients who received RT for right-sided breast cancer.2 Cardiac perfusion deficits appear as soon as six months following radiotherapy for breast cancer.3, 4 From five to ten years after adjuvant RT, the risk of arteriosclerosis of coronary arteries increases. These patients, more often than their counterparts not treated with radiation, had symptoms of ischemic heart disease including cardiac infarction.5
Some data shows that technological advances, such as better imaging and dose calculation and distribution in RT of breast cancer, which led to the decrease of dose given to healthy tissues with proper encompassing of the target volume, may reduce treatment-related cardiac morbidity. This is of crucial importance in the light of improvement of survival in breast cancer patients. Deep inspiration breath-hold RT (DIBH-RT) has been demonstrated to reduce heart doses. DIBH-RT decreases low and high doses given to the heart, left anterior descending artery (LAD), and sometimes doses to the left lung with preservation of the dose coverage and distribution within the planning target volume (PTV).6, 7, 8, 9, 10, 11, 12, 13 However, data about factors that may impact the dosimetric benefit from DIBH-RT are scarce.6, 12, 13, 14, 15 The routine use of this technique increases the treatment cost because of the significant increase in the staff workload and the necessity of acquiring and servicing new equipment. This is also a demanding technique for patients, which requires some degree of cooperation, effort and engagement for the procedure to be performed properly. Special measures to ensure the reproducibility and stability of DIBH-RT, such as monitoring of treatment by a real-time 3-dimensional (3D) surface imaging system, increase the time of treatment delivery and represent an additional burden not only for RT staff but also for patients. All of these factors are of special importance in the busy RT department, where each increase of the workload dedicated to one group of patients may reduce the number of treated patients and lengthen their waiting time.
2. Aim
Therefore, we decided to carry out a prospective study to evaluate the extent of dosimetric benefit from the use of DIBH-RT technique in patients with left breast cancer in relation to the patient-related factors. We tried to identify the anatomical characteristics thresholds to select patients for whom DIBH-RT will not be used. We expected that the results of this study would enable improvements in the selection of patients for routine use of DIBH-RT in the future without having to prepare two comparative treatment plans for free breathing (FB) and DIBH-RT.
3. Patients and methods
3.1. Patients selection
We intended to include in the study 30 consecutive patients with early stage left breast cancer referred for adjuvant RT following BCS who agreed to be treated with this technique and participate in the study. Inclusion criteria were: age between 18 and 70 years, invasive ductal carcinoma staged pT1–T2, N0–N1a, M0 or ductal carcinoma in situ and signature confirming informed consent to participation in the study. Excluded patients were those who did not agree to participate, who were unable to cooperate in the DIBH training or who had respiratory function impairment that precluded them from deep inspiration maintenance.
3.2. Treatment planning
Included patients were given instructions and were trained by dedicated RT staff to calmly breathe and hold their deep inspiration for about 20 s. The patients then underwent a FB and DIBH computed tomography (CT) scan. Two separate treatment plans were prepared: one for FB and one for DIBH. Contouring of clinical target volume (CTV) was performed according to published guidelines.16, 17 PTV was generated by expanding CTV by 6 mm in all directions, but not closer than 5 mm to the skin in both CTs. Critical structures (heart, LAD, lung, skin) were contoured without margins. Delineation of the heart and LAD was performed according to published guidelines.18 All delineations in this study were done by one person (BC).
The prescribed dose was 39.9 Gy in 15 fractions during 3 weeks. Patients were treated with tangential conformal fields with a multileaf collimator using 6 MV photons. The field-in-field technique was used to avoid hotspots. There were no special requirements for fields geometry and shape. Boost for the lumpectomy cavity was also prescribed if indicated; however, this part of treatment was realized at FB and was not subject of this study. Two plans were compared with regard to the covering of PTV by 93% isodose, maximum dose for LAD (LADmax), volume of heart receiving 20 Gy (V20Heart), mean heart dose (MHD), and volume of left lung receiving 20 Gy (V20L.lung). Patients were treated with DIBH if there was an improvement of at least one evaluated dose metric in the DIBH treatment plan compared with the FB treatment plan. Improvement in the DIBH treatment plan was defined arbitrarily as a relative reduction by at least 20% of any studied dose metrics compared with the FB plan without clinically significant worsening of other parameters as judged by treating physician and maintained covering of PTV with a 93% isodose. Patients that had no sufficient improvement of treatment plan or were not able to hold deep inspiration steadily were treated according to the FB plan. Additionally, relative improvement by 50% of evaluated dose metrics was assessed.
3.3. DIBH-RT technique
Treatment was realized using 6 MV photons on the linear accelerator equipped with a real-time 3D surface imaging system (AlignRT; Vision RT Ltd, London, UK) for guidance in DIBH-RT. Electronic portal imaging was performed for verification of bony anatomy before the first fraction and then weekly. The AlignRT system does not cause additional irradiation for patients and uses video-based stereoscopic image capturing. Before dose delivery, the AlignRT was used for setup verification by fast surface registration for alignment and coregistration with CT simulation with DIBH reference surface. This system was then used for monitoring the breath hold during treatment. Treatment was delivered only if the breath hold was kept at the predefined level. Threshold for translational error measured in three perpendicular axes (left-right, cranio-caudal, and anterior–posterior was fixed at 4 mm. Threshold for rotational error was fixed at 3°.
3.4. Statistical methods
For all analyzed dose metrics, the means, medians, range of values, and frequencies for non-continuous values were calculated. The differences (Δ) of these values for FB and DIBH plans were compared using a paired T-test. The data distribution was assessed using the χ2 test. Correlations of differences in relevant dose metrics with patient related parameters (body mass index [BMI], age, PTV, cardiac contact distance [CCD] defined as the maximum length of contact between the heart and chest wall on the left side of the sternum, volume of lung at FB) were analyzed using the Spearman rank correlation. Furthermore, receiver-operating characteristic (ROC) analysis was performed to select the threshold of values of patient-related parameters that may predict the lack of predefined dosimetric benefit for the heart and LAD of the DIBH over FB. A p value < 0.05 was considered statistically significant.
4. Results
Between June 2014 and June 2015, 37 eligible patients were offered the possibility of participation in the study. Two patients did not agree to participate and four were unable to cooperate efficiently during breath-hold training. Finally, 31 patients were included in the final analysis of dosimetric comparison of DIBH and FB and are the subjects of the analysis. The age of the analyzed patients ranged from 24 to 70 years (mean, 55.5 years). Their BMI ranged from 17.1 to 37.8 (mean, 25.7); PTV ranged from 409 cc to 2043 cc (mean, 770 cc); CCD ranged from 2.5 cm to 10.0 cm (mean, 5.5 cm), and total lung volume at FB ranged from 2055 cc to 3991 cc (mean, 2835 cc). There was no difference in the covering of PTV by 93% isodose in DIBH and FB plans. In 30 (97%) of 31 compared pairs of plans, the predefined relative improvement of 20% was obtained for at least one evaluated dose metric without clinically significant worsening of the other parameters, i.e. in 29, 29, 26, and 7 cases for MHD, V20Heart, LADmax, and V20L.lung, respectively. Most patients (22/30) had relative improvement of 20% in three out of four analyzed dosimetric parameters. For one, two, and four patients this improvement was seen respectively for one, two, and four analyzed parameters. Improvement by 50% was achieved in 20, 29, 18 cases for MHD, V20Heart, LADmax, respectively, but none of the patients showed any V20L.lung improvement.
There was worsening of V20L.lung in 10 cases and LADmax in two cases in DIBH plans compared with FB plans. Mean Δ of analyzed parameters for DIBH compared to FB were: for MHD, 1.5 Gy; for V20Heart, 3.02% (18.08 cc); for LADmax., 18.5 Gy; for V20L.lung, 1.71%. All differences were statistically significant, p < 0.001 (Table 1).
Table 1.
Comparison of dose metrics in FB-RT and DIBH-RT.
| Dose metric | FB-RT Mean [range] |
DIBH-RT Mean [range] |
Δ of mean value of dose metric [range of Δ] in FB-RT and DIBH-RT | Mean of relative improvement of Δ value [range] in FB-RT and DIBH-RT (in %) |
|---|---|---|---|---|
| MHD in Gy | 2.57 [0.66–7.92] | 1.06 [0.60–1.73] | 1.51 [0.06–6.45] p < 0.001 |
51.80 [9.10–81.40] |
| V20heart in Gy | 3.31 [0.00–7.03] | 0.28 [0.00–1.96] | 3.02 [0.00–6.59] p < 0.001 |
90.5 [0.00–100.00] |
| LADmax in Gy | 28.99 [1.18–38.91] | 10.50 [1.17–34.02] | 18.49 [−3.29 to 36.68] p < 0.001 | 52.40 [−105.4 to 96.9] |
| Volume of left lung receiving 20 Gy in % | 13.06 [6.84–24.44] | 11.35 [5.59–16.64] | 1.71% [−2.71 to 8.70] p = 0.001 |
9.8 [−23.60 to 43.30] |
MHD – mean heart dose, V20heart – volume of heart receiving 20 Gy, LADmax – maximum dose given to left anterior descending artery (LAD), FB-RT – free breathing radiotherapy, DIBH-RT – in deep inspiration breath-hold radiotherapy, Gy – gray, p – statistical significance.
ΔMHD, ΔV20Heart, and ΔLADmax correlated positively with CCD, BMI, but not age; PTV correlated positively with ΔMHD and ΔV20Heart. A negative correlation was found between ΔMHD, ΔV20Heart, ΔLADmax and the volume of lungs at the FB (Table 2).
Table 2.
Values of Spearman coefficients of correlation of Δ value of dose metrics for heart and LAD in FB-RT and DIBH-RT with patients’ related parameters; correlations values for which p value was <0.05 are shown in bold.
| Δ MHD | ΔV20heart | ΔLADmax | |
|---|---|---|---|
| BMI | 0.44 | 0.42 | 0.41 |
| CCD | 0.71 | 0.70 | 0.38 |
| PTV | 0.51 | 0.48 | 0.14 |
| Age | −0.05 | 0.02 | −0.03 |
| Volume of lung on FB | −0.58 | −0.60 | −0.36 |
MHD – mean heart dose, V20heart – volume of heart receiving 20 Gy, LADmax – maximum dose given to left anterior descending artery (LAD), BMI – body mass index, CCD – cardiac contact distance, PTV – planning target volume, FB – free breathing.
ROC analysis identified some threshold values of BMI, PTV, CCD above which all patients achieved the relative benefit of 20% or 50% in analyzed dosimetric parameters (Table 3). However, these threshold values cannot be used for selection of patients for planning of RT at FB only, because some patients whose values of BMI, PTV or CCD were below the threshold point achieved dosimetric benefit, too (Fig. 1, Fig. 2, Fig. 3).
Table 3.
Results of ROC analysis for threshold values of BMI, PTV, CCD, lung volume at FB above which all patients achieved the relative benefit of 20% or 50% in analyzed dose metrics; threshold values for which p value was <0.05 are shown in bold.
| Parameter | Threshold values for relative benefit ≥20% of improvement of dose metric |
Threshold values for relative benefit ≥50% of improvement of dose metric |
||||
|---|---|---|---|---|---|---|
| Δ MHD | Δ V20Heart | ΔLADmax | Δ MHD | Δ V20Heart | ΔLADmax | |
| BMI (kg/m2) Threshold value |
22.3 | 22.3 | 24.8 | 27.6 | 22.3 | 26.0 |
| AUC (95% CI) | 0.88 (0.75–1.0) | 0.80 (0.66–0.94) | 0.73 (0.50–0.96) | 0.76 (0.58–0.93) | 0.80 (0.66–0.94) | 0.63 (0.43–0.83) |
| CCD (cm) Threshold value |
2.9 | 2.9 | 3.8 | 5.7 | 2.9 | 3.0 |
| AUC (95% CI) | 0.99 (0.96–1.0) | 0.95(0.87–0.91) | 0.61 (0.26–0.97) | 0.76 (0.57–0.94) | 0.95 (0.87–1.0) | 0.45 (0.22–0.67) |
| PTV (cm3) Threshold value |
577.7 | 445.6 | – | 703.4 | 445.64 | – |
| AUC (95% CI) | 0.81 (0.63–0.99) | 0.90 (0.79–1.00) | 0.85 (0.71–0.98) | 0.90 (0.79–1.00) | ||
| FB lung volume (cm3) Threshold value |
2485.7 | 2589.6 | 2377.2 | 2909.6 | 2589.6 | 2804.8 |
| AUC (95% CI) | 0.58 (0.24–0.91) | 0.65 (0.48–0.82) | 0.54 (0.31–0.78) | 0.74 (0.55–0.93) | 0.65 (0.48–0.82) | 0.56 (0.35–0.76) |
MHD – mean heart dose; V20Heart – volume of heart receiving 20 Gy; LADmax – maximum dose for LAD; FB – free breathing; LAD – left anterior descending artery; AUC – area under the curve; CI – confidence interval.
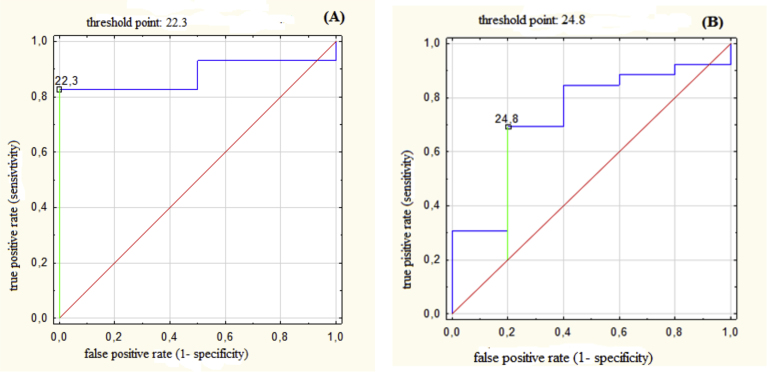
Fig. 1.
(A) ROC for body mass index (BMI) with threshold value of 22.3 kg/m2 predicting probability of benefit in mean heart dose (MHD) larger than relative improvement by 20%; AUC = 0.88 (95% CI: 0.75–1.00), p < 0.001. (B) ROC for body mass index (BMI) with threshold value of 24.8 kg/m2 predicting probability of benefit in maximum dose for left anterior descending artery (LADmax) larger than relative improvement by 20%; AUC = 0.73 (95% CI: 0.50–0.96), p = 0.049.
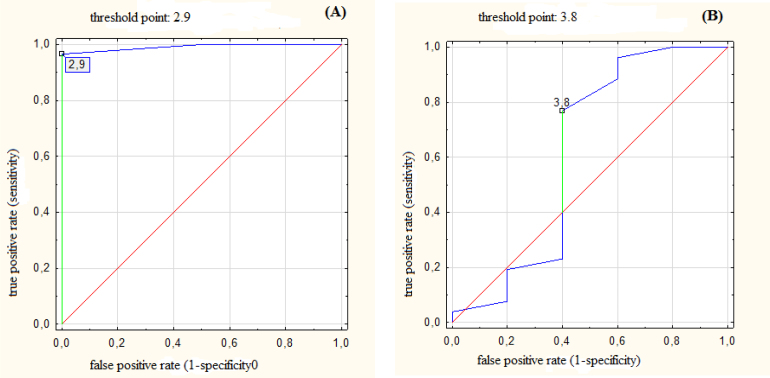
Fig. 2.
(A) ROC for cardiac contact distance (CCD) with threshold value of 2.9 cm predicting probability of benefit in mean heart dose (MHD) larger than relative improvement by 20%; AUC = 0.99 (95% CI: 0.96–1.00), p < 0.001. (B) ROC for cardiac contact distance (CCD) with threshold value of 3.8 cm predicting probability of benefit in maximum dose for left anterior descending artery (LADmax) larger than relative improvement by 20%; AUC = 0.61 (95% CI: 0.26–0.97), p = 0.53.
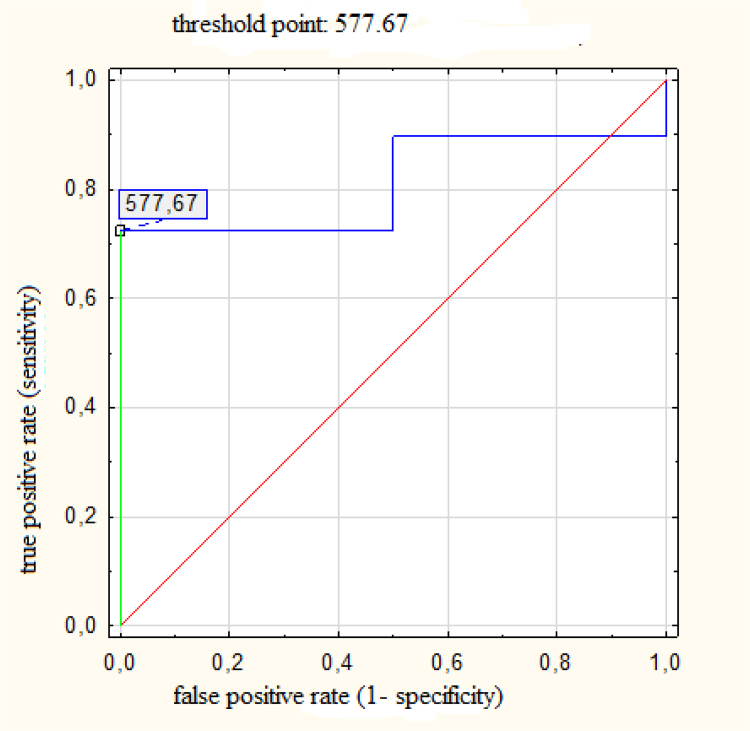
Fig. 3.
ROC for planning target volume (PTV) with threshold value of 577 cm3 predicting probability of benefit in mean heart dose (MHD) larger than relative improvement by 20%; AUC = 0.81 (95% CI: 0.63–0.98), p < 0.001.
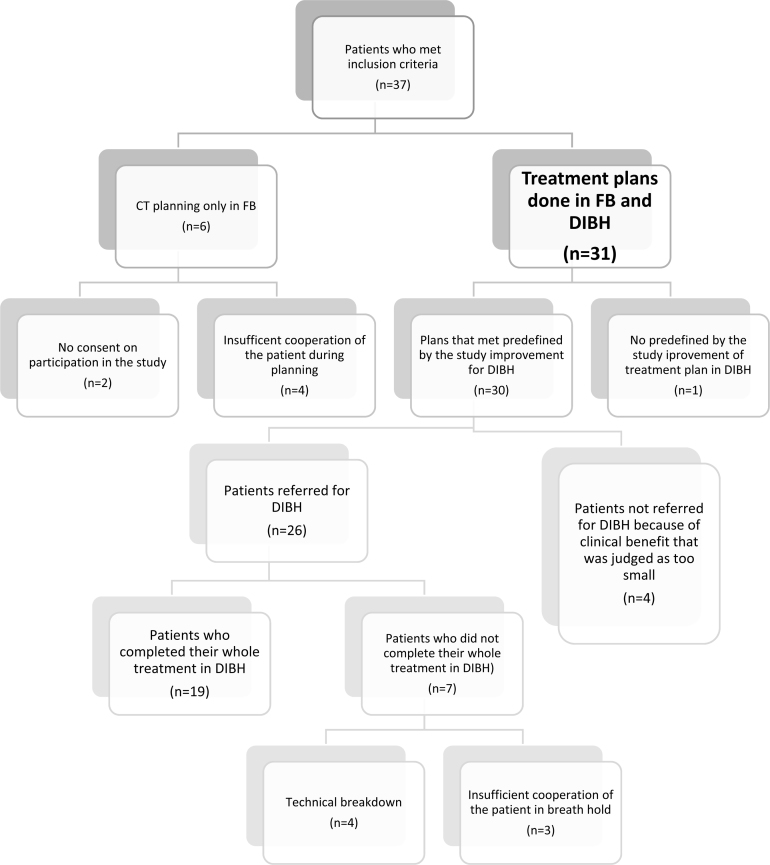
Despite the fulfillment of the predefined plan's improvement criteria in 30 patients, 26 patients (87%) were referred for DIBH-RT and four (13%) were not. In these four cases, this relative improvement by 20% was judged by the treating physician as very unlikely to bring any clinical benefit, because the dose metrics for the heart and LAD in FB were already very small, i.e. MHD ≤0.3 Gy and LADmax ≤2.2 Gy. Seven of the 26 patients did not complete their whole course of RT in DIBH. Some part of or whole RT was realized in FB, in four patients because of technical breakdown and in three patients because of their problems with cooperation in the maintenance of steady breath hold during treatment (predefined duration of breath hold not sufficient and/or Align-RT thresholds exceeded). Finally, 19 (61%) of 31 patients completed the whole course of RT in DIBH. A CONSORT flow diagram is presented in Fig. 4.
Fig. 4.
CONSORT flow diagram.
5. Discussion
As in other studies, we found a statistically significant improvement in dose given to the heart and LAD in patients treated with DIBH compared to FB. In all but one of the 31 analyzed treatment plans, we found a predefined level of dosimetric benefit in DIBH-RT. Unfortunately, we were not able to show the threshold values for patient-related factors that may help select patients who are not likely to benefit from DIBH-RT. Despite an increase in the probability of greater dosimetric benefit with higher BMI, longer adherence of the heart to the thoracic wall (CCD), larger PTV and smaller volume of lung at FB, we cannot exclude any patient from the use of the studied method because statistical analysis did not allow for such an exclusion. We did not find unambiguous threshold points for non-beneficial use of DIBH. All patients may benefit from the DIBH-RT at the extent predefined in our study as a presumed benefit. The anatomical characteristics of the patients cannot preclude them from the referral to DIBH-RT.
Only 63% (19/30) of patients that had improvement in the DIBH treatment plan actually completed the planned treatment with the use of DIBH. This reflects difficulties that may be encountered in the introduction into clinical practice of such a demanding technique in a busy RT department. Four (11%) out of 35 patients that initially consented to participate in the study were not able to hold their breath in deep inspiration efficiently and were not qualified for the DIBH-RT at the planning stage. Additionally, three (11%) out of 26 referred for DIBH-RT following their demonstrated dosimetric benefit of the DIBH-RT plan in comparison with FB-RT plan did not complete their treatment using the studied technique because they were not able to keep their breath efficiently throughout the whole treatment course. Therefore, our result indicates that about 20% of breast cancer patients would not be compliant with the use of this technique. We do not have a detailed information on patients’ compliance with DIBH in most studies. In a study without age restriction in referral for DIBH-RT, only 40% of 45 available patients completed their RT with breath hold. Older patients were not compliant with the use of this technique.19 Our age limit for inclusion into the study was 70 years, but we did not find any correlation between dosimetric benefit and age. Age alone cannot be a selection criterion for DIBH-RT. In the UK HeartSpare study, which prospectively evaluated voluntary DIBH with active-breathing coordinator DIBH, all 23 included patients completed their treatment.9 In a Dutch study, all 19 included patients completed their DIBH treatment; however, a preselection criteria for the study were not detailed.20 Certainly, we need more data from prospective studies on what is an exact rate of compliance with the studied technique.
Four out of 30 patients with relative improvement dose metrics by 20%, which was considered in the study as a dosimetric benefit, were finally not referred for DIBH-RT by a treating physician. These were cases with very low doses given to the heart and LAD in FB, for which an improvement of 20% was judged as not clinically relevant. This arbitrary admitted threshold of the benefit is a main limitation of our study. Reduction of dose by 20% seems relevant in the case of the delivery of higher doses. However, in the case of very low doses given to the heart and LAD in the FB technique, it is not surprising that in practice, a predefined dose reduction by 20% was considered as not worth the workload dedicated to the performance of this technique. On the other hand, we found that a majority of patients achieved relative dosimetric benefit above 50% in MHD, V20Heart and LADmax, which justifies the use of the technique. In the UK HeartSpare study, in which this technique was implemented at the national level, the referral for DIBH-RT was dedicated to patients with any heart inside the 50% isodose in FB-RT planning CT with standard tangential fields.9, 21 Such an approach would exclude patients with favorable anatomy and quite good heart and LAD sparing on FB from the use of this costly and time-consuming technique. On the other hand, one may argue that in the light of the suggestions that there is no threshold of dose reduction to the heart and LAD for which a clinical benefit disappears, it is difficult to set an arbitrary, but firm, value of dose constraints that preclude patients from further dose reduction attempts. Even a small dose decrease given to these critical structures may reduce late cardiac and vascular morbidity.5, 13, 22
Our attempt to identify the threshold values of patient-related factors that preclude the dosimetric benefit of DIBH over FB failed. There was no published data on this issue at the study design stage. Recently, Register et al.14 demonstrated in the retrospective study on 64 patients with left breast cancer that no anatomical surrogate, except a small heart volume in field (HVIF) in FB was related to minimal benefit from DIBH. We did not include HVIF into factors examined for influence on the outcome. The HVIF is not a pure anatomical characteristic, but a combination of the anatomy and treatment related factors, and its evaluation required the preparation of a FB treatment plan. We were looking for patient-related parameters that would prevent us from preparing two sets of treatment plans so that the workload could be reduced in the treatment preparation. Nevertheless, the smaller CCD may indirectly indicate a lower HVIF. Notably, the CCD correlated positively with a higher Δ of all cardiac and LAD evaluated dose metrics which may be in line with the relationship of HVIF with DIBH benefit. Hjelstuen et al.15 found a negative correlation between a total lung volume (TLV) at FB and MHD and V25Heart. They demonstrated that 2200 cc and lower TLV evaluated by pulmonary function tests are predictors for dosimetric benefit for DIBH-RT; thus, it was suggested that some patients should be referred directly for DIBH-RT while others may be treated at FB. In our study, despite a negative correlation of lung volume at FB with doses given to the heart, no unambiguous specific threshold was identified to enable the selection of patients for exclusion from DIBH-RT.
We found that some patients had worsening of V20L.lung and LADmax in DIBH-RT plans compared with FB plans. To our knowledge this problem was not pointed out in other studies on this issue. This was probably due to the reluctance to publish negative results.
In our series, the mean dose given to the heart in FB was 2.58 Gy, which is lower than an average mean dose of 4.1 Gy reported for left tangential fields in a supine position without breathing control in a systematic review of heart doses published during 2003–2013. There was significant variability among European countries with mean doses given to the heart with a lowest dose published from the UK (1.6 Gy) and the highest from Germany (6.5 Gy). The average mean heart dose reported from Poland was 3.2 Gy (standard deviation, 1.6 Gy), which is in line with our result.23 Such a large variability in the reported heart doses between countries may reflect differences in the anatomy of the patients treated, in the RT techniques and quality assurance and also, in great part, probably in the delineation of the heart. It was shown that the use of delineation guidelines reduces inter-observer variability in the delineation of the heart and LAD.24 Standardized guidelines for critical structures delineation have been introduced to the common practice over the last 5 years; therefore, the lack of guidelines might have contributed largely to the different results of mean heart doses reported in a systematic review by a Taylor et al.23 Even if there is an argument that we may already spare significantly heart doses with careful planning for FB RT, we demonstrated that even with relatively low doses given to the heart in the FB technique, we were still able to improve results by further using breathing control. To maintain a long-term improvement of survival with RT for breast cancer, we should use all means that lead to the reduction of doses given to critical structures providing proper irradiation of the target volume.
6. Conclusions
In the light of proven dosimetric benefit with DIBH and absence of factors that may negatively select patients for the referral to the use of DIBH-RT, every organizational effort should be done in implementation of this technique to all patients with left breast cancer. Based on our data, we cannot firmly identify a subgroup of patients that should not be referred for DIBH-RT.
Conflict of interest
None declared.
Financial disclosure
There was no financial support for the conduct of the research and/or preparation of the article.
Ethical approval
Ethical approval was obtained from the local Ethics Committee. All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed consent was obtained from all individual participants included in the study.
References
- 1.Clarke M., Collins R., Darby S. Effects of radiotherapy and differences in the extent of surgery for early breast cancer on local recurrence and 15-year survival: on overview of the randomized trials. Lancet. 2005;366:2087–2106. doi: 10.1016/S0140-6736(05)67887-7. [DOI] [PubMed] [Google Scholar]
- 2.Offersen B., Hojris I., Overgaard M. Radiation-induced heart morbidity after adjuvant radiotherapy of early breast cancer – is it still an issue? Radiother Oncol. 2011;100:157–159. doi: 10.1016/j.radonc.2011.08.007. [DOI] [PubMed] [Google Scholar]
- 3.Marks L.B., Yu X., Prosnitz R.G. The incidence and functional consequences of RT-associated cardiac perfusion defects. Int J Radiat Oncol Biol Phys. 2005;63:214–223. doi: 10.1016/j.ijrobp.2005.01.029. [DOI] [PubMed] [Google Scholar]
- 4.Zellars R., Bravo P.E., Tryggestad E. SPECT analysis of cardiac perfusion changes after whole-breast/chest wall radiation therapy with or without active breathing coordinator: results of a randomized phase 3 trial. Int J Radiat Oncol Biol Phys. 2014;88:778–785. doi: 10.1016/j.ijrobp.2013.12.035. [DOI] [PubMed] [Google Scholar]
- 5.Darby S.C., Ewertz M., McGale P. Risk of ischemic heart disease in women after radiotherapy for breast cancer. N Engl J Med. 2013;368:87–98. doi: 10.1056/NEJMoa1209825. [DOI] [PubMed] [Google Scholar]
- 6.Gierga D.P., Turcotte J.C., Sharp G.C., Sedlacek D.E., Cotter C.R., Taghian A.G. A voluntary breath – hold treatment technique for the left breast with unfavorable cardiac anatomy using surface imaging. Int J Radiat Oncol Biol Phys. 2012;84:e663–e668. doi: 10.1016/j.ijrobp.2012.07.2379. [DOI] [PubMed] [Google Scholar]
- 7.Jarvis L.A., Maxim P.G., Horst C.K. Deep inspiration breath hold reduces dose the left ventricle and proximal left anterior descending artery during radiotherapy for left-side breast cancers. J Cancer Therapy. 2012;3:673–679. [Google Scholar]
- 8.Pedersen A.N., Korreman S., Nystrom H., Specht L. Breathing adapted radiotherapy of breast cancer: reduction of cardiac and pulmonary doses using voluntary inspiration breath-hold. Radiother Oncol. 2004;72:53–60. doi: 10.1016/j.radonc.2004.03.012. [DOI] [PubMed] [Google Scholar]
- 9.Bartlett F.R., Colgan R.M., Carr K. The UK HeartSpare study: randomised evaluation of voluntary deep-inspiratory breath-hold in women undergoing breast radiotherapy. Radiother Oncol. 2013;108:242–247. doi: 10.1016/j.radonc.2013.04.021. [DOI] [PubMed] [Google Scholar]
- 10.Nissen H.D., Appel A.L. Improved heart, lung and target dose with deep inspiration breath hold in a large clinical series of breast cancer patients. Radiother Oncol. 2013;106:8–32. doi: 10.1016/j.radonc.2012.10.016. [DOI] [PubMed] [Google Scholar]
- 11.Remouchamps V.M., Vicini F.A., Sharpe M.B., Kestin L.L., Martinez A.A., Wong J.W. Significant reductions in heart and lung doses using deep inspiration breath hold with active breathing control and intensity-modulated radiation therapy for patients treated with locoregional breast irradiation. Int J Radiat Oncol Biol Phys. 2003;55:392–406. doi: 10.1016/s0360-3016(02)04143-3. [DOI] [PubMed] [Google Scholar]
- 12.Bruzzaniti V., Abate A., Pinnaro P. Dosimetric and clinical advantages of deep inspiration breath-hold (DIBH) during radiotherapy of breast cancer. J Exp Clin Cancer Res. 2013;32:88. doi: 10.1186/1756-9966-32-88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Smyth L.M., Knight K.A., Aarons Y.K., Wasiak J. The cardiac dose-sparing benefits of deep inspiration breath-hold in left breast irradiation: a systematic review. J Med Radiat Sci. 2015;62:66–73. doi: 10.1002/jmrs.89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Register S., Takita C., Reis I., Zhao W., Amestoy W., Wright J. Deep inspiration breath-hold technique for left-sided breast cancer: an analysis of predictors for organ-at-risk sparing. Med Dosim. 2015;40:89–95. doi: 10.1016/j.meddos.2014.10.005. [DOI] [PubMed] [Google Scholar]
- 15.Hjelstuen M.H., Mjaaland I., Vikström J., Madebo T., Dybvik K.I. Pulmonary function tests – an easy selection method for respiratory-gated radiotherapy in patients with left-sided breast cancer. Acta Oncol. 2015;54:1025–1031. doi: 10.3109/0284186X.2014.990107. [DOI] [PubMed] [Google Scholar]
- 16.Radiation Therapy Oncology Group (RTOG) 2011. Breast cancer atlas for radiation therapy planning: consensus definitions. Available from: http://www.rtog.org/ [Google Scholar]
- 17.Nielsen M.H., Berg M., Pedersen A.N. Delineation of target volumes and organs at risk in adjuvant radiotherapy of early breast cancer: national guidelines and contouring atlas by the Danish Breast Cancer Cooperative Group. Acta Oncol. 2013;52:703–710. doi: 10.3109/0284186X.2013.765064. [DOI] [PubMed] [Google Scholar]
- 18.Feng M., Moran J.M., Koelling T. Development and validation of a heart atlas to study cardiac exposure to radiation following treatment for breast cancer. Int J Radiat Oncol Biol Phys. 2011;79:10–18. doi: 10.1016/j.ijrobp.2009.10.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jacobson G.M., Watson C.N., Zhang J. Mean radiation dose to the heart in patients with left breast cancer with and without breath-hold technique. J Clin Oncol. 2014;32 [suppl. 26; abstr. 85] [Google Scholar]
- 20.Borst G.R., Sonke J.J., Den Hollander S. Clinical results of image-guided deep inspiration breath hold breast irradiation. Int J Radiat Oncol Biol Phys. 2010;78:1345–1351. doi: 10.1016/j.ijrobp.2009.10.006. [DOI] [PubMed] [Google Scholar]
- 21.Bartlett F.R., Donovan E.M., McNair H.A. The UK HeartSpare study (stage II): multicenter evaluation of a voluntary breath-hold technique. Radiother Oncol. 2015;115:S186. doi: 10.1016/j.radonc.2014.11.018. [DOI] [PubMed] [Google Scholar]
- 22.Sardaro A., Petruzzelli M.F., D’Errico M.P., Grimaldi L., Pili G., Portaluri M. Radiation-induced cardiac damage in early left breast cancer patients: risk factors, biological mechanisms, radiobiology, and dosimetric constraints. Radiother Oncol. 2012;103:133–142. doi: 10.1016/j.radonc.2012.02.008. [DOI] [PubMed] [Google Scholar]
- 23.Taylor C.W., Wang Z., Maculay E., Jagsi R., Duane F., Darby S.C. Exposure of the heart in breast cancer radiation therapy: a systematic review of heart doses published during 2003 to 2013. Int J Radiat Oncol Biol Phys. 2015;93:845–853. doi: 10.1016/j.ijrobp.2015.07.2292. [DOI] [PubMed] [Google Scholar]
- 24.Lorenzen E.L., Taylor C.W., Maraldo M. Inter-observer variation in delineation of the heart and left anterior descending coronary artery in radiotherapy for breast cancer: a multi-centre study from Denmark and the UK. Radiother Oncol. 2013;108:254–258. doi: 10.1016/j.radonc.2013.06.025. [DOI] [PubMed] [Google Scholar]