Abstract
Sachse et al. highlight work that reveals a Na+-dependent inactivation mechanism in the Na+/K+ pump.
In virtually all mammalian cells, the traditional roles of the Na+/K+ pump include establishing and maintaining transcellular gradients of Na+ and K+ and modulating steady-state gradients of H+ and Ca2+. By regulating intracellular Na+, [Na+]i, the Na+/K+ pump also makes a major contribution to cellular volume control, although some aspects of this modulation of osmolarity are not completely understood (Aronson et al., 2003). Additional roles of the Na+/K+ pump arise from the fact that it is electrogenic: specifically, 3 Na+ are extruded from the cell for every 2 K+ that are taken up. The resulting small net outward current is functionally important, as is the dependence of this pump on [Na+]i, which can change rapidly and substantially. In this issue of The Journal of General Physiology, Lu and Hilgemann reveal that [Na+]i usually remains relatively constant in healthy adult ventricular myocytes and that the decline in Na+/K+ pump current during continuous stimulation is caused by an inactivation mechanism.
In many tissues, including epithelia, endothelia, and the atria and conduction system of the heart, the net outward current generated by the Na+/K+ pump operating through a very high input resistance is sufficient to generate a hyperpolarizing influence of ∼5 mV. Moreover, the fundamental physicochemical properties of this ATP-requiring pump are such that it can contribute to the after-potential profile in a variety of nerve cells, especially following periods of intense firing (Rang and Ritchie, 1968; but see Wallén et al., 2007). Similarly, this pump current can result in a significant and quite long-lasting hyperpolarization and change in excitability after bouts of relatively high heart rate in the cardiac conduction system. This has been termed overdrive suppression in these cardiac Purkinje fibers (cf, Bocchi and Vassalle, 2008). A somewhat similar phenomenon may contribute to postrepolarization refractoriness in mammalian atrial myocytes in settings such as paroxysmal fibrillation (cf, Grandi et al., 2011). Many of these Na+/K+ pump–induced (or regulated) phenomena can be explained by the pronounced dependence of the turnover rate of the predominant isoforms of this active transporter on the precise levels of [Na+]i (Despa and Bers, 2007; Han et al., 2009). However, it is now known that several critical aspects of this regulation are based on the exact microanatomical localization and tethering of both the α and the β subunits of the predominant Na+/K+ pump isoforms. In some cells, there is evidence that the Na+/K+ pump is colocalized with other Na+-dependent antiporters (e.g., Na+/Ca2+ exchangers) and that the integral membrane proteins may function interdependently (cf, Clancy et al., 2015).
[Na+]i and the late Na+ current in the heart
The importance of [Na+]i as a primary regulator of the Na+/K+ pump has recently been brought into focus by work suggesting that a slowly inactivating or late component of the same Na+ current, which produces action potential depolarization and is essential for regenerative excitability, does not fully inactivate or turn off in certain cell types, e.g., cardiac Purkinje fibers and some “pacemaker neurons” (cf, Ahern et al., 2016). As a result, the total Na+ influx far exceeds the Na+ entry that corresponds to the action potential upstroke (Giles and Carmeliet, 2016; Makielski, 2016). Interestingly, the late Na+ current INa-L can be detected in healthy human ventricular myocytes, where it contributes to the plateau of the action potential and lengthens action potential duration by perhaps 10% at physiological heart rates (Yang et al., 2015). Importantly, this same current is enhanced in a variety of tissues, including the heart during hypoxia, ischemia, progressive type I or type II diabetes and conditions of reduced (acidic) pH (Swietach et al., 2015). Moreover, several point mutations in both cardiac and nerve Na+ channels that produce or contribute to arrhythmogenesis, or enhance CNS epileptic activity, do so by slowing or removing Na+ current inactivation and thus augmenting INa-L (Belardinelli et al., 2015). In the mammalian heart, including human atria, ventricles, and the conduction system, the downstream consequences of this extra Na+ influx are thought to be quite significant. This is based on evidence that this transmembrane Na+ influx does not lead to a Na+ concentration increase that is distributed uniformly in the myoplasm, but rather remains localized and therefore concentrated in a spatially restricted subsarcolemmal area, which has been denoted “the fuzzy space” (cf, Carmeliet, 1992; Verdonck et al., 2004). An important consequence of this working hypothesis is that [Na+]i can, in principle, increase quickly and substantially. The resulting reduction in the electrochemical gradient for Na+ could reduce and possibly reverse the Na+/Ca2+ exchanger, thus giving rise to abnormally high and long lasting intracellular Ca2+ ([Ca2+]i). This increase could alter contractility (Eisner, 1990; Eisner et al., 2009; Ramirez et al., 2011; Aronsen et al., 2013) and may cause a marked tendency to initiate and maintain abnormal rhythms. These arrhythmias are denoted “early or late” afterdepolarizations depending on whether they arise at the plateau of the action potential or in diastole (i.e., from the resting potential). Key parts of this scenario remain unproven (Cardona et al., 2016; Clark and Giles, 2016). However, it is very clear that drugs that selectively reduce or completely block INa-L have significant antiarrhythmic activity in both animal models and phase I clinical trials (Belardinelli et al., 2015; Yang et al., 2015).
The late Na+ current does not change [Na+]i
A paper from the Hilgemann group (Lu and Hilgemann, 2017) in this issue of JGP presents important new information concerning the relationship between Na+ influx, related changes in Na+/K+ activity, and the volume of distribution for Na+ ions in healthy adult ventricular myocytes. Many of these findings challenge the fundamental tenets of the prevalent working hypothesis, which states that INa-L increases [Na+]i. Specifically, their firm conclusion is that INa-L, even when activated at super normal levels, does not significantly change [Na+]i. In fact, their principal finding is that, although Na+/K+ pump turnover rate and hence electrogenic current is regulated strongly by [Na+]i, the Na+/K+ pump current declines even at a fixed value of [Na+]i within the physiological range (6–12 mM). Lu and Hilgemann (2017) conclude that this decline results from the development of inactivation due to failure of this integral membrane protein to be able to transition from one of the critically important steps in the reaction scheme that is essential for this coupled Na+ “out” for K+ “in” active transport. Their insights are made possible by clever and rigorous applications of sophisticated patch clamp recordings and perfusion methods that this group have previously developed and perfected (Hilgemann et al., 1992). Their approach consistently provides high-resolution records (obtained at 37°C) of small transmembrane currents for relatively long time periods in conjunction with sequential and rapid changes in patch perfusion and intracellular dialysis. In addition, high-resolution measurements of cell capacitance provide new information concerning intramembrane charge movements, which can be related to individual steps in the Na+/K+ ion exchange transport cycle (Lu et al., 2016).
Illustration of background currents in a human atrium myocyte
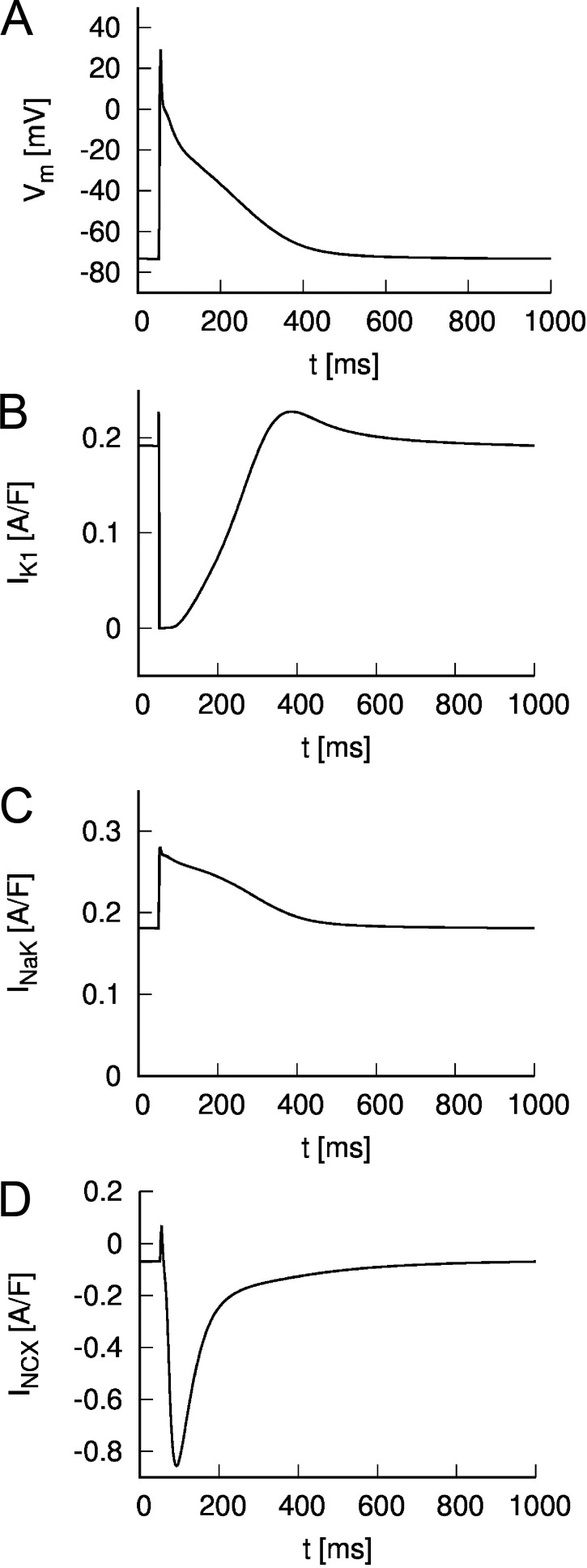
As an introduction to the main findings in Lu and Hilgemann (2017), it is useful to recall the relative sizes and temporal relationships of the main background currents in a human heart myocyte. This information is illustrated in Fig. 1. The chosen example is a human atrial action potential computed using the relatively recent and comprehensive mathematical model published by Grandi et al. (2011). Fig. 1 A shows a nonconducted or membrane action potential generated by this idealized in silico human atrial myocyte. Fig. 1 B shows the associated or underlying inwardly rectifying K+ current. Note that at rest (or in diastole), this current has a steady outward level, but that during the action potential this current is reduced dramatically and in a nonlinear fashion (cf, Giles and Noble, 2016). The electrogenic Na+/K+ pump current is shown in Fig. 1 C. Two components are noteworthy: (1) there is a maintained or steady electrogenic outward current even in diastole, and (2) the action potential waveform results in an additional outward pump current due to the intrinsic voltage dependence of this integral membrane protein. For completeness, the Na+/Ca2+ exchanger current is shown in Fig. 1 D. In this figure, each of the currents is expressed in terms of the respective current density (A/F). As a result, these values can be related directly to the data (expressed as pA/pF) that are presented in Lu and Hilgemann (2017). However, we note that the action potential and the underlying transmembrane currents in human atrium and mouse ventricular myocytes differ substantially.
Figure 1.
Simulated action potential and currents of a human atrial myocyte. The model of Grandi et al. (2011) was used to calculate the (A) membrane voltage and underlying currents at a pacing rate of 1 Hz. The action potential was triggered by a stimulus current at t = 50 ms. The inward rectifier K+ current is shown in B. Currents through the Na+-K+ pump and Na+-Ca2+ exchanger are displayed in C and D, respectively.
Significant new findings and insights
The results obtained by Lu and Hilgemann (2017) define and clarify the short- and long-term relationships between maintained transmembrane Na+ influx, arising from veratridine-modified (and therefore slowly inactivating or noninactivating) Na+ channels, and related increases in [Na+]i at a physiological temperature of 37°C. In marked distinction to much of the literature concerning the effects of INa-L, these authors find very little or no increase in [Na+]i provided that the Na+/K+ pump is operative at physiological turnover rates and expression levels (see also Cardona et al., 2016). This finding is put in context and rationalized using an appropriate model of the electrogenic Na+/K+ pump coupled to equations for veratridine-modified Na+ channels, together with detailed considerations and deductions about the actual volume of distribution for monovalent ions such as Na+ within an adult ventricular myocyte. The initial or baseline dataset confirms the well-known voltage and [Na+]i dependence of the Na+/K+ pump (De Weer et al., 1988; Hilgemann et al., 2006; Stanley et al., 2015) but also reveals a decline in this electrogenic current that the authors suggest and then demonstrate is caused by a novel and potentially important [Na+]i-dependent inactivation mechanism. Thus, when [Na+]i increases from its baseline value of ∼8 mM, the Na+/K+ pump and corresponding electrogenic current rapidly declines (Fig. 2 in Lu et al., 2016). Lu and Hilgemann (2017) explain this inactivation with reference to a well-established multistep Na+/K+ pump reaction mechanism. This new insight is based mainly on their high-resolution measurements of changes in total myocyte capacitance under conditions where all other ion channel and exchanger-mediated mechanisms have been blocked (see Fig. 4 in Lu and Hilgemann, 2017; and Figs. 2 and 3 in Lu et al., 2016). In aggregate, their primary data and semiquantitative analysis also suggest that this inactivation mechanism is voltage dependent. The novel findings in this paper clarify and further define key aspects of the cellular regulation of [Na+]i under physiological conditions and in important pathophysiological settings (cf, Despa and Bers, 2013; Makielski, 2016). In the mammalian myocardium, these include (a) some of the changes in action potential duration and contractility caused by maintained changes in heart rate (Carmeliet, 2006; Eisner et al., 2009) and (b) the precise electrolyte balance in the myocardium that can regulate the onset and duration of atrial fibrillation and its sensitivity to drug therapies (Grandi et al., 2011). It is also known that in heart failure, and in association with several different cardiomyopathies (including diabetes), the enhanced generation of intracellular reactive oxygen species can markedly augment INa-L, which has been suggested to result in increased [Na+]i (Belardinelli et al., 2015).
Opportunities for additional study and documentation
When these new findings and their potentially important implications are further documented and refined, it will be important to consider several factors. (a) The ventricular myocyte (in particular the mouse ventricular myocyte) has an extensive transverse-axial tubular system (Clark et al., 2001; Fraser et al., 2011; DiFranco et al., 2015). As is the case in skeletal muscle, this microanatomical structure corresponds to a restricted space where both diffusion and expression levels for key ion channels are significantly different from the surface membrane or sarcolemma. An important example of this is the expression of the relatively large and nonlinear K+ conductance that is mainly responsible for the resting potential in the mammalian ventricle (see Fig. 1 B). We note that Lu and Hilgemann (2017) use changes in [K+]o as the main driver or stimulus in their measurements of the Na+/K+ pump. The restricted diffusion in the extracellular space corresponding to the transverse tubule system can result in marked and long-lasting changes in localized [K+]o levels; and, in principle, this could alter the time course of the electrogenic Na+/K+ pump current. Moreover, the fact that these inwardly rectifying K+ channels can act as very sensitive sensors of [K+]o, and that their ion transfer or current voltage relationship is not only nonlinear but exhibits the so-called “crossover phenomenon” when [K+]o changes by as little as 1–2 mM, needs to be taken into account (Aronsen et al., 2015; Weiss, 2015). One way to do this would be to carry out a series of experiments using Purkinje cells from the cardiac conduction system because they are known to have little or no transverse tubule system.
(b) Future studies may also need to more explicitly take into account the presence of distinct isoforms of the Na+/K+ pump α and β subunits (cf, Despa and Bers, 2007; Clancy et al., 2015). Changes in the expression levels of either or both of these could alter important details of the inactivation process, which is a key finding in healthy mouse ventricular myocytes.
(c) Some of the classical literature concerning regulation of the Na+/K+ pump in red blood cells provides convincing evidence that ATP, the main energy source for this electrogenic transport mechanism, is highly localized (Chu et al., 2012). This possibility is addressed to some extent in the experiments in this paper. However, when these data are considered or applied in physiological or some pathophysiological contexts (e.g., the ischemic heart, or ischemia reperfusion), limitations of ATP supply and hence reduced pump activity may need to be considered.
(d) Without doubt, the most significant opportunity and challenge with respect to the data presented in this paper (and its extensive Supplemental material) concerns whether and the extent to which these solid, significant new findings apply to pathophysiological and/or chronic disease settings. In many of these, there is evidence that Na+/K+ pump activity changes and [Na+]i homeostasis is progressively altered; thus, these factors are considered to be important elements of the proarrhythmic and/or hypokinetic substrate (Hilgemann et al., 2006; Despa and Bers, 2013; Clancy et al., 2015; Makielski, 2016).
Overall, our assessment is that the Hilgemann laboratory have provided convincing evidence that there is no simple relationship between either physiological or augmented transmembrane Na+ influx and any measurable change in [Na+]i in healthy adult mouse ventricular myocytes. Rather, their results present a strong case that [Na+]i shows impressive stability as long as the expression levels and turnover rates of the Na+/K+ pump are maintained and [Ca2+]i is unchanged. The second new finding is that the same increases in [Na+]i that are well documented to enhance Na+/K+ pump turnover rate (and hence electrogenic current) can also introduce a relatively rapid, transient inhibition, termed “Na+-dependent inactivation.” Going forward, these findings will be useful and may be essential in contexts far beyond cardiac or CNS electrophysiology. Two important examples of this are as follows. (1) Several papers have identified markedly augmented slowly inactivating Na+ current in settings of sterile inflammation (Ward et al., 2006; Maingret et al., 2008). (2) It is apparent that there is an absolute requirement for the transformed target cells (usually fibroblasts) that are used as the key element in Regenerative Medicine to exhibit precise and long-lasting electrolyte and osmotic stability (Takahashi and Yamanaka, 2006; Takeuchi et al., 2006; Yamanaka, 2012; Jayawardena et al., 2015). Emerging procedures and clinical paradigms based on these transformed fibroblasts must take careful account of established knowledge and new information about [Na+]i, such as the dataset and insights provided in this paper.
Overall, in our view, the Hilgemann group has once again produced innovative, thorough, and comprehensive work that very likely will have long-term significance.
Acknowledgments
The W.R. Giles group receives financial support from the Canadian Institutes for Health Research, and F. Sachse is funded by the National Institutes of Health and the Nora Eccles Treadwell Foundation.
The authors declare no competing financial interests.
Eduardo Ríos served as editor.
References
- Ahern C.A., Payandeh J., Bosmans F., and Chanda B.. 2016. The hitchhiker’s guide to the voltage-gated sodium channel galaxy. J. Gen. Physiol. 147:1–24. 10.1085/jgp.201511492 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aronsen J.M., Swift F., and Sejersted O.M.. 2013. Cardiac sodium transport and excitation-contraction coupling. J. Mol. Cell. Cardiol. 61:11–19. 10.1016/j.yjmcc.2013.06.003 [DOI] [PubMed] [Google Scholar]
- Aronsen J.M., Skogestad J., Lewalle A., Louch W.E., Hougen K., Stokke M.K., Swift F., Niederer S., Smith N.P., Sejersted O.M., and Sjaastad I.. 2015. Hypokalaemia induces Ca2+ overload and Ca2+ waves in ventricular myocytes by reducing Na+,K+-ATPase α2 activity. J. Physiol. 593:1509–1521. 10.1113/jphysiol.2014.279893 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aronson P.S., Boron W.F., and Boulpaep E.M.. 2003. Physiology of membranes. In Medical Physiology. Boron W.F., and Boulpaep E.L., editors. Sanders, Philadelphia: 50–87. [Google Scholar]
- Belardinelli L., Giles W.R., Rajamani S., Karagueuzian H.S., and Shryock J.C.. 2015. Cardiac late Na+ current: proarrhythmic effects, roles in long QT syndromes, and pathological relationship to CaMKII and oxidative stress. Heart Rhythm. 12:440–448. 10.1016/j.hrthm.2014.11.009 [DOI] [PubMed] [Google Scholar]
- Bocchi L., and Vassalle M.. 2008. Characterization of the slowly inactivating sodium current INa2 in canine cardiac single Purkinje cells. Exp. Physiol. 93:347–361. 10.1113/expphysiol.2007.040881 [DOI] [PubMed] [Google Scholar]
- Cardona K., Trenor B., and Giles W.R.. 2016. Change in intracellular Na+ following enhancement of late Na+ current in virtual human ventricular myocytes. PLoS One. 11:e0167060 10.1371/journal.pone.0167060 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carmeliet E. 1992. A fuzzy subsarcolemmal space for intracellular Na+ in cardiac cells? Cardiovasc. Res. 26:433–442. 10.1093/cvr/26.5.433 [DOI] [PubMed] [Google Scholar]
- Carmeliet E. 2006. Action potential duration, rate of stimulation, and intracellular sodium. J. Cardiovasc. Res. 17:S2–S7. [DOI] [PubMed] [Google Scholar]
- Chu H., Puchulu-Campanella E., Galan J.A., Tao W.A., Low P.S., and Hoffman J.F.. 2012. Identification of cytoskeletal elements enclosing the ATP pools that fuel human red blood cell membrane cation pumps. Proc. Natl. Acad. Sci. USA. 109:12794–12799. 10.1073/pnas.1209014109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clancy C.E., Chen-Izu Y., Bers D.M., Belardinelli L., Boyden P.A., Csernoch L., Despa S., Fermini B., Hool L.C., Izu L., et al. 2015. Deranged sodium to sudden death. J. Physiol. 593:1331–1345. 10.1113/jphysiol.2014.281204 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark R.B., and Giles W.G.. 2016. Current-voltage relationship for late Na+ current in adult rat ventricular myocytes. In Na Channels from Phyla to Function. French R.J., and Noskov S., editors. Academic Press, Cambridge, MA: 451–478. 10.1016/bs.ctm.2016.04.002 [DOI] [PubMed] [Google Scholar]
- Clark R.B., Tremblay A., Melnyk P., Allen B.G., Giles W.R., and Fiset C.. 2001. T-tubule localization of the inward-rectifier K+ channel in mouse ventricular myocytes: a role in K+ accumulation. J. Physiol. 537:979–992. 10.1113/jphysiol.2001.012708 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Despa S., and Bers D.M.. 2007. Functional analysis of Na+/K+-ATPase isoform distribution in rat ventricular myocytes. Am. J. Physiol. Cell Physiol. 293:C321–C327. 10.1152/ajpcell.00597.2006 [DOI] [PubMed] [Google Scholar]
- Despa S., and Bers D.M.. 2013. Na+ transport in the normal and failing heart—remember the balance. J. Mol. Cell. Cardiol. 61:2–10. 10.1016/j.yjmcc.2013.04.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Weer P., Gadsby D.C., and Rakowski R.F.. 1988. Voltage dependence of the Na-K pump. Annu. Rev. Physiol. 50:225–241. 10.1146/annurev.ph.50.030188.001301 [DOI] [PubMed] [Google Scholar]
- DiFranco M., Hakimjavadi H., Lingrel J.B., and Heiny J.A.. 2015. Na,K-ATPase α2 activity in mammalian skeletal muscle T-tubules is acutely stimulated by extracellular K+. J. Gen. Physiol. 146:281–294. 10.1085/jgp.201511407 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisner D.A. 1990. The Wellcome prize lecture. Intracellular sodium in cardiac muscle: effects on contraction. Exp. Physiol. 75:437–457. 10.1113/expphysiol.1990.sp003422 [DOI] [PubMed] [Google Scholar]
- Eisner D.A., Dibb K.M., and Trafford A.W.. 2009. The mechanism and significance of the slow changes of ventricular action potential duration following a change of heart rate. Exp. Physiol. 94:520–528. 10.1113/expphysiol.2008.044008 [DOI] [PubMed] [Google Scholar]
- Fraser J.A., Huang C.L.H., and Pedersen T.H.. 2011. Relationships between resting conductances, excitability, and t-system ionic homeostasis in skeletal muscle. J. Gen. Physiol. 138:95–116. 10.1085/jgp.201110617 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giles W.R., and Carmeliet E.E.. 2016. This sodium current may be late, but it is important. Trends Cardiovasc. Med. 26:123–125. 10.1016/j.tcm.2015.06.003 [DOI] [PubMed] [Google Scholar]
- Giles W.R., and Noble D.. 2016. Rigorous phenotyping of cardiac iPSC preparations requires knowledge of their resting potential(s). Biophys. J. 110:278–280. 10.1016/j.bpj.2015.06.070 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grandi E., Pandit S.V., Voigt N., Workman A.J., Dobrev D., Jalife J., and Bers D.M.. 2011. Human atrial action potential and Ca2+ model: sinus rhythm and chronic atrial fibrillation. Circ. Res. 109:1055–1066. 10.1161/CIRCRESAHA.111.253955 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han F., Tucker A.L., Lingrel J.B., Despa S., and Bers D.M.. 2009. Extracellular potassium dependence of the Na+-K+-ATPase in cardiac myocytes: isoform specificity and effect of phospholemman. Am. J. Physiol. Cell Physiol. 297:C699–C705. 10.1152/ajpcell.00063.2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hilgemann D.W., Matsuoka S., Nagel G.A., and Collins A.. 1992. Steady-state and dynamic properties of cardiac sodium-calcium exchange. Sodium-dependent inactivation. J. Gen. Physiol. 100:905–932. 10.1085/jgp.100.6.905 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hilgemann D.W., Yaradanakul A., Wang Y., and Fuster D.. 2006. Molecular control of cardiac sodium homeostasis in health and disease. J. Cardiovasc. Electrophysiol. 17:S47–S56. 10.1111/j.1540-8167.2006.00383.x [DOI] [PubMed] [Google Scholar]
- Jayawardena T.M., Finch E.A., Zhang L., Zhang H., Hodgkinson C.P., Pratt R.E., Rosenberg P.B., Mirotsou M., and Dzau V.J.. 2015. MicroRNA induced cardiac reprogramming in vivo: evidence for mature cardiac myocytes and improved cardiac function. Circ. Res. 116:418–424. 10.1161/CIRCRESAHA.116.304510 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu F.-M., and Hilgemann D.W.. 2017. Na/K pump inactivation, subsarcolemmal Na measurements, and cytoplasmic ion turnover kinetics contradict restricted Na spaces in murine cardiac myocytes. J. Gen. Physiol. 149 10.1085/jgp.201711780 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu F.M., Deisl C., and Hilgemann D.W.. 2016. Profound regulation of Na/K pump activity by transient elevations of cytoplasmic calcium in murine cardiac myocytes. eLife. 5:e19267 10.7554/eLife.19267 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maingret F., Coste B., Padilla F., Clerc N., Crest M., Korogod S.M., and Delmas P.. 2008. Inflammatory mediators increase Nav1.9 current and excitability in nociceptors through a coincident detection mechanism. J. Gen. Physiol. 131:211–225. 10.1085/jgp.200709935 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Makielski J.C. 2016. Late sodium current: a mechanism for angina, heart failure, and arrhythmia. Trends Cardiovasc. Med. 26:115–122. 10.1016/j.tcm.2015.05.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramirez R.J., Sah R., Liu J., Rose R.A., and Backx P.H.. 2011. Intracellular [Na+] modulates synergy between Na+/Ca2+ exchanger and L-type Ca2+ current in cardiac excitation-contraction coupling during action potentials. Basic Res. Cardiol. 106:967–977. 10.1007/s00395-011-0202-z [DOI] [PubMed] [Google Scholar]
- Rang H.P., and Ritchie J.M.. 1968. On the electrogenic sodium pump in mammalian non-myelinated nerve fibres and its activation by various external cations. J. Physiol. 196:183–221. 10.1113/jphysiol.1968.sp008502 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanley C.M., Gagnon D.G., Bernal A., Meyer D.J., Rosenthal J.J., and Artigas P.. 2015. Importance of the voltage dependence of cardiac Na/K ATPase isozymes. Biophys. J. 109:1852–1862. 10.1016/j.bpj.2015.09.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swietach P., Spitzer K.W., and Vaughan-Jones R.D.. 2015. Na+ ions as spatial intracellular messengers for co-ordinating Ca2+ signals during pH heterogeneity in cardiomyocytes. Cardiovasc. Res. 105:171–181. 10.1093/cvr/cvu251 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi K., and Yamanaka S.. 2006. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell. 126:663–676. 10.1016/j.cell.2006.07.024 [DOI] [PubMed] [Google Scholar]
- Takeuchi A., Tatsumi S., Sarai N., Terashima K., Matsuoka S., and Noma A.. 2006. Ionic mechanisms of cardiac cell swelling induced by blocking Na+/K+ pump as revealed by experiments and simulation. J. Gen. Physiol. 128:495–507. 10.1085/jgp.200609646 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verdonck F., Mubagwa K., and Sipido K.R.. 2004. [Na+] in the subsarcolemmal ‘fuzzy’ space and modulation of [Ca2+]i and contraction in cardiac myocytes. Cell Calcium. 35:603–612. 10.1016/j.ceca.2004.01.014 [DOI] [PubMed] [Google Scholar]
- Wallén P., Robertson B., Cangiano L., Löw P., Bhattacharjee A., Kaczmarek L.K., and Grillner S.. 2007. Sodium-dependent potassium channels of a Slack-like subtype contribute to the slow afterhyperpolarization in lamprey spinal neurons. J. Physiol. 585:75–90. 10.1113/jphysiol.2007.138156 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ward C.A., Bazzazi H., Clark R.B., Nygren A., and Giles W.R.. 2006. Actions of emigrated neutrophils on Na+ and K+ currents in rat ventricular myocytes. Prog. Biophys. Mol. Biol. 90:249–269. 10.1016/j.pbiomolbio.2005.07.003 [DOI] [PubMed] [Google Scholar]
- Weiss J.N. 2015. Palpitations, potassium and the pump. J. Physiol. 593:1387–1388. 10.1113/jphysiol.2014.285924 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamanaka S. 2012. Induced pluripotent stem cells: past, present, and future. Cell Stem Cell. 10:678–684. 10.1016/j.stem.2012.05.005 [DOI] [PubMed] [Google Scholar]
- Yang P.C., Song Y., Giles W.R., Horvath B., Chen-Izu Y., Belardinelli L., Rajamani S., and Clancy C.E.. 2015. A computational modelling approach combined with cellular electrophysiology data provides insights into the therapeutic benefit of targeting the late Na+ current. J. Physiol. 593:1429–1442. 10.1113/jphysiol.2014.279554 [DOI] [PMC free article] [PubMed] [Google Scholar]