Abstract
Objective To systematically compare β lactam antibiotics with antibiotics active against atypical pathogens in the management of community acquired pneumonia.
Data sources Medline, Embase, Cochrane register of controlled trials, international conference proceedings, drug registration authorities, and pharmaceutical companies.
Review methods Double blind randomised controlled monotherapy trials comparing β lactam antibiotics with antibiotics active against atypical pathogens in adults with community acquired pneumonia. Primary outcome was failure to achieve clinical cure or improvement.
Results 18 trials totalling 6749 participants were identified, with most patients having mild to moderate community acquired pneumonia. The summary relative risk for treatment failure in all cause community acquired pneumonia showed no advantage of antibiotics active against atypical pathogens over β lactam antibiotics (0.97, 95% confidence interval 0.87 to 1.07). Subgroup analysis was undertaken in those with a specific diagnosis involving atypical pathogens. We found a significantly lower failure rate in patients with Legionella species who were treated with antibiotics active against atypical pathogens (0.40, 0.19 to 0.85). Equivalence was seen for Mycoplasma pneumoniae (0.60, 0.31 to 1.17) and Chlamydia pneumoniae (2.32, 0.67 to 8.03).
Conclusions Evidence is lacking that clinical outcomes are improved by using antibiotics active against atypical pathogens in all cause non-severe community acquired pneumonia. Although such antibiotics were superior in the management of patients later shown to have legionella related pneumonia, this pathogen was rarely responsible for pneumonia within the included trials. β lactam agents should remain the antibiotics of initial choice in adults with non-severe community acquired pneumonia.
Introduction
The optimal antibiotic therapy for community acquired pneumonia remains unclear. One of the barriers to better define treatment is the inability to accurately determine the part that the various micro-organisms play.1 In only a few cases is the causative organism identified, due to the poor yield from routine microbiological tests. Since it was first identified in 1881, Streptococcus pneumoniae has been considered the major cause of community acquired pneumonia.2 Its importance was supported by the reduction in mortality observed after the introduction of sulphonamides3 and later β lactam antibiotics.4
With improvements in diagnostic microbiology, it became apparent that other organisms seemed causative in community acquired pneumonia. Three of the more recently recognised ones (Mycoplasma pneumoniae, Legionella species, and Chlamydia pneumoniae) are now associated with the term atypical pathogen. Their major distinguishing feature is a lack of in vitro response to β lactam and sulphonamide antibiotics, rather than any differences to pneumococcal pneumonia in clinical presentation.5,6
The part that atypical organisms play and the need to provide specific antibiotic coverage for them in community acquired pneumonia is contentious. Recent guidelines vary.1,7-10 The single most important factor in this variance is the failure to produce level 1 evidence on which to base treatment recommendations. We carried out a meta-analysis to compare the efficacy of β lactam antibiotics with antibiotics active against atypical pathogens in adults with community acquired pneumonia to produce the level 1 evidence currently lacking.
Methods
We obtained relevant trials up to December 2003 from the Cochrane central register of controlled trials, Medline, and Embase using the broad search terms “explode pneumonia” and “explode antibiotic-agent” associated with standard limiters aimed to identify controlled trials. In addition we searched abstracts of conference proceedings, contacted registration authorities, searched the reference lists of review articles and retrieved studies, and contacted pharmaceutical companies that had carried out clinical trials on antibiotics active against atypical pathogens. We included studies regardless of date, language, or publication status.
Inclusion criteria were randomised double blind monotherapy trials comparing antibiotics active against atypical pathogens (fluoroquinolones, macrolides, and ketolides) with any β lactam antibiotic (penicillins and cephalosporins) in radiographically confirmed cases of community acquired pneumonia. The primary outcome of interest was failure to achieve clinical cure or improvement, as defined by each study. We excluded open label, non-comparative, and non-randomised studies owing to potential important bias from the subjective nature of the primary outcome variable. We also excluded studies with the option of adding an antibiotic active against atypical pathogens to β lactam therapy.
For the primary analysis we used the intention to treat or modified intention to treat populations (those with confirmed community acquired pneumonia who had received at least one dose of study drug). When both early and late end points were available, we used the earlier follow up time as the test of cure. We also reviewed the clinically evaluable per protocol population as well as all cause mortality. The criterion within each study report was used to define atypical pathogen diagnoses.
Data abstraction and quality
Two reviewers independently screened identified titles and abstracts without blinding to authorship or journal. Potentially relevant studies were obtained and the full text examined. All studies had complete blinding of investigators, participants, and outcome assessors thereby reducing the possibility of selection bias and detection bias. When important data were not reported, we contacted the author or pharmaceutical company that sponsored the study. Discrepancies between reviewers were resolved by discussion.
Study characteristics and quantitative data synthesis
We classified the studies by the name of the antibiotic active against atypical pathogens. Information was gathered for each study on participating countries, number of study sites, period (years) of study, study size, mean age, whether the intention to treat population was used for analysis, and the severity of the pneumonia (if available). Analysis was performed using meta-analytic software in Revman 4.2.3.
We expressed the results for the dichotomous outcome of failure to achieve clinical cure or improvement as relative risks with 95% confidence intervals. As there was no significant heterogeneity we pooled the data using the fixed effects model. Results were almost identical with the random effect model. We used a χ2 test to analyse heterogeneity, and we considered a P value of 0.05 or less as significant. Subgroup analysis was undertaken on participants with atypical pathogen diagnoses.
Results
We screened over 2000 studies and retrieved over 100 potentially eligible ones. We identified 20 studies that met our inclusion criteria.11-26 Four of the studies (30% of participants) were unpublished as at November 2004 and were obtained from the sponsoring pharmaceutical companies either directly or secondary to the finding of a conference proceedings (see bmj.com). Two small studies concerned children.11,12 We decided to exclude these from our meta-analysis given the distinct clinical context. Three blinded studies were also excluded, as the protocol included the option of adding an agent active against atypical pathogens to the β lactam therapy.27-29
The 18 included trials were carried out in more than 30 countries between 1980 and 2000 and included 6749 analysable participants (table). Overall, the trials used nine different fluoroquinolones, two macrolides, and one ketolide. Most study drugs were given orally, with only two of the earlier studies using intravenous therapy initially. Most of the studies listed specific exclusion criteria; a standard feature in most trials of community acquired pneumonia sponsored by pharmaceutical companies. Common exclusions included the requirement for parenteral antimicrobials at study entry in trials of oral antibiotics, hospital acquired or aspiration pneumonia, immunocompromised patients, and major hepatic or renal dysfunction. The specific inclusion and exclusion criteria resulted in participants who were younger and with a better prognostic risk profile than observational pneumonia cohorts.30
Table 1.
Characteristics of included studies comparing β lactam antibiotics with antibiotics active against atypical pathogens in patients with community acquired pneumonia
| Study drug | Reference, country | No of sites; year | Participants | Intention to treat | Intervention | Time of outcome assessment |
|---|---|---|---|---|---|---|
| Azithromycin | Kinasewitz 199113, United States | 28; pre-1990 | Adults (mean age 41); 119 randomised; 71 clinically analysable | Not available | Azithromycin for 5 days versus cefaclor for 10 days | Days 10-13 |
| Ciprofloxacin | Johnson 199614, United States | 19; 1990-3 | Adults (mean age 59); about 90% had community acquired pneumonia; 217 randomised | 217 patients | Intravenous ciprofloxacin versus intravenous ceftriaxone for 3-10 days | 0-7 days after end of treatment |
| Erythromycin | Macfarlane 198315, United Kingdom | One; 1980-1 | Adults (mean age 50); 122 randomised | 91 patients* | Intravenous erythromycin followed by oral erythromycin versus intravenous ampicillin followed by oral amoxicillin for 7 days | Day 13 |
| Gatifloxacin | Lode 200416, multinational | 73; 1997-8 | Adults (mean age 50); 462 randomised | 456 patients* | Gatifloxacin versus combined amoxicillin and clavulanic acid for 5-10 days | 1-3 days after end of treatment |
| Gatifloxacin | Study report 2000 (KF5501/16; see bmj.com), multinational | 123; 1999-2000 | Adults (mean age 52); 1061 randomised; 34% had severe community acquired pneumonia | 1051 patients* | Gatifloxacin versus amoxicillin for 7-10 days | Days 10-17 |
| Gemifloxacin | Leophonte 200417, multinational | 102; 1998-9 | Adults (mean age 54); 324 randomised; 16% had severe community acquired pneumonia | 320 patients* | Gemifloxacin versus combined amoxicillin and clavulanic acid for 7-10 days | Days 12-14 |
| Grepafloxacin | O'Doherty 199718, United Kingdom and Eire | 43; 1992-3 | Adults (mean age 55); 264 randomised; 225 clinically analysable | Not available | Grepafloxacin versus amoxicillin for 7-10 days | Days 28-42 |
| Grepafloxacin | Study report 1997 (106-92-201; see bmj.com); United States, United Kingdom, Eire | 83; 1992-6 | Adults; (mean age unavailable); 475 randomised | 475 patients | Grepafloxacin versus cefaclor for 7-10 days | Days 28-42 |
| Grepafloxacin | Study report 1999 (GFXB 3004; see bmj.com); multinational | 70; 1996-8 | Adults (median age 52); 370 randomised | 370 patients | Grepafloxacin versus amoxicillin for 10 days | 1-3 days after end of treatment |
| Levofloxacin | Study report 1994 (DR-3355/E05; see bmj.com); Europe | 40; 1992-3 | Adults (median age 61); 140 randomised, 16% had severe community acquired pneumonia | 123 patients* | Levofloxacin (two different doses) versus amoxicillin for 7-14 days | 0-2 days after end of treatment |
| Levofloxacin | Carbon 199919; multinational | 50; pre-1996 | Adults (mean age 41); 516 randomised | 516 patients | Levofloxacin (two different doses) versus combined amoxicillin and clavulanic acid for 7-10 days | 2-5 days after end of treatment |
| Moxifloxacin | Petitpretz 200120; multinational | 84; 1997-8 | Adults (mean age 51); 411 randomised | 408 patients* | Moxifloxacin versus amoxicillin for 10 days | 3-5 days after end of treatment |
| Sparfloxacin or erythromycin | Lode 199521; multinational | 124; 1990-2 | Adults (mean age 54); 808 randomised | 808 patients | Sparfloxacin versus erythromycin versus combined amoxicillin and clavulanic acid for 7-14 days; randomised 2:1:1 | Days 7-14 |
| Sparfloxacin | Aubier 199822; France, South Africa, Switzerland | 55; 1991-2 | Adults (mean age 42); 329 randomised | 329 patients | Sparfloxacin versus amoxicillin for 10-14 days | Days 14-21 |
| Sparfloxacin | Donowitz 199723; United States | 74; 1992-5 | Adults (mean age 49); 330 randomised | 330 patients | Sparfloxacin versus cefaclor for 10 days | Days 17-23 |
| Telithromycin | Hagberg 200224; multinational | 59; 1998-9 | Adults (median age 42); 404 randomised; 27% had prognostic severity index score ≥3) | 404 patients | Telithromycin versus amoxicillin for 10 days | Days 17-21 |
| Temafloxacin | Carbon 199225; France | 27; 1989-90 | Adults (mean age 55); 246 randomised | 243 patients* | Temafloxacin versus amoxicillin for 10 days | 1-3 days after end of treatment |
| Trovafloxacin | Tremolieres 199826; multinational | 44; 1995-6 | Adults (mean age 52); 342 randomised; 312 clinically analysable | Not available | Trovafloxacin versus amoxicillin for 7-10 days | Days 7-10 |
Modified intention to treat analysis.
Primary outcome of interest
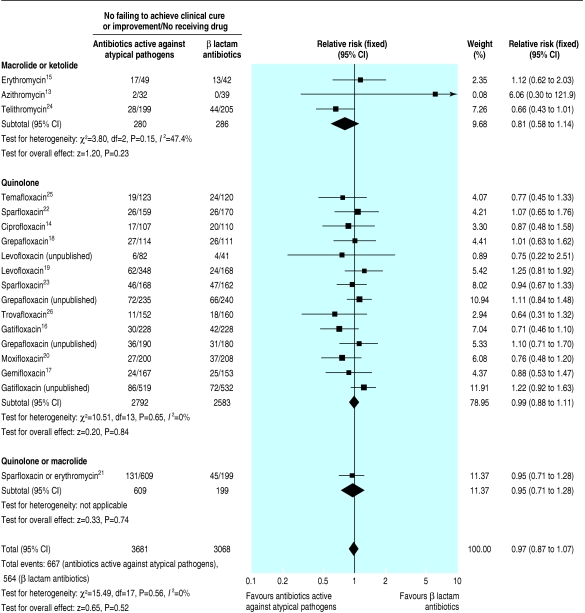
All trials reported the proportion of patients who failed to achieve clinical cure or improvement, an overall rate of 18%. We found no significant difference between treatments in any study or significant heterogeneity between studies. From a combined analysis of the studies (fig 1) we found no evidence that antibiotics active against atypical pathogens were superior to β lactam antibiotics (relative risk 0.97, 95% confidence interval 0.87 to 1.07). The same conclusion was drawn from separate analyses of the studies on macrolides and ketolides (0.81, 0.58 to 1.14) and fluoroquinolones (0.99, 0.88 to 1.11). We also compared the relative risk of the 10 published studies on fluoroquinolones (0.90, 0.77 to 1.04) with the four unpublished studies on fluoroquinolones (1.15, 0.96 to 1.37).
Fig 1.
Number of patients failing to achieve clinical cure or improvement with β lactam antibiotics compared with antibiotics active against atypical pathogens in all cause community acquired pneumonia
We analysed the data on all cause mortality separately; 130 deaths were reported (mortality 1.9%). We observed no differences in mortality between the study arms (relative risk 1.20, 0.84 to 1.71). This low mortality is in keeping with most patients having mild to moderate (non-severe) community acquired pneumonia.
Fifteen of the trials provided data on either the intention to treat population or the modified intention to treat population. Three studies reported only on the clinically evaluable population, although the overall dropout rate was less than 17%.13,18,26 As all the studies were blinded, we did not consider the lack of intention to treat data in these three studies as critical and we therefore included the data. The treatment effect (relative risk 0.97) was not altered when we excluded trials that did not use an intention to treat or modified intention to treat method. Similar results (0.93, 0.81 to 1.06) were obtained from a separate analysis on the clinically evaluable per protocol population (n = 5639), with the failure to achieve clinical cure or improvement reduced to 13%.
We decided to include one study (weighting 3.3%) where a small proportion (< 10%) of patients had nosocomial pneumonia.14 The result was not altered (relative risk 0.97) when we carried out a sensitivity analysis with these data excluded. The time point for assessment varied between studies. Sixteen of the studies had visits for end of treatment or for test of cure within 10 days of completion of the study drug, whereas two studies assessed patients at the end of follow up. We found no evidence of a secular trend of decreasing treatment effect in the β lactam arms, suggesting that there was no impact on treatment effect from the worldwide trend for increasing pneumococcal resistance.
Subgroup analysis
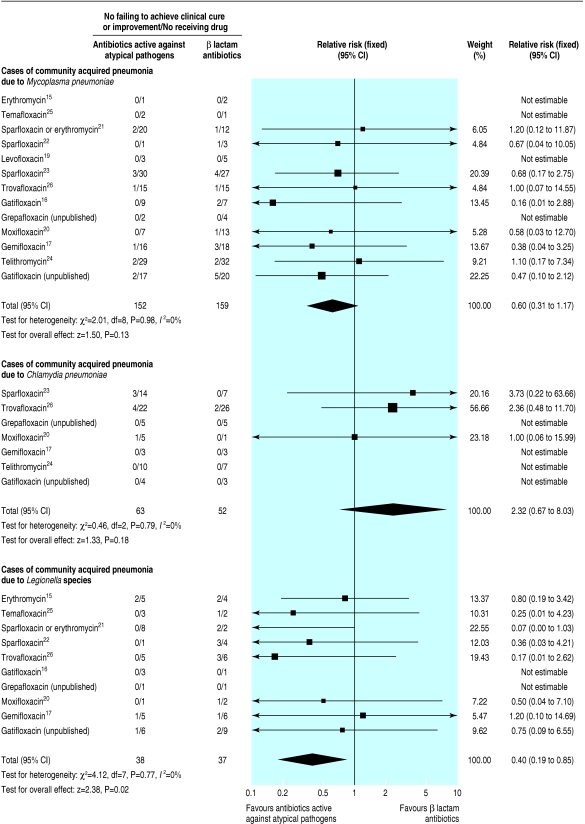
Overall, 311 patients (13 studies) were diagnosed as having M pneumoniae, 115 (seven studies) as having C pneumoniae, and 75 (10 studies) as having Legionella species (fig 2). We found no significant treatment effect in patients with M pneumoniae (relative risk 0.60, 0.31 to 1.17) or C pneumoniae (2.32, 0.67 to 8.03). In contrast, the failure rate from antibiotics active against atypical pathogens in patients with legionella was statistically lower (0.40, 0.19 to 0.85).
Fig 2.
Number of patients failing to achieve clinical cure or improvement with β lactam antibiotics compared with antibiotics active against atypical pathogens in confirmed cases of community acquired pneumonia related to Mycoplasma pneumoniae, Chlamydia pneumoniae, and Legionella species
Discussion
Data from our meta-analysis do not support the need for antibiotics that possess specific activity against atypical pathogens in the initial management of adults with mild to moderate community acquired pneumonia. A major strength of our study was the inclusion of only randomised prospective double blinded studies, thus appreciably avoiding bias. Although the patients were mostly recruited from hospital settings, the use of orally based regimens by many of the studies resulted in fewer patients with severe pneumonia. This is reflected by the low mortality. We are therefore not able to provide any guidance for the management of severe community acquired pneumonia, where the standard of care is currently intravenous antibiotic therapy.7
Despite this, our results are valuable in guiding the management of many adults with community acquired pneumonia. Although mortality is far more likely to occur in those with severe pneumonia as classified by prognostic indices for community acquired pneumonia, a large proportion (42% in the US derivation study30 and 53% in the international derivation study31) of admissions to hospital are patients with mild to moderate pneumonia (prognostic index scores 1-3). Mortality in patients admitted with prognostic index scores between 1 and 3 is 1.5%,30 similar to the rate seen in our study. In addition, most patients who remain in the primary care setting also have mild to moderate pneumonia.
We believe that our study has sufficient power to address adequately the study question, as reflected by the narrow confidence interval for relative risk (0.97, 0.87 to 1.07). If a relative risk of 0.97 was the true magnitude of benefit from using antibiotics active against atypical pathogens compared with β lactam antibiotics, then the number needed to treat of 185 for preventing one extra failure would not be seen as valuable by most respiratory physicians. This is particularly true when clinical failure may only indicate a slower time to resolution of symptoms, rather than severe morbidity or mortality.
The antibiotics active against atypical pathogens we reviewed were fluoroquinolones, macrolides, and ketolides. These agents have excellent in vitro activity against each of the three atypical organisms considered to cause community acquired pneumonia, with most having good coverage against S pneumoniae. The studies compared β lactam antibiotics in a variety of forms (cephalosporins, narrow spectrum penicillins, and combinations of β lactam and β lactamase inhibitor), which all lack activity against atypical pathogens. We chose to include studies on antibiotics no longer in general usage. Grepafloxacin, sparfloxacin, trovafloxacin, and temifloxacin are no longer actively marketed owing to their side effect profiles rather than lack of efficacy. The clinical response rates for these fluoroquinolones were no different from those currently approved by the US Food and Drug Administration (levofloxacin, gatifloxacin, moxifloxacin, and gemifloxacin).
In addition to looking at the results for all cause community acquired pneumonia, we reviewed specific therapeutic responses in patients whose pneumonia was considered to be related to the three atypical organisms. In total, 501 of the patients enrolled in these studies were diagnosed as having atypical pathogens. Each study defined the specific criteria on which these diagnoses were made although there was variability as to whether all, some, or none of the atypical organisms were sort after. Serology, the basis for nearly all of the diagnoses of atypical pathogens in the included studies, has the major drawbacks of variable sensitivity and specificity.32 As a result the diagnoses are uncertain, highlighting one of the reasons that the role of atypical pathogens has remained controversial. When antibiotics active against atypical pathogens were used, only pneumonia related to legionella showed a statistically significant improvement in outcome. This organism is uncommon (< 3%) in mild to moderate community acquired pneumonia.1,33 Our data suggest that coverage for the possibility of legionella is not warranted in the initial management of non-severe community acquired pneumonia.
Given the lack of in vitro activity of β lactam antibiotics, our finding of similar outcomes for M pneumoniae and C pneumoniae was unanticipated. Although confidence intervals were wide, we do not believe that lack of power necessarily explains these results, as the number of diagnoses were not small (311 and 115 patients for M pneumoniae and C pneumoniae, respectively), and failure rates were low (< 10%). Despite legionella being diagnosed on fewer occasions, differences in outcome were readily apparent, in contrast to M pneumoniae and C pneumoniae. Alternative explanations for our findings include incorrect diagnoses, self limiting infections, or asymptomatic infections associated with a coinfecting pathogen responsive to β lactam antibiotics. Each of these explanations is conceivable given the diagnostic difficulties, the reported high rate of atypical coinfections,34 and the ability to culture C pneumoniae from asymptomatic individuals.35-37 Another explanation is that using a specific time point to assess clinical outcome might have missed a more rapid response in one arm. Data on time to resolution of symptoms or length of hospital stay were, however, not available to us. Based on the low failure rates in both treatment arms and the lack of a significant treatment difference within the included studies, we suggest that the role of M pneumoniae and C pneumoniae in community acquired pneumonia may have been overplayed.
Several questions need to be asked before our findings can be extrapolated. Firstly, were the patients (over 6700) in our meta-analysis similar to cohorts with pneumonia not entered into clinical trials? As indicated there is a trend to inclusion of younger patients than seen in prospectively enrolled observational cohorts. In addition, because many of the studies used oral therapy, most of the patients had non-severe community acquired pneumonia. Therefore, although our data are only applicable to a subset of patients with community acquired pneumonia, this subset makes up a major proportion of patients with pneumonia. Our findings are at variance with the American Thoracic Society guidelines, which state that all populations with community acquired pneumonia should be treated for possible infection with atypical pathogens7; but our findings agree with the British Thoracic Society guidelines.1 The British guidelines consider S pneumoniae the most important target of initial antibiotic therapy and state that a policy aiming to always cover the atypical pathogens is inappropriate.
Although some may see the inclusion of unpublished material as a limitation in our study, we believe that it is a notable strength. It is unsurprising that four studies sponsored by the pharmaceutical industry (30% of the included patients) have not been published, given their rather unflattering treatment effect compared with the older, often out of patent, β lactam antibiotics.
Our results provide the best level of evidence currently available addressing the necessity for coverage of atypical pathogens in the initial management of community acquired pneumonia. In 2003, Oosterheert et al contended that data did not support the routine addition of macrolides or monotherapy with fluoroquinolones as standard care for patients admitted to hospital with community acquired pneumonia.38 The Swedish Infectious Diseases Society also suggested that the role of atypical pathogens might have been overestimated within current guidelines.39 Our results strongly support both these assertions. We have reviewed only initial therapy in this meta-analysis and emphasise that antibiotic treatment should always be reassessed in any patient who shows signs of deterioration or failure to improve. Guidelines based on retrospective studies40,41 should always be deemed inferior to level 1 evidence because it is impossible to control for the reasons why certain antibiotics are prescribed. The British Thoracic Society has astutely summed up the current situation: “There is clearly variation in medical practice with regard to licensing, availability, choice, dose, route of administration, and duration of treatment which is more a reflection of local custom and practice than robust scientific evidence.”1
What is already known on this topic
Recommendations for antibiotic treatment of community acquired pneumonia are based on expert opinion and in vitro data rather than data from clinical trials
North American and European guidelines vary as to whether initial antibiotic regimens should always include coverage for the atypical organisms Mycoplasma pneumoniae, Chlamydia pneumoniae, and Legionella species
What this study adds
Evidence is lacking that antibiotics active against atypical pathogens improve clinical outcomes in adults with non-severe community acquired pneumonia
β lactam antibiotics were not inferior to antibiotics active against atypical pathogens in patients with community acquired pneumonia related to Mycoplasma pneumoniae and Chlamydia pneumoniae
β lactam agents should remain the antibiotics of choice in the initial management of mild to moderate community acquired pneumonia, as currently advocated by the British Thoracic Society
In conclusion, our meta-analysis provides level 1 evidence contrary to the current American Thoracic Society guidelines for patients with community acquired pneumonia.7 Although we have confirmed the importance of specific therapy when legionella is confirmed, evidence is lacking that specific therapy is required for M pneumoniae or C pneumoniae. β lactam antibiotics should remain the initial choice in the management of non-severe community acquired pneumonia in adults given our results, the benefits of using narrower spectrum agents, and the cost advantages.
Supplementary Material
 Details of the four unpublished studies are on bmj.com
Details of the four unpublished studies are on bmj.com
We thank the pharmaceutical companies and study authors who provided access to unpublished study reports and assisted with data clarification.
Contributors: GDM conceived the study. GDM and MRO were involved in all stages of data collection, and data analysis. GDM and BA prepared the manuscript. GDM is guarantor.
Funding: MRO was funded by a summer studentship from the Waikato District health board.
Competing interests: GDM has received funding for research from Abbott, AstraZeneca, AventisPharma, Boehringer Ingelheim, GlaxoWellcome, ICOS, Merck&Co, Pfizer, and Roche. He has also been reimbursed by AventisPharma, Bristol-Myers Squibb, GlaxoWellcome, and Merck&Co for attending conferences. BA has received funding for research and conference attendance from the Future Forum, an educational cardiovascular forum funded by AstraZeneca.
Ethical approval: Not required.
References
- 1.British Thoracic Society Standards of Care Committee. BTS guidelines for the management of community acquired pneumonia in adults. Thorax 2001;56(suppl 4): IV1-64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Watson DA, Musher DM, Jacobson JW, Verhoef J. A brief history of the pneumococcus in biomedical research: a panoply of scientific discovery. Clin Infect Dis 1993;17: 913-24. [DOI] [PubMed] [Google Scholar]
- 3.Evans GM, Gaisford WF. Treatment of pneumonia with 2-(ρ-aminobenzenesulphonamido) pyridine. Lancet 1938;2: 14-9. [Google Scholar]
- 4.Tillett WS, Cambier MJ, McCormack JE. The treatment of lobar pneumonia and pneumococcal empyema with penicillin. Bull NY Acad Sci 1944;20: 142-78. [PMC free article] [PubMed] [Google Scholar]
- 5.Farr BW, Kaiser DL, Harrison BDW, Connolly CK. Prediction of microbial aetiology at admission to hospital for pneumonia from the presenting clinical features. Thorax 1989;44: 1031-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.File TM, Jr, Plouffe JF, Breiman RF, Skelton SK. Clinical characteristics of Chlamydia pneumoniae infection as the sole cause of community-acquired pneumonia. Clin Infect Dis 1999;29: 426-8. [DOI] [PubMed] [Google Scholar]
- 7.Niederman MS, Mandell LA, Anzueto A, Bass JB, Broughton WA, Campbell GD, et al. Guidelines for the management of adults with community-acquired pneumonia. Diagnosis, assessment of severity, antimicrobial therapy and prevention. Am J Respir Crit Care Med 2001;163: 1730-54. [DOI] [PubMed] [Google Scholar]
- 8.ERS Taskforce Report. Guidelines for the management of adults with community-acquired lower respiratory tract infections. Eur Resp J 1998;11: 986-91. [DOI] [PubMed] [Google Scholar]
- 9.Bartlett JG, Dowell SF, Mandell LA, File TM, Musher DM, Fine MJ. Practice guidelines for the management of community-acquired pneumonia in adults. Clin Infect Dis 2000;31: 347-82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mandell LA, Marrie TJ, Grossman RF, Chow AW, Hyland RH. Canadian guidelines for the initial management of community-acquired pneumonia: an evidence based update by the Canadian Infectious Diseases Society and the Canadian Thoracic Society. Clin Infect Dis 2000;31: 383-421. [DOI] [PubMed] [Google Scholar]
- 11.Harris JA, Kolohathis A, Campbell M, Cassell GH, Hammerschlag MR. Safety and efficacy of azithromycin in the treatment of community-acquired pneumonia in children. Pediatr Infect Dis J 1998;17: 865-71. [DOI] [PubMed] [Google Scholar]
- 12.Ferwerda A, Moll HA, Hop WCJ, Kouwenberg JM, Gi CVTP, Robben SGF, et al. Efficacy, safety and tolerability of 3 day azithromycin versus 10 day co-amoxiclav in the treatment of children with acute lower respiratory tract infections. J Antimicrob Chemother 2001;47: 441-6. [DOI] [PubMed] [Google Scholar]
- 13.Kinasewitz G, Wood RG. Azithromycin versus cefaclor in the treatment of acute bacterial pneumonia. Eur J Clin Microbiol Infect Dis 1991;10: 872-7. [DOI] [PubMed] [Google Scholar]
- 14.Johnson RH, Levine S, Traub SL, Echols RM, Haverstock D, Arnold E, et al. Sequential intravenous/oral ciprofloxacin compared with parenteral ceftriaxone in the treatment of hospitalised patients with community-acquired pneumonia Infectious. Dis Clin Pract 1996;5: 265-72. [Google Scholar]
- 15.Macfarlane JT, Finch RG, Ward MJ, Rose DH. Erythromycin compared with a combination of ampicillin and amoxycillin as initial therapy for adults with pneumonia including Legionnaires disease. J Infect 1983;7: 111-7. [DOI] [PubMed] [Google Scholar]
- 16.Lode H, Magyar P, Muir JF, Loos U, Kleutgens K, on behalf of the International Gatifloxacin Study Group. Once-daily oral gatifloxacin vs. three-times-daily co-amoxiclav in the treatment of patients with community-acquired pneumonia. Clin Microbiol Infect 2004;10: 512-20. [DOI] [PubMed] [Google Scholar]
- 17.Leophonte P, File T, Feldman C. Gemifloxacin once daily for 7 days compared to amoxicillin/clavulanic acid thrice daily for 10 days for the treatment of community-acquired pneumonia of suspected pneumococcal origin. Respir Med 2004:198: 708-20. [DOI] [PubMed] [Google Scholar]
- 18.O'Doherty B, Dutchman DA, Pettit R, Maroli A. Randomised, double-blind, comparative study of grepafloxacin and amoxycillin in the treatment of patients with community-acquired pneumonia. J Antimicrob Chemother 1997;40(suppl A): 73-81. [DOI] [PubMed] [Google Scholar]
- 19.Carbon C, Ariza H, Rabie W, Salvarezza C, Elkharrat D, Rangaraj M, et al. Comparative study of levofloxacin and amoxycillin/clavulanic acid in adults with mild-to moderate community acquired pneumonia. Clin Microbiol Infect 1999;5: 724-32. [Google Scholar]
- 20.Petitpretz PA, Arvis P, Marel M, Moita J, Urueta J, and the CAP5 Moxifloxacin Study Group. Oral moxifloxacin vs high-dosage amoxicillin in the treatment of mild-to-moderate, community-acquired, suspected pneumococcal pneumonia in adults. Chest 2001;119: 185-95. [DOI] [PubMed] [Google Scholar]
- 21.Lode HG, Garau J, Grassi C, Hosie J, Huchon G, Legakis N, et al. Treatment of community-acquired pneumonia: a randomized comparison of sparfloxacin, amoxycillin-clavulanic acid and erythromycin. Eur Respir J 1995;8: 1999-2007. [DOI] [PubMed] [Google Scholar]
- 22.Aubier M, Verster R, Regamey C, Geslin P, Vercken J. Once daily sparfloxacin versus high dosage amoxicillin in the treatment of community-acquired pneumococcal pneumonia in adults. Clin Infect Dis 1998;26: 1312-20. [DOI] [PubMed] [Google Scholar]
- 23.Donowitz GRB, Salisbury ML, Harman JP, Tipping CP, Urick DM, Talbot AE. Sparfloxacin versus cefaclor in the treatment of patients with community-acquired pneumonia: a randomized, double-masked, comparative, multicenter study. Clin Ther 1997;19: 936-53. [DOI] [PubMed] [Google Scholar]
- 24.Hagberg L, Torres A, van Rensburg D, Leroy B, Rangaraju M, Ruuth E. Efficacy and tolerability of once-daily telithromycin compared with high-dose amoxicillin for treatment of community-acquired pneumonia. Infection 2002;30: 378-86. [DOI] [PubMed] [Google Scholar]
- 25.Carbon C, Leophonte P, Petitpretz P, Chauvin J, Hazebroucq J. Efficacy and safety of temafloxacin versus those of amoxicillin in hospitalized adults with community-acquired pneumonia. Antimicrob Agents Chemother 1992;36: 833-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Trémolières F, de Kock F, Pluck N, Daniel R. Trovafloxacin versus high-dose amoxicillin (1 g three times daily) in the treatment of community-acquired bacterial pneumonia. Eur J Clin Microbiol Infect Dis 1998;17: 447-53. [DOI] [PubMed] [Google Scholar]
- 27.Fogarty C, Dowell ME, Ellison T, Vrooman PS, White BJ, Mayer H. Treating community-acquired pneumonia in hospitalised patients: gatifloxacin vs ceftriaxone/clarithromycin. J Respir Dis 1999;20: 11(suppl):60-9. [Google Scholar]
- 28.Food and Drug Administration new drug application file. Medical Officer review of CAP study 154-111. www.fda.gov/cder/foi/nda/97/020760a.htm (accessed 20 Nov 2004).
- 29.Food and Drug Administration new drug application file. Medical Officer review of CAP study 154-112. www.fda.gov/cder/foi/nda/97/020760a.htm (accessed 20 Nov 2004).
- 30.Fine MJ, Auble TE, Yealy DM, Hanusa BH, Weissfeld LA, Singer DE, et al. A prediction rule to identify low-risk patients with community-acquired pneumonia. N Engl J Med 1997;336: 243-50. [DOI] [PubMed] [Google Scholar]
- 31.Lim WS, van der Eerden MM, Laing R, Boersma WG, Karalus N, Town GI, et al. Defining community acquired pneumonia severity on presentation to hospital: an international derivation and validation study. Thorax 2003;58: 377-82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ferraro MJ. Limitations in laboratory diagnosis of community-acquired pneumonia and acute exacerbations of chronic bronchitis. Hosp Med 1997;33 (suppl 2): 14-7. [Google Scholar]
- 33.Yu VL, Greenberg RN, Zadeikis N, Stout JE, Khashab MM, Olson WH, et al. Levofloxacin efficacy in the treatment of community-acquired legionellosis. Chest 2004;125: 2135-9. [DOI] [PubMed] [Google Scholar]
- 34.Lieberman D, Schlaeffer F, Boldur I, Lieberman D, Horowitz S, Friedman MG, et al. Multiple pathogens in adult patients admitted with community-acquired pneumonia: a one-year prospective study of 346 consecutive patients. Thorax 1996;51: 179-84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.File TM Jr, Garau J, Blasi F, Chidiac C, Klugman K, Lode H, et al. Guidelines for empiric antimicrobial prescribing in community-acquired pneumonia. Chest 2004;125: 1888-901. [DOI] [PubMed] [Google Scholar]
- 36.Kauppinen MT, Saikku P, Kujala P, Herva E, Syrjala H. Clinical picture of community acquired Chlamydia pneumoniae pneumonia requiring hospital treatment: a comparison between chlamydial and pneumococcal pneumonia. Thorax 1996;51: 185-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mundy LM, Oldach D, Auwaerter PG, Gaydos CA, Moore RD, Bartlett JG, et al. Implications for macrolide treatment in community-acquired pneumonia. Hopkins CAP Team. Chest 1998;113: 1201-6. [DOI] [PubMed] [Google Scholar]
- 38.Oosterheert JJ, Bonten MJM, Hak E, Schneider MME, Hoepelman IM. How good is the evidence for the recommended empirical antimicrobial treatment of patients hospitalized because of community-acquired pneumonia? A systematic review. J Antimicrob Chemother 2003;52: 555-63. [DOI] [PubMed] [Google Scholar]
- 39.Hedlund J, Ortqvist A. Management of patients with community-acquired pneumonia treated in hospital in Sweden. Scand J Infect Dis 2002;34: 887-92. [DOI] [PubMed] [Google Scholar]
- 40.Gleason PP, Meehan TP, Fine JM, Galusha DH, Fine MJ. Associations between initial antimicrobial therapy and medical outcomes for hospitalized elderly patients with pneumonia. Arch Intern Med 1999;159: 2562-72. [DOI] [PubMed] [Google Scholar]
- 41.Houck PM, MacLehose RF, Niederman MS, Lowery JK. Empiric antibiotic therapy and mortality among Medicare pneumonia inpatients in 10 western states: 1993, 1995, and 1997. Chest 2001;119: 1420-6. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.