Abstract
Although new heptatis C virus treatments have increased efficacy and improved safety profiles, they also come with risk. We describe a 66-year-old white man with Child-Pugh A cirrhosis secondary to heptatis C virus genotype 3, who suffered from an acute kidney injury after treatment with sofosbuvir and daclatasvir. Kidney biopsy demonstrated evidence of acute tubular interstitial nephritis consistent with a drug reaction. A trial of steroid therapy was effective, and his creatinine continues to improve significantly. Our case is the first report in the literature that highlights potentially serious interstitial nephritis associated with sofosbuvir and daclatasvir use.
Introduction
Acute interstitial nephritis (AIN) is a frequent cause of acute kidney failure, occurring in 15–27% of renal biopsies performed for this indication.1 This under-reported condition is frequently clinically diagnosed by noting improvement in renal function after cessation of possible offending medications. It is only after failure to improve that patients undergo kidney biopsy and the diagnosis is confirmed. New direct-acting antiviral agents have generally been well received as safe and efficacious therapies for hepatitis C virus (HCV) infection; however, physicians should be vigilant for drug-induced kidney injury at all times, especially when using newer agents because adverse effects may not be known yet.
Case Report
A 66-year-old white man with Child-Pugh A cirrhosis secondary to HCV genotype 3 on a background of atrial fibrillation (AF), hypertension, and gout was found to have an acute kidney injury during treatment. For several years, his gout had been stable on allopurinol, perindopril, and atenolol with reasonable blood pressure control and rate-controlled AF. His weight was 89.6 kg, and his serum creatinine ranged from 50 µmol/L to 63 µmol/L during the past few years. There was no significant family or social history.
His liver fibroscan demonstrated median stiffness (66.4 kpa) consistent with cirrhosis. In light of the HCV genotype 3 infection treatment naivety and compensation, he was started on sofosbuvir-daclatasvir for a total of 24 weeks. His viral load was <12 IU/mL at his 4-week review, and he was tolerating his medications well, troubled only by fatigue that was thought to be related to his disease.
At week 22 of treatment, his creatinine rose to 91 µmol/L, with complaints of worsened fatigue but no evidence of liver decompensation on examination. Treatment was continued for its full course of 24 weeks. A repeat test requested 2 weeks prior to his sustained virological response at 12 weeks (SVR12) showed a creatinine of 284 µmol/L. On his SVR12 visit, his creatinine had risen to 377 µmol/L and he was immediately admitted to hospital for further evaluation.
On admission, he complained of generalized joint pain, anorexia, and persistent fatigue. He denied eye or infective symptoms. Medication review revealed no drug use other than previously described, and he denied using nonsteroidal anti-inflammatory agents, antibiotics, and over-the-counter medications or supplements. Physical examination revealed blood pressure 150/70 mm Hg and an asymptomatic diffuse maculopapular rash over the central face extending over the malar region, nose, and forehead. The remainder of his examination was unremarkable. Relevant laboratory investigations demonstrated mild compensated anion-gap acidosis, hemoglobin 116 g/L, mean corpuscular volume 92 fL, and creatinine 382 µmol/L. Inflammatory markers including C-reactive protein and white cell count were not elevated. Urine analysis showed 10–40 leukocytes/high power field, >100 erythrocytes/high power field, and proteinuria ranging from nephrotic to non-nephrotic with no growth on repeated cultures. The leukocyte count was too low to perform an accurate eosinophil count. There was no gross hematuria, complement C3 and C4 levels were normal, and autoimmune screens (Rh factor, antinuclear antibody, anti–double-stranded DNA antibodies, anti-neutrophil cytoplasmic antibodies, extractable nuclear antigens) were all negative.
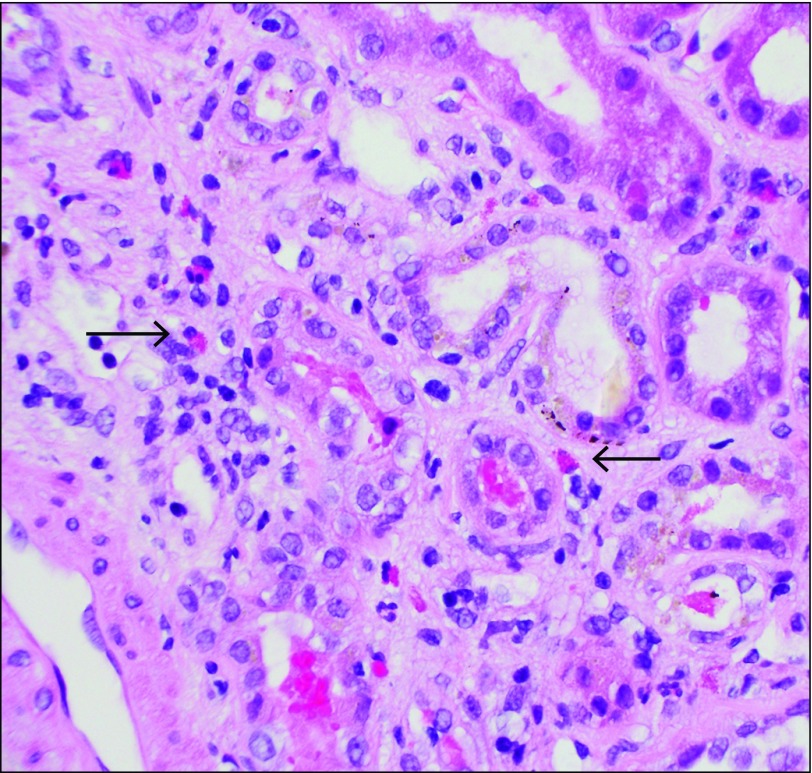
Allopurinol and perindopril were discontinued, and no improvement was seen with intravenous hydration. The patient remained vitally stable with normal urine output. A renal ultrasound showed normal kidneys with a mild diffuse increase in echogenicity. A kidney biopsy showed extensive tubulointerstitial nephritis with eosinophils (Figure 1). Other features included background mesangioproliferative immunoglobulin (Ig) A nephropathy with 1 crescent and no evidence of acute tubular necrosis or cryoglobulinemia (Figure 2). Electron microscopy demonstrated mesangial enlargement and sclerosis consistent with IgA nephropathy. In the clinical context, the reviewing pathologist and nephrologist made a diagnosis of drug-induced AIN.
Figure 1.
Kidney biopsy specimen showing intersitial eosinophils (arrows) at 400x magnification, typical of drug-induced acute interstitial nephritis.
Figure 2.
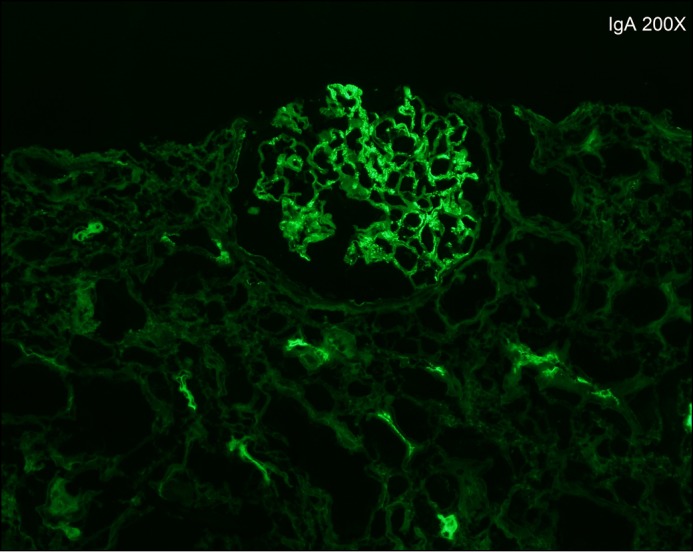
Immunofluorescence staining of kidney biopsy showing IgA deposits.
The patient’s creatinine continued to rise, and oral prednisolone (60 mg/d) was started. His creatinine peaked at 491 µmol/L and then improved, as did the facial rash. Due to fluid retention side-effects of steroid therapy, he required dose reduction to 40 mg/d and brief frusemide therapy during this period. He continues to be followed up with notable improvement of his creatinine to 284 µmol/L after weaning from immunosupression.
Discussion
The most common etiology of AIN is drugs (75% of cases), however, infections, systemic diseases, and idiopathic causes can also be responsible. Renal manifestations appear as little as 1 day after medication use or as much as several months later with some medications.1 The classic triad of fever, rash, and eosinophilia occurred together in only a minority of patients.1 Features such as arthralgias, microhematuria, leukocyturia, and proteinuria are common.1
Our patient did not have physical or laboratory indications of infection or systemic disease. The temporal relationship of use of sofosbuvir-daclatasvir, rising creatinine levels, development of facial maculopapular rash, arthalgias, and anorexia are strongly suggestive of sofosbuvir-daclatasvir-induced AIN. Although our patient had been using allopurinol, which has been known to cause AIN, for >7 years at a renally adjusted dose of 200 mg daily, his dose was reduced to 100 mg early upon deterioration and was immediately stopped on hospital admission. It is unlikely to be responsible for his acute presentation.
Biopsy findings of elements characteristic of AIN were key to the diagnosis. The additional features of IgA nephropathy are not uncommon in cirrhotic individuals, and the strong association is well known, in particular when secondary to HCV.2-5 Despite high frequency, most patients are apparently healthy individuals with no clinical signs of glomerular disease.5-7
Only one case of biopsy-proven AIN associated with direct-acting anti-virals ledipasvir-sofosbuvir exists in the literature.8 Another published example involves the concomitant use of ledipasvir-sofosbuvir and tenofovir in a patient with HCV and human immunodeficiency virus coinfection.9 The authors postulated that the kidney injury was secondary to the known interaction between ledipasvir and tenofovir disoproxil fumarate, resulting in increased tenofovir exposure. It was thought that tenofovir caused the kidney damage. The kidney biopsy showed evidence of both acute tubular necrosis and AIN with a relatively clear temporal relationship. The common sofosbuvir link in all 3 cases suggests that the AIN component was in fact related to sofosbuvir. We found no published reports of kidney injury related to daclatasvir and therefore believe sofosbuvir is the most likely culprit in our patient’s AIN.
From our review of the literature, this is the first biopsy-proven case where use of sofosbuvir and daclatasvir for treatment of HCV genotype 3 was associated with AIN. Physicians should be vigilant for drug-induced kidney injury at all times, especially when using newer agents because the full range of effects may not be known.
Disclosures
Author contributions: T. Ashraf wrote the manuscript, reviewed the literature, and is the article guarantor. W. Majoni revised and approved the manuscript.
Financial disclosure: None to report.
Informed consent was obtained for this case report.
References
- 1. Praga M, González E. Acute interstitial nephritis. Kidney Int. 2010;77(11):956–61. [DOI] [PubMed] [Google Scholar]
- 2. Newell GC. Cirrhotic glomerulonephritis: Incidence, morphology, clinical features, and pathogenesis. Am J Kidney Dis. 1987;9:183–90. [DOI] [PubMed] [Google Scholar]
- 3. Sinniah R. Heterogeneous IgA glomerulonephropathy in liver cirrhosis. Histopathology. 1984;8:947–62. [DOI] [PubMed] [Google Scholar]
- 4. Sancho J, Egido J, Sánchez-Crespo M, Blasco R. Detection of monomeric and polymeric IgA containing immune complexes in serum and kidney from patients with alcoholic liver disease. Clin Exp Immunol. 1982;47:327–35. [PMC free article] [PubMed] [Google Scholar]
- 5. McGuire B, Julian B, Eckhoff D, et al. Brief communication: Glomerulonephritis in patients with hepatitis C cirrhosis undergoing liver transplantation. Ann Intern Med. 2006;144(10):735–41. [DOI] [PubMed] [Google Scholar]
- 6. Waldherr R, Rambausek M, Duncker W, Ritz E. Frequency of mesangial IgA deposits in a non-selected autopsy series. Nephrol DialTransplant. 1989;4(11):943–6. [DOI] [PubMed] [Google Scholar]
- 7. Suzuki K, Honda K, Tanabe K, Toma H, Nihei H, Yamaguchi Y. Incidence of latent mesangial IgA deposition in renal allograft donors in japan. Kidney Int. 2003;63(6):2286–94. [DOI] [PubMed] [Google Scholar]
- 8. Wanchoo R, Thakkar J, Schwartz D, Jhaveri K. Harvoni (ledipasvir with sofosbuvir)-induced renal injury. Am J Gastroenterol. 2016;111(1):148–9. [DOI] [PubMed] [Google Scholar]
- 9. Bunnell K, Vibhakar S, Glowacki R, Gallagher M, Osei A, Huhn G. Nephrotoxicity associated with concomitant use of ledipasvir-sofosbuvir and tenofovir in a patient with hepatitis C virus and human immunodeficiency virus coinfection. Pharmacotherapy. 2016;36(9):e148–e53. [DOI] [PubMed] [Google Scholar]