Maedler and Ardestani discuss recent work from Chau et al. on the role of mTOR for β cell survival in diabetes.
Abstract
The pathways regulating pancreatic β cell survival in diabetes are poorly understood. Here, Chau et al. (2017. J. Cell Biol. https://doi.org/10.1083/jcb.201701085) demonstrate that mTOR regulates the apoptotic machinery through binding to the ChREBP–Mlx complex to suppress TXNIP, thereby protecting pancreatic β cells in the diabetic setting by inhibiting oxidative stress and mitochondrial dysfunction.
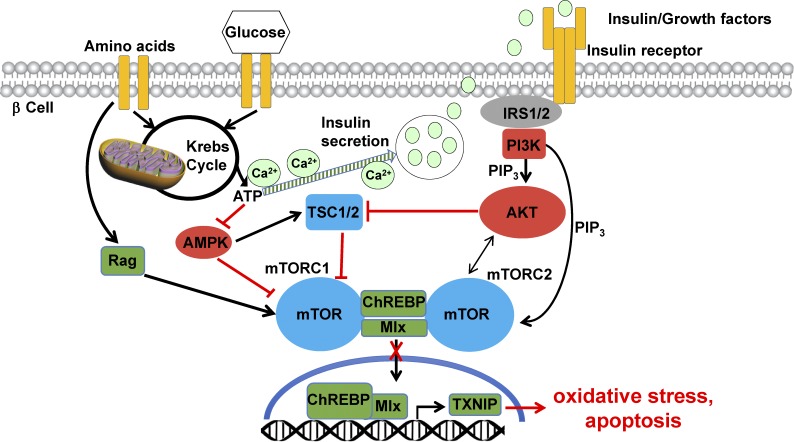
Pancreatic β cell failure, characterized by a loss of function and mass of the insulin-producing β cells, is a hallmark of both type 1 and type 2 diabetes. β cell function continues to decline during current therapies, which highlights the need for improved therapeutic approaches as well as for a better understanding of the molecular changes underlying β cell loss in diabetes. Apoptosis as well as impaired regeneration of β cells are major contributing factors to the reduced β cell mass in diabetes (Ardestani et al., 2014). Pancreatic β cells are highly vulnerable to apoptotic stimuli because of their relatively low expression of antiapoptotic enzymes and the presence of several pattern recognition receptors, which primes them for cell death under chronic metabolic and proinflammatory conditions. Furthermore, human β cells have a limited proliferative capacity. Therefore, the identification of cellular signaling pathways that regulate survival, proliferation, and regeneration of pancreatic β cells, together with an in-depth knowledge of their mechanisms of action is a prerequisite for the discovery of new drugs for β cell–directed therapies in diabetes. Because of the complexity of this metabolic disease and that multiple counter-regulating cellular pathways are in place during metabolic deterioration and adaptation, it has been difficult to control diabetes and to maintain β cell survival and function. One adaptive mechanism, which is very active in pancreatic β cells, is the mTOR signaling pathway. mTOR is an evolutionarily conserved serine/threonine kinase and nutrient-responsive regulator of cell growth, which forms two functionally and structurally distinct complexes, mTOR complex-1 (mTORC1) and mTOR complex-2 (mTORC2). mTOR is the core of a growth factor– and nutrient-sensing network. Diverse nutrients, but also mitogens, growth factors, stress, oxygen, and energy supply, stimulate the activation of mTOR complexes to regulate cell development, growth, proliferation, viability, and metabolism via controlling multiple downstream intracellular processes (Saxton and Sabatini, 2017). Consistent with the role of mTOR in coordinating cellular metabolism, in vivo physiological studies have revealed that the mTOR pathway is critical for proper glucose homeostasis and metabolic regulation at the cellular and organismal level (Saxton and Sabatini, 2017). Extracellular glucose levels and signals from nutrients need to be sensed to fine-tune insulin secretion by β cells in response to the current metabolic demand. Within β cells, glucose and nutrient metabolism activate mTORC1, while secreted insulin as well as other growth factors act via the insulin signaling pathway in the β cell to activate mTORC2 (Fig. 1).
Figure 1.
mTOR is the essential signaling pathway for nutrient sensing and apoptosis protection in the β cell. Nutrients such as amino acids and glucose activate both mTOR complexes in the β cell via interconnected pathways. mTORC1 is activated directly, whereas secreted insulin (induced by glucose metabolism and the raise in intracellular Ca2+) acts indirectly through mTORC2. mTORC1 is activated through Rag GTPase and inhibited by TSC1/2 and AMPK (elevated ATP/AMP derived from the Krebs cycle and mitochondrial respiration suppress AMPK). mTORC2 is activated by growth factors and insulin through the insulin receptor IRS1/2–PI3K–PIP3 pathway and is required for full activation of prosurvival kinase AKT. Under physiological conditions, mTOR binds to the ChREBP–Mlx complex and keeps it in the cytosol. mTOR can also dissociate the ChREBP–Mlx complex or prevent their interaction. In both scenarios, mTOR prevents the ChREBP–Mlx shuttling to the nucleus and binding to the TXNIP promoter. Subsequently, TXNIP transcription and β cell apoptosis are suppressed. In the absence of mTOR, the ChREBP–Mlx complex binds to the TXNIP promoter, which leads to elevated TXNIP levels, oxidative stress, and apoptosis.
mTOR has been reported to regulate β cell survival and proliferation under physiological conditions both in vitro and in vivo. mTOR is one of the major signaling complexes responsible for β cell growth, plasticity, and adaptation in response to both cell-autonomous and systemic growth signals in the context of nutrients and endocrine factors (Barlow et al., 2013). In this issue a well-designed study by Chau et al. provides the underlying mechanisms for the connection of mTOR deficiency to β cell death and the progression of diabetes. Chau et al. (2017) initially show that genetic disruption of mTOR selectively in pancreatic β cells leads to defective glucose-induced insulin secretion and glucose intolerance as a result of impaired β cell survival and reduced β cell mass. Further analysis of islets isolated from β cell–specific mTOR knockout (β-mTORKO) mice as well as an mTOR-deficient β cell line revealed that mitochondrial membrane potential and respiration is compromised, which leads to impaired ATP production, lower intracellular Ca2+, and elevated ROS production. These findings link loss of mTOR signaling to impaired mitochondrial activity, β cell apoptosis, and insulin production. As a lack of mTOR promotes β cell apoptosis, Chau et al. (2017) hypothesized that mTOR deficiency may regulate β cell survival, function, and glucose homeostasis under diabetic conditions. Injection of the β cell toxin streptozotocin (STZ) induced progressive hyperglycemia and impaired glucose tolerance in WT control mice, whereas in the β-mTORKO mice blood glucose levels were further increased and glucose tolerance and glucose-induced insulin secretion were severely impaired. Further reduced β cell survival and loss of compensatory hyperplasia led to pancreatic β cell mass reduction (Chau et al., 2017). These data suggest that β cell–specific ablation of mTOR exacerbated progressive hyperglycemia and further potentiated glucose intolerance and impaired insulin secretion in the STZ mouse model of β cell destruction and diabetes. These defects are β cell specific; Chau et al. (2017) reproduced earlier findings from pharmacologic mTOR inhibition by rapamycin (Barlow et al., 2013) in β cell–specific mTOR-deficient mice. Islets are smaller and exhibit reduced β cell mass together with defective glucose-stimulated insulin secretion. This is in line with the loss of important factors for β cell proliferation, i.e., cyclin D2 and D3 and Cdk4 upon mTOR inhibition (Balcazar et al., 2009).
Interestingly, Chau et al. (2017) identified that mTOR functions as an apoptosis-protecting signal in β cells by interfering with the activation of Thioredoxin-interacting protein (TXNIP), a key regulator of the intracellular redox state. Loss of mTOR induces TXNIP at both the mRNA and protein level and amplifies TXNIP up-regulation in stressed β cells challenged with STZ or increased glucose concentrations. TXNIP is up-regulated under diabetogenic conditions and linked to insulin resistance as well as β cell failure through its participation in the activation of the NLP3 inflammasome under chronic glucotoxicity in β cells (Zhou et al., 2010). But how could the regulated glucose/nutrient sensing by mTOR prevent such diabetic TXNIP activation? Under physiological conditions, all mTOR complex partners are present in the β cell, together with the carbohydrate-responsive element binding protein (ChREBP), which is responsible for the transcriptional regulation of TXNIP. Pharmacological and genetic disruption of mTOR signaling up-regulates ChREBP. Furthermore, STZ- and glucose-induced ChREBP is further increased in the absence of mTOR in β cells. Reciprocally, mTOR overexpression diminishes stress-induced TXNIP and ChREBP induction together with the protection from β cell death, suggesting an mTOR-dependent regulation of the TXNIP–ChREBP signaling axis with an impact on β cell survival. Mechanistically, Chau et al. (2017) showed that mTOR can bind to the ChREBP–Mlx complex and keeps it in the cytosol. Consequently, transcriptional activity and expression of coordinated genes is low. This was shown for TXNIP as mTOR depletion enhances ChREBP–Mlx binding to the TXNIP promoter in the nucleus, inducing its expression, which leads to oxidative stress and β cell apoptosis. These findings suggest that functional mTOR suppresses TXNIP and balances β cell survival by promoting ChREBP–Mlx cytoplasmic retention through a direct protein–protein interaction. Also, coimmunoprecipitation studies indicate that mTOR depletion increases the ChREBP–Mlx interaction, illustrating that mTOR might also negatively regulate TXNIP induction by disrupting the ChREBP–Mlx complex. Interestingly, upon diabetogenic stimuli, ChREBP–Mlx translocates into the nucleus, whereas mTOR remains in the cytosol. In the complete absence of mTOR, TXNIP is freed from its inhibitory regulatory mechanism imposed by mTOR, which leads to an uncontrolled redox state, oxidative stress, and massive β cell death (Fig. 1). Importantly, ChREBP as well as TXNIP are up-regulated in pancreatic autopsy sections from patients with type 2 diabetes; this might be an important mechanism by which hyperactivation of the ChREBP–TXNIP signaling axis contributes to the β cell pathogenesis of diabetes (Chau et al., 2017). As ChREBP coordinates the transcriptional activity of many other genes, it will be interesting to see which other genes are regulated through the mTOR–ChREBP–Mlx interaction under physiological and pathophysiological conditions.
The two different mTOR complexes, mTORC1 and mTORC2, have specific substrates and thus elicit distinct downstream signaling events to regulate several key cellular processes. mTORC1 is activated by growth factors (e.g., insulin and IGF-1), nutrients (amino acids and glucose), and cellular energy levels (high ATP/AMP ratio). In contrast, mTORC2 is primarily activated by extracellular stimuli such as growth factors and insulin. Although the major function of mTORC1 is to regulate the intracellular metabolic state by shifting catabolic to growth-promoting anabolic metabolism, promoting the synthesis of proteins, lipids, and nucleotides, mTORC2 regulates cell proliferation and survival as well as cytoskeleton dynamics and organization by phosphorylating a subset of AGC family kinases such as AKT, SGK1, and PKC (Saxton and Sabatini, 2017). Using siRNA-mediated silencing of raptor or rictor, integral components of mTORC1 and mTORC2, respectively, Chau et al. (2017) showed that loss of raptor but not rictor induces TXNIP and ChREBP, suggesting mTORC1-dependent regulation (Chau et al., 2017). The remaining question is, which of the mTOR complexes modulates the ChREBP–Mlx interaction and its nuclear translocation? We still do not know which mTOR complex regulates mitochondrial activity, oxidative stress, insulin secretion, and β cell apoptosis in this context. The focus of future studies should be the further investigation of the precise mechanisms through which mTORC1 and/or mTORC2 affects TXNIP–ChREBP signaling and β cell homeostasis.
Diabetes manifests when β cells fail to secrete adequate insulin levels. Healthy pancreatic islets coordinate nutrient availability and hormone secretion to maintain glucose homeostasis. β cells continuously measure glucose levels and respond within seconds (when blood glucose levels are elevated) with pulsatile bursts of insulin. But the system is not always sustainable; dysregulation of hormone production in the pancreas leads to hyperglycemia and vice versa. Insufficient levels of insulin are produced by the overworked and failing β cells, which then are unable to maintain a normoglycemic balance and diabetes manifests. This occurs when β cells had to respond to chronically elevated glucose levels and other metabolic stimuli (e.g., free fatty acids and amino acids) over a prolonged time. Cells fail to adaptively increase insulin secretion, a phenomenon termed “β cell decompensation.” Although glucose is needed as an important energy source for all cells of the human body, too much of it can cause stress for β cells, which respond initially with activation of defense pathways, but later undergo apoptosis through oxidative stress, ER stress, and inflammation (Leibowitz et al., 2010). Although physiological mTORC1 activation is essential for the maintenance of β cell homeostasis and insulin secretion, as discussed by Chau et al. (2017), chronic inappropriate hyperactivation of mTORC1 seems to play a central role in β cell failure by inducing progressive loss of β cell mass and function. mTORC1 is highly up-regulated in β cells under gluco/lipotoxicity in vitro and in islets from widely used mouse models of type 2 diabetes as well as from patients with type 2 diabetes and is paralleled with impaired β cell survival and function (Shigeyama et al., 2008; Bachar et al., 2009; Yuan et al., 2017). mTORC1 inhibition in β cells prevents β cell death and enhances insulin secretion (Bachar et al., 2009; Yuan et al., 2017). High glucose-induced mTORC1 activation (as demonstrated by phosphorylation of its downstream proteins including S6K1 and S6) is also confirmed by the study from Chau et al. (2017). Consistently, although constitutive activation of mTORC1 by β cell–specific deletion of TSC2, a negative regulator of mTORC1, shows increased β cell mass in the early phase of their life, mice become hyperglycemic and severely hypo-insulinemic as a result of a dramatic loss of β cells at an older age because of chronic mTORC1 activation (Shigeyama et al., 2008). A similar biphasic effect is elicited by Rapamycin treatment. Whereas acute pharmacological suppression of the mTORC1 pathway restores insulin signaling and glucose homeostasis in vitro and in vivo, the opposite is seen during prolonged exposure and, thus, chronic mTORC1 and mTORC2 suppression (Barlow et al., 2013). Thus, under physiological conditions mTOR is of utmost importance for β cell function and survival. Nevertheless, targeting the mTOR pathway for therapy of diabetes is critical, as many mTORC1-regulated processes, such as impaired insulin signaling (caused by several negative loops), defective autophagy, and profound unresolvable ER stress, prevent the β cell from proper adaptation to long-term over-nutrition and metabolic pressure (Yuan et al., 2017).
The aforementioned studies, including the one by Chau et al. (2017), make it clear that the metabolic cellular control by mTORC is more Than Only Recognizing Comestibles; its stimulation is essential for a physiological control and balance of β cell metabolism, growth, survival, and insulin secretion. However, chronic mTORC1 activation might play an important role in the failure of β cells to adapt to a chronic overnutrition and metabolic demand, which leads to a progressive loss of functional β cell mass in type 2 diabetes.
Acknowledgments
We apologize to our colleagues whose work we could not cite because of length restrictions.
We acknowledge the support of our projects from Juvenile Diabetes Research Foundation International, the Deutsche Forschungsgemeinschaft, and the European Foundation for the Study of Diabetes.
The authors declare no competing financial interests.
References
- Ardestani A., Paroni F., Azizi Z., Kaur S., Khobragade V., Yuan T., Frogne T., Tao W., Oberholzer J., Pattou F., et al. 2014. MST1 is a key regulator of β cell apoptosis and dysfunction in diabetes. Nat. Med. 20:385–397. 10.1038/nm.3482 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bachar E., Ariav Y., Ketzinel-Gilad M., Cerasi E., Kaiser N., and Leibowitz G.. 2009. Glucose amplifies fatty acid-induced endoplasmic reticulum stress in pancreatic β-cells via activation of mTORC1. PLoS One. 4:e4954 10.1371/journal.pone.0004954 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balcazar N., Sathyamurthy A., Elghazi L., Gould A., Weiss A., Shiojima I., Walsh K., and Bernal-Mizrachi E.. 2009. mTORC1 activation regulates beta-cell mass and proliferation by modulation of cyclin D2 synthesis and stability. J. Biol. Chem. 284:7832–7842. 10.1074/jbc.M807458200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barlow A.D., Nicholson M.L., and Herbert T.P.. 2013. Evidence for rapamycin toxicity in pancreatic β-cells and a review of the underlying molecular mechanisms. Diabetes. 62:2674–2682. 10.2337/db13-0106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chau G.C., Im D.U., Kang T.M., Bae J.M., Kim W., Pyo S., Moon E.-Y., and Um S.H.. 2017. mTOR controls ChREBP transcriptional activity and pancreatic β cell survival under diabetic stress. J. Cell Biol. 10.1083/jcb.201701085 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leibowitz G., Bachar E., Shaked M., Sinai A., Ketzinel-Gilad M., Cerasi E., and Kaiser N.. 2010. Glucose regulation of β-cell stress in type 2 diabetes. Diabetes Obes. Metab. 12(Suppl 2):66–75. 10.1111/j.1463-1326.2010.01280.x [DOI] [PubMed] [Google Scholar]
- Saxton R.A., and Sabatini D.M.. 2017. mTOR signaling in growth, metabolism, and disease. Cell. 168:960–976. 10.1016/j.cell.2017.02.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shigeyama Y., Kobayashi T., Kido Y., Hashimoto N., Asahara S., Matsuda T., Takeda A., Inoue T., Shibutani Y., Koyanagi M., et al. 2008. Biphasic response of pancreatic β-cell mass to ablation of tuberous sclerosis complex 2 in mice. Mol. Cell. Biol. 28:2971–2979. 10.1128/MCB.01695-07 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan T., Rafizadeh S., Gorrepati K.D., Lupse B., Oberholzer J., Maedler K., and Ardestani A.. 2017. Reciprocal regulation of mTOR complexes in pancreatic islets from humans with type 2 diabetes. Diabetologia. 60:668–678. 10.1007/s00125-016-4188-9 [DOI] [PubMed] [Google Scholar]
- Zhou R., Tardivel A., Thorens B., Choi I., and Tschopp J.. 2010. Thioredoxin-interacting protein links oxidative stress to inflammasome activation. Nat. Immunol. 11:136–140. 10.1038/ni.1831 [DOI] [PubMed] [Google Scholar]