Abstract
Previous research has shown that the ventral medial prefrontal cortex (vmPFC) and hippocampus (Hipp) are critical for extinction memory. Basal forebrain (BF) cholinergic input to the vmPFC and Hipp is critical for neural function in these substrates, which suggests BF cholinergic neurons may be critical for extinction memory. In order to test this hypothesis, we applied cholinergic lesions to different regions of the BF and observed the effects these lesions had on extinction memory. Complete BF cholinergic lesions induced contextual fear memory generalization, and this generalized fear was resistant to extinction. Animals with complete BF cholinergic lesions could not acquire cued fear extinction. Restricted cholinergic lesions in the medial septum and vertical diagonal bands of Broca (MS/vDBB) mimicked the effects that BF cholinergic lesions had on contextual fear memory generalization and acquisition of fear extinction. Cholinergic lesions in the horizontal diagonal band of Broca and nucleus basalis (hDBB/NBM) induced a small deficit in extinction of generalized contextual fear memory with no accompanying deficits in cued fear extinction. The results of this study reveal that MS/vDBB cholinergic neurons are critical for inhibition and extinction of generalized contextual fear memory, and via this process, may be critical for acquisition of cued fear extinction. Further studies delineating neural circuits and mechanisms through which MS/vDBB cholinergic neurons facilitate these emotional memory processes are needed.
Keywords: Recall, hippocampus, basal forebrain, acetylcholine, fear conditioning
INTRODUCTION
Cued extinction memory can be defined as remembering to inhibit conditioned fear (Quirk et al., 2006) and is often studied in the Pavlovian fear conditioning paradigm by testing levels of conditioned fear responding after fear extinction training (Bouton et al., 2006; Quirk et al., 2006). The ventral medial prefrontal cortex (vmPFC), hippocampus (Hipp), and certain amygdala nuclei are critical for extinction memory. Extinction memory is dependent on the infralimbic cortex (IL) (Milad and Quirk, 2002; Sierra-Mercado et al., 2011), and IL input to the intercalated region of the amygdala (ITC) and the lateral central nucleus (CeL) is critical for extinction memory (Likhtik et al., 2008; Quirk et al., 2003; Royer and Pare, 2002). The basolateral amygdala (BLA) is also critical for extinction memory (Berlau and McGaugh, 2006; Chhatwal et al., 2005; Sierra-Mercado et al., 2011), and hippocampal input to the BLA and prelimbic cortex (PL) is critical for gating cued extinction memory retrieval (Orsini et al., 2011; Sotres-Bayon et al., 2012). Within these systems a number of molecular processes and circuits are critical for extinction memory (for reviews see Herry and Johansen, 2014; Orsini and Maren, 2012).
Basal forebrain (BF) cholinergic input to the Hipp and neocortex has been implicated in mediating neural function within these substrates. Cholinergic neurons in the medial septum and vertical limb of the diagonal band of Broca (MS/vDBB) that project to the Hipp are critical for molecular signaling cascades in the Hipp that facilitate contextual memory formation (Tronson et al., 2009) as well as decrements in conditioned stimulus (CS) processing (Baxter et al., 1997). Cholinergic neurons in the nucleus basalis magnocellularis (NBM) and horizontal limb of the diagonal band of Broca (hDBB) are critical for increments in CS processing (Chiba et al., 1995), cortical arousal (Jones, 2003; Manns et al., 2000a), sustained attentional performance (McGaughy et al., 2005; Sarter et al., 2001), and motor cortex plasticity (Conner et al., 2003). Given these findings, BF cholinergic input to Hipp and vmPFC may be critical for extinction memory. Previous studies examining the role of BF cholinergic neurons in fear extinction did not examine conditioned fear responding after extinction training (Knox and Berntson, 2006; Tronson et al., 2009). Thus, the role of BF cholinergic neurons in extinction memory remains unknown.
We tested the hypothesis that BF cholinergic neurons are critical for cued extinction memory by examining the effects of selective BF cholinergic lesions on fear and extinction memory in the Pavlovian fear conditioned freezing paradigm (Figure 1). The results of this study demonstrate that rats with MS/vDBB cholinergic neurons generalize contextual fear memory, have deficits in extinguishing generalized contextual fear memory, and cannot acquire cued fear extinction.
Figure 1.

Experimental design used in this study.
MATERIALS AND METHODS
Animals
Fifty-five male Sprague-Dawley rats, weighing 226–250g, were obtained from Charles River Inc. (Portage MI) and dually housed on a 12 hour light-dark cycle with lights on at 6:00 a.m. and off at 6:00 p.m. They had ad lib access to water and were given a food restricted diet of 23g of rat chow (Purina RMH3000) per day, which is the supplier’s recommended diet for rats of the size used in this experiment (LabDiet, St. Louis MO). Animals weights were checked daily and all animals placed on this food restricted diet displayed normal weight gain (data not shown). Experiments were approved by the Institutional Laboratory Animal Care and Use Committee of The University of Delaware and in accordance with the National Institute of Health guidelines for the treatment of animals.
Surgery
We adopted previously described protocols to induce selective BF cholinergic lesions (Conner et al., 2003; Frick et al., 2004; Knox and Berntson, 2006). Rats were administered xylazine (12mg/kg, subcutanteously) and general anesthesia was induced using 5% isoflurane in air. Rats were then placed in a stereotaxic apparatus (David Kopf, Tujunga CA) and maintained at a surgical plane using .5 – 2% isoflurane in air. Trephine holes were drilled in the skull to allow for insertion of a 5μL Hamilton syringe with a 26 gauge needle into relevant regions of the BF. The selective immunotoxin 192-IgG saporin (Advanced Targeting Systems, San Diego CA) was used to induce selective BF cholinergic lesions. This cholinergic toxin was infused into the BF at a concentration of .2μg/μL, which at this concentration results in selective BF cholinergic loss (Baxter et al., 1997; Conner et al., 2003; Frick et al., 2004; Knox and Berntson, 2006). To target BF cholinergic neurons that project to the Hipp and medial cortical substrates, we infused .2μL the cholinergic toxin into the MS/vDBB (AP = .84 mm, ML = ± .5 mm, DV = −7.0 and 7.8 mm). To target BF cholinergic neurons that project to the neocortex we infused .3μL of the cholinergic toxin into the hDBB (AP = −.24mm, ML = ±1.8 mm, DV = −8.0mm) and .5μL of the toxin into the NBM (AP = −1.4mm, ML = ±3.1mm, DV = −7.0mm). Sham lesions were accomplished by infusion of an equivalent volume of phosphate buffered saline (PBS) into BF regions. In Experiment 1 complete BF lesions were accomplished by infusing the toxin into all BF regions (BF-lesion). Restricted cholinergic lesions were accomplished by infusing the toxin into the MS/vDBB (MS/vDBB-lesion), or hDBB and NBM (hDBB/NBM-lesion). All placements were made relative to Bregma and were referenced from the atlas of Paxinos and Watson (1998).
Fear conditioning and extinction training sessions
All sessions were conducted in identical rodent observation chambers constructed of aluminum and Plexiglas (30 × 24 × 21 cm; MED Associates, St. Albans, VT), situated in sound-attenuating chambers and located in an isolated room. Fear conditioning and extinction training was conducted as previously described (Knox et al., 2012). Briefly, a 10s auditory conditioned stimulus (CS, 2kHz, 80 dB) co-terminated with a footshock unconditioned stimulus (UCS, 1s, 1mA) five times in a distinct context (i.e. fear conditioning context). One day after fear conditioning, cued extinction training started and consisted of 30 CS-only presentations in a distinct context (i.e. extinction context). Three hours after cued extinction training, rats were exposed to the extinction context for one hour. Adopting this procedure lowers baseline freezing in an extinction test (Chang et al., 2009; laboratory observation). One day after extinction training all animals were tested for extinction in the extinction context by presenting 10 CSs. For all behavioral sessions there was a 210s baseline period and an inter-trial interval (ITI) of 60s. Creation of different contexts was done by manipulating auditory, visual, tactile, and odor stimulation as previously described (Knox et al., 2012). Freezing during baseline periods prior to extinction training and testing, as well as freezing during re-exposure to the extinction context, served as measures of contextual fear memory generalization (i.e. the extent to which animals would freeze in a context that was never paired with a footshock after fear conditioning).
Immunohistochemistry and histology
One day after the extinction test, rats were rapidly decapitated and brains were removed, frozen in isopentane that was chilled on dry ice, and stored in a −80 °C freezer until further processing. Brains were then maintained at −13 °C in a cryostat and sections through the mPFC, MS/vDBB, hDDB, NBM, and Hipp were taken at 30μm, mounted onto superfrost glass slides, and then stored in a −80 °C freezer until further processing. Glass slides with mPFC and Hipp sections were treated for visualization of acetylcholinesterase (AChE) in order to measure the cholinergic fiber loss in these brain regions. These regions were selected because they receive differential innervation from BF cholinergic neurons, with MS/vDBB cholinergic neurons innervating the Hipp and medial cortical regions and hDBB/NBM cholinergic neurons innervating the frontoparietal cortex and PFC (Mesulam et al., 1983a; Woolf et al., 1984). AChE histology was conducted as previously described (Tago et al., 1986), with some modifications. Briefly, slides were fixed for 2–3 hours in 4% paraformaldehyde in .2M PBS, rinsed with .1 M maleate buffer (pH 6.0), and incubated for 45 minutes in a solution consisting of 5 mg of acetylthiocholine iodide, 112 mg sodium citrate, 25 mg copper sulfate, and 164 mg potassium ferricyanide in .1 M maleate buffer. Sections were then rinsed in .01M Tris buffered saline (TBS) and incubated for 10 minutes in a solution consisting of .05 g diaminobenzidine (DAB), .375 g nickel ammonium sulfate, and .6 μL of a .1% H2O2 solution in TBS. Slides were then rinsed with TBS, dehydrated in ethanol, left overnight in xylene, and coverslipped using DPX mountant (Sigma-Aldrich Inc).
Immunocytochemistry was used to visualize choline acetyltransferase (ChAT) cell loss in BF regions. Slides were fixed for 2–3 hours in 4% paraformaldehyde solution, and then incubated in .1% Triton X-100 in TBS. Slides were then incubated in a 3% goat serum solution in TBS. Slides were then washed in TBS and exposed to a primary rabbit ChAT polyclonal antibody (Millipore Inc.) at a concentration of 1:1000 overnight at 4°C. After this slides were washed in TBS and visualization of the ChAT primary antibody was accomplished using an ABC kit (Vector Lab, Burlingame CA) with a goat anti-rabbit IgG secondary antibody according to the manufacturer’s instructions. Sections were then dehydrated in ethanol, left overnight in xylene, and coverslipped using DPX mountant. MS, vDBB, hDDB, and NBM sections were also treated to visualize Nissl substance in order determine if excitotoxic BF lesions were observed. In a subset of lesion and control rats (sampled from all experiments), MS/vDBB sections were treated for visualization of glutamate acid decarboxylase (GAD) using a rabbit polyclonal antibody (GAD-65, Santa Cruz Biotechnology Inc. Santa Cruz CA), ABC kit with a goat anti-rabbit IgG secondary antibody, and methods similar to that described for visualization of ChAT (see above). This was done to determine if non-cholinergic cell loss was observed with surgical lesions.
DATA ANALYSIS
AChE sections were imaged at 2.5× using a Leitz Dialux 20 microscope with attached 20MB Cannon Rebel T5i camera. AChE fiber density was then scored in ImageJ. The optical density (OD) of AChE fiber staining was compared to OD values in white matter. All values were then normalized relative to OD values of sham rats. These normalized OD scores were then subjected to t-test (lesion vs. sham) for each brain region analyzed.
Freezing was scored using Anymaze software (Stoelting Inc., Kiel WI) as previously described (Knox et al., 2012) and averaged across CS presentation and a corresponding ITI (e.g. CS1 and ITI1), averaged into blocks of two trial for extinction training, or averaged into six minute blocks for the context re-exposure session. Freezing during all behavioral sessions was subjected to a surgery × trial or block factor design. Main and simple effects were analyzed using analysis of variance (ANOVA) while post hoc comparisons were performed using t-test with Bonferroni corrections applied where appropriate. Certain t-tests were planned contrasts, and thus Bonferroni corrections were not applied to these analyses. A p-value of less than .05 was used as the statistical criterion of significance.
Levels of freezing during the baseline periods of extinction training and extinction testing were used to assess whether there was any generalized contextual fear (i.e. freezing in a context that was never paired with a footshock) with any of our experimental treatments. Freezing during re-exposure to the extinction context also served as a measure of contextual fear memory generalization.
RESULTS
ChAT, Nissl, and GAD staining
Figure 2 shows representative sections from all experiments in this study. Infusing 192-IgG toxin into the BF resulted in loss of ChAT-positive cells without producing an excitotoxic lesion or loss of GAD-positive cells. These findings demonstrated that our surgical procedure was successful at inducing selective cholinergic lesions in the BF.
Figure 2.
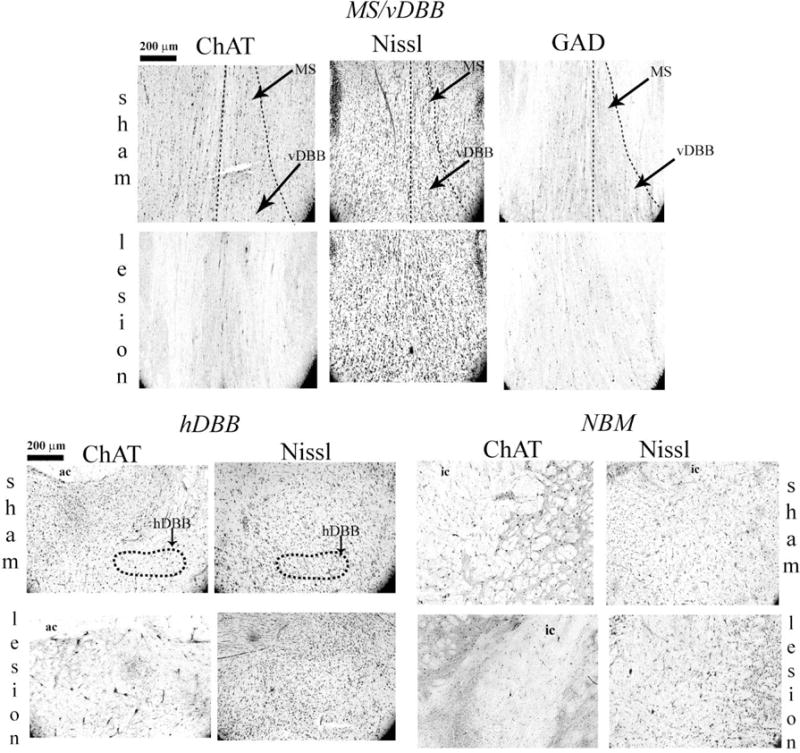
Immunohistochemistry from representative sections in all experiments in this study. Infusion of 192 IgG saporin into the BF resulted in loss of ChAT positive cells in the BF, without general cellular damage or loss of GAD positive cells. The MS, vDBB, and hDBB are illustrated in outline. Clusters of ChAT positive cells around the internal capsule (ic) comprise the NBM. Ac – anterior commissure. Images were captured at 10× magnification.
Experiment 1: BF cholinergic lesions induce contextual fear memory generalization, impair extinction of generalized contextual fear memory, and impair acquisition of cued fear extinction
The results of Experiments 1 are illustrated in Figure 3A. Even when there were no AChE fibers present in a brain region, the OD values of cortical and hippoampal tissue was always greater than OD value of white matter. Thus, the normalized OD scores for lesion rats never approached zero. Nevertheless, scoring AChE sections in ImageJ revealed that BF-lesion rats (n = 7) had extensive AChE loss in the ACC [t(10) = 5.696, p < .001], PL [t(10) =3.287, p = .008], frontoparietal cortex [t(10) = 4.624, p = .001, dentate gyrus (DG) [t(10) = 5.696, p < .001], CA3 [t(10) = 7.115, p < .001], and CA1[t(10) = 7.467, p < .001] relative to BF-sham rats (n = 6). There was also AChE loss in the retrosplenial cortex, though this loss was not quantified. There was no observed AChE loss in the IL [t(10) = .51, p = .621].
Figure 3.
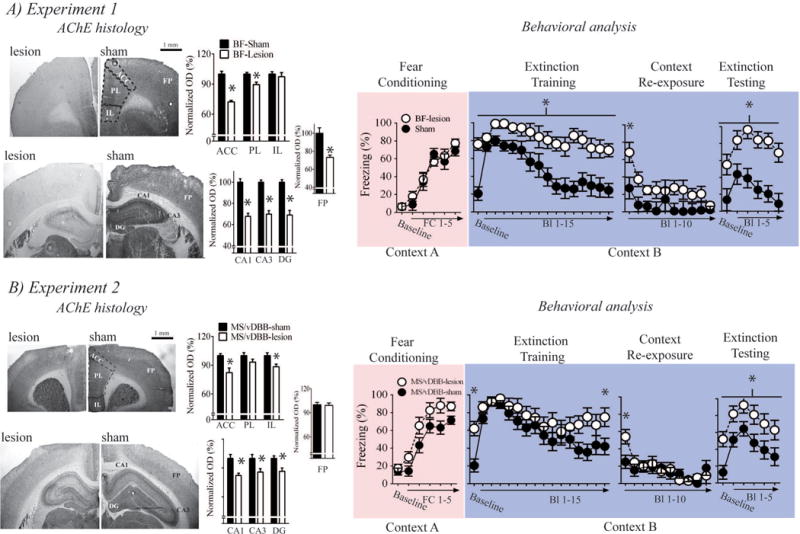
Effects of BF and MS/vDBB cholinergic lesions on contextual fear memory generalization and extinction memory. A) Complete BF cholinergic lesions resulted in extensive AChE loss in the neocortex and hippocampus. BF cholinergic lesions also induced contextual fear memory generalization, impaired extinction of this generalized contextual fear memory, and impaired acquisition of cued fear extinction. B) MS/vDBB cholinergic lesions resulted in selective AChE loss to the dHipp and mPFC. These lesions also induced context fear memory generalization, impaired extinction of generalized contextual fear memory, and impaired acquisition of cued fear extinction. Bl- Block, FC – fear conditioning, frontopariental cortex - FP.
Both BF-lesion and BF-sham rats acquired fear conditioning in an equivalent manner [main effect of fear conditioning trial: F(5,50) = 71.303, p < .001; main surgery term: F(1,10) = .209, p = .657]. BF-lesion rats displayed enhanced freezing during the baseline and all CS presentations of the extinction training session [main effect of surgery: F(1,11) = 24.823, p < .001]. This finding suggests that BF-lesions induced contextual fear memory generalization, which can be defined as enhanced freezing to a novel context after fear conditioning. The BF-lesion treatment also impaired acquisition of cued fear extinction. BF-lesion rats displayed enhanced freezing during the first block of trials when they were re-exposed to the extinction context [surgery × context exposure block interaction on the linear trend: F(1,11) = 6.847, p = .024]. However, both BF-lesion and BF-sham rats eventually showed equivalent levels of freezing at the end of the context re-exposure session [t(11) = .928, p = .373], which suggests BF-lesion rats could eventually inhibit generalized contextual fear memory.
Similar to what was observed during extinction training, BF-lesion rats displayed higher levels of freezing during baseline and all CS presentation in the extinction test [main effect of surgery: F(1,11) = 27.348, p < .001]. These results suggest that contextual fear memory generalization induced by complete BF cholinergic lesions were resistant to extinction and rats with these lesions could not acquire cued fear extinction.
Experiment 2: MS/vDBB cholinergic lesions induce contextual fear memory generalization, impair extinction of generalized contextual fear memory, and impair acquisition of cued fear extinction
The results of Experiment 2 are illustrated in Figure 3B. MS/vDBB-lesion rats (n = 9) had extensive AChE loss in the DG [t(17) = 3.129, p = .006], CA1 [t(17) = 3.676, p = .002], CA3 [t(17) = 2.635, p = .017], ACC [t(17) = 3.511, p = .003], and IL [t(17) = 2.503, p = .023] relative to MS/vDBB-sham rats (n = 11). There was no AChE loss in the PL [t(17) = 1.546, p = .14] or frontoparietal cortex [t(17) = .19, p = .892].
All rats acquired fear conditioning [main effect of fear conditioning trial: F(5,90) = 84.325, p < .001]. MS/vDBB-lesion rats appeared to have enhanced acquisition of fear conditioning, but this effect only approached statistical significance [main surgery term: F(1,18) = 4.387, p = .051].
MS/vDBB-lesion rats displayed enhanced freezing during the baseline period of the extinction training session [planned contrast: t(18) = 3.84, p = .001], which suggests that MS/vDBB-lesions induced contextual fear memory generalization. All rats acquired cued fear extinction [main effect of trial: F(15,270) = 10.837, p < .001], but acquisition of cued extinction was impaired in MS/vDBB-lesion rats [planned contrast: Block 15 of extinction training session – t(18) = 2.349, p = .03]. Planned contrast of freezing during Block 1 of re-exposure to the extinction context revealed that contextual freezing was enhanced in MS/vDBB-lesion rats [t(18) = 2.568, p = .019]. There was also a significant linear trend analysis for contextual freezing during re-exposure to the extinction context [F(1,18) = 19.786, p < .001], and all rats had similar levels of freezing during the last block of the extinction context re-exposure session. This suggests that MS/vDBB-lesion rats could eventually inhibit generalized contextual fear memory.
Planned contrasts of baseline freezing during the extinction test revealed a statistically significant comparison [lesion vs. sham: t(18) = 3.258, p = .004] with MS/vDBB-lesion rats displaying higher levels of contextual freezing. MS/vDBB-lesion rats also displayed higher levels of freezing during all CS presentations of the extinction test [main effect of surgery: F(1,18) = 7.533, p =.013]. These results suggest that contextual fear memory generalization induced by MS/vDBB cholinergic lesions were resistant to extinction and rats with these lesions could not acquire cued fear extinction.
Experiment 3: hDBB/NBM cholinergic lesions have no effects on extinction memory
The results of Experiment 3 are illustrated in Figure 4. hDBB/NBM-lesion rats (n = 11) had extensive AChE loss in the ACC [t(18) = 3.55, p = .002], PL [t(18) = 2.667, p = .016], and frontoparietal cortex [t(18) = 3.907, p = .001] relative to hDBB/NBM-sham rats (n = 10). No AChE loss was observed in the DG [t(18) = .869, p = .396], CA3 [t(18) = .388, p = .703], CA1 [t(18) = .852, p = .406], or IL [t(18) = 1.726, p = .101].
Figure 4.
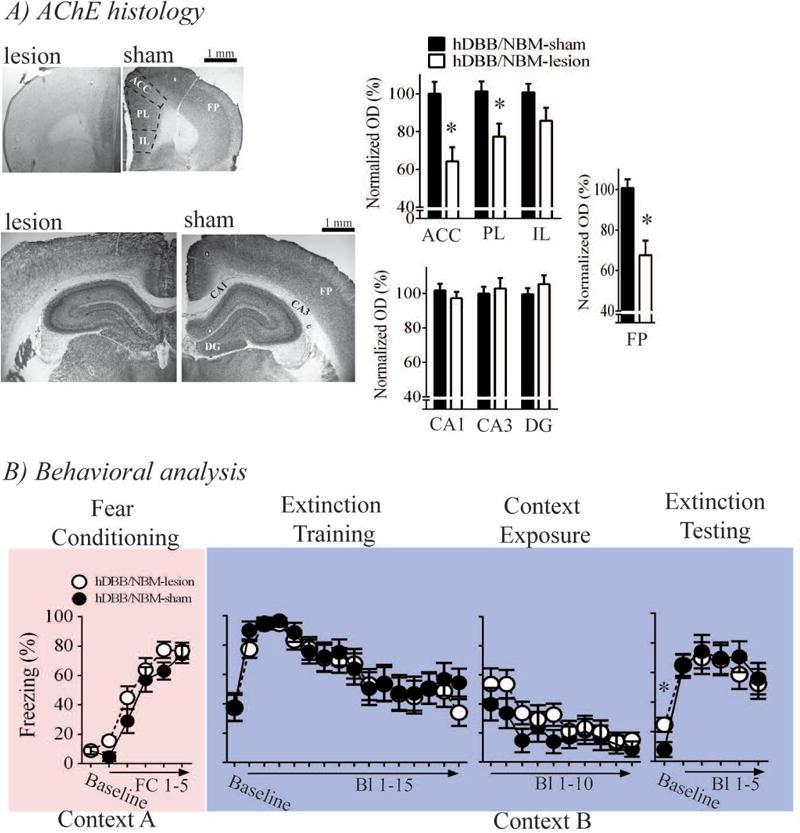
Effects of hDBB/NBM cholinergic lesions on contextual fear memory generalization and extinction memory. A) hDBB/NBM cholinergic lesions resulted in selective AChE loss to the FP and PFC, and B) induced a mild impairment in extinction of generalized contextual fear memory. These lesions, however, had no effects on cued fear or extinction memory.
Both hDBB/NBM-lesion and hDBB/NBM-sham rats acquired fear conditioning in an equivalent manner. This was revealed by a significant main effect of fear conditioning trial [F(5,95) = 84.919, p < .001], but no main effect of surgery [F(1,19) = 1.974, p =.176] or surgery × trial interaction [F(5,95) = .993, p =.426].
Planned contrast of baseline freezing during extinction training were not statistically significant [t(19) = .041, p = .968]. Upon CS presentation, freezing in all rats increased, but then decreased over the course of the extinction training session [main effect of trial on quadratic trend: F(1,19) = 7.541, p = .013], which suggests all rats expressed cued fear and acquired fear extinction. Planned contrast of freezing during the last block of the extinction training session was not significant [block 15 –t(19) = 1.554, p = .137]. Taken together these findings suggest that hDBB/NBM cholinergic lesions had no effects on contextual fear memory generalization or acquisition of cued fear extinction. Planned contrast of freezing during Block 1 of the extinction context re-exposure session was not significant [t(19) = .899, p = .38]. Freezing during re-exposure to the extinction context decreased across the session [linear trend analysis: F(1,19) = 21.814, p < .001] and this effect was equivalent between lesion and sham rats [main surgery term: F(1,19) = 1.41, p = .25; surgery × block term: F(9,171) = .65, p = .723]. These findings reinforce the earlier findings that hDBB/NBM cholinergic lesions do not induce contextual fear memory generalization.
Planned contrast of baseline freezing between hDBB/NBM-lesion and sham rats revealed a significant effect [t(19) = 2.415, p = .026]. While levels of contextual freezing during the baseline period of the extinction test was low in both hDBB/NBM-lesion and hDBB/NBM-sham rats, this statistical analysis suggest that retaining extinction of generalized contextual fear memory was impaired by hDBB/NBM cholinergic lesions. hDBB/NBM cholinergic lesions had no effects on cued freezing during the extinction test [main surgery term: F(1,19) = .003, p = .954; surgery × extinction test: F(5,95) = 1.164, p = .333]. These results suggest that hDBB/NBM cholinergic lesions had no effects on cued fear extinction.
DISCUSSION
The results of this study demonstrate that BF cholinergic neurons are critical for inhibiting generalized contextual fear memory, extinction of generalized contextual fear memory, and acquisition of cued fear extinction. Furthermore, the results specifically implicate MS/vDBB cholinergic neurons as critical for these emotional memory processes. Current definitions of the fear extinction circuit include IL input to the ITC and CeL (Likhtik et al., 2008; Quirk et al., 2003; Royer and Pare, 2002; Vidal-Gonzalez et al., 2006). Inhibitory GABAergic neuron projections from the vmPFC to the lateral amygdala and ventral hippocampal projections to the PL and BLA have also been implicated (Courtin et al., 2014; Orsini et al., 2011; Sotres-Bayon et al., 2012). Given the findings of this study, it would appear that MS/vDBB cholinergic neurons are also part of the fear extinction circuit in the rodent brain.
The exact mechanisms by which MS/vDBB cholinergic neurons facilitate inhibition of generalized contextual fear memory and acquisition of extinction memory remain to be determined. It is unlikely that generalized contextual fear memory induced by MS/vDBB cholinergic lesions were due to increases in unconditioned fear/anxiety expression or enhancements in contextual fear conditioning, because neither process is affected by BF cholinergic lesions (Frick et al., 2004; Knox and Berntson, 2006). However, the effects of MS/vDBB cholinergic lesions on generalized contextual fear memory could have led to deficits in acquisition of cued fear extinction. Enhanced stress generated from enhanced fear prior to cued fear extinction training disrupts acquisition of cued extinction memory (Chang and Maren, 2009; Maren and Chang, 2006). Thus, enhanced generalized contextual fear memory observed in MS/vDBB-lesion rats could have led to deficits in acquisition of cued fear extinction. Retrieval of contextual fear memory suppresses retrieval of cued extinction memory (Bouton et al., 2006). Generalized contextual fear memory induced by MS/vDBB lesions did not extinguish, which resulted in relatively high baseline levels of freezing prior to extinction testing. Thus, MS/vDBB cholinergic lesions may have impaired retrieval of cued fear extinction memory as well. MS/vDBB cholinergic lesions resulted in loss of cholinergic input to the Hipp and mPFC; two brain regions critical for contextual processing during emotional learning and memory (Bouton et al., 2006; Corcoran et al., 2005; Zelikowsky et al., 2013). This further supports the hypothesis that deficits in contextual memory processing contributed to deficits in acquisition of cued fear extinction in MS/vDBB-lesion rats. Of course, further research is needed to test this hypothesis as alternative hypotheses are possible. For example, MS/vDBB cholinergic lesions could directly impair acquisition of the CS-no UCS extinction rule.
Through what neural processes might MS/vDBB cholinergic neurons facilitate inhibition of generalized contextual fear memory and acquisition of extinction? Synchronization of neural activity between the Hipp and mPFC is critical for spatial learning and memory (Baeg et al., 2003; Griffin et al., 2007; Hallock and Griffin, 2014; Hallock et al., 2013), which raises the possibility that synchronized neural activity between the Hipp and mPFC could be critical for contextual memory formation during fear learning. If this is true, then MS/vDBB cholinergic lesions could impair contextual memory formation during fear learning by impairing synchronized neural activity between the Hipp and mPFC during fear learning. In turn, this could lead to generalized contextual fear memory, deficits in extinction of generalized contextual fear memory, and deficits in acquisition of cued fear extinction. While interesting, this hypothesis needs testing. This is especially so, because desychronization of theta coupling between the Hipp and mPFC, as well as Hipp and BLA, during acquisition of extinction memory and extinction memory testing are associated with robust extinction memory recall in mice (Lesting et al., 2011). This empirical finding is somewhat counter to the proposed hypothesis.
Increased hippocampal input to the PL and BLA suppresses fear extinction expression (Orsini et al., 2011; Sotres-Bayon et al., 2012). MS/vDBB cholinergic lesions could impair Hipp input to the PL and/or BLA during acquisition and retrieval of fear extinction. Such an effect would impair cued fear extinction. Further research examining this possibility is also needed.
Summary
The results of this study demonstrated that MS/vDBB cholinergic neurons are critical for inhibiting generalized contextual fear memory, extinguishing generalized contextual fear memory, and acquiring cued fear extinction. Further research is needed to better understand neural circuits, receptors, and molecular mechanisms through which MS/vDBB cholinergic neurons modulate these emotional processes.
Targeting the MS/vDBB in Experiments 1 and 2 resulted in AChE loss in the Hipp, but AChE loss in the IL was only observed in Experiment 2. Also, it would appear that hDBB and/or NBM cholinergic neurons project to the PL, because PL AChE loss was observed in Experiments 1 and 3, but not Experiment 2. These findings suggest that BF cholinergic input to the mPFC may be more differentiated than previously thought. The relevance of this differential projection pattern to BF cholinergic and mPFC function needs to be investigated.
Acknowledgments
We would like to thank all of the undergraduate students who helped make this study a possibility. We would also like to thank William Schreiber for his help with this study. This research was funded by start-up funding provided by the University of Delaware to D.K.
Grant sponsor – n/a
Grant number – n/a
Footnotes
None of the authors of this manuscript have any conflict of interest.
References
- Baeg EH, Kim YB, Huh K, Mook-Jung I, Kim HT, Jung MW. Dynamics of population code for working memory in the prefrontal cortex. Neuron. 2003;40(1):177–88. doi: 10.1016/s0896-6273(03)00597-x. [DOI] [PubMed] [Google Scholar]
- Baxter MG, Holland PC, Gallagher M. Disruption of decrements in conditioned stimulus processing by selective removal of hippocampal cholinergic input. J Neurosci. 1997;17(13):5230–6. doi: 10.1523/JNEUROSCI.17-13-05230.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berlau DJ, McGaugh JL. Enhancement of extinction memory consolidation: the role of the noradrenergic and GABAergic systems within the basolateral amygdala. Neurobiol Learn Mem. 2006;86(2):123–32. doi: 10.1016/j.nlm.2005.12.008. [DOI] [PubMed] [Google Scholar]
- Bouton ME, Westbrook RF, Corcoran KA, Maren S. Contextual and temporal modulation of extinction: behavioral and biological mechanisms. Biol Psychiatry. 2006;60(4):352–60. doi: 10.1016/j.biopsych.2005.12.015. [DOI] [PubMed] [Google Scholar]
- Chang CH, Knapska E, Orsini CA, Rabinak CA, Zimmerman JM, Maren S. Fear extinction in rodents. Curr Protoc Neurosci Chapter 8: Unit8 23. 2009 doi: 10.1002/0471142301.ns0823s47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang CH, Maren S. Early extinction after fear conditioning yields a context-independent and short-term suppression of conditional freezing in rats. Learn Mem. 2009;16(1):62–8. doi: 10.1101/lm.1085009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chhatwal JP, Myers KM, Ressler KJ, Davis M. Regulation of gephyrin and GABAA receptor binding within the amygdala after fear acquisition and extinction. J Neurosci. 2005;25(2):502–6. doi: 10.1523/JNEUROSCI.3301-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiba AA, Bucci DJ, Holland PC, Gallagher M. Basal forebrain cholinergic lesions disrupt increments but not decrements in conditioned stimulus processing. J Neurosci. 1995;15(11):7315–22. doi: 10.1523/JNEUROSCI.15-11-07315.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conner JM, Culberson A, Packowski C, Chiba AA, Tuszynski MH. Lesions of the Basal forebrain cholinergic system impair task acquisition and abolish cortical plasticity associated with motor skill learning. Neuron. 2003;38(5):819–29. doi: 10.1016/s0896-6273(03)00288-5. [DOI] [PubMed] [Google Scholar]
- Corcoran KA, Desmond TJ, Frey KA, Maren S. Hippocampal inactivation disrupts the acquisition and contextual encoding of fear extinction. J Neurosci. 2005;25(39):8978–87. doi: 10.1523/JNEUROSCI.2246-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Courtin J, Chaudun F, Rozeske RR, Karalis N, Gonzalez-Campo C, Wurtz H, Abdi A, Baufreton J, Bienvenu TC, Herry C. Prefrontal parvalbumin interneurons shape neuronal activity to drive fear expression. Nature. 2014;505(7481):92–6. doi: 10.1038/nature12755. [DOI] [PubMed] [Google Scholar]
- Frick KM, Kim JJ, Baxter MG. Effects of complete immunotoxin lesions of the cholinergic basal forebrain on fear conditioning and spatial learning. Hippocampus. 2004;14(2):244–54. doi: 10.1002/hipo.10169. [DOI] [PubMed] [Google Scholar]
- Griffin AL, Eichenbaum H, Hasselmo ME. Spatial representations of hippocampal CA1 neurons are modulated by behavioral context in a hippocampus-dependent memory task. J Neurosci. 2007;27(9):2416–23. doi: 10.1523/JNEUROSCI.4083-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hallock HL, Griffin AL. Spatial working memory deficits accompany reductions in hippocampal-prefrontal synchrony following inactivation of the ventral midline thalamic reuniens and rhomboid nuclei 2014 November 14–19th. Washington DC: [Google Scholar]
- Hallock HL, Wang A, Shaw CL, Griffin AL. Transient inactivation of the thalamic nucleus reuniens and rhomboid nucleus produces deficits of a working-memory dependent tactile-visual conditional discrimination task. Behav Neurosci. 2013;127(6):860–6. doi: 10.1037/a0034653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herry C, Johansen JP. Encoding of fear learning and memory in distributed neuronal circuits. Nat Neurosci. 2014;17(12):1644–54. doi: 10.1038/nn.3869. [DOI] [PubMed] [Google Scholar]
- Jones BE. Arousal systems. Front Biosci. 2003;8:s438–51. doi: 10.2741/1074. [DOI] [PubMed] [Google Scholar]
- Knox D, Berntson GG. Effect of nucleus basalis magnocellularis cholinergic lesions on fear-like and anxiety-like behavior. Behavioral Neuroscience. 2006;120(2):307–12. doi: 10.1037/0735-7044.120.2.307. [DOI] [PubMed] [Google Scholar]
- Knox D, Nault T, Henderson C, Liberzon I. Glucocorticoid Receptors And Extinction Retention Deficits In The Single Prolonged Stress Model. Neuroscience. 2012;223:163–173. doi: 10.1016/j.neuroscience.2012.07.047. [DOI] [PubMed] [Google Scholar]
- Lesting J, Narayanan RT, Kluge C, Sangha S, Seidenbecher T, Pape HC. Patterns of coupled theta activity in amygdala-hippocampal-prefrontal cortical circuits during fear extinction. PLoS One. 2011;6(6):e21714. doi: 10.1371/journal.pone.0021714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Likhtik E, Popa D, Apergis-Schoute J, Fidacaro GA, Pare D. Amygdala intercalated neurons are required for expression of fear extinction. Nature. 2008;454(7204):642–5. doi: 10.1038/nature07167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manns ID, Alonso A, Jones BE. Discharge properties of juxtacellularly labeled and immunohistochemically identified cholinergic basal forebrain neurons recorded in association with the electroencephalogram in anesthetized rats. J Neurosci. 2000a;20(4):1505–18. doi: 10.1523/JNEUROSCI.20-04-01505.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maren S, Chang CH. Recent fear is resistant to extinction. Proc Natl Acad Sci U S A. 2006;103(47):18020–5. doi: 10.1073/pnas.0608398103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGaughy J, Koene RA, Eichenbaum H, Hasselmo ME. Cholinergic deafferentation of the entorhinal cortex in rats impairs encoding of novel but not familiar stimuli in a delayed nonmatch-to-sample task. J Neurosci. 2005;25(44):10273–81. doi: 10.1523/JNEUROSCI.2386-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mesulam MM, Mufson EJ, Levey AI, Wainer BH. Cholinergic innervation of cortex by the basal forebrain: cytochemistry and cortical connections of the septal area, diagonal band nuclei, nucleus basalis (substantia innominata), and hypothalamus in the rhesus monkey. J Comp Neurol. 1983a;214(2):170–97. doi: 10.1002/cne.902140206. [DOI] [PubMed] [Google Scholar]
- Milad MR, Quirk GJ. Neurons in medial prefrontal cortex signal memory for fear extinction. Nature. 2002;420(6911):70–4. doi: 10.1038/nature01138. [DOI] [PubMed] [Google Scholar]
- Orsini CA, Kim JH, Knapska E, Maren S. Hippocampal and prefrontal projections to the basal amygdala mediate contextual regulation of fear after extinction. J Neurosci. 2011;31(47):17269–77. doi: 10.1523/JNEUROSCI.4095-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orsini CA, Maren S. Neural and cellular mechanisms of fear and extinction memory formation. Neurosci Biobehav Rev. 2012;36(7):1773–802. doi: 10.1016/j.neubiorev.2011.12.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paxinos G, Watson C. The Rat Brain in Stereotaxic Coordinates. San Diego: Academic Press; 1998. [Google Scholar]
- Quirk GJ, Garcia R, Gonzalez-Lima F. Prefrontal mechanisms in extinction of conditioned fear. Biol Psychiatry. 2006;60(4):337–43. doi: 10.1016/j.biopsych.2006.03.010. [DOI] [PubMed] [Google Scholar]
- Quirk GJ, Likhtik E, Pelletier JG, Pare D. Stimulation of medial prefrontal cortex decreases the responsiveness of central amygdala output neurons. J Neurosci. 2003;23(25):8800–7. doi: 10.1523/JNEUROSCI.23-25-08800.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Royer S, Pare D. Bidirectional synaptic plasticity in intercalated amygdala neurons and the extinction of conditioned fear responses. Neuroscience. 2002;115(2):455–62. doi: 10.1016/s0306-4522(02)00455-4. [DOI] [PubMed] [Google Scholar]
- Sarter M, Givens B, Bruno JP. The cognitive neuroscience of sustained attention: where top-down meets bottom-up. Brain Res Brain Res Rev. 2001;35(2):146–60. doi: 10.1016/s0165-0173(01)00044-3. [DOI] [PubMed] [Google Scholar]
- Sierra-Mercado D, Padilla-Coreano N, Quirk GJ. Dissociable roles of prelimbic and infralimbic cortices, ventral hippocampus, and basolateral amygdala in the expression and extinction of conditioned fear. Neuropsychopharmacology. 2011;36(2):529–38. doi: 10.1038/npp.2010.184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sotres-Bayon F, Sierra-Mercado D, Pardilla-Delgado E, Quirk GJ. Gating of fear in prelimbic cortex by hippocampal and amygdala inputs. Neuron. 2012;76(4):804–12. doi: 10.1016/j.neuron.2012.09.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tago H, Kimura H, Maeda T. Visualization of detailed acetylcholinesterase fiber and neuron staining in rat brain by a sensitive histochemical procedure. J Histochem Cytochem. 1986;34(11):1431–8. doi: 10.1177/34.11.2430009. [DOI] [PubMed] [Google Scholar]
- Tronson NC, Schrick C, Guzman YF, Huh KH, Srivastava DP, Penzes P, Guedea AL, Gao C, Radulovic J. Segregated populations of hippocampal principal CA1 neurons mediating conditioning and extinction of contextual fear. J Neurosci. 2009;29(11):3387–94. doi: 10.1523/JNEUROSCI.5619-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vidal-Gonzalez I, Vidal-Gonzalez B, Rauch SL, Quirk GJ. Microstimulation reveals opposing influences of prelimbic and infralimbic cortex on the expression of conditioned fear. Learn Mem. 2006;13(6):728–33. doi: 10.1101/lm.306106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woolf NJ, Eckenstein F, Butcher LL. Cholinergic systems in the rat brain: I. projections to the limbic telencephalon. Brain Research Bulletin. 1984;13(6):751–84. doi: 10.1016/0361-9230(84)90236-3. [DOI] [PubMed] [Google Scholar]
- Zelikowsky M, Bissiere S, Hast TA, Bennett RZ, Abdipranoto A, Vissel B, Fanselow MS. Prefrontal microcircuit underlies contextual learning after hippocampal loss. Proc Natl Acad Sci U S A. 2013;110(24):9938–43. doi: 10.1073/pnas.1301691110. [DOI] [PMC free article] [PubMed] [Google Scholar]


