Abstract
Plastid-made biopharmaceuticals treat major metabolic or genetic disorders, including Alzheimer’s, diabetes, hypertension, hemophilia, and retinopathy. Booster vaccines made in chloroplasts prevent global infectious diseases, such as tuberculosis, malaria, cholera, and polio, and biological threats, such as anthrax and plague. Recent advances in this field include commercial-scale production of human therapeutic proteins in FDA-approved cGMP facilities, development of tags to deliver protein drugs to targeted human cells or tissues, methods to deliver precise doses, and long-term stability of protein drugs at ambient temperature, maintaining their efficacy. Codon optimization utilizing valuable information from sequenced chloroplast genomes enhanced expression of eukaryotic human or viral genes in chloroplasts and offered unique insights into translation in chloroplasts. Support from major biopharmaceutical companies, development of hydroponic production systems, and evaluation by regulatory agencies, including the CDC, FDA, and USDA, augur well for advancing this novel concept to the clinic and revolutionizing affordable healthcare.
Keywords: chloroplast genome, oral drug delivery, plant vaccines/biopharmaceuticals, oral tolerance, mucosal/systemic immunity
INTRODUCTION
Protein drugs are prohibitively expensive and are not affordable for >90% of the global population, as one-third earns less than $2 per day (69, 71). The cost of the top 10 biologic drugs sold in 2013 exceeds the gross domestic product of 75% of all countries (139). Insulin, which is needed to treat the global diabetes epidemic, has been available for several decades and yet is unaffordable for more than 90% of the global population (69, 71). Beyond pricing policies of biopharmaceutical companies, there are major technological hurdles in production and delivery of protein drugs. Current production systems use fermentation facilities, which cost $500–$900 million (47, 121) and require prohibitively expensive purification of host proteins (69, 71). In addition, purified proteins are highly unstable and require cold storage, transportation, and sterile injections (69, 71). Furthermore, current vaccines use killed or attenuated viruses or bacteria, which can revert to virulence or recombine with other pathogens and result in vaccine-derived diseases (10, 11, 15). Indeed, the World Health Organization (WHO) has recommended withdrawal of oral polio vaccine (OPV) type 2 since April 2016 because of vaccine-associated paralytic poliomyelitis in recipients of OPV (16). OPV reverted to virulence by recombination with other enteroviruses or point mutations (10). In order to address these concerns, it is important to develop new strategies for production and delivery of protein drugs to prevent and treat metabolic/genetic disorders or infectious diseases.
Almost two decades ago, the field of chloroplast genetic engineering blossomed with several major publications on the high levels of insecticidal proteins expressed in plants, with Bt crystals in chloroplasts (31) and maternal inheritance of the most widely used herbicide resistance gene in genetically modified (GM) crops (19). Because these studies were demonstrated in the model plant tobacco, there was a great need to validate these traits in economically important crops. This was accomplished by creating transplastomic plants via somatic embryogenesis, first demonstrated in carrot (68); carrot is highly salt-sensitive, and transplastomic carrots could grow on 400–500 mM NaCl, which is the highest level of salt tolerance reported in the published literature. This ushered in a new era that led to the creation of transplastomic soybean plants with high expression of insecticidal proteins or herbicide resistance genes but that were free of antibiotic resistance genes (33, 34). Because this work was performed by industry (Bayer CropScience), there was excitement that these findings would advance toward commercial development, but this second-generation technology could not compete with nuclear GM crops already in the marketplace. Recent reports of resistant pests, new restrictions imposed by the United States Department of Agriculture (USDA) on planting Bt corn (61, 147), and European regulations on antibiotic-free GM crops (30) may encourage the utilization of transplastomic plants to enhance transgene expression and improve public perception of GM plants. In parallel, further technical advances are needed to transform chloroplast genomes of cereal crops, a technology that has been elusive for decades. This advance may require a better understanding of the stability of the chloroplast genome in mature leaves (90), identification of new selectable markers, and optimization of somatic embryogenesis in cereals.
Although GM crops meet the needs of farmers to confer insect or herbicide resistance with minimal levels of expression, nuclear genetic engineering has not yet been successful for molecular farming applications, which require much higher levels of transgene expression. Therefore, the transplastomic approach was developed to capture this niche, taking advantage of high-level expression and transgene containment capabilities. In addition, maternal inheritance of transgenes, which minimizes or eliminates escape via pollen, and the harvest of leaves that express protein drugs prior to flowering offer total transgene containment. The first biopharmaceutical protein and vaccine antigen were developed 15 years ago in transplastomic tobacco (22, 122). In the past decade, significant progress has been made in developing expression of therapeutic proteins in chloroplasts of edible plants (lettuce) and advance their oral delivery in a dose-dependent manner using lyophilized plant cells in order to eliminate prohibitively expensive fermentation, purification, cold storage, and transportation and develop affordable drugs to prevent or treat human infectious and inherited diseases. Further advances have been made recently in commercial-scale production of protein drugs in cGMP facilities approved by the US Food and Drug Administration (FDA) (124) as well as in the evaluation of their efficacy and toxicology in large animal models.
In this novel protein drug production and delivery concept using transplastomic plants, human blood proteins or vaccine antigen genes are cloned from different genomes and are stably integrated into the plastid genome of edible plants by bombarding chloroplast vectors using the gene gun. When all copies of plastid genomes in each cell are modified (i.e., homoplasmy is achieved), protein drugs can be expressed at high levels. For example, proinsulin can be expressed as high as 70% of total leaf protein (9, 107). When plant cells are lyophilized, ground powder in capsules can be stored for several months or years at room temperature without protein drugs losing their efficacy (69, 72, 74, 124). When lyophilized plant cells are orally delivered, the plant cell wall protects protein drugs from acids and enzymes in the stomach, which are incapable of breaking down all glycosidic bonds (40, 84). However, when intact plant cells reach the gut, commensal bacteria release cellulases and lyse plant cells, releasing protein drugs into the gut lumen (71, 81, 145). When suitable tags are fused to protein drugs, they cross the gut epithelium and are delivered into circulation (9, 72, 117), the immune system (3, 16, 29, 66, 74, 102–105, 118, 123, 124, 135, 141), or even across the blood-brain barrier (BBB) (65) or blood-retinal barrier (BRB) (119). Although these protein drug delivery concepts were developed a decade ago (81), delivery to specific targets has since advanced further (145). Most recently, this concept has been successfully employed to treat diabetes (9, 72, 105), hypertension (117), Alzheimer’s disease (65), hemophilia (118, 124, 135, 141), Pompe disease (123), and retinopathy (119). This approach has also been successful in developing protection against several infectious diseases (3, 16, 29, 66, 74). Commercial-scale production in a cGMP facility has also been recently achieved, facilitating further clinical development (124).
In this review, we capture recent advances in creation and production of transplastomic plants to treat human genetic or metabolic disorders or to protect against infectious diseases. In addition, we discuss potential challenges in expressing eukaryotic human or viral genes in prokaryotic chloroplasts and how these challenges can be addressed using modern genomic, proteomic, and ribosome profiling studies. Targeted proteomic quantification by mass spectrometry is yet another new tool used to deliver precise and repetitive doses of protein drugs made in plants, especially for quantitation of insoluble proteins without the need for purification. Future directions in the delivery of low-cost protein drugs or virus- and cold chain–free vaccines against infectious diseases are discussed.
CHLOROPLAST GENETIC ENGINEERING CONCEPTS
The plastid genome encodes ~80–100 proteins, all rRNAs, and ~30 of 64 tRNAs (23). It is organized into four major regions: a small single-copy (SSC) region, a large single-copy (LSC) region, and two copies of an inverted repeat (IR) region. Among 800 sequenced chloroplast genomes, DNA sequences between IR regions within the same chloroplast genome are identical without a single nucleotide variation (23). This is due to the copy correction mechanism, which ensures that changes made to one copy of the IR are duplicated in the other (19, 49); however, this mechanism is poorly understood. Although most reports assume that the plastid genome is circular, evidence of linear plastid genomes also exists. The percentage of circular versus linear genomes is highly debated (90), and further studies are needed to resolve this matter. Plastid genomes and genes have retained many prokaryotic-like features: Codon usage is similar to that found in prokaryotes (16, 26, 70, 125), and few protein-coding genes contain introns (23, 28, 106, 109).
In pioneering studies, introduction of foreign genes into chloroplasts was performed in isolated chloroplasts with the goal of reintroducing modified chloroplasts back into protoplasts. Indeed, green chloroplasts were introduced into albino protoplasts, and variegated plants were regenerated through this approach. Therefore, the first approach for introduction of foreign genes into chloroplasts was done using isolated chloroplasts that could survive for longer periods outside plant cells (20, 24). However, the invention of the gene gun provided a major breakthrough to introduce foreign DNA using tungsten particles coated with chloroplast vectors and gun powder to propel them into plant cells (18); gun powder was subsequently replaced with helium to enhance the safety of this particle delivery system (146). The first generation of chloroplast vectors utilized chloroplast origin of replication and demonstrated autonomous replication of introduced vectors for prolonged periods (27). Although this approach is now pursued in synthetic biology for introduction of large synplastomes, introduction of short segments of DNA was subsequently pursued using transgene integration by stable recombination.
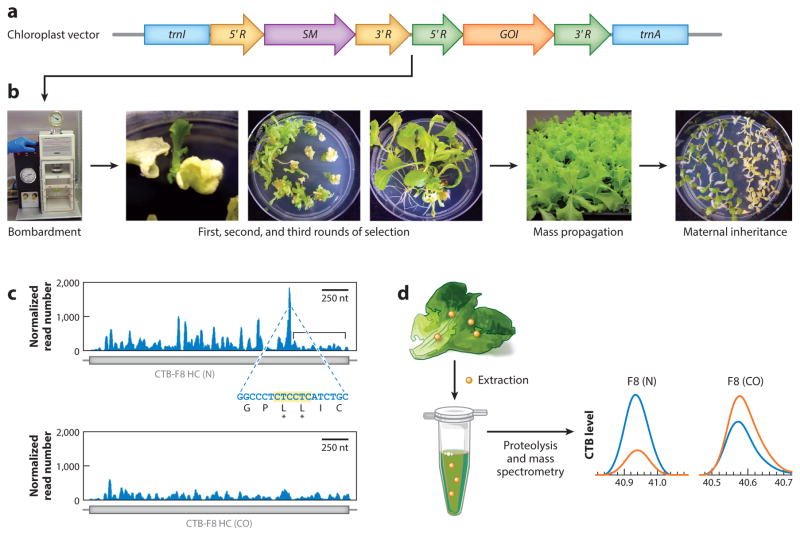
Unlike nuclear transgene integration, which occurs via nonhomologous recombination, plastid genome transformation occurs only through double homologous recombination utilizing a homolog of E. coli RecA (61, 100). In principle, a transgene cassette could be engineered to integrate anywhere, but the integration site is critical for maximal gene expression. On the genomic scale, LSC regions and IRs have both been tested, and transgenes inserted into IRs express at considerably higher levels (23). Historically, two integration sites have been used: transcriptionally active spacer regions, which lie within operons in the same strand, or transcriptionally silent spacer regions, which are on the strand opposite transcribed genes (61). The most widely used integration site is between trnA and trnI in the 16S rRNA operon, but the regions between rbcL and accD or rps12 and trnV are also used (23, 61). A direct comparison demonstrated a 25-fold increase in transgene expression when the same expression cassette was integrated into the transcriptionally active trnA/trnI site versus the rbcL/accD silent spacer regions (67), possibly because of multiple endogenous/heterologous promoters and introns within trnI/trnA genes to facilitate foreign transcript processing. Therefore, a large majority of transplastomic studies in the published literature utilize this site for transgene integration (Figure 1a) (23).
Figure 1.
Concepts of chloroplast transformation and characterization of transplastomic plants. (a) Representation of a chloroplast vector containing the most commonly used flanking sequences for homologous recombination, trnI and trnA (blue). The selectable marker (SM) gene ( purple) and gene of interest (GOI; orange) are regulated by promoters and 5′ untranslated regions (UTRs) (5′ R; yellow and green) to enhance transcription and translation and by 3′ UTRs (3′R; yellow and green) to enhance transcript stability. (b) The vector is bombarded into leaves with a gene gun. Homoplasmy is achieved after three rounds of selection on antibiotic-containing media, followed by mass propagation and verification of maternal inheritance. (c) Ribosome profiling demonstrates that ribosome stalling (blue peaks) occurs throughout a native human gene, Clotting Factor VIII (F8) (N; top), and especially in a region with consecutive lysines, but is substantially reduced when codons are optimized for plastid expression (CO; bottom). The bracketed region in the top graph indicates decreased ribosome occupancy of the transcript after the stalling site; this region is absent from the codon-optimized transcript, indicating increased translation of the entire open reading frame. (d ) Quantification of a therapeutic protein expressed in chloroplasts. Protein is extracted from transplastomic plants ( yellow dots), and the extract is subjected to proteolysis and mass spectrometry by parallel reaction monitoring. Levels of cholera toxin B (CTB) from the fusion protein (orange curves) are compared with standards (blue curves) and show higher levels in plants expressing codon-optimized human FVIII than in plants expressing the native gene. Panels c and d adapted with permission from Reference 70.
Sequencing of more than 800 plastid genomes has shown that intergenic spacer regions are highly variable (23); among Solanaceae members, only four intergenic spacer regions are conserved, including the trnA and trnI regions (21), and not a single one is conserved among cereal plastid genomes (112). Because even a few mismatches between plastid vector sequences and the host genome can dramatically reduce transformation efficiency (107), this variability underscores the need for complete plastid genome sequences to generate species-specific vectors that maximize the number of independent transformation events.
Because of different codon preferences, sequences of eukaryotic genes of interest (GOIs; Figure 1a) should be optimized for plastid codon usage. The psbA gene has a unique codon bias that may have evolved to promote more efficient translation (85), so it has been used as the basis for several codon optimization methods (26, 70). Because plastid gene expression is primarily regulated at the post-transcriptional level (6), selection of 5′ and 3′ ntranslated regions (UTRs) (Figure 1a) is also crucial for promoting maximal gene expression. In particular, the stem-loop secondary structure of the 5′ UTR stabilizes mRNAs and promotes efficient translation, likely through specific interactions with RNA-binding proteins (RBPs) (8, 52, 107, 150). The 3′ UTR facilitates mRNA stability of plastid-expressed genes (128). Because the psbA gene is both transcribed and translated in a light-dependent manner, and it is the most highly translated gene in the plastome, psbA 5′ and 3′ UTRs have been widely used to promote high-level expression of foreign genes in chloroplasts (23, 36, 61, 150). Indeed, after the use of the psbA promoter and UTR in the first chloroplast vector (27), it is still the most widely used regulatory sequence in transplastomic studies, especially in studies that report the highest levels of transgene expression (23). However, use of heterologous psbA regulatory sequences decreases efficiency of translation because of reduction in sequence-specific binding between endogenous RBPs and their targets (107).
Various studies have demonstrated that intercistronic processing is not required for efficient translation of many polycistronic mRNAs (31, 97, 149). In particular, a recent in-depth study analyzed ribosome footprints on chloroplast mRNAs and found that in a dicistronic mRNA containing open reading frames (ORFs) for the atpB and atpE genes, mutations affecting translation of the upstream atpB gene had no effect on atpE (149). This result and others show that ORFs are translated independently, even on the same cistron (6, 149).
As mentioned above, chloroplast vectors are delivered using biolistics (Figure 1b) (136). Plastids that successfully incorporate foreign DNA are selectively enriched to achieve homoplasmy, which is essential for stable transgene expression (61). Including an origin of replication (ori) in the transgene cassette (27), which promotes replication of chloroplast vectors prior to integration, helps to achieve homoplasmy even during the first round of selection (49, 61). Additionally, inserting transgenes into the IR doubles the number of transgene copies owing to copy correction, rapidly advancing transplastomic lines to homoplasmy (49, 61).
Generally, homoplasmy is reached after multiple rounds of selection on antibiotic-containing media (Figure 1b). The most commonly used selectable marker (SM) gene (Figure 1a) is aadA, which confers resistance to spectinomycin and streptomycin (45, 127); these antibiotics selectively inhibit plastid translation. However, interest in developing antibiotic-free selectable markers has increased recently because of the stringent requirements for GM plants (61). Antibiotic-free selectable markers include an anthranilate synthase mutant that grows on a toxic tryptophan analog (7), a bacterial D–amino acid oxidase for dual positive or negative selection of transplastomic tobacco (43), and a bacterial isopentenyl transferase that can be used to select transplastomic plants on cytokinin-free media (35). A bacterial gene conferring herbicide resistance (4-hydroxyphenylpyruvate dioxygenase) has also been used as an antibiotic-free selectable marker in both tobacco and soybean (33). Other efforts included the use of the betaine aldehyde dehydrogenase (BADH) gene, whose product detoxifies betaine aldehyde by converting it to glycine betaine (25). In transplastomic carrots, BADH conferred salt-stress tolerance and turned yellow plastids green (68). However, almost all antibiotic-free selectable markers discussed above are only suitable for the second round of selection, and further research is needed to identify new selectable marker genes.
An additional strategy is marker gene excision, which can be accomplished through direct repeat-mediated recombination (30, 58) or the use of the CRE-lox system (17). More recently, the site-specific Bxb1 recombinase has been used with attP/attB sites to precisely excise DNA from the plastid genome in the widely used trnI/trnA region, making it a valuable tool for future studies (116). Regardless of the selection method used, when homoplasmy is achieved mass propagation can proceed (Figure 1b). Maternal inheritance of the plastid genome in most species (Figure 1b) makes transgenes easier to contain and also lowers regulatory barriers to bringing transplastomic technology to market.
EXPRESSION OF EUKARYOTIC GENES IN PROKARYOTIC PLASTIDS
Plastid transformation technology promotes expression of high levels of recombinant proteins; prokaryotic or short human genes are highly expressed in chloroplasts (up to 70% of total leaf protein) (31, 107). However, low expression of larger human proteins is a major challenge in clinical translation of therapeutic proteins (70, 71, 117, 118). Likewise, expression of viral vaccine antigens is quite variable (82). Codon optimization based on AT content or codon usage has been performed to address this concern (26, 42, 78, 87). Whereas some studies reported an increase in transgene expression after codon optimization, others observed negligible enhancement. Most importantly, these studies tested only small eukaryotic coding sequences, and with limited mechanistic studies, they are not useful in solving major challenges in expression of large human proteins. In vitro studies of heterologous genes in tobacco chloroplasts have indicated that translation efficiencies of individual codons do not always correlate with codon usage (87). Therefore, there is a critical need for understanding and monitoring chloroplast translation. Ribosome profiling is a new tool to study in vivo protein translation dynamics with codon-level resolution; the technology analyzes translation using deep sequencing of ribosome-protected mRNA fragments to map ribosomes on a genome-wide scale (59, 60, 149). High-resolution, gene-specific ribosome density profiles show how variations in translation rate and effects such as ribosomal pausing modulate protein synthesis and folding (60, 61). Several studies have demonstrated in vivo ribosome behavior in maize chloroplasts (148, 149). Therefore, ribosome profiling has been used recently to study expression of transgenes in chloroplasts (Figure 1c) (70). Ribosome footprints did not increase proportionately with enhanced translation of transgenes or even decrease in codon optimized genes, but this will serve as a useful tool in diagnosing rate-limiting steps. For example, ribosome pause sites at rare codons in native human genes were eliminated upon codon optimization.
Accurate quantitation of target proteins and peptides in complex biological systems is critical to a diverse range of research and clinical applications (143). Some common immunoassays such as ELISA cannot be used to quantify insoluble proteins due to aggregation or formation of multimers, but accurate quantification is essential for drug delivery. Thus, targeted methods for sensitive and specific protein quantification are needed. Targeted proteomic quantification by mass spectrometry by parallel reaction monitoring (PRM) has become a powerful tool for relative and absolute protein quantitation; it provides high specificity for the target protein, high sensitivity for detection, and high-throughput quantification with confident targeted peptide confirmation (99). PRM has been successfully applied to validate the relative abundance of proteins in human sera (64, 101) and to study post-translational modifications (120, 131, 132), but it has not been utilized for plant-derived protein drugs. However, it has been successfully used recently to monitor doses of protein drugs expressed in chloroplasts (Figure 1d ) (70). PRM analysis facilitated accurate quantitation of foreign proteins based on normalization of stable isotope-labeled standard (SIS) peptides or housekeeping chloroplast proteins, thereby eliminating limitations of western blots in protein transfer, denaturation, solubility, or stability.
Inducing immunity or tolerance only requires presentation of linear peptide epitopes to the immune system. However, expressing functional proteins in chloroplasts often requires additional post-translational modifications. Recent studies have shown that human blood proteins with disulfide bonds, such as insulin and interferon, are properly folded and are fully functional in chloroplasts (2, 9). Chloroplasts can also assemble multimeric disulfide bonded structures such as the nontoxic cholera toxin B (CTB) subunit, which can then bind to GM1 receptors to facilitate drug delivery across the gut epithelium (discussed below) (3, 9, 16, 29, 66, 72, 74, 104, 105, 117–119, 123, 124, 135). Similarly, the human papillomavirus (HPV) L1 protein has been shown to self-assemble into virus-like particles (VLPs) within tobacco chloroplasts (39, 138), and aggregates of VLPs were observed in chloroplast extracts from transplastomic lettuce expressing the prM/E protein from dengue virus (62). Furthermore, protein disulfide isomerase/thioredoxin expression has been shown to enhance folding and assembly of human serum albumin, which contains 17 disulfide bonds, within chloroplasts (111). Cyclization in addition to three disulfide bonds is required for antimicrobial activity of retrocyclin-101, and both these modifications are observed in chloroplasts (51, 77). Indeed, the need for post-translational modifications is a major limitation of chemically synthesized peptides, and chloroplasts are the only biological systems known to make retrocyclin with antimicrobial activity (51). In addition, protein lipidation and N-terminal methionine excision occur in chloroplasts (15, 44, 122). However, the chloroplast is an N-glycosylation–free compartment, which offers unique advantages and disadvantages (15). There are examples in which glycosylation sites in human biopharmaceuticals such as IGF-1 are inactivated to obtain fully functional proteins (53, 94), making plastids a feasible platform for their synthesis without the need to modify glycosylation sites (15).
ORAL DRUG DELIVERY CONCEPTS
Administering biopharmaceuticals by injection has disadvantages, including reduced patient compliance, possible infection at the injection site, the need for refrigerated storage and transportation, and the requirement for skilled personnel to administer the medication (69, 71, 73). Oral delivery of these drugs (Figure 2a) could ameliorate or eliminate these drawbacks and revolutionize health-care. A major challenge associated with oral delivery of therapeutic proteins is degradation by acids and proteases in the stomach (15, 61). Bioencapsulating proteins within plant cells protects them in the stomach and facilitates their entry into the gut, where commensal microbes break down the cell wall and release the protein drug into the intestinal lumen (Figure 2b) (15, 40, 61, 71, 81, 84, 145). Translocating the released protein from the intestine to the immune or circulatory system requires fusing it with a transmucosal carrier protein (81, 145). The most widely used carrier protein is CTB. Functional CTB is a homopentamer that enters target cells by binding the GM1 ganglioside, which is present in membranes of intestinal epithelial cells, neurons, and immune cells, among others (5, 37, 76, 81). Binding to GM1 causes retrograde trafficking of CTB through the cell and into the ER (5, 73). Alternatively, the entire CTB-GM1 complex may be transcytosed across the intestinal epithelium (113). Because functional CTB is pentameric, a hinge is engineered into the fusion protein to prevent steric hindrance and facilitate the formation of CTB pentamers (9, 29, 65, 72, 81, 117–119, 123, 135). Additionally, a protease cleavage site is included between CTB, or any other carrier peptide, to promote the delivery of only the therapeutic protein or antigen to the target tissue following uptake. Furin is the most commonly used cleavage site because it is a ubiquitous protease present in most cell types and results in complete cleavage without leaving any amino acid residue on the therapeutic protein (9, 29, 65, 72, 81, 117–119, 123, 135).
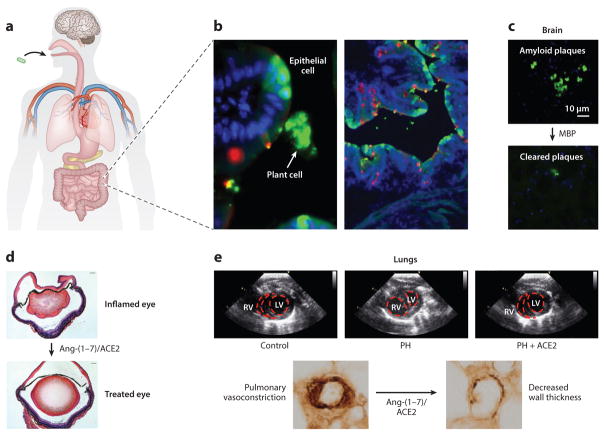
Figure 2.
Efficacy of chloroplast-made human blood proteins. (a) Capsules containing lyophilized plant cells expressing human blood proteins are orally delivered. (b) Bioencapsulation of blood proteins within the plant cell wall protects them in the stomach until they reach the gut. (Left) Close-up image of intact plant cells (arrow) expressing cholera toxin B (CTB)-fused green fluorescent protein (GFP) that passed through the stomach and reached the small intestine without any disruption. Intestinal epithelial cells take up GFP after lysis of plant cells by gut microbes. (Right) Widespread GFP uptake by gut epithelial cells through binding of CTB to GM1 receptors. Panel b adapted with permission from Reference 145. (c) CTB-fused myelin basic protein (MBP) crossed the blood-brain barrier and cleared amyloid plaques in Alzheimer’s brain tissue. Panel c adapted with permission from Reference 65. (d ) CTB-fused angiotensin (Ang)-(1–7) or angiotensin-converting enzyme 2 (ACE2) crossed the blood-retinal barrier, restored retinal folding, and reduced ocular inflammation. Panel d adapted with permission from Reference 119. (e) CTB-fused Ang-(1–7) or ACE2 orally delivered to rats after induction of pulmonary hypertension (PH) arrested disease progression, improved right heart function by reducing the size of the right ventricle (RV) (top), and decreased pulmonary wall thickness and lung injury (bottom). Abbreviation: LV, left ventricle. Panel e adapted with permission from Reference 117.
Because GM1 is expressed in many tissue types, and particularly in neurons, therapeutic CTB fusion proteins can cross the BBB and BRB; recent studies have demonstrated that functional, orally delivered CTB-fused proteins bioencapsulated in plant cells can cross the BBB or BRB and clear amyloid plaques associated with Alzheimer’s disease (Figure 2c) (65) or alleviate symptoms of retinopathy (Figure 2d ) (119). However, the broad distribution of GM1 also means that nontarget cells and tissues could take up CTB fusion proteins (145). Precision targeting of orally delivered therapeutic proteins could mitigate this problem but requires the identification and characterization of new cell- and tissue-specific carrier peptides. A recent study aimed at identifying such proteins represents an important step (145). In this study, authors used a dendritic cell peptide (DCpep) as a dendritic cell (DC)-specific carrier protein, opening the door to targeting therapeutic proteins specifically to the immune system (145). Authors also reported that a carrier peptide derived from the protein transduction domain (PTD) of the pancreatic and duodenal homeobox-1 protein not only delivered its GFP cargo more efficiently than CTB but also specifically targeted GFP to nonimmune cells (145). Therefore, PTD could be used to deliver therapeutic proteins orally without eliciting an immune response (145). Further in vivo testing of these peptides, identification of additional peptides, and even custom-designed novel peptides should expand the portfolio of available carrier proteins for highly specific oral drug delivery. Mechanistic aspects of protein drug delivery across the gut, BRB, or cell membranes are discussed in depth in a recent review (71).
CHLOROPLAST GENOME ENGINEERING TO CONFER TOLERANCE
Oral tolerance is the suppression of immune responses to an antigen by prior oral administration of the same antigen. Detailed mechanisms of oral tolerance have been reviewed elsewhere (15, 144), but we provide a brief conceptual overview here. Induction of antigen-specific regulatory T cells (Tregs) is a key component of the tolerance mechanism (142, 144). Secretion of interleukin-10 (IL-10) and transforming growth factor-β (TGF-β) by Tregs induces apoptosis or cell cycle arrest in effector T cells and blocks co-stimulation and maturation of DCs, which shifts DCs into tolerogenic function; when immature DCs present antigens to T cells in the absence of inflammation, tolerance is induced (75, 86). Thus, the interaction between Tregs and DCs plays a major role in oral tolerance (15). The frequency of oral antigen administration is critical; multiple antigen feedings are more effective than a single feeding in inducing oral tolerance in inflammatory immune responses and autoimmune disease models (50, 91, 142). Moreover, continuous feeding correlates with enhanced production of IL-10 and TGF-β (140). Age, immunological status, antigen dose, structure, and the form of the antigen fed affect oral tolerance induction (91). Antigen conjugation to transmucosal carrier proteins such as CTB greatly reduces required antigen concentration and the number of doses needed for oral tolerance induction and has been used for immunotherapy in several models of autoimmune diseases, type I allergies, and even phase I/phase II human clinical trials in patients with Behçet’s disease (98, 126).
Oral tolerance induction could also benefit those who suffer from genetic disorders that cause the reduction or loss of essential proteins or enzymes. Treatment for these disorders frequently involves injection of life-saving replacement proteins/enzymes, but neutralizing antibodies are developed against the injected therapeutic protein, at a minimum rendering it useless and at worst causing fatal anaphylaxis (12, 32, 61, 63). In some cases, antibody titer can be reduced through repeated, long-term exposure to the therapeutic protein [immune tolerance induction (ITI)], but this treatment is expensive and is not always effective (12, 61, 63, 73). Oral tolerance induction can be used to prevent inhibitor formation, as described below for model diseases.
Hemophilia
Hemophilia is an X-linked bleeding disorder caused by mutations in coagulation factor VIII (FVIII, hemophilia A) or coagulation factor IX (FIX, hemophilia B) and can affect as many as 1 in 5,000 males (12). Patients that develop neutralizing antibodies (inhibitors) against coagulation factors can be treated through ITI, but only 70% of hemophilia A and 30% of hemophilia B patients respond (63). To combat this problem, plant-based systems to promote oral tolerance have been developed for both hemophilia A and B. For hemophilia B, transplastomic tobacco plants were generated that expressed pentameric CTB-fused FIX, and powdered, frozen leaf tissue was orally administered to hemophiliac mice for two months; after the first month, injection of FIX began. Fatal anaphylaxis occurred in >75% of control animals that were either unfed or fed wild-type tissue, but >90% of animals fed CTB-FIX survived (135). Although FIX injection caused inhibitor formation after feeding stopped, when CTB-FIX feeding resumed, inhibitor levels dropped, demonstrating that feeding CTB-FIX can both prevent and reverse inhibitor formation (135). However, long-term tolerance required repetitive feeding of plant cells (141). In a follow-up study, CTB-FIX was expressed in lettuce chloroplasts and was produced at commercial scale in a cGMP hydroponic facility (124). Lyophilized lettuce cells expressing CTB-FIX maintained their efficacy with proper folding and assembly of CTB-FIX up to 2 years when stored at ambient temperature (124). Inhibitor titers in hemophilia B mice fed CTB-FIX were ~15-fold lower than control mice after FIX injections, and no mice that were fed CTB-FIX experienced anaphylaxis in response to FIX injection (124). To treat inhibitor formation in hemophilia A, transplastomic plants expressing CTB fused with the FVIII heavy chain (HC) or the C2 domain of the light chain were generated. Feeding powdered, lyophilized plant cells to hemophilia A mice prior to intravenous FVIII treatment suppressed inhibitor formation by ~sevenfold (118). In animals with previous exposure to FVIII, inhibitor levels were reduced three- to sevenfold after feeding CTB-FVIII plant cells when compared with controls, indicating that oral delivery of CTB-FVIII can not only prevent but also reverse inhibitor formation in hemophilia A mice (118).
Pompe Disease
Pompe disease is a lysosomal storage disorder caused by reduction or loss of the enzyme acid α-glucosidase (GAA), leading to the accumulation of glycogen in lysosomes (32). Currently, the only available treatment is enzyme replacement therapy (ERT); without it, patients with the most severe symptoms could die within a year (32). However, ~25% of Pompe patients are at high risk for formation of inhibitors, which can hinder treatment and even worsen the disease when enzyme replacement therapy continues in the presence of inhibitors (32). To facilitate oral tolerance to GAA, transplastomic tobacco plants were created that expressed CTB fused with a shortened version of GAA that contained all known antigenic epitopes (123). Full-length GAA was toxic to chloroplasts, and transplastomic lines could not be generated (123). CTB-GAA-NT (N-terminal 410 amino acids) when expressed in chloroplasts assembled into pentamers (123). Pompe mice were fed 1.5 μg CTB-GAA twice per week for a month before beginning weekly injections (500 μg GAA/mouse). Despite heavy GAA challenge—a 330-fold higher dose than that orally delivered using plant cells—IgG antibody formation was suppressed ~threefold when compared with control mice (123). These results demonstrate that plant-made, orally delivered antigens can promote oral tolerance to a variety of antigenic molecules and represent a step toward improving outcomes and quality of life for patients that require replacement therapy.
CHLOROPLAST GENOME ENGINEERING TO PREVENT OR TREAT METABOLIC OR GENETIC DISORDERS
Oral tolerance induction does not require delivered proteins to be functional; effective immune suppression requires only that T cell epitopes be present (118, 123, 124, 135, 141). By contrast, disease treatment requires functional proteins. Production, mechanism of action, and efficacy of plant-made functional biopharmaceuticals have recently been reviewed in detail (71), but here we give several examples of functional, orally delivered plant-made biopharmaceuticals that effectively treat metabolic or genetic disorders.
Alzheimer’s Disease
Alzheimer’s disease is an irreversible neurodegenerative disorder that affects more than five million people in the United States, making it the sixth most common cause of death and the third most common in older adults (88). It is the most common cause of dementia, and the only treatment is symptom management; no treatment is currently available to halt or reverse its progression (83, 88). A key aspect of Alzheimer’s pathology is deposition of extracellular aggregates (plaques) of the β-amyloid (Aβ) peptide (129). These plaques may initiate the disease and have been suggested as high-priority therapeutic targets (129). One potential therapy involves myelin basic protein (MBP), which cleaves Aβ peptides in vitro (79). Although delivering molecules such as MBP across the BBB is very challenging, CTB served as an effective carrier because of GM1 in the BBB. MBP was fused to CTB and expressed in chloroplasts (65). In ex vivo studies on brain slices, CTB-MBP reduced amyloid plaque size up to 60% in mice and 47% in postmortem human Alzheimer’s disease patients (Figure 2c) (65). Most importantly, in a mouse model of Alzheimer’s disease, feeding mice with ~30 μg of chloroplast-made CTB-MBP three times per week for three months reduced amyloid levels up to 70% in brains and 60% in retina (65). This study is the first report showing the successful oral delivery of a therapeutic protein across BBB and BRB (83). The mechanism of protein drug delivery across the BBB is described in depth elsewhere (71).
Retinopathy
Globally, ~285 million people are visually impaired, and retinopathy accounts for at least 2 million of these cases (92). Hundreds of thousands of others are affected by inflammatory retinal diseases (55). Both carry a high risk for blindness if left untreated. A good therapeutic target to treat retinal inflammation is the renin-angiotensin system (RAS) (41). The RAS consists of two axes with opposing effects: the proinflammatory ACE/AngII/AT1 axis and the anti-inflammatory/vasoprotective ACE2/Ang-(1–7)/Mas axis (110). To treat ocular inflammation, two components of the anti-inflammatory axis, ACE2 and Ang-(1–7), were separately fused with CTB and introduced into the tobacco plastid genome. Proteins encoded by both constructs interacted with GM1 in vitro, and up to 5 h after oral delivery, they were detected in sera and retinas of treated mice, confirming their ability to cross the BRB (119). In particular, animals fed with plant material containing ~0.05 mg of CTB-ACE2 showed 40% more ACE2 activity in serum and 20% more ACE2 activity in retinas than mice given wild-type material (119). More importantly, mice fed with plant cells containing ~0.25 mg of CTB-Ang-(1–7) or 0.05 mg of CTB-ACE2 showed reduced symptoms of both induced and autoimmune ocular inflammation, demonstrating the efficacy of this system to treat retinopathy (Figure 2d ) (119).
Hypertension
Pulmonary hypertension (PH) is an inflammatory disease characterized by elevated blood pressure in arteries between the heart and lungs. It compromises right heart function, is estimated to affect more than 100 million people worldwide, and is associated with a <60% three-year survival rate (57, 114). Current treatments are prohibitively expensive, are frequently administered via injection, and have remained essentially unchanged over time; even the newest therapies work on the same three pathways that have been targeted for years (56). Despite recent clinical advances, the high death rate associated with PH warrants new therapeutics (56). To that end, a novel treatment targeting the RAS is currently being developed. Feeding mice with plant cells containing 0.05 mg of CTB-ACE2 or 0.5 mg of CTB-Ang-(1–7) led to a 37% or twofold increase of these respective proteins in circulation and protected rats from chemically induced PH (117). Oral delivery of CTB-ACE2 or CTB-Ang-(1–7) could halt or reverse the progression of PH, improve right heart function, and decrease pulmonary wall thickness and lung injury (Figure 2e) (117). A combination therapy, in which either 0.025 mg of ACE2 with 0.25 mg of Ang-(1–7) or 0.05 mg of ACE2 with 0.5 mg of Ang-(1–7) was also tested, proved more effective than either one alone, with the latter combination showing up to a 25% greater reduction of symptoms (117). The mechanism underlying this improvement involves ACE2 and Ang-(1–7) reducing the abundance of transcripts encoding inflammatory cytokines and autophagic markers (up to ~fivefold) in affected tissue (117).
Diabetes
Diabetes is rapidly becoming an epidemic; in the United States, it was the seventh leading cause of death in 2013, and the number of people suffering from it quadrupled between 1980 and 2012 (14). In 2012, the total cost of diabetes treatment in the United States was $245 billion, including $69 billion due to missed work, disability, and premature death (13). In the face of such staggering numbers, the need for effective methods of prevention and treatment is clear.
Diabetes treatment frequently involves injection of insulin, which is expensive, has a short shelf life, and requires refrigeration (73). Orally delivered, plant-made diabetes medication costs less and eliminates the need for cold chain and injections, encouraging patient compliance (73). In one case, a cleavable form of CTB-fused proinsulin was incorporated into plastid genomes of lettuce or tobacco, and resulting CTB-proinsulin was fed to mice. Oral delivery of CTB-proinsulin (0.5 mg) derived from lettuce or tobacco could significantly lower blood glucose levels to an extent similar to that of clinical insulin injections, demonstrating that the fusion protein is functional in vivo (9). At reported expression levels, producing proinsulin in transplastomic tobacco could yield up to 20 million daily insulin doses per acre of tobacco per year. In a second strategy, exendin-4 (EX4), which is a glucagon-like peptide that modulates glucose levels in a glucose-dependent manner, was expressed in plant cells as a CTB fusion protein. Oral delivery of plant material containing ~200 μg of CTB-EX4 showed glucose-lowering ability similar to that of injected commercial EX4, an ~22% reduction 90 min after gavage/injection, indicating that the protein is functional and setting the stage for further development of these important therapeutics (Figure 2f ) (72). Additionally, orally delivered CTB-proinsulin expressed in tobacco chloroplasts prevented pancreatic insulitis in nonobese diabetic mice by inducing oral tolerance (105), meaning that oral delivery of CTB-proinsulin could prevent pancreatic insulitis in type 1 diabetes.
CHLOROPLAST GENOME ENGINEERING FOR VACCINE PRODUCTION
Traditional vaccines consist of inactivated or live attenuated pathogens, and although they have saved millions of lives, they are not entirely safe. Viruses present in live attenuated vaccines could revert to virulence, show antigenic variability between species, sometimes have low levels of immunogenicity, and may transfer genes to wild-type strains (15). For example, in 2006, an outbreak of type 2 vaccine-derived polio (VDPV2) was detected in Nigeria (38). Subsequently, it became endemic in Africa and still persists today. Phylogenetic analysis of the P1/capsid region sequences of isolates from the 403 cases reported between 2005 and 2011 showed that of 23 independent VDPV2 emergences, at least 7 established circulating lineage groups (11). Because of the prevalence of VDPV2 outbreaks over those derived from the other two Sabin strains, the WHO has mandated the removal of trivalent OPV from use (11, 16). As a replacement, the WHO recommends the use of either bivalent OPV, which contains only Sabin strains 1 and 3, or inactivated poliovirus vaccine (IPV), which is administered intradermally (16, 89). However, replacement of OPV with IPV is considerably more expensive than OPV, and although it induces systemic immunity, it fails to induce adequate mucosal immunity (16). Because polio spreads through contaminated water and food and multiplies in the intestine, which houses the largest immune tissue in the body, establishment of mucosal immunity through secretion of IgA is critical to controlling the spread of this disease; establishing a robust immune response at the site of pathogen attack can stop infection before it starts (16, 115). Therefore, there is a clear need for new approaches to improve current vaccines, and chloroplast-derived subunit vaccines, which have no risk of mutation or recombination, offer great potential for protection against infectious diseases.
Current Chloroplast-Made Vaccines
Orally administered plant-made oral vaccines have been shown to successfully induce immunity in animal and human models by promoting both humoral and cell-mediated immunity (Figure 3a) (15, 130). Importantly, antibodies produced by oral immunization (IgA) can neutralize pathogens on the mucosal surface before they can cause infection (115), making oral vaccination a key tool in combating infectious disease. To date, nearly 50 subunit vaccine candidates have been expressed in chloroplasts; these reports have been extensively reviewed recently (15, 82, 137), but we discuss a few representative cases here. Several studies have reported the expression and immunogenicity of HIV epitopes in mouse models (103, 104), and a dengue polyprotein expressed in lettuce chloroplasts forms VLPs in planta (62). Bacterial vaccines include one for anthrax that resulted in 100% protection against a lethal challenge in mice (Figure 3b) (66), a plague vaccine that protected >80% of mice from a lethal Yersinia pestis challenge (Figure 3b) (3), a cholera vaccine that provided 100% protection against a cholera toxin challenge for approximately half the life span of the average mouse (Figure 3b) (29), and a functional tuberculosis subunit vaccine (74). A chloroplast-produced malaria vaccine also completely inhibited the growth of the parasite in a mouse model (29).
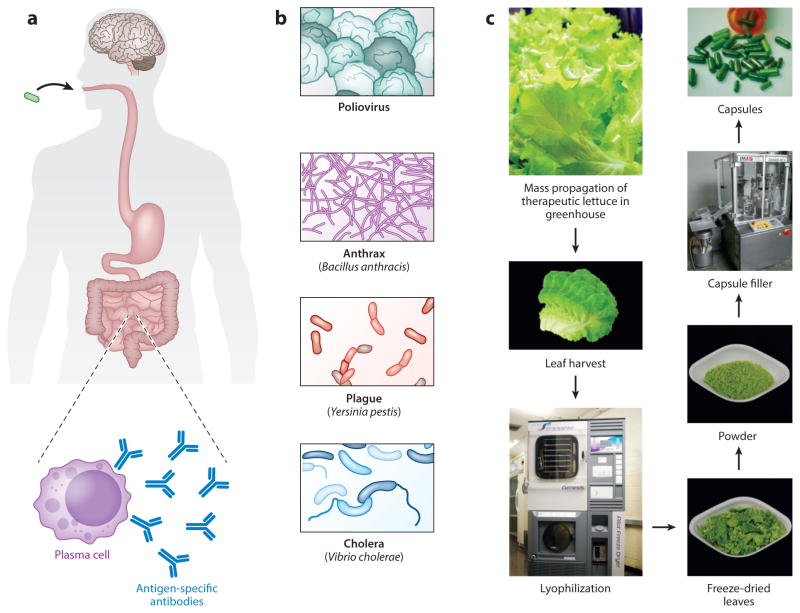
Figure 3.
Vaccination with vaccine antigens made in chloroplasts prevents human infectious diseases; industrial-scale production of chloroplast-made human therapeutic proteins. (a) Orally administered antigens are taken up in the gut and captured by antigen-presenting cells, such as dendritic cells (DCs), inducing antigen-specific T and B cells (plasma cells) that secrete antibodies against various pathogens. Panel a redrawn with permission from Reference 15. (b) Examples of diseases for which functional chloroplast-made vaccines have been developed. Animal models fed with chloroplast-made vaccine antigens produced antibodies and conferred protective immunity against viral (polio), bacterial (anthrax, plague), and toxin (cholera) challenges. (c) Workflow for industrial-scale production of plastid-made pharmaceuticals. Lettuce plants expressing therapeutic proteins are propagated in an industrial hydroponic greenhouse, and leaves are harvested and lyophilized in a programmed freeze dryer to maintain sublimation temperature below −20°C. Freeze-dried leaves are ground to a fine powder and used to fill capsules that can be stored for years while maintaining their efficacy. Panel c adapted with permission from Reference 15.
Most recently, members of the Daniell lab, in collaboration with the Centers for Disease Control and Prevention (CDC), have addressed the growing world health problem of cVDPV (circulating vaccine-derived poliovirus) (16). Because most outbreaks of cVDPV are derived from the type 2 poliovirus, whose wild form has been eradicated, the WHO now recommends that the current OPV, which contains all three strains of poliovirus, be replaced with a form that contains only types 1 and 3 (10). However, short supplies of IPV, which is required for immune priming prior to OPV administration, are delaying this effort. A complete switch from OPV to IPV and/or subunit vaccines would be the ideal way to address the global polio problem. To that end, the Daniell lab generated transplastomic plants expressing the viral protein 1 (VP1) subunit from type 1 poliovirus, which is responsible for all wild poliovirus infections in 2014 (96), in chloroplasts and used lyophilized plant tissue as an oral booster vaccine (16). Remarkably, in mice primed with IPV, oral delivery of this booster vaccine promoted production of neutralizing antibodies against all three poliovirus serotypes and elicited both systemic (IgG) and mucosal (IgA) immune responses (16). Without priming, antibodies were still produced, but they could not neutralize the virus, demonstrating the importance of the temporal sequence of vaccine administration: Initial systemic priming and mucosal boosting are important to promote immunity and prevent tolerance. Regardless, this study shows promise for eventual global eradication of polio.
Future studies on plant-based oral vaccines should consider appropriate antigen formulations and immunization protocols to ensure antigen stability through the alimentary tract and a balance between immunity and oral tolerance, such as targeting pivotal antigen-presenting cells (APCs), optimizing oral antigen delivery, dosage, and feeding frequency, and coadministration with a mucosal adjuvant (4, 95). However, currently, very few adjuvants are approved for use in humans. These adjuvants include aluminum salts, squalene, and monophosphoryl lipid A (MPL) and are approved for use in the United States by the FDA (133, 134), as well as in the European Union, for new vaccines (48). Using plant-derived substances such as squalene and saponin as adjuvants may enhance immune responses while quelling concerns about exposure to heavy metals. Indeed, efforts are already under way to engineer plants to produce higher levels of squalene (93).
CLINICAL DEVELOPMENT OF CHLOROPLAST-MADE BIOPHARMACEUTICALS
Using plastid genome engineering to produce therapeutic proteins and vaccines has several major advantages over conventional systems, which require fermentation, purification, and cold storage (61). Transplastomic plants can accumulate much higher levels of transgene product (9, 73, 107), allowing delivery of higher/more concentrated doses. Oral delivery of intact plant cells eliminates the need for fermentation, purification, and sterile delivery of therapeutic proteins (3, 9, 16, 29, 39, 65, 66, 71, 73, 74, 102–105, 117–119, 123, 124, 135), substantially reducing production costs. Lyophilization further reduces costs by increasing the concentration of therapeutic proteins, which reduces the amount of material needed per dose (72, 74, 119, 123, 124); permitting stable, long-term storage at ambient temperatures—sometimes for as long as two years—without loss of efficacy, thereby breaking the cold chain barrier (72, 74, 119, 124); and eliminating microbial contamination (72).
Maternal inheritance of chloroplasts (Figure 1b) (19) and harvesting leaves prior to flowering make transplastomic plant material easy to contain, mitigating concerns over transgene escape and making it easier to comply with government regulations (30, 61). Consistent with this, the USDA Animal and Plant Health Inspection Service (APHIS) issued a certification stating that transplastomic plants do not contain plant pest components and therefore do not fit the definition of a regulated article under 7 CFR part 340 (15, 69). This certification permitted transportation of >300 distinct transplastomic lines across state borders and demonstrated the comparative ease with which transplastomic plants can comply with government regulations (15).
Advantages associated with plant-made biopharmaceuticals have been recognized by the United States government, whose Defense Advanced Research Projects Agency (DARPA) funded construction of four pilot-scale commercial hydroponic plant-made-biopharmaceutical facilities (54). The initial investment was in Fraunhofer CMB, which was constructed with a 100 kg/day processing capacity and has since been used for pilot studies on industrial-scale hydroponic production of transplastomic therapeutic plants (54, 124). After Fraunhofer, additional projects were funded in Kentucky, North Carolina, and Texas (54). In particular, the Texas (Caliber) site is a scalable facility that can expand by adding proprietary pods, which contain hydroponic growth racks and specialized LED lighting to grow Nicotiana benthamiana for transient expression of recombinant therapeutic proteins (54). The Caliber facility, which went from groundbreaking to final product (an H1N1 influenza subunit vaccine) in <2 years, can now process 3,500 kg of biomass per week with its automated system (54). Additionally, the FDA recently approved taliglucerase alfa (ELELYSO® ), an injectable drug made in carrot cells, to treat Gaucher disease (46, 108). However, except Fraunhofer, these platforms still require purification of expressed proteins.
Bringing orally deliverable, cost-effective plant-made biopharmaceuticals to the clinic requires high expression levels in edible species and production of the material in compliance with cGMP standards (54, 61). Recently, some of these challenges have begun to be surmounted in the treatment of hemophilia when transplastomic lettuce plants expressing CTB-FIX were grown at an industrial scale and in accordance with cGMP standards at Fraunhofer’s hydroponics facility (Figure 3c) (124). Using 1,000 ft2 of growth space per year, ~870 kg of CTB-FIX lettuce (~43.5 kg dry weight) could be grown (124). At these levels, the system could be used to produce 24,000–36,000 doses of CTB-FIX for 20-kg pediatric hemophilia patients (124). This report is the first to show feasibility of industrial-scale production of orally deliverable therapeutic plant-made bio-pharmaceuticals and sets the stage for the clinical advancement of this system.
FUTURE DIRECTIONS
Plastid genome engineering can advance the plant-made biopharmaceutical field while alleviating some of the public concerns about GM plants. The first industrial-scale production of therapeutic transplastomic plants (124) is a critical proof-of-concept that this platform can be commercialized and used to revolutionize healthcare, and support from pharmaceutical companies is providing the expertise and infrastructure needed to efficiently move plant-made biopharmaceuticals into clinical trials. FDA approval of the first plant-made biopharmaceutical made in plant cells for human use marks the beginning of a new era in which more plant-made biopharmaceuticals may enter the clinic, providing affordable medication to prevent or treat many diseases.
However, there are still limitations to this system that must be overcome before its potential can be fully realized. First, the plant-made-biopharmaceutical field needs to continue moving away from tobacco-based studies and focus on clinically acceptable edible plants. Although transplastomic tobacco has proven a useful tool, toxic alkaloids will make it difficult, if not impossible, for it to advance beyond preliminary animal studies. Lettuce is emerging as an alternative system that yields consistent, reproducible results (9, 29, 61, 62, 74, 80, 105, 107, 124). Additional investigations of plastid transformation and expression in nongreen plastids such as tomato fruits and potato tubers, as well as plastid transformation of cereals, which has remained elusive, could further boost the utility of plastid-made plant-made biopharmaceuticals. However, it may never be feasible for nongreen plastids to exceed transgene expression levels achieved in chloroplasts because of the low expression levels of native chloroplast genes.
To bring transplastomic therapeutic plants to the clinic, marker-free plastid engineering systems must be developed. The most desirable selectable markers would utilize the plant’s own genes and metabolic pathways (30). Alternatively, the plastid’s highly active homologous recombination machinery (30) or the use of exogenous recombinases could be exploited to excise a selectable marker after achieving homoplasmy (17, 116). In particular, using Bxb1 recombinase and attP/attB sites to precisely remove DNA from within the trnI/trnA intergenic spacer shows great promise for this widely used site (116). Development and optimization of this technology in edible crops will be critical to facilitate the approval of plant-made biopharmaceuticals for public use.
Targeted oral delivery of therapeutic proteins could also revolutionize the field. CTB has yielded foundational studies, but the ubiquity of GM1 means that CTB-fused therapeutic peptides lack specificity. Targeting therapeutic proteins to specific cell or tissue types should minimize delivery to nontarget tissues. Engineering a protease cleavage site will ensure the cleavage of fused tags and release of mature functional therapeutic proteins (81). In this context, recent advances in identification of specific tags to deliver proteins to immune modulatory or nonimmune cells hold great promise to induce an immune response when desired or avoid the immune system while delivering functional plasma proteins.
The field of chloroplast-made vaccines has also experienced tremendous progress. In addition to cGMP facilities producing plant-made influenza vaccines (54), a chloroplast-made, orally delivered polio vaccine has proven effective against this devastating disease (16), providing a viable alternative to the current OPV. Unfortunately, this system does not eliminate the need for immune priming by injection and therefore still requires the cold chain (16). Elimination of the cold chain in oral delivery of vaccines will require further research into enhancement of oral priming. Additionally, incorporating plant-made adjuvants such as squalene into these vaccine formulations could boost immunity at minimal cost. However, a recent study has demonstrated that metabolic engineering, including that aimed at producing adjuvants such as squalene, could have unintended effects on unrelated pathways, affecting the abundance of as many as ~19,000 transcripts and ~120 metabolites (93). In this case, these large effects were likely mediated by metabolites, but proteins may also play a role (93). These results indicate that as researchers strive to produce additional therapeutic proteins in plants and bring plant-made biopharmaceuticals into the clinic, they should also be aware of potential off-target effects in engineered plants (93).
Of all of the >800 sequenced chloroplast genomes, only ~60 represent crop genera (23). Published chloroplast genome sequences encompass economically important species. Most of these studies were not funded by the National Science Foundation’s Plant Genome Research Program. Although research on model organisms provides useful scientific insights, this discrepancy between model and crop species highlights the need for additional funding to increase resources related to economically important crop plants.
Although plastid-made therapeutic proteins have not arrived in the clinic, the field is now closer than it has ever been to making orally delivered plant-made biopharmaceuticals a reality. Key pilot studies have demonstrated the feasibility of this platform, and new cutting-edge technologies may provide the final push needed to move chloroplast-made pharmaceuticals from the lab bench to the bedside.
Acknowledgments
Research reported from the Daniell lab was supported by the Bill and Melinda Gates Foundation (OPP1031406); NIH grants R01 HL107904, R01 HL109442, R01 GM63879, R01 EY024564; several USDA and DOE grants; and funding from industry, including Bayer and Novo Nordisk to Henry Daniell. He thanks all coauthors and collaborators from his laboratory for their valuable contributions to advance this field.
Glossary
- Homoplasmy
elimination of any unmodified chloroplast genome in a genetically modified plant cell
- Blood-brain barrier (BBB)
highly selective permeability barrier separating circulating blood from brain; allows penetration of small molecules but not protein drugs
- Blood-retinal barrier (BRB)
highly selective permeability barrier separating circulating blood from eye; allows penetration of small molecules but not protein drugs
- Copy correction
maintenance of 100% identical DNA sequence between the inverted repeat regions of the plastid genome
- Cholera toxin B (CTB)
binds GM1 receptors on cell membranes and facilitates delivery of fusion proteins through endocytosis
- GM1
ganglioside present in lipid rafts in membranes of many cell types (especially neurons). Binds CTB, causing endocytosis of this complex
- Protein transduction domain (PTD)
a small cationic peptide that can penetrate cell membranes without specific receptors
- Oral tolerance
the process by which the immune system is prevented from responding to native or foreign antigen
- Interleukin-10 (IL-10)
also known as human cytokine synthesis inhibitory factor (CSIF), IL-10 is an anti-inflammatory and key immunosuppressive cytokine
- Transforming growth factor-β (TGF-β)
an immunosuppressive cytokine involved in induction of tolerance
- Renin-angiotensin system (RAS)
involved in cardiovascular homeostasis and pathogenesis of inflammation and autoimmune dysfunction. Imbalance causes pulmonary hypertension, retinal diseases, and muscular dystrophy
- Subunit vaccines
vaccines derived from one or more specific antigenic regions of a pathogen but free from the entire organism
Footnotes
DISCLOSURE STATEMENT
Although there is no financial conflict of interest to report, the corresponding author is an inventor on numerous patents reporting expression of human therapeutic proteins in chloroplasts.
LITERATURE CITED
- 1.Adeniji JA, Faleye TO. Enterovirus C strains circulating in Nigeria and their contribution to the emergence of recombinant circulating vaccine-derived polioviruses. Arch Virol. 2015;160:675–83. doi: 10.1007/s00705-014-2322-x. [DOI] [PubMed] [Google Scholar]
- 2.Arlen PA, Falconer R, Cherukumili S, Cole AM, Oishi KK, Daniell H. Field production and functional evaluation of chloroplast-derived interferon-α2b. Plant Biotechnol J. 2007;5:511–25. doi: 10.1111/j.1467-7652.2007.00258.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Arlen PA, Singleton M, Adamovicz JJ, Ding Y, Davoodi-Semiromi A, Daniell H. Effective plague vaccination via oral delivery of plant cells expressing F1-V antigens in chloroplasts. Infect Immun. 2008;76:3640–50. doi: 10.1128/IAI.00050-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Azegami T, Yuki Y, Kiyono H. Challenges in mucosal vaccines for the control of infectious diseases. Int Immunol. 2014;26:517–28. doi: 10.1093/intimm/dxu063. [DOI] [PubMed] [Google Scholar]
- 5.Baldauf KJ, Royal JM, Hamorsky KT, Matoba N. Cholera toxin B: one subunit with many pharmaceutical applications. Toxins. 2015;7:974–96. doi: 10.3390/toxins7030974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Barkan A. Expression of plastid genes: organelle-specific elaborations on a prokaryotic scaffold. Plant Physiol. 2011;155:1520–32. doi: 10.1104/pp.110.171231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Barone P, Zhang XH, Widholm JM. Tobacco plastid transformation using the feedback-insensitive anthranilate synthase [α]-subunit of tobacco (ASA2) as a new selectable marker. J Exp Bot. 2009;60:3195–02. doi: 10.1093/jxb/erp160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bowman SM, Patel M, Yerramsetty P, Mure CM, Zielinski AM, et al. A novel RNA binding protein affects rbcL gene expression and is specific to bundle sheath chloroplasts in C4 plants. BMC Plant Biol. 2013;13:138. doi: 10.1186/1471-2229-13-138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Boyhan D, Daniell H. Low-cost production of proinsulin in tobacco and lettuce chloroplasts for injectable or oral delivery of functional insulin and C-peptide. Plant Biotechnol J. 2011;9:585–98. doi: 10.1111/j.1467-7652.2010.00582.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Burns CC, Diop OM, Sutter RW, Kew OM. Vaccine-derived polioviruses. J Infect Dis. 2014;210:S283–93. doi: 10.1093/infdis/jiu295. [DOI] [PubMed] [Google Scholar]
- 11.Burns CC, Shaw J, Jorba J, Bukbuk D, Adu F, et al. Multiple independent emergences of type 2 vaccine–derived polioviruses during a large outbreak in northern Nigeria. J Virol. 2013;87:4907–22. doi: 10.1128/JVI.02954-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Carr ME, Tortella BJ. Emerging and future therapies for hemophilia. J Blood Med. 2015;6:245–55. doi: 10.2147/JBM.S42669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cent. Dis. Control Prev. National diabetes statistics report: estimates of diabetes and its burden in the United States. Atlanta, GA: US Dep. Health Hum. Serv; 2014. http://www.cdc.gov/diabetes/pubs/statsreport14/national-diabetes-report-web.pdf. [Google Scholar]
- 14.Cent. Dis. Control Prev. Diabetes report card 2014. Atlanta, GA: US Dep. Health Hum. Serv; 2015. http://www.cdc.gov/diabetes/pdfs/library/diabetesreportcard2014.pdf. [Google Scholar]
- 15.Chan H-T, Daniell H. Plant-made oral vaccines against human infectious diseases: Are we there yet? Plant Biotechnol J. 2015;13:1056–70. doi: 10.1111/pbi.12471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chan H-T, Xiao Y, Weldon WC, Oberste SM, Chumakov K, Daniell H. Cold chain and virus free chloroplast-made booster vaccine to confer immunity against different poliovirus serotypes. Plant Biotechnol J. 2016;14:2190–200. doi: 10.1111/pbi.12575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Corneille S, Lutz K, Svab Z, Maliga P. Efficient elimination of selectable marker genes from the plastid genome by the CRE-lox site-specific recombination system. Plant J. 2001;27:171–78. doi: 10.1046/j.1365-313x.2001.01068.x. [DOI] [PubMed] [Google Scholar]
- 18.Daniell H. Foreign gene expression in chloroplasts of higher plants mediated by tungsten particle bombardment. Methods Enzymol. 1993;217:536–56. doi: 10.1016/0076-6879(93)17088-m. [DOI] [PubMed] [Google Scholar]
- 19.Daniell H, Datta R, Varma S, Gray S, Lee S-B. Containment of herbicide resistance through genetic engineering of the chloroplast genome. Nat Biotechnol. 1998;16:345–48. doi: 10.1038/nbt0498-345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Daniell H, Krishnan M, Uma Bai V, Gnanam A. An efficient and prolonged in vitro translational system from cucumber etioplasts. Biochem Biophys Res Commun. 1986;135:248–55. doi: 10.1016/0006-291x(86)90969-1. [DOI] [PubMed] [Google Scholar]
- 21.Daniell H, Lee S-B, Grevich J, Saski C, Quesada-Vargas T, et al. Complete chloroplast genome sequences of Solanum bulbocastanum, Solanum lycopersicum and comparative analyses with other Solanaceae genomes. Theor Appl Genet. 2006;112:1503–18. doi: 10.1007/s00122-006-0254-x. [DOI] [PubMed] [Google Scholar]
- 22.Daniell H, Lee S-B, Panchal T, Wiebe PO. Expression of the native cholera toxin B subunit gene and assembly as functional oligomers in transgenic tobacco chloroplasts. J Mol Biol. 2001;311:1001–9. doi: 10.1006/jmbi.2001.4921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Daniell H, Lin C-S, Yu M, Chang W-J. Chloroplast genomes: diversity, evolution, and applications in genetic engineering. Genome Biol. 2016;17:134. doi: 10.1186/s13059-016-1004-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Daniell H, McFadden BA. Uptake and expression of bacterial and cyanobacterial genes by isolated cucumber etioplasts. PNAS. 1987;84:6349–53. doi: 10.1073/pnas.84.18.6349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Daniell H, Muthukumar B, Lee S-B. Marker free transgenic plants: engineering the chloroplast genome without the use of antibiotic selection. Curr Genet. 2001;39:109–16. doi: 10.1007/s002940100185. [DOI] [PubMed] [Google Scholar]
- 26.Daniell H, Ruiz G, Denes B, Sandberg L, Langridge W. Optimization of codon composition and regulatory elements for expression of human insulin like growth factor-1 in transgenic chloroplasts and evaluation of structural identity and function. BMC Biotechnol. 2009;9:33. doi: 10.1186/1472-6750-9-33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Daniell H, Vivekananda J, Nielsen BL, Ye GN, Tewari KK, Sanford JC. Transient foreign gene expression in chloroplasts of cultured tobacco cells after biolistic delivery of chloroplast vectors. PNAS. 1990;87:88–92. doi: 10.1073/pnas.87.1.88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Daniell H, Wurdack KJ, Kanagaraj A, Lee S-B, Saski C, Jansen RK. The complete nucleotide sequence of the cassava (Manihot esculenta) chloroplast genome and the evolution of AtpF in Malpighiales: RNA editing and multiple losses of a group II intron. Theor Appl Genet. 2008;116:723–37. doi: 10.1007/s00122-007-0706-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Davoodi-Semiromi A, Schreiber M, Nalapalli S, Verma D, Singh ND, et al. Chloroplast-derived vaccine antigens confer dual immunity against cholera and malaria by oral or injectable delivery. Plant Biotechnol J. 2010;8:223–42. doi: 10.1111/j.1467-7652.2009.00479.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Day A, Goldschmidt-Clermont M. The chloroplast transformation toolbox: selectable markers and marker removal. Plant Biotechnol J. 2011;9:540–53. doi: 10.1111/j.1467-7652.2011.00604.x. [DOI] [PubMed] [Google Scholar]
- 31.De Cosa B, Moar W, Lee S-B, Miller M, Daniell H. Overexpression of the Bt cry2Aa2 operon in chloroplasts leads to formation of insecticidal crystals. Nat Biotechnol. 2001;19:71–74. doi: 10.1038/83559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Desnick RJ, Schuchman EH. Enzyme replacement therapy for lysosomal diseases: lessons from 20 years of experience and remaining challenges. Annu Rev Genom Hum Genet. 2012;13:307–35. doi: 10.1146/annurev-genom-090711-163739. [DOI] [PubMed] [Google Scholar]
- 33.Dufourmantel N, Dubald M, Matringe M, Canard H, Garcon F, et al. Generation and characterization of soybean and marker-free tobacco plastid transformants over-expressing a bacterial 4-hydroxyphenylpyruvate dioxygenase which provides strong herbicide tolerance. Plant Biotechnol J. 2007;5:118–33. doi: 10.1111/j.1467-7652.2006.00226.x. [DOI] [PubMed] [Google Scholar]
- 34.Dufourmantel N, Tissot G, Goutorbe F, Garçon F, Muhr C, et al. Generation and analysis of soybean plastid transformants expressing Bacillus thuringiensis Cry1Ab protoxin. Plant Mol Biol. 2005;58:659–68. doi: 10.1007/s11103-005-7405-3. [DOI] [PubMed] [Google Scholar]
- 35.Dunne A, Maple-Grødem J, Gargano D, Haslam RP, Napier JA, et al. Modifying fatty acid profiles through a new cytokinin-based plastid transformation system. Plant J. 2014;80:1131–38. doi: 10.1111/tpj.12684. [DOI] [PubMed] [Google Scholar]
- 36.Eibl C, Zou Z, Beck A, Kim M, Mullet J, Koop HU. In vivo analysis of plastid psbA, rbcL and rpl32 UTR elements by chloroplast transformation: tobacco plastid gene expression is controlled by modulation of transcript levels and translation efficiency. Plant J. 2003;19:333–45. doi: 10.1046/j.1365-313x.1999.00543.x. [DOI] [PubMed] [Google Scholar]
- 37.Eidels L, Proia RL, Hart DA. Membrane receptors for bacterial toxins. Microbiol Rev. 1983;47:596–620. doi: 10.1128/mr.47.4.596-620.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Famulare M, Hu H. Extracting transmission networks from phylogeographic data for epidemic and endemic diseases: Ebola virus in Sierra Leone, 2009 H1N1 pandemic influenza and polio in Nigeria. Int Health. 2015;7:130–38. doi: 10.1093/inthealth/ihv012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Fernández-San Millán A, Ortigosa SM, Hervás-Stubbs S, Corral Martínez P, Seguí-Simarro JM, et al. Human papillomavirus L1 protein expressed in tobacco chloroplasts self-assembles into virus-like particles that are highly immunogenic. Plant Biotechnol J. 2008;6:427–41. doi: 10.1111/j.1467-7652.2008.00338.x. [DOI] [PubMed] [Google Scholar]
- 40.Flint HJ, Bayer EA, Rincon MT, Lamed R, White BA. Polysaccharide utilization by gut bacteria: potential for new insights from genomic analysis. Nat Rev Microbiol. 2008;6:121–31. doi: 10.1038/nrmicro1817. [DOI] [PubMed] [Google Scholar]
- 41.Giese MJ, Speth RC. The ocular renin-angiotensin system: a therapeutic target for the treatment of ocular disease. Pharmacol Ther. 2014;142:11–32. doi: 10.1016/j.pharmthera.2013.11.002. [DOI] [PubMed] [Google Scholar]
- 42.Gisby MF, Mellors P, Madesis P, Ellin M, Laverty H, et al. A synthetic gene increases TGF-β3 accumulation by 75-fold in tobacco chloroplasts enabling rapid purification and folding into a biologically active molecule. Plant Biotechnol J. 2011;9:618–28. doi: 10.1111/j.1467-7652.2011.00619.x. [DOI] [PubMed] [Google Scholar]
- 43.Gisby MF, Mudd EA, Day A. Growth of transplastomic cells expressing D–amino acid oxidase in chloroplasts is tolerant to D-alanine and inhibited by D-valine. Plant Physiol. 2012;160:2219–26. doi: 10.1104/pp.112.204107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Glenz K, Bouchon B, Stehle T, Wallich R, Simon MM, et al. Production of a recombinant bacterial lipoprotein in higher plant chloroplasts. Nat Biotechnol. 2006;24:76–77. doi: 10.1038/nbt1170. [DOI] [PubMed] [Google Scholar]
- 45.Goldschmidt-Clermont M. Transgenic expression of aminoglycoside adenine transferase in the chloroplast: a selectable marker for site-directed transformation of chlamydomonas. Nucleic Acids Res. 1991;19:4083–89. doi: 10.1093/nar/19.15.4083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Grabowski GA, Golembo M, Shaaltiel Y. Taliglucerase alfa: an enzyme replacement therapy using plant cell expression technology. Mol Genet Metab. 2014;112:1–8. doi: 10.1016/j.ymgme.2014.02.011. [DOI] [PubMed] [Google Scholar]
- 47.Grabowski H, Cockburn I, Long G. The market for follow-on biologics: How will it evolve? Health Aff. 2006;25:1291–01. doi: 10.1377/hlthaff.25.5.1291. [DOI] [PubMed] [Google Scholar]
- 48.Granell A, Fernández-del-Carmen A, Orzáez D. In planta production of plant-derived and non-plant-derived adjuvants. Expert Rev Vaccines. 2010;9:843–58. doi: 10.1586/erv.10.80. [DOI] [PubMed] [Google Scholar]
- 49.Guda C, Lee S-B, Daniell H. Stable expression of a biodegradable protein-based polymer in tobacco chloroplasts. Plant Cell Rep. 2000;19:257–62. doi: 10.1007/s002990050008. [DOI] [PubMed] [Google Scholar]
- 50.Guetard D, Greco R, Cervantes Gonzalez M, Celli S, Kostrzak A, et al. Immunogenicity and tolerance following HIV-1/HBV plant-based oral vaccine administration. Vaccine. 2008;26:4477–85. doi: 10.1016/j.vaccine.2008.06.059. [DOI] [PubMed] [Google Scholar]
- 51.Gupta K, Kotian A, Subramanian H, Daniell H, Ali H. Activation of human mast cells by retrocyclin and protegrin highlight their immunomodulatory and antimicrobial properties. Oncotarget. 2015;6:28573–87. doi: 10.18632/oncotarget.5611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Hammani K, Cook WB, Barkan A. RNA binding and RNA remodeling activities of the half-a-tetratricopeptide (HAT) protein HCF107 underlie its effects on gene expression. PNAS. 2012;109:5651–56. doi: 10.1073/pnas.1200318109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Hede MS, Salimova E, Piszczek A, Perlas E, Winn N, et al. E-peptides control bioavailability of IGF-1. PLOS ONE. 2012;7:e51152. doi: 10.1371/journal.pone.0051152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Holtz BR, Berquist BR, Bennett LD, Kommineni VJM, Munigunti RK, et al. Commercial-scale biotherapeutics manufacturing facility for plant-made biopharmaceuticals. Plant Biotechnol J. 2015;13:1180–90. doi: 10.1111/pbi.12469. [DOI] [PubMed] [Google Scholar]
- 55.Horai R, Caspi RR. Retinal inflammation: uveitis/uveoretinitis. In: Pang I-H, Clark AF, editors. Neuromethods. Vol. 46. New York: Springer; 2009. pp. 207–25. [Google Scholar]
- 56.Humbert M, Ghofrani H-A. The molecular targets of approved treatments for pulmonary arterial hypertension. Thorax. 2016;71:73–83. doi: 10.1136/thoraxjnl-2015-207170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Humbert M, Sitbon O, Chaouat A, Bertocchi M, Habib G, et al. Survival in patients with idiopathic, familial and anorexigen-asociated pulmonary arterial hypertension in the modern management era. Circulation. 2010;122:156–63. doi: 10.1161/CIRCULATIONAHA.109.911818. [DOI] [PubMed] [Google Scholar]
- 58.Iamtham S, Day A. Removal of antibiotic resistance genes from transgenic tobacco plastids. Nat Biotechnol. 2001;18:1172–2000. doi: 10.1038/81161. [DOI] [PubMed] [Google Scholar]
- 59.Ingolia NT. Ribosome profiling: new views of translation, from single codons to genome scale. Nat Rev Genet. 2014;15:205–13. doi: 10.1038/nrg3645. [DOI] [PubMed] [Google Scholar]
- 60.Ingolia NT, Ghaemmaghami S, Newman JR, Weissman JS. Genome-wide analysis in vivo of translation with nucleotide resolution using ribosome profiling. Science. 2009;324:218–23. doi: 10.1126/science.1168978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Jin S, Daniell H. The engineered chloroplast genome just got smarter. Trends Plant Sci. 2015;20:622–40. doi: 10.1016/j.tplants.2015.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Kanagaraj AP, Verma D, Daniell H. Expression of dengue-3 premembrane and envelope polyprotein in lettuce chloroplasts. Plant Mol Biol. 2011;76:323–33. doi: 10.1007/s11103-011-9766-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Kempton CL, Meeks SL. Toward optimal therapy for inhibitors in hemophilia. Blood. 2014;124:3365–72. doi: 10.1182/blood-2014-05-577643. [DOI] [PubMed] [Google Scholar]
- 64.Kim YJ, Gallien S, El-Khoury V, Goswami P, Sertamo K, et al. Quantification of SAA1 and SAA2 in lung cancer plasma using the isotype-specific PRM assays. Proteomics. 2015;15:3116–25. doi: 10.1002/pmic.201400382. [DOI] [PubMed] [Google Scholar]
- 65.Kohli N, Westerveld DR, Ayache AC, Verma A, Shil P, et al. Oral delivery of bioencapsulated proteins across blood-brain and blood-retinal barriers. Mol Ther. 2014;22:535–46. doi: 10.1038/mt.2013.273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Koya V, Moayeri M, Leppla SH, Daniell H. Plant-based vaccine: mice immunized with chloroplast-derived anthrax protective antigen survive anthrax lethal toxin challenge. Infect Immun. 2005;73:8266–74. doi: 10.1128/IAI.73.12.8266-8274.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Krichevsky A, Meyers B, Vainstein A, Maliga P, Citovsky V. Autoluminescent plants. PLOS ONE. 2010;5:e15461. doi: 10.1371/journal.pone.0015461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Kumar S, Dhingra A, Daniell H. Plastid-expressed betaine aldehyde dehydrogenase gene in carrot cultured cells, roots, and leaves confers enhanced salt tolerance. Plant Physiol. 2004;136:2843–54. doi: 10.1104/pp.104.045187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Kwon K-C, Daniell H. Low-cost oral delivery of protein drugs bioencapsulated in plant cells. Plant Biotechnol J. 2015;13:1017–22. doi: 10.1111/pbi.12462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Kwon K-C, Chan H-T, León IR, Williams-Carrier R, Barkan A, Daniell H. Codon optimization to enhance expression yields insights into chloroplast translation. Plant Physiol. 2016;172:62–77. doi: 10.1104/pp.16.00981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Kwon K-C, Daniell H. Oral delivery of protein drugs bioencapsulated in plant cells. Mol Ther. 2016;24:1342–50. doi: 10.1038/mt.2016.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Kwon K-C, Nityanandam R, New JS, Daniell H. Oral delivery of bioencapsulated exendin-4 expressed in chloroplasts lowers blood glucose level in mice and stiumulates insulin secretion in β-TC6 cells. Plant Biotechnol J. 2013;11:77–86. doi: 10.1111/pbi.12008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Kwon K-C, Verma D, Singh ND, Herzog R, Daniell H. Oral delivery of biopharmaceuticals, autoantigens and vaccine antigens bioencapsulated in plant cells. Adv Drug Deliv Rev. 2013;65:782–99. doi: 10.1016/j.addr.2012.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Lakshmi PS, Verma D, Yang X, Lloyd B, Daniell H. Low cost tuberculosis vaccine antigens in capsules: expression in chloroplasts, bio-encapsulation, stability and functional evaluation in vitro. PLOS ONE. 2013;8:e54708. doi: 10.1371/journal.pone.0054708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Lamichhane A, Azegamia T, Koyonoa H. The mucosal immune system for vaccine development. Vaccine. 2014;32:6711–23. doi: 10.1016/j.vaccine.2014.08.089. [DOI] [PubMed] [Google Scholar]
- 76.Ledeen RW, Wu G. The multi-tasked life of GM1 ganglioside, a true factotum of nature. Trends Biochem Sci. 2015;40:407–18. doi: 10.1016/j.tibs.2015.04.005. [DOI] [PubMed] [Google Scholar]
- 77.Lee SB, Li B, Jin S, Daniell H. Expression and characterization of antimicrobial peptides Retrocyclin-101 and Protegrin-1 in chloroplasts to control viral and bacterial infections. Plant Biotechnol J. 2011;9:100–15. doi: 10.1111/j.1467-7652.2010.00538.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Lenzi P, Scotti N, Alagna F, Tornesello ML, Pompa A, et al. Translational fusion of chloroplast-expressed human papillomavirus type 16 L1 capsid protein enhances antigen accumulation in transplastomic tobacco. Transgenic Res. 2008;17:1091–102. doi: 10.1007/s11248-008-9186-3. [DOI] [PubMed] [Google Scholar]
- 79.Liao M-C, Ahmed M, Smith SO, Van Nostrand WE. Degradation of amyloid βprotein by purified myelin basic protein. J Biol Chem. 2009;284:28917–25. doi: 10.1074/jbc.M109.050856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Lim S, Ashida H, Watanabe R, Inai K, Kim Y-S, et al. Production of biologically active human thioredoxin 1 protein in lettuce chloroplasts. Plant Mol Biol. 2011;76:335–44. doi: 10.1007/s11103-011-9745-5. [DOI] [PubMed] [Google Scholar]
- 81.Limaye A, Koya V, Samsam M, Daniell H. Receptor-mediated oral delivery of a bioencapsulated green fluorescent protein expressed in transgenic chloroplasts into the mouse circulatory system. FASEB J. 2006;20:959–61. doi: 10.1096/fj.05-5134fje. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Lössl AG, Waheed MT. Chloroplast-derived vaccines against human diseases: achievements, challenges and scopes. Plant Biotechnol J. 2011;9:527–39. doi: 10.1111/j.1467-7652.2011.00615.x. [DOI] [PubMed] [Google Scholar]
- 83.Mäger I, Roberts TC, Wood MJA, El Andaloussi S. From gut to brain: bioencapsulated therapeutic protein reduces amyloid load upon oral delivery. Mol Ther. 2014;22:485–86. doi: 10.1038/mt.2014.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Martens EC, Lowe EC, Chiang H, Pudlo NA, Wu M, et al. Recognition and degradation of plant cell wall polysaccharides by two human gut symbionts. PLOS Biol. 2011;9:e1001221. doi: 10.1371/journal.pbio.1001221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Morton BR. Chloroplast DNA codon usage: evidence for selection at the psbA locus based on tRNA availability. J Mol Evol. 1993;37:273–80. doi: 10.1007/BF00175504. [DOI] [PubMed] [Google Scholar]
- 86.Mucida D, Kutchukhidze N, Agustin Erazo A, Russo M, Lafaille JJ, et al. Oral tolerance in the absence of naturally occurring Tregs. J Clin Investig. 2005;115:1923–33. doi: 10.1172/JCI24487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Nakamura M, Sugiura M. Translation efficiencies of synonymous codons are not always correlated with codon usage in tobacco chloroplasts. Plant J. 2007;49:128–34. doi: 10.1111/j.1365-313X.2006.02945.x. [DOI] [PubMed] [Google Scholar]
- 88.Natl. Inst. Aging. Alzheimer’s disease fact sheet. Bethesda, MD: NIA; 2015. https://www.nia.nih.gov/alzheimers/publication/alzheimers-disease-fact-sheet. [Google Scholar]
- 89.Okayasu H, Sutter RW, Jafari HS, Takane M, Aylward RB. Affordable inactivated poliovirus vaccine: strategies and progress. J Infect Dis. 2014;210:S459–64. doi: 10.1093/infdis/jiu128. [DOI] [PubMed] [Google Scholar]
- 90.Oldenburg DJ, Bendich AJ. DNA maintenance in plastids and mitochondria of plants. Front Plant Sci. 2015;6:883. doi: 10.3389/fpls.2015.00883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Oliveira RP, Santiago AF, Ficker SM, Gomes-Santos AC, Faria AM. Antigen administration by continuous feeding enhances oral tolerance and leads to long-lasting effects. J Immunol Methods. 2015;421:36–43. doi: 10.1016/j.jim.2015.02.005. [DOI] [PubMed] [Google Scholar]
- 92.Pascolini D, Mariotti SPM. Global estimates of visual impairment: 2010. Br J Ophthalmol. 2012;96:614–18. doi: 10.1136/bjophthalmol-2011-300539. [DOI] [PubMed] [Google Scholar]
- 93.Pasoreck EK, Su J, Silverman IS, Gosai SJ, Gregory BD, et al. Terpene metabolic engineering via nuclear or chloroplast genomes profoundly and globally impacts off-target pathways through metabolite signaling. Plant Biotechnol J. 2016;14:1862–75. doi: 10.1111/pbi.12548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Philippou A, Barton ER. Optimizing IGF-1 for skeletal muscle therapeutics. Growth Horm IGF Res. 2014;24:157–63. doi: 10.1016/j.ghir.2014.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Pniewski T, Kapusta J, Bociazg P, Wojciechowicz J, Kostrzak A, et al. Low-dose oral immunization with lyophilized tissue of herbicide-resistant lettuce expressing hepatitis B surface antigen for prototype plant-derived vaccine tablet formulation. J Appl Genet. 2011;52:125–36. doi: 10.1007/s13353-010-0001-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Glob. Polio Erad. Initiat. Fact sheet: vaccine-derived poliovirus. Geneva, Switz: Glob. Polio Erad. Initiat; 2015. http://polioeradication.org/wp-content/uploads/2016/09/CVDPVFactSheetMarch2015.pdf. [Google Scholar]
- 97.Quesada-Vargas T, Ruiz ON, Daniell H. Characterization of heterologous multigene operons in transgenic chloroplasts. Transcription, processing, and translation. Plant Physol. 2005;138:1746–62. doi: 10.1104/pp.105.063040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Rask C, Holmgren J, Fredriksson M, Nordstrom I, Sun JB, Czerkinsky C. Prolonged oral treatment with low doses of allergen conjugated to cholera toxin B subunit suppresses immunoglobulin E antibody responses in sensitized mice. Clin Exp Allergy. 2000;30:1024–32. doi: 10.1046/j.1365-2222.2000.00849.x. [DOI] [PubMed] [Google Scholar]
- 99.Rauniyar N. Parallel reaction monitoring: a targeted experiment performed using high resolution and high mass accuracy mass spectrometry. Int J Mol Sci. 2015;16:28566–81. doi: 10.3390/ijms161226120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Reiss BB. Homologous recombination and gene targeting in plant cells. Int Rev Cytol. 2003;228:85–139. doi: 10.1016/s0074-7696(03)28003-7. [DOI] [PubMed] [Google Scholar]
- 101.Ronsein GE, Pamir N, von Halle PD, Kim DS, Oda MN, et al. Parallel reaction monitoring (PRM) and selected reaction monitoring (SRM) exhibit comparable linearity, dynamic range and precision for targeted quantitative HDL proteomics. J Proteom. 2015;113:388–99. doi: 10.1016/j.jprot.2014.10.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Rosales-Mendoza S, Alpuche-Solis AG, Soria-Guerra RE, Moreno-Fierros L, Martínez-González L, et al. Expression of an Escherichia coli antigenic fusion protein comprising the heat labile toxin B subunit and the heat stable toxin, and its assembly as a functional oligomer in transplastomic tobacco plants. Plant J. 2009;57:45–54. doi: 10.1111/j.1365-313X.2008.03666.x. [DOI] [PubMed] [Google Scholar]
- 103.Rubio-Infante N, Govea-Alonso DO, Alpuche-Solís ÁG, García-Hernández AL, Soria Guerra RE, et al. A chloroplast-derived C4V3 polypeptide from the human immunodeficiency virus (HIV) is orally immunogenic in mice. Plant Mol Biol. 2012;78:337–49. doi: 10.1007/s11103-011-9870-1. [DOI] [PubMed] [Google Scholar]
- 104.Rubio-Infante N, Govea-Alonso DO, Romero-Maldonado A, García-Hernández AL, Ilhuicatzi-Alvarado D, et al. A plant-derived multi-HIV antigen induces broad immune responses in orally immunized mice. Mol Biotechnol. 2015;57:662–74. doi: 10.1007/s12033-015-9856-3. [DOI] [PubMed] [Google Scholar]
- 105.Ruhlman T, Ahangari R, Devine A, Samsam M, Daniell H. Expression of cholera toxin B–proinsulin fusion protein in lettuce and tobacco chloroplasts: oral administration protects against development of insulitis in non-obese diabetic mice. Plant Biotechnol J. 2007;5:495–510. doi: 10.1111/j.1467-7652.2007.00259.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Ruhlman T, Lee S-B, Jansen RK, Hostetler JB, Tallon LJ, et al. Complete plastid genome sequence of Daucus carota: implications for biotechnology and phylogeny of angiosperms. BMC Genom. 2006;7:222. doi: 10.1186/1471-2164-7-222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Ruhlman T, Verma D, Samson N, Daniell H. The role of heterologous chloroplast sequence elements in transgene integration and expression. Plant Physiol. 2010;152:2088–104. doi: 10.1104/pp.109.152017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Rybicki E. Plant-based vaccines against viruses. Virol J. 2014;11:205. doi: 10.1186/s12985-014-0205-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Samson N, Bausher MG, Lee S-B, Jansen RK, Daniell H. The complete nucleotide sequence of the coffee (Coffea arabica L.) chloroplast genome: organization and implications for biotechnology and phylogenetic relationships amongst angiosperms. Plant Biotechnol J. 2007;5:339–53. doi: 10.1111/j.1467-7652.2007.00245.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Santos RAS, Ferreira AJ, Verano-Braga T, Bader M. Angiotensin-converting enzyme 2, angiotensin-(1–7) and Mas: new players of the renin-angiotensin system. J Endocrinol. 2012;216:R1–17. doi: 10.1530/JOE-12-0341. [DOI] [PubMed] [Google Scholar]
- 111.Sanz-Barrio R, Millán AF, Corral-Martínez P, Seguí-Simarro JM, Farran I. Tobacco plastidial thioredoxins as modulators of recombinant protein production in transgenic chloroplasts. Plant Biotechnol J. 2011;9:639–50. doi: 10.1111/j.1467-7652.2011.00608.x. [DOI] [PubMed] [Google Scholar]
- 112.Saski C, Lee S-B, Fjellheim S, Guda C, Jansen RK, et al. Complete chloroplast genome sequences of Hordeum vulgare, Sorghum bicolor and Agrostis stolonifera, and comparative analyses with other grass genomes. Theor Appl Genet. 2007;115:571–90. doi: 10.1007/s00122-007-0567-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Saslowsky DE, te Welscher YM, Chinnapen DJ-F, Wagner JS, Wan J, et al. Ganglioside GM1-mediated transcytosis of cholera toxin bypasses the retrograde pathway and depends on the structure of the ceramide domain. J Biol Chem. 2013;288:25804–9. doi: 10.1074/jbc.M113.474957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Schermuly RT, Ghofrani HA, Wilkins MR, Grimminger F. Mechanisms of disease: pulmonary arterial hypertension. Nat Rev Cardiol. 2011;8:433–55. doi: 10.1038/nrcardio.2011.87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Shakya AK, Chowdhury MYE, Tao W, Gill HS. Mucosal vaccine delivery: current state and a pediatric perspective. J Control Release. 2016;240:394–413. doi: 10.1016/j.jconrel.2016.02.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Shao M, Kumar S, Thomson JG. Precise excision of plastid DNA by the large serine recombinase Bxb1. Plant Biotechnol J. 2014;12:322–29. doi: 10.1111/pbi.12139. [DOI] [PubMed] [Google Scholar]
- 117.Shenoy V, Kwon K-C, Rathinasabapathy A, Lin S, Jin G, et al. Oral delivery of angiotensin-converting enzyme 2 and angiotensin-(1–7) bioencapsulated in plant cells attenuates pulmonary hypertension. Hypertension. 2014;64:1248–59. doi: 10.1161/HYPERTENSIONAHA.114.03871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Sherman A, Su J, Lin S, Wang X, Herzog RW, Daniell H. Suppression of inhibitor formation against FVIII in a murine model of hemophilia A by oral delivery of antigens bioencapsulated in plant cells. Blood. 2014;124:1659–68. doi: 10.1182/blood-2013-10-528737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Shil PK, Kwon C-K, Zhu P, Verma A, Daniell H, Li Q. Oral delivery of ACE2/Ang(1–7) bioen-capsulated in plant cells protects against experimental uveitis and autoimmune uveoretinitis. Mol Ther. 2014;22:2069–82. doi: 10.1038/mt.2014.179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Sowers JL, Mirfattah B, Xu P, Tang H, Park IY, et al. Quantification of histone modifications by parallel-reaction monitoring: a method validation. Anal Chem. 2015;87:10006–14. doi: 10.1021/acs.analchem.5b02615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Spök A, Karner S, Stein AJ, Rodríguez-Cerezo E. Plant molecular farming. Opportunities and challenges. JRC Sci Tech Rep, Joint Res Cent, Inst Prospect Technol Stud, Seville, Spain 2008 [Google Scholar]
- 122.Staub JM, Garcia B, Graves J, Hajdukiewicz PT, Hunter P, et al. High-yield production of a human therapeutic protein in tobacco chloroplasts. Nat Biotechnol. 2000;18:333–38. doi: 10.1038/73796. [DOI] [PubMed] [Google Scholar]
- 123.Su J, Sherman A, Doerfler PA, Byrne BJ, Herzog RW, Daniell H. Oral delivery of acid alpha glucosidase epitopes expressed in plant chloroplasts suppresses antibody formation in treatment of Pompe mice. Plant Biotechnol J. 2015;13:1023–32. doi: 10.1111/pbi.12413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Su J, Zhu L, Sherman A, Wang X, Lin S, et al. Low cost industrial production of coagulation factor IX bioencapsulated in lettuce cells for oral tolerance induction in hemophilia B. Biomaterials. 2015;70:84–93. doi: 10.1016/j.biomaterials.2015.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Sugiura M. Plastid mRNA translation. In: Maliga P, editor. Methods of Molecular Biology. Vol. 1132. New York: Springer; 2014. pp. 73–91. [DOI] [PubMed] [Google Scholar]
- 126.Sun JB, Czerkinsky C, Holmgren J. Mucosally induced immunological tolerance, regulatory T cells and the adjuvant effect by cholera toxin B subunit. Scand J Immunol. 2010;71:1–11. doi: 10.1111/j.1365-3083.2009.02321.x. [DOI] [PubMed] [Google Scholar]
- 127.Svab Z, Maliga P. High-frequency plastid transformation in tobacco by selection for a chimeric aadA gene. PNAS. 1993;90:913–17. doi: 10.1073/pnas.90.3.913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Tangphatsornruang S, Birch-Machin I, Newell CA, Gray JC. The effect of different 3′ untranslated regions on the accumulation and stability of transcripts of a gfp transgene in chloroplasts of transplastomic tobacco. Plant Mol Biol. 2011;76:385–96. doi: 10.1007/s11103-010-9689-1. [DOI] [PubMed] [Google Scholar]
- 129.Taylor JP, Hardy J, Fischbeck KH. Toxic proteins in neurodegenerative disease. Science. 2002;296:1991–95. doi: 10.1126/science.1067122. [DOI] [PubMed] [Google Scholar]
- 130.Thanavala Y, Mahoney M, Pal S, Scott A, Richter L, et al. Immunogenicity in humans of an edible vaccine for hepatitis B. PNAS. 2005;102:3378–82. doi: 10.1073/pnas.0409899102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Thomas S, Harlan R, Chen J, Aiyetan P, Liu Y, et al. Multiplexed targeted mass spectrometry–based assays for the quantification of N-linked glycosite-containing peptides in serum. Anal Chem. 2015;87:10830–38. doi: 10.1021/acs.analchem.5b02063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Tsuchiya H, Tanaka K, Saeki Y. The parallel reaction monitoring method contributes to a highly sensitive polyubiquitin chain quantification. Biochem Biophys Res Commun. 2013;436:223–29. doi: 10.1016/j.bbrc.2013.05.080. [DOI] [PubMed] [Google Scholar]
- 133.US Food Drug Admin. Common ingredients in US licensed vaccines. Silver Spring, MD: US Food Drug Admin; 2014. http://www.fda.gov/BiologicsBloodVaccines/SafetyAvailability/VaccineSafety/ucm187810.htm. [Google Scholar]
- 134.US Food Drug Admin. FDA approves first seasonal influenza vaccine containing an adjuvant. Silver Spring, MD: US Food Drug Admin; 2015. http://www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/ucm474295.htm. [Google Scholar]
- 135.Verma D, Maghimi B, LoDuca PA, Singh HD, Hoffman BE, et al. Oral delivery of bioencapsuated coagulation factor IX prevents inhibitor formation and fatal anaphylaxis in hemophilia B mice. PNAS. 2010;107:7101–6. doi: 10.1073/pnas.0912181107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Verma D, Samson NP, Koya V, Daniell H. A protocol for expression of foreign genes in chloroplasts. Nat Protoc. 2008;3:739–58. doi: 10.1038/nprot.2007.522. [DOI] [PubMed] [Google Scholar]
- 137.Waheed MT, Ismail H, Gottschamel J, Mirza B, Lössl AG. Plastids: the green frontiers for vaccine production. Front Plant Sci. 2015;6:1005. doi: 10.3389/fpls.2015.01005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Waheed MT, Thönes N, Müller M, Hassan SW, Razavi NM, et al. Transplastomic expression of a modified human papillomavirus L1 protein leading to the assembly of capsomeres in tobacco: a step towards cost-effective second-generation vaccines. Transgenic Res. 2011;20:271–82. doi: 10.1007/s11248-010-9415-4. [DOI] [PubMed] [Google Scholar]
- 139.Walsh G. Biopharmaceutical benchmarks 2014. Nat Biotechnol. 2014;32:992–1000. doi: 10.1038/nbt.3040. [DOI] [PubMed] [Google Scholar]
- 140.Wang X, Sherman A, Liao G, Leong KW, Daniell H, et al. Mechanism of oral tolerance induction to therapeutic proteins. Adv Drug Deliv Rev. 2013;65:759–73. doi: 10.1016/j.addr.2012.10.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Wang X, Su J, Sherman A, Rogers GL, Liao G, et al. Plant-based oral tolerance to hemophilia therapy employs a complex immune regulatory response including LAP+CD4+ T cells. Blood. 2015;125:2418–27. doi: 10.1182/blood-2014-08-597070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Wang X, Terhorst C, Herzog RW. In vivo induction of regulatory T cells for immune tolerance in hemophilia. Cell Immunol. 2015;301:18–29. doi: 10.1016/j.cellimm.2015.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Wasinger VC, Zeng M, Yau Y. Current status and advances in quantitative proteomic mass spectrometry. Int J Proteom. 2013;2013:180605. doi: 10.1155/2013/180605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Weiner HL, da Cunha AP, Quintana F, Wu H. Oral tolerance. Immunol Rev. 2011;241:241–59. doi: 10.1111/j.1600-065X.2011.01017.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Xiao Y, Kwon K-C, Hoffman BE, Kamesh A, Jones NT, et al. Low cost delivery of proteins bioencapsulated in plant cells to human non-immune or immune modulatory cells. Biomaterials. 2016;80:68–79. doi: 10.1016/j.biomaterials.2015.11.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Ye GN, Daniell H, Sanford JC. Optimization of delivery of foreign DNA into higher plant chloroplasts. Plant Mol Biol. 1990;15:809–20. doi: 10.1007/BF00039421. [DOI] [PubMed] [Google Scholar]
- 147.Yoon CK. EPA announces new rules on genetically altered corn. New York Times. 2000 Jan 17; http://www.nytimes.com/2000/01/17/us/epa-announces-new-rules-on-genetically-altered-corn.html.
- 148.Zoschke R, Barkan A. Genome-wide analysis of thylakoid-bound ribosomes in maize reveals principles of cotranslational targeting to the thylakoid membrane. PNAS. 2015;112:E1678–87. doi: 10.1073/pnas.1424655112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Zoschke R, Watkins KP, Barkan A. A rapid ribosome profiling method elucidates chloroplast ribosome behavior in vivo. Plant Cell. 2013;25:2265–75. doi: 10.1105/tpc.113.111567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Zou Z, Eibl C, Koop H-U. The stem-loop region of the tobacco psbA 5′ UTR is an important determinant of mRNA stability and translation efficiency. Mol Gen Genom. 2003;269:340–49. doi: 10.1007/s00438-003-0842-2. [DOI] [PubMed] [Google Scholar]