Abstract
Therapies with both immunomodulatory and neuroprotective properties are thought to have the greatest promise in reducing the severity and progression of multiple sclerosis (MS). Several reactive oxygen (ROS) and reactive nitrogen species (RNS) are implicated in inflammatory-mediated damage to the central nervous system (CNS) in MS and its animal model, experimental autoimmune encephalomyelitis (EAE). TEMPOL (4-hydroxy-2,2,6,6-tetramethylpiperidine-N-oxyl) is a stable nitroxide radical with potent antioxidant activity. The goal of our studies was to investigate the immunomodulatory effects and therapeutic potential of orally-delivered TEMPOL in the mouse EAE model. Mice receiving TEMPOL chow ad libitum for 2 weeks prior to induction of active EAE showed delayed onset and reduced incidence of disease compared to control-fed animals. Reduced disease severity was associated with limited microglial activation and fewer inflammatory infiltrates. TEMPOL’s effects were immunomodulatory, not immunosuppressive: T cells produced less interferon-γ and tumor necrosis factor-α, and TEMPOL-fed mice exhibited a shift towards TH2-type antibody responses. Both myeloid and myeloid-dendritic cells of TEMPOL-fed EAE animals had significantly lower levels of MHC class II expression than controls; CD40 was also significantly reduced. TEMPOL administration was associated with an enrichment of CD8+ T cell populations and CD4+FoxP3+regulatory populations. TEMPOL reduced the severity of clinical disease when administered after the induction of disease, and also after the onset of clinical symptoms. To exclude effects on T cell priming in vivo, TEMPOL was tested with the passive transfer of encephalitogenic T cells and was found to reduce the incidence and peak severity of disease. Protection was associated with reduced infiltrates and a relative sparing of neurofilaments and axons. The ability of oral TEMPOL to reduce inflammation and axonal damage and loss demonstrate both anti-inflammatory and protective properties, with significant promise for the treatment of MS and related neurological disorders.
Keywords: EAE, multiple sclerosis, nitroxide, inflammation, neurodegeneration
Introduction
Multiple sclerosis (MS) is a chronic inflammatory and demyelinating neurodegenerative disorder, characterized pathologically by central nervous system (CNS) alterations in the vasculature, inflammatory infiltrates, demyelination, glial scarring, oligodendrocyte loss, and axonal damage and loss(Frohman et al., 2006). The irreversible axonal loss and neurodegeneration are thought to be the major correlate of chronic disability (Bjartmar and Trapp, 2003) and likely starts early in disease (Ferguson et al., 1997; Trapp et al., 1998). Approved disease modifying therapies (DMTs) target inflammatory processes and fall into two groups: those well tolerated but with partial efficacy, or those with greater efficacy and increased risk profiles (Kieseier et al., 2011; Stuve, 2009). Development of orally efficacious, safe, and well-tolerated therapeutics with immunomodulatory and neuroprotective properties remains a priority.
In experimental autoimmune encephalomyelitis (EAE), the mouse model of MS, autoreactive T cells that produce pro-inflammatory cytokines including tumor necrosis factor α (TNFα), interferon-γ (IFNγ), interleukin (IL)-17, and granulocyte macrophage colony stimulating factor (GM-CSF), travel to the CNS to cause disease (Sospedra and Martin, 2005). Both CD4 and CD8 T cells may contribute directly to pathogenesis via direct contact, but phenotypical and molecular profiles vary and define pathogenic or suppressive/regulatory roles (Hauser et al., 2008; Johnson et al., 2010; Racke, 2009). While demyelination and associated axonal loss appear secondary to inflammation (Trapp et al., 1998), neurodegeneration seen prior to inflammatory cell infiltration in EAE and the progressive loss of function after the inflammatory phase has subsided (Hobom et al., 2004; Qi et al., 2006) seem distinct. Recent findings implicate mitochondrial dysfunction secondary to energy imbalance and increases in reactive oxygen species (ROS) as sources of oxidative damage not directly attributable to inflammation (Mahad et al., 2015; Mahad et al., 2009; Su et al., 2009).
Emerging evidence implicates ROS and reactive nitrogen species (RNS), focusing on superoxide, nitric oxide, and their intermediates, as contributors to several mechanisms underlying the pathogenesis of MS and EAE (Gilgun-Sherki et al., 2004; Gonsette, 2008a). ROS drive morphological alterations that promote leukocyte traffic across the blood-brain barrier (BBB) (Schreibelt et al., 2006; Van der Goes et al., 2001). Infiltrated leukocytes produce ROS that induce myelin phagocytosis and, therein, myelin breakdown by macrophages, oligodendrocyte damage, as well as axonal and neuronal injury and loss (Smith et al., 1999; Smith et al., 2001; van Meeteren et al., 2004). Microglia and neurons generate peroxynitrite, a principal mediator of the oxidative stress and excitotoxicity that drives neurodegenerative processes in MS (Gonsette, 2008b; Torreilles et al., 1999).
Nitroxide radicals exhibit antioxidant activity and have been reported to react with both ROS and RNS, directing a shift from their use as biophysical tools to potential therapeutics (Soule et al., 2007b). Nitroxides are flexible, cycling via redox transformation between the oxidation states of nitroxide radical, hydroxylamine, and the oxoammonium cation (Fig. 1A)(Soule et al., 2007a), as they interact with biological oxidants and reductants. TEMPOL (4-hydroxy-2,2,6,6-tetramethylpiperidine-N-oxyl) is a small (MW 172Da) stable nitroxide radical that can readily permeate biological membranes (Bonini et al., 2002), allowing for significant tissue and intracellular accumulation. Indeed, TEMPOL exerts beneficial effects in animal models of shock, hypertension, diabetes, ischemia-reperfusion injury, spinal cord injury, traumatic brain injury (Deng-Bryant et al., 2008; Kato et al., 2003; Schnackenberg and Wilcox, 2001; Sledzinski et al., 1995; Thiemermann, 2003), and neurodegenerative models for Alzheimer’s and Parkinson’s disease (Ghosh et al., 2008; Lipman et al., 2006). The current study demonstrates that oral TEMPOL limits CNS inflammation both prophylactically and therapeutically in the most established models of MS. Through immunomodulation and deviation away from an inflammatory phenotype, TEMPOL reduces expression of inflammatory mediators and markers both outside and within the target organ, while enriching for regulatory populations. In this regard, oral TEMPOL affords neuroprotection through several distinct immunomodulatory mechanisms that have not previously been characterized or associated with modes of action for nitroxide radicals. Ultimately, the overall outcome is substantial protection from disability in a setting of neuroinflammation.
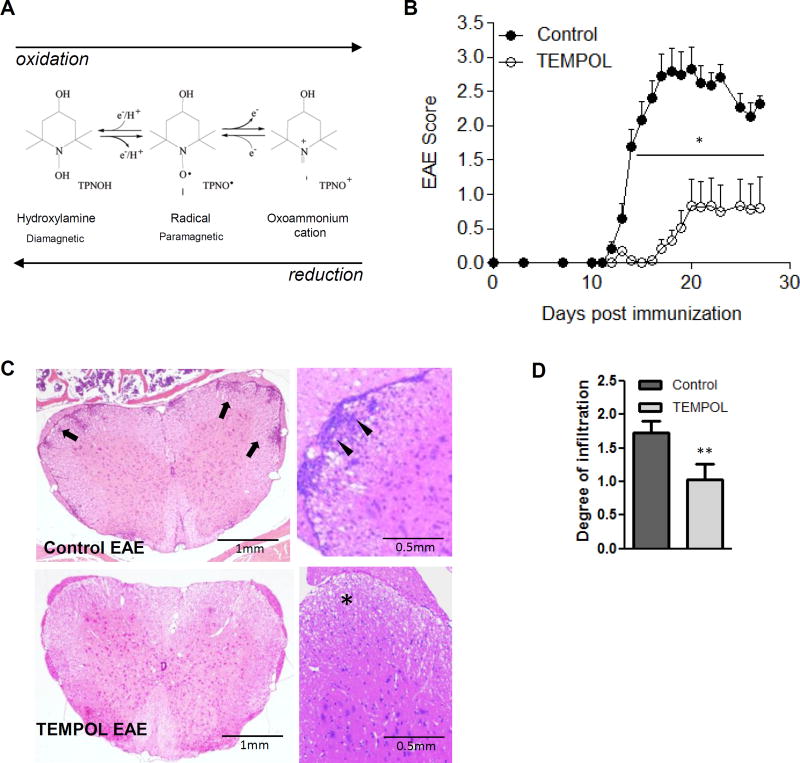
Figure 1. The nitroxide compound TEMPOL (4-hydroxy-2,2,6,6-tetramethylpiperidine-N-oxyl) given orally reduces clinical disease in EAE.
The five- or six-membered rings (Fig. 1A) contain a nitrogen atom – the molecule bound to the nitrogen dictates the various properties of the compound. Conversion of nitroxide radical to hydroxylamine or oxoammonium cation occurring in vivo are shown. Nitroxides exist in vivo in equilibrium between the nitroxide radical form [detected by electron paramagnetic resonance studies (EPR) and the reduced “hydroxylamine” form (not detected by EPR)]. Fig. 1B. C57BL/6J animals maintained on TEMPOL chow (●) for 2 weeks prior to induction of EAE show resistance to the induction of active EAE compared to animals on control chow (○). n = 15 animals/group, 4/15 TEMPOL-fed animals vs. 14/15 control fed animals presented with a limp tail or greater during the course of the experiment. TEMPOL-fed animals are resistant to induction of chronic EAE as shown by reduced incidence and overall disease burden. One representative of four experiments with similar results is shown; p < 0.01 unpaired two-tailed T test comparing daily disease scores of control to TEMPOL-fed animals. Inflammatory infiltrates and axonal loss are reduced in mice fed TEMPOL prophylactically. In an experiment with similar results to Fig. 1B, tissues were taken at day 21 for histological analyses (Fig.1C). Control-fed animals show significant infiltrates stemming from the meninges and infiltrating the parenchyma of the white matter (arrows). (D) The degree of leukocyte infiltration in TEMPOL-fed mice was significantly lower than in controls. n = 5 mice per treatment group were analyzed, each with 8–10 levels spread over the entire cord examined per mouse. **p = 0.002 Mann-Whitney Rank Sum test.
Methods
EAE induction
All experiments were carried out in compliance with the Guide for the Care and Use of Laboratory Animal Resources (1996), the Canadian Council on Animal Care guidelines and approved by the NINDS Animal Care and Use and UBC Animal Care Committees. Female C57BL/6J (Jackson Labs) or SJL/J (Harlan Laboratories) mice were acclimatized at least one week prior to study and then used as models of active chronic and passive relapsing-remitting model of MS, respectively. 8–10 week old C57BL6/J mice were immunized with 200 μg MOG35-55 (MEVGWYRSPFSRVVHLYRNGK; Stanford Pan Facility, Stanford CA) as described previously (Quandt et al., 2012). For passive disease, animals were immunized with 75 µg PLP139-151 (HCLGKWLGHPDKF, Stanford Pan Facility) and draining lymph node (LN) cells enriched with PLP before transfer to healthy recipients as described (Anderson et al., 2004). Animals were monitored daily for clinical signs at least through the first attack of disease and through the first days of recovery (typically day 30–35) or longer in chronic studies to day 45 or 60 according to the scoring table and clinical symptoms outlined in Table 1.
Table 1. Scoring of clinical symptoms of disability in EAE mice.
Animals were assessed daily and scored according to the above clinical presentation of symptoms/disability.
| Score | Presentation/clinical symptoms |
|---|---|
| 0 | No disease |
| 1 | Limp tail |
| 2 | Hindlimb paresis mild; 2.5 severe paresis |
| 3 | Single hindlimb paralysis; 3.5 both hindlimbs paralyzed |
| 4 | Hindlimb paralysis, single forelimb paresis; 4.5 hindlimb paralysis and paresis in both forelimbs |
| 5 | No mobility/moribund |
Quantification of TEMPOL
Mice that were given TEMPOL in their drinking water at a concentration of 58 mM lived longer secondary to lower tumor incidence and maintained a normal healthy weight, while sucrose-fed controls gained substantial weight (Mitchell et al., 2003). Powdered TEMPOL was uniformly mixed with mouse chow by a “cold press” technique (Bio-Serv, Frenchtown, NJ) at 10 g/kg of chow (the equivalent to 58 mM supplied in drinking water) and was found to reduce body weight without toxicity, decrease cancer, and extend survival when administered after nonlethal total body radiation (Mitchell et al., 2012). This dose was applied in our study. Purina 4501 chow (Bio-Serv) with or without 10 g/kg TEMPOL (Sigma Aldrich, St. Louis, MO) was given ad libitum. For detection of TEMPOL in blood and tissue samples, animals were euthanized with CO2 and analyzed as described previously (Hyodo et al., 2006). Blood and whole brain tissue samples were diluted and homogenized and ferricyanide added prior to measurement on a Varian E-9 X-band electron paramagnetic resonance spectrometer and compared to standard curves.
Histology
Animals were perfused following anesthesia with a Ketamine/Xylazine mixture (87.5 mg/kg Ketamine and 12.5 mg/kg Xylazine) prior to thoracotomy and PBS perfusion via the left ventricle followed by 4% paraformaldehyde (PFA) in PBS. The brain and spinal column were removed to 10% neutral buffered formalin for 5 days. The entire spinal column from each animal was trimmed, decalcified, and cut into ten equal lengths of 5 mm. Six µm sections were cut starting at the “top” of each section and stained with hematoxylin and eosin (H&E), Luxol fast blue (LFB, for myelin), and silver Bielshowsky (axonal) staining (Histoserve, Gaithersburg, MD). Sections were scored according to the number and degree of infiltration as outlined in Table 2.
Table 2. Scoring of inflammatory infiltrates in tissues.
The number of infiltrates and degree of infiltration were scored according to the scale above. Cross sections of 8–10 different levels of the spinal cord, spanning the entire spinal column, were assessed for each mouse.
| Score | Presentation/clinical symptoms |
|---|---|
| 0 | No infiltrates |
| 1 | 1–2 mild infiltrates (10–25 cells)-little/no invasion of parenchyma |
| 2 | 3+ mild infiltrates, +/−infiltration of parenchyma |
| 3 | 1–2 moderate infiltrates (25–50 cells), 1–2 mild infiltrates/little infiltration |
| 4 | 3 or more moderate infiltrates, or 1 severe/broad infiltrate (50+ cells) with extensive infiltration to parenchyma present |
| 5 | 2 or more severe/broad parenchymal infiltrates with or without additional mild/moderate infiltrates extending into parenchyma |
Flow cytometry
PBS-perfused spinal cords were flushed from columns and a single cell suspension representing infiltrates from the entire cord in 30% Percoll (GE Healthcare, Uppsala Sweden) were layered over 70% Percoll and mononuclear cells were recovered and stained with antibodies in staining buffer (5% FBS in PBS, 0.05% sodium azide) and run on a BD FacsCalibur for analysis with CellQuest software (BD).
Lymphoid organ cell culture
Animals were anesthetized using CO2 followed by cervical dislocation. Spleens and inguinal, brachial, and axillary LN were removed and single cell preparations red cell-depleted with Ammonium-Chloride-Potassium Lysing Buffer (Life Technologies, Carlsbad, CA). Cells were immediately set into culture or stained for flow cytometry as above. Intracellular staining with Foxp3 and IgG2a isotype control antibodies was performed using the Mouse Regulatory T Cell Staining Kit #2 (eBioscience, San Diego, CA). Cells were examined on a Miltenyi MacsQuant flow cytometer and analyzed using FlowJo (version 7.6.5; Treestar, Ashland, OR) to compare the median fluorescence intensity (MFI) of markers.
Leukocytes were seeded with MOG35-55, anti-CD3 (clone 2C11, purified from mouse ascites; NCI, Frederick, MD) or Concanavalin A (Con A; Sigma-Aldrich, Oakville, ON) at 0.2 μg/mL. Cell proliferation was measured after 72 hours of 0.5 μCi tritiated thymidine incorporation (Perkin Elmer, Waltham, MA). Plates were harvested on a Tomtec IIIM harvester (Tomtec, Hamden, CT) and incorporated radioactivity was measured on a Wallac Trilux Microbeta Scintillation Counter (Perkin Elmer). For cytokine analysis cells were seeded with 20 μg/mL MOG35-55, 0.125 μg/mL anti-CD3, or 0.2 μg/mL Con A. Supernatants were collected at 65 hours and evaluated using DuoSet ELISA Development kits (R&D Systems, Minneapolis, MN) or the TNFα Ready-SET-Go! ELISA kit (eBioscience, San Diego, California).
MOG-specific antibody ELISA
Blood collected from the submandibular vein was processed for serum and stored at −20°C. Micro-titre plates (Greiner Bio-one, Munroe, NC) were coated with 2 μg/mL MOG35-55 prior to incubation with sera. Secondary antibodies [goat anti-mouse IgG-peroxidase (Jackson ImmunoResearch Laboratories Inc., West Grove, PA), goat anti-mouse IgG1-biotin, IgG2a-biotin, or IgG2c-biotin (Sigma-Aldrich)]) and streptavidin-HRP enabled chromogenic conversion of TMB and quantification as for cytokine ELISAs.
Neurofilament ELISA
A sandwich ELISA to detect normal neurofilament H (phosphorylated heavy chain) levels was performed as previously described (Petzold and Shaw, 2007). SMI35R monoclonal antibody (to neurofilament heavy chain, Covance Princeton, NJ)-coated microtitre plates were incubated with 15 µg of spinal cord protein; protein was detected with rabbit polyclonal anti-neurofilament H antibody (N-4142; Sigma) and horseradish peroxidase-conjugated anti-rabbit immunoglobulin.
Statistical evaluation
Statistical evaluation of significant differences between groups was performed using one-way analysis of variance (ANOVA) with subsequent multiple comparison analysis. When comparing TEMPOL to control groups in EAE disease or for infiltrates, neurofilament, or antibody we applied the Mann-Whitney nonparametric ranking test or T test when data were normally distributed. Significance is reported as *** p < 0.001, ** p < 0.01, and * p < 0.05. For flow cytometry population and cell surface marker analyses we applied Bonferroni corrections for the populations analyzed and for the separate T cell subset analyses. All analyses were performed using SigmaPlot (Version 11.0, Systat Software, Inc.) and graphs prepared with GraphPad Prism 5.
Results
TEMPOL protects against actively-induced EAE
Animals were placed on TEMPOL or control chow for 2 weeks, immunized, and euthanized 10 days later to quantify TEMPOL. The hydroxylamine (reduced) form of TEMPOL could be detected at 3.0 ±1.9 to 5.0 ±1.5 nmol/kg tissue in the brains and 10.6 ±4.9 to 40.4 ±14.5 nmol/mL (µM) blood in C57BL/6J and SJL/J animals respectively (n = 3 per group). TEMPOL reduced the incidence (27% vs. 93% in control-diet animals) and delayed disease onset (Fig. 1B). Average daily scores were lower in TEMPOL-fed mice compared to controls, from day 13 and on. TEMPOL decreased the total disease burden/cumulative disease over the course of the experiment (5.8 ± 2.6 vs. 29.9 ± 2.8 in control diet animals).
Histologically, there were fewer immune cells within spinal cords of TEMPOL-fed animals (Fig. 1C). TEMPOL-fed EAE mice showed fewer infiltrates extending into the parenchyma and lesser involvement of parenchymal vessels compared to controls (Fig. 1C; 1.73 ± 0.19 in control vs. 1.04 ± 0.24 in TEMPOL-fed animals p = 0.002, Fig. 1D). Infiltrates were absent in 40% of TEMPOL-fed animals examined at 10 different levels of the spinal cord; every control animal showed infiltrates in more than 50% of levels examined (data not shown)..
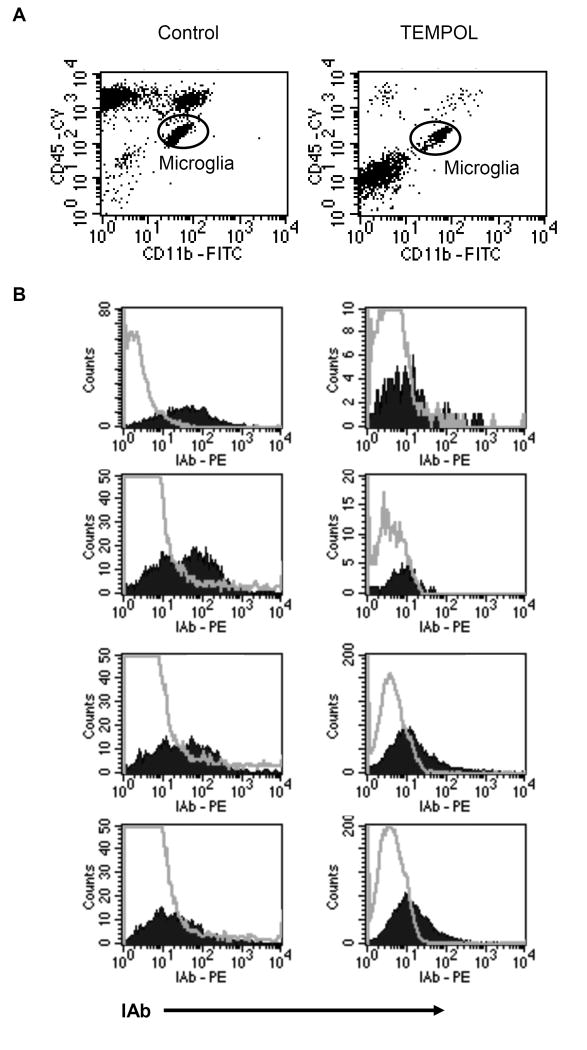
Flow analysis at peak disease revealed increased infiltrating cells (monocytes, T cells, and B cells) in control versus TEMPOL-fed EAE animals (Fig. 2A). In the recovered CD45 population of CD45 dim (CNS resident) and CD45-hi (CNS infiltrating) cells, CD45hiinfiltrating leukocytes comprised the majority of cells in spinal cords of control EAE animals (55 to 69% of CD45 positive cells) compared to TEMPOL-fed animals where the primary population recovered (51 to 67%) were CD45-dim-resident microglia. Each sample of CD11b high/CD45 dim microglia from control-fed EAE animals showed greater IAb/MHC class II than levels of the isotype control (Fig. 2B), whereas no samples from TEMPOL-fed animals showed IAb staining greater than that of the isotype control.
Figure 2. Microglia from spinal cords of TEMPOL-fed EAE mice show reduced microglial activation.
CD45 dim/CD11b high microglial cells from spinal cords of control or TEMPOL-fed EAE mice were examined for expression of MHC class II IAb. Spinal cords from 8 mice at peak disease were analyzed from each group (cords from 2 mice in each group were pooled for n = 4 per treatment group). 4 of 4 TEMPOL-fed groups showed low IAb compared to 3 of 4 control-fed EAE groups with higher MHC class II expression. Hollow histograms represent isotype controls; filled histograms represent IAb staining on gated microglial population.
Immunomodulation in TEMPOL-fed animals
In both LN and spleens of TEMPOL-fed EAE mice, ex vivo proliferative responses to MOG, anti-CD3, or Con A (Fig. 3A,B) were comparable to controls. Ex vivo proliferation was also examined using cells isolated from age-matched healthy mice administered control or TEMPOL chow for 28 days. Similar to EAE mice, polyclonal responses to Con A or anti-CD3 measured ex vivo were not reduced by TEMPOL administration (data not shown).
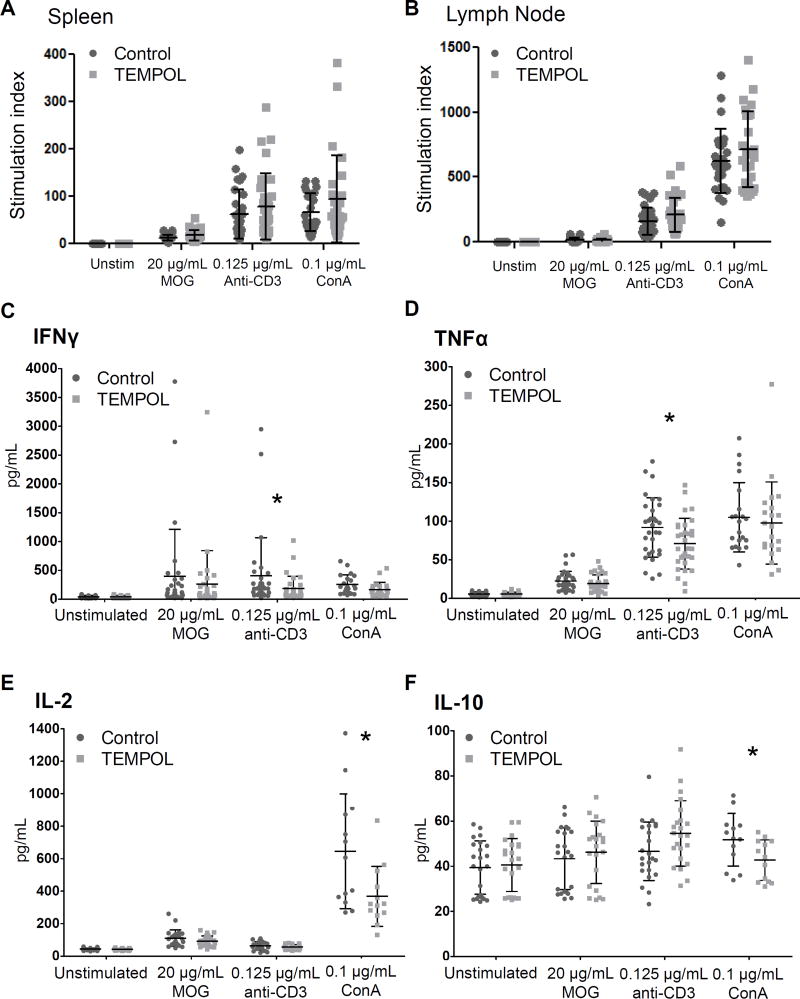
Figure 3. Autoreactive and polyclonal T cell responses measured ex vivo are similar in control and TEMPOL-fed EAE animals while cytokine production is altered by TEMPOL in LN cells isolated from EAE mice.
Spleen cells (A) and draining LN (B) were isolated from control (circles) or TEMPOL-fed (squares) EAE mice 14 days following disease induction and compared for proliferative responses by thymidine incorporation after 48 hours. Proliferation was normalized to proliferation of unstimulated cells (the stimulation index, or SI) for each mouse before pooling. LN cells were treated as indicated (C–F), and supernatants collected at 65 hours and cytokines measured by ELISA. Data is pooled from 5 experiments of 5–8 mice (n = 31) per chow group; each point represents a single mouse, with bars ±SD. *p < 0.05, Mann-Whitney rank sum test, control vs. TEMPOL.
Both IFNγ and TNFα production by anti-CD3-stimulated cells was significantly lower in isolates from TEMPOL-fed EAE animals (Fig. 3C,D). GM-CSF production also tended to be lower (p = 0.090) but was not significant (data not shown). Interestingly, IL-10 production also tended to be greater by TEMPOL-fed animals (p = 0.087). Responses to Con A were also significantly different between control and TEMPOL-fed animals: production of IL-2 and IL-10 by cells from TEMPOL-fed animals was significantly lower than controls (Fig. 3E,F). While IFNγ production also tended to be lower in TEMPOL-fed animals, (p = 0.059), IL-17 levels were similar between groups and IL-4 and TGF-β1 were also similar but below the limit of detection.
Fewer differences were noted in spleen samples; lower IL-2 production by MOG-stimulated cells from TEMPOL-fed animals was the only significant difference (p = 0.035). Cytokine production was also examined in supernatants from spleens of healthy mice fed a control or TEMPOL diet; only TGF-β1 levels were different (lower) in TEMPOL-fed animals following the addition of Con A to cells (p = 0.018).
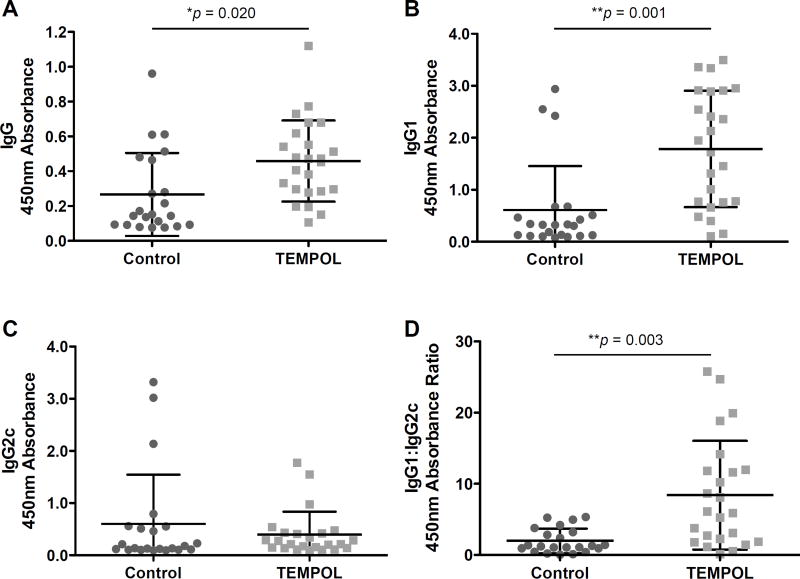
The generation of MOG-specific antibodies is thought to contribute to disease in rodent models of MS (Dittel, 2000), and to a greater extent the disease process in MS (Reindl et al., 1999). TEMPOL-fed EAE mice showed increased levels of MOG-specific serum IgG (Fig. 4A). This increase was predominantly in the IgG1 isotype group typically associated with a TH2 response (Fig. 4B). Similarly, the IgG2c levels associated with more pro-inflammatory TH1 responses tended to be reduced in TEMPOL-fed animals (Fig. 4C), and drove IgG1:IgG2c ratios (Fig. 4D).
Figure 4. TEMPOL administration enhances immunosuppressive MOG-specific antibody responses.
Sera were prepared from blood drawn from EAE animals at Day 24 – 28 and compared for overall IgG response (A) as well as isotype-specific responses (B – C) and presented as an IgG1:IgG2c ratio as a generalized ratio of Th2 to Th1 type responses. Results were pooled from 3 experiments, n = 22 mice per chow group. Bars represent the mean 450 nm absorbance with error bars ±SD. *p < 0.05, **p < 0.01, ***p < 0.001. Mann-Whitney Rank Sum test, control vs. TEMPOL.
TEMPOL alters T cell subset distributions
While no significant differences were observed in the relative proportions of T, B, myeloid, dendritic, or NK subsets between control or TEMPOL-fed EAE animals (Table 3), TEMPOL-fed mice had increased numbers of CD8+ T cells and decreased numbers of CD4+ T cell populations. Similarly, the spleen population of EAE mice which was predominantly CD4+ (56.6 vs. 32.1% CD8+) in control-fed mice, showed significant increases in CD8+ T cells with 50.7 vs. 37.7% CD4+ T cells present.
Table 3. Composition of organ populations is altered in control vs. TEMPOL-fed EAE animals.
Cells were isolated 10 days after EAE induction in animals on control or TEMPOL feed for 14 days prior to immunization. Analyses were performed on flow cytometry data by selecting the live cells based on forward and side scatter, then selecting the CD45+ leukocyte population, then subsequently gating the described populations as a percentage of CD45+ cells. Values represent average results of 2 pooled experiments (n = 13 mice total per feed group) for lymphoid organs ±SD, and 8 animals per group, brain and spinal cord pooled 2 to 1 (n = 4 per group).
| Control Average % |
TEMPOL Average % |
P value | |
|---|---|---|---|
| Lymph node | |||
| B cells (CD19+) | 55.6 ±3.4 | 54.6 ±4.1 | 0.441 |
| Myeloid cells (CD11b+CD11c−) | 1.1 ±0.6 | 1.8 ±0.8 | 0.051 |
| Dendritic cells (CD11c+CD11b−) | 1.3 ±0.2 | 1.3 ±0.2 | 0.681 |
| Myeloid DCs (CD11b+CD11c+) | 2.0 ±0.5 | 2.3 ±0.3 | 0.045* |
| NK cells (NK1.1+) | 4.0 ±0.7 | 4.5 ±0.5 | 0.043* |
| T cells (CD3+) | 37.9 ±4.4 | 38.2 ±2.9 | 0.538 |
| % CD4+ T cells | 53.3 ±3.3 | 46.7 ±2.3 | <0.001* |
| % CD8+ T cells | 40.1 ±2.1 | 46.4 ±2.2 | <0.001* |
| % CD4+CD25+Foxp3+ T cells | 9.7 ±2.6 | 12.3 ±2.5 | 0.027* |
| Spleen | |||
| B cells (CD19+) | 36.3 ±7.3 | 34.2 ±6.3 | 0.719 |
| Myeloid cells (CD11b+CD11c−) | 26.1 ±8.0 | 29.4 ±5.0 | 0.442 |
| Dendritic cells (CD11c+CD11b−) | 1.5 ±0.3 | 1.4 ±0.3 | 0.259 |
| Myeloid DCs (CD11b+CD11c+) | 3.1 ±1.4 | 2.9 ±0.4 | 0.858 |
| NK cells (NK1.1+) | 4.9 ±1.5 | 4.7 ±2.4 | 0.305 |
| T cells (CD3+) | 19.7 ±8.0 | 17.6 ±4.3 | 0.918 |
| % CD4+ T cells | 56.6 ±3.6 | 50.7 ±4.2 | 0.001* |
| % CD8+ T cells | 32.1 ±3.2 | 37.7 ±4.0 | 0.001* |
| % CD4+CD25+Foxp3+ T cells | 10.2 ±2.2 | 8.8 ±1.1 | 0.045* |
| CNS – brain & spinal cord | |||
| # cells recovered (x106) | 1.8 ±0.2 | 1.0 ±0.1 | |
| CD45 high leukocytes | 62.0 ±8.8 | 28.1 ±6.5 | 0.006* |
| CD11b | 32.7 ±8.6 | 53.7 ±5.5 | 0.027* |
| CD3 | 57.7 ±8.0 | 39.3 ±5.5 | 0.031* |
Mann-Whitney Rank Sum test with uncorrected p values are shown for each subset analyzed comparing control to TEMPOL fed animals, *p < 0.050. Bolded values are those withstanding Bonferroni correction for six populations analysed in the spleen and lymph node (p < 0.0083) and correction for four populations analyzed in the CNS (p < 0.0125).
More CNS infiltrates were recovered from control-fed EAE mice than those fed TEMPOL (Table 3). Control-fed animals had greater proportions of CD45 cells and CD11b and CD3+ T cell populations also tended to be greater than in TEMPOL-fed mice. Notably, the CD8 enrichment apparent in lymphoid organs was also detected in CNS isolates, where CD4/CD8 ratios dropped significantly from 4.9 ±0.8 to 1.7 ±0.1 in TEMPOL-fed animals.
CD4+ Treg cell (CD4+CD25+Foxp3+) populations tended to increase from 9.7 to 12.3% of CD4+ T cells in LN of TEMPOL-fed compared to control-fed EAE mice. CD8+ Treg cells also tended to be higher in LN, but still made up less than 1% of the CD8+ T cell population (data not shown). This increase in regulatory populations was not observed in the spleen, where the numbers of Treg cells in the CD4+ subset were slightly lower in TEMPOL-fed animals than controls (8.8 vs. 10.2% of the CD4+ population respectively, p = 0.045). Notably, CD8 T cells and CD4 Treg populations were both significantly greater in TEMPOL-fed than control-fed healthy mice (Table 4).
Table 4. TEMPOL-fed healthy mice show altered distributions of lymphocyte subsets compared to controls.
Lymphoid organs were harvested and cells prepared following 28 days on control or TEMPOL feed. Values represent pooled results from 2 experiments, ±SD, with 13 mice per feed group.
| Control Average % |
TEMPOL Average % |
P value | |
|---|---|---|---|
| Lymph node | |||
| % CD4+ T cells | 49.4 ±1.6 | 45.1 ±3.2 | 0.001* |
| % CD8+ T cells | 42.1 ±1.1 | 47.1 ±2.6 | <0.001* |
| % CD4+CD25+Foxp3+ T cells | 8.9 ±0.9 | 11.7 ±1.2 | <0.001* |
| Spleen | |||
| % CD4+ T cells | 52.9 ±3.2 | 47.7 ±3.0 | 0.001* |
| % CD8+ T cells | 40.9 ±3.2 | 46.5 ±2.9 | <0.001* |
| % CD4+CD25+Foxp3+ T cells | 7.0 ±0.6 | 6.1 ±0.6 | 0.003* |
Mann-Whitney Rank Sum test with uncorrected p values are shown for each subset analyzed in comparing control to TEMPOL fed animals, *p < 0.05. Bolded values are those withstanding Bonferroni correction for three populations analysed p < 0.0167.
TEMPOL influences antigen presenting and co-stimulatory molecule expression
Expression of MHC class II, CD40, CD80, and CD86 was examined in myeloid cells, lymphoid DCs, and myeloid DCs in spleens and LN of control and TEMPOL-fed EAE mice. In the spleen, both MHC II and CD40 were significantly lower in TEMPOL-fed EAE animals than control-fed EAE animals. These changes were noted in both myeloid as well as myeloid-derived dendritic cells. In draining LN of TEMPOL-fed EAE mice, CD11b+CD11c− myeloid cells and CD11b+CD11c+ myeloid DC from TEMPOL-fed mice also tended to have lower MHC II expression compared to control-fed mice.
CD86 expression tended to be higher in myeloid cells of both the LN and spleen of TEMPOL-fed animals. Notably, CD80 expression was also significantly increased with TEMPOL in myeloid DC within LN. MHC I expression was also studied, but was not found to be altered (data not shown).
Surface molecule expression was also studied in healthy mice on control or TEMPOL chow. There were no differences observed in LN, but MHC II also tended to be lower in TEMPOL-fed spleens compared to controls in CD11b+/CD11c− myeloid 133.75 ±15.0 vs. 108.5 ± 21.1, p=0.010) and also in CD11b+/CD11c+ myeloid-derived DC (5746.4 ± 715.3 vs. 4843.8 ± 1118.8 p=0.035).
TEMPOL limits disability when given after disease induction
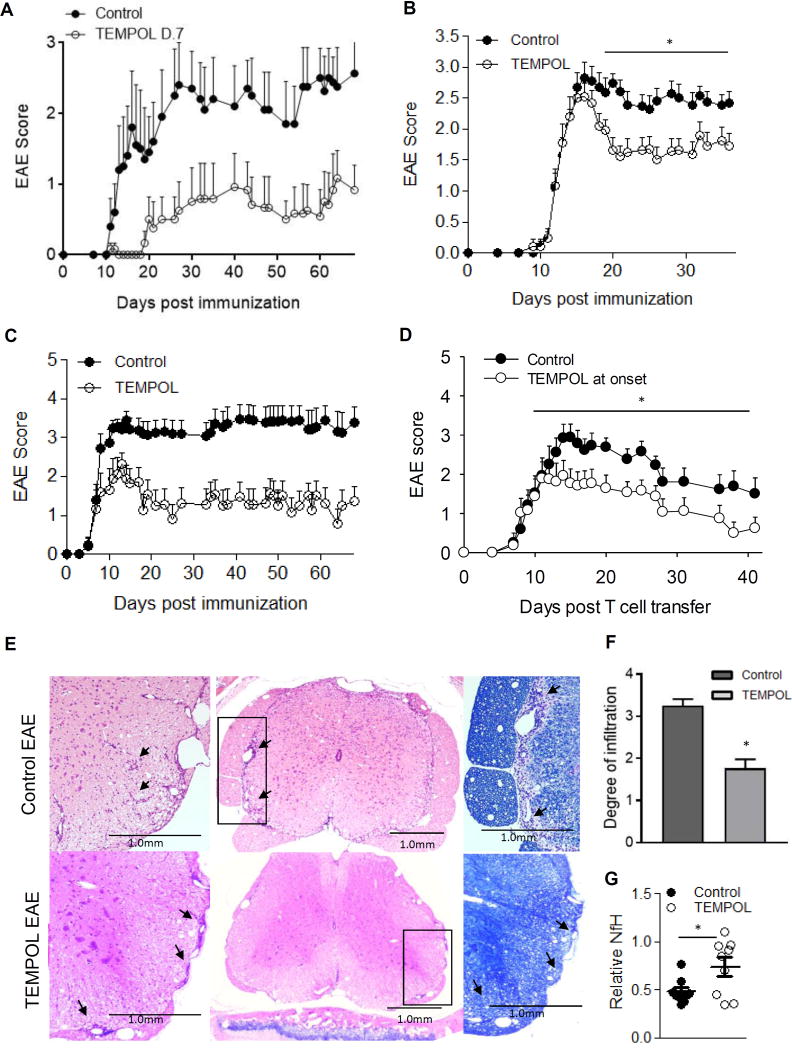
Animals fed TEMPOL starting 7 days after immunization (Fig. 5A) on average had milder disease (0.52 ± 0.63 vs. 1.72 ± 0.89, p = 0.03) and a lower peak disease severity (1.09 ± 1.0 vs. 3.0 ± 1.58; p = 0.05) than controls. TEMPOL also reduced disease incidence compared to controls (50 vs. 100% respectively). To test therapeutic efficacy, a subset of EAE animals were switched to TEMPOL chow at the onset of clinical symptoms (first sign of a limp tail or paresis; grade 1 or greater) (Fig. 5B). Cumulative disease scores from the start of TEMPOL administration through to experiment end were reduced in TEMPOL-fed animals (36.9 ± 2.9 vs. 50.3 ± 4.3 in controls; p = 0.004) consistent with the reduction in average disease score over the same time (1.8 ± 0.1 in TEMPOL-fed vs. 2.5 ± 0.2 in controls, p = 0.004). Average disease scores differed significantly between control and TEMPOL-fed animals from day 19 until the study end (p < 0.05).
Figure 5. TEMPOL administration limits clinical disease in both active and passive models of EAE.
Actively-induced EAE C57BL/6J mice initiated TEMPOL-chow at (A) 7 days post immunization. The average disease severity and peak disease severity were both significantly reduced in TEMPOL-fed animals. N = 6 animals per group, points represent the average disease severity with error bars ±SEM; 1 of 3 representative experiments. (B) TEMPOL administration after the onset of clinical symptoms (limp tail or greater in >60% of mice (day 14 post immunization) reduced disease severity. n = 17 mice per group, 1 of 3 representative experiments is shown. Values shown correspond to the mean ± SEM. (C) Two weeks of TEMPOL-chow prior to the transfer of encephalitogenic T cells reduced incidence and severity of disease. TEMPOL administration (○) or control chow (●). Data points represent the average clinical disease score ±SEM with n = 10 per group. 1 of 3 representative experiments is shown. (D) Passively induced EAE in SJL animals was treated with control or TEMPOL chow at the onset of symptoms (day 10). Bars represent the average of n = 13 and 12 for control and TEMPOL groups respectively, ±SEM. Average disease severity was significantly different over the time from disease onset to study end. *p = 0.026 Mann-Whitney rank sum test. Infiltrates in animals from (C) were reduced in TEMPOL versus control-fed animals (E,F). SJL/J animals induced for passive EAE were examined at peak disease (day 18) for assessment of inflammatory infiltrates. Animals on control chow were compared to animals on TEMPOL chow for two weeks prior to T cell transfer. Spinals cords from four animals per group were examined at nine similar levels spanning the entire cord and scored as outlined in Material & Methods. The scores from each animal were pooled and the two groups compared by unpaired t-test. Bars represent the average of n = 36 levels per group ± SEM. *p = 0.03. Furthermore, regions of myelin pallor (as shown by reduced luxol fast blue staining) were typically not observed in TEMPOL-fed animals. (G) TEMPOL administration reduces the degree of neuronal loss or damage. Spinal cord neurofilament levels were measured in spinal cord homogenates from control EAE or TEMPOL-fed EAE mice in (C) by ELISA for neurofilament H. Results represent the mean relative healthy phosphorylated or heavy neurofilament levels (n = 9 per group) ±SEM, *p = 0.03 when TEMPOL (filled bars) were compared with control (open bars) EAE animals.
To distinguish the protective effects of TEMPOL from effects on the generation of autoreactive T cells, passive disease was examined following the transfer of primed encephalitogenic T cells (Fig. 5C) into animals that received TEMPOL feed for two weeks and through the duration of the study. While the day of onset was similar, the peak severity of disease was significantly reduced in TEMPOL-fed mice (3.85 vs. 2.81 respectively, p = 0.011). Protection lasted 45 days, whereby TEMPOL reduced the average cumulative disease score by more than half (78.8 vs. 33.9 in control-fed mice, p = 0.00046). To further examine the potential for TEMPOL to treat established disease, we also performed a study where animals were transferred from control to TEMPOL feed after the onset of clinical disease (Fig. 5D). TEMPOL did not significantly reduce the cumulative disease scores over the time of this shorter study (28.38 ± 6.07 vs. 42.65 ± 6.04, p = 0.068 with TEMPOL vs. control chow respectively), but similar to the treatment of established active disease in Fig.5B average disease scores were significantly lower in TEMPOL-fed animals than controls (1.38 ± 0.27 vs. 2.31 ± 0.28, p = 0.026) over the treatment period.
Infiltrates were assessed at or near peak disease in an experiment similar to that in Fig. 5C. TEMPOL-fed animals showed fewer infiltrates extending beyond the meninges or involving the parenchyma (Fig. 5E). Typical infiltrates in controls extended well beyond the meninges (3.25 ± 0.15), were numerous throughout the cord, and were typically associated with areas of reduced Luxol fast blue-stained myelin (see arrows) (Fig. 5F). In contrast, TEMPOL-fed animals had fewer infiltrates overall (1.76 ± 0.21, p < 0.0001), rarely extending beyond the meningeal interface (see arrows) and with relative preservation of myelin staining. Neurofilaments are major proteins of neurons and are particularly concentrated in axons. To assess whether there was a relative loss of axons, the relative amount of healthy or normal neurofilament H (phosphorylated/heavy neurofilament of axons) was measured (Fig. 5G). TEMPOL-fed animals showed significantly higher levels of neurofilament H in their spinal cords, suggesting that this axon-associated protein was preserved in these mice compared to control fed EAE animals (p = 0.03).
Discussion
Our study is the first to show that oral administration of the antioxidant TEMPOL, alone, both prophylactically and therapeutically limit CNS autoimmune disease and damage in the most established models of MS. Animals on TEMPOL were spared from severe clinical disease, and showed little evidence of microglial activation, one of the earliest indicators of insult to the CNS that often precedes infiltration and clinical disease in EAE (Kreutzberg, 1996; Ponomarev et al., 2005). Our studies demonstrate potent immunomodulatory properties of TEMPOL that have not been described previously. At doses associated with efficacy in a complex disease model, we describe immune deviation from an inflammatory phenotype evidenced by cytokine shifts, antibody profile changes, and reductions in antigen-presenting molecules. Additionally, TEMPOL administration is associated with changes in T cell subsets, most notably an increase in CD4+ regulatory T cell populations.
Our studies clearly show that TEMPOL does not limit adaptive immune responses, but instead alters the nature of that response. Immune-mediated diseases can often be treated with immunosuppressive therapies, which function by globally limiting immune responses (Bach, 1993). While effective, immunosuppression leaves a patient vulnerable to potentially life-threatening infections. Mitoxantrone (Novantrone), for example, suppresses proliferation of T cells, B cells, and macrophages (Hartung et al., 2002). Teriflunomide (Aubagio) is a newer oral MS therapy that also inhibits T cell proliferation and activation (Warnke et al., 2009). Both ameliorate EAE, (Edan et al., 2004) yet while teriflunomide is relatively safe compared to mitoxantrone in terms of toxicity, it still carries a risk of opportunistic infections (Warnke et al., 2009). TEMPOL does not limit antigen-specific responses, and may indeed enhance CD8+ T cell-mediated responses. Notably, TEMPOL enhanced T cell proliferation in vitro at doses matching those measured in animals benefitting from oral TEMPOL (data not shown) and is similar to uric acid, which was also found to be beneficial in EAE (Kean et al., 2000).
Fundamentally, the immune deviation observed following TEMPOL administration is the central driver of its immunomodulatory and protective properties. TEMPOL was associated with lesser IFNγ, TNFα, and TGF-β1 production in EAE animals. IFNγ and TNFα promote an inflammatory TH1-type immune response that leads to ROS and RNS production, cytotoxicity, and tissue injury (Kopf et al., 2010). Both IFNγ and TNFα have been implicated in disease progression in MS, as well as in other immune-mediated diseases(Benveniste and Benos, 1995). In the CNS, TNFα is observed within active lesions and in the CSF of people with MS, and is associated with BBB disruption (Benveniste and Benos, 1995). In vitro, TNFα has been shown to mediate damage to myelin and oligodendrocytes (Selmaj and Raine, 1988). IFNγ induces expression of MHC II, and has been shown to exacerbate MS when administered to patients (Benveniste and Benos, 1995).
Collectively, decreased production of these cytokines indicates a prototypical shift from a pro-inflammatory TH1 response towards a more immunosuppressive or TH2 type response. Driving such a shift has long been proposed to have beneficial effects in MS, and is the functional mechanism of the immunomodulatory therapy glatiramer acetate (Oreja-Guevara et al., 2012). Our cytokine data are supported by the observed differences in MOG-specific serum IgG antibodies: TEMPOL-fed EAE mice had increased levels of the TH2-associated IgG1 and decreased levels of the TH1-associated IgG2c isotype (Stevens et al., 1988).
TEMPOL’s ability to alter the cytokine response and the proinflammatory setting would also explain reductions in the inflammatory potential of innate immune cells that orchestrate inflammatory responses both within and outside the CNS. Limiting microglial activation within the CNS, as we have shown with lower MHC II expression on microglia, indicates that TEMPOL limits activation of the “first line” of CNS respondents. It is also known that altering expression of the surface markers involved in T cell activation can impact the subsequent proliferation and differentiation of activated T cells (Chitnis and Khoury, 2003). Both myeloid and myeloid DC populations from TEMPOL-fed EAE mice exhibited decreased MHC II expression. Decreased MHC II expression by APCs would lead to decreased activation of CD4+ T cells, and interestingly is considered a key mechanism of action for interferon beta-1b drugs (Betaseron, Extavia) that are used in MS, where it limits IFNγ-induced expression of MHC II on endothelial cells in the brain (Huynh et al., 1995).
Coincident with a less inflammatory environment in both cell phenotype and cellular mediators of inflammation, TEMPOL administration is clearly associated with a diminished capacity to generate CD4+ T helper cells that typically modulate CNS autoimmunity in EAE and importantly, instead drives increases in regulatory populations that limit disease. Treg cells are known to be immunosuppressive, limiting TH1 cell development and production of pro-inflammatory factors by secreting IL-10 and TGF-β1 (Marie et al., 2005). A recent study demonstrated that a novel saline therapy upregulated CD4+CD25+Foxp3+ Treg cells in ex vivo experiments by way of nitric oxide suppression, resulting in suppression of TH1 and TH17 cells and a shift towards a TH2 response (Mondal et al., 2012) that seemed to underlie its ability to protect against adoptively transferred EAE. Overall, our observed relative increase in Treg cells provides further support that TEMPOL influences a shift away from harmful autoimmunity in EAE.
Within the T cell compartment, a significant increase in CD8+ T cell proportions was observed in lymphoid organs from TEMPOL-fed animals. This foreseeably results from the observed decrease in MHC II expression by APCs in those same TEMPOL-fed animals, where lesser MHC II would limit the potential for CD4+ T cell responses. CD8+ cytotoxic T cells are “killer” cells that destroy other cells that are infected with intracellular pathogens and protect against spontaneous malignant tumours, as they are able to detect abnormal cells (Andersen et al., 2006). In general increases in CD8+ T cells could provide a “boost” to the immune system in the form of enhanced protection against pathogens. An increase in CD8+ T cells may even explain other benefits associated with TEMPOL, as CD8+ T cells are primary players in anti-tumor responses and mice on long term TEMPOL are less likely to develop tumours, and therein live longer than control-fed mice (Mitchell et al., 2003).
Importantly, TEMPOL’s effects are not limited to an influence on the priming of pathogenic autoreactive T cells. Our study of disease in SJL animals, where encephalitogenic cells are primed outside of the animal, demonstrates TEMPOL’s ability to afford protection against activated autoreactive populations known to traffic to, and thereby illicit damage and disease within the CNS. T cells can mediate neuronal/axonal damage directly, or facilitate pathology through recruitment of macrophages and their complement of oxidative stressors and other soluble mediators. In this passive model, it has been well-characterized that changes in the BBB and alterations within the CNS attributed to autoreactive T cells begin to occur as early as 5–7 days post T cell transfer, well before the onset of clinical symptoms (Chakrabarty et al., 2003; Smorodchenko et al., 2007; Wuerfel et al., 2007). Preliminary studies in our lab show that initiating oral TEMPOL as early as 7 days post disease induction, differences are observed as soon as 4 days later by CNS microarray analysis of control vs. TEMPOL-fed animals, suggesting TEMPOL is able to alter the earliest events in CNS-specific autoimmunity (data not shown).
Indeed, other groups have shown TEMPOL to be well-tolerated and orally efficacious in animals, with potential health benefits beyond the treatment of disease (Mitchell et al., 2003). While our study documents immunomodulatory properties of TEMPOL in the periphery and suggests they occur within the CNS, additional mechanisms may also serve to limit CNS damage. In our study, TEMPOL was detected in tissues after 24 days on TEMPOL chow, but was not examined earlier. i.p. administration of TEMPOL (24 mg/kg) in a demyelinating mouse hepatitis virus model resulted in TEMPOL measurements around 15 µm by 10 minutes after administration (Tsuhako et al., 2010). Lowering of TEMPOL to 3 μM in EAE mice was deemed secondary to TEMPOL’s reacting with radicals resulting from viral infection. In the same study, TEMPOL administered daily i.p. as well as in the drinking water from the time of viral inoculation showed a reduction in subsequent demyelinating disease severity in this model of encephalomyelitis (Tsuhako et al., 2010). The authors observed reduced mortality associated with lower viral load, reduced infiltration, and less BBB breakdown, but this efficacy was only demonstrated prophylactically. This reduction may be attributed to alterations in the anti-viral response or protection at the level of the CNS; the study design did not allow for distinction. In this model of disease, with rapid significant morbidity and mortality secondary to viral attack, it is difficult to discern which steps in viral pathogenesis are of direct relevance to MS.
Administration of TEMPOL orally is not only simple but appears to evade several of the deleterious effects observed following i.v. or bolus injections of TEMPOL. In studies requiring a high dose i.v. administration to achieve therapeutic efficacy, a significant lowering of blood pressure is noticed. One study has described the ability of a similar nitroxide radical, Tempamine, to modulate autoantigen-driven EAE. Importantly, TEMPOL was only effective prophylactically and only when encapsulated in a liposomal structure delivered i.v. (Kizelsztein et al., 2009). Free Tempamine had no effect, thought to be quickly eliminated from plasma. After optimization, they showed only a modest ability to limit the development of mild disease in a therapeutic manner, and ultimately found methylprednisolone to be far superior to the Tempamine formulation (Turjeman et al., 2015). Notably, Tempamine reduced the proliferation of LN cells isolated from EAE mice and assayed in vitro, and is perhaps an important difference in mechanisms of action between the two formulations.
The separation of anti-inflammatory and neuroprotective effects of TEMPOL in EAE models is simplified, if one considers neurodegeneration to occur largely only secondary to inflammation. Our studies clearly show that limiting inflammation limits disability in this model. Notably, the efficacy of TEMPOL in models of “classic” or less inflammation-associated neurodegenerative disease is already well described (Ghosh et al., 2008; Lipman et al., 2006). With the ability to permeate cell membranes and scavenge superoxide and peroxynitrite, TEMPOL could indeed limit inflammatory demyelinating disease by scavenging both inflammatory by-products in the tissue and free radicals within cells experiencing mitochondrial dysfunction. Evidence for a pathogenic role for ROS in MS and EAE stems from the beneficial effects of antioxidants on disease models (Carlson and Rose, 2006). ROS play important physiological roles in cellular regulatory processes (Fialkow et al., 2007), but oxygen metabolites such as superoxide (O2−), hydroxyl radical (OH•), and hydrogen peroxide (H2O2) can react with any oxidizable compound, damaging proteins, carbohydrates, nucleic acids (causing mutations), and lipids (causing cell wall damage). CNS cells, including microglia and neurons, generate peroxynitrite (ONOO−), which is increasingly viewed as the principal mediator of oxidative stress and excitotoxicity driving the neurodegenerative processes apparent in MS (Gonsette, 2008b; Torreilles et al., 1999).
In cellular analyses, TEMPOL has well-characterized SOD mimetic and O2− scavenging activity (Krishna et al., 1992; Mitchell et al., 1990) with an ability to catalytically convert the O2− radical to H2O2 and O2. Moreover, TEMPOL protects cells against damage from exposure to hypoxanthine/xanthine oxidase or H2O2 in vitro with greater efficacy than exogenously supplemented catalase or SOD itself (Samuni et al., 1991a; Samuni et al., 1991b). As well it can rapidly decompose ONOO−-derived free radicals nitrogen dioxide and carbonate to which ONOO−-mediated tissue damage in MS are attributed (Carroll et al., 2000). Given the evidence for increased levels of ROS and RNS in MS either prior to or secondary to inflammatory processes, or secondary to mitochondrial dysfunction occurring independent of inflammation (Andrews et al., 2005), oral TEMPOL becomes a strong therapeutic candidate in MS as it achieves fully effective concentrations in tissues where it could exert antioxidant and protective actions (Wilcox, 2010). Such protective effects at the level of the target tissue may indeed contribute to the ability of TEMPOL to treat established EAE in both active and passive models of EAE.
The most desirable therapies for MS would stop disease development at the earliest stages, limiting inflammation and the related axonal loss and neurodegeneration that are associated with disability, but also have the potential to limit disease processes already underway. In these regards, the oral administration of TEMPOL provides a favourable alternative to current therapies, and thus represents an important therapeutic agent for further evaluation in the treatment of MS.
Table 5. Markers associated with co-stimulation are altered in lymph node and spleens of TEMPOL-fed EAE mice.
The median fluorescence intensity (MFI) was calculated for each surface marker, and values from 2 experiments (13 mice per feed group) were pooled for statistical analysis and reported ±SD
| Control Average MFI |
TEMPOL Average MFI |
P value | |
|---|---|---|---|
| Lymph Node | |||
| CD11b+CD11c− | |||
| MHC II | 1113.6 ±289.5 | 821.8 ±298.3 | 0.021* |
| CD80 | 211.1 ±24.0 | 295.5 ±126.3 | <0.001* |
| CD86 | 130.5 ±38.7 | 176.1 ±60.3 | 0.033* |
| CD11b+CD11c+ | |||
| MHC II | 5817.0 ±1334.9 | 4523.0 ±748.0 | 0.021* |
| CD80 | 441.2 ±43.6 | 544.7 ±212.9 | 0.024* |
| Spleen | |||
| CD11b+CD11c− | |||
| MHC II | 122.4 ±60.4 | 87.9 ±8.5 | <0.001* |
| CD40 | 119.6 ±14.4 | 103.8 ±7.8 | 0.008* |
| CD86 | 69.5 ±19.6 | 88.4 ±28.3 | 0.040* |
| CD11b+CD11c+ | |||
| MHC II | 4660.6 ±1552.4 | 3188.6 ±1471.9 | 0.005* |
| CD40 | 455.1 ±80.0 | 352.7 ±61.4 | 0.002* |
Mann-Whitney Rank Sum test with uncorrected p values are shown for each marker where *p < 0.05 in comparing control to TEMPOL fed animals. Bolded values are those withstanding Bonferroni correction for four costimulatory markers analysed p < 0.0125.
Highlights.
The nitroxide radical TEMPOL given orally limits clinical disease in models of MS
TEMPOL reduced inflammatory infiltrates and preserved neurofilaments
TEMPOL lowered costimulatory molecule expression and enriched suppressor cells
Immunomodulation and therapeutic efficacy combine as a promising oral MS therapy
Acknowledgments
This study was supported by a biomedical operating grant to JQ from the Multiple Sclerosis Society of Canada (MSSOC) with partial support for early preclinical studies from the Intramural program of the National Institute for Neurological Disorders and Stroke, National Institutes of Health to JQ while in the Neuroimmunology Branch. JQ is the recipient of an MSSOC Donald Paty Career Development Award and SN was the recipient of an MSSOC Masters Studentship Award.
We are grateful to the NINDS and UBC veterinary and technical staff for exceptional support pertaining to animal care. Dr. Axel Petzold provided guidance in the application of the neurofilament ELISA. Daphne de Launay and Lixin Zhou provided technical assistance with extraction of tissues. Fuminori Hyodo assisted with determination of TEMPOL levels in serum and tissues from study mice.
Abbreviations
- CFA
complete Freunds adjuvant
- EAE
experimental autoimmune encephalomyelitis
- MOG
Myelin Oligodendroglial Protein
- TEMPOL
4-hydroxy-2,2,6,6-tetramethylpiperidine-N-oxyl
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Author Contributions
SN contributed to the isolation of immune cells from study animals and performed the in vitro assays, cytokine ELISAs, and flow cytometry assessments along with subsequent analyses to address the immunomodulatory properties of TEMPOL and contributed to the first draft of the manuscript. JH assisted with EAE induction, serum isolations and monitoring of animals including the isolation and processing of tissues for immunohistochemistry and preliminary flow cytometric analyses. VB performed the MOG-specific ELISA. XL performed the neurofilament ELISA. JBM, HFM and MK contributed to the study design and interpretation related to the application, delivery and measurement f TEMPOL. JQ planned all experiments and procedures to assess the immunomodulatory and protective effects of TEMPOL in EAE; JQ performed all of the EAE inductions, animal scoring, designed the experimental approaches, oversaw the data collection and analyses, and interpretation of results in addition to preparing the complete manuscript as a whole and critically reviewing data and interpretations.
Potential Conflicts of Interest: The authors declare that they have no conflict of interest to report.
References
- Andersen MH, Schrama D, Thor Straten P, Becker JC. Cytotoxic T cells. J Invest Dermatol. 2006;126:32–41. doi: 10.1038/sj.jid.5700001. [DOI] [PubMed] [Google Scholar]
- Anderson SA, Shukaliak-Quandt J, Jordan EK, Arbab AS, Martin R, McFarland H, Frank JA. Magnetic resonance imaging of labeled T-cells in a mouse model of multiple sclerosis. Ann Neurol. 2004;55:654–659. doi: 10.1002/ana.20066. [DOI] [PubMed] [Google Scholar]
- Andrews HE, Nichols PP, Bates D, Turnbull DM. Mitochondrial dysfunction plays a key role in progressive axonal loss in Multiple Sclerosis. Med Hypotheses. 2005;64:669–677. doi: 10.1016/j.mehy.2004.09.001. [DOI] [PubMed] [Google Scholar]
- Bach JF. Immunosuppressive therapy of autoimmune diseases. Trends Pharmacol Sci. 1993;14:213–216. doi: 10.1016/0165-6147(93)90211-2. [DOI] [PubMed] [Google Scholar]
- Barnes MJ, Griseri T, Johnson AM, Young W, Powrie F, Izcue A. CTLA-4 promotes Foxp3 induction and regulatory T cell accumulation in the intestinal lamina propria. Mucosal Immunol. 2013;6:324–334. doi: 10.1038/mi.2012.75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benveniste EN, Benos DJ. TNF-alpha- and IFN-gamma-mediated signal transduction pathways: effects on glial cell gene expression and function. FASEB J. 1995;9:1577–1584. doi: 10.1096/fasebj.9.15.8529837. [DOI] [PubMed] [Google Scholar]
- Bjartmar C, Trapp BD. Axonal degeneration and progressive neurologic disability in multiple sclerosis. Neurotox Res. 2003;5:157–164. doi: 10.1007/BF03033380. [DOI] [PubMed] [Google Scholar]
- Bonini MG, Mason RP, Augusto O. The Mechanism by which 4-hydroxy-2,2,6,6-tetramethylpiperidene-1-oxyl (tempol) diverts peroxynitrite decomposition from nitrating to nitrosating species. Chem Res Toxicol. 2002;15:506–511. doi: 10.1021/tx015571z. [DOI] [PubMed] [Google Scholar]
- Carlson NG, Rose JW. Antioxidants in multiple sclerosis: do they have a role in therapy? CNS Drugs. 2006;20:433–441. doi: 10.2165/00023210-200620060-00001. [DOI] [PubMed] [Google Scholar]
- Carroll RT, Galatsis P, Borosky S, Kopec KK, Kumar V, Althaus JS, Hall ED. 4-Hydroxy-2,2,6,6-tetramethylpiperidine-1-oxyl (Tempol) inhibits peroxynitrite-mediated phenol nitration. Chemical research in toxicology. 2000;13:294–300. doi: 10.1021/tx990159t. [DOI] [PubMed] [Google Scholar]
- Chakrabarty A, Emerson MR, LeVine SM. Heme oxygenase-1 in SJL mice with experimental allergic encephalomyelitis. Mult Scler. 2003;9:372–381. doi: 10.1191/1352458503ms928oa. [DOI] [PubMed] [Google Scholar]
- Chitnis T, Khoury SJ. Role of costimulatory pathways in the pathogenesis of multiple sclerosis and experimental autoimmune encephalomyelitis. J Allergy Clin Immunol. 2003;112:837–849. doi: 10.1016/j.jaci.2003.08.025. quiz 850. [DOI] [PubMed] [Google Scholar]
- Deng-Bryant Y, Singh IN, Carrico KM, Hall ED. Neuroprotective effects of tempol, a catalytic scavenger of peroxynitrite-derived free radicals, in a mouse traumatic brain injury model. J Cereb Blood Flow Metab. 2008;28:1114–1126. doi: 10.1038/jcbfm.2008.10. [DOI] [PubMed] [Google Scholar]
- Dittel BN. Evidence that Fas and FasL contribute to the pathogenesis of experimental autoimmune encephalomyelitis. Arch Immunol Ther Exp (Warsz) 2000;48:381–388. [PubMed] [Google Scholar]
- Edan G, Morrissey S, Le Page E. Rationale for the use of mitoxantrone in multiple sclerosis. J Neurol Sci. 2004;223:35–39. doi: 10.1016/j.jns.2004.04.017. [DOI] [PubMed] [Google Scholar]
- Ferguson B, Matyszak MK, Esiri MM, Perry VH. Axonal damage in acute multiple sclerosis lesions. Brain. 1997;120(Pt 3):393–399. doi: 10.1093/brain/120.3.393. [DOI] [PubMed] [Google Scholar]
- Fialkow L, Wang Y, Downey GP. Reactive oxygen and nitrogen species as signaling molecules regulating neutrophil function. Free Radic Biol Med. 2007;42:153–164. doi: 10.1016/j.freeradbiomed.2006.09.030. [DOI] [PubMed] [Google Scholar]
- Frohman EM, Racke MK, Raine CS. Multiple sclerosis--the plaque and its pathogenesis. N Engl J Med. 2006;354:942–955. doi: 10.1056/NEJMra052130. [DOI] [PubMed] [Google Scholar]
- Ghosh MC, Tong WH, Zhang D, Ollivierre-Wilson H, Singh A, Krishna MC, Mitchell JB, Rouault TA. Tempol-mediated activation of latent iron regulatory protein activity prevents symptoms of neurodegenerative disease in IRP2 knockout mice. Proc Natl Acad Sci U S A. 2008;105:12028–12033. doi: 10.1073/pnas.0805361105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilgun-Sherki Y, Melamed E, Offen D. The role of oxidative stress in the pathogenesis of multiple sclerosis: the need for effective antioxidant therapy. J Neurol. 2004;251:261–268. doi: 10.1007/s00415-004-0348-9. [DOI] [PubMed] [Google Scholar]
- Gonsette RE. Neurodegeneration in multiple sclerosis: the role of oxidative stress and excitotoxicity. J Neurol Sci. 2008a;274:48–53. doi: 10.1016/j.jns.2008.06.029. [DOI] [PubMed] [Google Scholar]
- Gonsette RE. Oxidative stress and excitotoxicity: a therapeutic issue in multiple sclerosis? Mult Scler. 2008b;14:22–34. doi: 10.1177/1352458507080111. [DOI] [PubMed] [Google Scholar]
- Hartung HP, Gonsette R, König N, Kwiecinski H, Guseo A, Morrissey SP, Krapf H, Zwingers T (MIMS), M.i.M.S.S.G. Mitoxantrone in progressive multiple sclerosis: a placebo-controlled, double-blind, randomised, multicentre trial. Lancet. 2002;360:2018–2025. doi: 10.1016/S0140-6736(02)12023-X. [DOI] [PubMed] [Google Scholar]
- Hauser SL, Waubant E, Arnold DL, Vollmer T, Antel J, Fox RJ, Bar-Or A, Panzara M, Sarkar N, Agarwal S, Langer-Gould A, Smith CH. B-cell depletion with rituximab in relapsing-remitting multiple sclerosis. N Engl J Med. 2008;358:676–688. doi: 10.1056/NEJMoa0706383. [DOI] [PubMed] [Google Scholar]
- Hobom M, Storch MK, Weissert R, Maier K, Radhakrishnan A, Kramer B, Bahr M, Diem R. Mechanisms and time course of neuronal degeneration in experimental autoimmune encephalomyelitis. Brain Pathol. 2004;14:148–157. doi: 10.1111/j.1750-3639.2004.tb00047.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huynh HK, Oger J, Dorovini-Zis K. Interferon-beta downregulates interferon-gamma-induced class II MHC molecule expression and morphological changes in primary cultures of human brain microvessel endothelial cells. J Neuroimmunol. 1995;60:63–73. doi: 10.1016/0165-5728(95)00054-6. [DOI] [PubMed] [Google Scholar]
- Hyodo F, Matsumoto K, Matsumoto A, Mitchell JB, Krishna MC. Probing the intracellular redox status of tumors with magnetic resonance imaging and redox-sensitive contrast agents. Cancer Res. 2006;66:9921–9928. doi: 10.1158/0008-5472.CAN-06-0879. [DOI] [PubMed] [Google Scholar]
- Johnson TA, Jirik FR, Fournier S. Exploring the roles of CD8(+) T lymphocytes in the pathogenesis of autoimmune demyelination. Semin Immunopathol. 2010;32:197–209. doi: 10.1007/s00281-010-0199-7. [DOI] [PubMed] [Google Scholar]
- Kato N, Yanaka K, Hyodo K, Homma K, Nagase S, Nose T. Stable nitroxide Tempol ameliorates brain injury by inhibiting lipid peroxidation in a rat model of transient focal cerebral ischemia. Brain Res. 2003;979:188–193. doi: 10.1016/s0006-8993(03)02918-4. [DOI] [PubMed] [Google Scholar]
- Kean RB, Spitsin SV, Mikheeva T, Scott GS, Hooper DC. The peroxynitrite scavenger uric acid prevents inflammatory cell invasion into the central nervous system in experimental allergic encephalomyelitis through maintenance of blood-central nervous system barrier integrity. J Immunol. 2000;165:6511–6518. doi: 10.4049/jimmunol.165.11.6511. [DOI] [PubMed] [Google Scholar]
- Kieseier BC, Wiendl H, Hartung HP, Leussink VI, Stuve O. Risks and benefits of multiple sclerosis therapies: need for continual assessment? Curr Opin Neurol. 2011;24:238–243. doi: 10.1097/WCO.0b013e32834696dd. [DOI] [PubMed] [Google Scholar]
- Kizelsztein P, Ovadia H, Garbuzenko O, Sigal A, Barenholz Y. Pegylated nanoliposomes remote-loaded with the antioxidant tempamine ameliorate experimental autoimmune encephalomyelitis. J Neuroimmunol. 2009;213:20–25. doi: 10.1016/j.jneuroim.2009.05.019. [DOI] [PubMed] [Google Scholar]
- Kopf M, Bachmann MF, Marsland BJ. Averting inflammation by targeting the cytokine environment. Nat Rev Drug Discov. 2010;9:703–718. doi: 10.1038/nrd2805. [DOI] [PubMed] [Google Scholar]
- Kreutzberg GW. Microglia: a sensor for pathological events in the CNS. Trends Neurosci. 1996;19:312–318. doi: 10.1016/0166-2236(96)10049-7. [DOI] [PubMed] [Google Scholar]
- Krishna MC, Grahame DA, Samuni A, Mitchell JB, Russo A. Oxoammonium cation intermediate in the nitroxide-catalyzed dismutation of superoxide. Proc Natl Acad Sci U S A. 1992;89:5537–5541. doi: 10.1073/pnas.89.12.5537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lipman T, Tabakman R, Lazarovici P. Neuroprotective effects of the stable nitroxide compound Tempol on 1-methyl-4-phenylpyridinium ion-induced neurotoxicity in the Nerve Growth Factor-differentiated model of pheochromocytoma PC12 cells. Eur J Pharmacol. 2006;549:50–57. doi: 10.1016/j.ejphar.2006.08.022. [DOI] [PubMed] [Google Scholar]
- MacPhee IA, Turner DR, Yagita H, Oliveira DB. CD80(B7.1) and CD86(B7.2) do not have distinct roles in setting the Th1/Th2 balance in autoimmunity in rats. Scand J Immunol. 2001;54:486–494. doi: 10.1046/j.1365-3083.2001.00998.x. [DOI] [PubMed] [Google Scholar]
- Mahad DH, Trapp BD, Lassmann H. Pathological mechanisms in progressive multiple sclerosis. The Lancet Neurology. 2015;14:183–193. doi: 10.1016/S1474-4422(14)70256-X. [DOI] [PubMed] [Google Scholar]
- Mahad DJ, Ziabreva I, Campbell G, Lax N, White K, Hanson PS, Lassmann H, Turnbull DM. Mitochondrial changes within axons in multiple sclerosis. Brain. 2009;132:1161–1174. doi: 10.1093/brain/awp046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marie JC, Letterio JJ, Gavin M, Rudensky AY. TGF-beta1 maintains suppressor function and Foxp3 expression in CD4+CD25+ regulatory T cells. J Exp Med. 2005;201:1061–1067. doi: 10.1084/jem.20042276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell JB, Anver MR, Sowers AL, Rosenberg PS, Figueroa M, Thetford A, Krishna MC, Albert PS, Cook JA. The antioxidant tempol reduces carcinogenesis and enhances survival in mice when administered after nonlethal total body radiation. Cancer Res. 2012;72:4846–4855. doi: 10.1158/0008-5472.CAN-12-1879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell JB, Samuni A, Krishna MC, DeGraff WG, Ahn MS, Samuni U, Russo A. Biologically active metal-independent superoxide dismutase mimics. Biochemistry. 1990;29:2802–2807. doi: 10.1021/bi00463a024. [DOI] [PubMed] [Google Scholar]
- Mitchell JB, Xavier S, DeLuca AM, Sowers AL, Cook JA, Krishna MC, Hahn SM, Russo A. A low molecular weight antioxidant decreases weight and lowers tumor incidence. Free Radic Biol Med. 2003;34:93–102. doi: 10.1016/s0891-5849(02)01193-0. [DOI] [PubMed] [Google Scholar]
- Mondal S, Martinson JA, Ghosh S, Watson R, Pahan K. Protection of Tregs, suppression of Th1 and Th17 cells, and amelioration of experimental allergic encephalomyelitis by a physically-modified saline. PLoS One. 2012;7:e51869. doi: 10.1371/journal.pone.0051869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oreja-Guevara C, Ramos-Cejudo J, Aroeira LS, Chamorro B, Diez-Tejedor E. TH1/TH2 Cytokine profile in relapsing-remitting multiple sclerosis patients treated with Glatiramer acetate or Natalizumab. BMC Neurol. 2012;12:95. doi: 10.1186/1471-2377-12-95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petzold A, Shaw G. Comparison of two ELISA methods for measuring levels of the phosphorylated neurofilament heavy chain. J Immunol Methods. 2007;319:34–40. doi: 10.1016/j.jim.2006.09.021. [DOI] [PubMed] [Google Scholar]
- Ponomarev ED, Shriver LP, Maresz K, Dittel BN. Microglial cell activation and proliferation precedes the onset of CNS autoimmunity. J Neurosci Res. 2005;81:374–389. doi: 10.1002/jnr.20488. [DOI] [PubMed] [Google Scholar]
- Qi X, Lewin AS, Sun L, Hauswirth WW, Guy J. Mitochondrial protein nitration primes neurodegeneration in experimental autoimmune encephalomyelitis. J Biol Chem. 2006;281:31950–31962. doi: 10.1074/jbc.M603717200. [DOI] [PubMed] [Google Scholar]
- Quandt JA, Huh J, Baig M, Yao K, Ito N, Bryant M, Kawamura K, Pinilla C, McFarland HF, Martin R, Ito K. Myelin Basic Protein-Specific TCR/HLA-DRB5*01:01 Transgenic Mice Support the Etiologic Role of DRB5*01:01 in Multiple Sclerosis. J Immunol. 2012;189:2897–2908. doi: 10.4049/jimmunol.1103087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Racke MK. Immunopathogenesis of multiple sclerosis. Ann Indian Acad Neurol. 2009;12:215–220. doi: 10.4103/0972-2327.58274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reindl M, Linington C, Brehm U, Egg R, Dilitz E, Deisenhammer F, Poewe W, Berger T. Antibodies against the myelin oligodendrocyte glycoprotein and the myelin basic protein in multiple sclerosis and other neurological diseases: a comparative study. Brain. 1999;122(Pt 11):2047–2056. doi: 10.1093/brain/122.11.2047. [DOI] [PubMed] [Google Scholar]
- Samuni A, Mitchell JB, DeGraff W, Krishna CM, Samuni U, Russo A. Nitroxide SOD-mimics: modes of action. Free Radic Res Commun. 1991a;12–13(Pt 1):187–194. doi: 10.3109/10715769109145785. [DOI] [PubMed] [Google Scholar]
- Samuni A, Winkelsberg D, Pinson A, Hahn SM, Mitchell JB, Russo A. Nitroxide stable radicals protect beating cardiomyocytes against oxidative damage. J Clin Invest. 1991b;87:1526–1530. doi: 10.1172/JCI115163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schnackenberg CG, Wilcox CS. The SOD mimetic tempol restores vasodilation in afferent arterioles of experimental diabetes. Kidney Int. 2001;59:1859–1864. doi: 10.1046/j.1523-1755.2001.0590051859.x. [DOI] [PubMed] [Google Scholar]
- Schreibelt G, Musters RJ, Reijerkerk A, de Groot LR, van der Pol SM, Hendrikx EM, Dopp ED, Dijkstra CD, Drukarch B, de Vries HE. Lipoic acid affects cellular migration into the central nervous system and stabilizes blood-brain barrier integrity. J Immunol. 2006;177:2630–2637. doi: 10.4049/jimmunol.177.4.2630. [DOI] [PubMed] [Google Scholar]
- Selmaj KW, Raine CS. Tumor necrosis factor mediates myelin and oligodendrocyte damage in vitro. Ann Neurol. 1988;23:339–346. doi: 10.1002/ana.410230405. [DOI] [PubMed] [Google Scholar]
- Sledzinski Z, Wozniak M, Antosiewicz J, Lezoche E, Familiari M, Bertoli E, Greci L, Brunelli A, Mazera N, Wajda Z. Protective effect of 4-hydroxy-TEMPO, a low molecular weight superoxide dismutase mimic, on free radical toxicity in experimental pancreatitis. Int J Pancreatol. 1995;18:153–160. doi: 10.1007/BF02785889. [DOI] [PubMed] [Google Scholar]
- Smith KJ, Kapoor R, Felts PA. Demyelination: the role of reactive oxygen and nitrogen species. Brain Pathol. 1999;9:69–92. doi: 10.1111/j.1750-3639.1999.tb00212.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith KJ, Kapoor R, Hall SM, Davies M. Electrically active axons degenerate when exposed to nitric oxide. Ann Neurol. 2001;49:470–476. [PubMed] [Google Scholar]
- Smorodchenko A, Wuerfel J, Pohl EE, Vogt J, Tysiak E, Glumm R, Hendrix S, Nitsch R, Zipp F, Infante-Duarte C. CNS-irrelevant T-cells enter the brain, cause blood-brain barrier disruption but no glial pathology. Eur J Neurosci. 2007;26:1387–1398. doi: 10.1111/j.1460-9568.2007.05792.x. [DOI] [PubMed] [Google Scholar]
- Sospedra M, Martin R. Immunology of multiple sclerosis. Annu Rev Immunol. 2005;23:683–747. doi: 10.1146/annurev.immunol.23.021704.115707. [DOI] [PubMed] [Google Scholar]
- Soule BP, Hyodo F, Matsumoto K, Simone NL, Cook JA, Krishna MC, Mitchell JB. The chemistry and biology of nitroxide compounds. Free Radic Biol Med. 2007a;42:1632–1650. doi: 10.1016/j.freeradbiomed.2007.02.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soule BP, Hyodo F, Matsumoto K, Simone NL, Cook JA, Krishna MC, Mitchell JB. Therapeutic and clinical applications of nitroxide compounds. Antioxid Redox Signal. 2007b;9:1731–1743. doi: 10.1089/ars.2007.1722. [DOI] [PubMed] [Google Scholar]
- Stevens TL, Bossie A, Sanders VM, Fernandez-Botran R, Coffman RL, Mosmann TR, Vitetta ES. Regulation of antibody isotype secretion by subsets of antigen-specific helper T cells. Nature. 1988;334:255–258. doi: 10.1038/334255a0. [DOI] [PubMed] [Google Scholar]
- Stuve O. Knowns and unknowns in the future of multiple sclerosis treatment. J Neurol Sci. 2009;287(Suppl 1):S30–36. doi: 10.1016/S0022-510X(09)71298-5. [DOI] [PubMed] [Google Scholar]
- Su KG, Banker G, Bourdette D, Forte M. Axonal degeneration in multiple sclerosis: the mitochondrial hypothesis. Curr Neurol Neurosci Rep. 2009;9:411–417. doi: 10.1007/s11910-009-0060-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thiemermann C. Membrane-permeable radical scavengers (tempol) for shock, ischemia-reperfusion injury, and inflammation. Crit Care Med. 2003;31:S76–84. doi: 10.1097/00003246-200301001-00011. [DOI] [PubMed] [Google Scholar]
- Torreilles F, Salman-Tabcheh S, Guerin M, Torreilles J. Neurodegenerative disorders: the role of peroxynitrite. Brain Res Brain Res Rev. 1999;30:153–163. doi: 10.1016/s0165-0173(99)00014-4. [DOI] [PubMed] [Google Scholar]
- Trapp BD, Peterson J, Ransohoff RM, Rudick R, Mork S, Bo L. Axonal transection in the lesions of multiple sclerosis. N Engl J Med. 1998;338:278–285. doi: 10.1056/NEJM199801293380502. [DOI] [PubMed] [Google Scholar]
- Tsuhako MH, Augusto O, Linares E, Chadi G, Giorgio S, Pereira CA. Tempol ameliorates murine viral encephalomyelitis by preserving the blood-brain barrier, reducing viral load, and lessening inflammation. Free Radic Biol Med. 2010;48:704–712. doi: 10.1016/j.freeradbiomed.2009.12.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turjeman K, Bavli Y, Kizelsztein P, Schilt Y, Allon N, Katzir TB, Sasson E, Raviv U, Ovadia H, Barenholz Y. Nano-Drugs Based on Nano Sterically Stabilized Liposomes for the Treatment of Inflammatory Neurodegenerative Diseases. PLoS One. 2015;10:e0130442. doi: 10.1371/journal.pone.0130442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van der Goes A, Wouters D, Van Der Pol SM, Huizinga R, Ronken E, Adamson P, Greenwood J, Dijkstra CD, De Vries HE. Reactive oxygen species enhance the migration of monocytes across the blood-brain barrier in vitro. FASEB J. 2001;15:1852–1854. doi: 10.1096/fj.00-0881fje. [DOI] [PubMed] [Google Scholar]
- van Meeteren ME, Hendriks JJ, Dijkstra CD, van Tol EA. Dietary compounds prevent oxidative damage and nitric oxide production by cells involved in demyelinating disease. Biochem Pharmacol. 2004;67:967–975. doi: 10.1016/j.bcp.2003.10.018. [DOI] [PubMed] [Google Scholar]
- van Meeteren ME, Teunissen CE, Dijkstra CD, van Tol EA. Antioxidants and polyunsaturated fatty acids in multiple sclerosis. Eur J Clin Nutr. 2005;59:1347–1361. doi: 10.1038/sj.ejcn.1602255. [DOI] [PubMed] [Google Scholar]
- Warnke C, Meyer zu Hörste G, Hartung HP, Stüve O, Kieseier BC. Review of teriflunomide and its potential in the treatment of multiple sclerosis. Neuropsychiatr Dis Treat. 2009;5:333–340. [PMC free article] [PubMed] [Google Scholar]
- Wilcox CS. Effects of tempol and redox-cycling nitroxides in models of oxidative stress. Pharmacol Ther. 2010;126:119–145. doi: 10.1016/j.pharmthera.2010.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wuerfel J, Tysiak E, Prozorovski T, Smyth M, Mueller S, Schnorr J, Taupitz M, Zipp F. Mouse model mimics multiple sclerosis in the clinico-radiological paradox. Eur J Neurosci. 2007;26:190–198. doi: 10.1111/j.1460-9568.2007.05644.x. [DOI] [PubMed] [Google Scholar]