Abstract
Maternal and neonatal tetanus is still a substantial but preventable cause of mortality in many developing countries. Case fatality from these diseases remains high and treatment is limited by scarcity of resources and effective drug treatments. The Maternal and Neonatal Tetanus Elimination Initiative, launched by WHO and its partners, has made substantial progress in eliminating maternal and neonatal tetanus. Sustained emphasis on improvement of vaccination coverage, birth hygiene, and surveillance, with specific approaches in high-risk areas, has meant that the incidence of the disease continues to fall. Despite this progress, an estimated 58 000 neonates and an unknown number of mothers die every year from tetanus. As of June, 2014, 24 countries are still to eliminate the disease. Maintenance of elimination needs ongoing vaccination programmes and improved public health infrastructure.
Introduction
Elimination of maternal and neonatal tetanus is a key area in global public health policy. Although much progress has been made in reduction of the incidence of maternal and neonatal tetanus during the past two decades, it remains a leading cause of preventable neonatal mortality in some countries.1,2 Without medical care, mortality from neonatal tetanus is close to 100%, often exceeding 50% even with hospital care.3–5
Maternal immunisation has resulted in 82% of today’s newborn babies being protected from tetanus.6 Since the 2007 Lancet Seminar1 on this subject, 24 additional countries have achieved elimination status (35 out of the 59 countries targeted had achieved elimination as of June, 2014), although this still falls short of the outcome predicted in the previous seminar of all but 11 countries having eliminated the disease by 2009. Partial elimination of maternal and neonatal tetanus has been validated in India, Ethiopia, and Indonesia. 161 million women of childbearing age have been targeted with tetanus toxoid supplementary immunisation activities and 128 million have received two doses of tetanus toxoid.7
Epidemiology
Tetanus is caused by the contamination of wounds with Clostridium tetani spores in individuals without protective circulating antibodies. In maternal tetanus, infection occurs after abortion, miscarriages, or unhygienic delivery practices, whereas neonatal tetanus infection usually occurs through the umbilical stump after delivery. Inadequate maternal vaccination and poor perinatal hygiene contribute to the occurrence of the disease. Unlike many other infectious diseases, tetanus elimination needs continuing vaccination programmes because the tetanus spores are widespread in soil and faeces throughout the world.
The case definition for confirmed neonatal tetanus is “a neonate with the normal ability to suck and cry during the first two days of life, and between 3 and 28 days of age cannot suck normally and becomes stiff or has spasms (ie, jerking of the muscles)”.8 In 1988, the World Health Assembly passed a resolution to eliminate neonatal tetanus by the year 2000, a disease then estimated to kill roughly 800 000 neonates a year, causing 6·7 deaths per 1000 livebirths. Elimination is defined as fewer than one case per 1000 livebirths in every district in every country. By the end of 1999, 57 countries (mainly in Asia and Africa; figure 1) were still to achieve the target and a renewed initiative was launched by WHO and its partners, UNICEF and the UN Population Fund.9 The deadline for elimination was extended to 2005 and the endpoint was changed to include the elimination of maternal tetanus because both are prevented by the same measures.
Figure 1. Global maternal and neonatal tetanus elimination.
Reproduced from reference 7, by permission of WHO.
Maternal tetanus is defined as tetanus during pregnancy or within 6 weeks of the end of pregnancy (birth, miscarriage, or abortion). No formal reporting system exists for maternal tetanus, although elimination is assumed to occur with neonatal tetanus elimination. Implementation of the Maternal and Neonatal Tetanus Elimination Initiative has involved three main strategies: immunisation, birth hygiene, and surveillance.10
Immunisation of women who are pregnant or of childbearing age reduces neonatal tetanus mortality by an estimated 94% (95% CI 80–98).11,12 Data from rural North India suggest that 16% of neonatal deaths (78 632 cases per year) can be attributed to the failure to receive two doses of tetanus toxoid.13 In addition to routine immunisation during childhood, along with boosters every decade, the Maternal and Neonatal Tetanus Elimination Initiative uses two main immunisation programmes.7,9 The original approach was to strengthen the routine immunisation of women during pregnancy (see “Prevention” section). However, in many areas, this method alone was insufficient and led to the development of supplementary immunisation activities (SIAs). These normally involve the immunisation of all women of childbearing age in areas at high risk of maternal and neonatal tetanus, and use various approaches, such as school-based programmes or community-based initiatives. In Nepal, immunisation coverage increased from 45% of women receiving the recommended dose of tetanus toxoid to more than 80% after SIA activities during 2000–04.14 Similar coverage rates were reported in Papua New Guinea in a programme targeting 1·6 million women.15 Because tetanus arises from a combination of poor birth hygiene and deficient immunisation, immunisation initiatives are likely to have the biggest effect in communities with high rates of home births or births with traditional attendants.
Most births in countries of low and middle income occur outside hospital facilities. Data from 2005 to 2012 show only 60% of births in countries of lower middle income occur with skilled birth attendants.16 In Africa, this proportion is less than 50%. Studies in Africa and Asia show that interventions to improve education and birth hygiene have a substantial effect on maternal and neonatal tetanus, even without vaccination programmes.17 In 2012, China was validated as having eliminated maternal and neonatal tetanus but, unlike other countries, much of this success has been ascribed to improved birth hygiene and increased inhospital delivery rates, without specific vaccination schemes. Safe motherhood strategies modelled by UNICEF were promoted alongside education and improvements in infrastructure. By 2011, 98% of babies were born in hospital, compared with 65% when the programme began in 2000.18,19
Neonatal tetanus is vastly under-reported: many births occur at home and unknown numbers of births and deaths are never recorded. One of the major challenges in the Maternal and Neonatal Tetanus Initiative has been accurate validation of local and national elimination. Strengthening of local reporting systems has been prioritised and methods such as education of local community workers and traditional birth attendants, and engagement of the private sector have been used. Lot-quality assurance and cluster surveys have been used to assess endpoints more accurately at a district level. Typically, districts within a country that are most likely to perform poorly are chosen. Such surveys are subject to limitations, with selection bias likely to lead to overestimation of vaccination coverage, as the same population is likely to be missed.20 Cases of neonatal tetanus are likely to be under-reported.21 In some areas, improvements in the speed, accuracy, and detail of data collection have been achieved by linking neonatal tetanus to other surveillance programmes, such as the acute flaccid paralysis monitoring system.14,21 Improved data dissemination and the availability of additional detail, such as geographical and demographic information, has enabled the design and implementation of SIA programmes to target at-risk populations more effectively.15
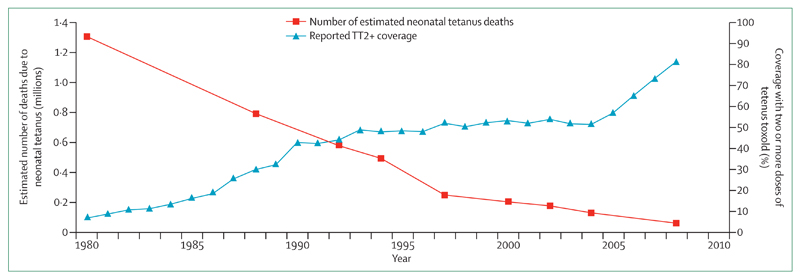
To date, 24 countries are yet to eliminate maternal and neonatal tetanus, and the most recent strategic plan aims to achieve tetanus elimination by 2015.10,22 Since 1987, global deaths from neonatal tetanus have fallen from an estimated 787 000 deaths in 1987 to 58 000 in 20107 (figure 2). The proportion of neonatal deaths caused by tetanus fell from 14% in 1993 to 1·7% in 2008.2
Figure 2. Global progress towards maternal and neonatal tetanus elimination.
Reproduced from Blencowe and colleagues.11
Maternal tetanus was estimated to kill 15 000–30 000 women per year in 1993.23 No recent data are available but its incidence is likely to have fallen substantially because of the increase in maternal vaccination coverage, although in some countries, it remains an important preventable cause of morbidity and mortality. For example, in a recent analysis of 8220 livebirths at one hospital in Nigeria, 5·4% of maternal deaths were due to puerperal tetanus.24 The ongoing elimination of maternal and neonatal tetanus represents a continuing challenge. The most recent strategic plan aims to vaccinate 49 million women through SIAs in 2013 and 2014.10 To achieve this goal, geographically remote areas must be reached, political barriers removed, and social issues addressed. Good maternal health services are central to maternal and neonatal tetanus prevention. The authors of a large study of women from Kenya, Namibia, Nepal, and India concluded that gender inequality was still significantly associated with unequal access to reproductive health care, including tetanus prevention, so this is an issue that still remains to be addressed.25
Abortion-related tetanus
Abortion-related tetanus is a specific problem, with a higher associated mortality compared with tetanus arising from other portals of entry.4 Reliable data regarding the incidence of postabortion tetanus are not available. Studies in Bangladesh in the late 1990s suggested that 55% of tetanus-related deaths in women and girls aged 10–50 years were due to postabortion tetanus, and national data showed that 35% of pregnancy-related tetanus deaths were due to abortion.26 Fauveau and colleagues,23 in an analysis of 1101 cases of maternal tetanus in developing countries, concluded that 27% of cases were secondary to abortion.
Traditional abortion methods commonly involve high-risk practices, such as the insertion of sticks, herbs, or roots into the cervix, or the use of unsterile surgical instruments. An estimated 22 million unsafe abortions occurred in 2008.27 Women who use these methods are unlikely to seek conventional health care and thus might miss pregnancy-linked vaccination services. Adolescents might be too young to access maternal programmes. Although standard infant immunisation is likely to confer protective antibodies to a sufficient level until antenatal boosters are given, adolescents will be at risk of tetanus in areas where infant immunisation is deficient. In one study done in the 1990s,28 serum concentrations of antitetanus IgG were undetectable in 27% of Nigerian adolescents who reported having had at least one abortion. WHO guidance on standards for abortion, including sterilisation procedures designed to protect against tetanus, provides no recommendations on concomitant immunisation strategies.29
Pathophysiology
Tetanus is caused by the toxin produced by the Gram-positive bacillus C tetani, which is an obligate anaerobe. Their spores, by contrast, are highly resistant and can tolerate air, extremes of temperature, and common disinfectants. Spores of C tetani are mainly found in human and animal faeces, soil, and manure, and have been isolated throughout the world. Environmental factors, such as flooding and typhoons, can increase the number of C tetani spores in the soil, potentially increasing the risk of tetanus infection after natural disasters.30 The spores enter the body through contamination of both deep and superficial wounds, and can transform in anaerobic conditions. The bacteria do not multiply in healthy tissue with normal oxygen tensions, but are able to grow and multiply in the low-oxygen-tension environment in devitalised or necrotic tissue.
Strains of C tetani can be differentiated by variations in flagellar antigens and their ability to produce tetanus toxin. Genome sequencing has revealed the presence of multiple virulence factors, including tetanolysin O, haemolysin, and fibronectin, as well as proteins involved in cell-wall binding.31 Only bacteria capable of producing tetanus toxin are able to cause disease. Tetanus toxin is encoded on plasmid pE88, which also encodes the gene for its direct transcription regulator.32 The origin of this plasmid is unclear because much of its sequence is unique to C tetani.31
Tetanus toxin is one of the most potent toxins identified, with a median human lethal dose of less than 2·5 ng/kg.33 Similar to all clostridial toxins, it exerts its effect by very specific action on one of the soluble N-ethylmaleimide-sensitive fusion attachment protein receptor proteins involved in synaptic vesicle release.34 However, unlike most botulinum toxins, which mainly act at the neuromuscular junction, tetanus toxin is transported into the CNS. This ability to travel retrogradely inside neurons has led to recent interest in tetanus toxin as a means of mapping neuronal connections or as a vehicle for transporting therapeutic agents into the CNS.35
The toxin is produced as a single-chain, 150-kDa protein, which is then cleaved to produce a heavy and light chain of 100 and 50 kDa, respectively. The toxin is released during the stationary phase of bacterial growth or after cell lysis. The two subchains remain linked by a disulphide bond. The N-terminal domain of the heavy chain is involved in translocation of the light chain into the cytosol from an endosome. The C terminus is further subdivided into two parts, with the C-terminal subsection of the C-terminus being necessary for binding and internalisation into the neuron.36,37
Tetanus toxin enters the nervous system at the neuromuscular junction after binding to polysialogangliosides and other molecules in lipid rafts in the neuronal membrane.37,38 The toxin is transported in the neuron along the same pathways as brain-derived neurotrophic factor, p75 neurotrophin receptor, and tropo-myosin-related kinase B.39 The toxin is then trancytosed to preganglionic inhibitory interneurons via as yet unclear mechanisms.35 The N terminus of the heavy chain triggers translocation of the light chain into the cytoplasm.40 The light chain has zinc-dependent endopeptidase activity and cleaves vesicle-associated monophosphate 2 (synaptobrevin), preventing its action in synaptic vesicle docking and neurotransmitter release.34 Tetanus toxin therefore inhibits presynaptic inhibitory interneurons, resulting in disinhibition of motor neuron discharge.
Clinical features
The clinical signs and symptoms of tetanus result from disinhibition of motor neuron discharge, causing hypertonus and spasm of skeletal muscle. In severe forms, additional autonomic nervous system dysfunction can occur. Distinct stages of disease progression are noted and correlate with toxin uptake and action. The incubation period is defined as the time between wound contamination and the first symptom, and is the period during which toxin release and transport occurs. The period of onset is the time during which generalised muscle spasms subsequently develop.
In neonatal tetanus, infection occurs via the umbilical cord, which can become contaminated during cutting with unsterile instruments at birth or because of substances being applied to the cord (eg, animal faeces), meaning that the incubation period is equal to the age at which symptoms develop. The median incubation period is 5–7 days (range 3–24) after birth.3,4 Neonates present with refusal to feed, with difficulty in opening the mouth due to trismus.41 Sucking then stops and facial muscle spasm can produce risus sardonicus. The hands often become clenched, along with dorsiflexion of the feet and increased muscle tone, progressing to rigidity and opisthotonus (spasm of spinal extensors). Spasms of the limbs develop early, initially provoked by physical, auditory, or visual stimuli, but eventually occurring spontaneously.
In most cases, maternal tetanus presents as a generalised form, affecting all muscle groups. Incubation periods in older children and adults are usually longer than in neonates, with means of 7–10 days (range 2–38).42–44 The period of onset is typically 24–72 h. Both periods of time are shorter the more severe the disease.4,42,44 Maternal tetanus arising from internal entry sites (postpartum, postabortional, or intramuscular injection) are associated with higher mortality than tetanus arising from other entry sites.4,45,46
Proximal muscle groups are usually affected first in maternal tetanus and, similar to neonatal tetanus, initial symptoms invariably include trismus,43 with risus sardonicus often evident. Pharyngeal and laryngeal muscle involvement results in airway compromise at an early stage. Respiration is further compromised by tension and spasm in respiratory muscles. Opisthotonus also occurs and can result in spinal fractures.47,48
Autonomic nervous system dysfunction occurs in severe tetanus, usually becoming apparent during the second week of illness. Cardiovascular manifestations are the most common and include labile blood pressure, tachycardia, bradycardia, and arrhythmias. Circulating concentrations of noradrenaline and adrenaline, as indicated by urinary excretion, are higher in tetanus compared with those measured in other critical illnesses, and normal cardiovascular regulation is impaired.49,50 Direct catecholamine-related necrosis has been shown at autopsy and tumour necrosis factor α-associated dysfunction has also been implicated in the pathophysiology.51 Electrocardiograms of 33 patients in Dakar (Senegal) showed more than one abnormality in 93% of patients, despite normal echocardiography.52
Localised forms of tetanus can occur, which are generally milder with a better prognosis, although localised cephalic tetanus is associated with increased airway and respiratory complications.
Mortality from maternal and neonatal tetanus is high. Adult mortality rates up to 52% are reported in Asia and Africa.42,43,53–56 Neonatal mortality is even higher, ranging from 3% to 88% in these regions.57–61 Most tetanus occurs in rural areas. Mortality is highest in patients not admitted to hospital, and delayed admission to hospital is associated with worse outcomes in neonatal tetanus.62–64 Facilities for ventilation and intensive care are associated with improved outcome in both neonatal and non-neonatal tetanus.45,65,66 However, with improved respiratory support, cardiovascular and other respiratory complications then become apparent.45,65,67,68
Low birthweight (especially <2·5 kg), young age at presentation, fever, generalised rigidity, and risus sardonicus are associated with worse outcome in neonatal tetanus.58 A meta-analysis of 4535 cases69 of neonatal tetanus (from studies published between 1974 and 2011) concluded that low birthweight and age at onset were the most important prognostic factors (low birthweight: odds ratio [OR] 2·09, 95% CI 1·29–3·37), with the combination of a low birthweight of less than 2·5 kg and an age at onset of younger than 6 days most significant (OR 6·8, 95% CI 2·42–19·11). In maternal tetanus, rapid progression (short incubation and period of onset), an internal entry site, and underlying disease are all significant indicators of worse prognosis.42–44
Tetanus can take 6–8 weeks to resolve completely, with spasms often lasting 2–3 weeks.70–72 Both adults and neonates can need ventilation for several weeks (median 23 days, range 17–60) and intensive care for longer.65,70 Data for long-term sequelae are scarce. In patients in a hospital in northwestern Tanzania, Chalya and colleagues42 reported that 8·6% of survivors were discharged with permanent disability, such as a persistent vegetative state, limb amputations, and gait abnormality. Neurological impairments of persistent rigidity and memory loss were described in a study of Bangledeshi patients, with a total of three of 75 patients discharged with permanent disability.43 Muscle rigidity was reported in all 45 surviving patients at discharge in a study in Thailand.67
Few studies have examined sequelae of neonatal tetanus, but complication rates are likely to be even higher. The authors of one Kenyan study found that 20–40% of survivors of neonatal tetanus had evidence of brain damage, manifesting as microcephaly and mild neurological, developmental, or behavioural problems.73 Complications of cerebral palsy, cognitive delay, and deafness were described in 20% of survivors in one Nigerian case series.62 These complications might be caused by the hypoxia and hypoglycaemia commonly detected during the clinical course.
Diagnosis
The diagnosis of tetanus is clinical, and criteria for neonatal tetanus diagnosis are described in the previous section. The differential diagnosis of neonatal tetanus includes birth asphyxia, hypoglycaemia, hypocalcaemic tetany, and seizures. Few conditions truly mimic generalised maternal tetanus.
C tetani can be cultured from entry sites with oxygen-reduced blood agar or meat broth,74 but facilities are often not available and interventions should not be delayed. Bedside inoculation of media has been reported to improve detection rates. A serum antitetanus IgG concentration higher than >0·1 IU/mL (taken before antitoxin is given) is accepted as sufficient protection against tetanus infection and would make the clinical diagnosis less likely.75,76 Bioassay or PCR detection of tetanus toxin in plasma or wound exudates can also be used, although these are rarely available in most settings.74,77
Management
Management of maternal and neonatal tetanus involves toxin neutralisation, bacterial elimination, and symptomatic control, with supportive care. Most data for tetanus treatment are derived from observation or studies in adults. Evidence to support most therapies used in treatment is scarce. Since the publication of Roper and colleagues’ Seminar in 2007,1 there have been only four randomised controlled trials of treatment in tetanus (three in adults78–80 and one in neonates81), involving a total of 190 patients, and there have been no trials of novel therapies or regimens. In the same period, two meta-analyses have been published, with one including only one study82 and the other including three,83 two of which were published after 2007. Research into effective treatments is hampered by the settings in which most tetanus occurs—often rural and remote, and in low or low-middle income countries with few resources—and the unwillingness of funding bodies to support research into a vaccine-preventable disease.
Although comprehensive accounts of tetanus treatment in adults have been published, including the management of tetanus in pregnancy,68,84,85 there are few accounts of specialist neonatal tetanus care.86
Antibiotics
Intravenous penicillin and metronidazole are first-line treatments in both maternal and neonatal tetanus. Antibiotic sensitivity testing is not routine, but a study of 45 isolates of C tetani in Vietnam74 revealed all isolates to be sensitive to metronidazole and penicillin, but resistant to co-trimoxazole. Prolonged infection occurred in this study despite antibiotic treatment because C tetani grows in devitalised anaerobic conditions in which antibiotic penetration is poor.
Parenteral antitoxin
Antitoxin reduces mortality. Outcomes do not differ between patients treated with equine-derived antitetanus serum or human tetanus immunoglobulin.87 Globally, equine-derived antitetanus serum is most widely used, although human tetanus immunoglobulin is preferred, as recommended by the Centers for Disease Control and Prevention88 and the UK Health Protection Agency.75 Current doses are based on studies done in the 1960s, but dose regimens remain controversial.89 Blake and colleagues87 found that there was no difference in outcome in those receiving less than 500 IU of tetanus immunoglobulin compared with higher doses. The difficulties caused by restricted availability of tetanus immunoglobulin supplies have led to new guidance stating that human normal immunoglobulin can be used in its place.75 This decision is based on theoretical considerations after assay of antitoxin IgG concentrations in a few commercially produced products, and prospective comparative trials or studies on generalisability to other preparations have not been done.
Intrathecal antitoxin
Administration of antitoxin via the intrathecal route has been of interest for several decades. This route provides a means of neutralising toxin within the nervous system and increasing the intrathecal concentration of antitoxin compared with concentrations achieved after intramuscular administration. In a 2006 meta-analysis including 12 clinical trials involving a total of 942 patients, Kabura and colleagues90 suggested that intrathecal administration was beneficial (relative risk 0·71, 95% CI 0·62–0·81. However, heterogeneity and methodological differences, such as the variable use of adjunctive steroids, means that these results should be interpreted with caution.
More recently, a randomised controlled trial81 found reduced mortality and hospital stay in neonates in Pakistan randomly assigned to intrathecal lyophilised human immunoglobulin delivered by lumbar puncture in addition to normal care of intramuscular equine antitetanus serum (mortality rate 1/32 vs 8/32, p=0·026; mean hospital stay 10·0 [SD 2·1] vs 13·2 [SD 2·6] days, p<0·001). In 42 adult patients in Togo,80 a regimen of 1500 IU heterologous immunoglobulin administered intrathecally via the suboccipital route was compared with 9000 IU heterologous antitetanus serum given intramuscularly and subcutaneously. The intrathecal group also received intravenous metronidazole, which was not necessarily given to the controls. Results showed a reduction in mortality in the intrathecal group (11·7 vs 52·0%, p=0·007), with improved recovery rates, as indicated by mobilisation and spasm reduction at 48 h (p<0·001). Miranda-Filho and colleagues91 used 1000 IU lyophilised human immunoglobulin to treat 58 patients older than 12 years in Brazil via intrathecal (suboccipital or lumbar) routes combined with 3000 IU immunoglobulin administered intramuscularly and compared the outcome with that of 62 patients randomly assigned to intramuscular immunoglobulin alone. Although mortality rates were not different between the two groups, hospital stay and progression of disease were reduced in the intrathecal group and treatment with intrathecal antitoxin significantly reduced costs of both intensive care unit and inhospital stay.92
One side-effect reported during intrathecal administration was mild headache91 and reversible paraparesis was reported on one occasion only.93
Symptom control and supportive care
In many developing countries, chlorpromazine and phenobarbitone (nasogastric or intravenous administration) remain the mainstays of treatment for neonatal tetanus because they are affordable sedatives. Intravenous diazepam is used widely in neonates and adults to control spasms. Without ventilatory facilities, drugs such as intramuscular paraldehyde are used for further spasm control.
Magnesium
Magnesium sulphate has muscle relaxant, vasodilatory, and negative chronotropic properties, and offers a potentially simple and cheap method of treating tetanus. Initial data from observational case series in adults showed that careful titration of dose against patellar reflex can allow spasms to be controlled without the need for mechanical ventilation.71 However, a randomised, controlled trial of 195 patients72 and more recent smaller trials79 and case series70,94 have not supported this sole use other than in milder cases of tetanus, although magnesium does reduce the need for other muscle relaxants and improves cardiovascular stability. A 2012 meta-analysis of three trials was unable to show any reduction in mortality in patients treated with magnesium compared with placebo or diazepam therapy, or to draw conclusions on the effects on hospital stay or the need for ventilatory support because of large methodological differences in the studies reviewed.83 We were unable to find any randomised controlled trials on the use of magnesium in neonatal tetanus.
Prevention
Prevention and elimination of maternal and neonatal tetanus is achieved with a combination of vaccination and improvement in perinatal care. WHO has made clear recommendations regarding the immunisation of women and provision of clean deliveries 95 and standards for ongoing neonatal tetanus surveillance have been published.96
Unimmunised pregnant women or those with no documentation of immunisation should receive two doses of tetanus toxoid given 1 month apart, with the first dose as early as possible in pregnancy. Further doses of toxoid should be given in subsequent pregnancies (or at intervals of at least a year) up to a total of five doses, a level considered sufficient to ensure life-long protection.76 WHO’s recommendations are shown in the table.97 In areas deemed to confer a high risk of neonatal tetanus, additional immunisation with three spaced doses of tetanus toxoid is recommended for all women of childbearing age.
Table. WHO recommendations for tetanus immunisation to prevent maternal and neonatal tetanus97.
| Recommendation | |
|---|---|
| Routine vaccination | |
| Infancy (<1 year) | Primary series of three doses (DTP3: DTwP or DTaP) |
| 4–7 years | Tetanus toxoid-containing booster |
| 12–15 years | Tetanus toxoid-containing booster |
| Adults (eg, first pregnancy or military service) | Tetanus toxoid booster—ie, total of 6 doses. For those receiving their first tetanus vaccine as adolescents or adults, a total of five appropriately spaced doses |
| Pregnant women with inadequate or unknown vaccination history | Two doses of tetanus toxoid-containing vaccine. An effort is made to complete a total course of five vaccinations (eg, postnatal visits and subsequent pregnancies) |
| All women of childbearing age in high-risk areas for maternal and neonatal tetanus | Three doses of tetanus toxoid, usually during a 12-month period |
DTP3=diphtheria, tetanus, pertussis. DTwP=tetanus toxoid combined with diphtheria toxoid and whole-cell pertussis.
DTaP=tetanus toxoid combined with diphtheria toxoid and acellular pertussis vaccines.
Maternal antitetanus antibodies are passively transferred to the fetus and protect the baby for the first few months of life. HIV infection is associated with reduced response to maternal tetanus vaccination.98,99 Malaria and HIV might interfere with the transfer of tetanus antibodies to the fetus, although the evidence is conflicting, with some studies reporting a reduction in passive antibody transfer in HIV and malaria100 but others reporting no change.101,102 The largest and most recent study103 analysed 704 pairs of maternal–cord-paired serum samples in Kenya and reported a 52% (95% CI 30–67) reduction in antitetanus antibody concentrations in neonates with HIV-infected mothers and a 48% (95% CI 26–62) reduction in cases with active chronic or past placental malaria. The use of less sensitive methods for identification of placental malaria and absence of adjustment for maternal tetanus vaccination status in the studies above101 compared with the recent study103 could explain the difference in findings. Optimum tetanus immunisation regimens have not been defined for pregnant women with HIV or malaria, irrespective of past immunisation status.
A systematic review104 examining evidence for the hygiene interventions recommended by WHO95,105 reported that only low-quality evidence exists to support the use of handwashing and cord antimicrobial applications as methods of reducing neonatal tetanus. WHO advocates the use of six clean measures to improve birth hygiene: clean birth surface, clean hands, clean perineum, cord cutting, cord tying, and cord care. In the absence of good quality data, a Delphi expert consensus104 concluded that clean birth practices at home could prevent 30% (IQR 20–30) of neonatal tetanus deaths, increasing to 35% (IQR 30–40) if skilled attendants were present and 40% (IQR 30–50) if the birth took place in a health-care facility with postnatal measures. These analyses are supported by a recent case-control study106 investigating the use of clean delivery kits in Pakistan, the authors of which concluded that, irrespective of birth attendant training, in a setting of low vaccination coverage, the use of specialist clean delivery kits prevented about a quarter of cases of neonatal tetanus.
Maintaining maternal and neonatal tetanus elimination
Much of the success of the Maternal and Neonatal Tetanus Elimination Initiative lies with specifically designed local methods to increase vaccination coverage or improve birth hygiene. High-risk areas are often geographically remote, with poor infrastructure and often political instability, making the delivery of basic programmes challenging. Continuing elimination needs vaccination and surveillance programmes to be maintained. As childhood vaccination (diphtheria, tetanus, pertussis; DTP3) coverage improves, the reliance on vaccination of pregnant women as a primary means of prevention of maternal and neonatal tetanus should be reduced. However, subsequent booster doses are necessary and in countries with less developed public health programmes or fewer children in secondary education, other methods of providing adolescent booster doses might be needed.
Conclusion
Although cases of neonatal tetanus have reduced substantially in the past two decades, 24 countries are yet to eliminate this preventable condition. Insufficient recent data are available for the incidence and outcomes of maternal tetanus, although its incidence is assumed to have fallen. Prevention of maternal and neonatal tetanus must remain the main goal for tetanus programmes, which is now linked to the Millennium Development Goals: Millennium Development Goal 4 aims to reduce mortality in children younger than 5 years by two-thirds between 1990 and 2015; Goal 5 seeks to reduce the maternal mortality ratio by three-quarters and ensure universal access to reproductive health in the same time period.
Nevertheless, the disease is still common in many countries. Maintenance of elimination status needs ongoing efforts and, in many countries, public health programmes remain vulnerable to conflicts or natural disasters. Countries that have achieved elimination are unlikely to have completely eradicated the disease and tetanus continues to affect older children and adult men. Research into effective therapies and prevention is still necessary. The effects of HIV and malaria on the effectiveness of immunisation of pregnant women need to be taken into consideration in public health programmes and need further study. Cost-effective treatments that can be used in less developed settings are still needed. Intrathecal immunoglobin and intravenous magnesium sulphate might reduce mortality, duration of hospital stay, and health-care cost, but no evidence exists that other supportive treatments improve outcome.
Search strategy and selection criteria.
We identified publications via searches of the Medline, PubMed, and Cochrane databases using the search terms “tetanus”, “neonatal tetanus”, “maternal and neonatal tetanus”, and “maternal tetanus”. We also searched the WHO website using these terms. Additionally, we obtained further articles from citations within articles retrieved during the initial search, as well as from personal collections. In reviewing advances since the previous Lancet Seminar, we have given preference to recent publications; however, in many areas, there is a lack of timely or reliable data, and many recent publications rely on much older data. As such, we gave preference to articles published after 2006, but did not exclude commonly referenced and highly regarded older publications. We included review articles and book chapters to provide more detailed information. We have not done a systematic review of all papers using grading systems to assess the quality of published sources and recommendations.
Acknowledgments
CRN is funded by the Wellcome Trust and CLT is funded by the Li Ka Shing Foundation.
Footnotes
Contributors
CLT, NJB, and CRN were all involved in researching, writing, and revising this Seminar.
Declaration of interests
We declare no competing interests.
Contributor Information
Dr C Louise Thwaites, Oxford University Clinical Research Unit, Hospital for Tropical Diseases, Ho Chi Minh City, Vietnam.
Dr Nicholas J Beeching, Liverpool School of Tropical Medicine, and Tropical and Infectious Disease Unit, Royal Liverpool University Hospital, and National Institute for Health Research Health Protection Research Unit in Emerging and Zoonotic Infections, Liverpool, UK.
Prof Charles R Newton, Kenya Medical Research Institute – Wellcome Trust Collaborative Programme, Kilifi, Kenya, and Department of Psychiatry, University of Oxford, Oxford, UK.
References
- 1.Roper MH, Vandelaer JH, Gasse FL. Maternal and neonatal tetanus. Lancet. 2007;370:1947–59. doi: 10.1016/S0140-6736(07)61261-6. [DOI] [PubMed] [Google Scholar]
- 2.Black RE, Cousens S, Johnson HL, et al. and the Child Health Epidemiology Reference Group of WHO and UNICEF Global, regional, and national causes of child mortality in 2008: a systematic analysis. Lancet. 2010;375:1969–87. doi: 10.1016/S0140-6736(10)60549-1. [DOI] [PubMed] [Google Scholar]
- 3.Rai R, Singh DK. Neonatal tetanus: a continuing challenge. Indian J Pediatr. 2012;79:1648–50. doi: 10.1007/s12098-011-0666-8. [DOI] [PubMed] [Google Scholar]
- 4.Patel JC, Mehta BC. Tetanus: study of 8,697 cases. Indian J Med Sci. 1999;53:393–401. [PubMed] [Google Scholar]
- 5.Chavada VK. To study the clinico-epidemiological factors of tetanus cases admitted in a tertiary care hospital for the last 10 years. J Clin Diagn Res. 2010;4:2649–51. [Google Scholar]
- 6.UNICEF. The state of the world’s children. [accessed July 4, 2014];2013 http://www.unicef.org/sowc2013.
- 7.WHO. Maternal and neonatal tetanus (MNT) elimination. [accessed July 4, 2014]; http://www.who.int/immunization/diseases/MNTE_initiative/en.
- 8.WHO. WHO-recommended surveillance standard of neonatal tetanus. [accessed July 4, 2014]; http://www.who.int/immunization/monitoring_surveillance/burden/vpd/surveillance_type/active/NT_Standards/en.
- 9.WHO, UNICEF, UN Population Fund. Maternal and neonatal tetanus elimination by 2005. Strategies for achieving and maintaining elimination. Geneva: World Health Organization; 2000. [Google Scholar]
- 10.WHO. Achieving and sustaining maternal and neonatal tetanus elimination. Strategic plan 2012–2015. Geneva: World Health Organization; 2012. [Google Scholar]
- 11.Blencowe H, Lawn J, Vandelaer J, Roper M, Cousens S. Tetanus toxoid immunization to reduce mortality from neonatal tetanus. Int J Epidemiol. 2010;39(suppl 1):i102–09. doi: 10.1093/ije/dyq027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Demicheli V, Barale A, Rivetti A. Vaccines for women to prevent neonatal tetanus. Cochrane Database Syst Rev. 2013;5:CD002959. doi: 10.1002/14651858.CD002959.pub2. [DOI] [PubMed] [Google Scholar]
- 13.Singh A, Pallikadavath S, Ogollah R, Stones W. Maternal tetanus toxoid vaccination and neonatal mortality in rural north India. PLoS One. 2012;7:e48891. doi: 10.1371/journal.pone.0048891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Vandelaer J, Partridge J, Suvedi BK. Process of neonatal tetanus elimination in Nepal. J Public Health (Oxf) 2009;31:561–65. doi: 10.1093/pubmed/fdp039. [DOI] [PubMed] [Google Scholar]
- 15.Datta SS, Barnabas R, Sitther A, et al. Three cases of neonatal tetanus in Papua New Guinea lead to development of national action plan for maternal and neonatal tetanus elimination. Western Pac Surveill Response J. 2013;4:40–43. doi: 10.5365/WPSAR.2013.4.1.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.WHO. World health statistics 2013. Geneva: World Health Organization; 2012. [Google Scholar]
- 17.Meegan ME, Conroy RM, Lengeny SO, Renhault K, Nyangole J. Effect on neonatal tetanus mortality after a culturally-based health promotion programme. Lancet. 2001;358:640–41. doi: 10.1016/S0140-6736(01)05787-7. [DOI] [PubMed] [Google Scholar]
- 18.Liu X, Yan H, Wang D. The evaluation of “Safe Motherhood” program on maternal care utilization in rural western China: a difference in difference approach. BMC Public Health. 2010;10:566. doi: 10.1186/1471-2458-10-566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.WHO. Expanded programme on immunization. [accessed July 4, 2014]; http://www.wpro.who.int/immunization/news/china_achieves_mnte/en.
- 20.Cutts FT, Izurieta HS, Rhoda DA. Measuring coverage in MNCH: design, implementation, and interpretation challenges associated with tracking vaccination coverage using household surveys. PLoS Med. 2013;10:e1001404. doi: 10.1371/journal.pmed.1001404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lambo JA, Nagulesapillai T. Neonatal tetanus elimination in Pakistan: progress and challenges. Int J Infect Dis. 2012;16:e833–42. doi: 10.1016/j.ijid.2012.07.015. [DOI] [PubMed] [Google Scholar]
- 22.UNICEF. Elimination of maternal and neonatal tetanus. [accessed July 4, 2014]; http://www.unicef.org/health/index_43509.html.
- 23.Fauveau V, Mamdani M, Steinglass R, Koblinsky M. Maternal tetanus: magnitude, epidemiology and potential control measures. Int J Gynaecol Obstet. 1993;40:3–12. doi: 10.1016/0020-7292(93)90765-o. [DOI] [PubMed] [Google Scholar]
- 24.Okusanya BO, Aigere EO, Abe A, Ibrahim HM, Salawu RA. Maternal deaths: initial report of an on-going monitoring of maternal deaths at the Federal Medical Centre Katsina, Northwest Nigeria. J Matern Fetal Neonatal Med. 2013;26:885–88. doi: 10.3109/14767058.2013.765851. [DOI] [PubMed] [Google Scholar]
- 25.Namasivayam A, Osuorah DC, Syed R, Antai D. The role of gender inequities in women ’ s access to reproductive health care: a population-level study of Namibia, Kenya, Neapl, and India. Int J Womens Health. 2012;4:351–64. doi: 10.2147/IJWH.S32569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rochat R, Akhter HH. Tetanus and pregnancy-related mortality in Bangladesh. Lancet. 1999;354:565. doi: 10.1016/s0140-6736(98)05193-9. [DOI] [PubMed] [Google Scholar]
- 27.WHO. Unsafe abortion: global and regional estimates of the incidence of unsafe abortion and associated mortality in 2008. 6th edn. Geneva: WHO; 2011. [Google Scholar]
- 28.Brabin L, Fazio-Tirrozzo G, Shahid S, et al. Tetanus antibody levels among adolescent girls in developing countries. Trans R Soc Trop Med Hyg. 2000;94:455–59. doi: 10.1016/s0035-9203(00)90139-1. [DOI] [PubMed] [Google Scholar]
- 29.WHO. Safe abortion: technical and policy guidance for health systems. 2nd edn. Geneva: World Health Organization; 2012. [PubMed] [Google Scholar]
- 30.Huang SW, Chan JP, Shia WY, Shyu CL, Tung KC, Wang CY. The utilization of a commercial soil nucleic acid extraction kit and PCR for the detection of Clostridium tetanus and Clostridium chauvoei on farms after flooding in Taiwan. J Vet Med Sci. 2013;75:489–95. doi: 10.1292/jvms.12-0271. [DOI] [PubMed] [Google Scholar]
- 31.Bruggemann H, Baumer S, Fricke WF, et al. The genome sequence of Clostridium tetani, the causative agent of tetanus disease. Proc Natl Acad Sci USA. 2003;100:1316–21. doi: 10.1073/pnas.0335853100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Finn CW, Jr, Silver RP, Habig WH, Hardegree MC, Zon G, Garon CF. The structural gene for tetanus neurotoxin is on a plasmid. Science. 1984;224:881–84. doi: 10.1126/science.6326263. [DOI] [PubMed] [Google Scholar]
- 33.Gill DM. Bacterial toxins: a table of lethal amounts. Microbiol Rev. 1982;46:86–94. doi: 10.1128/mr.46.1.86-94.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Schiavo G, Benfenati F, Poulain B, et al. Tetanus and botulinum-B neurotoxins block neurotransmitter release by proteolytic cleavage of synaptobrevin. Nature. 1992;359:832–35. doi: 10.1038/359832a0. [DOI] [PubMed] [Google Scholar]
- 35.Salinas S, Schiavo G, Kremer EJ. A hitchhiker’s guide to the nervous system: the complex journey of viruses and toxins. Nat Rev Microbiol. 2010;8:645–55. doi: 10.1038/nrmicro2395. [DOI] [PubMed] [Google Scholar]
- 36.Swaminathan S. Molecular structures and functional relationships in clostridial neurotoxins. FEBS J. 2011;278:4467–85. doi: 10.1111/j.1742-4658.2011.08183.x. [DOI] [PubMed] [Google Scholar]
- 37.Fishman PS, Carrigan DR. Motoneuron uptake from the circulation of the binding fragment of tetanus toxin. Arch Neurol. 1988;45:558–61. doi: 10.1001/archneur.1988.00520290094020. [DOI] [PubMed] [Google Scholar]
- 38.Chen C, Fu Z, Kim JJ, Barbieri JT, Baldwin MR. Gangliosides as high affinity receptors for tetanus neurotoxin. J Biol Chem. 2009;284:26569–77. doi: 10.1074/jbc.M109.027391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Deinhardt K, Salinas S, Verastegui C, et al. Rab5 and Rab7 control endocytic sorting along the axonal retrograde transport pathway. Neuron. 2006;52:293–305. doi: 10.1016/j.neuron.2006.08.018. [DOI] [PubMed] [Google Scholar]
- 40.Pirazzini M, Rossetto O, Bertasio C, et al. Time course and temperature dependence of the membrane translocation of tetanus and botulinum neurotoxins C and D in neurons. Biochem Biophys Res Commun. 2013;430:38–42. doi: 10.1016/j.bbrc.2012.11.048. [DOI] [PubMed] [Google Scholar]
- 41.Dey AC, Saha L, Shahidullah M. Risk factors, morbidity and mortality of neonatal tetanus. Mymensingh Med J. 2011;20:54–58. [PubMed] [Google Scholar]
- 42.Chalya PL, Mabula JB, Dass RM, Mbelenge N, Mshana SE, Gilyoma JM. Ten-year experiences with tetanus at a tertiary hospital in northwestern Tanzania: a retrospective review of 102 cases. World J Emerg Surg. 2011;6:20. doi: 10.1186/1749-7922-6-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Feroz AHM, Rahman MH. A ten-year retrospective study of tetanus at a teaching hospital in Bangladesh. J Bangladesh Coll Phys Surg. 2007;25:62–69. [Google Scholar]
- 44.Thwaites CL, Yen LM, Glover C, et al. Predicting the clinical outcome of tetanus: the tetanus severity score. Trop Med Int Health. 2006;11:279–87. doi: 10.1111/j.1365-3156.2006.01562.x. [DOI] [PubMed] [Google Scholar]
- 45.Thwaites CL, Yen LM, Nga NT, et al. Impact of improved vaccination programme and intensive care facilities on incidence and outcome of tetanus in southern Vietnam, 1993–2002. Trans R Soc Trop Med Hyg. 2004;98:671–77. doi: 10.1016/j.trstmh.2004.01.008. [DOI] [PubMed] [Google Scholar]
- 46.Manga NM, Dia NM, Ndour CT, et al. Maternal tetanus in Dakar from 2000 to 2007. Med Trop (Mars) 2010;70:97–98. (in French) [PubMed] [Google Scholar]
- 47.Wilson TJ, Orringer DA, Sullivan SE, Patil PG. An L-2 burst fracture and cauda equina syndrome due to tetanus. J Neurosurg Spine. 2012;16:82–85. doi: 10.3171/2011.7.SPINE11335. [DOI] [PubMed] [Google Scholar]
- 48.Davis PR, Rowland HA. Vertebral fractures in west Africans suffering from tetanus: a clinical and osteological study. J Bone Joint Surg Br. 1965;47:61–71. [PubMed] [Google Scholar]
- 49.Sykora M, Diedler J, Veltkamp R, Steiner T. Autonomic impairment in tetanus: delayed baroreflex involvement. J Neurol Sci. 2008;270:201–04. doi: 10.1016/j.jns.2008.02.008. [DOI] [PubMed] [Google Scholar]
- 50.Thwaites CL, Yen LM, Cordon SM, et al. Urinary catecholamine excretion in tetanus. Anaesthesia. 2006;61:355–59. doi: 10.1111/j.1365-2044.2006.04580.x. [DOI] [PubMed] [Google Scholar]
- 51.Pomara C, Neri M, Riezzo I, Turillazzi E, Fineschi V. Autonomic nervous system instability, tetanic necrosis of the heart and myocardial TNFalpha expression in a tetanus fatal case. Int J Cardiol. 2009;136:e54–57. doi: 10.1016/j.ijcard.2008.05.007. [DOI] [PubMed] [Google Scholar]
- 52.Soumaré M, Seydi M, Ndour CT, Diack KC, Diop BM, Kane A. Cardiovascular events in the course of tetanus: a prospective study on 30 cases in the infectious diseases clinic, in the Fann teaching hospital, Dakar. Med Mal Infect. 2005;35:450–54. doi: 10.1016/j.medmal.2005.09.005. (in French) [DOI] [PubMed] [Google Scholar]
- 53.Marulappa VG, Manjunath R, Mahesh Babu N, Maligegowda L. A ten year retrospective study on adult tetanus at the Epidemic Disease (ED) Hospital, Mysore in southern India: a review of 512 cases. J Clin Diagn Res. 2012;6:1377–80. doi: 10.7860/JCDR/2012/4137.2363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Adekanle O, Ayodeji O, Olatunde L. Tetanus in a rural setting of south-western Nigeria: a ten-year retrospective study. Libyan J Med. 2009;4:78–80. doi: 10.4176/081125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Amare A, Yami A. Case-fatality of adult tetanus at Jimma University Teaching Hospital, southwest Ethiopia. Afr Health Sci. 2011;11:36–40. [PMC free article] [PubMed] [Google Scholar]
- 56.Muteya MM, Kabey AK, Lubanga TM, Tshamba HM, Nkoy AM. Prognosis of tetanus patients in the intensive care unit of Provincial Hospital Jason Sendwe, Lubumbashi, DR Congo. Pan Afr Med J. 2013;14:93. doi: 10.11604/pamj.2013.14.93.2180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Ertem M, Cakmak A, Saka G, Ceylan A. Neonatal tetanus in the south-eastern region of Turkey: changes in prognostic aspects by better health care. J Trop Pediatr. 2004;50:297–300. doi: 10.1093/tropej/50.5.297. [DOI] [PubMed] [Google Scholar]
- 58.Basu S, Paul DK, Ganguly S, Chandra PK. Risk factors for mortality from neonatal tetanus: 7 years experience in north Bengal, India. Ann Trop Paediatr. 2006;26:233–39. doi: 10.1179/146532806X120336. [DOI] [PubMed] [Google Scholar]
- 59.Mwaniki MK, Gatakaa HW, Mturi FN, et al. An increase in the burden of neonatal admissions to a rural district hospital in Kenya over 19 years. BMC Public Health. 2010;10:591. doi: 10.1186/1471-2458-10-591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Fetuga BM, Ogunlesi TA, Adekanmbi FA. Risk factors for mortality in neonatal tetanus: a 15-year experience in Sagamu, Nigeria. World J Pediatr. 2010;6:71–75. doi: 10.1007/s12519-010-0010-9. [DOI] [PubMed] [Google Scholar]
- 61.Amar-Singh HS. Neonatal tetanus in Malaysia. Med J Malaysia. 2009;64:1–2. [PubMed] [Google Scholar]
- 62.Mchil Ugwu GI. Neonatal tetanus in Warri Niger Delta: a ten year retrospective study. Cont J Med Res. 2010;4:3–7. [Google Scholar]
- 63.Peterside O, Duru CO, George B. Neonatal tetanus at the Niger Delta University Teaching Hospital: a 5 year retrospective study. Int J Pediatr Neonatol. 2012;14 [Google Scholar]
- 64.Mishra K, Basu S, Kumar D, Dutta AK, Kumar P, Rath B. Tetanus - still a scourge in the 21st century: a paediatric hospital-based study in India. Trop Doct. 2012;42:157–59. doi: 10.1258/td.2012.110445. [DOI] [PubMed] [Google Scholar]
- 65.Jeena PM, Coovadia HM, Gouws E. Risk factors for neonatal tetanus in KwaZulu-Natal. S Afr Med J. 1997;87:46–48. [PubMed] [Google Scholar]
- 66.Brauner JS, Vieira SR, Bleck TP. Changes in severe accidental tetanus mortality in the ICU during two decades in Brazil. Intensive Care Med. 2002;28:930–35. doi: 10.1007/s00134-002-1332-4. [DOI] [PubMed] [Google Scholar]
- 67.Pornchai S, Chutarat S, Kitti L, Suwanna S, Kanitpong P. Tetanus: a retrospective study of clinical presentations and outcomes in a medical teaching hospital. J Med Assoc Thai. 2009;92:315–19. [PubMed] [Google Scholar]
- 68.Gibson K, Bonaventure Uwineza J, Kiviri W, Parlow J. Tetanus in developing countries: a case series and review. Can J Anaesth. 2009;56:307–15. doi: 10.1007/s12630-009-9058-1. [DOI] [PubMed] [Google Scholar]
- 69.Lambo JA, Anokye EA. Prognostic factors for mortality in neonatal tetanus: a systematic review and meta-analysis. Int J Infect Dis. 2013;17:e1100–10. doi: 10.1016/j.ijid.2013.05.016. [DOI] [PubMed] [Google Scholar]
- 70.Karanikolas M, Velissaris D, Marangos M, Karamouzos V, Fligou F, Filos KS. Prolonged high-dose intravenous magnesium therapy for severe tetanus in the intensive care unit: a case series. J Med Case Reports. 2010;4:100. doi: 10.1186/1752-1947-4-100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Attygalle D, Rodrigo N. Magnesium as first line therapy in the management of tetanus: a prospective study of 40 patients. Anaesthesia. 2002;57:811–17. doi: 10.1046/j.1365-2044.2002.02698_6.x. [DOI] [PubMed] [Google Scholar]
- 72.Thwaites CL, Yen LM, Loan HT, et al. Magnesium sulphate for treatment of severe tetanus: a randomised controlled trial. Lancet. 2006;368:1436–43. doi: 10.1016/S0140-6736(06)69444-0. [DOI] [PubMed] [Google Scholar]
- 73.Barlow JL, Mung’Ala-Odera V, Gona J, Newton CR. Brain damage after neonatal tetanus in a rural Kenyan hospital. Trop Med Int Health. 2001;6:305–08. doi: 10.1046/j.1365-3156.2001.00705.x. [DOI] [PubMed] [Google Scholar]
- 74.Campbell JI, Lam TM, Huynh TL, et al. Microbiologic characterization and antimicrobial susceptibility of Clostridium tetani isolated from wounds of patients with clinically diagnosed tetanus. Am J Trop Med Hyg. 2009;80:827–31. [PubMed] [Google Scholar]
- 75.Public Health England. Tetanus: information for health professionals. [accessed July 4, 2014]; http://www.hpa.org.uk/webc/HPAwebFile/HPAweb_C/1194947374762.
- 76.Borrow R, Balmer P, Roper MH. Geneva: World Health Organization; 2006. The immunological basis for immunization series. Module 3: tetanus update 2006. [Google Scholar]
- 77.Nagao K, Mori T, Sawada C, Sasakawa C, Kanezaki Y. Detection of the tetanus toxin gene by polymerase chain reaction: a case study. Jpn J Infect Dis. 2007;60:149–50. [PubMed] [Google Scholar]
- 78.Ali G, Kamal M, Khan AN. Comparison of the efficacy of magnesium sulphate and diazepam in the control of tetanus spasm. J Postgrad Med Inst. 2011;25:106–110. [Google Scholar]
- 79.Osalusi BS, Ogun SA, Ogunniyi A, Kolapo KO. Comparison of the efficacy of magnessium sulphate and diazepam in the control of tetanus spasms. Sci Res Essays. 2008;3:571–76. [Google Scholar]
- 80.Wateba M, Diop S, Nichols S, et al. Intrathecal therapy with 1 500 UI of antitetanic serum and 1.5 g of intravenous metronidazole: prognosis of tetanus in hospitalized patients in Togo. Santé. 2008;18:125–29. doi: 10.1684/san.2008.0115. (in French) [DOI] [PubMed] [Google Scholar]
- 81.Ahmad A, Qaisar I, Naeem M, Mazhar AU, Ashfaq M. Intrathecal anti-tetanus human immunoglobulin in the treatment of neonatal tetanus. J Coll Physicians Surg Pak. 2011;21:539–41. [PubMed] [Google Scholar]
- 82.Hemilä H, Koivula T. Vitamin C for preventing and treating tetanus. 2013;11:CD006665. doi: 10.1002/14651858.CD006665.pub3. [DOI] [PubMed] [Google Scholar]
- 83.Rodrigo C, Samarakoon L, Fernando SD, Rajapakse S. A meta-analysis of magnesium for tetanus. Anaesthesia. 2012;67:1370–74. doi: 10.1111/anae.12020. [DOI] [PubMed] [Google Scholar]
- 84.Sheffield JS, Ramin SM. Tetanus in pregnancy. Am J Perinatol. 2004;21:173–82. doi: 10.1055/s-2004-828605. [DOI] [PubMed] [Google Scholar]
- 85.Okoromah CN, Lesi FE. Diazepam for treating tetanus. Cochrane Database Syst Rev. 2004;1:CD003954. doi: 10.1002/14651858.CD003954.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Newton C. Tetanus. In: Mabey D, Perry G, Weber M, Whitty CJM, editors. Principles of medicine in Africa. 4th edn. Cambridge: Cambridge University Press; 2013. pp. 345–50. [Google Scholar]
- 87.Blake PA, Feldman RA, Buchanan TM, Brooks GF, Bennett JV. Serologic therapy of tetanus in the United States, 1965–1971. JAMA. 1976;235:42–44. [PubMed] [Google Scholar]
- 88.Centers for Disease Control and Prevention. Tetanus. In: Atkinson W, Hamborsky J, Stanton A, Wolfe CS, editors. Epidemiology and prevention of vaccine-preventable diseases. Washington DC: Public Health Foundation; 1997. pp. 291–300. [Google Scholar]
- 89.Nation NS, Pierce NF, Adler SJ, Chinnock RF, Wehrle PF. Tetanus; the use of human hyperimmune globulin in treatment. Calif Med. 1963;98:305–07. [PMC free article] [PubMed] [Google Scholar]
- 90.Kabura L, Ilibagiza D, Menten J, Van den Ende J. Intrathecal vs. intramuscular administration of human antitetanus immunoglobulin or equine tetanus antitoxin in the treatment of tetanus: a meta-analysis. Trop Med Int Health. 2006;11:1075–81. doi: 10.1111/j.1365-3156.2006.01659.x. [DOI] [PubMed] [Google Scholar]
- 91.Miranda-Filho Dde B, Ximenes RA, Barone AA, Vaz VL, Vieira AG, Albuquerque VM. Randomised controlled trial of tetanus treatment with antitetanus immunoglobulin by the intrathecal or intramuscular route. BMJ. 2004;328:615. doi: 10.1136/bmj.38027.560347.7C. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Miranda-Filho DB, Ximenes RA, Siqueira-Filha NT, Santos AC. Incremental costs of treating tetanus with intrathecal antitetanus immunoglobulin. Trop Med Int Health. 2013;18:555–63. doi: 10.1111/tmi.12091. [DOI] [PubMed] [Google Scholar]
- 93.Robert R, Rouffineau J, Cremault A, et al. Reversible paraplegia following intrathecal injection of high doses of human gammaglobulins in the treatment of low-grade tetanus. 4 cases. Presse Med. 1984;13:1947–49. (in French) [PubMed] [Google Scholar]
- 94.Mathew PJ, Samra T, Wig J. Magnesium sulphate for treatment of tetanus in adults. Anaesth Intensive Care. 2010;38:185–89. doi: 10.1177/0310057X1003800128. [DOI] [PubMed] [Google Scholar]
- 95.WHO. Making pregnancy safer: the critical role of the skilled attendant: a joint statement by WHO, ICM and FIGO. Geneva: WHO; 2004. [Google Scholar]
- 96.WHO. WHO recommended surveillance standards. Second edition. Geneva: World Health Organization; [Google Scholar]
- 97.WHO. Weekly Epidemiological Record. Wkly Epidemiol Rec. 2006;81:197–208. [Google Scholar]
- 98.Bonetti TC, Succi RC, Weckx LY, Tavares-Lopes L, de Moraes-Pinto MI. Tetanus and diphtheria antibodies and response to a booster dose in Brazilian HIV-1-infected women. Vaccine. 2004;22:3707–12. doi: 10.1016/j.vaccine.2004.03.023. [DOI] [PubMed] [Google Scholar]
- 99.Dieye TN, Sow PS, Simonart T, et al. Immunologic and virologic response after tetanus toxoid booster among HIV-1- and HIV-2-infected Senegalese individuals. Vaccine. 2001;20:905–13. doi: 10.1016/s0264-410x(01)00383-8. [DOI] [PubMed] [Google Scholar]
- 100.de Moraes-Pinto MI, Almeida AC, Kenj G, et al. Placental transfer and maternally acquired neonatal IgG immunity in human immunodeficiency virus infection. J Infect Dis. 1996;173:1077–84. doi: 10.1093/infdis/173.5.1077. [DOI] [PubMed] [Google Scholar]
- 101.de Moraes-Pinto MI, Verhoeff F, Chimsuku L, et al. Placental antibody transfer: influence of maternal HIV infection and placental malaria. Arch Dis Child Fetal Neonatal Ed. 1998;79:F202–05. doi: 10.1136/fn.79.3.f202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Okoko B, Wesuperuma L, Ota MO, et al. Influence of placental malaria infection and maternal hypergammaglobulinaemia on materno-foetal transfer of measles and tetanus antibodies in a rural west African population. J Health Popul Nutr. 2001;19:59–65. [PubMed] [Google Scholar]
- 103.Cumberland P, Shulman CE, Maple PA, et al. Maternal HIV infection and placental malaria reduce transplacental antibody transfer and tetanus antibody levels in newborns in Kenya. J Infect Dis. 2007;196:550–57. doi: 10.1086/519845. [DOI] [PubMed] [Google Scholar]
- 104.Blencowe H, Cousens S, Mullany LC, et al. Clean birth and postnatal care practices to reduce neonatal deaths from sepsis and tetanus: a systematic review and Delphi estimation of mortality effect. BMC Public Health. 2011;11(suppl 3):S11. doi: 10.1186/1471-2458-11-S3-S11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.World Health Organization. Essential newborn care: report of a technical working group. [accessed July 14, 2014];1994 Available: http://helid.digicollection.org/es/d/Js2892e.
- 106.Raza SA, Avan BI. Disposable clean delivery kits and prevention of neonatal tetanus in the presence of skilled birth attendants. Int J Gynaecol Obstet. 2013;120:148–51. doi: 10.1016/j.ijgo.2012.07.030. [DOI] [PubMed] [Google Scholar]