Abstract
Objective/Background
Biomarker(s) for prediction of the future progression rate of abdominal aortic aneurysms (AAA) may be useful to stratify the management of individual patients. AAAs are associated with features of systemic inflammation and endothelial dysfunction. Flow mediated dilatation (FMD) of the brachial artery is a recognised non-invasive measurement for endothelial function. We hypothesised that FMD is a potential biomarker of AAA progression and reflects the temporal changes of endothelial function during AAA progression.
Methods
In a prospectively recruited cohort of patients with AAAs (Oxford Abdominal Aortic Aneurysm Study), AAA size was recorded by antero-posterior diameter (APD) (outer to outer) on ultrasound. Annual AAA progression was calculated by (ΔAPD/APD at baseline)/(number of days lapsed/365 days). FMD was assessed at the same time as AAA size measurement. Analyses of data were performed in the overall cohort, and further in subgroups of AAA by size (small: 30–39 mm; moderate: 40–55 mm; large: > 55 mm).
Results
FMD is inversely correlated with the diameter of AAAs in all patients (n = 162, Spearman’s r = −.28, p < .001). FMD is inversely correlated with AAA diameter progression in the future 12 months (Spearman’s r = −.35, p = .001), particularly in the moderate size group. Furthermore, FMD deteriorates during the course of AAA surveillance (from a median of 2.0% at baseline to 1.2% at follow-up; p = .004), while surgical repair of AAAs (n = 50 [open repair n = 22, endovascular repair n = 28)] leads to an improvement in FMD (from 1.1% pre-operatively to 3.8% post-operatively; p < .001), irrespective of the type of surgery.
Conclusion
FMD is inversely correlated with future AAA progression in humans. FMD deteriorates during the natural history of AAA, and is improved by surgery. The utility of FMD as a potential biomarker in the context of AAA warrants further investigation.
Keywords: Abdominal aortic aneurysm, Biomarkers, Flow mediated dilatation, AAA Progression
Introduction
Abdominal aortic aneurysms (AAAs) can result in rupture and death if left untreated. Existing international guidelines recommend a threshold of 55 mm for elective surgical repair in men. In clinical practice, patients with AAAs < 55 mm are typically kept under surveillance by regular ultrasound until the AAA expands above the threshold.1 However, the landmark clinical trials that led to the establishment of these guidelines also demonstrated the progression rate of AAAs vary between individuals, and the majority of patients (~70%) with an initial AAA diameter < 55 mm will progress and require surgery within 5 years.2,3 Biomarkers of future AAA progression can improve clinical management. Those with AAAs that will be fast growing may benefit from surgery earlier than the existing 55 mm threshold. However, such a biomarker is, as yet, not available.
AAAs are associated with features of systemic inflammation and endothelial dysfunction.4–6 Endothelial dependent vasomotion has been widely used as a surrogate marker of endothelial function. Flow mediated dilatation (FMD) was first reported by Celermajer et al. to be a repeatable, non-invasive physiological assessment for the quantification of systemic endothelial function.7 It has since become a widely recognised and applied method for the assessment of endothelial function.8
FMD is measured following the generation of a vasodilatory stimulus of a distal vascular bed, most commonly the forearm.8 In practice this involves the application of a blood pressure cuff on the forearm, which is then inflated to supra-peak systolic pressure to generate a short period of blood flow occlusion. The brachial artery is imaged continuously using ultrasound to detect any changes in arterial diameter induced by shear stress from changes in blood flow. This test is frequently coupled with the pharmacological supplementation of nitroglycerin to assess the endothelium independent vasodilatory response, which serves as a comparator for FMD mediated through the endogenous release of nitric oxide.9
Since the first report by Knipp et al., which showed impaired FMD in patients with AAA compared with healthy volunteers or those with peripheral occlusive arterial disease,6 two other small case series (n ≤ 66) further reported the inverse association between FMD and the diameter of AAAs.4,5 Further, it has been shown that vascular intervention for occlusive arterial disease, either in the form of endovascular intervention or surgical bypass,10,11 can lead to significant improvements in brachial artery FMD. In the broader literature for cardiovascular disease, endothelial dysfunction has also been shown to correlate with the severity of atherosclerotic arterial disease, and is a potentially reversible risk factor.12 However, no study has yet examined the changes of FMD during the course of AAA progression, or the effect of operative intervention on FMD.
We investigated the role of FMD as a potential biomarker of AAA by assessments of FMD during the natural history in individuals with AAAs.
Methods
Participant cohort
The Oxford Abdominal Aortic Aneurysm (OxAAA) study is designed to investigate longitudinally the natural history of AAAs. The study received full regulatory and ethics approval from the Oxford University and Oxford University Hospitals (OUH) National Health Service (NHS) Foundation Trust (Ethics Ref: 13/SC/0250). Every participant provided written consent. Participants were recruited at the John Radcliffe Hospital, which is part of the OUH NHS Foundation Trust.
We recruited patients with infrarenal AAAs (aortic diameter ≥ 30 mm) under surveillance and those that had an incidentally diagnosed AAA requiring surgery. To capture a study cohort that reflects the characteristics of modern day patients with AAA, we only excluded patients with active neoplasms, renal failure on dialysis, or where long-term participation in the study would not be reasonable (e.g., unwillingness to return to OUH for follow-up assessments, or the inability to tolerate the FMD protocol). Participants in the surveillance group were approached after being identified through the OUH regional vascular unit surveillance database by the clinical team, followed by an invitation to take part in the study. If patients chose to participate, their study visits were synchronised with their regularly scheduled surveillance appointments in order to minimise their inconvenience.
Participants in the operative stream were typically assessed at the time of pre-operative assessment or during their hospital admission for surgery. This stream also included patients who were part of the surveillance programme and progressed through to the surgical threshold. After surgery, patients routinely returned for a post-operative assessment in the outpatient clinic between 8 and 12 weeks, when the research assessment was also repeated.
For each study assessment, the participant was required to fast for 6 hours before the study (except their regular medications) and to refrain from caffeinated beverages and cigarette smoking for 24 hours prior to their visits. Demographic data were collected at the first research appointment and changes in medications and comorbidities were recorded at each subsequent appointment. Clinical blood tests were performed by the OUH biochemistry laboratory.
AAA size and subgroups
For each participant, AAA size was obtained as the clinical test by the NHS AAA surveillance, performed by a certified vascular technologist in the vascular laboratory. AAA size was defined by the maximum antero-posterior (AP) diameter (outer to outer). For this study, an AAA was defined by AP diameter > 30 mm. Further subgroups were defined according to the AP diameter (APD) into small (30–39 mm), moderate (40–55 mm), and large (> 55 cm) AAAs. The annual growth rate of AAA during surveillance was calculated by: (ΔAPD/APD at baseline)/(number of days lapsed/365 days).
For the analyses pertaining to future growth rate, we focused on those with AAAs < 55 mm at the time of recruitment. We reasoned that beyond this point, biomarkers of future AAA progression should have little impact on existing clinical practice: those with large AAAs (> 55 mm) should have been referred for surgery promptly, and those who do not undergo surgery beyond this size are likely to be affected by other confounding factors. The distinction was set between the subgroup of small and moderate size AAAs, as the previous clinical trials on early surgery versus surveillance for AAAs used 40–55 mm as the size criteria for inclusion.13 On the other hand, patients with small AAAs (30–39 mm) are unlikely to be offered surgery, even in a clinical trial setting.
Ultrasound image acquisition and analyses
The research appointments for each patient took place on the same visit as the NHS AAA surveillance scan, during daytime hours. Ultrasound imaging of the brachial artery was then performed (CX50, L12-3 probe; Phillips, Eindhoven, The Netherlands) to record flow mediated and nitroglycerin mediated responses according to an established protocol.9 For detailed descriptions of the FMD procedure and analysis please see Appendix S1 (Supplementary Material). Briefly, the procedure was performed in a dimly lit, quiet room, with the patient in the supine position. Electrocardiogram (ECG) tabs were applied to enable ECG gated acquisition of images. The brachial artery in the right arm was identified by ultrasound and the baseline measurement obtained. Supra-systolic pressure was applied to the forearm via the blood pressure cuff for 5 minutes to achieve blood flow occlusion. The diameter of the brachial artery was recorded at specific time points during flow occlusion and after the release of the blood pressure cuff to assess flow mediated changes in diameter. For the assessment of nitroglycerin mediated dilatation (NMD), 400 μg of glyceryl trinitrate was administered sublingually at the end of the FMD protocol followed by further recording of the brachial artery diameter.
Analysis of flow mediated dilatation (FMD) or constriction (FMC) were performed by assessors blinded to the clinical characteristics of each patient using the Vascular Research Tool (Version 6.7.4; Medical Imaging Applications, Coralville, IA, USA) with adherence to standardised operating procedures. The percentage change in FMD (FMD%) or FMC (FMC%) and NMD (NMD%) was calculated by: (Δbrachial artery diameter before and after flow stimuli/brachial artery diameter at baseline). For the calculation of FMD% and NMD%, the maximum diameter of brachial artery during the respective measurement period was used. For the calculation of FMC%, the minimum diameter of the brachial artery was used.
Reproducibility of the FMD analysis was assessed by repeated scans by two different assessors on 10 healthy individuals under the same conditions, with a 30 minute rest between scans. Intra-observer variation was assessed by re-analyses of randomly selected scans by the same assessor. The coefficient of reproducibility and intra-observer variation for FMD analysis were 5% and 6%, respectively. Our experience is consistent with the published data, which demonstrate the acceptable reproducibility of FMD measurements for short- and medium-term evaluations of FMD.14
Statistical analysis
Statistical analyses were performed in GraphPad Prism Version 6.01 (GraphPad Inc., La Jolla, CA, USA). Exploratory data analysis was performed for the initial examination of the dataset. Summary statistics are presented as mean ± SD or median (interquartile range [IQR]), depending on the normality of distribution. We opted to use nonparametric tests for all comparative analyses (Wilcoxon matched pairs signed rank test [WMPST], Mann–Whitney test, Kruskal–Wallis test, and Spearman rank correlation), as many variables demonstrated non-Gaussian distributions. No transformation of data was performed.
Results
Participant characteristics
From October 2013, 162 patients with AAAs were recruited (147 men, 15 women). Median AAA diameter was 50 mm (IQR 40–57 mm). The mean ± SD age of participants was 75 ± 7 years at the time of consent. The majority were ex-smokers (66%) and 19% were current smokers. A history of symptomatic atherosclerotic arterial disease was prevalent in this group (40% ischaemic heart disease; 20% peripheral arterial disease; 12% cerebral vascular disease). The majority of participants reported a prior diagnosis of arterial hypertension (65%) and hypercholesterolaemia (59%). However, these were well controlled by long-term medical therapy (antihypertensive(s): 71%; statin: 75%; antiplatelet(s): 62%), as reflected by their controlled systolic blood pressure/diastolic blood pressure (138/79 ± 17/12 mmHg) and overall normal cholesterol profiles (median 3.9 mmol/L [IQR 3.3–4.7 mmol/L], < 5.2 mmol/L in 82% of participants) at the time of recruitment. Seventeen per cent of the participants reported a history of diabetes mellitus, 19% had chronic respiratory disease, 19% had treated neoplasms, and 24% had chronic kidney disease with an estimated glomerular filtration rate of < 60 (Table 1). There was no correlation between FMD at baseline with any demographic variables except the AP diameter of AAA (Table S1; Supplementary Material).
Table 1.
Summary of characteristics of participants at the baseline assessment.
| All participants | AAA (30–39 mm) | AAA (40–55 mm) | AAA (> 55 mm) | Comparison between subgroups (p) | |
|---|---|---|---|---|---|
| Male (n) | 162 (147) | 40 (36) | 72 (62) | 50 (49) | .08 |
| Mean ± SD age at consent (y) | 75 ± 7 | 73 ± 8 | 76 ± 7 | 75 ± 8 | .22 |
| Median (IQR) AAA size (mm) | 50 (40–57) | 35 (32–37) | 49 (43–52) | 61 (58–67) | < .001 |
| Mean ± SD height (m) | 1.73 ± 0.08 | 1.74 ± 0.08 | 1.74 ± 0.09 | 1.71 ± 0.07 | .72 |
| Mean ± SD weight (kg) | 83 ± 15 | 83 ± 15 | 84 ± 14 | 81 ± 17 | .40 |
| Median (IQR) BMI | 27 (24–30) | 26 (24–31) | 28 (25–31) | 26 (24–29) | .32 |
| Mean ± SD blood pressure (SBP/DBP) | 138/79 ± 17/12 | 139/77 ± 16/13 | 141/80 ± 18/12 | 134/79 ± 18/12 | .33/.28 |
| Smoking status, n (%) | |||||
| Current smoker | 30 (19) | 6 (15) | 13 (18) | 11 (22) | .39 |
| Past history of smoking (> 1 mo) | 106 (65) | 28 (70) | 48 (67) | 30 (60) | .31 |
| Never smoked | 26 (16) | 6 (15) | 11 (15) | 9 (18) | .69 |
| History of ischaemic heart disease, n (%) | 64 (40) | 16 (40) | 28 (39) | 20 (40) | .95 |
| MI/ACS | 50 (31) | 14 (35) | 23 (32) | 13 (26) | .35 |
| Stable angina | 29 (18) | 9 (23) | 13 (18) | 7 (14) | .30 |
| Coronary intervention/bypass | 48 (30) | 14 (35) | 21 (29) | 13 (26) | .36 |
| History of PAD, n (%) | 32 (20) | 7 (18) | 17 (24) | 8 (16) | .79 |
| History of cerebral arterial disease | 20 (12) | 6 (15) | 8 (11) | 6 (12) | .69 |
| History of hypertension | 106 (65) | 28 (70) | 47 (65) | 31 (62) | .26 |
| History of hypercholesterolaemia | 95 (59) | 19 (48) | 43 (60) | 33 (66) | .51 |
| Median (IQR) TC (mmol/L) | 3.9 (3.3–4.7) | 4.2 (3.6–4.8) | 3.9 (3.2–4.8) | 3.6 (3.2–4.4) | .09 |
| Median (IQR) HDL (mmol/L) | 1.1 (1.0–1.4) | 1.1 (1.0–1.5) | 1.1 (1.0–1.4) | 1.2 (0.9–1.3) | .43 |
| Median (IQR) LDL (mmol/L) | 2.0 (1.6–2.8) | 2.2 (1.8–2.8) | 2.0 (1.6–3.0) | 1.9 (1.5–2.5) | .29 |
| Median (IQR) TG (mmol/L) | 1.3 (0.9–1.8) | 1.3 (0.9–1.8) | 1.4 (0.9–1.8) | 1.2 (0.8–2.0) | .69 |
| History of DM, n (%) | 27 (17) | 3 (8) | 14 (19) | 10 (20) | .18 |
| Mean HbA1C (%) | 5.9 | 5.7 | 5.9 | 5.6 | .75 |
| Oral antihyperglycaemics | 20 (12) | 2 (5) | 10 (14) | 8 (16) | .13 |
| Insulin (n) | 1 | 0 | 0 | 1 | .21 |
| CKD (eGFR < 60), n (%) | 39 (24) | 7 (18) | 17 (24) | 15 (30) | .17 |
| Median (IQR) creatinine μmol/L | 84 (73–104) | 82 (70–101) | 80 (69–97) | 97 (77–110) | .14 |
| Chronic respiratory disease, n (%) | 31 (19) | 4 (10) | 15 (21) | 12 (24) | .10 |
| Family history of AAA, n (%) | 32 (20) | 6 (15) | 16 (22) | 10 (20) | .59 |
| History of treated neoplasms, n (%) | 30 (19) | 6 (15) | 12 (17) | 12 (24) | .28 |
| Regular medication, n (%) | |||||
| Aspirin | 94 (58) | 22 (55) | 44 (61) | 28 (56) | .97 |
| Thienopyridine/ | 18 (11) | 6 (15) | 10 (14) | 3 (6) | .17 |
| cyclopentyltriazolopyrimidine | |||||
| Oral anticoagulants | 21 (13) | 4 (10) | 7 (10) | 10 (20) | .14 |
| Statin | 121 (75) | 27 (68) | 56 (78) | 38 (76) | .39 |
| β-Blocker | 64 (40) | 14 (35) | 30 (42) | 20 (40) | .66 |
| ACE inhibitor/ARB | 99 (61) | 28 (70) | 43 (60) | 28 (56) | .18 |
| Median (IQR) CRP (mg/L) | 2.7 (1.2–7.5) | 1.8 (0.9–7.8) | 3.6 (1.2–6.8) | 2.8 (1.6–8.2) | .34 |
| Median (IQR) FMD (%) | 1.7 (0.6–3.6) | 2.7 (1.5–5.0) | 1.8 (0.5–3.4) | 1.2 (0.3–2.6) | < .001 |
Note. Data are n (%) unless otherwise indicated. AAA = abdominal aortic aneurysm; IQR = interquartile range; BMI = body mass index; SBP = systolic blood pressure; DBP = diastolic blood pressure; MI = myocardial infarction; ACS = acute coronary syndrome; PAD = peripheral arterial disease; TC = total cholesterol; HDL = high density lipoprotein; LDL = low density lipoprotein; TG = triglycerides; DM = diabetes mellitus; HbA1C = glycated haemoglobin; CKD = chronic kidney disease; eGFR = estimated glomerular filtration rate; ARB = angiotensin II receptor blocker; CRP = C-reactive protein; FMD = flow mediated dilatation.
Note. For variables that demonstrate Gaussian distribution, mean ± SD are presented. For variables that demonstrate non-Gaussian distribution, median and (interquartile range) are presented. Comparison between subgroups of abdominal aortic aneurysms (AAA) were performed using Kruskale–Wallis test for continuous variables, and chi-square test for trend for categorical data. BMI = body mass index; SBP = systolic blood pressure; DBP = diastolic blood pressure; MI = myocardial infarction; ACS = acute coronary syndrome; HDL = high density lipoprotein; LDL = low density lipoprotein; HbA1C = glycated haemoglobin; eGFR = estimated glomerular filtration rate; ACE = angiotensin converting enzyme; ARB = angiotensin II receptor blocker; FMD = flow mediated dilatation.
Correlation between FMD and AAA diameter
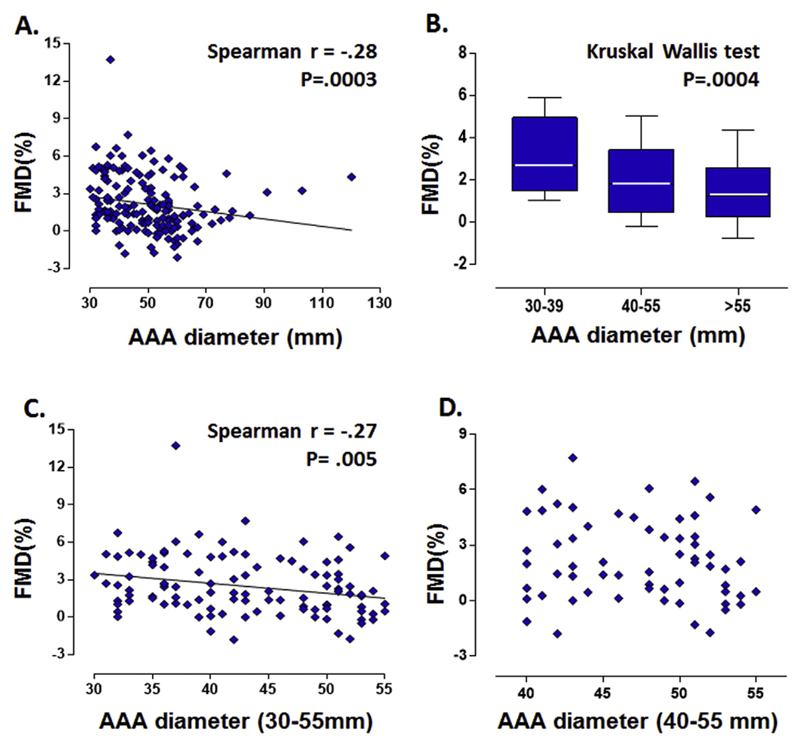
Among all participants, there was a weak significant inverse correlation between the diameter of AAA and FMD at baseline assessment (Spearman’s r = −.28, p < .001) (Fig. 1A). There was also a significant reduction of FMD across the different size groups of AAAs (small AAAs: 30–39 mm; moderate AAAs: 40–55 mm; large AAAs: > 55 mm) with the median FMD being 2.7%, 1.8%, and 1.2%, respectively (Kruskale–Wallis test p < .001) (Fig. 1B). This significant inverse correlation between AAA diameter and FMD was still present when the large AAAs (> 55 mm) were excluded from the analyses (Spearman’s r = −.27, p = .005) (Fig. 1C). However, such correlation was no longer observed within the subgroup of moderate size AAAs (40–55 mm) (Spearman’s r = −.1, p = .4) (Fig. 1D), or in the small size AAAs (30–39 mm) (Spearman’s r = .04, p = .8, data not shown). These findings highlight the importance of targeted analyses of AAA subgroups defined by relevance to clinical practice.
Figure 1.
Correlation between flow mediated dilatation (FMD) and size of abdominal aortic aneurysm (AAA). There is a significant inverse correlation between the size of AAAs and FMD of forearm brachial artery at the initial assessment. (A) The percentage change in brachial artery diameter after stimuli is presented as FMD(%) (Spearman’s r = −.28, p < .001). (B) There is also a significant reduction of FMD across the different size groups of AAAs (Kruskale–Wallis test p < .001). (C) This significant negative correlation between AAA size and FMD is still present when the large AAAs (> 55 mm) were excluded from the analyses (Spearman’s r = −.27, p = .005). (D) However, such correlation is not observed within the group of moderate sized AAAs (40–55 mm) (Spearman’s r = −.1, p = .4).
Correlations between baseline AAA diameter and future 12 month AAA growth
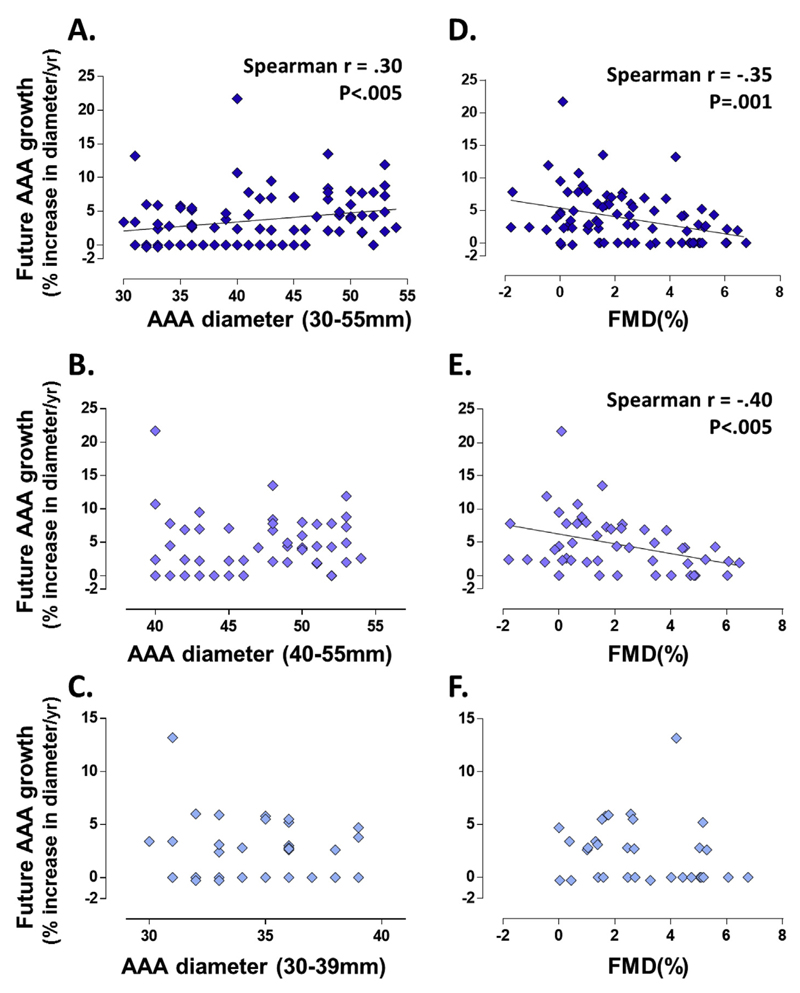
Eighty-eight of the participants with small to moderate sized AAAs (30–55 mm) under surveillance were reassessed at 12 months. The median duration of follow-up for this group was 365 days (IQR 343–381 days). The median progression rate over this period for this combined group of patients was 2.8% per year (IQR 0–5.7%/year). There was an overall significant weak correlation between the baseline diameter of AAAs and the growth rate in the future 12 months (Spearman’s r = .30, p < .005) (Fig. 2A).
Figure 2.
Correlations between baseline abdominal aortic aneurysm (AAA) diameter, flow mediated dilatation (FMD), and future 12 month AAA growth. Participants with small and moderate sized AAAs (30–55 mm) under surveillance were reassessed at 12 months. The median duration of follow-up for this group was 365 days (interquartile range [IQR] 343–381 days). Annual AAA growth was calculated by [(ΔAP diameter/antero-posterior diameter at baseline)/(number of days lapsed/365days)]. The median progression rate over this period for this group of patients was 2.8% per year (IQR 0–5.7%/year). (A) There is a significant correlation between the baseline diameter of AAAs and the growth rate in the future 12 months (Spearman’s r = .30, p < .005). (B) The correlation between baseline AAA diameter and future growth rate was no longer observed in the moderate sized AAA group (40–55 mm) (Spearman’s r = .12, p = .39). (C) Within the subgroup of small sized AAAs (30–39 mm), there was no correlation between baseline diameter and future growth rate either (Spearman’s r = −.02, p = .9). (D) During the same follow-up period, FMD at baseline correlated significantly with the future growth rate. This significant correlation was observed in both small and moderate sized AAAs (30–55 mm) (Spearman’s r = −.35, p < .005). (E) There was also a significant correlation between FMD and future 12 month growth in the moderate sized AAAs (40–55 mm) (Spearman’s r = −.40, p < .005). (F) In contrast, there was no correlation between FMD and future 12 month growth in the small AAAs (30–39 mm) (Spearman’s r = −.17, p = .34). This evidence suggests FMD to be a predictive marker for future AAA growth, particularly in the moderate sized AAAs.
When the participants were further stratified according to AAA diameter (30–39 mm, 40–55 mm), the median growth rate over the following 12 month period differed significantly between the small sized AAAs (30–39 mm) and the moderate sized AAAs (40–55 mm) (2.6% vs. 4.2%, Manne–Whitney test p < .01). Further examination of the AAA subgroups showed no correlation between baseline diameter versus the future growth rate in either the moderate sized AAAs (40–55 mm) (Spearman’s r = .12, p = .39) (Fig. 2B) or in the subgroup of small sized AAAs (30–39 mm) (Spearman’s r = −.02, p = .9) (Fig. 2C).
These observations suggest the significant correlation observed in Fig. 2A to be underpinned by the overall disparate growth rates observed between the groups of small (30–39 mm) and moderate (40–55 mm) AAAs, whereas within these subgroups of small and moderate sized AAAs the baseline diameter has no predictive value on its future growth rate. This evidence suggests that baseline diameter alone is not a useful predictor of future growth rates when patients are stratified into small and moderate size AAA subgroups.
Correlations between FMD and future AAA growth
During the same follow-up period, FMD at baseline correlated significantly with the future growth rate. This significant weak correlation was observed in both small and moderate sized AAAs (30–55 mm) (Spearman’s r = −.35, p < .005) (Fig. 2D). Within the subgroups there was also a significant and moderate correlation between FMD and future 12 month growth in the moderate sized AAAs (40–55 mm) (Spearman’s r = −.40, p < .005) (Fig. 2E). In contrast, there was no correlation between FMD and future 12 month growth in the small AAAs (30–39 mm) (Spearman’s r = −.17, p = .34) (Fig. 2F). This evidence suggests FMD may be a predictive marker for future AAA growth, particularly in the moderate sized AAAs.
FMD deteriorates during AAA progression, and is improved by AAA surgery
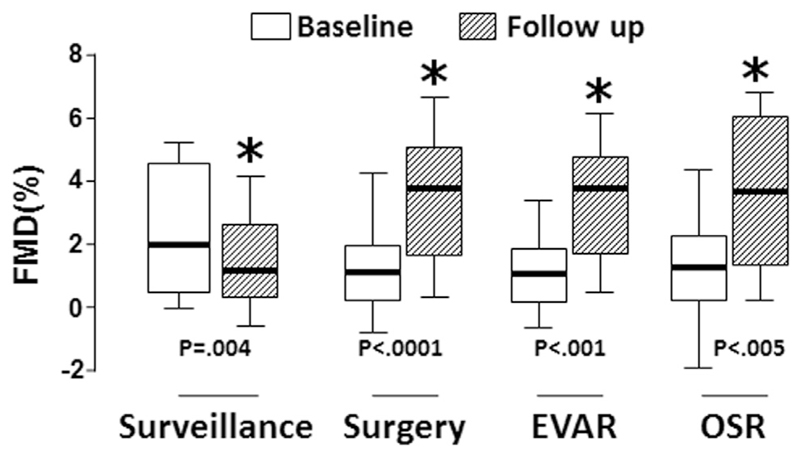
FMD reduced significantly between assessments during AAA surveillance (from a median of 2.0% at baseline to 1.2% at follow-up, WMPST p = .004). FMD was measured in 50 patients prior to AAA surgery, and re-measured at 8–12 weeks after. FMD improved significantly after surgical repair (from 1.1% pre-operatively to 3.8% post-operatively, WMPST p < .001), irrespective of the type of surgery performed (endovascular repair [EVAR] n = 28; open surgical repair [OSR] n = 22) (Fig. 3).
Figure 3.
Flow mediated dilatation (FMD) deteriorates during abdominal aortic aneurysm (AAA) progression, and is improved by AAA surgery. FMD reduced significantly between assessments during AAA surveillance (from a median of 2.0% at baseline to 1.2% at follow-up, Wilcoxon matched pairs signed rank test [WMPST] p = .004). FMD was measured in 50 patients prior to AAA surgery, and repeated 8–12 weeks afterwards. FMD improved significantly after surgical repair (from 1.1% pre-operatively to 3.8% post-operatively, WMPST p < .001), irrespective of the type of surgery performed (endovascular repair [EVAR] n = 28; open surgical repair [OSR] n = 22).
NMD and AAA progression
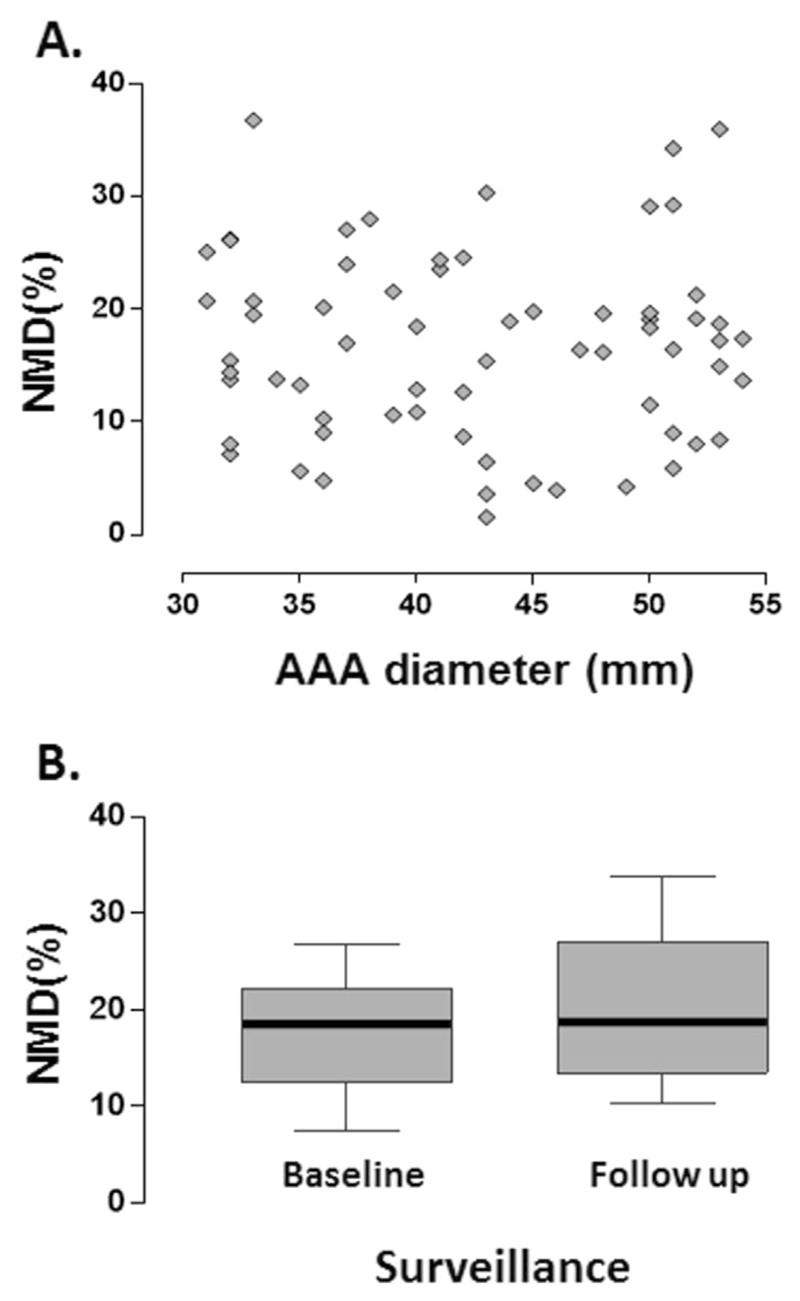
The administration of nitroglycerin can result in immediate side effects, such as hypotension and headache. In this cohort of elderly patients with medical comorbidities, these symptoms can be challenging to manage. For each study, the NMD protocol was therefore not performed when there was concern of significant hypotension after administration of nitroglycerin (resting systolic blood pressure < 100 mmHg, resting pulse rate < 60/minute) or previous reactions to nitroglycerin. Additional confirmation of consent was obtained from each participant.
NMD was performed in 76 of the participants. There was no correlation between AAA diameter and NMD (Spearman’s r = −.08, p = .49) (Fig. 4A). NMD did not deteriorate under AAA surveillance (Fig. 4B). Ethically, it was not possible to carry out the NMD protocol in most patients with large AAAs scheduled for surgery, as the side effect of hypotension in this setting could potentially have complicated the clinical management. As such, no comparison could be made for the pre- and post-operative NMD measurements.
Figure 4.
Nitroglycerin mediated dilatation (NMD) and abdominal aortic aneurysm (AAA) progression. (A) There was no correlation between AAA size and NMD. (B) NMD did not deteriorate under AAA surveillance. Ethically, the NMD protocol could not be carried out in most patients with large AAAs scheduled for surgery, as the side effect of hypotension in this setting could potentially have complicated clinical management. As such, no comparison could be made for the pre- and post-operative NMD measurements.
Low flow mediated constriction and the progression of AAA
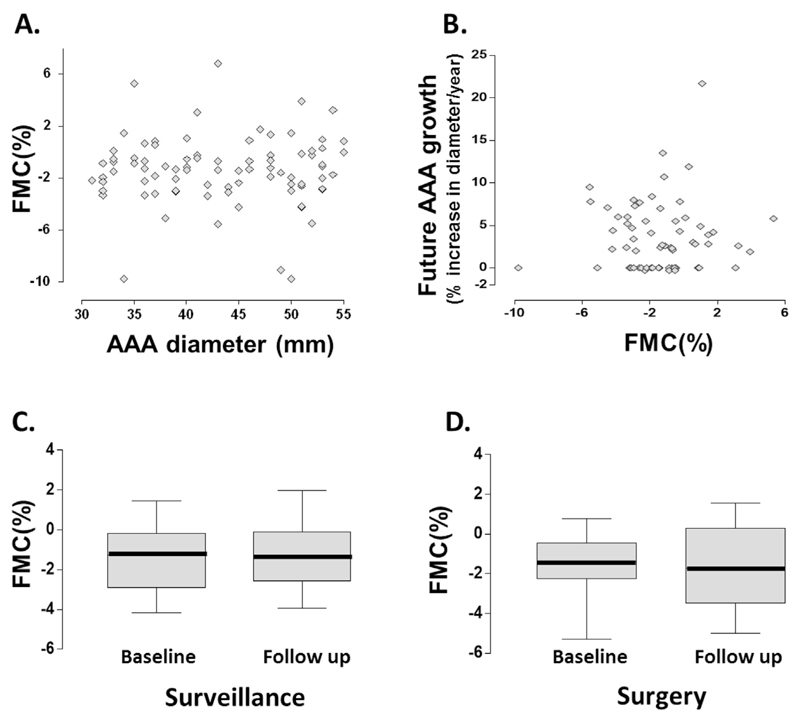
The process of occluding the brachial artery induces a low flow mediated constriction (FMC), which reflects the basal vasoconstrictive tone conferred by the release of endothelin.15 To gain mechanistic insights into the observations on FMD and AAA progression, the FMC response of brachial artery at the same time points was further examined, as an extension to the FMD protocol (n = 96). It was hypothesised that the significant changes in FMD observed were driven by the derangement in vasoconstrictive tone in these patients, thereby masking the otherwise normal FMD response. However, there was no correlation between FMC and AAA diameter (Spearman’s r = −.03, p = .74) (Fig. 5A) or future growth rate of AAA (Spearman’s r = 0.4, p = .76) (Fig. 5B). FMC did not change during the natural history of AAA progression (WMPST p = .8) (Fig. 5C), and is unaffected by AAA surgery (WMPST p = .8) (Fig. 5D).
Figure 5.
Low flow mediated constriction and the progression of abdominal aortic aneurysm (AAA). The low flow mediated constriction (FMC) response of brachial artery at the same time points was investigated, as an extension to the FMD protocol. We hypothesised that the significant changes in FMD observed were driven by the derangement in vasoconstrictive tone in these patients, thereby masking the otherwise normal FMD response. However, there was no correlation between (A) FMC and AAA size or (B) future growth rate of AAA. (C) FMC did not change during the natural history of AAA progression (Wilcoxon matched pairs signed rank test [WMPST] p = .8), and (D) is unaffected by AAA surgery (WMPST p = .8).
Discussion
In addition to being the largest prospectively recruited cohort to date that examines FMD in the context of human AAAs, this study demonstrates these novel findings: FMD correlates with future growth in AAAs; FMD deteriorates during AAA progression; FMD improves after the surgical treatment of AAAs. These findings suggest FMD could potentially be a novel biomarker of AAA progression and warrants further validation by external cohorts.
Biomarkers of AAA progression can have a significant impact on clinical practice in the era of personalised medicine. The existing surgical threshold is based on evidence generated by trials conducted more than two decades ago,16,17 which relies on size (> 55 mm) criteria in order to inform operative decisions. This approach has intrinsic shortcomings, as aneurysm size is not an absolute predictor of the risk of rupture, and the rate of AAA progression varies greatly between individuals.2,3 There is a small (~1%) annual risk of rupture in those with moderate sized AAAs (40–55 mm), and the majority of patients (~70%) with an initially small/moderate sized AAA will progress and require surgery within 5 years.2,3 This is an important consideration in ageing populations, as patients are also being diagnosed with AAAs and undergoing interventions at an increasingly old age.18 Interventions in older patients comes with an increase in peri-operative risk.19,20 Biomarker(s) of future AAA progression may therefore enable the stratification of patients who may benefit from surgery earlier than the existing size threshold.
The size of an AAA is conventionally regarded as a predictor of its subsequent rate of progression. We also observed a positive correlation between AAA diameter and future growth rate in AAAs measuring < 55 mm in diameter. However, it is important to note that the assessment of associations between “baseline AAA diameter” versus “future AAA growth” is inherently confounded by the fact that these two variables are both derivatives of AAA diameter and therefore are covariates by default. Interestingly, within the moderate sized AAA (40–55 mm) group, the diameter at baseline was no longer predictive of the future AAA growth, but FMD was predictive. In contrast, within the patients with small sized AAA (30–39 mm) neither the diameter nor FMD was predictive of future AAA growth. These findings suggest the utility of FMD as a potential biomarker of future AAA progression will be most fruitful in the 40–55 mm AAA group.
The moderate size (40–55 mm) AAA group is particularly important in terms of relevance to clinical practice. All the landmark trials that compared the efficacy of surgery for “small” AAAs have used 40 mm as the threshold for inclusion in the trials (UKSAT, ADAM, CAESAR, PIVOTAL).13 Although some of the small AAAs (< 40 mm) may also be fast growing in the subsequent year(s). We reasoned that surgeons would be unlikely to offer surgery to patients when the AAA was < 40 mm, even in the setting of a trial. The potential of FMD as a biomarker of future AAA progression in this subgroup of moderate sized (40–55 mm) AAAs is therefore particularly important. Further, within the small AAA group, only three patients who progressed to moderate sized AAAs within the 12 month follow-up period were observed, and they all started with the baseline size of 38 mm or more. In terms of clinical applicability, this evidence would support the role of FMD as an adjunctive tool in addition to diameter measurement for the monitoring of patients with moderate sized AAAs.
This is also the first time a study has demonstrated the deterioration of FMD during the natural history of AAA surveillance, which is reversible by AAA surgery, irrespective of the surgical technique. FMD is a recognised surrogate marker for systemic endothelial function.8 Given the key role of endothelial dysfunction in predisposing to atherosclerotic arterial disease and subsequent cardiovascular morbidities,21 this study provides novel insights into why patients with AAAs have higher risks of developing incident cardiovascular morbidities compared with their age and sex matched contemporaries without AAAs.22–24 The finding that surgical treatment of AAA (OSR or EVAR) results in an improvement in FMD suggests the aneurysm itself to be a nidus of stimulus causing the deterioration of FMD observed during its natural history. We hypothesise that intraluminal thrombus is the source of such biological signals, as it is present in significant volumes in most AAAs and contains biologically active cells.25,26 This hypothesis requires further investigation.
Although a deterioration of FMD has been shown to occur with the normal ageing process (at a rate of approximately 0.2% per year),27 the deterioration in FMD observed in this cohort over the course of AAA surveillance is greater than would be expected by ageing alone. In addition, the improvement of FMD demonstrated in the interventional group further strengthens the case against age related deterioration of FMD as a significant confounder. In the Framingham Heart Study, FMD was systematically measured in 2,883 participants (mean age 61 years). The mean FMD% in this cohort was 3.3 ± 3%.28 The present data suggest that AAA surgery restores FMD a level similar to their contemporaries.
There are other variables that could potentially cause a derangement in FMD, in particular the traditional cardiovascular risk factors. A body of literature indicates that medications commonly used for cardiovascular risk prevention, such as angiotensin converting enzyme inhibitors and statins, can improve endothelial function.29–31 In this cohort no association between FMD and baseline characteristics was observed. This may be owing to the patients being on established long-term optimal medical therapy for secondary risk factor prevention, evidenced by the clinically normal blood pressure and cholesterol profile as a cohort. In this study design, it cannot be ruled out that temporary medication changes during the peri-operative period (e.g., anaesthetic and analgesic agents) may have short-term effects on FMD. However, the patients are mostly discharged home within 1 week of OSR, or a couple of days after EVAR. Therefore, the effect of these peri-operative changes in medications should be temporary and should not affect the measurement of FMD taken 8–12 weeks after surgery, as the patients would have reverted to their pre-existing established medication regimens upon their discharge from the hospital.
This study also provides some mechanistic insight to the changes of FMD observed in this disease context. It is shown that this derangement in flow mediated vasodilatory response is not due to alterations in the basal vasoconstrictive state, as there was no difference in FMC in any of the comparisons. To consolidate this mechanistic observation, plasma endothelin-1 level was measured in patients who demonstrated individual improvements in FMD after AAA surgery (n = 29) by enzyme immune assay (R&D Systems, Abingdon, UK). No differences in endothelin-1 were observed after surgery (data not shown). This is consistent with the observed FMC results.
Conduct of FMD studies is not without challenges. It is imperative for every research team to perform quality assurance exercises to ensure there is good reproducibility of the study results. To achieve this, detailed standard operating procedures for data acquisition and analysis were developed and adhered to (see Appendix S2; Supplementary Material) In addition, an automated timer pre-set with a voice prompt for every step during the FMD study was devised. This tool simplified the FMD study and can be operated on any Android device (Impetus Plus App, Google Play; the FMD protocol pre-set is available upon request.)
These novel findings on FMD as a potential biomarker for AAA warrant further validation by external cohorts. It will be of value for future studies to examine how time course data on FMD (e.g., serial assessments repeated annually) relate to subsequent AAA growth in individuals. It is also important to reiterate that novel biomarkers of AAA progression are unlikely to replace the role of ultrasound scans in the detection and monitoring of AAAs. Rather, such biomarkers would serve as an adjunctive tool to individualise either the surveillance interval or the timing for surgery.
Conclusion
FMD is inversely correlated with future AAA progression in humans. FMD deteriorates during the natural history of AAA, and can be improved by surgery. These observations indicate FMD to be a potentially novel biomarker in the context of AAA, and warrant further validation.
Supplementary Material
Appendix A. Supplementary Data
Supplementary data related to this article can be found at http://dx.doi.org/10.1016/j.ejvs.2017.03.001.
What this Paper Adds.
Abdominal aortic aneurysm (AAA) is a complex disease with multifactorial contributions towards disease progression. Biomarkers for AAA progression will be useful to better inform the surveillance interval and threshold for surgery in individual patients. This study provides novel evidence to support the potential utility of flow mediated dilatation (FMD) as a biomarker in the context of AAA disease: FMD deteriorates during the natural history of AAA growth; FMD is inversely correlated with future AAA growth, and can be improved by AAA surgery. These findings warrant further investigation.
Acknowledgement
The authors acknowledge the support of the following: Professor Paul Leeson and Jonathan Diesch at Clinical Cardiovascular Research Facility, RDM Cardiovascular Medicine, University of Oxford; Medical Sciences Division, University of Oxford Medical Research Fund; Jackie Walton Vascular Studies Unit, Oxford University Hospitals NHS Foundation Trust; Oxford Regional Vascular Services Unit, Oxford University Hospitals NHS Foundation Trust; Oxford Biomedical Research Centre Research Capacity Fund; Academy of Medical Sciences UK. Contributors to the OxAAA Study: Alexios Antonopoulos, Charalambos Antoniades, Keith M Channon, Rafael Perera, Katherine Hurst, Ioan Milosevic, and the Oxford Regional Vascular Services Unit (Chris R Darby, Alison Halliday, Linda J Hands, Patrick Lintott, Tim R Magee, Andrew Northeast, Jeremy Perkins, Ediri Sideso).
Funding
None.
Footnotes
Conflict of Interest
None.
References
- 1.Chaikof EL, Brewster DC, Dalman RL, Makaroun MS, Illig KA, Sicard GA, et al. SVS practice guidelines for the care of patients with an abdominal aortic aneurysm: executive summary. J Vasc Surg. 2009;50:880–96. doi: 10.1016/j.jvs.2009.07.001. [DOI] [PubMed] [Google Scholar]
- 2.Moxon JV, Parr A, Emeto TI, Walker P, Norman PE, Golledge J. Diagnosis and monitoring of abdominal aortic aneurysm: current status and future prospects. Curr Probl Cardiol. 2010;35:512–48. doi: 10.1016/j.cpcardiol.2010.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rutherford RB. Open versus endovascular stent graft repair for abdominal aortic aneurysms: an historical view. Semin Vasc Surg. 2012;25:39–48. doi: 10.1053/j.semvascsurg.2012.03.005. [DOI] [PubMed] [Google Scholar]
- 4.Medina F, de Haro J, Florez A, Acin F. Relationship between endothelial dependent vasodilation and size of abdominal aortic aneurysms. Ann Vasc Surg. 2010;24:752–7. doi: 10.1016/j.avsg.2009.11.011. [DOI] [PubMed] [Google Scholar]
- 5.Sung SH, Wu TC, Chen JS, Chen YH, Huang PH, Lin SJ, et al. Reduced number and impaired function of circulating endothelial progenitor cells in patients with abdominal aortic aneurysm. Int J Cardiol. 2013;168:1070–7. doi: 10.1016/j.ijcard.2012.11.002. [DOI] [PubMed] [Google Scholar]
- 6.Knipp BS, Peterson DA, Rajagopalan S, Kehrer C, Ford JW, D’Alecy LG, et al. Impaired vasoreactivity despite an increase in plasma nitrite in patients with abdominal aortic aneurysms. J Vasc Surg. 2002;35:363–7. doi: 10.1067/mva.2002.121069. [DOI] [PubMed] [Google Scholar]
- 7.Celermajer DS, Sorensen KE, Gooch VM, Spiegelhalter DJ, Miller OI, Sullivan ID, et al. Non-invasive detection of endothelial dysfunction in children and adults at risk of atherosclerosis. Lancet. 1992;340:1111–5. doi: 10.1016/0140-6736(92)93147-f. [DOI] [PubMed] [Google Scholar]
- 8.Flammer AJ, Anderson T, Celermajer DS, Creager MA, Deanfield J, Ganz P, et al. The assessment of endothelial function: From research into clinical practice. Circulation. 2012;126:753–67. doi: 10.1161/CIRCULATIONAHA.112.093245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Leeson P, Thorne S, Donald A, Mullen M, Clarkson P, Deanfield J. Non-invasive measurement of endothelial function: Effect on brachial artery dilatation of graded endothelial dependent and independent stimuli. Heart. 1997;78:22–7. doi: 10.1136/hrt.78.1.22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Husmann M, Dorffler-Melly J, Kalka C, Diehm N, Baumgartner I, Silvestro A. Successful lower extremity angioplasty improves brachial artery flow-mediated dilation in patients with peripheral arterial disease. J Vasc Surg. 2008;48:1211–6. doi: 10.1016/j.jvs.2008.06.039. [DOI] [PubMed] [Google Scholar]
- 11.Unal O, Karatepe O, Ugurlucan M, Koc B, Filizcan U, Aksoy M. Effects of lower extremity revascularization on the endothelial functions measured with noninvasive brachial artery flow-mediated dilatation. Ann Vasc Surg. 2011;25:969–74. doi: 10.1016/j.avsg.2011.02.013. [DOI] [PubMed] [Google Scholar]
- 12.Bailey MA, Griffin KJ, Sohrabi S, Whalley DJ, Johnson AB, Baxter PD, et al. Plasma thrombin-antithrombin complex, prothrombin fragments 1 and 2, and d-dimer levels are elevated after endovascular but not open repair of infrarenal abdominal aortic aneurysm. J Vasc Surg. 2013;57:1512–8. doi: 10.1016/j.jvs.2012.12.030. [DOI] [PubMed] [Google Scholar]
- 13.Filardo G, Powell JT, Martinez MA, Ballard DJ. Surgery for small asymptomatic abdominal aortic aneurysms. Cochrane Database Syst Rev. 2015 doi: 10.1002/14651858.CD001835.pub4. Cd001835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Charakida M, de Groot E, Loukogeorgakis SP, Khan T, Luscher T, Kastelein JJ, et al. Variability and reproducibility of flow-mediated dilatation in a multicentre clinical trial. Eur Heart J. 2013;34:3501–7. doi: 10.1093/eurheartj/eht223. [DOI] [PubMed] [Google Scholar]
- 15.Gori T, Parker JD, Münzel T. Flow-mediated constriction: further insight into a new measure of vascular function. Eur Heart J. 2011;32:784–7. doi: 10.1093/eurheartj/ehq412. [DOI] [PubMed] [Google Scholar]
- 16.Lederle FA, Wilson SE, Johnson GR, Reinke DB, Littooy FN, Acher CW, et al. Immediate repair compared with surveillance of small abdominal aortic aneurysms. N Engl J Med. 2002;346:1437–44. doi: 10.1056/NEJMoa012573. [DOI] [PubMed] [Google Scholar]
- 17.United Kingdom Small Aneurysm Trial Participants. Long-term outcomes of immediate repair compared with surveillance of small abdominal aortic aneurysms. N Engl J Med. 2002;346:1445–52. doi: 10.1056/NEJMoa013527. [DOI] [PubMed] [Google Scholar]
- 18.Choke E, Vijaynagar B, Thompson J, Nasim A, Bown MJ, Sayers RD. Changing epidemiology of abdominal aortic aneurysms in england and wales: Older and more benign? Circulation. 2012;125:1617–25. doi: 10.1161/CIRCULATIONAHA.111.077503. [DOI] [PubMed] [Google Scholar]
- 19.Hamel MB, Henderson WG, Khuri SF, Daley J. Surgical outcomes for patients aged 80 and older: morbidity and mortality from major noncardiac surgery. J Am Geriatr Soc. 2005;53:424–9. doi: 10.1111/j.1532-5415.2005.53159.x. [DOI] [PubMed] [Google Scholar]
- 20.Hicks CW, Obeid T, Arhuidese I, Qazi U, Malas MB. Abdominal aortic aneurysm repair in octogenarians is associated with higher mortality compared with nonoctogenarians. J Vasc Surg. 2016;64:956–965.e951. doi: 10.1016/j.jvs.2016.03.440. [DOI] [PubMed] [Google Scholar]
- 21.Deanfield JE, Halcox JP, Rabelink TJ. Endothelial function and dysfunction. 2007 doi: 10.1161/CIRCULATIONAHA.106.652859. [DOI] [PubMed] [Google Scholar]
- 22.Freiberg MS, Arnold AM, Newman AB, Edwards MS, Kraemer KL, Kuller LH. Abdominal aortic aneurysms, increasing infrarenal aortic diameter, and risk of total mortality and incident cardiovascular disease events: 10-year follow-up data from the cardiovascular health study. Circulation. 2008;117:1010–7. doi: 10.1161/CIRCULATIONAHA.107.720219. [DOI] [PubMed] [Google Scholar]
- 23.Newman AB, Arnold AM, Burke GL, O’Leary DH, Manolio TA. Cardiovascular disease and mortality in older adults with small abdominal aortic aneurysms detected by ultrasonography: the cardiovascular health study. Ann Intern Med. 2001;134:182–90. doi: 10.7326/0003-4819-134-3-200102060-00008. [DOI] [PubMed] [Google Scholar]
- 24.Sohrabi S, Wheatcroft S, Barth JH, Bailey MA, Johnson A, Bridge K, et al. Cardiovascular risk in patients with small and medium abdominal aortic aneurysms, and no history of cardiovascular disease. Br J Surg. 2014;101:1238–43. doi: 10.1002/bjs.9567. [DOI] [PubMed] [Google Scholar]
- 25.Adolph R, Vorp DA, Steed DL, Webster MW, Kameneva MV, Watkins SC. Cellular content and permeability of intraluminal thrombus in abdominal aortic aneurysm. J Vasc Surg. 1997;25:916–26. doi: 10.1016/s0741-5214(97)70223-4. [DOI] [PubMed] [Google Scholar]
- 26.Cassimjee I, Lee R, Patel J. Inflammatory mediators in AAA, aortic aneurysms. InTech. 2017 doi: 10.5772/65961. [DOI] [Google Scholar]
- 27.Celermajer DS, Sorensen KE, Spiegelhalter DJ, Georgakopoulos D, Robinson J, Deanfield JE. Aging is associated with endothelial dysfunction in healthy men years before the age-related decline in women. J Am Coll Cardiol. 1994;24:471–6. doi: 10.1016/0735-1097(94)90305-0. [DOI] [PubMed] [Google Scholar]
- 28.Benjamin EJ, Larson MG, Keyes MJ, Mitchell GF, Vasan RS, Keaney JF, et al. Clinical correlates and heritability of flow-mediated dilation in the community: the Framingham heart study. Circulation. 2004;109:613–9. doi: 10.1161/01.CIR.0000112565.60887.1E. [DOI] [PubMed] [Google Scholar]
- 29.Mancini GB, Hartigan PM, Bates ER, Sedlis SP, Maron DJ, Spertus JA, et al. Angiographic disease progression and residual risk of cardiovascular events while on optimal medical therapy: Observations from the courage trial. Circ Cardiovasc Interv. 2011;4:545–52. doi: 10.1161/CIRCINTERVENTIONS.110.960062. [DOI] [PubMed] [Google Scholar]
- 30.Treasure CB, Klein JL, Weintraub WS, Talley JD, Stillabower ME, Kosinski AS, et al. Beneficial effects of cholesterol-lowering therapy on the coronary endothelium in patients with coronary artery disease. N Engl J Med. 1995;332:481–7. doi: 10.1056/NEJM199502233320801. [DOI] [PubMed] [Google Scholar]
- 31.Anderson TJ, Meredith IT, Yeung AC, Frei B, Selwyn AP, Ganz P. The effect of cholesterol-lowering and antioxidant therapy on endothelium-dependent coronary vasomotion. N Engl J Med. 1995;332:488–93. doi: 10.1056/NEJM199502233320802. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.