Abstract
Leishmania are a genus of sandfly-transmitted protozoan parasites that cause a spectrum of debilitating and often fatal diseases in humans throughout the tropics and subtropics. During the parasite life cycle, Leishmania survive and proliferate in highly hostile environments. Their survival strategies involve the formation of an elaborate and dense cell-surface glycocalyx composed of diverse stage-specific glycoconjugates that form a protective barrier. Phosphoglycans constitute the variable structural and functional domain of major cell-surface lipophosphoglycan and secreted proteophosphoglycans. In this paper, we discuss structural aspects of various phosphoglycans from Leishmania with the major emphasis on the chemical preparation of neoglycoconjugates (neoglycoproteins and neoglycolipids) based on Leishmania lipophosphoglycan structures as well as the immunological evaluation for some of them as potential anti-leishmaniasis vaccines.
Keywords: Leishmania, lipophosphoglycan, carbohydrates, neoglycoconjugates, glycoconjugate vaccines, chemical synthesis
Leishmania are a genus of sandfly-transmitted protozoan parasites that cause a spectrum of debilitating and often fatal diseases in humans throughout the tropics and subtropics. The disease (termed leishmaniasis) torments over 12 million people worldwide, making 2.4 million people disabled and causing about 60,000 deaths annually. The WHO estimations show that 350 million people are thought to be at risk from the disease [1-3]. Although leishmaniasis is most common in tropical regions, it has also been diagnosed in overseas travelers and U.S. Gulf War veterans [4] and has emerged as an opportunistic infection of HIV patients [5]. The geographical distribution and pathology of leishmaniasis varies according to the species of the parasite. For instance, Leishmania donovani causes “visceral leishmaniasis” (also known as kala azar), characterized by an enlarged liver and spleen, that is often fatal, whereas L. major and L. tropica (in the Old World) and L. mexicana species complex, consisting of L. mexicana, L. amazonensis, and L. venezuelensis mostly (in the New World) cause “cutaneous leishmaniasis” (known as oriental sore and characterized by self-limiting skin lesions) [1]. This form is the most common leishmanial disease, and is often fairly mild in comparison to the others. Widespread, chronic, non-ulcerative skin lesions resembling leprosy are indicative of “diffuse cutaneous leishmaniasis” [6, 7] that is commonly an effect of L. aethiopica (Old World) and L. mexicana species complex (New World) infection. “Mucocutaneous leishmaniasis” (or espundia) is due to L. (V.) braziliensis (Viannia subgenus) infection and most prevalent in Brazil, Bolivia, and Peru. It causes lesion in the mucous membranes, leading to devastation of tissues and extreme facial disfigurement [1, 8]. Secondary bacterial infections are common. Leishmania (L.) mexicana causes “Chiclero’s ulcer”, which can involve almost total destruction of the external ear.
The mechanism of infection [9] poses a serious challenge for therapeutic approaches. The vector for Leishmania is a sandfly of Phlebotomus (Old World) or Lutzomyia (New World) female species that inject the parasite when taking a blood meal. To initiate and propagate the infection, parasites then actively invade and reside within macrophages, the very part of the immune system that is designed to destroy them. The majority of the current anti-leishmanial drugs, such as Sb(V) based medications Pentostam and Glucantime or non-antimonial Amphotericin B, Paromomicin, and Miltefosine, seem to be inadequate due to factors such as cost, resistance, and toxic side effects [10-14]. Thus, a vaccine that would protect the human host by annihilating the parasites upon transfer from the insect vector would be welcome. Several different designs have been recently explored [15], but no generally applicable vaccine for leishmaniasis has yet come to light.
In principle, any unique feature Leishmania exposed on its cell surface could be investigated and exploited for the development of an anti-leishmaniasis vaccine [16]. Leishmania are digenetic organisms that alternate between the insect vector and a mammalian host. The most abundant macromolecule on the surface of the insect infectious stage of all Leishmania species is a complex glycoconjugate called lipophosphoglycan (LPG, see section “Cell-Surface and Secreted Phosphoglycans of Leishmania”). Although killed or attenuated parasites did not result a valid vaccine [15, 16], the LPG itself has been suggested to be a perspective vaccine candidate as the protective effect of the purified LPG fraction on mice challenged with L. major was demonstrated [17-19]. Here we discuss the preparation of synthetic neoglycoconjugates (neoglycoproteins and neoglycolipids) based on various elements of the LPG structure as potential anti-leishmaniasis vaccines as well as the immunological evaluation for some of them.
GLYCOCONJUGATE SYNTHETIC VACCINES
The carbohydrate vaccine technology is an area developing rapidly because of modern bioconjugation techniques [20-22], which allow an effective formation of carbohydrate–protein and carbohydrate–lipid conjugates. Traditional polysaccharide vaccines, i.e. highly purified immunogenic bacterial polysaccharides (usually capsular polysaccharides) [23, 24], have been associated with the short duration of the induced immunity and proved to be poorly immunogenic in infants and young children [25, 26]. That happens because the polysaccharide antigens are T-cell independent: they induce an immune response without the involvement of T-cells. The response lacks several important features that characterize the T-cell-dependent immune response, such as immunological memory, an antibodies class switch from IgM to IgG, and affinity maturation [23]. Conjugation of the bacterial polysaccharide to an immunogenic protein carrier (detoxified versions of strongly immunogenic proteins like diphtheria and tetanus toxins are often used) converts it to a T-cell-dependent antigen with enhanced immunogenicity. Thus, the new generation glycoconjugate vaccines against bacterial infections are immunogenic in infants and induce long lasting immunological memory resulting in a boostable response [27]. In 1990s, four anti-meningitis glycoconjugate vaccines against Haemophilus influenzae b (Hib) were introduced with great result: the Hib-induced meningitis has virtually been eradicated in countries with high immunization coverage [28]. Similarly, a tetravalent meningococcal glucoconjugate vaccine against Neisseria meningitidis serogroups A, C, Y, and W135 has recently been developed by Sanofi-Pasteur and licensed in the USA [29].
The glycan parts of modern glycoconjugate vaccines are, normally, native or functionalized bacterial capsular polysaccharides. These naturally derived carbohydrate polymers are heterogeneous mixtures that may include impurities and contaminants. The use of synthetic carbohydrate structures, which can be chemically produced as single compounds in a controlled manner without batch-to-batch variability, can eliminate these problems. Recently, a fully synthetic hexasaccharide conjugated to keyhole limpet hemocyanin (KLH) was shown to be an effective vaccine against prostate cancer [30]. The methodology has been used by Verez-Bencomo et al. [31] for the preparation of the first commercial synthetic anti-meningitis glycoconjugate vaccine against Hib. The results of clinical testing in the target population demonstrated that the glycovaccine containing fully synthetic phosphoglycans (which are fragments of capsular polysaccharides) is as effective as its natural counterpart. The vaccine was registered in Cuba in 2003 and is now part of the national immunization program. Similarly, a synthetic vaccine against Shigella dysenteriae type 1 based on a synthetic oligosaccharide conjugated to a protein was found to be more immunogenic than a glycoconjugate prepared from the native polysaccharide [32]. Synthetic preparation of the glycan part of the glycoconjugate vaccines also allows for chemical modifications of the structure that may not be possible to perform on the native material.
CELL-SURFACE AND SECRETED PHOSPHOGLYCANS OF Leishmania
During the parasite life cycle, which involves a promastigote stage(s) in the midgut of the insect vector and an amastigote stage in the phagolysosomes of the mammalian macrophage, Leishmania survive and proliferate in highly hostile environments. Their survival strategies involve the formation of an elaborate and dense cell-surface glycocalyx composed of diverse stage-specific glycoconjugates that form a protective barrier [33, 34]. Some of these macromolecules (including various phosphoglycans) were shown to be essential for virulence of the parasite [35-38].
LPG is a predominant cell-surface glycoconjugate of Leishmania promastigotes. Structurally, this is a poly(glycosyl phosphate) consisting of the β-Gal-(1→4)-α-Man-1→OPO3 repeating units (Fig. 1), where the nature of the X and Y substituents varies according to the species. Leishmania donovani [34, 39] and L. infantum synthesize a linear phosphoglycan, whereas those of L. mexicana [40, 41], L. major [42-44], and L. aethiopica [45] have branched structures bearing non-stoichiometric mono- and/or disaccharide components (X) linked at O3 of the β-D-Galp residue. In L. major, the composition of the side chains varies and depends on the developmental stage of the parasite. In the LPG produced by L. aethiopica there are also some additional α-D-Manp residues (Y) linked to D-mannose residues in the main chain. All LPG molecules are capped at the non-reducing terminus with an oligosaccharide phosphate unit (basically, GalMan3-phosphate, where some residues are non-stoichiometric; Fig. 1) and contain a glycan core (not shown) linked to an inositol-phospholipid anchor [33, 38] at the reducing end of the chain. The cap oligosaccharide structures appeared to be species- and stage-specific: GalMan3 and GalMan2 caps have been found in L. donovani and L. mexicana LPG [33, 40, 47], whereas for L. major LPG in promastigotes the cap was a Man2 disaccharide [34, 42] and in amastigotes a GalMan disaccharide [34].
Fig. 1.
Phosphoglycan region of lipophosphoglycans and proteophosphoglycans of Leishmania.
In addition to cell-surface LPG, Leishmania promastigotes produce a number of secreted glycoproteins, where a peptide core is modified (glycosylated) with LPG-type phosphoglycans [47, 48]. Leishmania donovani secretes an acid phosphatase (sAP) in which the peptide chains are heavily glycosylated on C-terminal serine/threonine domains. The glycans (which are, in fact, phosphoglycans) consist of the same disaccharide phosphate repeating units found in the corresponding LPG (Fig. 1) and are phosphodiester-linked to select serine residues [34]. All species of Leishmania promastigotes synthesize secreted filamentous proteophosphoglycan, which forms a highly viscous gel surrounding the parasite cells. Compositionally this proteophosphoglycan consists of 95% phosphoglycans in a species-specific manner (Fig. 1). The poly(glycosyl phosphate) chains are attached to serine residues of the peptide component via phosphodiester groups.
Hydrophilic phosphoglycans have been found in culture supernatants of Leishmania promastigotes and have been structurally characterized for L. major [49], L. mexicana [50], and L. donovani [51]. They are essentially the corresponding LPG molecules without the glycan core—inositol-phospholipid region.
Leishmania mexicana amastigotes synthesize large amounts of a macromolecular amastigote-specific proteophosphoglycan and secrete it (in mg/ml concentrations!) into the phagolysosome of the mammalian macrophage [46]. Some phosphoglycan chains are similar to those found in L. mexicana LPG, but the majority represent novel stage-specific highly branched structures (Fig. 1), including (1→3)-linked glucobiose and glucotriose moieties and long phosphorylated side chains. The phosphoglycans are attached to serine residues of the protein backbone, most likely through the phosphate groups. The amastigote-specific proteophosphoglycan is believed to activate the complement system via the mannose-binding pathway. It may also contribute to the binding of Leishmania to host cells and play a role in modulation of the biology of the infected macrophage [34].
CHEMICAL SYNTHESIS OF PHOSPHOGLYCANS OF Leishmania DESIGNED FOR FURTHER BIOCONJUGATION
The most distinctive part of Leishmania LPG is their variable phosphoglycan domain, made of β-Gal-(1→4)-α-Man-1→OPO3 phosphodisaccharide repeats linked to each other through a phosphodiester group between the anomeric hydroxyl of D-mannose of one repeat and the 6-OH group of D-galactose of the adjoining repeat (see section “Cell-Surface and Secreted Phosphoglycans of Leishmania”). The preparation of neoglycoconjugates based on phosphosaccharide repeats upholds the need to develop routes for the chemical synthesis of Leishmania phosphoglycans. The development of synthetic approaches to phosphoglycans is challenging, because the phosphoglycans are hydrolytically labile due to anomeric phosphodiester linkage present between the repeating units. Synthesis of anomeric phosphodiesters is complicated, as both the correct stereochemistry at C1 and the lability of anomeric phosphodiester linkages must be taken into consideration [52, 53].
The first successful syntheses of Leishmania phosphoglycans were achieved by the Dundee University group [54-56] by exploiting the H-phosphonate chemistry. The glycosyl H-phosphonate route was proven to be the method of choice for the efficient and reliable assembly of various phosphodiester linkages characteristic of natural phosphoglycans [53]. The L. donovani phosphoglycans 1-5 (linear chains, built up from disaccharide phosphate repeating units in (1→6)-phosphodiester linkage and varying in length and by the presence of the Man2-phosphate cap in 4 and 5; Fig. 2) were constructed in a stepwise (1-4) [54, 55] and a blockwise (5) [56] chain elongation manner.
Fig. 2.
Leishmania donovani phosphoglycan structures prepared by stepwise (1-4) and blockwise (5) chain elongation strategy.
All the prepared phosphoglycans contain a dec-9-enyl aglycone moiety as they were purposely designed [55] for (a) studying Leishmania biosynthetic enzymes and (b) further preparation of neoglycoconjugates, which could be tested as potential anti-leishmaniasis vaccines. The hydrophobicity of the dec-9-enyl chain has been exploited for assaying, first, the mannosylphosphate transferases [57, 58] and then the galactosyltransferases [59, 60] involved in the LPG biosynthesis in Leishmania. As for the bioconjugation, the high lability of the anomeric phosphodiester bridges in phosphoglycans was given the top priority while selecting techniques for the neoglycoconjugate preparation. It was decided to avoid the use of any condensing reagents (which could easily interfere with the phosphate groups and facilitate the cleavage of the glycosyl phosphate bonds) and profit from the ozonolysis of the double bond in the dec-9-enyl aglycone linker instead, thus transforming it into an aldehyde functionality, which could be coupled to a protein or a lipid carrier by reductive amination (see section “The Preparation of Neoglycoconjugates Based on Phosphosaccharide Repeats Using Ozonolysis/Reductive Amination Technique”).
The branched heptaglycosyl triphosphate phosphoglycan 6 (Fig. 3) from L. mexicana (containing β-D-glucose moiety in the side chain) was assembled via stepwise chain elongation [61]. A matching strategy was employed in the synthesis of the branched phosphoglycans 7-9 [62, 63] and 10-12 ([64, 65] and Yashunsky, D. V., and Nikolaev, A. V., unpublished data) from L. major containing β-D-Gal and/or β-D-Gal-(1→2)-β-D-Ara side chains (Fig. 3).
Fig. 3.
Leishmania mexicana (6) and L. major (7-12) phosphoglycans prepared by the Dundee University group.
Polymer-supported synthetic methodologies [66, 67] that employed glycosyl H-phosphonates for consecutive chain elongation have been also successfully developed for the preparation of Leishmania phosphoglycans as well as the H-phosphonate polycondensation strategy [67, 68].
THE PREPARATION OF NEOGLYCOCONJUGATES BASED ON PHOSPHOSACCHARIDE REPEATS USING OZONOLYSIS/REDUCTIVE AMINATION TECHNIQUE
As mentioned above, all the phosphoglycans designed and synthesized in Dundee were equipped with a dec-9-enyl aglycone linker to enable their future incorporation into neoglycoconjugates via oxidation of the terminal double bond. Ozonolysis of the phosphoglycans in methanolic solution afforded the corresponding 8-carbonyloctyl glycosides (or phosphosaccharide 8-carbonyloctyl diesters; Fig. 4), which were immediately used for coupling with a protein (or a lipid) carrier by reductive amination with NaCNBH3 ([69, 70] and Sizova, O. V., Nikolaev, A. V., and Ferguson, M. A. J., unpublished data). The conversion of the phosphoglycans to their 8-carbonyloctyl derivatives was shown to be complete and proceeds without destruction of the labile anomeric phosphodiesters [69]. Ozonolysed compounds were mixed with NaCNBH3 and a protein in a phosphate buffer solution to obtain a molar ratio of phosphosaccharide/protein NH2 group/NaCNBH3 of 1 : 3 (if another one is not specified) : 50. The prepared neoglycoconjugates were purified by gel filtration on a column of Superdex 200 HR (Amersham Biosciences, USA). Fractions containing the product were concentrated using ultrafiltration technique and Amicon centrifugal filters (Millipore, USA) (see the experimental procedures below). Following SDS-PAGE analysis showed that no starting protein was observed [69, 70].
Fig. 4.
Preparation and some characteristics of synthetic neoglycoconjugates based on Leishmania LPG phosphosaccharide repeats.
To develop the optimal conjugation conditions, first [69], the L. donovani phosphoglycan 4 (Fig. 2) was ozonolysed and coupled to ribonuclease A (RNase A; M = 13,690 Da, 10 amino groups) using a molar ratio of phosphosaccharide/protein amino group of 1 : 5. That gave a glycoconjugate containing 1.1 phosphosaccharide (hapten) chains per RNase A molecule (i.e. one chain of a hapten per 12,440 Da of protein). The ozonolysed disaccharide phosphate 1 (Fig. 2) was coupled to BSA as a carrier (M = 66,382 Da, 59 amino groups) using a molar ratio of phosphosaccharide/protein amino group of 1 : 1, thus producing a glycoconjugate containing eight hapten chains per molecule of BSA (i.e. one chain of a hapten per 8,300 Da of protein). Although this was around three-fold lower than that reported for oligosaccharide–BSA conjugates prepared using similar reductive amination techniques [71, 72], in the latter cases a large molar excess of oligosaccharide over BSA amino groups (about 200 : 1) was used. Since (a) the synthetic phosphoglycans are rather precious and (b) a relatively low hapten/protein ratio (like one chain of a hapten per 10 kDa of protein) may be desirable from an antigen-presentation point of view [69], the developed conditions were used for the preparation ([70] and Sizova, O. V., Nikolaev, A. V., and Ferguson, M. A. J., unpublished data) of Leishmania phosphoglycan–tetanus toxin fragment C conjugates 13-19 (Fig. 4).
The engineered tetanus toxin fragment C (TetC) [73] does not retain any toxicity, but it retains immunogenicity [74]. It was already tested on the vaccine market [75] and has the advantage of inducing a Th-1 response that is necessary for the killing Leishmania parasites [76, 77]. The synthetic phosphoglycans were coupled to TetC using a molar ratio of phosphosaccharide/protein amino group of 1 : 3 [69, 70]. Thus, three L. donovani–TetC glycoconjugates 13-15 were prepared from three different linear phosphoglycans 2-4, correspondingly. Ozonolysis of the branched L. mexicana phosphoglycan 6 (Fig. 3), followed by protein coupling produced the L. mexicana–TetC glycoconjugate 16, while the L. major–TetC glycoconjugates 17 and 18 were generated from the L. major phosphoglycans 9 and 12 (Fig. 3), respectively. The prepared conjugates were carrying from 3.37 to 4.85 phosphosaccharide haptens per molecule with, on average, one chain of a hapten per 13,300 Da of protein. The SDS-PAGE analysis of the prepared neoglycoproteins showed that no starting TetC protein was observed (Fig. 5).
Fig. 5.
SDS-PAGE analysis of TetC before and after its transformation into synthetic neoglycoproteins based on Leishmania LPG phosphosaccharide repeats.
The modified L. donovani–TetC glycoconjugates 19a and 19b (Fig. 4) were prepared from the synthetic C-glycosyl phosphono-analogue of the LPG fragment with a structure of β-Gal-(1→4)-α-Man-(1→CH2PO2H-6)-β-Gal-(1→4)-α-Man-1→CH2PO2H-O(CH2)8CH=CH2 21 [78]. Replacing the anomeric O1 in D-mannose residues with CH2 groups in 21 does not, probably, change much conformation of the molecule, but makes the C-glycosyl phosphono analogues significantly more stable chemically than parent phosphoglycans and resists the action of phosphatases as well. All of those make the phosphono analogues of the parasitic and bacteria phosphoglycans strong candidates for synthetic glycoconjugate vaccines [79, 80]. Ozonolysis of compound 21, followed by coupling to TetC with a molar ratio of phosphonosaccharide/protein amino group of 1 : 3 furnished the glycoconjugate 19a containing just two hapten chains per molecule, while the higher loaded conjugate 19b (with 9.7 hapten chains per molecule; Fig. 5) was prepared using a higher molar ratio of the phosphonosaccharide over TetC amino groups of 2 : 3.
Finally, the neoglycolipid 20 (Fig. 4) was synthesized [69] by coupling the ozonolysed phosphoglycan 4 and 1,2-di-O-hexadecyl-sn-glycero-3-phosphatidylethanolamine in a mixture of chloroform–methanol–water using a molar ratio of phosphosaccharide/amine/NaCNBH3 of 1 : 20 : 50. After purification on an Octyl-Sepharose column followed by Kieselgel 60, the glycoconjugate was isolated in 36% yield. For immunological studies, the neoglycolipids could be incorporated into liposomes that act as a carrier and as an adjuvant [81].
Experimental procedures for the preparation of neoglycoproteins 13-19 ([70] and Sizova, O. V., Nikolaev, A. V., and Ferguson, M. A. J., unpublished data). Ozonolysis of phosphosaccharides. Synthetic phosphosaccharides 2-4, 6, 9, 12, or 21 (as their ammonium or triethylammonium salts; 0.3-0.6 mg of a phosphosaccharide in one experiment) were dissolved in 1 ml of methanol or a mixture of methanol–water (5 : 1). The solution was cooled to −78°C and ozone (from ozone generator OZ06; Peak Scientific Ltd., Scotland) was bubbled (~5 min) into it via a sintered-glass bubbling tube until a pale blue colour persisted. Oxygen was then bubbled (~10 min) through the solution until it became colorless to eliminate excess O3. Dimethylsulfide (0.01 ml) was added to reduce the ozonide, and the solution was allowed to warm to room temperature and left for 2 h. The products (corresponding 8-carbonyloctyl phosphosaccharides) were dried by evaporation of methanol therefrom (twice) and used immediately for coupling with the protein.
Coupling of phosphosaccharides to TetC and rapid purification of neoglycoconjugates
The prepared 8-carbonyloctyl phosphosaccharides and NaCNBH3 were dissolved in 0.1 M phosphate buffer (0.54-0.70 ml, pH 7.4) and added to a TetC solution (0.06-0.15 ml) in the same buffer to obtain a molar ratio of phosphosaccharide/protein amino group/NaCNBH3 of 1 : 3 : 50 (for the preparation of the glycoconjugate 19b only, the ratio was taken as 2 : 3 : 50.) The mixture was incubated at 37°C for 72 h and then centrifuged at 13,000-14,000 rpm (30 min, 4°C). The prepared neoglycoproteins stayed in the supernatant solution and were purified by gel filtration on a column of Superdex 200 HR (void volume 8.5 ml) using 0.1 M phosphate buffer, pH 7.2, as eluant at a flow rate of 0.5 ml/min. Neoglycoproteins were detected with a UV detector at 280 nm; eluate of 1 min was collected into each tube. For each coupling experiment, the elution profile appeared to be as a single or one major peak, eluting in the area between 25 and 33 min. The appropriate fractions were combined (the total volume of the peak was about 2-3 ml). The glycoconjugate solutions were concentrated by ultrafiltration (5,000g, 30 min, 4°C) using an Amicon Ultra-0.5 Centrifugal Filter (Millipore) with an Ultracel-30 Membrane (30 kDa nominal molecular weight limit) and an Avanti centrifuge (Beckman, USA), thus providing samples of neoglycoproteins 13-19 in phosphate buffered saline (volume of each sample 0.30-0.89 ml; protein concentration 1.13-4.28 mg/ml). The following SDS-PAGE analysis of the samples showed that no starting TetC protein was observed (Fig. 5).
Oligosaccharide concentrations were determined by phenol–sulfuric acid assay [82] performed in triplicate. Protein concentrations were determined by the bicinchoninic acid assay (Pierce, USA) according to the manufacturer’s instructions. SDS-PAGE was performed for native and coupled (to phosphosaccharides) TetC using 8% gel and the discontinuous buffer system of Laemmli [83]. Gels were stained with Coomassie blue to detect protein bands.
IMMUNOLOGICAL STUDIES OF Leishmania SYNTHETIC NEOGLYCOCONJUGATES
The glycoconjugates 14 (L. donovani–TetC), 16 (L. mexicana–TetC), 17 (L. major–TetC with D-Gal side chains), and 18 (L. major–TetC with D-Ara→D-Gal side chains) were used by Bates and Rogers (University of Liverpool) as glycovaccines (without any adjuvants) to immunize BALB/c mice, which were then challenged by the bite of L. mexicana infected Lutzomyia longipalpis sand flies [84]. Intriguingly, only the L. mexicana–TetC glycovaccine 16 showed significant protection compared with the control TetC immunization, resulting in a 50% reduction in lesion size and a 94% (!) reduction in parasite burden. The other glycovaccines 14, 17, and 18, neither the L. mexicana phosphoglycan 6 (Fig. 3) alone, were unable to provide protection against infected fly bite challenge. Thus, that was the first (to the best of the authors [84] knowledge) demonstration that a synthetic glycoconjugate vaccine can have a direct antiparasite effect in leishmaniasis or any other parasitic disease. It seems that these glycovaccines may induce species and glycan structure-specific protection, although the ability of the L. mexicana–TetC glycovaccine 16 to protect against other species of Leishmania has not been tested yet.
THE PREPARATION OF NEOGLYCOCONJUGATES BASED ON THE TETRASACCHARIDE CAP STRUCTURE
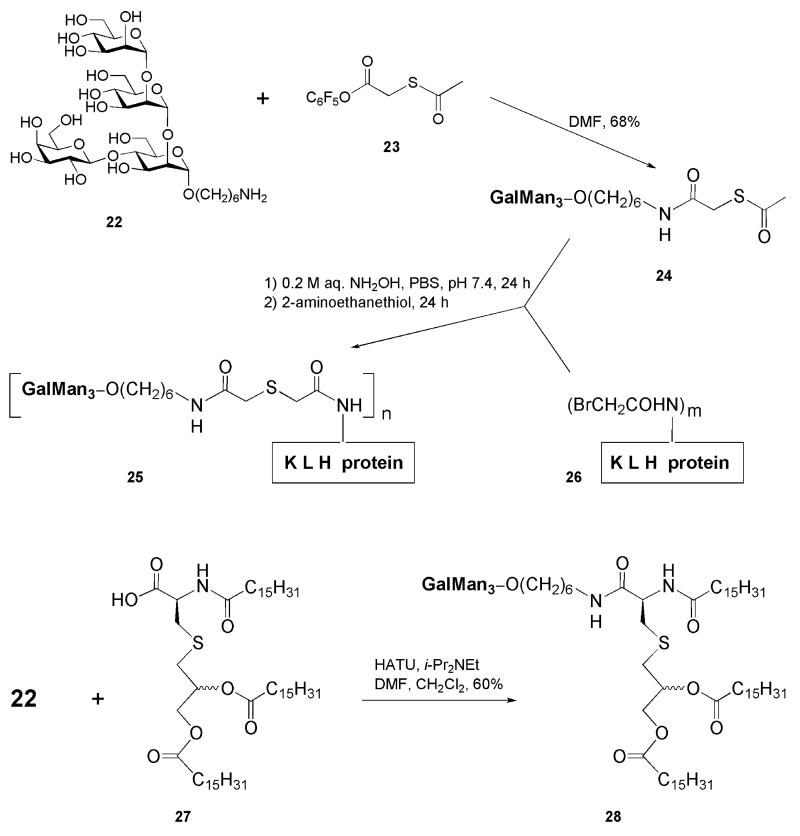
The Cambridge (Massachusetts, USA) group [16, 85] has designed and synthesized two potential Leishmania vaccine constructs based on a structure of the tetrasaccharide phosphate cap (GalMan3-phosphate) of LPG, while the phosphate group being omitted thus simplifying strongly the conjugation technique (see section “Chemical Synthesis of Phosphoglycans of Leishmania Designed for Further Bioconjugation”). Chemically prepared tetrasaccharide 22 (Fig. 6) was equipped with a 6-aminohexyl aglycone linker to facilitate further bioconjugation. The GalMan3 tetrasaccharide itself has previously been a subject of two synthetic approaches [86, 87].
Fig. 6.
Synthetic neoglycoconjugates based on the tetrasaccharide cap structure from Leishmania LPG.
Two neoglycoconjugates 25 and 28 (Fig. 6) were prepared by joining the tetrasaccharide antigen 22 with protein carrier KLH or with lipid immunostimulator tripalmitoyl-S-glycerylcysteine 27, respectively. The KLH proteins are known to be highly heterogeneous and have enormous molecular masses (4,500-13,000 kDa) as well as ill defined structures, but their immunogenic capabilities can be superior [88]. Prior to the conjugation, the protein itself was reacted with N-succinimidyl bromoacetate to form the N-(bromoacetyl)-modified carrier 26, while the 6-aminohexyl tetrasaccharide 22 was coupled with pentafluorophenyl (acetylsulfanyl)acetate 23 to give the tetrasaccharide 24 with masked SH functionally in the linker. Further coupling of 24 with the modified protein 26 in the presence of hydroxylamine was followed by capping of any remaining bromoacetyl groups with 2-aminoethanethiol to furnish the neoglycoprotein 25, which was purified by gel filtration on a Pd-10 column (Sephadex G-25). The reported conjugation ratio of the GalMan3 hapten chains to each KLH molecule was 55 : 1 (which is about one chain of the tetrasaccharide hapten per 82,000-236,000 Da of protein).
The lipopeptide N-palmitoyl-S-[(2R,2S)-2,3-di(palmitoyloxy)propyl]-L-cysteine 27 (tripalmitoyl-S-glycerylcysteine, Pam3Cys) has been already reported as a part of a fully synthetic vaccine [89]. It can work both as a carrier and an adjuvant [90] and is readily available by synthesis [91]. Coupling of lipopeptide 27 with the 6-aminohexyl tetrasaccharide 22 was performed in the presence of HATU and diisopropylethylamine and afforded the neoglycolipid 28 (60%) after purification on a silica column. Both synthetic glycovaccines have been tested for their immunological evaluation in BALB/c mice, but the results have not been reported yet.
FUTURE ADVANCES
Despite appreciable progress in the preparation of neoglycoconjugates based on synthetic cell-surface structures of Leishmania, there are still several unresolved issues that prevent the neoglycoconjugates from being routinely synthesized to furnish further immunological testing. Part of them is dealing with rapid and effective preparation of synthetic LPG fragments and, therefore, novel methods towards anomeric phosphodiesters are in demand. This may also include a search for novel activating (for the H-phosphonate condensation) and oxidizing reagents. The oxidation step appeared to be a problem for the preparation of some sterically hindered glycosyl phosphosaccharides [52, 53] as the intermediate H-phosphonic diester, instead of its smooth transformation to the phosphodiester, may undergo hydrolysis of the glycosidic linkage as a concurrent reaction.
We have already cited in the introduction to this review that any unique feature Leishmania expose on its cell surface could be investigated and exploited for the development of an anti-leishmaniasis vaccine [16]. As all LPG molecules contain at the reducing end of the chain a glycan core of the α-Gal-(1→6)-α-Gal-(1→3)-β-Galf-(1→3)-[α-Glc-1→PO3H-6)]-α-Man-(1→3)-α-Man-(1→4)-α-GlcNH2 structure, which, in turn, is (1→6)-linked to a 1-O-alkyl(C24-26 chain)-2-lyso-phosphatidyl-myo-inositol lipid anchor [33, 38], it seems valuable to prepare neoglycoconjugates based on the above structure. The preparation of the glycan core-myo-inositol conjugate has been recently reported [92, 93].
LPG molecules are expressed on the promastigote (i.e. insect’s midgut stage) membrane of all species of Leishmania [33, 40, 94], while their expression in amastigote parasites (existing in human phagolysosomes) significantly decreases, with an exception of L. major amastigotes [95]. At this stage, small molecules called glycoinositolphospholipids, which structures are, in some extent, similar to the above heptasaccharide glycan core (or part of it) linked to an inositolphospholipid anchor [33, 38, 44], remain the dominant surface structures. Thus, it looks worthy to prepare neoglycoconjugates based on the glycoinositolphospholipid structures and test them as potential anti-leishmaniasis vaccines.
Acknowledgments
We are indebted to Prof. M. A. J. Ferguson (Dundee University) for his interest, constant support, and encouragement. Tetanus toxin fragment C (TetC) was a generous gift from Prof. C. Watts (Dundee University). Financial support from the Royal Society (6-month Traveling Fellowship to the United Kingdom for O. V. S.) and the Wellcome Trust grant 083481 (for the infrastructure maintenance of the Division of Biological Chemistry and Drug Discovery within the University of Dundee) is gratefully acknowledged.
Abbreviations
- Hib
Haemophilus influenzae b
- KLH
keyhole limpet hemocyanin
- LPG
lipophosphoglycan
REFERENCES
- 1.Herwaldt BL. Lancet. 1999;354:1191–1199. doi: 10.1016/S0140-6736(98)10178-2. [DOI] [PubMed] [Google Scholar]
- 2.World Health Organisation http://www.who.int; WHO Leishmaniasis Factsheet: http:/www.who.int/leishmaniasis/disease_epidemiology/en/index.html.
- 3.Division of Parasitic Diseases, Centres for Disease Control and Prevention http://www.dpd.cdc.gov/DPDx/
- 4.Magill AJ, Grogl M, Gasser RA, Sun W, Oster CN. N. Engl. J. Med. 1993;328:1383–1387. doi: 10.1056/NEJM199305133281904. [DOI] [PubMed] [Google Scholar]
- 5.Alvar J, Canavate C, Gutierres-Solar B, Jimenez M, Laguna F, Lopez-Velez R, Molina R, Moreno J. Clin. Microbiol. Rev. 1997;10:298–319. doi: 10.1128/cmr.10.2.298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Health A to Z http://www.healthatoz.com/healthatoz/Atoz/common/standard/transform.jsp?requestURI=/healthatoz/Atoz/ency/leishmaniasis.jsp.
- 7.University of Texas Medical Branch, Graduate School of Biomedical Sciences http://www.gsbs.utmb.edu/microbook/ch082.htm.
- 8.Tropical Medicine Central resource http://tmcr.usuhs.mil/tmcr/chapter46/large46/46-16.jpg.
- 9.Reed SG, Scott P. Curr. Opin. Immunol. 1993;5:524–531. doi: 10.1016/0952-7915(93)90033-o. [DOI] [PubMed] [Google Scholar]
- 10.Berman JD. Clin. Infect. Dis. 1997;24:684–703. doi: 10.1093/clind/24.4.684. [DOI] [PubMed] [Google Scholar]
- 11.Nelson KG, Bishop JV, Ryan RO, Titus R. Antimicrob. Agents Chemother. 2006;50:1238–1244. doi: 10.1128/AAC.50.4.1238-1244.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Croft SL, Sundar S, Fairlamb AH. Clin. Microbiol. Rev. 2006;19:111–126. doi: 10.1128/CMR.19.1.111-126.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Faghihi G, Tavakoli-kai R. Clin. Exp. Dermatol. 2003;28:13–16. doi: 10.1046/j.1365-2230.2003.01169.x. [DOI] [PubMed] [Google Scholar]
- 14.Verma NK, Dey CS. Antimicrob. Agents Chemother. 2004;48:3010–3015. doi: 10.1128/AAC.48.8.3010-3015.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Modabber F. Ann. Trop. Med. Parasitol. 1995;89(Suppl. 1):83–88. doi: 10.1080/00034983.1995.11813017. [DOI] [PubMed] [Google Scholar]
- 16.Hewitt MC, Seeberger PH. J. Org. Chem. 2001;66:4233–4243. doi: 10.1021/jo015521z. [DOI] [PubMed] [Google Scholar]
- 17.McConville MJ, Bacic A, Mitchell GF, Handman E. Proc. Natl. Acad. Sci. USA. 1987;84:8941–8945. doi: 10.1073/pnas.84.24.8941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Russell DG, Alexander J. J. Immunol. 1988;140:1274–1279. [PubMed] [Google Scholar]
- 19.Moll H, Mitchell GF, McConville MJ, Handman E. Infect. Immun. 1989;57:3349–3356. doi: 10.1128/iai.57.11.3349-3356.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Stowell CP, Lee Y-C. Adv. Carbohydr. Chem. Biochem. 1980;37:225–281. doi: 10.1016/s0065-2318(08)60022-0. [DOI] [PubMed] [Google Scholar]
- 21.Magnusson G, Chernyak AY, Kihlberg J, Kononov LO. In: Neoglycoconjugates, Preparation and Applications. Lee Y-C, Lee RT, editors. Academic Press; San Diego: 1994. pp. 53–143. [Google Scholar]
- 22.Hermanson GT. Bioconjugate Techniques. 2nd Edn. Elsevier, Academic Press; London-Amsterdam-Burlington-San Diego: 2008. [Google Scholar]
- 23.Morley SL, Pollard AJ. Vaccine. 2002;20:666–687. doi: 10.1016/s0264-410x(01)00410-8. [DOI] [PubMed] [Google Scholar]
- 24.Gotschlich EC, Goldschneider I, Artenstein MS. J. Exp. Med. 1969;129:1367–1384. doi: 10.1084/jem.129.6.1367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gold R, Lepow ML, Goldschneider I, Draper TL, Gotschlich EC. J. Clin. Invest. 1975;56:1536–1547. doi: 10.1172/JCI108235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gold R, Lepow ML, Goldschneider I, Gotschlich EC. J. Infect. Dis. 1977;136:31–35. doi: 10.1093/infdis/136.supplement.s31. [DOI] [PubMed] [Google Scholar]
- 27.Jennings HJ, Sood RK. In: Neoglycoconjugates, Preparation and Applications. Lee Y-C, Lee RT, editors. Academic Press; San Diego: 1994. pp. 325–370. [Google Scholar]
- 28.Lindberg AA. Vaccine. 1999;17:28–36. [Google Scholar]
- 29.Girard MP, Preziosi MP, Aguado MT, Kieny MP. Vaccine. 2006;24:4692–4700. doi: 10.1016/j.vaccine.2006.03.034. [DOI] [PubMed] [Google Scholar]
- 30.Slovin SF, Ragupathi G, Adluri S, Ungers G, Terry K, Kim S, Spassova M, Bornmann WG, Fazzari M, Dantis L, Olkiewicz K, Lloyd KO, Livingston PO, Danishefsky SJ, Scher HI. Proc. Natl. Acad. Sci. USA. 1999;96:5710–5715. doi: 10.1073/pnas.96.10.5710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Veres-Bencomo V, et al. Science. 2004;305:522–525. doi: 10.1126/science.1095209. [DOI] [PubMed] [Google Scholar]
- 32.Pozsgay V, Chu C, Pannell L, Wolfe J, Robbins JB, Schneerson R. Proc. Natl. Acad. Sci. USA. 1999;96:5194–5197. doi: 10.1073/pnas.96.9.5194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.McConville MJ, Ferguson MAJ. Biochem. J. 1993;294:305–324. doi: 10.1042/bj2940305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Guha-Niyogi A, Sullivan DR, Turco SJ. Glycobiology. 2001;11:45R–59R. doi: 10.1093/glycob/11.4.45r. [DOI] [PubMed] [Google Scholar]
- 35.Turco SJ, Spath GF, Beverley SM. Trends Parasitol. 2001;17:223–226. doi: 10.1016/s1471-4922(01)01895-5. [DOI] [PubMed] [Google Scholar]
- 36.Spath GF, Lye LF, Segawa H, Sacks D, Turco SJ, Beverley SM. Science. 2003;301:1241–1243. doi: 10.1126/science.1087499. [DOI] [PubMed] [Google Scholar]
- 37.Rogers ME, Ilg T, Nikolaev AV, Ferguson MAJ, Bates P. Nature. 2004;430:463–467. doi: 10.1038/nature02675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Novozhilova NM, Bovin NV. Biochemistry (Moscow) 2010;75:686–694. doi: 10.1134/s0006297910060027. [DOI] [PubMed] [Google Scholar]
- 39.Turco SJ, Hull SR, Organdi PA, Sheperd SD, Homans SW, Dwek RA, Rademacher TW. Biochemistry. 1987;26:6233–6238. doi: 10.1021/bi00393a042. [DOI] [PubMed] [Google Scholar]
- 40.Ilg T, Etges R, Overath P, McConville MJ, Thomas-Oates JE, Thomas JR, Homans SW, Ferguson MAJ. J. Biol. Chem. 1992;267:6834–6840. [PubMed] [Google Scholar]
- 41.Ilg T, Overath P, Ferguson MAJ, Rutherford T, Campbell DG, McConville MJ. J. Biol. Chem. 1994;269:24073–24081. [PubMed] [Google Scholar]
- 42.McConville MJ, Thomas-Oates JE, Ferguson MAJ, Homans SW. J. Biol. Chem. 1990;265:19611–19623. [PubMed] [Google Scholar]
- 43.McConville MJ, Turco SJ, Ferguson MAJ, Sacks DL. EMBO J. 1992;11:3593–3600. doi: 10.1002/j.1460-2075.1992.tb05443.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ilg T, Stierhof YD, Craik D, Simpson R, Handman E, Bacic A. J. Biol. Chem. 1996;271:21583–21596. doi: 10.1074/jbc.271.35.21583. [DOI] [PubMed] [Google Scholar]
- 45.McConville MJ, Schnur LF, Jaffe C, Schneider P. Biochem. J. 1995;310:807–818. doi: 10.1042/bj3100807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ilg T, Craik D, Currie G, Multhaup G, Bacic A. J. Biol. Chem. 1998;273:13509–13523. doi: 10.1074/jbc.273.22.13509. [DOI] [PubMed] [Google Scholar]
- 47.Ilg T. Parasitol. Today. 2000;16:489–501. doi: 10.1016/s0169-4758(00)01791-9. [DOI] [PubMed] [Google Scholar]
- 48.Ilg T, Handman E, Stierhof Y-D. Biochem. Soc. Trans. 1999;27:518–525. doi: 10.1042/bst0270518. [DOI] [PubMed] [Google Scholar]
- 49.Handman E, Greenblatt CL, Goding J. EMBO J. 1984;3:2301–2306. doi: 10.1002/j.1460-2075.1984.tb02130.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ilg T, Stierhof YD, Wiese M, McConville MJ, Overath P. Parasitology. 1994;108:S63–S71. doi: 10.1017/s0031182000075739. [DOI] [PubMed] [Google Scholar]
- 51.Greis KD, Turco SJ, Thomas JR, McConville MJ, Homans SW, Ferguson MAJ. J. Biol. Chem. 1992;267:5876–5881. [PubMed] [Google Scholar]
- 52.Hansson J, Oscarson S. Curr. Org. Chem. 2000;4:535–564. [Google Scholar]
- 53.Nikolaev AV, Botvinko IV, Ross AJ. Carbohydr. Res. 2007;342:297–344. doi: 10.1016/j.carres.2006.10.006. [DOI] [PubMed] [Google Scholar]
- 54.Nikolaev AV, Rutherford TJ, Ferguson MAJ, Brimacombe JS. Bioorg. Med. Chem. Lett. 1994;4:785–788. [Google Scholar]
- 55.Nikolaev AV, Rutherford TJ, Ferguson MAJ, Brimacombe JS. J. Chem. Soc. Perkin Trans. 1. 1995:1977–1987. [Google Scholar]
- 56.Nikolaev AV, Rutherford TJ, Ferguson MAJ, Brimacombe JS. J. Chem. Soc. Perkin Trans. 1. 1996:1559–1566. [Google Scholar]
- 57.Brown GM, Millar AR, Masterson C, Brimacombe JS, Nikolaev AV, Ferguson MAJ. Eur. J. Biochem. 1996;242:410–416. doi: 10.1111/j.1432-1033.1996.0410r.x. [DOI] [PubMed] [Google Scholar]
- 58.Routier FH, Higson AP, Ivanova IA, Ross AJ, Tsvetkov YE, Yashunsky DV, Bates PA, Nikolaev AV, Ferguson MAJ. Biochemistry. 2000;39:8017–8025. doi: 10.1021/bi000371s. [DOI] [PubMed] [Google Scholar]
- 59.Ross AJ, Sizova OV, Nikolaev AV. Carbohydr. Res. 2006;341:1954–1964. doi: 10.1016/j.carres.2006.03.026. [DOI] [PubMed] [Google Scholar]
- 60.Sizova OV, Ross AJ, Ivanova IA, Borodkin VS, Ferguson MAJ, Nikolaev AV. ACS Chem. Biol. 2011 doi: 10.1021/cb100416j. doi: 10.1021/cb100416j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Higson AP, Tsvetkov YE, Ferguson MAJ, Nikolaev AV. J. Chem. Soc. Perkin Trans. 1. 1998:2587–2595. [Google Scholar]
- 62.Higson AP, Tsvetkov YE, Ferguson MAJ, Nikolaev AV. Tetrahedron Lett. 1999;40:9281–9284. [Google Scholar]
- 63.Higson AP, Ross AJ, Tsvetkov YE, Routier FH, Sizova OV, Ferguson MAJ, Nikolaev AV. Chem. Eur. J. 2005;11:2019–2030. doi: 10.1002/chem.200400563. [DOI] [PubMed] [Google Scholar]
- 64.Yashunsky DV, Higson AP, Ross AJ, Nikolaev AV. Carbohydr. Res. 2001;336:243–248. doi: 10.1016/s0008-6215(01)00274-9. [DOI] [PubMed] [Google Scholar]
- 65.Yashunsky DV, Higson AP, Sizova OV, Ferguson MAJ, Nikolaev AV. Abstr. Book 12th Europ. Carbohydrate Symp.; Grenoble, France. 2003; 2003. p. 116. Abstract OC074. [Google Scholar]
- 66.Ross AJ, Ivanova IA, Higson AP, Nikolaev AV. Tetrahedron Lett. 2000;41:2449–2452. [Google Scholar]
- 67.Ruhela D, Vishwakarma RA. J. Org. Chem. 2003;68:4446–4456. doi: 10.1021/jo0341867. [DOI] [PubMed] [Google Scholar]
- 68.Nikolaev AV, Chudek JA, Ferguson MAJ. Carbohydr. Res. 1995;272:179–189. doi: 10.1016/0008-6215(95)00067-4. [DOI] [PubMed] [Google Scholar]
- 69.Routier FH, Nikolaev AV, Ferguson MAJ. Glycoconj. J. 1999;16:773–780. doi: 10.1023/a:1007171613195. [DOI] [PubMed] [Google Scholar]
- 70.Sizova OV, Nikolaev AV, Ferguson MAJ. Abstr. Book 21st Int. Carbohydrate Symp.; Cairns, Australia. 2002; 2002. Abstract PP251. [Google Scholar]
- 71.Aspinall GO, Crane AM, Gammon DW, Ibrahim IH, Khare NK. Carbohydr. Res. 1991;216:337–355. doi: 10.1016/0008-6215(92)84172-o. [DOI] [PubMed] [Google Scholar]
- 72.Pozsgay VA. Glycoconj. J. 1993;10:133–141. doi: 10.1007/BF00737710. [DOI] [PubMed] [Google Scholar]
- 73.Makoff AJ, Ballantine SP, Smallwood AE, Fairweather NF. Biotechnology (NY) 1989;7:1043–1046. [Google Scholar]
- 74.Hewitt EW, Treumann A, Morrice N, Tatnell PJ, Kay J, Watts C. J. Immunol. 1997;159:4693–4699. [PubMed] [Google Scholar]
- 75.Kasper DL, Paoletti LC, Wessels MR, Guttormsen HK, Carey VJ, Jennings HJ, Baker CJ. J. Clin. Invest. 1997;98:23080–2314. doi: 10.1172/JCI119042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Bogdan C, Roelinghoff M. In: Concept in Vaccine Development. Kaufmann SHE, editor. Walter de Gruyter; Berlin-New-York; 1996. pp. 205–242. [Google Scholar]
- 77.Bogdan C, Roelinghoff M. Int. J. Parasitol. 1998;28:121–134. doi: 10.1016/s0020-7519(97)00169-0. [DOI] [PubMed] [Google Scholar]
- 78.Borodkin VS, Milne FC, Ferguson MAJ, Nikolaev AV. Tetrahedron Lett. 2002;43:7821–7825. [Google Scholar]
- 79.Teodorovic P, Slattegard R, Oscarson S. Org. Biomol. Chem. 2006;4:4485–4490. doi: 10.1039/b614038f. [DOI] [PubMed] [Google Scholar]
- 80.Torres-Sanches MI, Zaccaria C, Buzzi B, Miglio G, Lombardi G, Polito L, Russo G, Lay L. Chem. Eur. J. 2007;13:6623–6635. doi: 10.1002/chem.200601743. [DOI] [PubMed] [Google Scholar]
- 81.Beckman EM, Porcelli S, Morita CT, Behar SM, Furlong ST, Brenner MB. Nature. 1994;372:691–694. doi: 10.1038/372691a0. [DOI] [PubMed] [Google Scholar]
- 82.Dubois M, Gilles KA, Hamilton JK, Rebers PA, Smith F. Anal. Chem. 1956;28:350–356. [Google Scholar]
- 83.Laemmli UK. Nature. 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- 84.Rogers ME, Sizova OV, Ferguson MAJ, Nikolaev AV, Bates PA. J. Infect. Dis. 2006;194:512–518. doi: 10.1086/505584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Hewitt MC, Seeberger PH. Org. Lett. 2001;3:3699–3702. doi: 10.1021/ol016631v. [DOI] [PubMed] [Google Scholar]
- 86.Arasappan A, Fraser-Reid B. J. Org. Chem. 1996;61:2401–2406. [Google Scholar]
- 87.Upreti M, Ruhela D, Vishwakarma RA. Tetrahedron. 2000;56:6577–6584. [Google Scholar]
- 88.Aslam M, Dent A. Bioconjugation: Protein Coupling Techniques for the Biomedical Sciences. Macmillan Reference; London: 1998. p. 41. [Google Scholar]
- 89.Toyokuni T, Dean B, Cai S, Boivin D, Hakomori S-I, Singhal AK. J. Am. Chem. Soc. 1994;116:395–396. [Google Scholar]
- 90.Reichel F, Ashton PR, Boons G-J. Chem. Commun. 1997:2087–2088. [Google Scholar]
- 91.Wiesmuller H-K, Bessler WG, Jung G. Hoppe-Seylers Z. Physiol. Chem. 1983;364:593–606. doi: 10.1515/bchm2.1983.364.1.593. [DOI] [PubMed] [Google Scholar]
- 92.Ruda K, Lindberg J, Garegg PJ, Oscarson S, Konradsson P. J. Am. Chem. Soc. 2000;122:11072–11076. [Google Scholar]
- 93.Ruda K, Lindberg J, Garegg PJ, Oscarson S, Konradsson P. Tetrahedron. 2000;56:3969–3975. [Google Scholar]
- 94.Mahoney AB, Sacks DL, Saraiva E, Modi G, Turco SJ. Biochemistry. 1999;38:9813–9823. doi: 10.1021/bi990741g. [DOI] [PubMed] [Google Scholar]
- 95.Moody SF, Handman E, McConville MJ, Bacic A. J. Biol. Chem. 1993;268:18457–18466. [PubMed] [Google Scholar]