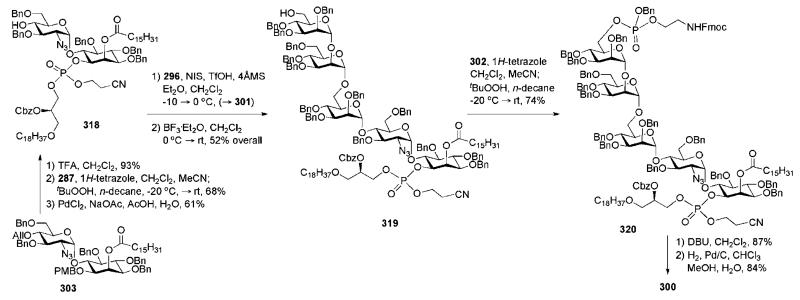
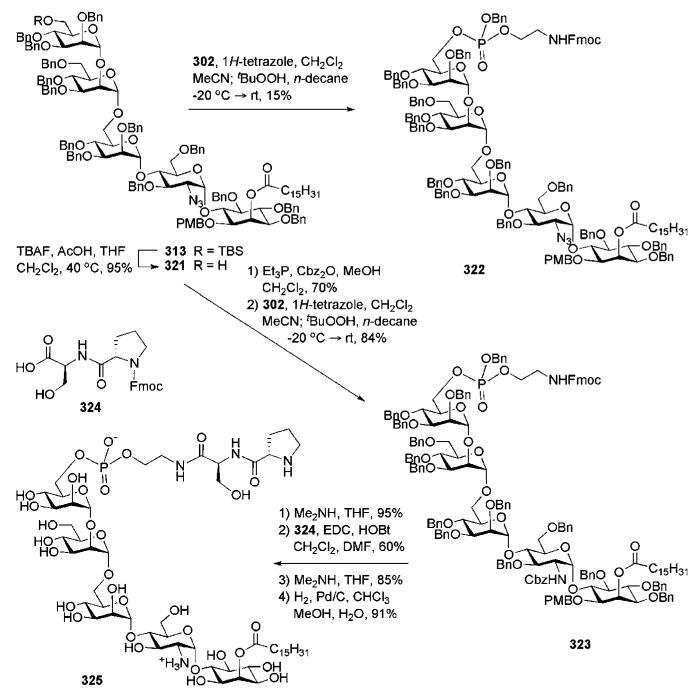
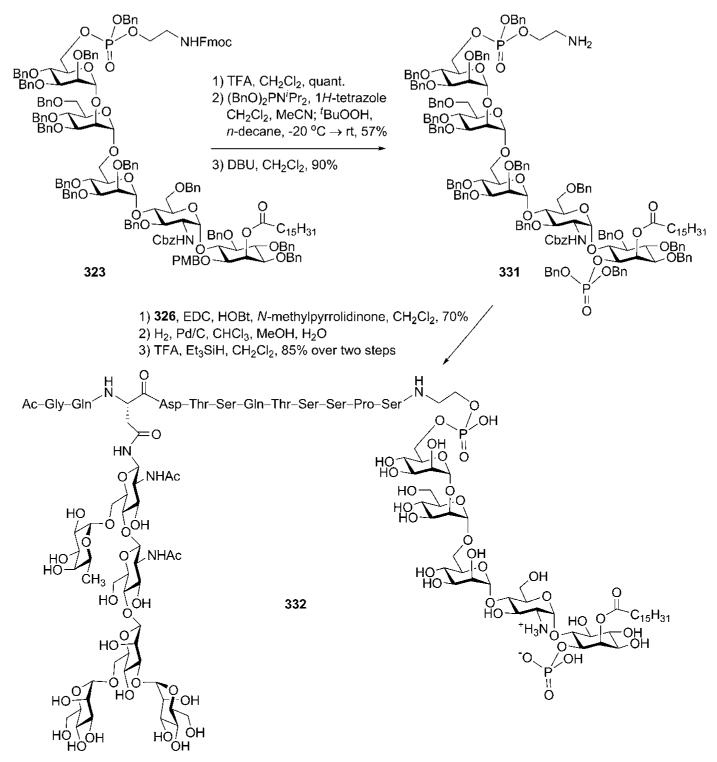
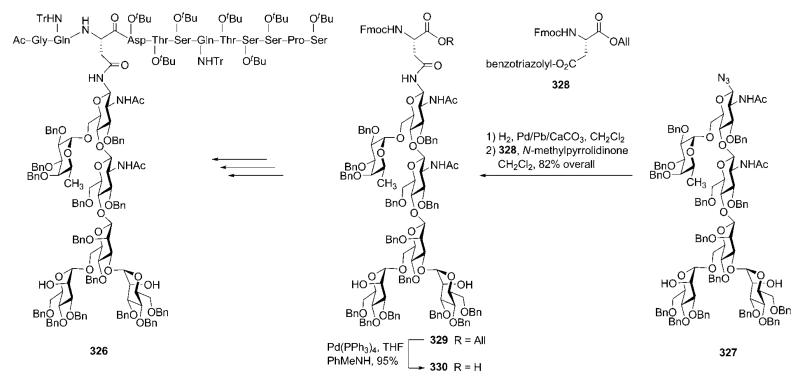
Abstract
Glycosylphosphatidylinositols (GPIs) are a class of natural glycosylphospholipids that anchor proteins, glycoproteins and lipophosphoglycans to the membrane of eukaryotic cells. GPI anchors are widely present in parasitic protozoa, where GPI-anchored mucins and phosphoglycans are abundant and form a dense protective layer (glycocalyx) on the surface of the parasites. This type of anchor appears to be present in these organisms with a much higher frequency than in higher eukaryotes. Since the first full assignment of a GPI structure in 1988, more than 50 glycosylphosphatidylinositols have been structurally characterised. The functions of GPI anchors (in addition to the clear one of linking the above biopolymers to membranes) have been extensively discussed. The high lateral mobility of GPIs and GPI-anchored polymers seems to actively facilitate the selective release of molecules from the cell surface and the exchange of membrane proteins between cells. There is also evidence that GPIs and/or their metabolites can act as secondary messengers, modulating biological events including insulin production, insulin-mediated signal transduction, cellular proliferation and cell–cell recognition. Their discovered role as mediators of regulatory processes makes the chemical preparation of these compounds and their analogues of great interest. This comprehensive review highlights the progress in the chemical synthesis of GPI anchors and related glycoconjugate structures from protozoan parasites, yeast and mammals in the last two decades. The synthesis of a structurally related prokaryotic glycoconjugate of Mycobacterium tuberculosis is also discussed.
1 Introduction
Glycosylphosphatidylinositols (GPIs) are a class of natural glycosylphospholipids that anchor proteins and glycoproteins (via their C-terminus) as well as lipophosphoglycans (via the reducing end of the chain) to the membrane of eukaryotic cells. GPI anchors are widely present in parasitic protozoa, which are the most diverse and amongst the most ancient group of organisms in the eukaryotic kingdom.1 This type of anchor is not unique to the protozoa, but it appears to be present in these organisms with a much higher frequency than in higher eukaryotes.2 Although some of the plasma membrane proteins of the parasites use transmembrane polypeptide anchors, most of their major cell-surface macromolecules are GPI-anchored.
The surface of protozoan parasites is covered with various glycoconjugates: glycoinositolphospholipids (GIPLs) and GPI-anchored glycoproteins (mucins) as well as GPI-anchored lipophosphoglycans (LPGs).2 The tremendous diversity of the glycoconjugates has implicated their critical importance in the life cycle of these organisms, often determining parasite survival and infectivity. The survival strategies of many protozoan parasites (Trypanosoma, Leishmania) involve the formation of an elaborate and dense cell-surface glycocalyx composed of diverse stage-specific glycoconjugates that form a protective barrier.3,4 The GIPLs form a dense layer on the surface of the parasite, with the GPI-anchored mucins and lipophosphoglycans projecting out and upwards from this layer.5
Historically, the first structural identification of a GPI anchor was that of Trypanosoma brucei variant surface glycoprotein (published in 1988 by Ferguson et al.6), followed by the structure of a GPI anchor of rat brain Thy-1 glycoprotein (published by Homans et al.7 in the same year). Since then, more than 50 GPI anchors have been structurally characterised, all having a common core structure of Manα1→4GlcNH2α1→6-myo-Ino1-OPO3-lipid.8
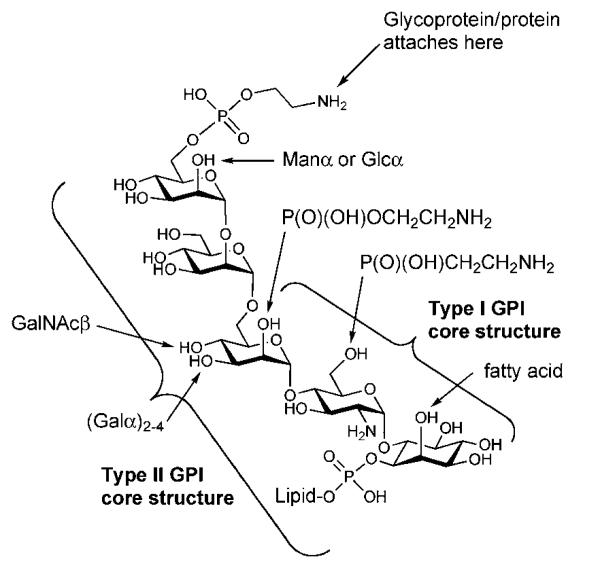
Two classes of GPI anchors have been identified (Fig. 1). Type I anchors have the above core structure and are usually found in protozoan parasites. These GPIs attach non-protein-bound glycoconjugates such as GIPLs and LPGs onto parasite cell membranes. Amongst the most complex structures is the lipophosphoglycan from Leishmania parasites, which is a predominant cell-surface glycoconjugate of Leishmania promastigotes.
Fig. 1.
Structure of glycosylphosphatidylinositol (GPI) anchors.
Type II anchors contain the expanded core structure, H2NC2H4OPO3–6Manα1→2Manα1→6Manα1→4GlcNH2α1→6-myo-Ino1–OPO3–lipid, and are found in mammals and lower eukaryotes including protozoan parasites and yeasts.2 Type II GPIs attach proteins or glycoproteins through their C-termini to the ethanolamine phosphate group at the non-reducing end of the glycan core. Many of type II GPIs diverge from their basic core structure and contain one or more species-specific side chains9 linked to specific positions of the core shown in Fig. 1. The side chains can comprise mono- or oligosaccharides, ethanolamine phosphate (specific for higher eukaryotes), 2-aminoethylphosphonate (specific for Trypanosoma cruzi GPIs) or an additional fatty acid residue. Another modification site is the lipid moiety, where various structures (diacylglycerol, acylalkylglycerol, lyso-alkylglycerol and ceramide) can be found.
The functions of GPI anchors (in addition to the clear one of linking the above biopolymers to membranes as well as their special importance on the parasitic cell-surface) have been extensively discussed.4,9–11 The high lateral mobility of GPIs and GPI-anchored polymers seems to actively facilitate the selective release of molecules from the cell surface and the exchange of membrane proteins between cells. There is also evidence that GPIs and/or metabolites of them can act as secondary messengers, modulating biological events including insulin production, insulin-mediated signal transduction, cellular proliferation and cell–cell recognition. Their discovered role as mediators of regulatory processes makes the chemical preparation of the compounds and their analogues of great interest. To date, two review papers describing the chemical synthesis of GPI-related derivatives have been published: the first by Gigg and Gigg in 199712 and the second by Guo and Bishop in 2004.13 Here, we discuss various methodologies and specific features for the chemical preparation of glycosylphosphatidylinositol anchors and related glyconjugates. We start our discourse from syntheses of GPIs from the lower eukaryotes including protozoan parasites and yeast, followed by the preparation of GPIs from mammals. The approaches developed for GPI syntheses have also been used for the synthesis of structurally related prokaryotic glycoconjugates, phosphatidylinositol mannosides of Mycobacterium tuberculosis, which are discussed in Section 11.
2 Chemical synthesis of glycosylphosphatidylinositols: general notes
GPIs are among the most complex classes of natural products, as they combine lipids, carbohydrates, myo-inositol and phosphate groups. Their structural complexity and recently discovered biological importance have inspired widespread chemical interest, and a number of synthetic approaches towards various GPIs (yeast, rat brain Thy-1, Trypanosoma brucei, Trypanosoma cruzi, Plasmodium falciparum, Toxoplasma gondii, Leishmania and sperm CD52) have been reported. Several problems are faced by those conducting GPI synthesis, such as synthesis of protected and optically pure myo-inositol derivatives, the stereoselective construction of the glycan core and the regioselective introduction of the side chains and phosphate moieties.
Positioning of side groups complicates the protecting group strategy. Positions on the glycan core have to be differentiated using orthogonal protecting groups that enable regioselective inclusion of the side groups (such as phosphoethanolamine and phospholipid) at later stages in the synthesis. Branching sugar chains also complicate the protecting group strategy of the main glycan backbone assembly and add a further problem of their (i.e. the branching sugars) stereoselective introduction. This can be affected by the nature of protecting groups on both the glycosyl donor and glycosyl acceptor and by the steric hindrance caused by other sugar residues or side groups in the oligosaccharide. If the right glycosidic linkage (α or β) is not obtained, this can result in a complete change to the synthetic strategy. In general, the stereochemistry of the glycosylation can be resolved by ingenious use of protecting groups as well as by varying glycosylation methods and conditions (e.g., experimentation with different promoters and solvents).
GPI structures are generally synthesized by linear or convergent means. The linear approach can be used to build the oligosaccharide from individual monosaccharides in a stepwise manner. This strategy relies on the selective activation of a monoglycosyl donor over the growing oligosaccharide chain. The convergent (or blockwise) approach constructs the oligosaccharide from smaller building blocks, which results in a fewer number of protecting group manipulations within the oligosaccharide chain.
Synthesis of the glycan core usually proceeds with the synthesis of an optically pure differentially protected myo-inositol derivative followed by the addition of appropriately protected α-linked d-glucosamine. The next stage involves the formation of the remaining glycan core either by linear or convergent means. The glycan core is then decorated with the ethanolamine phosphate and phospholipid moieties before the global deprotection to provide the final GPI structure.
3 Syntheses of a GPI anchor of Trypanosoma brucei
3.1 Combined linear-convergent synthesis by the Ogawa group
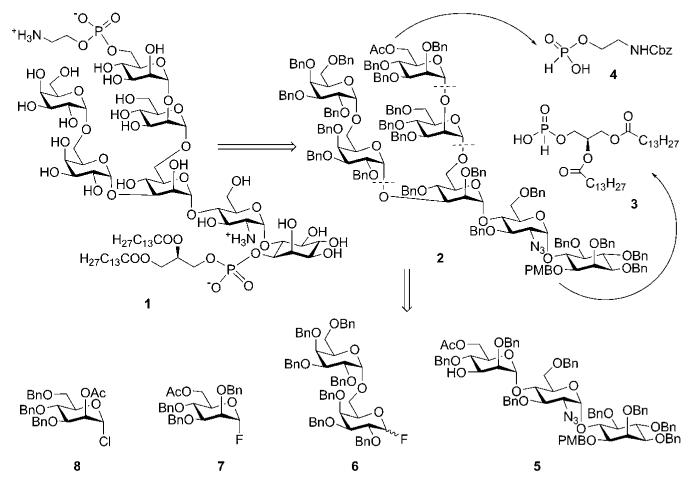
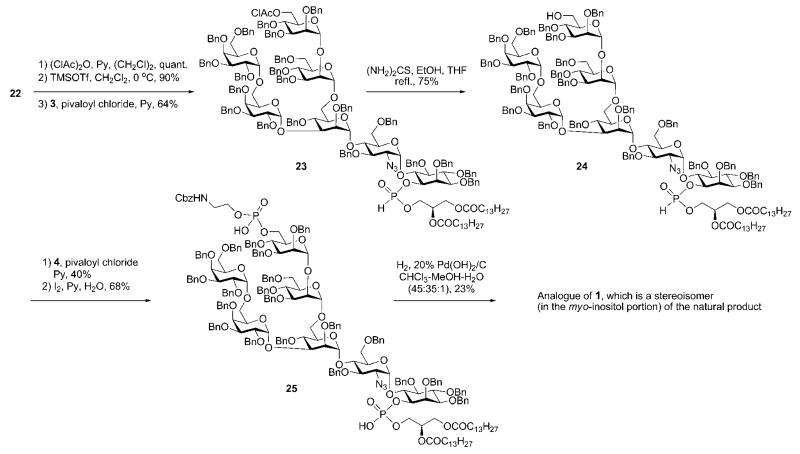
The first total synthesis of a glycosylphosphatidylinositol was achieved in 1991–1992 by Ogawa and co-workers, who published the synthesis of a GPI anchor of T. brucei variant surface glycoprotein.14,15 As indicated in Scheme 1, the synthetic plan for the preparation of a GPI anchor 1 was to assemble the glycan core 2 and then introduce phosphoethanolamine first, followed by diacylglycerol phosphate through the H-phosphonate precursors 4 and 3, correspondingly. Glycan core 2 was further disconnected into four smaller building blocks 5–8. This synthesis of a GPI anchor did not involve the preparation of the pseudodisaccharide (i.e., azidoglucose-inositol) block separately. Instead, the myo-inositol acceptor 12 (Scheme 2) was glycosylated with the disaccharide glycosyl fluoride 16 to form the pseudotrisaccharide 5.
Scheme 1.
Retrosynthesis of a GPI anchor of T. brucei (the Ogawa group).
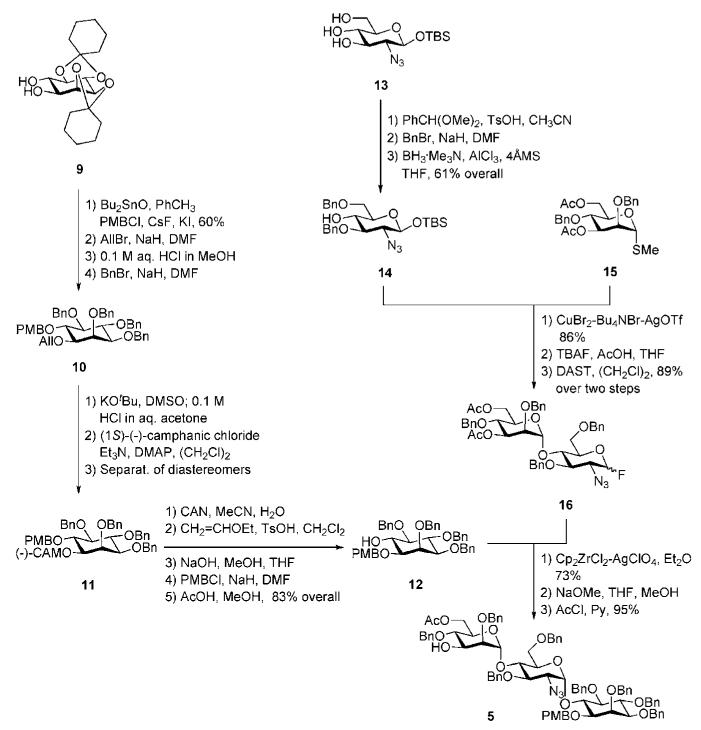
Scheme 2.
myo-Inositol enantiomeric resolution and the synthesis of a pseudotrisaccharide derivative (the Ogawa group).
The myo-inositol acceptor 12 was constructed (Scheme 2) starting from the previously synthesised racemic dicyclohexylidene derivative 9.16 The latter was subjected to regioselective 6-O-methoxybenzylation assisted by Bu2SnO, 1-O-allylation, followed by acidic removal of cyclohexylidene protecting groups and per-O-benzylation of the hydroxyl groups to afford racemate 10. This was O-deallylated, and treated with (1S)-(−)-camphanic acid chloride to afford the corresponding diastereomeric mixture of (1S)-(−)-camphanoyl-d,l-myo-inositols. Thus, enantiomeric resolution of myo-inositol was achieved by addition of a chiral (1S)-(−)-camphanate group17 and then separation of the diastereomeric mixture by silica gel chromatography. Conversion of the chiral myo-inositol derivative 11 to the required glycosyl acceptor 12 was readily achieved in 83% yield over 5 steps (O-demethoxybenzylation, 6-O-vinylation, hydrolysis of the camphanate, introduction of the p-methoxybenzyl group at 1-OH and then cleavage of the vinyl ether).
The glycobiosyl fluoride 16 (Scheme 2) was constructed from the methyl thiomannoside donor 1518 and azidoglucose acceptor 14 as described in Scheme 2. The acceptor 14 was obtained19 in 61% overall yield via sequential 4,6-O-benzylidenation of azidoglucoside 13, 3-O-benzylation and finally regioselective cleavage of benzylidene group. CuBr2–Bu4NBr–AgOTf-promoted glycosylation of 14 with methyl thiomannoside donor 15, followed by removal of the anomeric silyl group and conversion of the hemiacetal intermediate to the glycosyl fluoride, gave the glycobiosyl fluoride donor 16 (89%; α:β 2 : 3). Cp2ZrCl2–AgClO4 effected coupling of the glycosyl fluoride 16 to the myo-inositol acceptor 12, furnishing the corresponding α-linked pseudotrisaccharide in 73% yield (plus 20% of the β-anomer),19 in which the two acetyl groups were removed and the 6-hydroxyl of mannose was selectively acetylated to afford pseudotrisaccharide 5 in 95% yield.
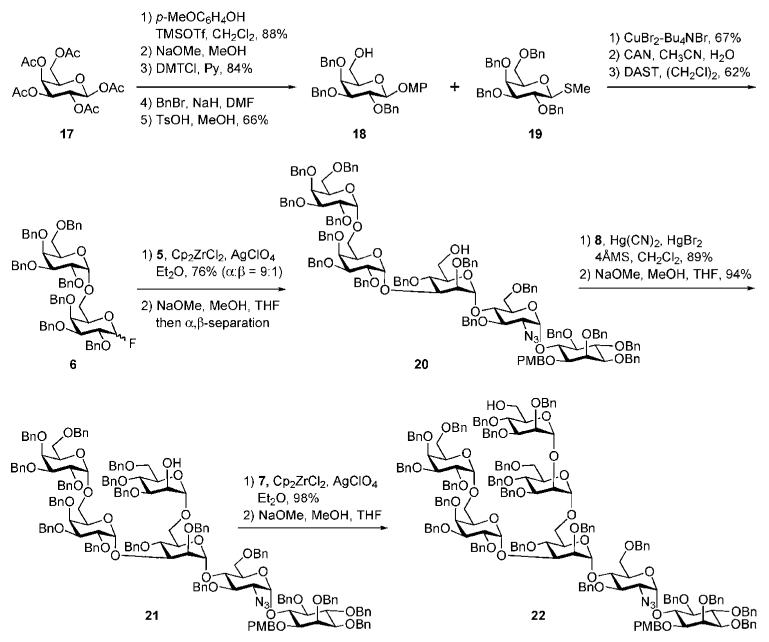
The galactobiose synthon 6 was constructed starting from peracetylated galactose 17 as depicted in Scheme 3. Glycosylation of p-methoxyphenol under the influence of trimethylsilyl trifluoromethanesulfonate (TMSOTf) with the donor 17, followed by sequential O-deacetylation, selective 6-O-tritylation with p,p′-dimethoxytrityl chloride (DMTCl), exhaustive O-benzylation and finally removal of the DMT group afforded the desired acceptor 18 in 51% overall yield. This was glycosylated with the thiogalactoside 1920 in presence of CuBr2–Bu4NBr to afford the corresponding (α1→6)-linked galactobiose, which was further subjected to cerium(IV) ammonium nitrate (CAN)-mediated cleavage of p-methoxyphenyl group followed by conversion of the hemiacetal intermediate to the glycosyl fluoride donor 6 in 62% yield (α:β = 2 : 3).
Scheme 3.
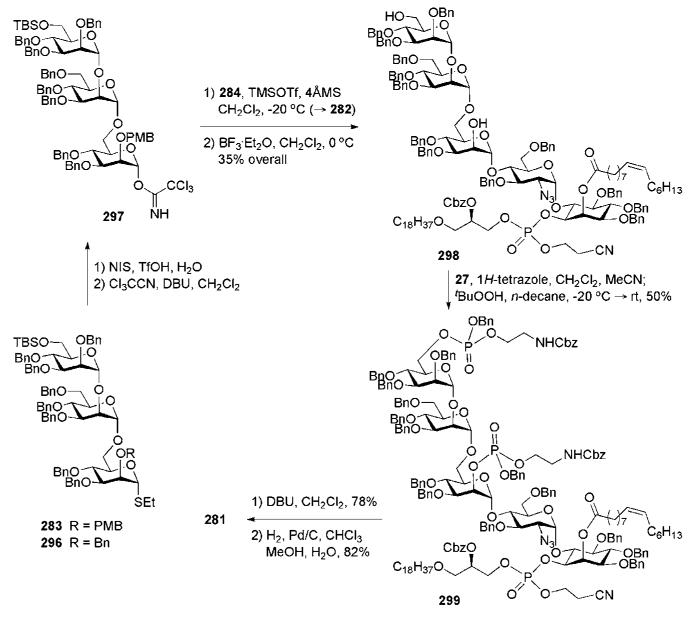
GPI branched glycan core assembly (the Ogawa group).
The tricky coupling between the galactobiose 6 and the pseudotrisaccharide 5 (Scheme 3) was achieved under the influence of Cp2ZrCl2–AgClO4 in 76% yield, forming preferentially the α-linked product (α:β = 9 : 1). After the removal of the acetyl group the required α-linked pseudopentasaccharide 20 was isolated. The latter was glycosylated with the mannosyl chloride 8 in the presence of a mixture of HgBr2 and Hg(CN)2 as promoter to offer the corresponding branched pseudohexasaccharide in 89% yield, in which the acetyl group was removed to give the acceptor 21. The branched pseudoheptasaccharide 22 was then obtained by glycosylation of 21 with the mannosyl fluoride 7 using the Cp2ZrCl2–AgClO4 mixture as promoter, followed by removal of the acetyl group.
Due to the problems associated with the CAN-assisted removal of the PMB group from C-1 of the myo-inositol unit after the introduction of the ethanolamine phosphate at C-6 in mannose-3, the synthesis was completed (Scheme 4) initially by introduction of the phospholipid and then addition of the ethanolamine phosphate moiety. 6-O-Chloroacetylation in mannose-3, then TMSOTf-mediated removal of the methoxybenzyl group at C-1 of myo-inositol followed by pivaloyl chloride-assisted condensation with the H-phosphonate 3, afforded the H-phosphonate 23. The chloroacetyl group of the latter compound was cleaved with thiourea to yield 24. This was then phosphitylated with N-Cbz-protected aminoethyl H-phosphonate 4 to give the corresponding pseudoheptasaccharide (40%; as a mixture of four diastereomers at P atoms), in which the two H-phosphonate moieties were then oxidized with iodine in aqueous pyridine to give the fully protected GPI 25 in a 68% yield. Finally, global deprotection by hydrogenolysis in the presence of Pd(OH)2/C in CHCl3–MeOH–H2O solution gave the first synthetic GPI anchor in 23% yield.
Scheme 4.
Successive phosphorylation steps and global deprotection (the Ogawa group).
It was later proved12,21,22 that the above synthetic study was based on the incorrect stereoisomer of the myo-inositol derivative 11 (Scheme 2), which was chosen by the Ogawa group erroneously, and thus provided the wrong stereoisomer of the glycosyl acceptor 12. Because of this misassignment of the absolute configuration of the myo-inositol derivatives used, the final deprotected synthetic GPI anchor appeared to be a stereoisomer (in the myo-inositol portion) of the natural T. brucei GPI anchor 1.
In summary, the first total synthesis of a GPI anchor was achieved by employing glycosyl fluoride, glycosyl chloride and thioglycoside derivatives as glycosyl donors, and benzyl groups as a permanent protecting group, as well as the use of H-phosphonate chemistry to introduce two different phosphodiester units.
3.2 Convergent synthesis by the Ley group
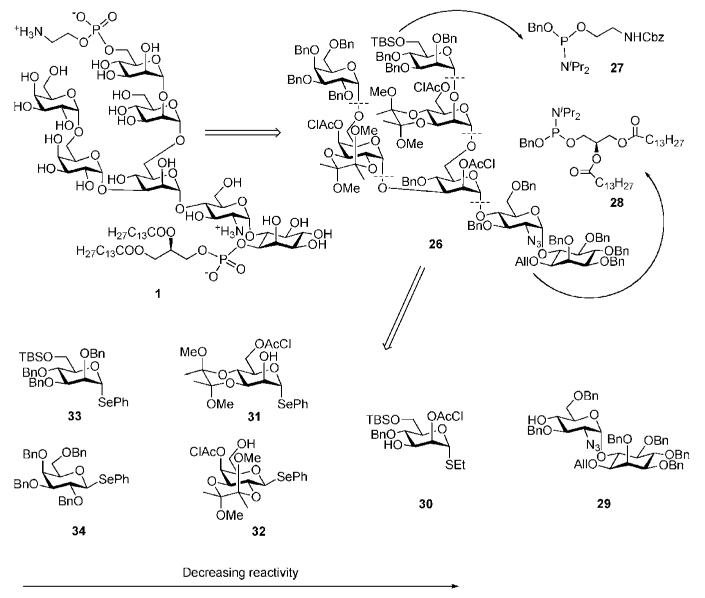
Synthesis of the GPI anchor 1 by the Ley group in 1998 appeared to be the first chemical preparation of a real GPI anchor of T. brucei variant surface glycoprotein.23,24 The synthetic strategy relied on the use of (i) butanediacetal (BDA) groups, chloroacetic esters and benzyl ethers as permanent protecting groups, (ii) selenoglycosides and thioglycosides as glycosyl donors that allowed as few manipulations on the oligosaccharide core as possible, and (iii) a chiral bis(dihydropyran) for enantiomeric resolution of myo-inositol. Ley brought together the 1,2-diacetal protecting group studies from his laboratory to simplify the puzzle of putting together an oligosaccharide chain.
As indicated in Scheme 5, the two phosphate substituents in the GPI anchor 1 were retrosynthetically disconnected as phosphoramidites 27 and 28, leaving corresponding protected glycan core 26 for further simplification. The group followed a convergent route for assembling the pseudoheptasaccharide 26. Protecting groups may exhibit a distinctive influence on the reactivity of glycosyl donors. The authors successfully used this effect for rapid assembly of the glycan core with just one protecting group manipulation needed. The core was assembled in just six steps from the six building blocks 29–34, taking advantage from the reactivity-tuning effects caused by protecting groups and the use of appropriate anomeric leaving groups.
Scheme 5.
Retrosynthesis of a GPI anchor of T. brucei (the Ley group).
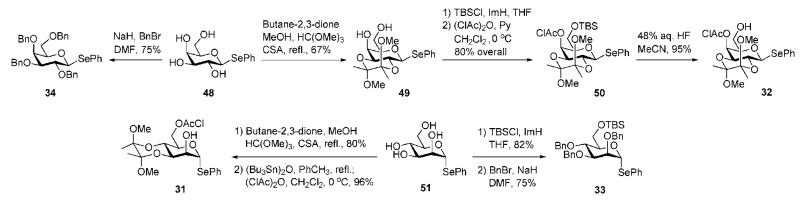
The anomeric PhSe group in compounds 31–34 has higher reactivity towards electrophilic activation than the EtS counterpart in 30 due to the greater polarisability of the selenium atom. The selenoglycosides 33 and 34 are more reactive than the selenoglycosides 31 and 32 due to the nature of the ‘arming–disarming’ effects of the protecting groups. The O-benzyl ‘arming’ groups in 33 and 34 enhance the reactivity of the donors, whereas the butanediacetal (BDA) and O-chloroacetyl groups in acceptors 31 and 32 exhibit deactivating (‘disarming’) effects on their anomeric leaving groups.
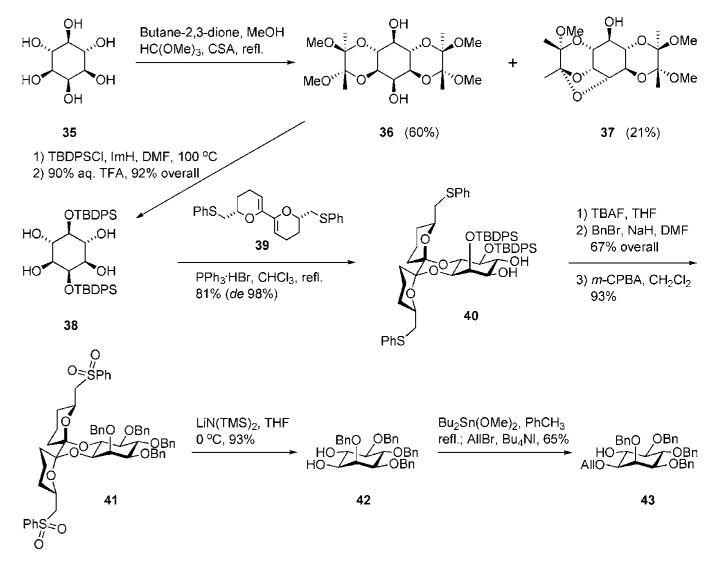
Desymmetrisation or chiral enantiomeric resolution of myo-inositol molecule remains a problem, despite significant efforts invested towards establishing methods to obtain enantiomerically pure myo-inositol derivatives. The Ley group reported a route to both chiral d-myo-inositols and l-myo-inositols employing a chiral bis(dihydropyran). This method was illustrated by the synthesis of d-myo-inositol derivative 43 (Scheme 6). Regioselective transformation of myo-inositol 35 to bis(dispoke) acetal 36 was easily achieved on treatment with butane-2,3-dione.25 The side product 37 was removed by simple recrystallization. It is important that butane-2,3-dione forms 1,2-trans-diacetal derivatives (i.e., involving di-equatorial 1,2-diols), contrasting with isopropylidene and benzylidene acetal formation, when 1,2-cis-acetals (i.e., involving axial/equatorial 1,2-diols) are the favoured products. Butane-2,3-dione initially adds to the most reactive hydroxyl group in 35 (i.e., the equatorial hydroxyl groups vicinal to the axial 2-hydroxyl) and then cyclizes with a vicinal equatorial OH to form a six-membered ring.
Scheme 6.
myo-Inositol chiral desymmetrisation (the Ley group).
O-Silylation of the 1,6:3,4-bis(diacetal) 36 with TBDPS chloride followed by removal of the BDA groups with aqueous TFA gave tetraol 38, which was desymmetrised with chiral bis(dihydropyran) 39 yielding 40 as a single diastereoisomer in 81% yield. This product was subsequently O-desilylated, per-O-benzylated and oxidized to form bis(phenylsulfone) 41. Exposure of 41 to lithium hexamethyldisilazide removed the dispiroacetal, furnishing the chiral diol 42, which in turn was selectively allylated on the C-1 hydroxyl group via dibutyltin acetal formation to offer the desired myo-inositol acceptor 43.
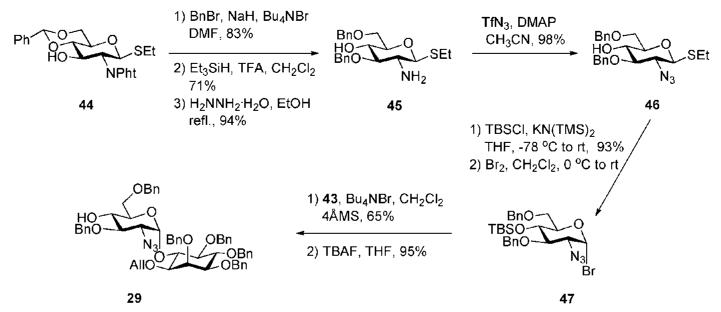
Building block 47 (Scheme 7) required for the construction of pseudodisacchride 29 was synthesised from the known phthalimide derivative 44.26 Sequential 3-O-benzylation, reductive benzylidene acetal ring opening and hydrazine hydrate-assisted cleavage of the phthalimido protection transform 44 into 2-amine 45. Further transformation into 2-azide 46 was accomplished (98%) employing triflic azide (TfN3) in the presence of DMAP. Initial O-silylation of the alcohol 46 with TBSCl followed by bromination gave glycosyl donor 47, which was coupled with myo-inositol acceptor 43 using Lemieux’s halide inversion protocol.27 This afforded only the desired α-linked pseudodisaccharide (65%), which on de-O-silylation furnished the pseudodisaccharide acceptor 29.
Scheme 7.
Synthesis of a pseudodisaccharide derivative (the Ley group).
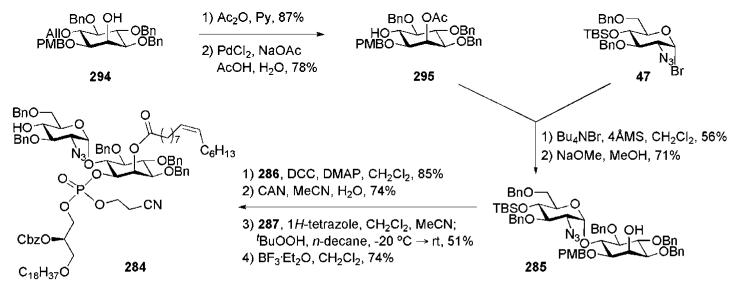
Readily available phenyl 1-selenogalactoside 4828,29 was converted into two required building blocks 32 and 34 employing standard procedures (Scheme 8). Consecutive 2,3-O-protection with butane-2,3-dione (→49), 6-O-silyation with TBSCl, O-chloroacylation (→50) and desilylation gave the acceptor 32. Conventional O-benzylation of the same tetraol 48 afforded the galactoside donor 34. The mannose building blocks 31 and 33 were synthesised from phenyl 1-selenomannoside 51.30 BDA protection of the 3,4-diol, followed by 6-O-chloroacetylation via 6-tributyltin ether formation produced the acceptor 31. Compound 33 was prepared by selective 6-O-silylation of 51, followed by per-O-benzylation.
Scheme 8.
Synthesis of d-galactose and d-mannose building blocks (the Ley group).
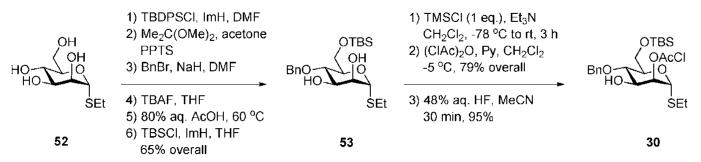
Synthesis of the central thiomannoside 30 (Scheme 9) required chloroacetate group at the 2-position to allow further anchimeric assistance and differentiation of 3- and 6-OH groups for regioselective glycosylation. The tetraol 52 was silylated at the 6-OH with TBDPSCl, the 2,3-diol was isopropylidene-protected and the 4-OH group was benzylated. Successive desilylation, acidic removal of acetonide and silylation at the 6-position with TBSCl gave the 2,3-diol 53 in 65% overall yield. Selective silylation of the equatorial 3-hydroxyl group with trimethylsilyl chloride (TMSCl) followed by standard introduction of the chloroacetyl group at 2-position and 3-O-desilylation furnished the desired middle building block 30.
Scheme 9.
Synthesis of a central d-mannose building block (the Ley group).
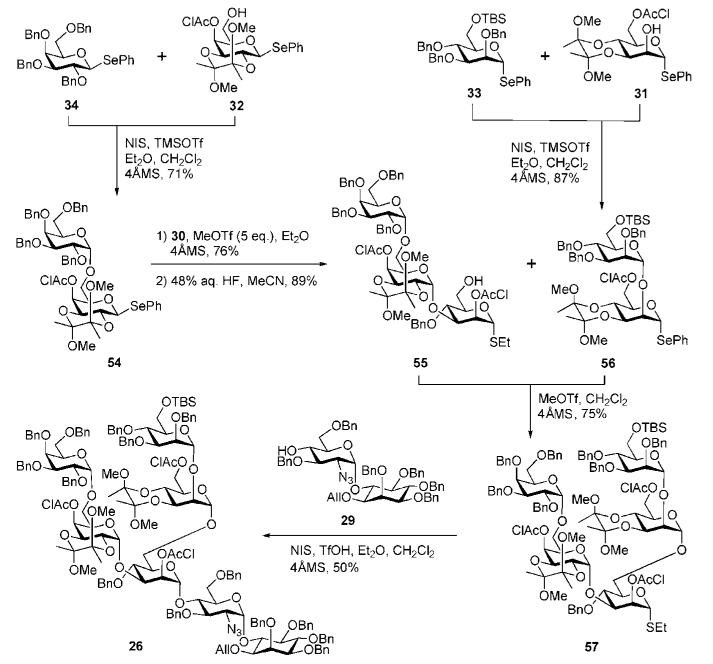
With all the building blocks in hand, the protected glycan core 26 was then assembled (Scheme 10). The assembly began with the stereoselective coupling of the fully benzylated donor 34 with the acceptor 32 under the influence of N-iodosuccinimide (NIS) and TMSOTf to afford the α-linked disaccharide fragment 54 (71%). The combined deactivating effects of the BDA and the chloroacetyl groups in 32 are crucial in avoiding any homocoupling. The thiomannoside acceptor 30 was then 3-O-glycosylated with the galactobioside donor 54 under the influence of methyl triflate (MeOTf) (76%) followed by 6-O-desilylation to furnish the requisite trisaccharide alcohol 55. Glycosylation of the acceptor 55 with mannobioside donor 56 (5 eq.) [prepared, in turn, by coupling of 33 and 31 (87%) in a similar fashion to the formation of compound 54] under the influence of MeOTf furnished the branched pentasaccharide 57 (75%). Finally, coupling of the pseudodisaccharide acceptor 29 and the pentasaccharide donor 57 was promoted with NIS/TfOH to offer the branched pseudoheptasaccharide core 26 (50%).
Scheme 10.
GPI branched glycan core assembly (the Ley group).
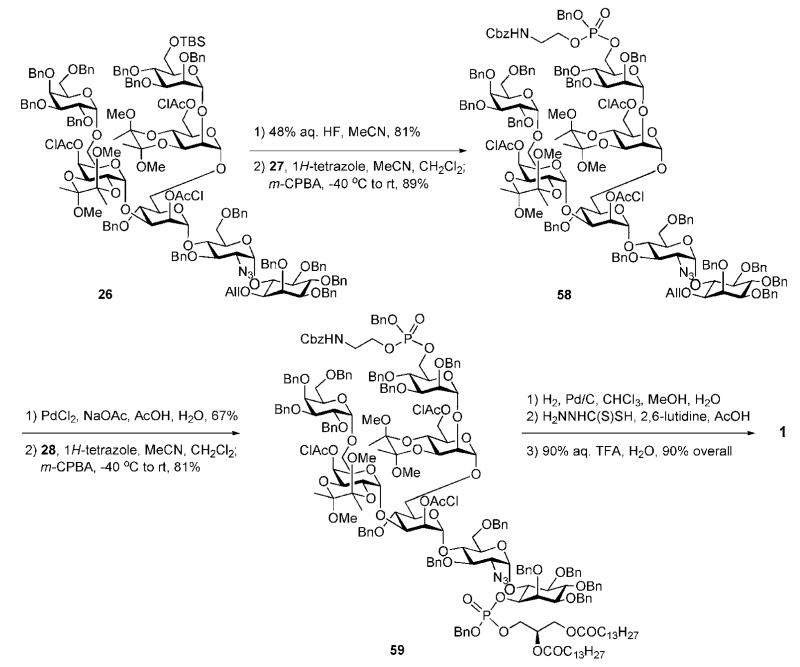
The glycan core 26 was further elaborated to the protected GPI anchor 59 (Scheme 11) by making use of phosphoramidite chemistry, which had been successfully applied earlier in syntheses of GPIs from yeast by Schmidt and co-workers31,32 and rat brain Thy-1 by Campbell and Fraser-Reid.33,34 Removal of the TBS group at the 6-position in mannose-3 with aq. HF enabled the introduction of the ethanolamine phosphate moiety via 1H-tetrazole-assisted phosphitylation with phosphoramidite 27 and subsequent oxidation of the formed phosphite triester with m-chloroperbenzoic acid (m-CPBA) to afford the phosphotriester 58 (89%). This was subjected to O-deallylation with PdCl2 and 1H-tetrazole-assisted phospholipidation with diacylglyceryl phosphoramidite 28, followed by oxidation with m-CPBA to furnish the fully protected compound 59 (81%) as a mixture of four diastereoisomers at the P atoms.
Scheme 11.
Successive phosphorylation steps and global deprotection (the Ley group).
A global deprotection sequence involving, first, palladiumcatalysed hydrogenolysis to remove the O-benzyl and the N-Cbz groups and to reduce the azide to an amino group, followed by O-dechloroacetylation and TFA-assisted deacetalisation gave the required GPI anchor 1 in 90% overall yield.
To summarise, Ley and co-workers developed an efficient and highly convergent synthetic strategy of T. brucei VSG GPI anchor 1, which is adaptable to the preparation of other GPI anchors. The strategy is based on (3 + 2 + 2) building block assembly for construction of the glycan core, use of BDA and chloroacetate protecting groups to tune the reactivity of leaving groups in the glycosyl donors, and the use of bis(dihydropyran) to desymmetrise myo-inositol.
4 Synthesis of a glycoconjugate of Toxoplasma gondii by the Schmidt group
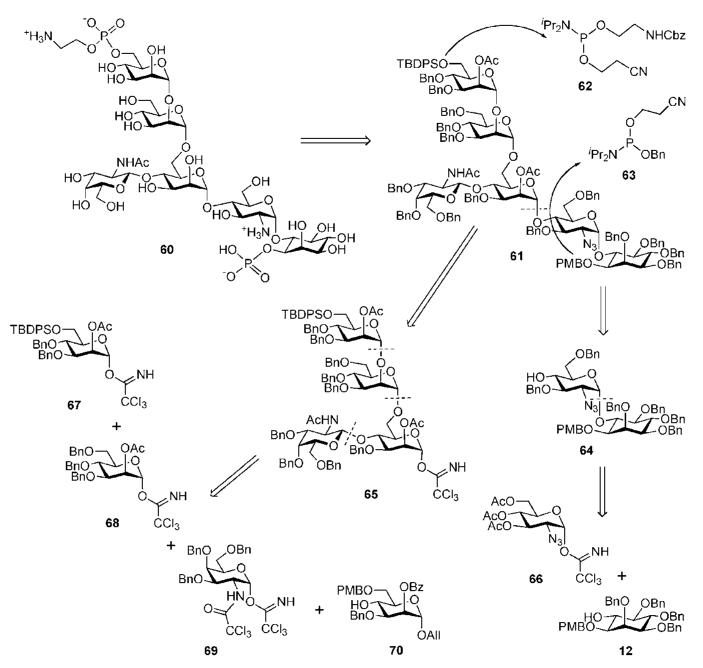
Toxoplasma gondii is an ubiquitous intracellular protozoan parasite. It is a causative agent for congenital infection (toxoplasmosis) and severe and often lethal encephalitis in the course of acquired immunodeficiency syndrome (AIDS). An oligosaccharide-containing small antigen has been illustrated to express immunological characteristics suitable for serological diagnosis of acute toxoplasmosis.35 The structure of this antigen was identified to be a family of protein-free GPI glycolipids, from which the structures of two core glycans (A and B, not shown here) were elucidated. Immunological studies revealed that only type B GPIs containing a Glcα1→4GalNAc side chain linked to the first Man moiety were recognised by sera from infected humans, suggesting that the unique glucose modification is essential for immunogenicity.36 In order to understand this phenomenon, synthesis of the type A GPI glycan of T. gondii (containing GalNAc side chain instead) 60 (Scheme 12) was required.
Scheme 12.
Retrosynthesis of a GPI-related glycoconjugate of T. gondii (the Schmidt group).
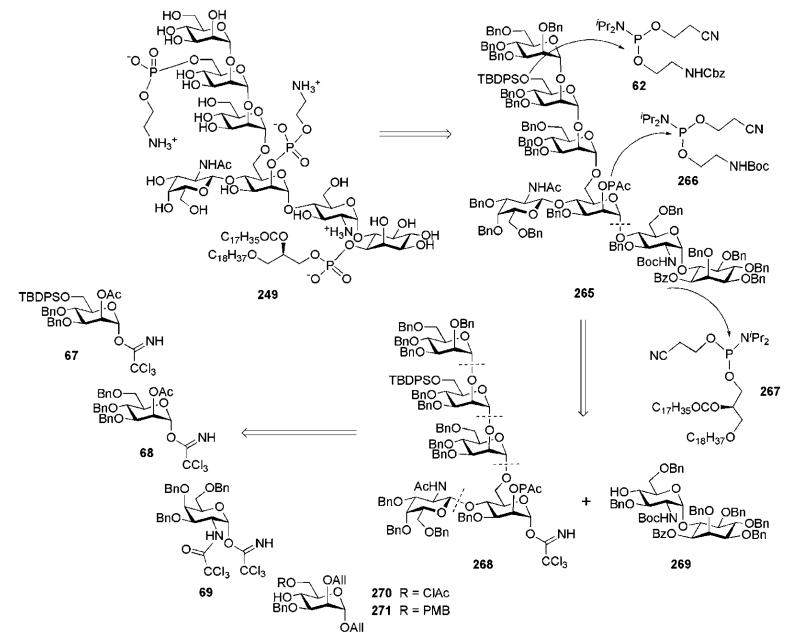
A convergent and versatile strategy for the synthesis of phosphorylated T. gondii GPI anchor pseudohexasaccharide 60 was developed by Schmidt and co-workers.37 They used a strategy that relies on late-stage phosphorylation (after the assembly of the glycan core 61) with N,N-diisopropylphosphoramidites 62 and 63, and the use of appropriate protecting groups (Scheme 12). The Schmidt group syntheses of GPI anchors rely, in general,31,32,37,38 on the trichloroacetimidate chemistry together with the participating effect of acetic esters at C-2 of the d-mannose glycosyl donors to confer α-selectivity in every glycosylation. In the retrosynthesis of the branched glycan core 61, the α-glycoside linkage between the central d-mannose and d-glucosamine units is disconnected, affording the building blocks 64 and 65. The tetrasaccharide 65 could be easily prepared from building blocks 67–70, while the synthesis of the pseudodisaccharide 64 is challenging.
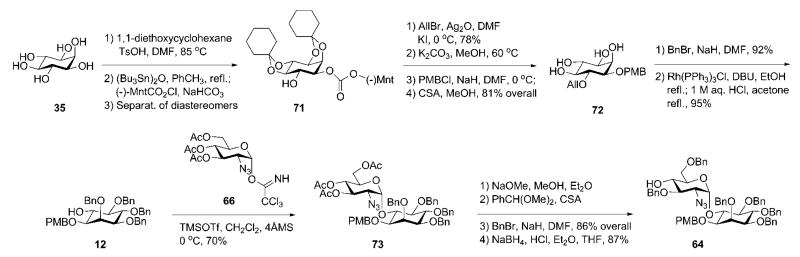
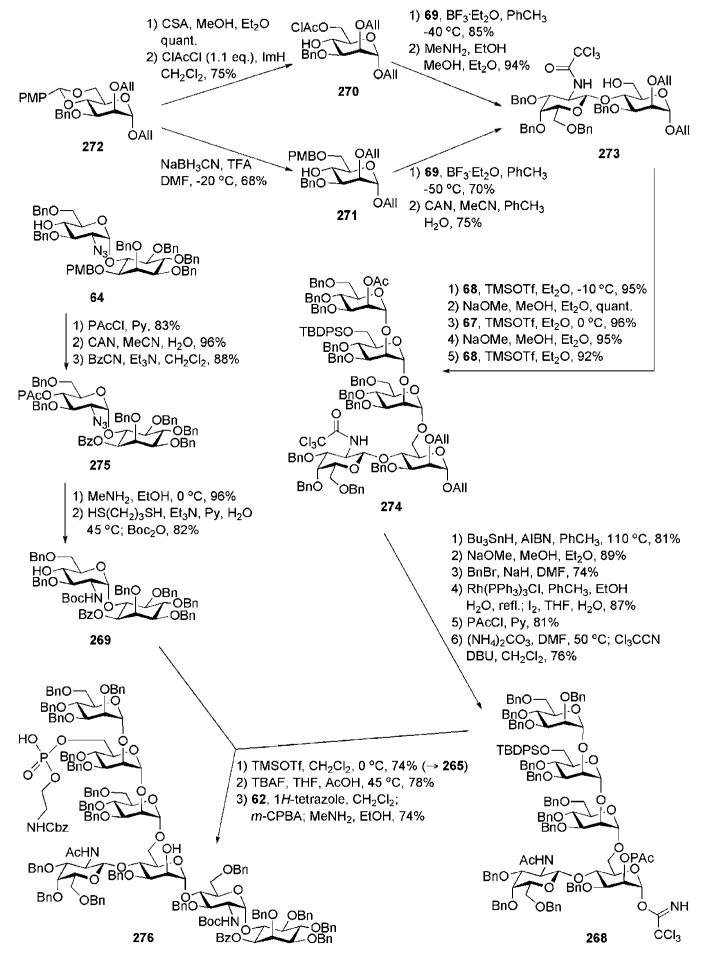
Synthesis of the pseudodisaccharide 64 is based upon the conversion of the chiral myo-inositol derivative 71 into the required product (Scheme 13). O-Cyclohexylidenation of myo-inositol 35,16,39 followed by enantiomeric resolution via addition of a chiral (−)-menthylformate group and then separation of diastereomers by crystallisation gave the desired d-myo-inositol derivative 71.31,40 Subsequent consecutive 6-O-allylation, replacement of the (−)-menthyloxycarbonyl group with p-methoxybenzyl group and finally acid-catalysed cleavage of the cyclohexylidene groups offered the required tetraol 72. Perbenzylation of OH groups in 72, followed by 6-O-deallylation using Wilkinson’s catalyst and acidic treatment, gave the 6-OH myo-inositol acceptor 12. The latter was glycosylated with the trichloroacetimidate donor 6641 in the presence of TMSOTf to furnish the desired (α1→6)-linked pseudodisaccharide 73 in 70% yield. The 2-azido group in 66 served as a non-participating group that assists in the formation of the α-glycoside linkage. Compound 73 was subjected to sequential de-O-acetylation, 4′,6′-O-benzylidenation, 3′-O-benzylation and finally reductive ring opening of the benzylidene acetal with NaBH4 in the presence of HCl, to furnish the pseudodisaccharide acceptor 64.
Scheme 13.
myo-Inositol enantiomeric resolution and the synthesis of a pseudodisaccharide derivative (the Schmidt group).
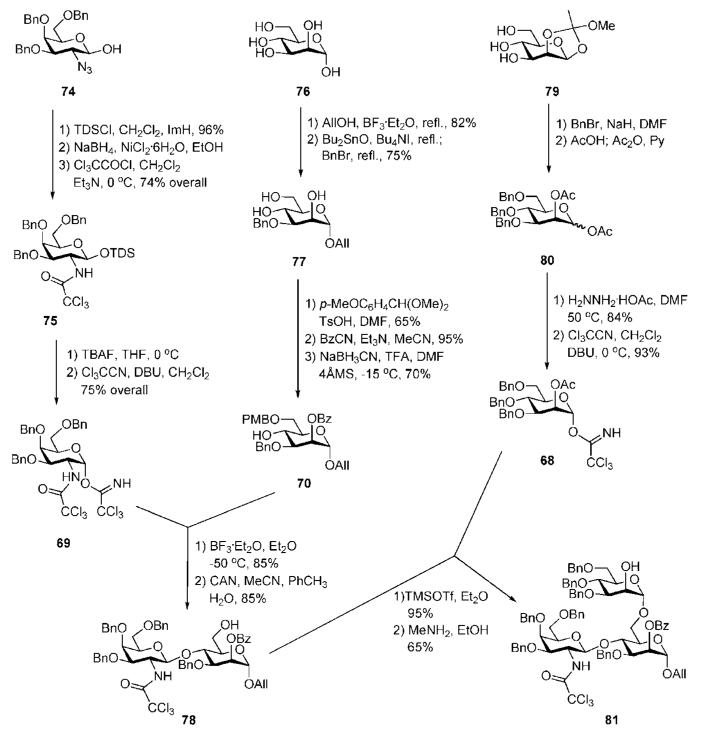
The branched trisaccharide 81 (Scheme 14) was assembled from three monosaccharide synthons, 68, 69 and 70. Mannosyl donor 68 was derived32 from 1,2-orthoester 79 (available from d-mannose)42 via the 1,2-di-O-acetate 80.43 Compound 70 was prepared from d-mannose 76 in five steps,44 while the d-galactosamine donor 69 was made from the 2-azido derivative 74.41
Scheme 14.
The synthesis of monosaccharide building blocks and assembly of a branched trisaccharide derivative (the Schmidt group).
Anomeric O-allylation of 76 and subsequent Bu2SnO-mediated selective 3-O-benzylation afforded the desired triol 77 in 61% overall yield. This was treated with p-methoxybenzaldehyde dimethylacetal to form 4,6-O-arylidene intermediate, which was 2-O-benzoylated with benzoyl cyanide. The 4,6-acetal ring was reductively opened using NaBH3CN–TFA, furnishing the 4-OH mannose derivative 70. The nature of protecting groups in the acceptor 70 provides the desired regio- and stereoselective glycosylation sequence and further transformation into a glycosyl donor, permitting later the α-selective glycosylation of the pseudodisaccharide 64.
The d-galactosamine donor 69 was derived45 from the azide 74 via silylation of the 1-OH group with thexyldimethylsilyl chloride [TDSCl; (2,3-dimethylbut-2-yl)dimethylsilyl chloride], reduction of the 2-azido group and trichloroacetylation of amine to furnish 75, which on successive 1-O-desilylation and anomeric O-trichloroacetimidation offered 69. The N-trichloroacetyl protecting group was introduced in order to ensure high glycosyl donor properties and anchimeric assistance for β-glycoside bond formation. The trichloroacetimidate 69 and the acceptor 70 were coupled under the influence of BF3·Et2O to afford stereoselectively the corresponding disaccharide (85%), in which the p-methoxybenzyl group was removed (→78) to enable TMSOTf-promoted coupling with the mannosyl donor 68, yielding the branched trisaccharide (95%) that was O-deacetylated to give the trisaccharide acceptor 81.
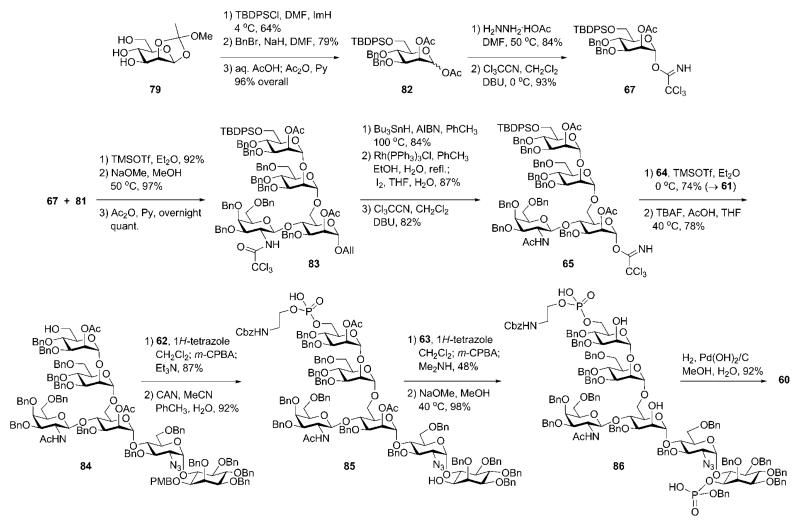
The third mannose building block 67 (Scheme 15) was derived32 from the orthoester 79. 6-O-Silylation, O-benzylation, subsequent acid-catalysed orthoester opening and then O-acetylation afforded 82. Selective anomeric deacetylation with hydrazinium acetate gave the hemiacetal, which was treated with trichloroacetonitrile in presence of DBU to give the trichloroacetimidate donor 67. TMSOTf-promoted stereoselective glycosylation of the branched trisaccharide acceptor 81 with the donor 67 gave the corresponding tetrasaccharide (92%), in which the benzoic ester on mannose-1 was then replaced with acetate via the standard O-deacylation–O-acetylation procedure (→83).
Scheme 15.
T. gondii GPI branched glycan core assembly, successive phosphorylation steps and global deprotection (the Schmidt group).
Transformation of the N-trichloroacetyl group of the tetrasaccharide 83 into the N-acetyl group was carried out by homolytic reduction and removal of the anomeric allyl group under the influence of Wilkinson’s catalyst, followed by the reaction of the hemiacetal with trichloroacetonitrile to afford trichloroacetimidate donor 65. The pseudodisaccharide 64 was then coupled onto the growing chain (→61, 74%) to furnish, after O-desilylation, the pseudohexasaccharide 84.
The introduction of phosphate residues onto 84 (Scheme 15) was achieved via 1H-tetrazole-assisted phosphitylation reactions. First, treatment with the phosphoramidite 62 furnished the phosphite intermediate, which was oxidised to the phosphotriester with m-CPBA followed by treatment with Et3N to remove the cyanoethyl P-protecting group. The following CAN-assisted removal of the p-methoxybenzyl group afforded the aminoethyl phosphate 85. Phosphorylation of 85 with benzylcyanoethyl-N,N-diisopropylaminophosphoramidite 63 in a similar fashion, followed by cyanoethyl cleavage with Me2NH and O-deacetylation, furnished the diphosphorylated compound 86, which by hydrogenolytic O-debenzylation afforded the target compound 60 in good yield and purity.
5 Synthesis of a glycoconjugate of Leishmania by the Konradsson–Oscarson group
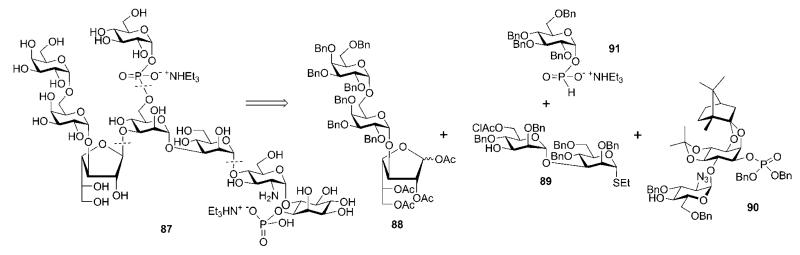
Two Swedish groups, those of Oscarson and Konradsson, jointly reported46 the synthesis of Leishmania lipophosphoglycan core phosphorylated heptasaccharyl myo-inositol 87 (Scheme 16) found in cell-surface structures of Leishmania parasites. The synthesis was accomplished using a convergent (3 + 2 + 2 + 1) synthetic strategy, in which the introduction of the α-d-glucose anomeric phosphodiester unit (due to its instability towards acidic conditions) was accomplished at a late stage, just before the global deprotection.
Scheme 16.
Retrosynthesis of a GPI-related glycoconjugate of Leishmania (the Konradsson–Oscarson group).
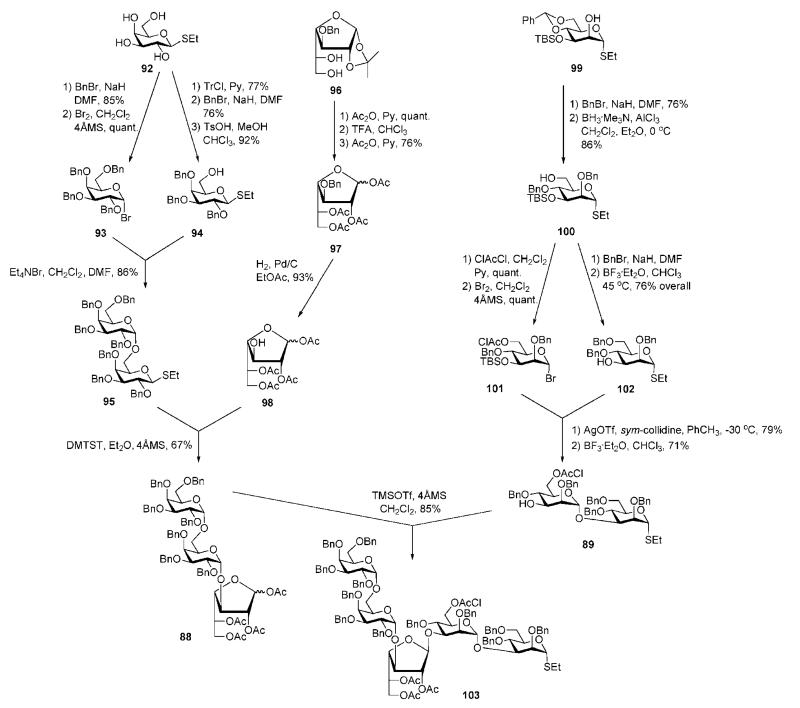
The preparation of the trisaccharide 88 and the disaccharide 89 building blocks is shown in Scheme 17. Ethyl 1-thio-β-d-galactopyranoside 92 was 6-O-tritylated and then per-O-benzylated, followed by acidic O-detritylation to yield derivative 94. Per-O-benzylation of 92 and replacement of the ethanethiol group with bromine gave the galactosyl bromide 93, which was coupled with 94 using the ‘halide inversion’ method27 to give the galactobiose disaccharide 95. The galactofuranose acceptor 98 was prepared from the 5,6-diol 96.47 Sequential O-acetylation, to preserve the furanose ring, TFA-assisted cleavage of isopropylidene acetal and O-acetylation gave 97. After hydrogenolysis, the acceptor 98 was glycosylated with the thioglycoside donor 95 under the influence of dimethyl(methylthio)sulfonium trifluoromethanesulfonate (DMTST) to yield the trisaccharide 88 (67%).
Scheme 17.
The synthesis of monosaccharide building blocks and assembly of a linear pentasaccharide (the Konradsson–Oscarson group).
The mannobiose disaccharide 89 was synthesised48 starting from mannose derivative 99.49 O-Benzylation followed by reductive benzylidene ring cleavage gave 100. This was 6-O-benzylated and 3-O-desilylated to furnish the acceptor 102. 6-O-Chloroacetylation of 100, followed by 1-bromination gave the mannosyl bromide 101, which was coupled with 102 in the presence of AgOTf to give the corresponding disaccharide (79%), from which the TBS group was removed to furnish 89. This was coupled with trisaccharide donor 88 under the influence of TMSOTf to yield the pentasaccharide 103 (85%).
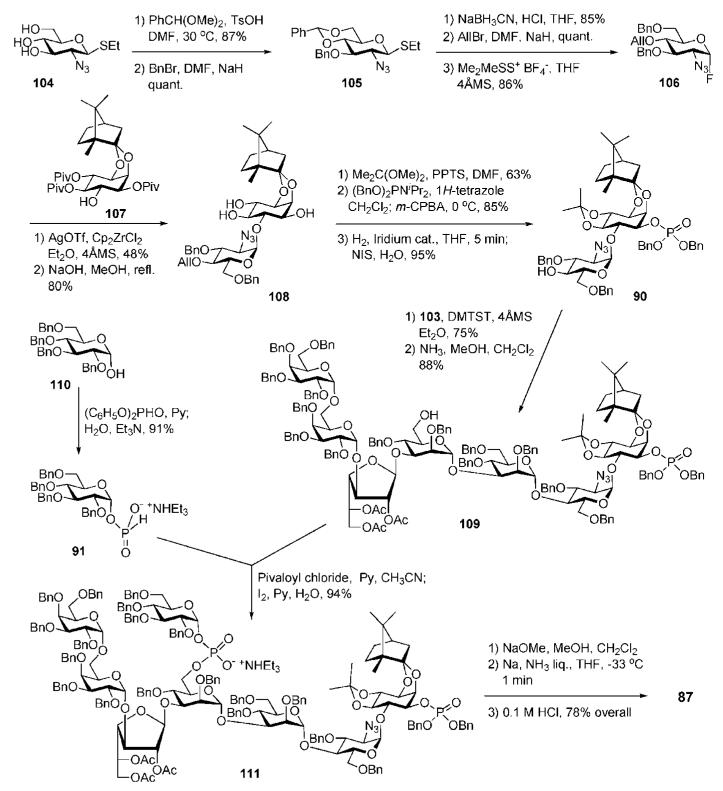
The myo-inositol-containing building block 90 (Scheme 18) was synthesised via AgOTf–dicyclopentadienylzirconium dichloride (Cp2ZrCl2)-mediated coupling (48%) of glycosyl fluoride 106 (acquired in five steps from ethyl 2-azido-2-deoxy-1-thio-β-d-glucopyranoside 104)50 to chiral myo-inositol derivative 107,51,52 followed by consecutive removal of pivaloyl goups (→108, 80%), 4,5-O-isopropylidene acetal introduction, phosphorylation of the 1-OH and, finally, removal of the allyl group. The pentasaccharide thioglycoside donor 103 was activated with DMTST in ether and coupled to the acceptor 90 to give the corresponding protected pseudoheptasaccharide (75%), from which the chloroacetyl group was removed to give 109.
Scheme 18.
GPI glycan core assembly (including phosphorylation steps) and global deprotection (the Konradsson–Oscarson group).
The pseudoheptasaccharide 109 was subjected to pivaloyl chloride mediated coupling with the α-d-glucopyranosyl H-phosphonate 91 (prepared by the reaction of the hemiacetal 110 with diphenyl phosphite)53 and subsequent oxidation with iodine to produce the protected compound 111 (94%). Three-step deprotection, i.e., standard O-deacetylation, then O-debenzylation by Birch reduction and subsequent acid hydrolysis of the acetals, gave the phosphorylated glycan-myo-inositol 87 in 78% yield.
6 Syntheses of GPI anchors and a glycoconjugate of Plasmodium falciparum
6.1 Linear synthesis of a GPI anchor by the Fraser-Reid group
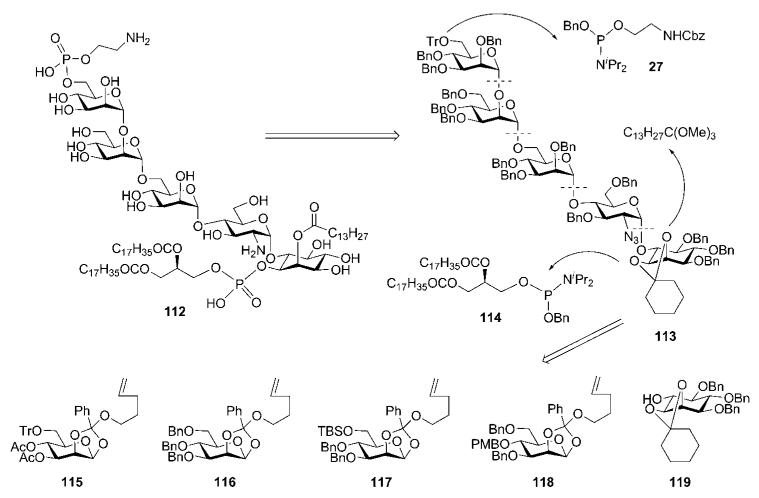
A step-by-step synthetic route for preparation of fully lipidated GPI anchor 112 present on the cell surface of the malaria pathogen P. falciparum was developed by Fraser-Reid and co-workers.54,55 The anchor in this case contains an additional lipid acyl (myristoyl) moiety at the axial 2-OH in myo-inositol. The synthetic strategy, which was based on the retrosynthetic analysis presented on Scheme 19, relies on the use of n-pentenyl orthoesters as glycosyl donors and on a appropriately protected carbohydrate core to allow site-specific deprotection, phosphorylation and acylation at appropriate stages. The strategy exploited a trityl ether and a cyclohexylidene acetal as orthogonal blocking groups, while the benzyl group was chosen for permanent O-protection. In the retrosynthesis, the protected glycan core 113 was assembled from d-mannose 1,2-orthoesters 115–118 and 6-O-unprotected myo-inositol acceptor 119. Late phosphorylation was achieved by making use of the phosphoramidites 27 and 114.
Scheme 19.
Retrosynthesis of a GPI anchor of P. falciparum (the Fraser-Reid group).
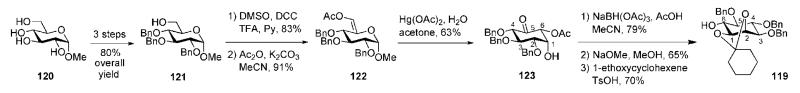
The Fraser-Reid group developed56 a novel methodology for the preparation of optically pure and differentially protected myo-inositol acceptor 119 starting from methyl glucoside 120 (Scheme 20). The procedure relied on the discovery57,58 that enol esters undergo the Ferrier reaction with excellent stereocontrol. First, the tetraol 120 was converted to the 6-OH derivative 121 and the primary hydroxyl group transformed into the aldehyde using Moffat oxidation conditions, followed by enol acetylation to afford the enol acetate 122. Lewis acid-promoted rearrangement of 122 employing Ferrier conditions59 furnished the corresponding cyclohexan-5-one 123, which was further subjected to consecutive reduction, O-deacetylation and 1,2-O-cyclohexylidenation to furnish the required 6-OH d-myo-inositol derivative 119.
Scheme 20.
De novo synthesis of an optically pure myo-inositol derivative (the Fraser-Reid group).
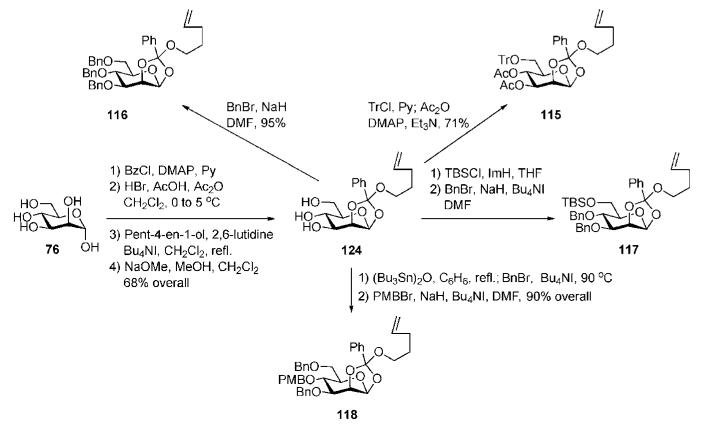
Building blocks 115–118 were synthesised (Scheme 21) from the readily available n-pentenyl 1,2-orthoester 124.60 Tin-mediated selective double benzylation of the C-6 and C-3 OH-groups in 124, followed by p-methoxybenzylation of the remaining 4-OH, afforded the donor 118. Conventional 6-O-tritylation of 124 followed by acetylation gave 115, while standard benzylation furnished 116, and consecutive 6-O-silylation and benzylation provided 117.
Scheme 21.
Synthesis of monosaccharide building blocks (the Fraser-Reid group).
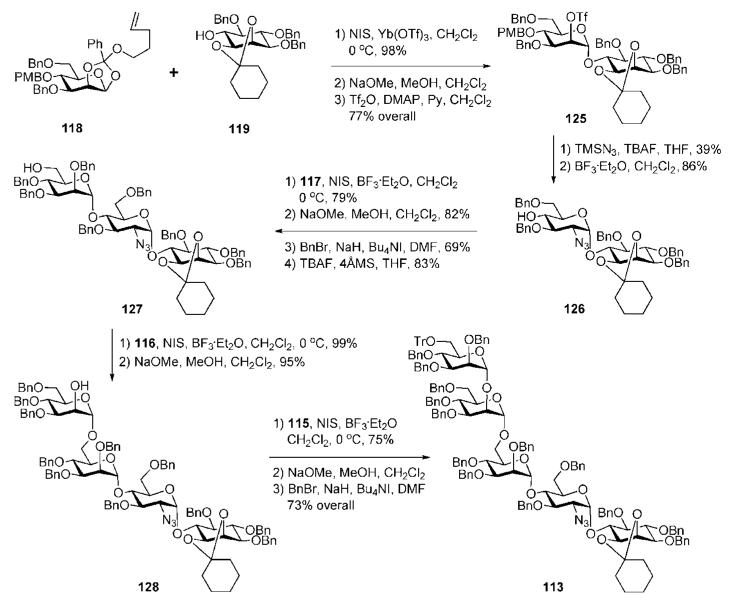
Step-by-step assembly of the glycan core 113 (Scheme 22) started from the glycosylation of 119 with the orthoester 118 in the presence of ytterbium(III) triflate and NIS to give the corresponding pseudodisaccharide (98%), in which the 2-benzoate group was replaced with the 2-triflate group to furnish the pseudodisaccharide 125. Conversion of the mannose-inositol 125 into the azidoglucose-inositol 126 was achieved via azide displacement61 of the 2-triflate, followed by BF3·Et2O-assisted removal of the p-methoxybenzyl group. Glycosylation of the acceptor 126 with the orthoester donor 117 under the influence of NIS and BF3·Et2O (79%), followed by replacement of the 2-benzoate with a 2-O-benzyl group and 6-O-desilylation furnished the pseudotrisaccharide 127. This was then coupled with the orthoester 116 to give the corresponding oligomer (99%), which on conventional debenzoylation afforded the requisite pseudotetrasaccharide 128. The latter was coupled with the donor 115 (75%), followed by subsequent O-deacylation and O-benzylation to furnish the desired pseudopentasaccharide 113.
Scheme 22.
GPI glycan core assembly (the Fraser-Reid group).
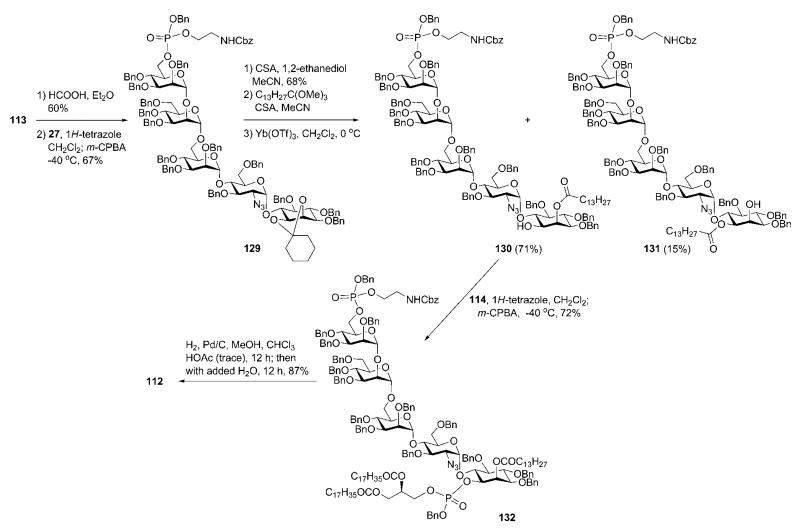
Mildly acidic removal of the trityl group in 113 (Scheme 23) enabled further O-phosphitylation with the phosphoramidite 27 and 1H-tetrazole, followed by oxidation to provide the N-Cbz-aminoethyl phosphotriester 129. Removal of the cyclohexylidene protection in 129, followed by myristoylation of the diol with trimethylorthomyristate,62 gave the corresponding 1,2-cyclic orthoester, which was opened efficiently and with good regioselectivity employing Yb(OTf)3 to provide 4.7 : 1 ratio of the axial 130 (71%, the required one) and the equatorial 131 (15%, the isomeric) myristic esters.
Scheme 23.
Successive phosphorylation and lipidation steps and global deprotection (the Fraser-Reid group).
The axial ester 130 was phospholipidated by making use of the diacylglyceryl phosphoramidite 11433 to provide the fully protected GPI 132 (72%), which on global deprotection by hydrogenolysis (first in an organic solvent followed by addition of water) gave the desired GPI anchor 112 in 87% yield.
6.2 Convergent synthesis of a GPI anchor by the Seeberger group
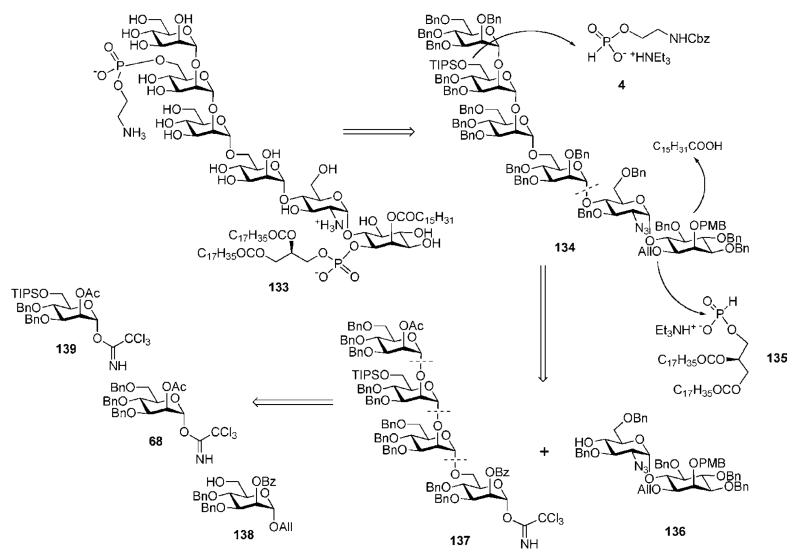
Seeberger and co-workers63 synthesized another GPI anchor 133 (Scheme 24) of P. falciparum with a structure similar to 112, but with an extra d-mannose residue (α1→2)-linked to mannose-3 and differing in the myo-inositol fatty acyl moiety.
Scheme 24.
Retrosynthesis of a GPI anchor of P. falciparum (the Seeberger group).
The glycan core 134 was assembled using a convergent (4 + 2) synthetic strategy via coupling of the tetramannose trichloracetimidate 137 and the pseudodisaccharide 136. The strategy exploited orthogonal protecting groups as PMB ether for the C-2 inositol acylation site, a TIPS ether for the ethanolamine phosphate in mannose-3, and an allyl ether for the phospholipid at C-1 of myo-inositol. Late phosphorylation was achieved by making use of the H-phosphonate derivatives 4 and 135.
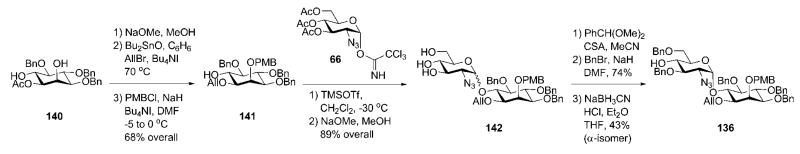
The pseudodisaccharide 136 (Scheme 25) was constructed starting from compound 140,56,64 used earlier by the Fraser-Reid group. Deacetylation of 140 and subsequent Bu2SnO-assisted regioselective 1-O-allylation and 2-O-methoxybenzylation gave the myo-inositol acceptor 141. This was glycosylated with the known trichloroacetimidate 6641,65 to give the corresponding pseudodisaccharide as inseparable anomeric mixture (α:β = 4 : 1), which was O-deacetylated to provide triol 142 (89%). Installation of 4′,6′-benzylidene acetal followed by 3′-O-benzylation and reductive opening of the 4′,6′-O-benzylidene protection with NaBH3CN–HCl gave the pseudodisaccharide, while the separation of the α- (136) and β-anomers became possible.
Scheme 25.
Synthesis of a pseudodisaccharide derivative (the Seeberger group).
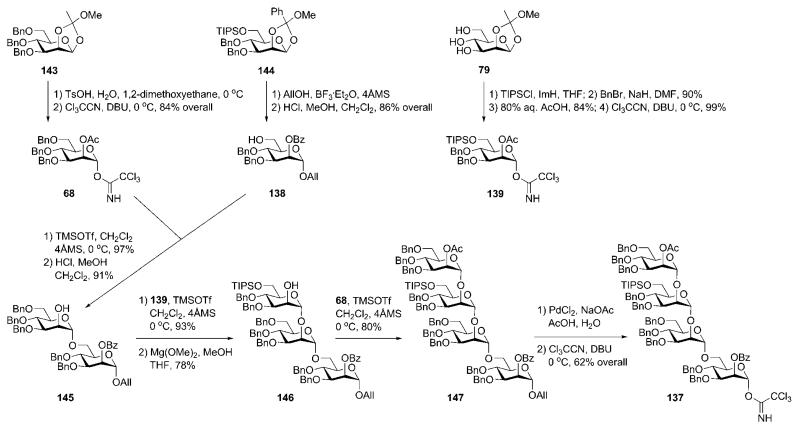
The tetrasaccharide block 13766,67 was formed (Scheme 26) by making use of trichloroacetimidate donors employing a similar approach to Schmidt (Section 4) towards the construction of (α1→2)-linked mannose residues. The mannose building blocks 68, 138 and 13964,68,69 were derived from the orthoesters 143, 144 and 79, respectively.
Scheme 26.
Synthesis of monosaccharide building blocks and assembly of a linear tetramannoside (the Seeberger group).
Construction of the tetramannoside 137 started with the TMSOTf-catalysed coupling of acceptor 138 with the donor 68 (91%), followed by removal of the acetic ester in presence of benzoate by acidic methanolysis74 to furnish the disaccharide 145. The latter was further elongated to the tetrasaccharide 147 employing iterative glycosylations with donors 139 [93%, followed by deacetylation with Mg(OMe)2 to give 146] and 68 (80%). Late removal of the anomeric allyl group and introduction of the trichloroacetimidate gave the tetrasaccharide glycosyl donor 137.
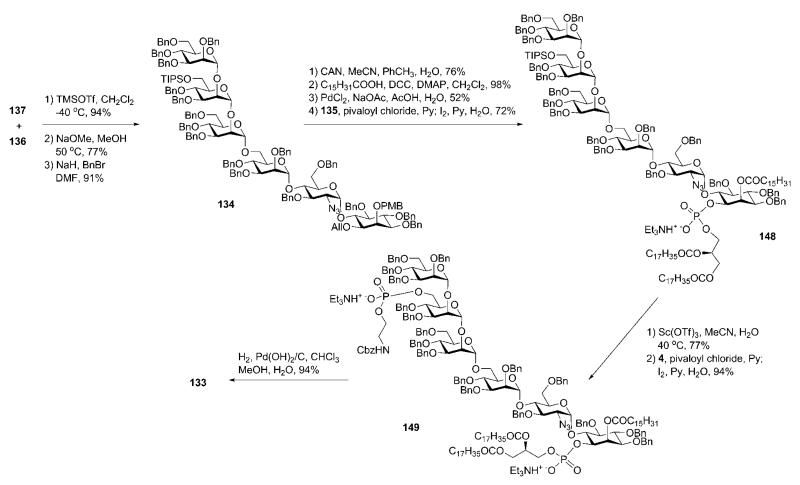
Stereoselective TMSOTf-catalysed coupling of 137 and the pseudodisaccharide acceptor 136 (Scheme 27) gave the corresponding pseudohexasaccharide (94%; the 2-O-benzoyl group in the donor 137 assisted in the α-selectivity), in which the O-acetyl and -benzoyl groups were replaced with O-benzyl to yield the protected glycan core 134. Oxidative cleavage of the PMB protecting group enabled the introduction of the palmitoyl moiety at O-2 of myo-inositol using DCC-assisted acylation. Subsequent removal of the allyl group and introduction of the phospholipid by pivaloyl chloride assisted phosphitylation with the H-phosphonate 135 followed by oxidation gave the phosphodiester 148 (72%). Finally, after removal of the silyl ether in mannose-3 with the help of Sc(OTf)3, the ethanolamine phosphate was installed in a similar manner using the H-phosphonate 4 to provide the fully protected GPI 149 (94%). Global deprotection was then achieved by hydrogenolysis over Pearlman’s catalyst to give the GPI anchor 133 in 94% yield.
Scheme 27.
GPI glycan core assembly, successive lipidation and phosphorylation steps and global deprotection (the Seeberger group).
6.3 Solid-phase synthesis of pseudohexasaccharide malarial toxin by the Seeberger group
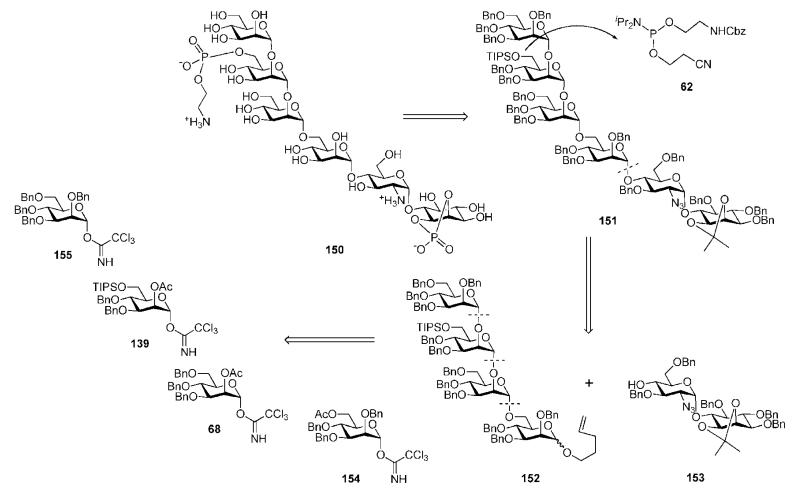
The group has also synthesized the phosphorylated pseudohexasaccharide 150 (Scheme 28) of the GPI anchor of P. falciparum, which they termed a ‘pseudohexasaccharide malarial toxin’,68 employing automated solid-phase synthesis for the preparation of the tetramannoside 152. The glycan core 151 was assembled utilising a (4 + 2) strategy via the coupling of a tetramannose trichloracetimidate made from 152 and the pseudodisaccharide 153. Late introduction of the phosphate groups was achieved using methyl dichlorophosphate and the phosphoramidite 62.
Scheme 28.
Retrosynthesis of a GPI-related glycoconjugate of P. falciparum (pseudohexasaccharide malarial toxin) (the Seeberger group).
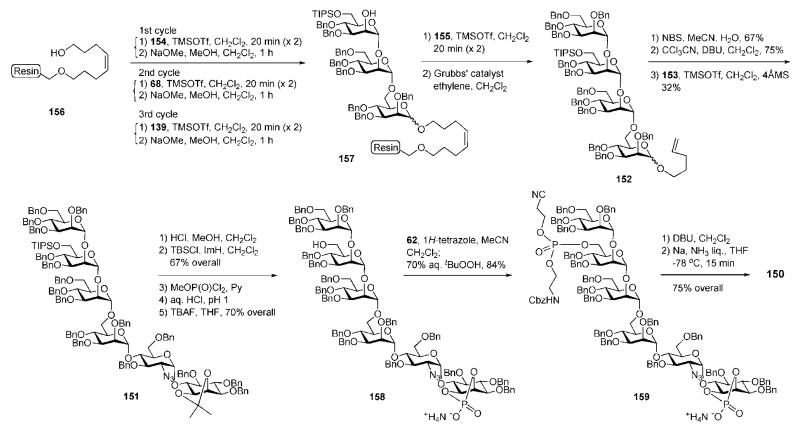
The tetramannoside 152 was assembled on the solid phase by making use of trichloroacetimidate mannosyl donors 68, 139, 154 and 155 and octenediol-functionalized Merrifield resin 156 (Scheme 29) as the first polymer-bound acceptor. Each chain elongation cycle relied on double glycosylations (using catalytic TMSOTf) to secure high coupling efficiencies followed by removal of the acetic ester with NaOMe. Three chain elongations of the acceptor 156 using, consecutively, the donors 154, 68 and 139 provided the polymer-bound acceptor 157, which on further glycosylation with the donor 155, followed by cleavage of the octendiol linker with Grubbs’ catalyst70 in an atmosphere of ethylene, afforded the n-pentenyl tetrasaccharide 152.
Scheme 29.
Solid-phase synthesis of a pseudohexasaccharide malarial toxin (the Seeberger group).
The aglycon moiety in 152 was then hydrolysed with NBS, and the hemiacetal was converted to the corresponding glycosyl trichloracetimidate prior to coupling with the pseudodisaccharide 153, thus forming the glycan core 151 (32%). Further protecting group remodelling released the 1,2-diol in the myo-inositol moiety, which was cyclophosphorylated with MeOP(O)Cl2, followed by O-demethylation with aqueous HCl. Subsequent standard O-desilylation on mannose-3 provided the glycan cyclophosphate 158 (70%). Final phosphorylation was achieved via 1H-tetrazole-catalysed coupling with the phosphoramidite 62 followed by oxidation with tert-BuOOH to give the protected compound 159 (84%). Cleavage of the cyanoethyl ester with DBU followed by Birch reduction for global deprotection gave the required phosphoglycan 150. The chemically synthesised compound 150 was then bound to a protein carrier, and the conjugate was tested as a malarial toxin glycovaccine in mice, which appeared to be substantially protected from death caused by malaria parasites.69
7 Syntheses of GPI anchors and a glycoconjugate of Trypanosoma cruzi
7.1 Convergent syntheses of GPI anchors by the Nikolaev group
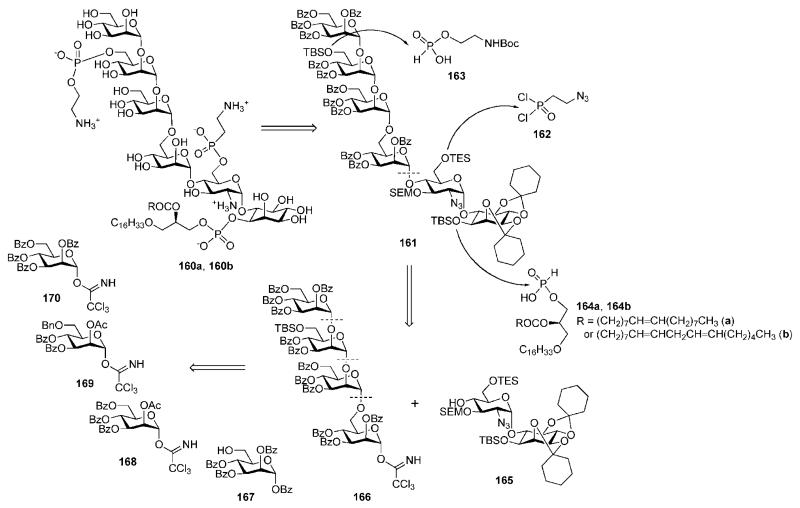
The protozoan parasite Trypanosoma cruzi is a causative agent of Chagas’ disease, which is widespread in South and Central America. Throughout the life cycle, T. cruzi produce both common and stage-specific GPI-anchored cell-surface macromolecules.5 Local release of GPI-anchored mucins by the bloodstream trypomastigote stage of the parasite is believed to be responsible for development of parasite-elicited inflammation causing cardiac and other pathologies associated with acute and chronic phases of Chagas’ disease. It has been discovered71 that the purified GPI fraction of T. cruzi trypomastigote mucins (trypomastigote GPI or tGPI) has extraordinary pro-inflammatory activities, comparable to those of bacterial lipopolysaccharide. The extreme biological activity was reportedly associated with the presence of unsaturated fatty acids in the sn-2 position of the alkylacylglycerophosphate moiety (structures 160a and 160b in Scheme 30). The content of fatty acid components in the biologically active tGPI anchor fraction was found to be: oleic acid (C18:1, 31%), linoleic acid (C18:2, 21%) and palmitic acid (C16:0, 37%). Nikolaev and co-workers recently reported chemical syntheses of T. cruzi tGPIs bearing oleic (160a) and linoleic (160b) acid moieties.
Scheme 30.
Retrosynthesis of GPI anchors of T. cruzi; synthetic strategy A (the Nikolaev group).
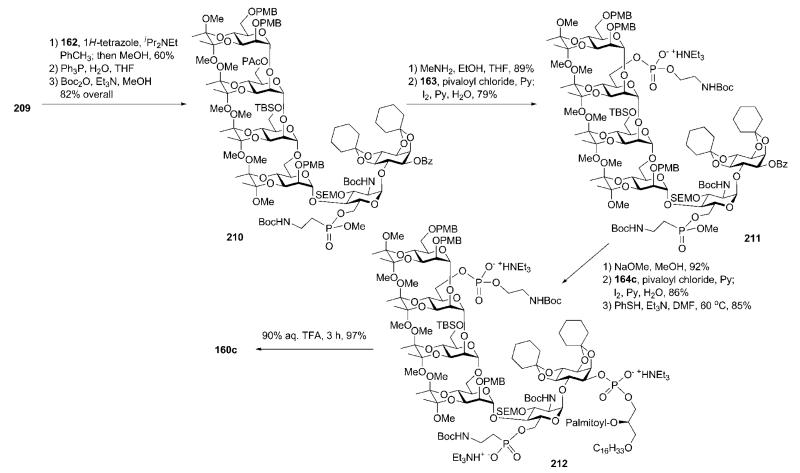
There are two major structural features distinguishing compounds 160a and 160b from the GPIs synthesised previously: (i) the presence of unsaturated fatty acids in the lipid moiety instead of saturated ones and (ii) the presence of a 2-aminoethylphosphonate at C-6 of the d-glucosamine moiety, which is a parasite-specific substituent for T. cruzi only. Since the presence of double bonds was not compatible with the use of benzyl ethers (widely used before) as permanent O-protecting groups, novel strategies were developed. Strategy A was designed to use mildbase-labile (esters) and acid-labile (acetals and N-Boc) permanent protecting groups, whereas Strategy B required acid-labile (diacetals, acetals and N-Boc) and fluoride-labile (primary and secondary TBS ethers) groups for this purpose. Strategy C was designed for acid-labile (diacetals, acetals, PMB ethers and N-Boc) permanent protecting groups only, and was used for the preparation of T. cruzi tGPI bearing a palmitic acid moiety (160c).
Synthetic strategy A. 72,75
The C-phosphonate and phosphate linkers in the GPI anchors 160a and 160b (Scheme 30) were retrosynthetically disconnected sequentially as phosphonodichloridate 162, ethanolamine H-phosphonate 163 and acylalkylglycerol H-phosphonates 164a and 164b, leaving the corresponding glycan core 161 for further simplification. The core was assembled in a (4 + 2) manner from benzoylated mannotetraose building block 166 and acetal-protected pseudodisaccharide 165. Various silyl ethers were employed as orthogonal blocking groups for C-6 of d-glucosamine (TES), C-6 of d-mannose-3 (primary TBS) and C-1 of myo-inositol (secondary TBS) to ensure further introduction of the P-containing esters. The tetramannoside 166, in turn, was assembled stepwise from four building blocks 167–170 in an upstream manner.
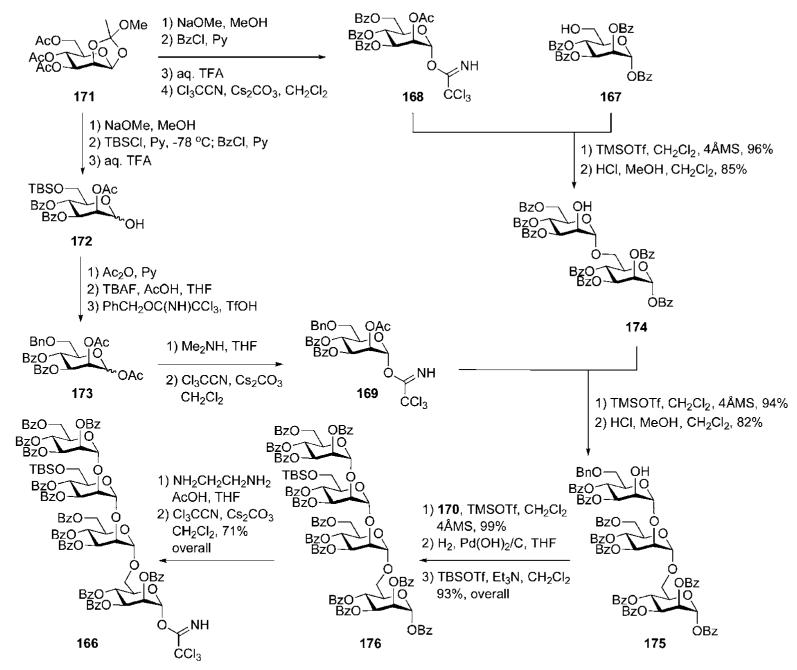
The acceptor 167 and the donor 170 were prepared from d-mannose in three basic steps (for each). The trichloroacetimidate donors 168 and 169 were again derived (Scheme 31) from d-mannose via the 1,2-orthoester 171.73 Successive O-deacetylation, 6-O-silylation, O-benzoylation and TFA-catalysed opening of the orthoester ring gave the hemiacetal 172, which was O-acetylated at the anomeric position, O-desilylated and then 6-O-benzylated with benzyl trichloroacetimidate to give 173. Anomeric O-deacetylation of 173, followed by treatment with Cl3CCN in the presence of Cs2CO3, furnished the trichloroacetimidate donor 169.
Scheme 31.
Synthesis of d-mannose building blocks and assembly of a linear tetramannoside; synthetic strategy A (the Nikolaev group).
Replacement of the acetyl groups with benzoates in the orthoester 171, followed by opening of the orthoester ring and reaction with Cl3CCN in the presence of Cs2CO3, offered the α-trichloroacetimidate donor 168. Coupling of 168 with the acceptor 167 under the influence of TMSOTf (Scheme 31), followed by selective removal of the lone acetyl group in the presence of benzoates with HCl in methanol–dichloromethane,74 gave the disaccharide acceptor 174, which on glycosylation with the trichloroacetimidate donor 169 and O-deacetylation produced the trisaccharide 175. One more glycosylation of 175 with 170, followed by replacement of the lone benzyl ether with the TBS protecting group, gave the tetrasaccharide 176, which was subjected to anomeric debenzoylation with ethylenediamine and trichloroacetimidation to furnish the tetrasaccharide building block 166.
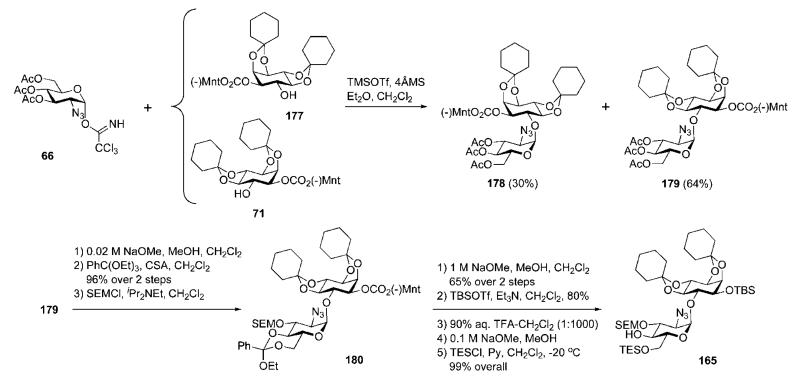
Synthesis of the novel pseudodisaccharide acceptor 165 (Scheme 32) was developed starting from glycosylation of the diastereomeric mixture of myo-inositol derivatives 177 and 71 (prepared first by Mayer and Schmidt)40 with the known glycosyl donor 66.41 This produced the required pseudodisaccharide 179 (d-d) in 64% yield, which appeared to be separable from the diastereoisomer 178 (d-l) (30%) by standard flash column chromatography. Manipulation of the protecting group pattern in 179 was achieved via consecutive mild selective de-O-acetylation in the presence of (−)-menthyl carbonate, 4′,6′-orthoesterification and introduction of the acid-labile trimethylsilylethoxymethyl (SEM) group at 3′-OH to give fully protected azidoglucose-inositol 180. Replacement of the (−)-menthylcarbonate with the TBS group, then mildly acidic ring opening of the 4′,6′-orthoester (thus forming a mixture of the 4′- and 6′-benzoates), followed by standard de-O-benzoylation and selective 6′-O-silylation with Et3SiCl, furnished compound 165.
Scheme 32.
Enantiomeric resolution of myo-inositol and the synthesis of a pseudodisaccharide derivative (the Nikolaev group; synthetic strategy A).
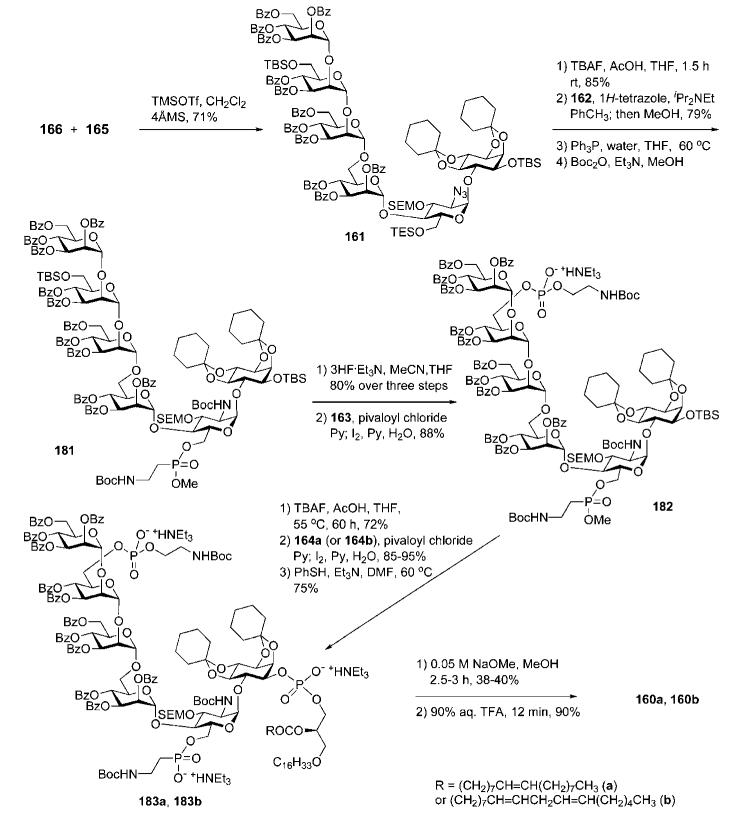
With both building blocks in hand, the assembly of the GPI glycan backbone was only one step away (Scheme 33). Glycosylation of the pseudodisaccharide acceptor 165 with the tetrasaccharide trichloroacetimidate donor 166 in the presence of the TMSOTf catalyst gave the pseudohexasaccharide 161 (71%). Selective cleavage of the TES ether with TBAF produced the corresponding 6′-OH derivative (85%). 1H-Tetrazole-assisted esterification of the 6′-OH group with phosphonodichloridate 162, followed by methanolysis, gave the corresponding phosphonic diester (79%), which was subjected to successive reduction of the two azido groups employing Staudinger conditions and N-protection with Boc anhydride to afford 181. Selective removal of the primary TBS group, phosphitylation with the H-phosphonate 163, and subsequent oxidation with iodine gave the phosphate-phosphonate block 182 (88%).
Scheme 33.
GPI glycan core assembly, successive phosphonylation and phosphorylation steps and global deprotection (the Nikolaev group; synthetic strategy A).
Cleavage of the secondary TBS group in the myo-inositol moiety enabled the final addition of the phospholipid via coupling with the corresponding acylalkylglycerol H-phosphonate (164a or 164b) to afford fully protected GPIs (85–95%), which were demethylated at the aminoethylphosphonate moiety to furnish 183a and 183b. Global deprotection involved first controlled O-debenzoylation, which provided corresponding debenzoylated GPIs (38–40%) isolated by flash column chromatography. Mild base treatment in polar solvent cleaved the benzoic esters preferentially, and left the fatty ester of the lipid mostly intact, probably because of micelle formation. Final removal of the acid-labile acetal and N-Boc protections with aqueous TFA gave the required products 160a and 160b.
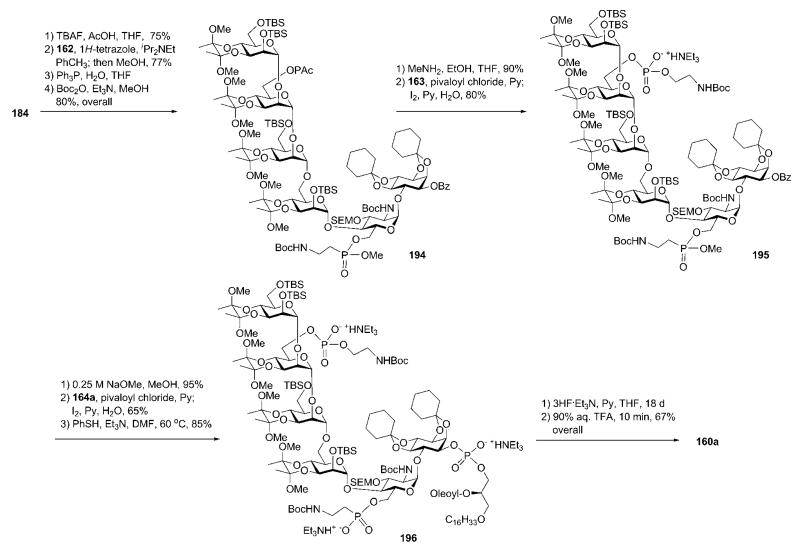
Synthetic strategy B.75
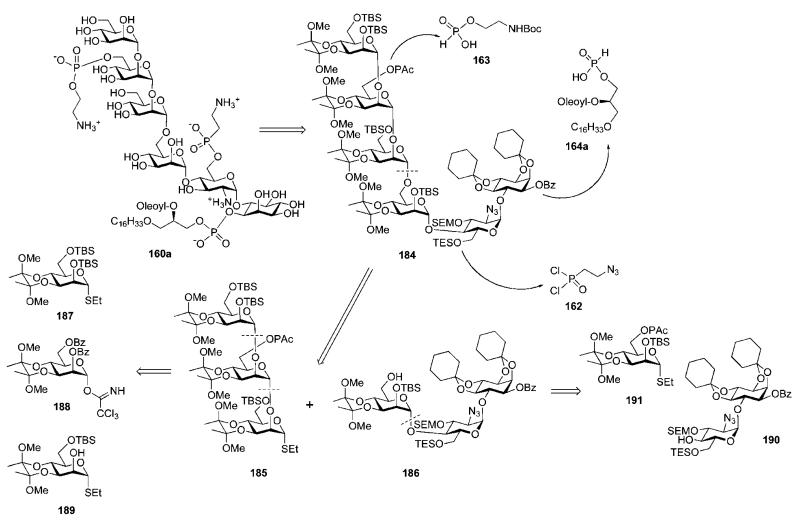
In the second approach, the pseudohexasaccharide core 184 (Scheme 34) was assembled by (3 + 3) coupling of derivatives 185 and 186. These building blocks were designed utilising acid- and fluoride-labile permanent protecting groups, whilst also using orthogonal protecting groups (TES ether at C-6 of d-glucosamine, phenoxyacetate at C-6 of d-mannose-3 and benzoic ester at C-1 of myo-inositol) required to achieve further ‘P-decoration’ at a later stage. The pseudotrisaccharide 186 was formed from the pseudodisaccharide 190 and the thiomannoside donor 191. The trimannoside 185 was made up of three mannosyl derivatives 187–189.
Scheme 34.
Retrosynthesis of GPI anchors of T. cruzi (the Nikolaev group; synthetic strategy B).
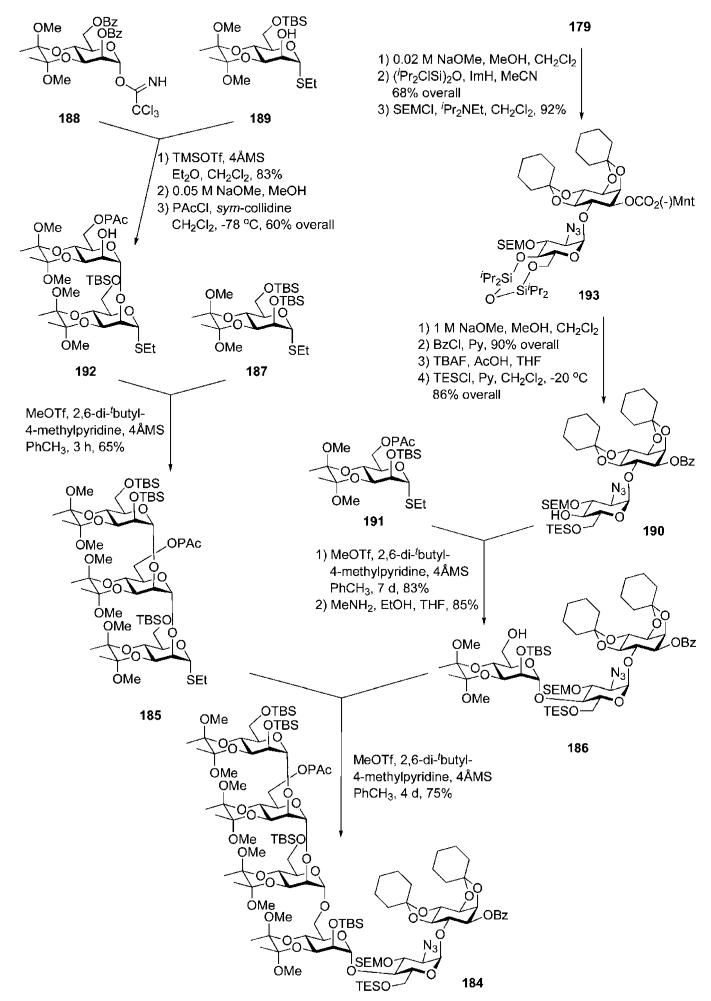
The trimannoside 185 was constructed (Scheme 35) by starting with coupling of the trichloroacetimidate 188 with the acceptor 189 (83%), followed by O-debenzyolation and 6-O-phenoxyacetylation to furnish the disaccharide acceptor 192, which on glycosylation with the thioglycoside 187 in the presence of MeOTf gave the trimannoside 185 (65%). The pseudotrisaccharide block 186 was assembled from the thiomannoside donor 191 and the azidoglucose-inositol derivative 190.
Scheme 35.
Synthesis of a linear trimannoside and a pseudotrisaccharide derivative and GPI glycan core assembly (the Nikolaev group; synthetic strategy B).
Compound 190, in turn, was made from the pseudodisaccharide 179 by manipulation of the protecting group pattern, as shown in Scheme 35. It was also discovered that more efficient separation of the diastereomers 178 and 179 (Scheme 32) could be achieved after addition of tetraisopropyldisiloxane (TIPDS) 4′,6′-O-protection.
Replacement of the (−)-menthyl carbonate with benzoate in TIPDS-protected derivative 193, followed by TIPDS cleavage (with TBAF) and selective 6′-O-silylation with TESCl furnished 190. Methyl triflate-promoted glycosylation of the pseudodisaccharide acceptor 190 with 191 in the presence of 2,6-di-tert-butyl-4-methylpyridine to avoid cleavage of the acid-labile groups, followed by removal of the phenoxyacetate (with MeNH2) gave the pseudotrisaccharide 186 (71% over two steps). The latter was coupled to the trimannoside 185 under the conditions described above to furnish the pseudohexasaccharide 184 (75%).
Selective removal of the TES ether in the azidoglucose moiety in 184 (Scheme 36), followed by introduction of the azidoethylphosphonate with 162 (77%) and conversion of the azido groups into Boc-protected amines (as performed in strategy A) gave the phosphonic diester 194. Cleavage of the phenoxyacetate, followed by introduction of the N-Boc-protected ethanolamine phosphate by the use of the H-phosphonate 163, gave the phosphate-phosphonate block 195 (80%). Removal of the benzoate in the myo-inositol moiety of 195 enabled the final addition of the phospholipid (using the H-phosphonate 164a, 65%), followed by demethylation (with thiophenol) to give the protected GPI anchor 196.
Scheme 36.
Successive phosphonylation and phosphorylation steps and global deprotection (the Nikolaev group; synthetic strategy B).
Total deprotection involved removal of the silyl ethers with 3HF·Et3N and cleavage of the acid-labile acetals and N-Boc groups with aqueous TFA to give the required product 160a.
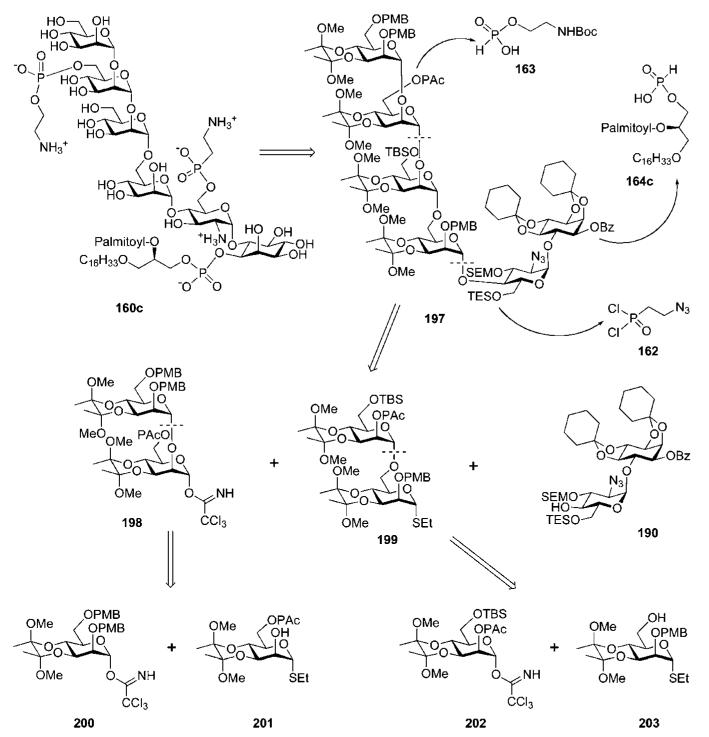
Synthetic strategy C.76
In the third approach, the glycan core 197 (Scheme 37) was retrosynthetically broken down into three major building blocks: the mannobiosides 198 and 199 and the pseudodisaccharide 190. The disaccharides 198 and 199 were further disconnected to the mannosyl derivatives 200–203. These building blocks were designed in such a way that only acid-labile protecting groups – such as cyclohexylidene and SEM acetals, butanediacetal (BDA), p-methoxybenzyl ethers and primary TBS ethers, which are known to be stable towards miscellaneous reaction conditions – were used for permanent OH-protection. Their simultaneous removal in the final deprotection step was therefore anticipated. The orthogonal protecting groups required to execute further ‘P-decoration’ of the glycan core 197 and to prepare the GPI anchor 160c were identical to those used in strategy B.
Scheme 37.
Retrosynthesis of a GPI anchor of T. cruzi (the Nikolaev group; synthetic strategy C).
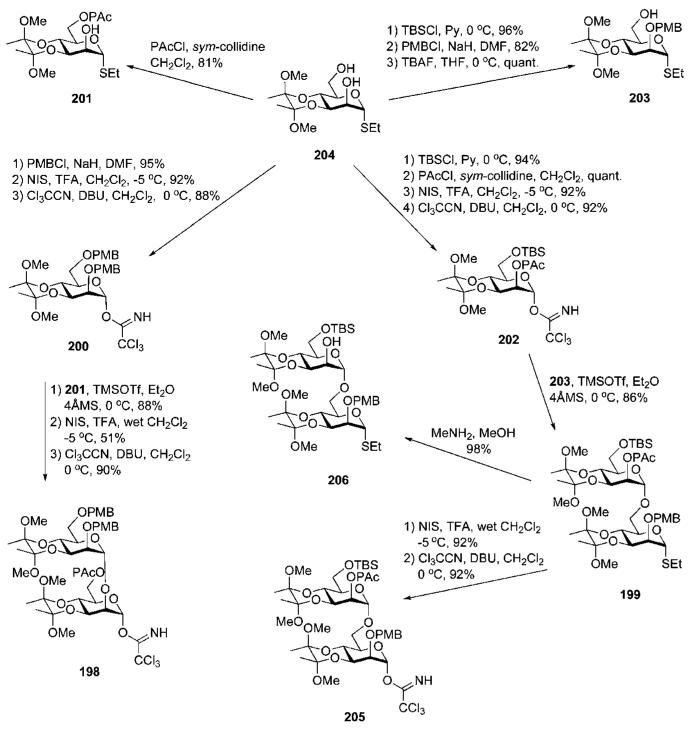
The derivatives 200–203 derived from BDA-protected thiomannoside 204 (Scheme 38) (prepared, in turn, by selective incorporation of the BDA motif at the 3,4-vicinal diol).77 Selective 6-O-silylation of 204 and subsequent benzylation with p-methoxybenzyl chloride and desilylation gave 203. 2,6-Di-O-methoxybenzylation of 204, followed by subsequent NIS–TfOH-mediated hydrolysis of the ethanethiol group and treatment with Cl3CCN in the presence of DBU, furnished the trichloroacetimidate 200.
Scheme 38.
Synthesis of d-mannose mono- and disaccharide building blocks (the Nikolaev group; synthetic strategy C).
Coupling of the donor 200 with acceptor 201 (prepared by selective 6-O-phenoxyacetylaton of 204) under the influence of TMSOTf gave the corresponding disaccharide (88%), which upon hydrolysis of the ethanethiol moiety and installation of the trichloroacetimidate gave the disaccharide glycosyl donor 198. The dimannoside building block 199 was assembled by TMSOTf-activated glycosylation of 203 with the trichloroacetimidate 202, prepared from 204 by successive 6-O-silylation, 2-O-phenoxyacetylation, anomeric deprotection and trichloroacetimidation. The protected mannobiose 199 intermediate was ideally suited to further glycan chain extension, either upstream or downstream.
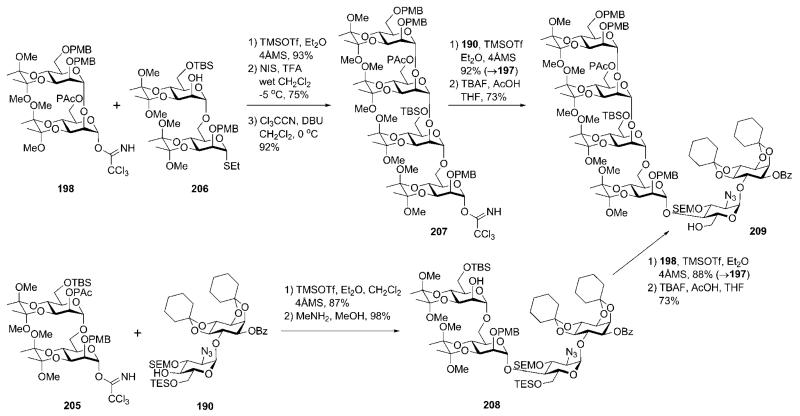
For upstream extension, a portion of the compound was transformed into the trichloroaceimidate donor 205. This was used for TMSOTf-promoted glycosylation of the pseudodisaccharide 190 (Scheme 39) to give the corresponding pseudotetrasaccharide (87%), in which the phenoxyacetyl group was removed to yield the acceptor 208. This was further glycosylated with the disaccharide donor 198 in the presence of TMSOTf to give the glycan core 197 (88%), from which the TES group was removed with TBAF to yield the pseudohexasaccharide 209. A second portion of 199 was converted (Scheme 38) to the acceptor 206 (required for downstream chain assembly) by removal of the phenoxyacetyl group. Coupling of 206 and the donor 198 (93%), followed by anomeric deprotection and trichloroacetimidation of the hemiacetal, afforded the tetrasaccharide trichloroacetimidate 207. The latter was coupled (TMSOTf) with the acceptor 190 (→197, 92%), followed by selective TES removal to afford the pseudohexasaccharide 209.
Scheme 39.
GPI glycan core assembly (the Nikolaev group; synthetic strategy C).
Successive ‘P-decoration’ of the glycan core (Scheme 40) was achieved as described above in strategy B. First, 1H-tetrazole-assisted phosphonylation of the alcohol function in 209 with phosphonodichloridate 162 followed by methanolysis afforded a phosphonic diester (60%), in which the azido groups were reduced with PPh3 and N-protected with Boc-anhydride to afford the phosphonylated block 210.
Scheme 40.
Successive phosphonylation and phosphorylation steps and global deprotection (the Nikolaev group; synthetic strategy C).
Removal of the PAc group in 210 and subsequent pivaloyl chloride assisted phosphitylation with the H-phosphonate 163, followed by oxidation of the intermediate with iodine, gave the phosphate-phosphonate block 211 (79%). Cleavage of the benzoic ester in the myo-inositol moiety enabled successful phospholipidation (86%) using the palmitoylhexadecylglycerol H-phosphonate 164c. The O-methyl group in the aminoethylphosphonate moiety was removed with thiophenol to afford 212 (85%). Global deprotection of 212 was successfully carried out in one step with 90% aqueous TFA to provide 160c in almost quantitative yield.
7.2 Convergent synthesis of a GPI anchor by the Vishwakarma group
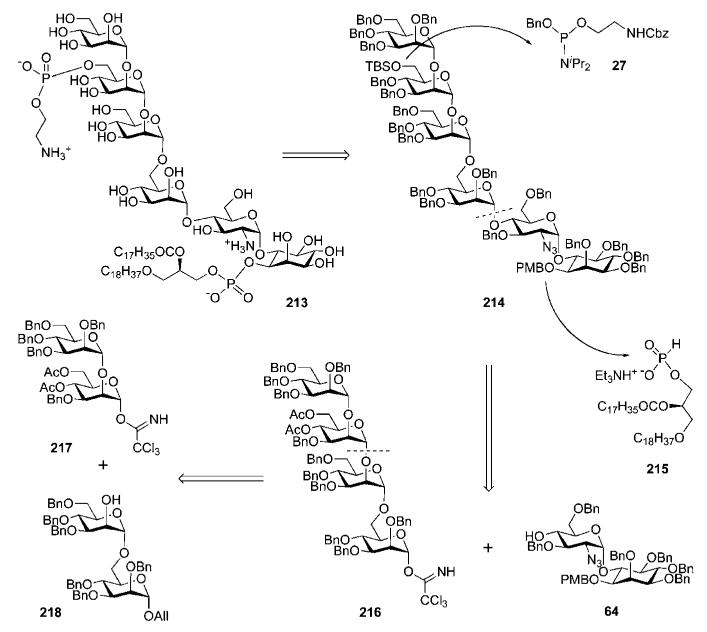
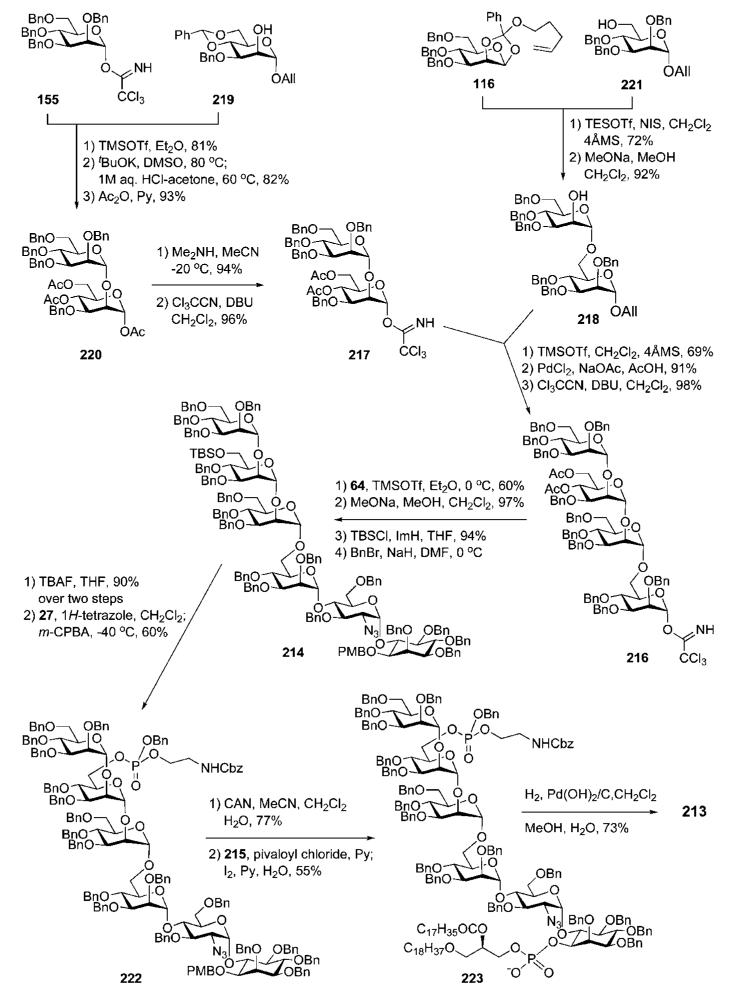
Vishwakarma and co-workers reported78 a downstream (2 + 2 + 2) approach to synthesise GPI anchor 213 of the T. cruzi IG7 antigen (Scheme 41) via the efficient construction of the glycan core 214 from the azidoglucose-inositol 64 and mannotetraose 216 intermediates. The tetramannoside 216 was retrosynthetically broken down into two disaccharide building blocks, 217 and 218, which were further disconnected to monosaccharide derivatives 116, 155, 219 and 221 (Scheme 42).
Scheme 41.
Retrosynthesis of a GPI anchor of T. cruzi (the Vishwakarma group).
Scheme 42.
GPI glycan core assembly, successive phosphorylation steps and global deprotection (the Vishwakarma group).
The assembly of the tetramannoside 216 (Scheme 42) began by coupling of the acceptor 219 with the trichloroacetimidate 155 to afford a disaccharide intermediate (81%); this was subjected to the anomeric O-allyl and the 4,6-O-benzylidene removal followed by O-acetylation to afford the disaccharide 220. Anomeric deacetylation followed by Schmidt activation (CCl3CN, DBU) provided the required glycosyl donor 217. Glycosylation of the acceptor 221 with the orthoester donor 116 in the presence of TESOTf–NIS (72%) followed by removal of the O-benzoyl group from position-2 gave the mannobioside 218. The latter was coupled with the donor 217 (69%), followed by consecutive anomeric deallylation and trichloroacetimidation to give the tetramannoside donor 216. TMSOTf-activated glycosylation of the pseudodisaccharide 6437 with compound 216 afforded the corresponding pseudohexasaccharide intermediate (60%).
Further standard O-deacetylation prior to selective silylation of the primary 6′′′′-OH and conventional benzylation of the 4′′′′-OH provided the glycan core 214. Sequential 6′′′′-O-desilylation (TBAF), 1H-tetrazole-assisted phosphitylation with N-Cbz-aminoethyl phosphoramidite 27 and oxidation with m-CPBA gave the phosphotriester 222 (60%). The PMB group was removed from position-1 of the myo-inositol moiety with CAN, followed by phospholipidation with 1-O-stearyl-2-O-stearoylglycerol H-phosphonate 215 to afford the protected conjugate 223 (55%), which on global deprotection by hydrogenolysis over the Pd(OH)2/C gave the target GPI anchor 213.
7.3 Synthesis of phosphorylated glycoconjugate by the Konradsson group
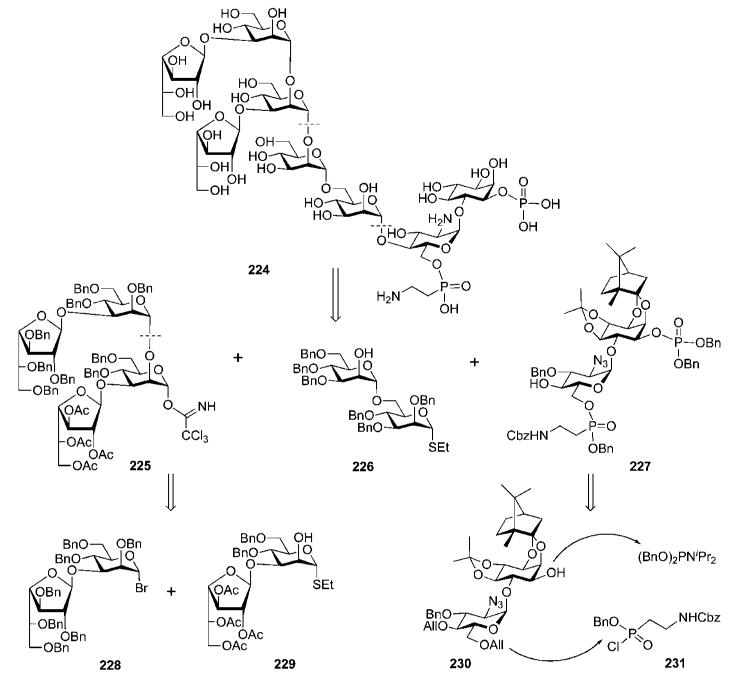
Hederos and Konradsson developed79,80 a convergent methodology for the preparation of the phosphorylated heptasaccharyl myo-inositol 224 (Scheme 43) found of the T. cruzi lipopeptidophosphoglycan.81 This glycan is attached to a ceramide lipid anchor and constitutes a major cell membrane component in the proliferative epimastigote stage of T. cruzi found in the insect vector.
Scheme 43.
Retrosynthesis of a GPI-related glycoconjugate of T. cruzi (the Konradsson group).
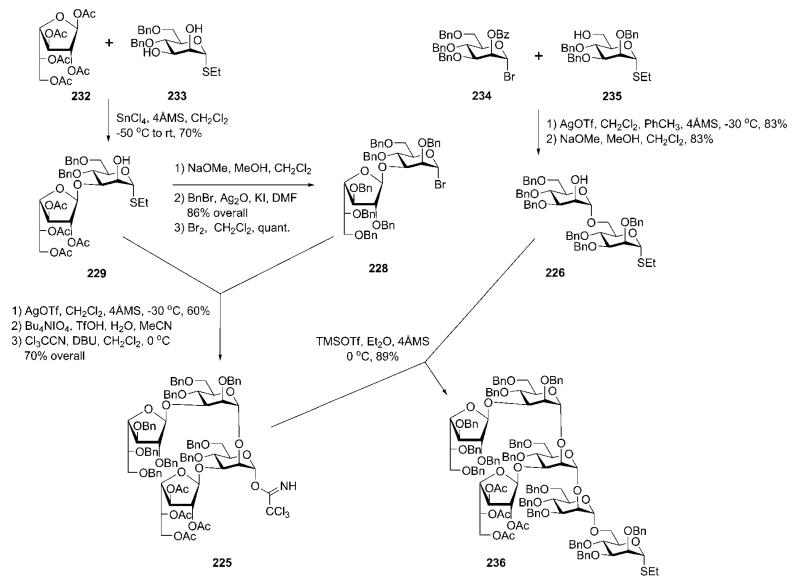
Structurally, the phosphoglycan 224 consists of the basic GPI sequence of the Man4GlcNH2Ino core with an extra 2-aminoethylphosphonic ester at C-6 of d-glucosamine, and two β-d-galactofuranose branches linked to the mannose-3 and mannose-4 residues. The synthetic plan relied on a (4 + 2 + 2) downstream assembly strategy and the use of tetrasaccharide 225 and disaccharide 226 building blocks, whereas the phosphonatephosphate-decorated pseudodisaccharide 227 was introduced just before the global deprotection steps. The tetrasaccharide block 225 was further disconnected into two smaller Galf-Man building blocks 228 and 229.
Construction of the branched hexasaccharide 236 (Scheme 44) began with regioselective glycosylation of the diol 23382 with the galactofuranose donor 232.83 The 2-O-acetyl participating group in the donor assists the reaction toward the formation of the target (β1→3)-linked disaccharide 229. The acetyl groups in compound 229 were then replaced with benzyl ethers prior to bromination to generate the glycosyl bromide donor 228.
Scheme 44.
Synthesis of mono- and disaccharide building blocks and assembly of a branched hexasaccharide (the Konradsson group).
Compound 228 was coupled with the acceptor 229 using AgOTf as promoter to give the corresponding tetrasaccharide (60%), in which the anomeric ethanethiol group was then replaced with the trichloroacetimidate group to furnish the tetrasaccharide donor 225. This was used in the glycosylation of the disaccharide 226 (prepared by condensation of the glycosyl bromide 23484 with the mannose acceptor 23585 followed by 2′-O-debenzoylation) using TMSOTf as a catalyst to give the branched hexasaccharide 236 (89%).
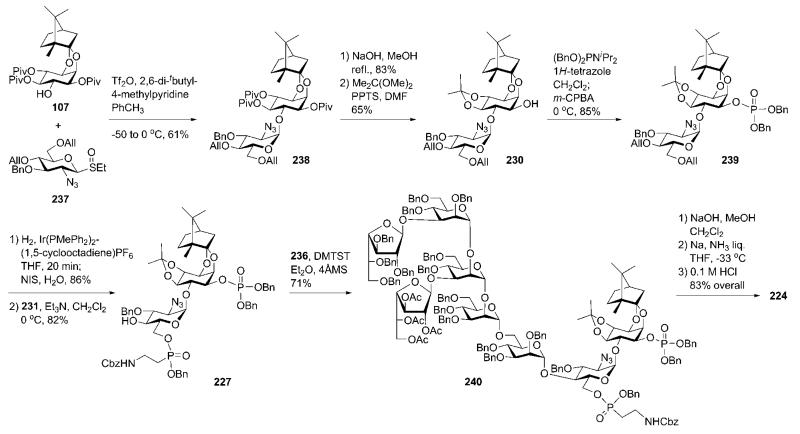
Glycosylation of chiral d-camphor acetal-protected myo-inositol acceptor 107 (Section 5) with the glycosyl sulfoxide donor 237 (Scheme 45) using triflic anhydride (Tf2O) as promoter gave the pseudodisaccharide 238. Protecting group manipulation by the removal of pivalic esters and introduction of the 4,5-O-isopropylidene acetal gave the required pseudodisaccharide synthon 230, which was further 1-O-phosphorylated (→239; 85%) using phosphoramidite chemistry.
Scheme 45.
GPI glycan core assembly (including successive phosphorylation and phosphonylation steps) and global deprotection (the Konradsson group).
The pseudodisaccharide phosphate 239 was O-deallylated using an iridium(I) catalyst and then regioselectively 6′-O-phosphonylated with the phosphonochloridate 231 to furnish the ‘P-decorated’ glycosyl acceptor 227 (82%). The latter was glycosylated with the branched hexasaccharide donor 236 utilising dimethyl(methylthio)sulfonium trifluoromethanesulfonate (DMTST) as a promoter to afford the fully protected pseudooctasaccharide derivative 240 (71%). Global deprotection of 240 started with O-deacetylation using Zemplén conditions, was followed by O-debenzylation with Na in liquid NH3 and concluded with removal of the acetals by acid hydrolysis, to provide the desired phosphoglycan 224 in 83% yield.
8 Synthesis of a GPI anchor of the yeast Saccharomyces cerevisiae by the Schmidt group
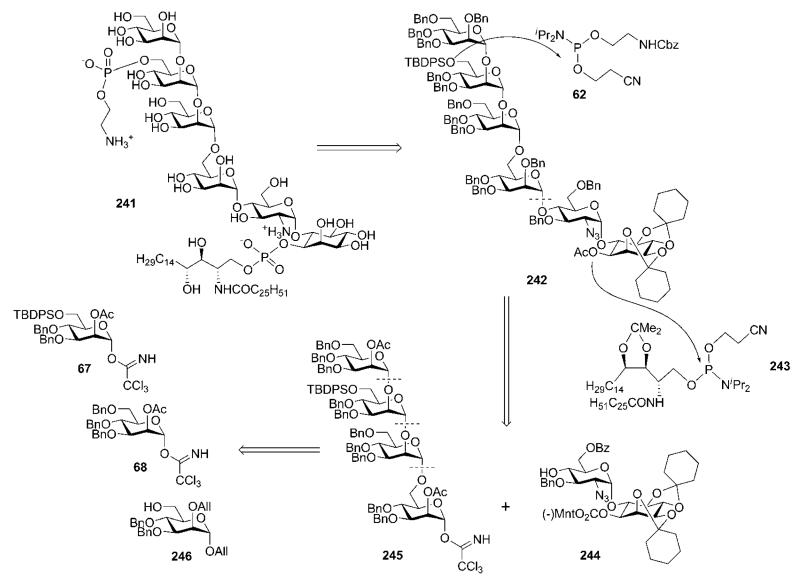
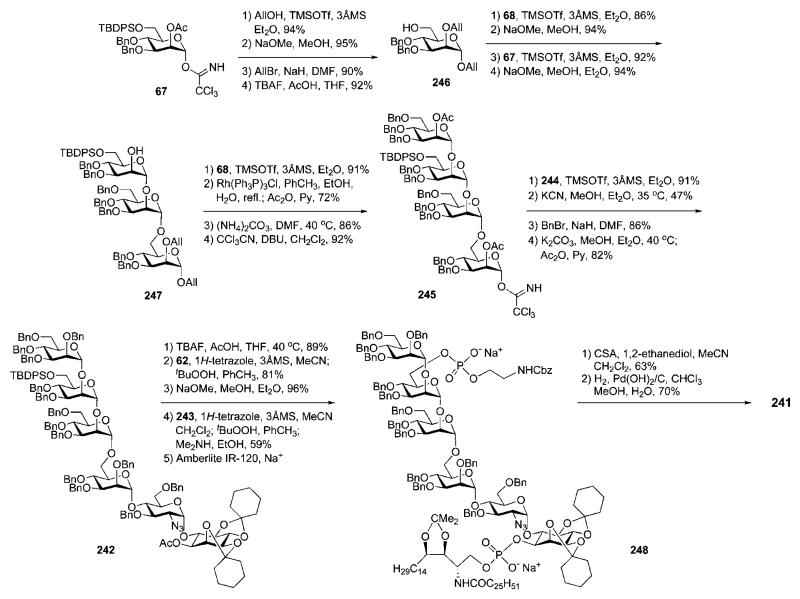
The preparation in 1994 of a GPI anchor 241 (Scheme 46) of the yeast S. cerevisiae by the Schmidt group31,32 was, historically, the second (see Section 3.1) successful total synthesis of a GPI anchor. The lipid moiety of this glycoconjugate is different from the other GPIs in this paper, as in GPI anchors of yeast, ceramide-containing phytosphingosine was found as a lipid instead of glycerolipids.86,87 With the synthesis of the structure 241, the Schmidt group established a convergent and versatile synthetic strategy that was later adopted in their syntheses of GPI anchors of T. gondii37 (Section 4) and rat brain Thy-138,88 (Section 9.2). The strategy relied on a (4 + 2) retrosynthetic disconnection of the glycan core 242 and making use of trichloroacetimidate chemistry, together with the participating effect of acetic esters at C-2 in d-mannopyranosyl donors to ensure the α-selectivity in every glycosylation step. After the assembly of the glycan core, the N,N-diisopropylphosphoramidites 62 and 243 (the synthesis of protected ceramide compound 243 is described in ref. 89) were used for the introduction of the phosphoethanolamine moiety and the ceramidecontaining phospholipid, respectively. The backbone 242 was further dissected to the pseudodisaccharide block 244 and the tetramannoside 245.
Scheme 46.
Retrosynthesis of a GPI anchor of the yeast Saccharomyces cerevisiae (the Schmidt group).
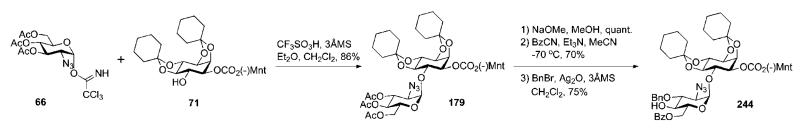
Synthesis of the pseudodisaccharide 244 (Scheme 47) was based on direct glycosylation of the chiral myo-inositol derivative 71 [prepared (see Scheme 13) by enantiomeric resolution of racemic 2,3:4,5-di-O-cyclohexylidene-myo-inositol 9 (Scheme 2)16,39 via selective addition of a chiral (−)-menthyl formate group at O-1 and then crystallisation of the desired product31,40] with the 2-azidoglucose donor 6641 in the presence of triflic acid to furnish the desired (α1→6)-linked pseudodisaccharide 179 in 86% yield.89 Compound 179 was subjected to sequential de-O-acetylation, 6-O-benzoylation with benzoyl cyanide and 3-O-benzylation using benzyl bromide in the presence of Ag2O, to give the pseudodisaccharide acceptor 244.
Scheme 47.
The synthesis of a pseudodisaccharide derivative (the Schmidt group).
The target α-linked tetramannoside 245 was assembled from the acceptor 246 and glycosyl donors 67 and 68 (Scheme 46). The glycosyl donors were readily available from d-mannose 1,2-orthoester 79 (see Section 4), while compound 246 was formed (Scheme 48) by the TMSOTf-catalysed coupling of the donor 67 with allyl alcohol, followed by sequential (and conventional) 2-O-deacetylation, 2-O-allylation and removal of the 6-O-silyl group. TMSOTf-promoted glycosylation of 246 with the glycosyl donor 68 (86%), followed by O-deacetylation and glycosylation with the trichloroacetimidate 67, gave the corresponding trisaccharide (92%), which on O-deacetylation gave the trimannoside acceptor 247.
Scheme 48.
GPI glycan core assembly, successive phosphorylation steps and global deprotection (the Schmidt group).
The tetrasaccharide glycosyl donor 245 was easily available by the following reaction sequence: glycosylation of 247 with the donor 68 (91%), removal of the allyl group at O-1 and O-2 of mannose-1 with Wilkinson’s catalyst, exhaustive O-acetylation and regioselective anomeric O-deacetylation (with ammonium carbonate) to enable installation of the trichloroacetimidate with trichloroacetonitrile in the presence of DBU. This was then coupled with the pseudodisaccharide 244 in the presence of TMSOTf to form stereoselectively the corresponding pseudohexasaccharide in 91% yield. Subsequent cleavage of all the acetic and benzoic esters with KCN in methanol and benzylation of the free OH-groups, followed by replacement of the 1-O-(−)-menthyloxycarbonate group (cleaved with K2CO3 in methanol) in the myo-inositol moiety with O-acetate afforded the target pseudohexasaccharide glycan core 242 ready for the installation of the phosphate residues.
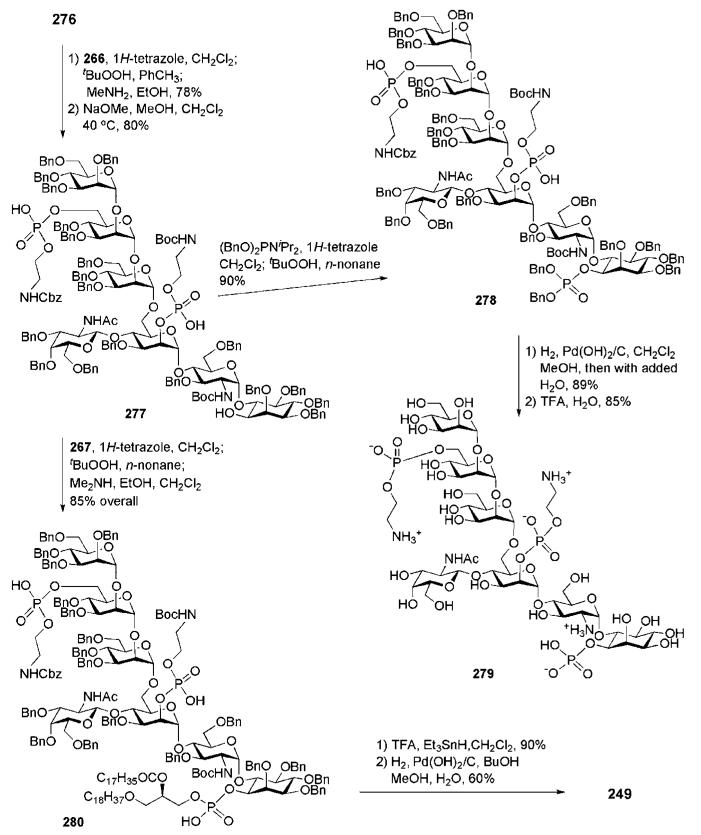
First, 6″″-O-desilylation of 242 with TBAF and 1H-tetrazole-activated phosphitylation with the phosphoramidite 62, followed by oxidation with tert-BuOOH, gave the corresponding protected ethanolamine phosphate derivative (81%). Simultaneous removal of the 1-O-acetate in the myo-inositol unit and the cyanoethyl P-protecting group was achieved (96%) with methanolic NaOMe prior to the final phospholipidation via 1H-tetrazole-catalysed coupling with the ceramide-containing phosphoramidite 243. Oxidation and P-deprotection (with Me2NH) then afforded the protected GPI compound 248 (59%). Global deprotection was achieved in two steps: first, acid-catalysed cleavage of the acetal protecting groups with 1,2-ethanediol (63%), followed by hydrogenolysis of the O-benzyl groups, the N-Cbz group and the azide to give the GPI anchor 241 (70%).
9 Syntheses of a GPI anchor of the rat brain Thy-1 glycoprotein
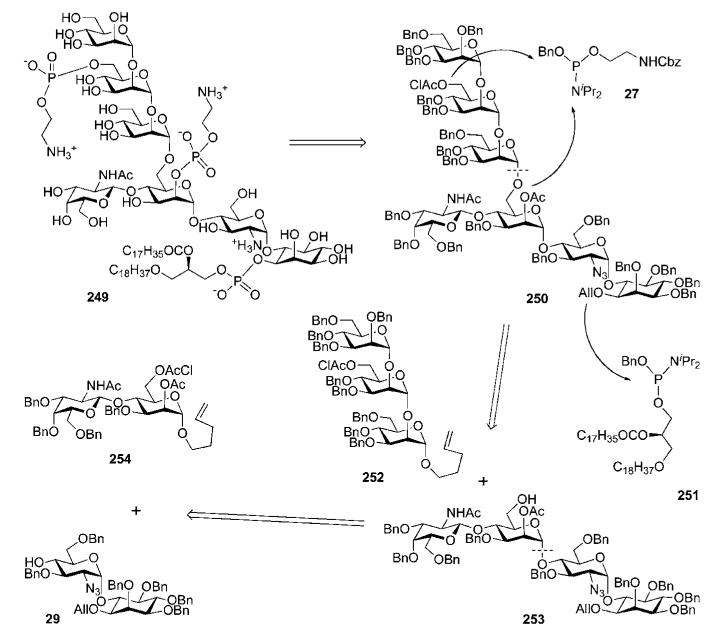
9.1 Convergent synthesis by the Fraser-Reid group
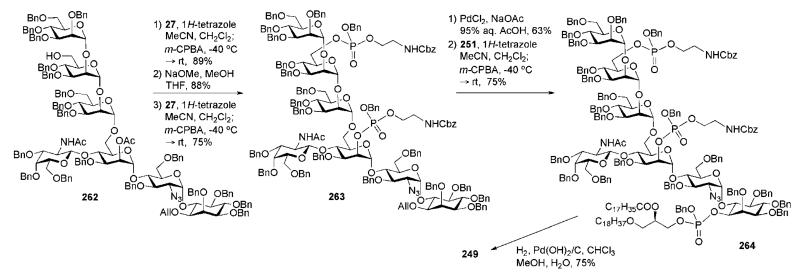
Before their elegant synthetic approach to the highly lipidated GPI anchor of P. falciparum was published54,55 in 2004 (Section 6.1), Fraser-Reid and co-workers reported34,90 in 1995 the synthesis of the highly phosphorylated GPI membrane anchor of the rat brain Thy-1 glycoprotein (which appeared to be the third total synthesis of a GPI anchor). Structurally, the rat brain Thy-1 anchor (compound 249, Scheme 49) has a basic type II GPI core (see Fig. 1) with extra 2-acetamido-2-deoxy-β-d-galactose and ethanolamine phosphate moieties both linked to mannose-1 and an extra α-d-mannose residue linked to mannose-3. The assembly of the GPI branched backbone 250 involved a (3 + 4) coupling of the trimannoside 252 and the pseudotetrasaccharide 253, which, in turn, was assembled from the GalNAc-Man block 254 and the pseudodisaccharide 29. Phosphoramidite chemistry and the synthons 27 and 251 were used for the later phosphorylation steps.
Scheme 49.
Retrosynthesis of a GPI anchor of rat brain Thy-1 glycoprotein (the Fraser-Reid group).
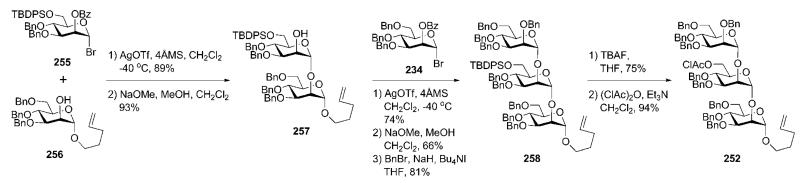
In contrast to their synthesis of P. falciparum GPI anchor that relied heavily on the use of n-pentenyl orthoester glycosyl donors, this strategy made use of a combination of n-pentenyl glycoside, glycosyl bromides and trichloroacetimidate donors. Construction of the trimannoside 252 (Scheme 50) commenced91 with coupling of the glycosyl bromide 255 and the pentenyl glycoside acceptor 256 in the presence of AgOTf (89%) to furnish (after NaOMe-catalysed removal of benzoate) the dimannoside 257. AgOTf-assisted glycosylation of compound 257 with the glycosyl bromide 234 gave the corresponding trimannoside (74%), in which the benzoate was replaced with a benzyl group (→258) and the silyl group was replaced with a chloroacetyl group to give 252 (this was to enable selective introduction of ethanolamine phosphate further along the synthetic process).
Scheme 50.
Synthesis of a linear trimannoside (the Fraser-Reid group).
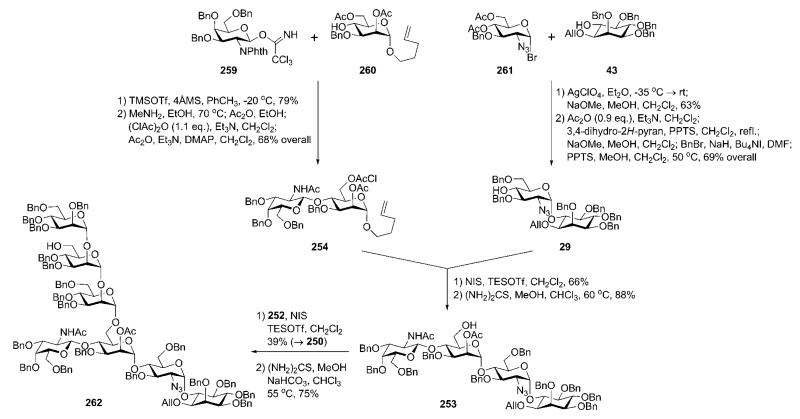
Assembly of the pseudotetrasaccharide 253 (Scheme 51) was split into two: assembly of the pseudodisaccharide 29, and coupling of this to the GalNAc-Man disaccharide donor 254. The latter compound was prepared90 via TMSOTf-catalysed glycosylation of the pentenyl mannoside acceptor 260 with the 2-phthalimidogalactosyl trichloroacetimidate donor 259 (79%; the 2-phthalimido group secured formation of the β-linkage only), followed by replacement of the N-phthalyl group with an N-acetyl group and successive introduction of the orthogonal 6-O-chloroacetyl (to enable further chain elongation to form glycan core 250) and 2-O-acetyl (to enable further introduction of the second ethanolamine phosphate) protecting groups in d-mannose.
Scheme 51.
GPI branched glycan core assembly (the Fraser-Reid group).
Synthesis of the pseudodisaccharide 29 involved AgClO4-promoted reaction of the myo-inositol acceptor 4334 with the glycosyl bromide 261,92 followed by standard O-deacetylation to give the corresponding α-linked pseudodisaccharide (63%; 22% of the β-linked isomer was also isolated), in which the 6″-OH group was then selectively benzylated. The selective 6″-O-benzylation (69% overall yield) was achieved through a reaction sequence that involved selective 6″-O-acetylation, 4″-O-tetrahydopyranylation, 6″-O-deacetylation, 6″-O-benzylation and, finally, cleavage of the acetal group by mildly acidic methanolysis. Thus prepared compound 29 was glycosylated with the disaccharide n-pentenyl donor 254 using NIS–TESOTf as an activator to afford a pseudotetrasaccharide (66%), which on thiourea-assisted removal of the chloroacetate gave the tetrasaccharide glycosyl acceptor 253, ready for glycosylation with the n-pentenyl trimannoside 252 to give the glycan core 250 (39%).90,91 The latter compound provided (Scheme 51) the branched pseudoheptasaccharide 262 once the chloroacetyl group in mannose-3 was removed.
The ethanolamine phosphates were sequentially introduced on the glycan core (Scheme 52) at O-6 of mannose-3 (89% yield) and then at O-2 of mannose-1 (75% yield) by making use of the phosphoramidite 27 and 1H-tetrazole followed by oxidation with m-CPBA to give the fully protected diphosphate 263, consisting of four diastereomers at P atoms. 1-O-Deallylation and subsequent 1H-tetrazole-mediated O-phospholipidation with the phosphoramidite 251 and oxidation (75%) gave the totally protected GPI anchor 264. This was deprotected in one step by hydrogenolysis over palladium catalyst to give the desired GPI anchor 249 in 75% yield.
Scheme 52.
Successive phosphorylation steps and global deprotection (the Fraser-Reid group).
9.2 Convergent synthesis by the Schmidt group
The Schmidt group has also contributed38,88 to the total synthesis of the rat brain Thy-1 GPI anchor 249 (Scheme 53), along with its non-lipidated water-soluble glycoconjugate analogue 279 (Scheme 55). The work followed on from the group’s two previous GPI syntheses [of S. cerevisiae31,32 (Section 8) and T. gondii37 (Section 4)] and relied on (i) use of trichloroacetimidate and phosphoramidite chemistry, and (ii) the combination of a convergent design with a sequential glycosylation strategy. The main difference in the GPIs structure of rat brain Thy-1 and T. gondii is that the former contains an extra d-mannose (α1→2)-linked to mannose-3 and an extra ethanolamine phosphate group at C-2 of mannose-1. This resulted in the design of the GalNAc-Man fragment being altered so that the ethanolamine phosphate side group could be added at a later stage. The assembly of the GPI branched spine 265 (Scheme 53) involved a (5 + 2) coupling of the pentasaccharide trichloroacetimidate 268 and the pseudodisaccharide 269. The glycan 268 was, in turn, further disconnected to monosaccharide donors 67–69 and acceptors 270 and 271 for stepwise chain elongation. In contrast to the synthesis of 249 by the Fraser-Reid group (see Section 9.1), three different P-cyanoethyl-protected synthons, 62, 266 and 267, were used for the phosphorylation steps.
Scheme 53.
Retrosynthesis of a GPI anchor of rat brain Thy-1 glycoprotein (the Schmidt group).
Scheme 55.
The final phosphorylation steps and global deprotection (the Schmidt group).
The required pentasaccharide donor 268 was formed starting from the d-mannose derivative 272 (Scheme 54), which was readily available from allyl 3-O-benzyl-α-d-mannopyranoside 77 (Scheme 14) in two steps.37,38 Compound 272 was converted to glycosyl acceptors 270 and 271, each of them to be further glycosylated with the galactosamine trichloroacetimidate 69. BF3·Et2O-promoted glycosylation of either of the 1,2-di-O-allyl intermediates furnished the corresponding disaccharides, which were further converted to the same glycosyl acceptor 273 after removal of the 6-O-protecting group, either by CAN-mediated oxidative cleavage of the PMB ether for the compound derived from 271, or MeNH2-assisted removal of the O-chloroacetyl group for the disaccharide prepared from 270. It was observed that using the 6-chloroacetate 270 instead of the 6-O-PMB protected 271 caused a dramatic increase of the overall yield and reduced the preparation time of 273. Construction of the branched pentasaccharide 274 proceeded sequentially and almost exactly as previously reported37 for the branched tetrasaccharide block 83 in the T. gondii GPI synthesis (Section 4). Glycosylation of the disaccharide 273 with the trichloroacetimidate 68 under TMSOTf catalysis (95%), removal of the 2-O-acetyl group, followed by glycosylation with the donor 67 (96%), and again 2-O-deacetylation and α-manosylation with 68 (92%) led to the required branched pentasacharide 274. All the mannosylation reactions were stereospecific due to the anchimeric effect of the 2-O-acetyl group, and very high-yielding due to the ‘arming’ effect of the O-benzyl protecting groups in the glycosyl donors.
Scheme 54.
GPI branched glycan core assembly and the first phosphorylation step (the Schmidt group).
After the formation of compound 274, the N-trichloroacetyl group was reduced with Bu3SnH and AIBN to the N-acetyl group, the 2-O-acetyl group in mannose-4 was replaced with an O-benzyl group, and the 1,2-di-O-allyl grouping in mannose-1 was cleaved using Wilkinson’s catalyst. After phenoxyacetylation of the 1,2-diol and anomeric deprotection, the trichloroacetimidate was installed at the anomeric position to afford the pentasaccharide donor 268 in good overall yield. 4-O-Phenoxyacetylation of the pseudodisaccharide 64 (used previously in the synthesis of T. gondii GPI) followed by protecting group remodelling gave first the fully protected derivative 275, which was subjected to successive removal of the phenoxyacetyl group with MeNH2, reduction of the azido group, and finally selective N-Boc protection of the free amino group to afford the pseudodisaccharide acceptor 269.
Glycosylation of compound 269 with the pentasaccharide donor 268 (Scheme 54) in the presence of TMSOTf afforded the branched glycan 265 (74%). The 6-O-TBDPS group in mannose-3 was cleaved with TBAF–AcOH prior to selective installation of the first ethanolamine phosphate. This was achieved by making use of 1H-tetrazole-catalysed phosphitylation with the phosphoramidite 62, followed by oxidation with m-CPBA and subsequent removal (in one step) of the 2-O-phenoxyacetate in mannose-1 and the cyanoethyl P-protecting group with MeNH2 to give the phosphodiester 276 (74%) ready for the next phosphorylation step.
The second ethanolamine phosphate was attached in a similar manner (Scheme 55) using the phosphoramidite 266 and tert-BuOOH for the oxidation step. Stepwise removal of the P-cyanoethyl group (with MeNH2; 78% over three steps) and the 1-O-benzoyl group (with NaOMe) provided the pseudoheptasaccharide diphosphate 277. This was first phosphorylated with dibenzyl-N,N-diisopropylaminophosphoramidite (in a similar fashion) to furnish the protected triphosphoglycan 278 (90%), which on hydrogenolytic O-debenzylation, followed by cleavage of N-Boc groups with aqueous TFA, afforded the non-lipidated water-soluble glycoconjugate 279.
Final phospholipidation of 277 was performed via 1H-tetrazole-catalysed coupling with the phosphoramidite 267, followed by oxidation (tert-BuOOH) and P-deprotection (Me2NH) to form the protected GPI compound 280 (85%). Global deprotection was completed in two steps: removal of the N-Boc protecting groups with TFA and Et3SnH as a scavenger (90%), followed by hydrogenolysis of the O-benzyl groups and the N-Cbz group over Pearlman’s catalyst to give the GPI anchor 249 (60%).
10 Syntheses of GPI anchors and related glycopeptide conjugates of sperm CD52 glycopeptide antigen by the Guo group
10.1 Synthesis of a GPI anchor containing three phosphate residues
Guo and co-workers have been interested in the chemical preparation of the CD52 antigen – a GPI-anchored glycopeptide antigen involved in the human reproduction and human immune recognition processes.93 The Guo group’s most recent achievement was the first synthesis of a highly phosphorylated and highly lipidated GPI anchor (compound 281, Scheme 56) bearing a phospholipid, two ethanolamine phosphate moieties and having a bulky acyl (palmitoyl) at the myo-inositol O-2 position that makes the inositol moiety more crowded.94,95 Earlier synthetic work by the Fraser-Reid group96 encountered acyl migration and steric hindrance for the phospholipid introduction at O-1 whilst working with a 2-O-acyl group in the myo-inositol moiety. In contrast to the majority of synthetic plans of GPI structures that have been already discussed, the Guo group’s synthetic design94,95 relied on the introduction of the phospholipid and lipid moieties in myo-inositol at an early stage and further introduction of the phosphoethanolamine functions (via the phosphoramidite 27) once the acylated and phospholipidated core 282 was constructed.
Scheme 56.
Retrosynthesis of the CD52 antigen GPI anchor containing three phosphate residues (the Guo group).
The glycan core 282 was retrosynthetically broken down into the trimannoside 283 and the acylated/phospholipidated pseudodisaccharide 284. The trisaccharide was further disconnected to simple monosaccharide derivatives 288–290, while the complex synthon 284 was built up from the differentially protected pseudodisaccharide 285, the phosphoramidite 287 and palmitoleic acid 286. The unsaturated fatty acid was introduced because, before the global deprotection, the double bond could be modified (e.g., oxidized) to produce some reactive intermediates97 that are useful for the preparation of various GPI intermediates. Alternatively, the reductive debenzylation at the final step could readily convert the 2-O-palmitoleoyl group to the palmitic ester, the natural acyl chain of the CD52 anchor.
As the target GPI 281 has three positions on the myo-inositol ring (O-1, O-2 and O-6) to be differentiated, a considerable amount of research was performed towards the synthesis of suitable myo-inositol derivatives. This resulted in an effective synthetic procedure for a novel differentially protected synthon 294 (Scheme 57).98,99 Enantiomeric resolution of the racemic 2,3:4,5-di-O-cyclohexylidene-(±)-myo-inositol 916,39 was achieved using the literature procedure100,101 via enantioselective enzymatic degradation of its 6-butyric ester (±)-myo-inositol 291. When the racemate (±)-291 was digested with porcine pancreatic lipase or cholesterol esterase, only one enantiomer (+)-291 was deacylated, providing the optically pure diol (−)-9 that was readily separable from the undigested 6-butyrate (−)-291. Subsequent basic deacylation of (−)-291 gave the optically pure enantiomer (+)-9. As the O-1 and O-6 positions have already been discriminated from the others in the diol (+)-9, it was transformed to 1,6-O-differentially protected/2-O-deprotected synthon 294 via a 6-step sequence of regioselective protections and deprotections in 32% overall yield. Because the myo-inositol molecule is symmetric and the chiral property of compound 9 was created by derivatisation, the authors accomplished the preparation of the same synthetic target 294 from the other enantiomer (−)-9 as well (in 42% overall yield) using a reverse 6-step sequence of synthetic transformations.98,99
Scheme 57.
myo-Inositol enantiomeric resolution (the Guo group).
The O-2 position in compound 294 was further acetylated (Scheme 58), followed by cleavage of the 6-allyl ether to furnish the glycosyl acceptor 295. The pseudodisaccharide 285 was prepared94,95 via Bu4NBr-promoted glycosylation of the acceptor 295 with the known24 2-azido-α-d-glucosyl bromide 47 (56%) and subsequent deacetylation. 2-O-Acylation of the derivative 285 with palmitoleic acid 286 in presence of DCC (85%), followed by oxidative removal of the 1-O-PMB group and then 1H-tetrazolecatalysed phosphorylation with the lipidated phosphoramidite 287 (51%), gave the corresponding acylated/phospholipidated pseudodisaccharide, which afforded the target synthon 284 after 4′-O-desilylation as a diastereomeric mixture at the P atom.
Scheme 58.
The synthesis of a pseudodisaccharide derivative and successive lipidation and phosphorylation steps (the Guo group).
The trimannoside building block 283 (Scheme 59), the preparation of which was not described in detail by the authors,94,95 was constructed from monosaccharide compounds 288–290 according to a procedure developed earlier for a similar structure 29699 (see Section 10.2). Glycosylation of the pseudodisaccharide acceptor 284 using the thioglycoside donor 283 and DMTST or methyl triflate as promoter failed to form the desired product, while the use of N-iodosuccinimide and silver triflate (NIS–AgOTf) would influence the double bond in the palmitoleoyl moiety. Therefore, compound 283 transformed into the trisaccharide trichloroacetimidate 297 via subsequent NIS–TfOH-assisted oxidative hydrolysis of the thioglycoside and then O-trichloroacetimidation of the formed hemiacetal.94 TMSOTf-catalysed glycosylation of the pseudodisaccharide 284 with the donor 297, followed by BF3·Et2O-mediated simultaneous removal of the TBS and PMB ethers, gave the pseudopentasaccharide 2″,6″″-diol 298 (35%) as two separable diastereomers at the P atom.
Scheme 59.
GPI glycan core assembly, the final double phosphorylation step and global deprotection (the Guo group).
One-step double phosphitylation of the diol 298 (of one isomer only) employing the N-Cbz-aminoethanol phosphoramidite 27 in the presence of 1H-tetrazole, followed by oxidation with tert-BuOOH gave the fully protected GPI anchor 299 (50%). Removal of the cyanoethyl P-protecting group with DBU (78%) and global deprotection via Pd-catalysed hydrogenolysis (82%) gave the desired fully phosphorylated and lipidated GPI anchor 281.
10.2 Synthesis of a GPI anchor containing two phosphate residues
This convergent and versatile synthesis of the fully functionalized GPI anchor 281 was accomplished as a result of the experience gained by the authors from their previous trials. Earlier attempts by the Guo group102 to adopt the traditional approach for the synthesis of the CD52 GPI anchor 300 (Scheme 60), by introducing the phospholipid at O-1 position at a late stage once the glycan core was assembled, seemed not to work. Compared to the structure 281 (Scheme 56), the GPI anchor 300 lacks the 2″-ethanolamine phosphate moiety. The initial synthetic strategy for compound 300 involved the final introduction of the 6″″-phosphoethanolamine function (via the N-Fmoc-protected phosphoramidite 302) once the 2-O-acylated-1-O-phospholipidated core 301 was constructed. It also relied102 on a traditional (3 + 2) assembly of a non-phosphorylated glycan core first, using the trimannoside 296 and the pseudodisaccharide 303, followed by phospholipidation with the O-Cbz-protected phosphoramidite 287 or its O-Bn-protected analogue 304. The trisaccharide 296 was further disconnected to simple monosaccharide derivatives 288, 289 and 235, while the synthon 303 was built up from the 2-azidoglucose donor 106 and the 2-O-palmitoylated myo-inositol acceptor 305.
Scheme 60.
Retrosynthesis of the CD52 antigen GPI anchor containing two phosphate residues (the Guo group).
The trimannoside building block 296 was constructed99 from two glycosyl halide donors 288 and 289 and a glycosyl acceptor 235, as depicted in Scheme 61. The glycosyl chloride 289 was obtained from the d-mannose 1,2-orthoester 171 following a reported procedure,103 while the glycosyl bromide 288 was formed from methyl α-d-mannoside 306 by successive perbenzylation, acetolysis (→307) and 1-bromination with HBr in acetic acid.
Scheme 61.
The synthesis of a linear trimannoside (the Guo group).
Treatment of the 1,6-diacetate 307 with ethanethiol and BF3·Et2O gave the corresponding thioglycoside, which provided the acceptor 235 upon conventional deacetylation. Coupling of the chloride 289 and the thiomannoside 235 in the presence of AgOTf as a promoter furnished, after basic deacetylation, the dimannoside acceptor 308. Subsequent AgOTf-activated glycosylation with the glycosyl bromide 288 gave the matching trimannoside (53%), in which the 6″-O-acetyl group was then replaced with the TBS ether to afford the required synthon 296.
The pseudodisaccharide synthon 303 (Scheme 62) was synthesised98,99 by coupling of the 2-O-lipidated myo-inositol derivative 305 with the 2-azido-d-glucosyl fluoride 106. The acceptor 305 was prepared by 2-O-acylation of the key myo-inositol synthon 294 (Scheme 57) with palmitic acid in presence of DCC and DMAP (>99%), followed by cleavage of the 6-allyl ether with PdCl2 and acetic acid. The donor 106 was derived from the 1,6-anhydro compound 309, obtained from the commercially available d-glucal according to a literature procedure.104,105 Five-membered ring-opening acetolysis of 309 provided the 1,6-diacetate 310. Subsequent anomeric O-deacetylation with BnNH2 and fluorination with DAST gave the glycosyl fluoride 311, which on replacement of the 6-O-acetyl with benzyl ether provided the target 106 in 75% yield. This was used in a glycosylation (promoted by AgOTf–hafnocene dichloride) of the acceptor 305 to give the α-linked pseudodisaccharide 303 (40%) along with its β-linked isomer (30%). Compound 303 was 4′-O-deallylated with PdCl2 and acetic acid to furnish the 2-O-lipidated pseudodisaccharide acceptor 312 (99%). It was found that the removal of the 4′-O-allyl group allowed better separation of 312 from its β-anomer.
Scheme 62.
The synthesis of lipidated pseudodisaccharide derivative including prior lipidation of myo-inositol (the Guo group).
Coupling of the thioglycoside donor 296 and the acceptor 312 in the presence of NIS–TfOH afforded the protected glycan core 313 (Scheme 63) in excellent yield.99 Further oxidative cleavage of the PMB ether at O-1 gave the pseudopentasaccharide 314. The phospholipidation reaction of 314 with the phosphoramidite 304 was performed under the established conditions102 in the presence of 1H-tetrazole, followed by oxidation with m-CPBA. Very much to the authors’ surprise, a cyclic phosphoramidate 315 was isolated as a major product (83%, as a diastereomeric mixture at the P atom), thus indicating the involvement of the azido group in the reaction. They proposed that this unwanted phenomenon was due to participation of the azido group in an intramolecular Staudinger-type reaction, which seemed to be possible in the adopted conformation (of the intermediate phosphite triester at O-1) forced by the steric interactions between the trimannoside segment and the long lipid chain at O-2.
Scheme 63.
GPI glycan core assembly and attempted phosphorylation steps (the Guo group).
The expectation that exclusion of the azido group involvement might solve the problem proved to be impractical. Reduction to amino group, which was then protected with N-Cbz functionality (→316), did not help to add the phospholipid. The pseudopentasaccharide 316 appeared to be totally unreactive towards the lipidated phosphoramidite 304, probably due to steric hindrance around the 1-OH group. This assumption was confirmed by the quantitative yield of the phosphite triester 317 obtained from phosphitylation of 316 under the same conditions with the less bulky phosphoramidite reagent (BnO)2PN(iso-Pr)2.
Trying to circumvent this problem, a novel synthetic strategy for the GPI anchor 300 was developed (Scheme 64). The acylated/phospholipidated glycan core 301 (Scheme 60) was now assembled102 via the phospholipidation of the pseudodisaccharide, and later addition of the trimannoside block. To this end, the acylated pseudodisaccharide synthon 303 was subjected to TFA-assisted removal of the 1-O-PMB group followed by 1H-tetrazole-catalysed reaction with the lipid phosphoramidite 287 and oxidation (68%). Further cleavage of the 4′-O-allyl group afforded the acylated/phospholipidated synthon 318 (as a diastereomeric mixture at P). NIS–TfOH-assisted glycosylation of the pseudodisaccharide 318 with the trimannoside donor 296 (→301) and subsequent BF3·Et2O-mediated removal of the 6″″-O-TBS group gave the pseudopentasaccharide 319 (52%) as two separable diastereomers at the P atom. Final phosphorylation employing the N-Fmoc-aminoethanol phosphoramidite 302 in the presence of 1H-tetrazole, followed by oxidation with tert-BuOOH, gave the fully protected GPI anchor 320 (74%).
Scheme 64.
The synthesis of lipidated and phosphorylated pseudodisaccharide derivative, GPI glycan core assembly, the final phosphorylation step and global deprotection (the Guo group).
Two-step deprotection involved removal of the cyanoethyl P-protecting and the Fmoc N-protecting groups with DBU (87%), and then global deprotection via Pd-catalysed hydrogenolysis (84%) to furnish the desired twice-phosphorylated and twice-lipidated GPI anchor 300.
10.3 Syntheses of CD52 glycopeptide conjugates
In order to benefit from the pseudopentasaccharide structure 313, which appeared to be unsuitable for the phospholipidation (see Scheme 63), the Guo group made use of it for the first syntheses of CD52 GPI-related glycopeptide conjugates.99,106 First, they synthesised the GPI core-anchored peptide 325 (Scheme 65), in which the amino group of the ethanolamine phosphate is acylated with a Pro-Ser dipeptide.99
Scheme 65.
The synthesis of a GPI core-anchored dipeptide conjugate (the Guo group).
After removal of the 6″″-O-TBS group in 313 with TBAF, attempted phosphorylation of 321 with the N-Fmoc-aminoethanol phosphoramidite 302 in the presence of 1H-tetrazole led to a slow and incomplete reaction, yielding, after oxidation, only 15% of the fully protected derivative 322. Once the azido group in 321 was reduced under Staudinger conditions (PEt3, MeOH) and transformed to the N-Cbz-protected amino group, the phosphorylation reaction with the same reagents was effective and produced the phosphotriester 323 (84%) as a diastereomeric mixture at the P atom. After mildly basic removal of the N-Fmoc group, the GPI fragment having a free amino group was coupled with the dipeptide Fmoc-Pro-Ser 324 using 1-(3-dimethylaminopropyl)-3-ethylcarbodiimide hydrochloride (EDC) and 1-hydroxybenzotriazole (HOBt) as condensing reagents to give the corresponding O,N-protected conjugate (60%). Two-step deprotection – cleavage of the N-Fmoc group with Me2NH (85%), and then global Pd-catalysed hydrogenolysis (91%) – furnished the desired GPI core-anchored dipeptide 325.
The phosphorylated/lipidated glycan core 323 was also elaborated in the synthesis of the complex GPI-anchored glycopeptide106 containing native CD52 dodecapeptide with one N-glycosylation site bearing a Man3FucGlcNAc2 glycoform (structure 332, Scheme 67). The O,N-protected glycopeptide synthon 326 (Scheme 66) was prepared first, starting from the N-glycan 327 with an azido group attached at C-1.107 After selective reduction to a glycosyl amine (using Lindlar catalyst), the latter was coupled to the allyl aspartate side-chain-activated ester 328, thus forming the N-glycosyl asparagine block 329 (82%). Cleavage of the allyl ester with Pd(PPh3)4 gave the asparagine derivative 330 ready for the glycopeptide assembly. The nonapeptide fragment (starting from the carboxy terminus) of the synthon 326 was assembled using N-Fmoc amino acids and conventional automatic solid-phase synthesis on the 2-chlorotrityl resin, which contained a highly acid-sensitive 2-chlorotrityl linker. Next, the N-glycosyl asparagine 330 was introduced manually using DCC and HOBt as condensing reagents, followed by N-deprotection (with piperidine) and manual introduction of the two final amino acids. The protected glycopeptide 326 was then freed from the resin by treatment with a mixture of AcOH, trifluoroethanol and CH2Cl2 (1 : 1 : 8).
Scheme 67.
Synthesis of the CD52 antigen GPI core-anchored glycopeptide conjugate (the Guo group).
Scheme 66.
Synthesis of the CD52 specific N-linked glycopeptide conjugate (the Guo group).
Before the conjugation of the structure 326 to the GPI core (Scheme 67), the phosphorylated/lipidated glycan 323 was deprotected at O-1 by acidic treatment prior to phosphorylation by making use of the phosphoramidite chemistry (57%). DBU-assisted removal of the N-Fmoc group afforded the protected 1,6″″-diphosphate 331 having a free amino group ready for the attachment of the glycopeptide 326. Amide bond formation was performed using EDC and HOBt as condensing reagents (as for the preparation of the glycodipeptide 325) to furnish the corresponding fully protected glycopeptide conjugate (70%). Two-step global deprotection was accomplished by catalytic hydrogenolysis to remove the benzyl ethers and esters as well as the N-Cbz group, followed by acidic removal of the peptide protecting groups, and provided the desired sperm CD52 GPI-anchored glycopeptide 332 (85%).
11 Synthesis of a phosphatidylinositol mannoside of Mycobacterium tuberculosis by the Seeberger group
Phosphatidylinositol mannosides (PIMs) are a major class of antigenic glycolipids found in the cell wall of the prokaryote M. tuberculosis, one of the most effective human pathogens and a causative agent of tuberculosis. PIMs contain a phosphatidyl myo-inositol anchor that is extended by mannosyltransferases to phosphatidylinositol oligomannosides of various lengths. The phosphatidylinositol tetramannoside (PIM4) is postulated to be a key biosynthetic intermediate and a precursor for the lipoarabinomannan, which is one of the major polymeric components of the bacterium glycocalyx.108,109 PIMs are also present in the mycobacterial cell wall, mostly in the form of PIM2 and PIM6, and elicit a variety of immune responces.110,111 Seeberger and co-workers recently reported64 the total synthesis of the PIM6 molecule, phosphatidylinositol mannoside 333 (Scheme 68).
Scheme 68.
Retrosynthesis of a phosphatidylinositol mannoside (PIM6) of M. tuberculosis (the Seeberger group).
The PIM6 structure 333 has a myo-inositol moiety glycosylated at O-6 with a mannopentaose glycan and at O-2 with 6-O-palmitoyl-α-d-mannopyranose. Palmitic acid is also present in the glycerophospholipid at the sn-2 position, while the primary position is acylated with chiral tuberculostearic acid [(R)-10-methyloctadecanoic acid], which was found to be the basic cell wall constituent of mycobacteria.112,113 The strategy adopted for the synthesis was similar to the one used by the group in their preparation of a GPI anchor of Plasmodium falciparum63,66,69 (Section 6.2) and relied on a (4 + 3) glycosylation of the branched pseudotrisaccharide 337 with the mannotetraose donor 336 to form (after protecting group remodelling) the glycan backbone 334, which was subsequently lipidated with palmitic acid and phospholipidated with the H-phosphonate 335. The building block 337 was further disconnected to the monosaccharide donor 338 and the pseudodisaccharide 339, while the tetrasaccharide 336 was prepared from the smaller synthons 68 and 145.
The assembly of the pseudotrisaccharide 337 (Scheme 69) commenced with the synthetic elaboration of enantiopure myo-inositol derivative 140 that was readily derived from methyl α-d-glucoside and used earlier by the authors64 and by the Fraser-Reid group.56 Protection of the 2,6-diol in 140 as the 1-ethoxyethyl ethers (EE), followed by consecutive 2-O-deacetylation, 2-O-allylation, acid hydrolysis of the two EE groups and regioselective reaction with 2-naphthylmethyl bromide (NAPBr) for orthogonal 2-O-protection, afforded compound 341. The 6-O-TIPS-protected glycosyl donor 338 was prepared from the β-d-mannose 1,2-orthobenzoic ester 340 (via the intermediate 144) in a manner similar to the preparation of the donors 68 and 139 (Scheme 26). TMSOTf-promoted coupling of 341 and 338 afforded the corresponding pseudodisaccharide with complete α-selectivity. Replacement of 6′-O-TIPS ether with the levulinoyl (Lev) group and DDQ-mediated removal of the 2-O-NAP protection furnished 339 ready for the subsequent 2-O-mannosylation. Glycosylation of 339 with the trichloroacetimidate 338 prior to the removal of the levulinic ester gave the pseudotrisaccharide 337 ready for coupling with the tetramannoside 336.
Scheme 69.
The synthesis of a branched pseudotrisaccharide derivative (the Seeberger group).
The fragment 336 was assembled (Scheme 70) by the route prescribed for construction of the tetramannoside 13763 (Scheme 26) with a minor modification. TMSOTf-assisted elongation of the disaccharide 14563,64 with the donor 68, followed by mildly acidic methanolysis74 to cleave the 2′-O-acetyl group, gave the trimannoside 342 (74%), which was extended to the corresponding tetramannoside (83%) using iterative mannosylation with the same donor. Subsequent replacement of the anomeric O-allyl group with the trichloroacetimidoyl group provided the desired mannotetraose donor 336.
Scheme 70.
PIM6 glycan core assembly, successive lipidation and phosphorylation steps and global deprotection (the Seeberger group).
TMSOTf-catalysed combination of 336 with 337 (91%) and conventional replacement of the acetic and benzoic esters with O-benzyl groups furnished branched pseudoheptasaccharide 334. Mildly acidic methanolytic cleavage of TIPS, esterification of the generated OH-group with palmitic acid and DCC, followed by removal of the 1-O-allyl group using a cationic cyclopentadienyl ruthenium(II) complex with quinaldic acid114 and pivaloyl chloride assisted phospholipidation with the H-phosphonate 335, afforded the fully protected glycoconjugate 343 (81%). Global O-debenzylation was then accomplished by hydrogenolysis over palladium catalyst to furnish the target phosphatidylinositol mannoside 333 in 76% yield.
12 Future advances
Despite the appreciable progress in the chemical synthesis of GPI anchors, there are still several unresolved issues that prevent these complex molecules from being routinely synthesised and, therefore, require special attention and experience:
Additional side chains in GPIs, or positional alteration in the side chains, results in total change to the whole plan, particularly in the selection of orthogonal protecting groups. Thus, advanced strategies in oligosaccharide synthesis115 could play an increasingly important role in the design and preparation of the compounds.
All the utilized synthetic plans are time-consuming; therefore, reduction of the number of steps is highly desirable. This is achievable by adopting and developing one-pot strategies116 that enable the construction of the glycan core by means of sequential multiple glycosylations under catalytic activation.
Installation and removal of protecting groups are the most time-consuming aspects of GPI glycan core synthesis. Minimizing their use by employing the ‘glycosyl donor–glycosyl acceptor match’ concept is a valuable goal (e.g., see Section 3.2), since there is no need to protect a hydroxyl that does not match the specific donor used.
A combined chemo-enzymatic approach to GPI anchors would be an interesting challenge. This would probably require chemical preparation of a pseudodisaccharide (or a pseudotrisaccharide), followed by further chain elongation with the help of recombinant glycosyltransferases, phospholipases, phosphoryltransferases and acyltransferases.
Acknowledgements
This work was supported by the Wellcome Trust project grant WT 067089 (including sponsorship of N. A.-M.) and grant WT 083481 for the Division infrastructure maintenance.
Biography

Andrei Nikolaev graduated from Lomonosov Moscow State University (Moscow) with an MSc, and obtained his PhD in the synthetic chemistry of saccharides under the legendary Nikolay Kochetkov at the Zelinsky Institute of Organic Chemistry (Moscow). This experience, and his postdoctoral studies with Klaus Jann at the Max-Planck-Institute of Immunobiology (Freiburg), crystallised his interest in the chemical preparation of biologically important carbohydrates, to be used as tools for biosynthetic, biochemical and immunological studies, and for the preparation of carbohydrate synthetic vaccines. In 1995 he was appointed as a Lecturer at Dundee University (UK), where he is currently a Reader. In 2007 he was distinguished with the Royal Society of Chemistry Medal and Award in Carbohydrate Chemistry “for the synthesis of complex carbohydrates of biological importance, enabling the study of their operation in Nature”.

Nawaf Al-Maharik received his MSc in chemistry from the Technische Hochschule Leuna-Merseburg (Germany). He obtained his PhD in synthetic chemistry of isoflavonoids under Tapio Hase and Kristtiina Wähälä at Helsinki University (Finland). He subsequently joined the group of Lars Engman at Uppsala University (Sweden), and in 2001 the group of Nigel Botting at St Andrews University (UK) to work on synthesis of 13C-labelled polyphenols. In 2006 he joined Andrei Nikolaev’s group at Dundee University to work towards the chemical preparation GPI anchors and other biologically important carbohydrates. In 2010 he joined David O’Hagan’s group in St Andrews to work on the synthesis of diastereoisomeric multi-vicinal fluoroalkanes. His research interests include the chemistry of natural products, tellurium- and fluorinecontaining compounds, 18F-labelled radiopharmaceuticals and carbohydrate chemistry.
Footnotes
Dedicated to the memory of Professor Vladimir N. Shibaev.
14 References
- 1.Sogin ML, Gunderson JH, Elwood HJ, Alonso RA, Peattie DA. Science. 1989;243:75–77. doi: 10.1126/science.2911720. [DOI] [PubMed] [Google Scholar]
- 2.McConville MJ, Ferguson MAJ. Biochem. J. 1993;294:305–324. doi: 10.1042/bj2940305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ferguson MAJ. Philos. Trans. R. Soc. London, Ser. B. 1997;352:1295–1302. doi: 10.1098/rstb.1997.0113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ferguson MAJ. J. Cell Sci. 1999;112:2799–2809. doi: 10.1242/jcs.112.17.2799. [DOI] [PubMed] [Google Scholar]
- 5.Almeida IC, Gazzinelli RT. J. Leukoc. Biol. 2001;70:467–477. [PubMed] [Google Scholar]
- 6.Ferguson MAJ, Homans SW, Dwek RA, Rademacher TW. Science. 1988;239:753–759. doi: 10.1126/science.3340856. [DOI] [PubMed] [Google Scholar]
- 7.Homans SW, Ferguson MAJ, Dwek RA, Rademacher TW, Anand R, Williams AF. Nature. 1988;333:269–272. doi: 10.1038/333269a0. [DOI] [PubMed] [Google Scholar]
- 8.Ferguson MAJ, Williams AF. Annu. Rev. Biochem. 1988;57:285–320. doi: 10.1146/annurev.bi.57.070188.001441. [DOI] [PubMed] [Google Scholar]
- 9.McConville MJ, Ralton JE. In: Glycopeptides and Related Compounds. Large DG, Warren CD, editors. Marcel Dekker, Inc.; New York: 1997. pp. 393–425. [Google Scholar]
- 10.Varma R, Mayor S. Nature. 1998;394:798–801. doi: 10.1038/29563. [DOI] [PubMed] [Google Scholar]
- 11.McConville MJ, Menon AK. Mol. Membr. Biol. 2000;17:1–16. doi: 10.1080/096876800294443. [DOI] [PubMed] [Google Scholar]
- 12.Gigg R, Gigg J. In: Glycopeptides and Related Compounds. Large DG, Warren CD, editors. Marcel Dekker, Inc.; New York: 1997. pp. 327–392. [Google Scholar]
- 13.Guo Z, Bishop L. Eur. J. Org. Chem. 2004:3585–3596. [Google Scholar]
- 14.Murakata C, Ogawa T. Tetrahedron Lett. 1991;32:671–674. [Google Scholar]
- 15.Murakata C, Ogawa T. Carbohydr. Res. 1992;235:95–114. doi: 10.1016/0008-6215(92)80081-b. [DOI] [PubMed] [Google Scholar]
- 16.Garegg PJ, Iversen T, Johansson R, Lindberg B. Carbohydr. Res. 1984;130:322–326. [Google Scholar]
- 17.Murakata C, Ogawa T. Tetrahedron Lett. 1990;31:2439–2442. [Google Scholar]
- 18.Ogawa T, Sasajima K. Tetrahedron. 1981;37:2787–2792. [Google Scholar]
- 19.Murakata C, Ogawa T. Carbohydr. Res. 1992;234:75–91. doi: 10.1016/0008-6215(92)85040-7. [DOI] [PubMed] [Google Scholar]
- 20.Koike K, Sugimoto M, Sato S, Ito Y, Nakabara Y, Ogawa T. Carbohydr. Res. 1987;163:189–208. doi: 10.1016/0008-6215(87)80181-7. [DOI] [PubMed] [Google Scholar]
- 21.Cottaz S, Brimacombe JS, Ferguson MAJ. J. Chem. Soc., Perkin Trans. 1. 1993:2945–2951. [Google Scholar]
- 22.Ogawa T. Chem. Soc. Rev. 1994;23:397–407. [Google Scholar]
- 23.Baeschlin DK, Chaperon AR, Charbonneau V, Green LG, Ley SV, Lücking U, Walther E. Angew. Chem., Int. Ed. 1998;37:3423–3428. doi: 10.1002/(SICI)1521-3773(19981231)37:24<3423::AID-ANIE3423>3.0.CO;2-I. [DOI] [PubMed] [Google Scholar]
- 24.Baeschlin DK, Chaperon AR, Green LG, Hahn MG, Ince SJ, Ley SV. Chem. Eur. J. 2000;6:172–186. doi: 10.1002/(sici)1521-3765(20000103)6:1<172::aid-chem172>3.0.co;2-5. [DOI] [PubMed] [Google Scholar]
- 25.Riley AM, Jenkins DJ, Potter BVL. Carbohydr. Res. 1998;314:277–281. [Google Scholar]
- 26.Nicolaou KC, Caulfield TJ, Kataoka H, Stylianides NA. J. Am. Chem. Soc. 1990;112:3693–3695. [Google Scholar]
- 27.Lemieux RU, Hendriks KB, Stick RV, James K. J. Am. Chem. Soc. 1975;97:4056–4062. [Google Scholar]
- 28.Zuurmond HM, van der Meer PH, van der Klein PAM, van der Marel GA, van Boom JH. J. Carbohydr. Chem. 1993;12:1091–1103. [Google Scholar]
- 29.Mallet A, Mallet JM, Sinay P. Tetrahedron: Asymmetry. 1994;5:2593–2608. [Google Scholar]
- 30.Grice P, Ley SV, Pietruszka J, Osborn HMI, Priepke HWM, Warriner SL. Chem. Eur. J. 1997;3:431–440. [Google Scholar]
- 31.Mayer TG, Kratzer B, Schmidt RR. Angew. Chem., Int. Ed. Engl. 1994;33:2177–2181. [Google Scholar]
- 32.Mayer TG, Schmidt RR. Eur. J. Org. Chem. 1999:1153–1165. [Google Scholar]
- 33.Campbell AS, Fraser-Reid B. Bioorg. Med. Chem. 1994;2:1209–1219. doi: 10.1016/s0968-0896(00)82072-6. [DOI] [PubMed] [Google Scholar]
- 34.Campbell AS, Fraser-Reid B. J. Am. Chem. Soc. 1995;117:10387–10388. [Google Scholar]
- 35.Striepen B, Zinecker CF, Damm JBL, Melgers PAT, Gerwig GJ, Koolen M, Vliegenthart JFG, Dubremetz J-F, Schwarz RT. J. Mol. Biol. 1997;266:797–813. doi: 10.1006/jmbi.1996.0806. [DOI] [PubMed] [Google Scholar]
- 36.Striepen B, Dubremetz J-F, Schwarz RT. Biochemistry. 1999;38:1478–1487. doi: 10.1021/bi981884q. [DOI] [PubMed] [Google Scholar]
- 37.Pekari K, Tailler D, Weingart R, Schmidt RR. J. Org. Chem. 2001;66:7432–7442. doi: 10.1021/jo015840q. [DOI] [PubMed] [Google Scholar]
- 38.Pekari K, Schmidt RR. J. Org. Chem. 2003;68:1295–1308. doi: 10.1021/jo026380j. [DOI] [PubMed] [Google Scholar]
- 39.Massy DJR, Wyss P. Helv. Chim. Acta. 1990;73:1037–1057. [Google Scholar]
- 40.Mayer TG, Schmidt RR. Liebigs Ann./Recl. 1997:859–863. [Google Scholar]
- 41.Grundler G, Schmidt RR. Liebigs Ann. Chem. 1984:1826–1847. [Google Scholar]
- 42.Kaur KJ, Hindsgaul O. Carbohydr. Res. 1992;226:219–231. doi: 10.1016/0008-6215(92)84069-5. [DOI] [PubMed] [Google Scholar]
- 43.Ponpipom MM. Carbohydr. Res. 1977;59:311–317. [Google Scholar]
- 44.Goebel M, Nothofer H-G, Ross G, Ugi I. Tetrahedron. 1997;53:3123–3134. [Google Scholar]
- 45.Tailler D, Ferrieres V, Pekari K, Schmidt RR. Tetrahedron Lett. 1999;40:679–682. [Google Scholar]
- 46.Ruda K, Lindberg J, Garegg PJ, Oscarson S, Konradsson P. J. Am. Chem. Soc. 2000;122:11067–11072. [Google Scholar]
- 47.Morris PE, Kiely DE. J. Org. Chem. 1987;52:1149–1152. [Google Scholar]
- 48.Ruda K, Lindberg J, Garegg PJ, Oscarson S, Konradsson P. Tetrahedron. 2000;56:3969–3975. [Google Scholar]
- 49.Garegg PJ, Olsson L, Oscarson SJ. J. Carbohydr. Chem. 1993;12:955–967. [Google Scholar]
- 50.Buskas T, Garegg PJ, Konradsson P, Maloisel J-L. Tetrahedron: Asymmetry. 1994;5:2187–2194. [Google Scholar]
- 51.Bruzik KS, Tsai MD. J. Am. Chem. Soc. 1992;114:6361–6374. [Google Scholar]
- 52.Garegg PJ, Konradsson P, Oscarson S, Ruda K. Tetrahedron. 1997;53:17727–17734. [Google Scholar]
- 53.Jankowska J, Sobkowski M, Stawinski J, Kraszewski A. Tetrahedron Lett. 1994;35:3355–3358. [Google Scholar]
- 54.Lu J, Jayaprakash KN, Fraser-Reid B. Tetrahedron Lett. 2004;45:879–882. [Google Scholar]
- 55.Lu J, Jayaprakash KN, Schlueter U, Fraser-Reid B. J. Am. Chem. Soc. 2004;126:7540–7547. doi: 10.1021/ja038807p. [DOI] [PubMed] [Google Scholar]
- 56.Jia ZJ, Olsson L, Fraser-Reid B. J. Chem. Soc., Perkin Trans. 1. 1998:631–632. [Google Scholar]
- 57.Bender SL, Budhu RJ. J. Am. Chem. Soc. 1991;113:9883–9885. [Google Scholar]
- 58.Estevez VA, Prestwich GD. J. Am. Chem. Soc. 1991;113:9885–9887. [Google Scholar]
- 59.Ferrier RJ, Middleton S. Chem. Rev. 1993;93:2779–2831. [Google Scholar]
- 60.Mach M, Schlueter U, Mathew F, Fraser-Reid B, Hazen KC. Tetrahedron. 2002;58:7345–7354. [Google Scholar]
- 61.Soli ED, Manoso AE, Patterson MC, DeShong P, Favor DA, Hirschmann R, Smith ABI. J. Org. Chem. 1999;64:3171–3177. doi: 10.1021/jo982302d. [DOI] [PubMed] [Google Scholar]
- 62.Presova M, Smrt J. Collect. Czech. Chem. Commun. 1989;54:487–497. [Google Scholar]
- 63.Liu X, Kwon Y-U, Seeberger PH. J. Am. Chem. Soc. 2005;127:5004–5005. doi: 10.1021/ja042374o. [DOI] [PubMed] [Google Scholar]
- 64.Liu X, Stocker BL, Seeberger PH. J. Am. Chem. Soc. 2006;128:3638–3648. doi: 10.1021/ja0565368. [DOI] [PubMed] [Google Scholar]
- 65.Orgueira HA, Bartolozzi A, Schell P, Litjens REJN, Palmacci ER, Seeberger PH. Chem. Eur. J. 2003;9:140–169. doi: 10.1002/chem.200390009. [DOI] [PubMed] [Google Scholar]
- 66.Seeberger PH, Soucy RL, Kwon Y-U, Snyder DA, Kanemitsu T. Chem. Commun. 2004:1706–1707. doi: 10.1039/b407323a. [DOI] [PubMed] [Google Scholar]
- 67.Kwon Y-U, Soucy RL, Snyder DA, Seeberger PH. Chem. Eur. J. 2005;11:2493–2504. doi: 10.1002/chem.200400934. [DOI] [PubMed] [Google Scholar]
- 68.Hewitt MC, Snyder DA, Seeberger PH. J. Am. Chem. Soc. 2002;124:13434–13436. doi: 10.1021/ja027538k. [DOI] [PubMed] [Google Scholar]
- 69.Schofield L, Hewitt MC, Evans K, Siomos M-A, Seeberger PH. Nature. 2002;418:785–789. doi: 10.1038/nature00937. [DOI] [PubMed] [Google Scholar]
- 70.Schwab P, Grubbs RH, Ziller JW. J. Am. Chem. Soc. 1996;118:100–110. [Google Scholar]
- 71.Almeida IC, Camargo MM, Procopio DO, Silva LS, Mehlert A, Travassos LR, Gazzinelli RT, Ferguson MAJ. EMBO J. 2000;19:1476–1485. doi: 10.1093/emboj/19.7.1476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Yashunsky DV, Borodkin VS, Ferguson MAJ, Nikolaev AV. Angew. Chem., Int. Ed. 2006;45:468–474. doi: 10.1002/anie.200502779. [DOI] [PubMed] [Google Scholar]
- 73.Dais P, Shing TKM, Perlin AS. Carbohydr. Res. 1983;122:305–313. [Google Scholar]
- 74.Byramova NE, Ovchinnikov MV, Backinowsky LV, Kochetkov NK. Carbohydr. Res. 1983;124:c8–c11. [Google Scholar]
- 75.Yashunsky DV, Borodkin VS, McGivern PG, Ferguson MAJ, Nikolaev AV. In: Frontiers in Modern Carbohydrate Chemistry. Demchenko AV, editor. American Chemical Society; Washington, DC: 2007. pp. 285–306. [Google Scholar]
- 76.Al-Maharik N, Nikolaev AV. 2008. unpublished results.
- 77.Baeschlin DK, Green LG, Hahn MG, Hinzen B, Ince SJ, Ley SV. Tetrahedron: Asymmetry. 2000;11:173–197. [Google Scholar]
- 78.Ali A, Gowda DC, Vishwakarma RA. Chem. Commun. 2005:519–521. doi: 10.1039/b414119a. [DOI] [PubMed] [Google Scholar]
- 79.Hederos M, Konradsson P. J. Org. Chem. 2005;70:7196–7207. doi: 10.1021/jo0508595. [DOI] [PubMed] [Google Scholar]
- 80.Hederos M, Konradsson P. J. Am. Chem. Soc. 2006;128:3414–3419. doi: 10.1021/ja057339b. [DOI] [PubMed] [Google Scholar]
- 81.de Lederkremer RM, Lima C, Ramirez MI, Ferguson MAJ, Homans SW, Thomas-Oates J. J. Biol. Chem. 1991;266:23670–23675. [PubMed] [Google Scholar]
- 82.Sarbajna S, Misra AK, Roy N. Synth. Commun. 1998;28:2559–2570. [Google Scholar]
- 83.Lerner LM. Carbohydr. Res. 1996;282:189–192. [Google Scholar]
- 84.Elie CJJ, Verduyn R, Dreef CE, Brounts DM, van der Marel GA, van Boom JH. Tetrahedron. 1990;46:8243–8254. [Google Scholar]
- 85.Ottosson H. Carbohydr. Res. 1990;197:101–107. [Google Scholar]
- 86.Conzelmann A, Puoti A, Lester RL, Desponds C. EMBO J. 1992;11:457–466. doi: 10.1002/j.1460-2075.1992.tb05075.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Fankhauser C, Homans SW, Thomas-Oates JE, McConville MJ, Desponds C, Conzelmann A, Ferguson MAJ. J. Biol. Chem. 1993;268:26365–26374. [PubMed] [Google Scholar]
- 88.Tailler D, Ferrieres V, Pekari K, Schmidt RR. Tetrahedron Lett. 1999;40:679–682. [Google Scholar]
- 89.Kratzer B, Mayer TG, Schmidt RR. Eur. J. Org. Chem. 1998:291–298. [Google Scholar]
- 90.Madsen R, Udodong UE, Roberts C, Mootoo DR, Konradsson P, Fraser-Reid B. J. Am. Chem. Soc. 1995;117:1554–1565. [Google Scholar]
- 91.Udodong UE, Madsen R, Roberts C, Fraser-Reid B. J. Am. Chem. Soc. 1993;115:7886–7887. [Google Scholar]
- 92.Hori H, Nishida Y, Ohrui H, Meguro H. J. Org. Chem. 1989;54:1346–1353. [Google Scholar]
- 93.Schroter S, Derr P, Conradt HS, Nimtz M, Hale G, Kirchoff C. J. Biol. Chem. 1999;274:29862–29873. doi: 10.1074/jbc.274.42.29862. [DOI] [PubMed] [Google Scholar]
- 94.Wu X, Guo Z. Org. Lett. 2007;9:4311–4313. doi: 10.1021/ol701870m. [DOI] [PubMed] [Google Scholar]
- 95.Wu X, Shen Z, Zeng X, Lang S, Palmer M, Guo Z. Carbohydr. Res. 2008;343:1718–1729. doi: 10.1016/j.carres.2008.03.033. [DOI] [PubMed] [Google Scholar]
- 96.Schlueter U, Lu J, Fraser-Reid B. Org. Lett. 2003;5:255–257. doi: 10.1021/ol0202161. [DOI] [PubMed] [Google Scholar]
- 97.Xue J, Pan Y, Guo Z. Tetrahedron Lett. 2002;43:1599–1602. [Google Scholar]
- 98.Xue J, Guo Z. Bioorg. Med. Chem. Lett. 2002;12:2015–2018. doi: 10.1016/s0960-894x(02)00301-3. [DOI] [PubMed] [Google Scholar]
- 99.Xue J, Shao N, Guo Z. J. Org. Chem. 2003;68:4020–4029. doi: 10.1021/jo034213t. [DOI] [PubMed] [Google Scholar]
- 100.Gou D, Liu Y, Chen C. Carbohydr. Res. 1992;234:51–64. [Google Scholar]
- 101.Lu P, Gou D, Shieh W, Chen C. Biochemistry. 1994;33:11586–11587. doi: 10.1021/bi00204a021. [DOI] [PubMed] [Google Scholar]
- 102.Xue J, Guo Z. J. Am. Chem. Soc. 2003;125:16334–16339. doi: 10.1021/ja0382157. [DOI] [PubMed] [Google Scholar]
- 103.Garegg PJ, Maron L. Acta Chem. Scand., Ser. B. 1979;B33:39–41. [Google Scholar]
- 104.Tailler D, Jacquinet J-C, Noirot A-M, Beau J-M. J. Chem. Soc., Perkin Trans. 1. 1992:3163–3165. [Google Scholar]
- 105.Xue J, Guo Z. Tetrahedron Lett. 2001;42:6487–6489. [Google Scholar]
- 106.Shao N, Xue J, Guo Z. Angew. Chem., Int. Ed. 2004;43:1569–1573. doi: 10.1002/anie.200353251. [DOI] [PubMed] [Google Scholar]
- 107.Guo Z, Nakahara Y, Nakahara Y, Ogawa T. Bioorg. Med. Chem. 1997;5:1917–1924. doi: 10.1016/s0968-0896(97)00126-0. [DOI] [PubMed] [Google Scholar]
- 108.Nigou M, Gilleron M, Puzo G. Biochimie. 2003;85:153–166. doi: 10.1016/s0300-9084(03)00048-8. [DOI] [PubMed] [Google Scholar]
- 109.Morita YS, Patterson JH, Billman-Jacobe H, McConville MJ. Biochem. J. 2004;378:589–597. doi: 10.1042/BJ20031372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Gilleron M, Ronet C, Mempel M, Monsarrat B, Gachelin G, Puzo G. J. Biol. Chem. 2001;276:34896–34904. doi: 10.1074/jbc.M103908200. [DOI] [PubMed] [Google Scholar]
- 111.Gilleron M, Quesniaux VFJ, Puzo G. J. Biol. Chem. 2003;278:29880–29889. doi: 10.1074/jbc.M303446200. [DOI] [PubMed] [Google Scholar]
- 112.Anderson RJ, Chargaff E. J. Biol. Chem. 1929;85:77–88. [Google Scholar]
- 113.Spielman MA. J. Biol. Chem. 1934;106:87–96. [Google Scholar]
- 114.Tanaka S, Saburi H, Ishibashi Y, Kitamura M. Org. Lett. 2004;6:1873–1875. doi: 10.1021/ol0493397. [DOI] [PubMed] [Google Scholar]
- 115.Huang X, Wang Z. In: Comprehensive glycoscience. From chemistry to system biology. Kamerling JP, Boons G-J, Lee YC, Suzuki A, Taniguchi N, Voragen AGJ, editors. Vol. 1. Elsevier; Amsterdam: 2007. pp. 379–412. [Google Scholar]
- 116.Parameswar AR, Demchenko AV. In: Progress in the synthesis of complex carbohydrate chains of plant and microbial polysaccharides. Nifantiev NE, editor. Transworld Research Network; Kerala, India: 2009. pp. 465–490. [Google Scholar]