Abstract
Brain aging is the known strongest risk factor for Alzheimer’s disease (AD). In recent years, mitochondrial deficits have been proposed to be a common mechanism linking brain aging to AD. Therefore, to elucidate the causative mechanisms of mitochondrial dysfunction in aging brains is of paramount importance for our understanding of the pathogenesis of AD, in particular its sporadic form. Cyclophilin D (CypD) is a specific mitochondrial protein. Recent studies have shown that F1FO ATP synthase oligomycin sensitivity conferring protein (OSCP) is a binding partner of CypD. The interaction of CypD with OSCP modulates F1FO ATP synthase function and mediates mitochondrial permeability transition pore (mPTP) opening. Here, we have found that increased CypD expression, enhanced CypD/OSCP interaction, and selective loss of OSCP are prominent brain mitochondrial changes in aging mice. Along with these changes, brain mitochondria from the aging mice demonstrated decreased F1FO ATP synthase activity and defective F1FO complex coupling. In contrast, CypD deficient mice exhibited substantially mitigated brain mitochondrial F1FO ATP synthase dysfunction with relatively preserved mitochondrial function during aging. Interestingly, the aging-related OSCP loss was also dramatically attenuated by CypD depletion. Therefore, the simplest interpretation of this study is that CypD promotes F1FO ATP synthase dysfunction and the resultant mitochondrial deficits in aging brains. In addition, in view of CypD and F1FO ATP synthase alterations seen in AD brains, the results further suggest that CypD-mediated F1FO ATP synthase deregulation is a shared mechanism linking mitochondrial deficits in brain aging and AD.
Keywords: Aging brain, Cyclophilin D, F1FO ATP synthase, mitochondria, oligomycin sensitivity conferring protein
Alzheimer’s disease (AD) is a heterogeneous chronic neurodegenerative disorder. The development of the familial form of AD is strongly associated with genetic risk factors [1]; while the etiology of its sporadic form still remains obscure [2]. Clinical observations and epidemiological studies have repeatedly identified that aging is the strongest risk factor for AD [3, 4]. Aging brain and AD share many similarities in brain structural changes and other brain pathologies, which seems to lend credibility to the hypothesis that brain aging and AD, particularly its sporadic form are mechanistically related [5]. Consistent with the mitochondrial cascade hypothesis of AD [6], mitochondrial defects have been highlighted to be a shared mechanism, in which brain aging and AD converge [5, 7, 8]. In this regard, the mechanisms of brain aging-related mitochondrial dysfunction are significant in assisting our understanding of the development of AD, particularly the development of sporadic AD.
Cyclophilin D (CypD. Gene name: Ppif), a protein specifically located in mitochondria, is a member of the prolyl isomerase (PPIase) family [9]. CypD has been linked with several diseases including AD and brain aging [8, 10]. A long-standing concept is that CypD facilitates the formation of mitochondrial permeability transition pore (mPTP), leading to compromised brain mitochondrial ATP production, increased reactive oxygen species (ROS) generation, and eventually neuronal death [9]. However, how CypD triggers mPTP formation is not clear until recently. It has been proposed that CypD interacts with mitochondrial F1FO ATP synthase oligomycin sensitivity conferring protein (OSCP) subunit [11, 12]. The interplay of CypD with OSCP uncouples this key mitochondrial enzyme, causing activated mPTP formation and lowered mitochondrial oxidative phosphorylation (OXPHOS) capacity [11, 12]. Therefore, CypD potentially causes mitochondrial defects through its impacts on F1FO ATP synthase at pathological states.
Mitochondrial F1FO ATP synthase is an essential mitochondrial enzyme for OXPHOS and plays a vital role in ATP production as well as the maintenance of mitochondrial membrane potential [13]. In addition, recent studies have revealed the novel role of F1FO ATP synthase in the formation of mPTP [11, 12, 14]. Previous studies have implicated the deregulation of F1FO ATP synthase in brain aging [15–17], suggesting the changes in this key mitochondrial enzyme are aging-related brain pathology. Of note, in our recent study we have determined that mitochondrial F1FO ATP synthase deregulation is a primary mitochondrial pathology in postmortem AD brains [7] along with upregulated CypD expression [8]. Given the impacts of CypD on F1FO ATP synthase functionality and the implication of CypD in the development of brain aging and AD [8], it would be intriguing to know whether CypD contributes to mitochondrial F1FO ATP synthase dysfunction during aging, thus serving as a common mechanism linking mitochondrial dysfunction in brain aging and AD.
In this study, we have found the expression levels of CypD increase in brain mitochondria in an age-dependent manner. In addition, the interaction of CypD with OSCP is enhanced while the expression level of OSCP is selectively reduced in brain mitochondria with age. To dissociate the impacts of CypD on F1FO ATP synthase in brain mitochondria from aging mice, we employed CypD deficient mice and found that the aging-related F1FO ATP synthase dysfunction was substantially ameliorated by the genetic depletion of CypD, resulting in preserved OXPHOS capacity, reduced ROS generation, and inhibited mPTP formation. Of note, the aging-related OSCP loss was dramatically attenuated by CypD depletion. Put together, our results suggest the role of CypD in mediating mitochondrial F1FO ATP synthase deregulation in aging brain. Furthermore, mitochondrial F1FO ATP synthase dysfunction constitutes a key aspect of aging-related mitochondrial deficits, which could be at least partly protected through the inhibition of CypD.
METHODS AND MATERIALS
Mice
Animal studies were approved and performed under the guidelines of Institutional Animal Care and Use Committee (IACUC) at the University of Texas at Dallas and National Institute of Health. Non-transgenic (nonTg) and Cyclophilin D homozygous null (Ppif−/−) strains of the same genetic background (B6/129) were used in this study. PCR was performed to identify the genotype of the strains using specific primers as described previously [18]. All mice used in this study were mixed genders and prepared by a research assistant. The researchers were blinded to the genotypes until the end of the experiments.
Mitochondrial isolation
Brain mitochondria were prepared as previously described [19]. Briefly, cortices were dissected from mouse brains and homogenized in ice-cold isolation buffer [225 mM mannitol, 75 mM sucrose, 2 mM K2PO4, 0.1% BSA, 5 mM Hepes, 1 mM EGTA (pH 7.2)] with a Dounce homogenizer (Wheaton). After a centrifugation at 1,300 × g for 5 min to remove blood and cell debris, the supernatant was layered on 15% Percoll (GE) and centrifuged at 8,000 ×g for 10 min. The pellet was collected and resus-pended in isolation buffer with 0.02% Digitonin (Sigma-Aldrich) and then subjected to the second centrifugation at 8,000 ×g for another 10 min. The pellet was then washed by centrifugation to remove digitonin and the resultant pellet was resuspended in ice-cold isolation buffer without EGTA for experiments. Protein concentrations were measured using Bradford assay for protein detection (BioRad).
Mitochondrial oxygen consumption assay
Oxygen consumption was measured polarographically using a temperature regulated Clark-type oxygen electrode (Oxytherm, Hansatech) [20]. Respiration control ratio (RCR) for the freshly isolated brain mitochondria was calculated by triggering oxygen consumption in the presence of Glutamate/Malate (5 mM) and ADP (300 μM), and by taking the ratio of state III over state IV respirations. ATP:O ratios were expressed with the ratio of ADP consumed over oxygen consumption. Oligomycin insensitive respiration was calculated by adding 1 μM oligomycin at state IV respirations. The ratio of Oligomycin state (So) over state III was used to calculate oligomycin insensitive respiration by mitochondria.
Immunoblotting
Immunoblotting was performed to quantify the expression levels of various proteins in brain mitochondrial lysates [20]. Samples were prepared by lysing mitochondrial fractions with RIPA buffer as previously described [19]. Proteins were separated in 12% Bis-Tris Gel (NuPAGE, Life Technologies) and then transferred to PVDF membrane (ImmunBlot Membrane, BioRad). Membranes were blocked with 5% non-fat dry milk (Labscientific Inc.) for 1 h at room temperature and probed with appropriate primary antibodies overnight at 4°C; followed by the incubation with the appropriate secondary antibody for 1 h at room temperature. Proteins were detected using ECL (Clarity Substrate, BioRAD) and imaged using a Chemidoc system (BioRAD). The following antibodies were used in the experiments: anti-Cyclophilin D (Calbiochem, 1:5,000), anti-Tom 40 (Santa Cruz, 1:500), and for all the major F1FO ATP synthase subunits: anti-OSCP (Santa Cruz Biotechnologies, 1:5,000), anti-ATP5A (Santa Cruz Biotechnologies, 1:5,000), anti-ATP5B (Santa Cruz Biotechnologies, 1:5,000), anti-ATP6 (Protein-Tech, 1:5000), anti-ATP5F1 (Santa Cruz Biotechnologies, 1:500), anti-ATP5C1 (Santa Cruz Biotechnologies, 1:500), and anti-ATP synthase C (Abcam, 1:5000). The intensities of the immunoreactive bands were analyzed using NIH ImageJ software.
Mitochondrial ATP synthesis
Freshly isolated brain mitochondria were used to perform mitochondrial ATP synthesis using Luminescence ATP detection Assay Kit (Abcam) to measure ATP production. 25 μg mitochondria were energized by glutamate/malate (5 mM) followed by the addition of ADP (200 μM). ATP production was measured following the manufacturer’s instructions. Luminescence was detected using a Microplate Reader (Synergy Mx., Biotek) with Gen5 software. Readings were converted using standard curve and expressed in fold change.
Mitochondrial superoxide assay
Freshly prepared brain mitochondria (15 μg) were incubated with glutamate/malate (5 mM) and ADP (200 μM) for 10 min and then permeablized with 0.1% triton-X. The produced superoxide was detected using Amplex Red reagent (Invitrogen Amplex UltraRed) by reading fluorescence excitations on a Microplate Reader (Synergy Mx., Biotek).
Mitochondrial swelling assay
Mitochondrial swelling was performed as previously described [20]. Mitochondria were energized by Glutamate/Malate (5 mM) in Assay Buffer (150 mM KCl and 2 mM K2HPO4 at pH 7.2). Mitochondrial swelling was triggered by adding Ca2+ at the concentration of 500 nM/mg mitochondria. The optical density at 540 nm (OD540 nm) was recorded by a spectrophotometer (Ultrospect 2100, Amersham Biosciences). Data were expressed as percentage of initial OD value.
F1FO ATP synthase catalytic activity assay
F1FO ATP synthase enzymatic activity was measured spectrophotometrically using NADH-linked ATP regenerating system as previously described [7, 21]. Briefly, mitochondria of appropriate amount were placed in the assay buffer [100 mM Tris-HCl (pH 7.4), 2 mM MgCl2, 50 mM KCl, 0.2 mM EDTA, 0.23 mM NADH, and 1 mM Phosphoenolpyruvate]. The reaction was triggered by the addition of 0.4 M ATP-Mg and recorded on a spectrophotometer (Ultrospect 2100, Amersham Biosciences) at OD340 nm for a total of 600 s at 10-s intervals and expressed in fold change.
F1FO ATP synthase coupling assay
The coupling assay was performed as previously described [7, 22]. Mitochondrial fractions were treated with various concentrations of oligomycin A for 15 min at room temperature before conducting ATP synthase catalytic activity assay.
Co-immunoprecipitation
Co-immunoprecipitation was performed as previously described [7, 8]. Brain mitochondrial isolates (0.5 mg protein) were lysed in Co-IP lysis buffer (50 mM Tris-HCl, 150 mM NaCl, 1 mM EDTA, 0.5% NP-40, 5% glycerol, and 1X Protease inhibitor (Cal-biochem), pH 7.4) by keeping on ice for 30 min, followed by 7 freeze and thaw cycles and pelleted at 12,500 g at 4°C. The supernatant was used to immunoprecipitate OSCP using anti-OSCP (Santa Cruz Biotechnologies 0.5 μg IgG/100 μg protein) overnight at 4°C. Preimmuned IgG at the same concentration was used as the negative control. Prepared immuno-complex was incubated with pre-cleaned Protein agarose A/G (Pierce) for 2 h at room temperature. Immunoblot was conducted by using anti-CypD (Calbiochem, 1:1,000).
Statistical analysis
Two-way ANOVA followed by Bonferroni post hoc analysis or Student t tests wherever appropriate were used for repeated measure analysis on SPSS software (IBM software). The distribution and variance were normal and similar in all groups. p < 0.05 was considered significant. All data were expressed as the mean ± s.e.m.
RESULTS
CypD expression levels are increased in brain mitochondria with age
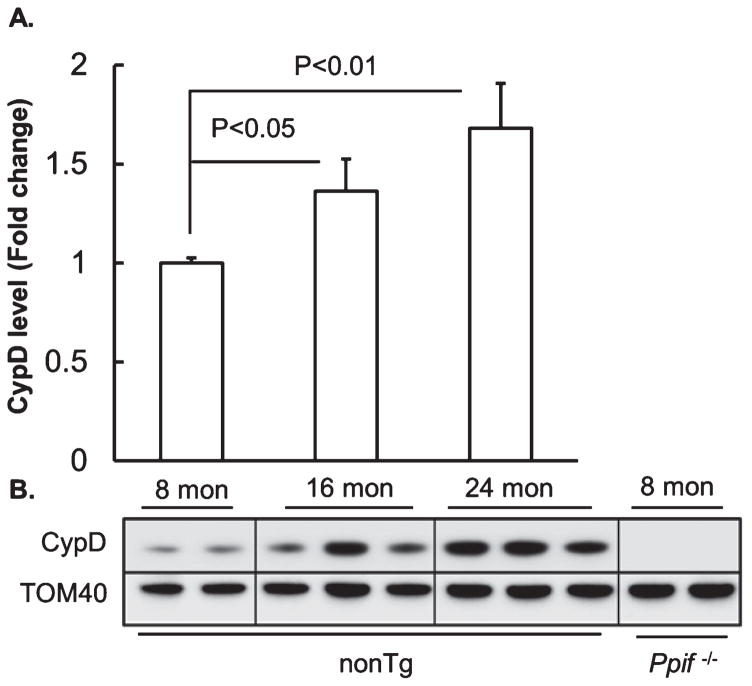
To determine whether there is an age-effect on the expression level of CypD in brain mitochondria from our experimental mice, we prepared brain mitochondria from nonTg mice at 8, 16, and 24 months of age (mimicking young, middle-aged, and aging stages, respectively), and subjected the purified brain mitochondria to the detection of CypD levels by immunoblotting. Translocase of outer membrane 40 KDa Subunit (TOM40) was employed as the loading control. Quantitative analysis showed that brain mitochondria from the middle-aged mice demonstrated a significant increase in the expression level of CypD in comparison to their counterpart from the young nonTg mice (Fig. 1A, B); while the upregulation of CypD expression levels was even greater in aging mice (Fig. 1A, B). The results suggest that the expression levels of CypD in brain mitochondria are elevated with brain aging, which conforms to our previous findings showing age-dependent brain CypD elevation in cognitively normal human subjects [8].
Fig. 1.
Increased CypD expression levels in brain mitochondria with age. A) Densitometric quantification of CypD expression in brain mitochondria from 8-, 16-, and 24-month-old nonTg mice. N = 8 for 8-month-old, 10 for 16-month-old, and 10 for 24-month-old nonTg mice. B) Representative immunoreactive bands of CypD. Tom 40 was used to determine the loading amount of mitochondrial fractions.
CypD/OSCP interaction is enhanced in brain mitochondria with age
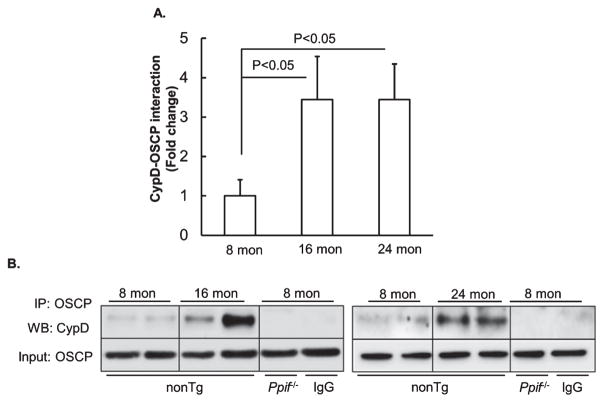
Recent studies have reported that mitochondrial F1FO ATP synthase OSCP subunit is the binding partner of CypD; and the interplay of OSCP and CypD disrupts F1FO ATP synthase stability leading to compromised ATP production and activated mPTP formation [11, 14, 23, 24]. Since brain mitochondrial CypD expression level is increased with age, we thus ask whether the interaction of CypD and OSCP would be promoted in aging mouse brain. To address this question, we purified brain mitochondria from 8-, 16-, and 24-month-old nonTg mice and subjected them to the co-immunoprecipitation of CypD and OSCP by using specific antibody against OSCP followed by the immunoblot to detect CypD. The CypD deficient (Ppif−/−) mice were used as the negative control. As expected, our densitometry analysis showed significantly increased CypD/OSCP complex in brain mitochondria from middle-aged and aging nonTg mice in comparison to those from the young mice (Fig. 2A, B), suggesting CypD and OSCP interaction is enhanced in aging. The complex of OSCP and CypD was detected in brain mitochondria from nonTg mice but not in those from CypD deficient mice (Fig. 2B). Furthermore, when OSCP antibody was replaced by nonimmune IgG to precipitate OSCP in nonTg mitochondria (Fig. 2B), the immunoreactive bands of CypD disappeared, indicating the specificity of OSCP and CypD interaction.
Fig. 2.
Increased CypD and OSCP interaction in brain mitochondria with age. A) Coimmunoprecipitation of OSCP and CypD in brain mitochondria from 8-, 16-, and 24-month-old nonTg mice. N = 8 mice per group. B) Representative immunoreactive bands of CypD (Upper panel) and input OSCP (Lower panel). CypD deficient brain mitochondria were used as a negative control. Nonimmune IgG was used to replace CypD antibody to determine the specificity of the immunoprecipitation.
CypD promotes selective loss of OSCP in brain mitochondria with age
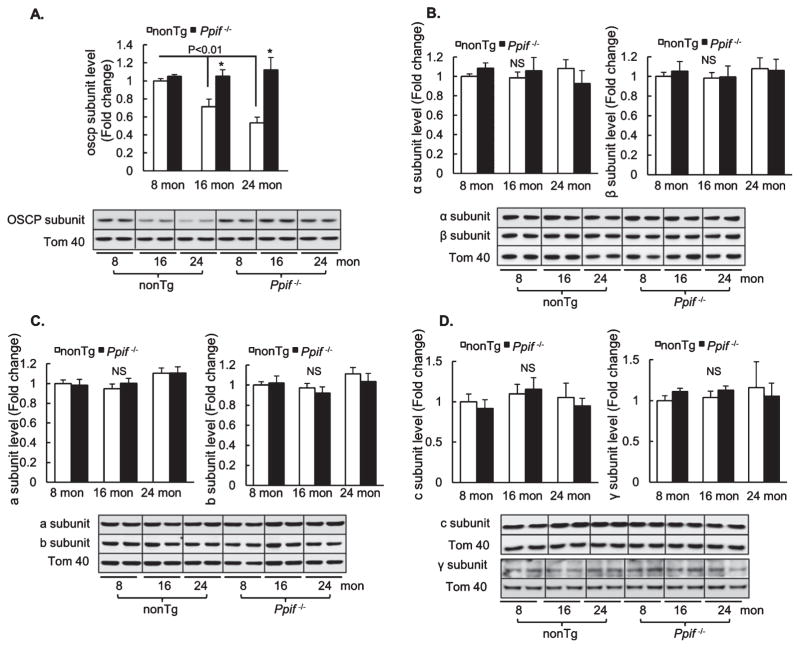
Given increased OSCP/CypD interaction in aged mice as aforementioned, it would be intriguing to know whether the expression level of OSCP in brain mitochondria is altered with age. Purified brain mitochondria from 8-, 16-, and 24-month-old nonTg and Ppif−/−mice were subjected to immunoblotting to detect the expression levels of OSCP as well as of other major subunits of mitochondrial F1FO ATP synthase including a, b, c, γ, α, and β subunits. TOM40 was used as the loading control. The analysis of the densitometry of the immunoreactive bands showed that middle-aged and aging mice had a significant reduction in the expression level of OSCP in comparison to that in young nonTg mice (Fig. 3A). In addition, our immunoblotting data showed only a single immunoreactive band of OSCP at ~23 KD, indicating that at the sample preparation condition CypD-bound OSCP has been completely dissociated from the complex; and the OSCP that was detected by the specific antibody against it was all at “free” state. Therefore, the data suggest that loss of OSCP is the result of age-effect. However, the expression levels of the other major F1FO ATP synthase subunits exhibited no difference across the nonTg mice at different ages (Fig. 3B–D), implying that OSCP expression is selectively affected during aging.
Fig. 3.
Selective loss of OSCP in brain mitochondria with age. Densitometric quantification of the expression levels of OSCP (A), α (B), β (B), a (C), b (C), c (D), and γ (D) subunits in brain mitochondria of 8-, 16-, and 24-month-old nonTg and Ppif−/− mice. N = 6–10 mice per group. The lower panels are representative immunoreactive bands of indicated proteins. Tom40 was used as the loading control. *p < 0.05 versus age-matched nonTg counterpart.
Surprisingly, CypD deficient mice displayed preserved OSCP levels even at 24 months (Fig. 3A). Of note, there was no significant difference in OSCP levels between young nonTg and Ppif−/− mice (Fig. 3A). Furthermore, CypD deficient mice showed no detectable aging-associated changes in the expression levels of other tested mitochondrial F1FO ATP synthase major subunits, which were also comparable to those in the age-matched nonTg mice (Fig. 3B–D). Collectively, the results seem to suggest that CypD is a potentiating factor underlying the selective loss of OSCP in aging.
CypD affects brain mitochondrial F1FO ATP synthase function in aging mice
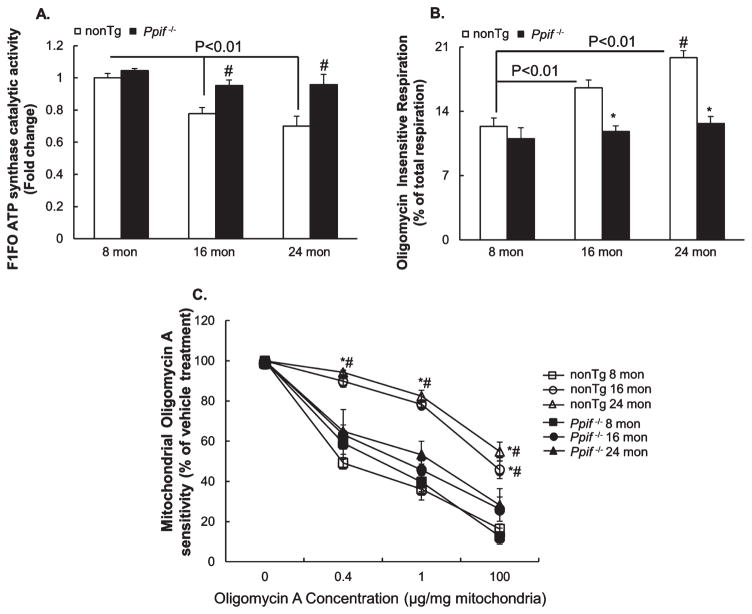
To determine the impacts of CypD on mitochondrial F1FO ATP synthase in aging brain, we first measured the catalytic activity of the enzyme. To dissociate the impacts of CypD on F1FO ATP synthase, we also adopted the age- and gender-matched Ppif−/− mice in the experiments. Our results showed that brain mitochondria from nonTg mice exhibited a significant decline in their F1FO ATP synthase enzymatic activity in an age-dependent manner (Fig. 4A); whereas the aging-related decrease in F1FO ATP synthase enzymatic activity was substantially mitigated by CypD depletion (Fig. 4A). Furthermore, in comparison to nonTg mice at young age, CypD depletion did not demonstrate detectable genotypic effect on the catalytic activity of this enzyme (Fig. 4A).
Fig. 4.
The deleterious effect of CypD on brain mitochondrial F1FO ATP synthase function in aging mice. A) Fold change in ATP synthase catalytic activity of brain mitochondria from nonTg and Ppif−/− mice at 8, 16, and 24 months of age. N = 4–5 per group. #p < 0.05 versus age-matched nonTg counterpart. B) Oligomycin insensitive respiration of brain mitochondria from nonTg and CypD deficient mice at the indicated ages. N = 5–7 per group. #p < 0.05 versus 16-month-old nonTg. *p < 0.05 versus age-matched nonTg counterpart. C) F1FO ATP synthase coupling assay performed on brain mitochondria from nonTg and CypD deficient mice at the indicated ages. N = 4–5 mice per group. *p < 0.01 versus age matched Ppif−/− mice. #p < 0.01 versus nonTg at 8 months.
Next, given the primary function of OSCP is to sustain the coupling state of F1FO complex we examined F1FO ATP synthase proton-flow coupling [7, 25] by measuring oligomycin A insensitive respiration on a Clark electrode. Our results showed that nonTg mice displayed remarkably increased oligomycin A insensitive respiration in an age-dependent manner (Fig. 4B); whereas such aging-associated change was significantly mitigated by CypD depletion (Fig. 4B). The impact of CypD on the coupling state of F1FO ATP synthase in aging brain was further supported by the mitochondrial coupling assay. As shown in Fig. 4C, when compared to their own activity at 0 μg oligomycin A per mg mitochondrial protein, all the mitochondrial fractions exhibited F1FO ATP synthase inhibition at the increment of oligomycin A. However, the F1FO ATP synthase inhibition by oligomycin A was drastically blunted in brain mitochondria from 16-and 24-month-old nonTg mice (Fig. 4C); while the aging-related phenotypic change was protected by CypD deficiency (Fig. 4C). Taken together, our results suggest that mitochondria from aging brains undergo mitochondrial F1FO ATP synthase dysfunction including decreased catalytic activity and increased F1FO complex uncoupling, which is at least partly associated with the effect of CypD.
CypD-mediated mitochondrial F1FO ATP synthase deregulation contributes to brain mitochondrial dysfunction in aging mice
Given mitochondrial F1FO ATP synthase is a key OXPHOS enzyme [26], we examined whether CypD-mediated F1FO ATP synthase deregulation contributes to compromised mitochondrial OXPHOS efficiency. To address this question, we purified brain mitochondria from nonTg and CypD deficient mice at 8, 16, and 24 months of age and used the mitochondria for the assay of mitochondrial oxygen consumption on a Clark electrode. Mitochondrial respiratory control ratio (RCR), the ratio of State III to State IV respiration was measured and compared. At 8 months of age, there was no significant genotype-related difference in mitochondrial RCR (Fig. 5A). However, brain mitochondria from middle-aged to aging nonTg mice displayed a significant decline in their RCRs in comparison to that of their counterpart at young age (Fig. 5A); whereas, such aging-related mitochondrial RCR decrease was substantially mitigated by CypD deficiency (Fig. 5A). To determine the oxygen use efficiency, we then compared ATP:O ratio. Similar to the changes of mitochondrial RCR, brain mitochondria from the older mice demonstrated remarkably decreased ATP:O ratio (Fig. 5B); while the aging-related ATP:O ratio reduction in nonTg mice was attenuated by the depletion of CypD (Fig. 5B). Indeed, the aforementioned mitochondrial changes may be the results of conjoint defects of mitochondrial electron transfer chain (ETC) and F1FO ATP synthase. To gain the direct association of decreased OXPHOS capacity with F1FO ATP synthase deregulation in aging mice, we examined mitochondrial ATP synthesis, which is predominantly related to the function of F1FO ATP synthase. Brain mitochondria from 16- and 24-month-old nonTg mice displayed significantly reduced ability in converting ADP into ATP in comparison to those from young nonTg mice (Fig. 5C); whereas CypD deficient mice showed little age-effect on ATP synthesis (Fig. 5C). The results indicate that CypD-mediated F1FO ATP synthase deregulation is an involving factor in compromised mitochondrial OXPHOS efficacy in aging.
Fig. 5.
The deleterious effect of CypD-mediated F1FO ATP synthase deregulation on brain mitochondrial function in aging mice. A) RCRs of brain mitochondria from nonTg and Ppif−/− mice at 8, 16, and 24 months of age. N = 6–8 mice per group. *p < 0.05 versus age-matched nonTg counterparts. B) ATP: O ratio of brain mitochondria from nonTg and Ppif−/− mice. N = 6–8 mice per group. #p < 0.05 versus age-matched nonTg counterparts. C) ATP synthesis of brain mitochondria from nonTg and Ppif−/− mice. N = 4–5 mice per group. #p < 0.05 versus age-matched nonTg counterparts. D) Ca2+-induced mitochondrial swelling assay performed on brain mitochondria from nonTg and Ppif−/− mice. The results were plotted using percentage decrease in initial OD540 readings. N = 6–8 mice per group. *p < 0.01 versus all the other groups with Ca2+ treatment. #p < 0.01 versus age-matched Ppif−/− mice with Ca2+ treatment. E) The effect of CypD on ROS production in aging mice. ROS production was examined by measuring Amplex Red intensity on freshly isolated brain mitochondria from nonTg and Ppif−/− mice. N = 4–5 mice per group.
Recent studies have highly accentuated the mPTP forming role of deregulated F1FO ATP synthase. CypD is a well-known key regulator of mPTP [9] probably through its interaction with F1FO ATP synthase OSCP subunit [11, 12]. Therefore, increased CypD expression levels as well as enhanced CypD and OSCP interaction may confer susceptibility to the opening of mPTP. By examining mPTP formation using calcium-induced mitochondrial swelling that reflects the propensity of mPTP opening [9], we have found that brain mitochondria from nonTg mice demonstrated increased response to calcium induction in an age-dependent manner (Fig. 5D) indicating that sensitized mPTP formation is an aging-related brain mitochondrial alteration. However, the mitochondrial swelling at the same amount of calcium exposure was significantly retarded by CypD depletion with much less age-effect (Fig. 5D), indicating the association of CypD-mediated F1FO ATP synthase deregulation and the aging-related mPTP activation.
Compromised mitochondrial OXPHOS as well as sensitized mPTP formation are closely associated with increased ROS production [9, 27]. To determine the influence of CypD-mediated F1FO ATP synthase deregulation on mitochondrial ROS production in aging brains, brain mitochondria from 8-, 16- and 24-month-old nonTg and CypD deficient mice were subjected to the assay for mitochondrial superoxide levels. Purified brain mitochondria were energized by glutamate and malate followed by permeabilization to measure the levels of superoxide by using Amplex Red, which is a sensitive indicator of superoxide [28]. The results showed that there was no genotypic difference between the two types of mice in the levels of mitochondrial superoxide at the mouse age of 8 months (Fig. 5E). However, brain mitochondria from nonTg mice exhibited an age-dependent increase in superoxide generation, which was substantially attenuated by CypD deficiency (Fig. 5E).
DISCUSSION
With age, the incidence of AD substantially increases [29, 30], which suggests the relationship of brain aging and AD and thus instigates the exploration of common molecular mechanisms linking these two pathological scenarios. Based on the findings of many similarities in mitochondrial pathology between AD and brain aging as well as the encouraging data collected from cybrid cells generated using mitochondria from sporadic AD patients, Swerd-low and colleagues have formed the mitochondrial cascade hypothesis for sporadic AD [5, 31]. They propose that a functional threshold of mitochondria is the turning point splitting brain aging and AD. Thereafter, the comparative studies on the development of mitochondrial pathology in these two pathological states have received increasing attention. In this study, we have focused on CypD-mediated F1FO ATP synthase deregulation and determined its deleterious impact on brain mitochondrial function during aging, which has never been comprehensively studied. Considering our previous studies on CypD-mediated mitochondrial dysfunction and mitochondrial F1FO ATP synthase alterations in AD-related pathological settings [7, 8], our results suggest that CypD-mediated F1FO ATP synthase deregulation is at least one potential common mechanism of mitochondrial dysfunction linking AD and brain aging.
CypD is a conservative protein in evolution and is abundant in mitochondria. Its physiological function has not been fully elucidated, yet its pathological relevance to neurodegeneration has been well documented [8, 32, 33]. Our previous studies [8, 10] as well as others [34] have noticed increased CypD expression in brain mitochondria from AD cases as well as AD animal models. The blockade of CypD has demonstrated significant protection on mitochondrial function in AD-relative pathological settings [8, 10]. In this study, we have found increased CypD in brain mitochondria from aging mice suggesting that CypD elevation is a primary aging effect. The findings of increased CypD in brain mitochondria during aging have raised an intriguing question, that is, by what mechanism does CypD exerts its effect on mitochondria to promote mitochondrial deficits during aging. Previous studies have predominantly attributed the deleterious effect of CypD to the activation of mPTP formation [9].
mPTP is generally accepted to be a pathological event of mitochondria with the major consequences including the rupture of the outer mitochondrial membrane, decreased OXPHOS activity, collapsed mitochondrial membrane potential, and increased mitochondrial ROS generation and release [9]. However, ever since the description of mPTP, its molecular identity has become a major scientific problem. CypD is the only determined regulator of mPTP so far. Adenine nucleotide translocase (ANT) and voltage-dependent anion channel (VDAC) were proposed to be the major components of mPTP. But this concept was challenged in later studies showing that the depletion of ANT or VDAC does not affect mPTP opening [35–37]. Recent studies have reported the role of uncoupled mitochondrial F1FO ATP synthase in the formation of mPTP [14]. The conductance of uncoupled c rings in inner mitochondrial membrane and the blockade of mPTP opening by the downregulation of c subunits strongly indicate that uncoupled F1FO ATP synthase constitutes mPTP through the empty c rings [14]. Furthermore, it has been shown that the uncoupling of F1FO ATP synthase is closely associated with the interaction of CypD with OSCP subunit, which may disrupt the coupling state of this enzyme [11, 12]. In this context, emerging evidence implicates the influence of CypD on F1FO ATP synthase via OSCP. Here, our results showed that increased CypD expression and enhanced CypD and OSCP interaction in brain mitochondria from aging mice. These alterations coincide with uncoupling of this enzyme and increased mitochondrial response to calcium-induced swelling, which indicate sensitized mPTP formation. In addition, mitochondrial OXPHOS efficacy also decreased during aging. This may be associated with the dissipation of proton gradient due to altered mitochondrial inner membrane permeability caused by mPTP over-activation. However, the decreased capacity of F1FO ATP synthase in converting ADP to ATP suggests that the deactivation of this key mitochondrial enzyme also contributes to lowered OXPHOS efficiency with age. Increased ROS generation is the result of mitochondrial OXPHOS deficits, eventually disrupting intracellular redox balance causing oxidative stress, which is a characteristic brain pathology in aging brain and AD [38]. Here, we have also found that brain mitochondria from the aging mice demonstrated enhanced superoxide production along with the deregulation of F1FO ATP synthase. Put together, such changes implicate that the alterations of this key mitochondrial enzyme stand at the nexus of aging-associated mitochondrial dysfunction. Indeed, a previous study showed increased F1FO complex dissociation in brain mitochondria from aging rats [39], which is in agreement with our findings that F1FO ATP synthase dysfunction is an aging phenotype in brains. Importantly, we have found that such aging-related F1FO ATP synthase changes were significantly ameliorated by the genetic depletion of CypD. This serves as strong evidence that CypD is an involving factor contributing to F1FO ATP synthase dysfunction and the resultant mitochondrial defects during aging.
Another intriguing finding of this study that merits discussion is the age-dependent decrease in OSCP expression. OSCP locates in the peripheral stalk of F1FO ATP synthase and plays a critical role in tethering F1 and FO as well as in holding F1 subunits [40, 41]. By measuring the major subunits of F1FO ATP synthase including a and c subunits of FO, γ, α, and β subunits of F1, and b and OSCP subunits of the peripheral stalk, we did not observe significant change in other major subunits except OSCP. This result has two implications. Firstly, the loss of OSCP is not likely to be the result of decreased F1FO ATP synthase in aging brain; and secondly, the selective loss of OSCP is a primary aging-effect in the brains. The result conforms to our previous findings that OSCP is selectively lost among the F1FO ATP synthase major subunits in postmortem AD brains [7], further indicating a potential link between aging brain and AD. However, our finding has raised a scientific issue of the regulation of OSCP expression in aging brain. Indeed, the loss of proteins including mitochondrial proteins during aging has been observed in previous studies [42–44]. Genomic DNA damage, abnormal protein modifications, and enhanced proteolysis are thought to contribute to the loss of proteins in aging brains [42–44]. Interestingly, in this study we have found the selective loss of OSCP closely accompanies CypD upregulation and the depletion of CypD preserves OSCP expression levels during aging. Although it cannot be concluded that CypD elevation is the triggering factor for OSCP loss, the concomitant changes of CypD and OSCP in aging brain implicate the potential link between the regulations of the expressions levels of the two proteins, which needs our future investigation. It should be noted that a previous study has found increased γ, α, and β subunits in brains from aging rats [17]; while we failed to find any aging-effect on these subunits in aging mice. This disparity may arise from the use of different species of animal models. Further comparative studies may help to answer this question.
In summary, in this study we have revealed a novel mechanism of mitochondrial defects in aging brain. Our data suggest that decreased OXPHOS capacity, increased oxidative stress, and sensitized mPTP opening, which are characteristics of aging brain, have a strong association with CypD-mediated mitochondrial F1FO ATP synthase deregulation (Fig. 6). The inhibition of CypD ameliorates the aging-associated F1FO ATP synthase alterations as well as the resultant mitochondrial dysfunction, further implicating the damage-causing role of CypD and CypD-mediated F1FO ATP synthase dysfunction during aging. In addition, in view of the similar changes of CypD and brain mitochondrial F1FO ATP synthase seen in the AD brains, our data seem to suggest that CypD-mediated F1FO ATP synthase deregulation may constitute a common molecular mechanism of mitochondrial dysfunction between brain aging and AD. This forms the groundwork for our future studies on determining whether such change contributes to the transition from brain aging to AD under certain conditions. Nevertheless, this study provides us with a detailed mechanistic pathway through which CypD imposes its effect on mitochondria during aging and suggests that the suppression of CypD is a potential therapeutic strategy for the treatment of brain aging.
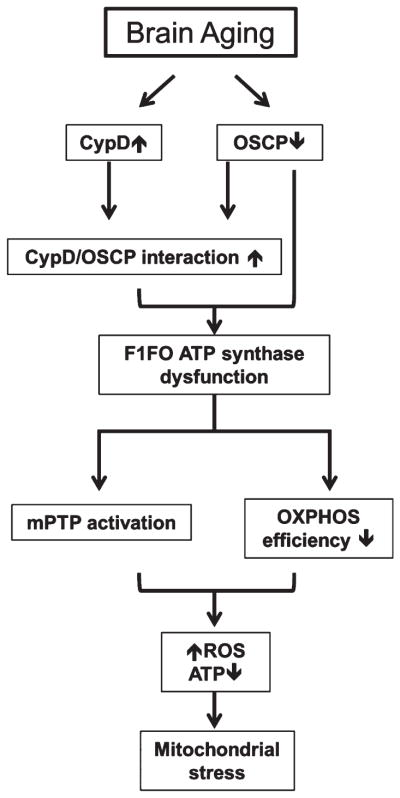
Fig. 6.
Schematic summary. Increased CypD expression, enhanced CypD and OSCP interaction as well as selectively decreased OSCP expression are aging-related brain mitochondrial alterations. These changes cause mitochondrial F1FO ATP synthase dysfunction, which results in sensitized mPTP formation and compromised mitochondrial OXPHOS capacity leading to lowered ATP production, activated ROS production, and eventually severe mitochondrial stress during aging.
Acknowledgments
This study is supported by research funding from NIH (R00AG037716, R01AG053588), Alzheimer’s Association (NIRG-12-242803, AARG-16-442863), NSFC (31271145, 81200847), and SDNSF (JQ201318).
Authors’ disclosures available online (http://j-alz.com/manuscript-disclosures/16-0822r1).
References
- 1.Veugelen S, Saito T, Saido TC, Chavez-Gutierrez L, De Strooper B. Familial Alzheimer’s disease mutations in presenilin generate amyloidogenic Abeta peptide seeds. Neuron. 2016;90:410–416. doi: 10.1016/j.neuron.2016.03.010. [DOI] [PubMed] [Google Scholar]
- 2.Querfurth HW, LaFerla FM. Alzheimer’s disease. N Engl J Med. 2010;362:329–344. doi: 10.1056/NEJMra0909142. [DOI] [PubMed] [Google Scholar]
- 3.Braak H, Braak E, Bohl J, Reintjes R. Age, neurofibrillary changes, A beta-amyloid and the onset of Alzheimer’s disease. Neurosci Lett. 1996;210:87–90. doi: 10.1016/0304-3940(96)12668-9. [DOI] [PubMed] [Google Scholar]
- 4.Morrison JH, Baxter MG. The ageing cortical synapse: Hallmarks and implications for cognitive decline. Nat Rev Neurosci. 2012;13:240–250. doi: 10.1038/nrn3200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Swerdlow RH. Brain aging, Alzheimer’s disease, and mitochondria. Biochim Biophys Acta. 2011;1812:1630–1639. doi: 10.1016/j.bbadis.2011.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Swerdlow RH, Khan SM. The Alzheimer’s disease mitochondrial cascade hypothesis: An update. Exp Neurol. 2009;218:308–315. doi: 10.1016/j.expneurol.2009.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Beck SJ, Guo L, Phensy A, Tian J, Wang L, Tandon N, Gauba E, Lu L, Pascual JM, Kroener S, Du H. Deregulation of mitochondrial F1FO-ATP synthase via OSCP in Alzheimer’s disease. Nat Commun. 2016;7:11483. doi: 10.1038/ncomms11483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Du H, Guo L, Fang F, Chen D, Sosunov AA, McKhann GM, Yan Y, Wang C, Zhang H, Molkentin JD, Gunn-Moore FJ, Vonsattel JP, Arancio O, Chen JX, Yan SD. Cyclophilin D deficiency attenuates mitochondrial and neuronal perturbation and ameliorates learning and memory in Alzheimer’s disease. Nat Med. 2008;14:1097–1105. doi: 10.1038/nm.1868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Baines CP, Kaiser RA, Purcell NH, Blair NS, Osinska H, Hambleton MA, Brunskill EW, Sayen MR, Gottlieb RA, Dorn GW, Robbins J, Molkentin JD. Loss of cyclophilin D reveals a critical role for mitochondrial permeability transition in cell death. Nature. 2005;434:658–662. doi: 10.1038/nature03434. [DOI] [PubMed] [Google Scholar]
- 10.Du H, Guo L, Zhang W, Rydzewska M, Yan S. Cyclophilin D deficiency improves mitochondrial function and learning/memory in aging Alzheimer disease mouse model. Neurobiol Aging. 2011;32:398–406. doi: 10.1016/j.neurobiolaging.2009.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Giorgio V, Bisetto E, Soriano ME, Dabbeni-Sala F, Basso E, Petronilli V, Forte MA, Bernardi P, Lippe G. Cyclophilin D modulates mitochondrial F0F1-ATP syn-thase by interacting with the lateral stalk of the complex. J Biol Chem. 2009;284:33982–33988. doi: 10.1074/jbc.M109.020115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chinopoulos C, Adam-Vizi V. Modulation of the mitochondrial permeability transition by cyclophilin D: Moving closer to F(0)-F(1) ATP synthase? Mitochondrion. 2012;12:41–45. doi: 10.1016/j.mito.2011.04.007. [DOI] [PubMed] [Google Scholar]
- 13.Zhou A, Rohou A, Schep DG, Bason JV, Montgomery MG, Walker JE, Grigorieff N, Rubinstein JL. Structure and conformational states of the bovine mitochondrial ATP synthase by cryo-EM. Elife. 2015;4:e10180. doi: 10.7554/eLife.10180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Alavian KN, Beutner G, Lazrove E, Sacchetti S, Park HA, Licznerski P, Li H, Nabili P, Hockensmith K, Graham M, Porter GA, Jr, Jonas EA. An uncoupling channel within the c-subunit ring of the F1FO ATP synthase is the mitochondrial permeability transition pore. Proc Natl Acad Sci U S A. 2014;111:10580–10585. doi: 10.1073/pnas.1401591111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lam PY, Yin F, Hamilton RT, Boveris A, Cadenas E. Elevated neuronal nitric oxide synthase expression during ageing and mitochondrial energy production. Free Radic Res. 2009;43:431–439. doi: 10.1080/10715760902849813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Daum B, Walter A, Horst A, Osiewacz HD, Kuhlbrandt W. Age-dependent dissociation of ATP synthase dimers and loss of inner-membrane cristae in mitochondria. Proc Natl Acad Sci U S A. 2013;110:15301–15306. doi: 10.1073/pnas.1305462110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nicoletti VG, Tendi EA, Lalicata C, Reale S, Costa A, Villa RF, Ragusa N, Giuffrida Stella AM. Changes of mitochondrial cytochrome c oxidase and FoF1 ATP synthase subunits in rat cerebral cortex during aging. Neurochem Res. 1995;20:1465–1470. doi: 10.1007/BF00970595. [DOI] [PubMed] [Google Scholar]
- 18.Du H, Guo L, Fang F, Chen D, Sosunov AA, McKhann GM, Yan Y, Wang C, Zhang H, Molkentin JD. Cyclophilin D deficiency attenuates mitochondrial and neuronal perturbation and ameliorates learning and memory in Alzheimer’s disease. Nat Med. 2008;14:1097–1105. doi: 10.1038/nm.1868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Du H, Guo L, Zhang W, Rydzewska M, Yan S. Cyclophilin D deficiency improves mitochondrial function and learning/memory in aging Alzheimer disease mouse model. Neurobiol Aging. 2011;32:398–406. doi: 10.1016/j.neurobiolaging.2009.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Du H, Guo L, Yan S, Sosunov AA, McKhann GM, Yan SS. Early deficits in synaptic mitochondria in an Alzheimer’s disease mouse model. Proc Natl Acad Sci U S A. 2010;107:18670–18675. doi: 10.1073/pnas.1006586107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pullman ME, Penefsky HS, Datta A, Racker E. Partial resolution of the enzymes catalyzing oxidative phosphorylation I. Purification and properties of soluble, dinitrophenol-stimulated adenosine triphosphatase. J Biol Chem. 1960;235:3322–3329. [PubMed] [Google Scholar]
- 22.Cortes-Hernandez P, Vazquez-Memije ME, Garcia JJ. ATP6 homoplasmic mutations inhibit and destabilize the human F1F0-ATP synthase without preventing enzyme assembly and oligomerization. J Biol Chem. 2007;282:1051–1058. doi: 10.1074/jbc.M606828200. [DOI] [PubMed] [Google Scholar]
- 23.Giorgio V, von Stockum S, Antoniel M, Fabbro A, Fogolari F, Forte M, Glick GD, Petronilli V, Zoratti M, Szabo I, Lippe G, Bernardi P. Dimers of mitochondrial ATP synthase form the permeability transition pore. Proc Natl Acad Sci U S A. 2013;110:5887–5892. doi: 10.1073/pnas.1217823110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Halestrap AP. The C ring of the F1FO ATP synthase forms the mitochondrial permeability transition pore: A critical appraisal. Front Oncol. 2014;4:234. doi: 10.3389/fonc.2014.00234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Antoniel M, Giorgio V, Fogolari F, Glick GD, Bernardi P, Lippe G. The oligomycin-sensitivity conferring protein of mitochondrial ATP synthase: Emerging new roles in mitochondrial pathophysiology. Int J Mol Sci. 2014;15:7513–7536. doi: 10.3390/ijms15057513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yin F, Boveris A, Cadenas E. Mitochondrial energy metabolism and redox signaling in brain aging and neurodegeneration. Antioxid Redox Signal. 2014;20:353–371. doi: 10.1089/ars.2012.4774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Reddy PH, Beal MF. Amyloid beta, mitochondrial dysfunction and synaptic damage: Implications for cognitive decline in aging and Alzheimer’s disease. Trends Mol Med. 2008;14:45–53. doi: 10.1016/j.molmed.2007.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chinta SJ, Rane A, Yadava N, Andersen JK, Nicholls DG, Polster BM. Reactive oxygen species regulation by AIF- and complex I-depleted brain mitochondria. Free Radic Biol Med. 2009;46:939–947. doi: 10.1016/j.freeradbiomed.2009.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Evans DA, Funkenstein HH, Albert MS, Scherr PA, Cook NR, Chown MJ, Hebert LE, Hennekens CH, Taylor JO. Prevalence of Alzheimer’s disease in a community population of older persons. Higher than previously reported. JAMA. 1989;262:2551–2556. [PubMed] [Google Scholar]
- 30.Polvikoski T, Sulkava R, Rastas S, Sutela A, Niinisto L, Notkola IL, Verkkoniemi A, Viramo P, Juva K, Haltia M. Incidence of dementia in very elderly individuals: A clinical, neuropathological and molecular genetic study. Neuroepidemiology. 2006;26:76–82. doi: 10.1159/000090252. [DOI] [PubMed] [Google Scholar]
- 31.Swerdlow RH, Khan SM. A mitochondrial cascade hypothesis for sporadic Alzheimer’s disease. Med Hypotheses. 2004;63:8–20. doi: 10.1016/j.mehy.2003.12.045. [DOI] [PubMed] [Google Scholar]
- 32.Thomas B, Banerjee R, Starkova NN, Zhang SF, Calingasan NY, Yang L, Wille E, Lorenzo BJ, Ho DJ, Beal MF, Starkov A. Mitochondrial permeability transition pore component cyclophilin D distinguishes nigrostriatal dopaminergic death paradigms in the MPTP mouse model of Parkinson’s disease. Antioxid Redox Signal. 2012;16:855–868. doi: 10.1089/ars.2010.3849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Forte M, Gold BG, Marracci G, Chaudhary P, Basso E, Johnsen D, Yu X, Fowlkes J, Rahder M, Stem K, Bernardi P, Bourdette D. Cyclophilin D inactivation protects axons in experimental autoimmune encephalomyelitis, an animal model of multiple sclerosis. Proc Natl Acad Sci U S A. 2007;104:7558–7563. doi: 10.1073/pnas.0702228104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Manczak M, Mao P, Calkins MJ, Cornea A, Reddy AP, Murphy MP, Szeto HH, Park B, Reddy PH. Mitochondria-targeted antioxidants protect against amyloid-beta toxicity in Alzheimer’s disease neurons. J Alzheimers Dis. 2010;20(Suppl 2):S609–S631. doi: 10.3233/JAD-2010-100564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Baines CP, Kaiser RA, Sheiko T, Craigen WJ, Molkentin JD. Voltage-dependent anion channels are dispensable for mitochondrial-dependent cell death. Nat Cell Biol. 2007;9:550–555. doi: 10.1038/ncb1575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Krauskopf A, Eriksson O, Craigen WJ, Forte MA, Bernardi P. Properties of the permeability transition in VDAC1(−/−) mitochondria. Biochim Biophys Acta. 2006;1757:590–595. doi: 10.1016/j.bbabio.2006.02.007. [DOI] [PubMed] [Google Scholar]
- 37.Kokoszka JE, Waymire KG, Levy SE, Sligh JE, Cai J, Jones DP, MacGregor GR, Wallace DC. The ADP/ATP translocator is not essential for the mitochondrial permeability transition pore. Nature. 2004;427:461–465. doi: 10.1038/nature02229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Reddy VP, Zhu X, Perry G, Smith MA. Oxidative stress in diabetes and Alzheimer’s disease. J Alzheimers Dis. 2009;16:763–774. doi: 10.3233/JAD-2009-1013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Frenzel M, Rommelspacher H, Sugawa MD, Dencher NA. Ageing alters the supramolecular architecture of OxPhos complexes in rat brain cortex. Exp Gerontol. 2010;45:563–572. doi: 10.1016/j.exger.2010.02.003. [DOI] [PubMed] [Google Scholar]
- 40.Ruhle T, Leister D. Assembly of F1F0-ATP synthases. Biochim Biophys Acta. 2015;1847:849–860. doi: 10.1016/j.bbabio.2015.02.005. [DOI] [PubMed] [Google Scholar]
- 41.Carbajo RJ, Kellas FA, Yang JC, Runswick MJ, Montgomery MG, Walker JE, Neuhaus D. How the N-terminal domain of the OSCP subunit of bovine F1FO-ATP synthase interacts with the N-terminal region of an alpha subunit. J Mol Biol. 2007;368:310–318. doi: 10.1016/j.jmb.2007.02.059. [DOI] [PubMed] [Google Scholar]
- 42.Nkuipou-Kenfack E, Koeck T, Mischak H, Pich A, Schanstra JP, Zurbig P, Schumacher B. Proteome analysis in the assessment of ageing. Ageing Res Rev. 2014;18:74–85. doi: 10.1016/j.arr.2014.09.002. [DOI] [PubMed] [Google Scholar]
- 43.Sherratt MJ. Tissue elasticity and the ageing elastic fibre. Age (Dordr) 2009;31:305–325. doi: 10.1007/s11357-009-9103-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Stoll EA, Cheung W, Mikheev AM, Sweet IR, Bielas JH, Zhang J, Rostomily RC, Horner PJ. Aging neural progenitor cells have decreased mitochondrial content and lower oxidative metabolism. J Biol Chem. 2011;286:38592–38601. doi: 10.1074/jbc.M111.252171. [DOI] [PMC free article] [PubMed] [Google Scholar]