Abstract
Spodoptera litura (S. litura) is one of the most serious agricultural insect pests worldwide. Takeout (TO) is involved in a variety of physiological and biochemical pathways and performs various biological functions. We characterized 18 S. litura TO genes and investigated their differential responses to insecticides and sex pheromones. All predicted TO proteins have two Cysteines that are unique to the N-terminal of the TO family proteins and contain four highly conserved Prolines, two Glycines, and one Tyrosine. The expression levels of seven TO genes in the male antennae were higher than those in the female antennae, although the expression levels of 10 TO genes in the female were higher than those in the male. We investigated the effects of the sex pheromone and three insecticides, that is, chlorpyrifos (Ch), emamectin benzoate (EB), and fipronil (Fi), on the expression levels of the TO genes in the antennae. The results showed that the insecticides and sex pheromone affect the expression levels of the TO genes. One day after the treatment, the expression levels of SlTO15 and SlTO4 were significantly induced by the Ch/EB treatment. Two days after the S. litura moths were treated with Fi, the expression of SlTO4 was significantly induced (28.35-fold). The expression of SlTO10 changed significantly after the Ch and EB treatment, although the expression of SlTO12 and SlTO15 was inhibited by the three insecticides after two days of treatment. Our results lay a foundation for studying the role of TO genes in the interaction between insecticides and sex pheromone.
Keywords: Spodoptera litura, sex pheromone, chlorpyrifos, emamectin benzoate, fipronil
The Takeout (TO) genes encode a family of insect-specific proteins that were first discovered in Drosophila (Sarovblat et al. 2000). The TO protein usually contains ∼250 amino acids. The TO protein is a type of secretory protein that has an 18-amino acid signal peptide at the N-terminal. Most TO proteins have two highly conserved Cysteine (Cys) residues at the N-terminal (Sarovblat et al. 2000, Du et al. 2003, Dauwalder et al. 2002, Fujikawa et al. 2006, So et al. 2000, Zhu et al. 2008); Wojtasek et al. have demonstrated that the two Cys residues at the N-terminal of Juvenile hormone binding protein (JHBP) are key amino acids for disulphide bond formation (Wojtasek and Prestwich 1995). JHBP shares a high homology with TO, suggesting that the TO family proteins may have characteristics that are similar to those of the ligand (Wojtasek and Prestwich 1995). The crystal structure of Epiphyas postvittana TO1 (EpTO1) is a hollow barrel-like structure comprising hydrophobic amino acids and a disulphide bond that is formed by two Cys at the N-terminal (Hamiaux et al. 2009), suggesting that EpTO1 binds the ligand at the top of the protein, carries it to the target cell and then releases it at the bottom of the protein, which indicates that some TO proteins function through binding and transportation (Hamiaux et al. 2009).
The TO protein has been shown to participate in a variety of physiological and biochemical pathways and perform various biological metabolic functions. Drosophila TO proteins have been shown to deliver time and food signals for feeding, metabolism, and other activities (Sarovblat et al. 2000). It is also known that the Drosophila TO gene is expressed at low levels in rhythmic mutants and is regulated by the CLK-CYC (Clock-Cycle) hetero-dimer (So et al. 2000). It has been reported that the physiological rhythmic transcription factor PARP1 (Pdp1ε) regulates the expression of the TO gene (Dauwalder et al. 2002). The TO gene can be activated by low levels of Pdp1ε (Benito et al. 2010). The TO gene can also improve the sensitivity of taste nerves to carbohydrates and functions during starvation (Meunier et al. 2007). In Drosophila melanogaster, Manduca sexta, Anopheles gambiae, and Bombyx mori, the nutrient level has been shown to regulate the TO genes (Meunier et al. 2007, Du et al. 2003, Justice et al. 2003, Saito et al. 2006). The TO genes were expressed in the head, the fat body and other parts of the male fruit-fly but not in the female fruit-fly (Dauwalder et al. 2002). TO affects male courtship behavior by processing sex-biased signals (Dauwalder et al. 2002). The TO gene in the termite Reticulitermes flavipes, deviate, is expressed in various tissues, such as the thorax and head, and is also involved in trail-following behavior (Schwinghammer et al. 2011). Hagai et al. speculated that juvenile hormone (JH) mediates the regulation of TO genes via the development-related factors of the Italian honey bee Apis mellifera (Hagai et al. 2007). The expression level of the TO gene in Adenophora glauca was significantly higher than that in migratory adults (Zhu et al. 2008). The TO gene in Drosophila is also involved in the regulation of longevity (Bauer et al. 2010). The TO gene in Locusta migratoria manilensis plays crucial roles in the transformation from mutual exclusion to mutual attraction as the population increases from a low density to a high density (Guo et al. 2011). The receptivity of the chemical receptors in Helicoverpa armigera is influenced by the JH (Angioy et al. 1983), suggesting that the TO genes may endogenously perform the hormonal functions of lipophilic ligands that act through hydrophobic receptors in lymphocytes and are released into helper cells.
Spodoptera litura (Lepidoptera: Noctuidae, S. litura) is a widely distributed crop pest that has a significant impact on the productivity of economic crops (Meagher et al. 2008). The resistance and cross-resistance of S. litura to insecticides make it a more difficult pest to control (Rehan and Freed 2014). Identifying the correlation between the TO gene and insecticides can lay a foundation for the future development of a strategy to control S. litura. Sex pheromones are the key to the courtship behavior of S. litura (Lin et al. 2015). It has also been reported that the TO gene is associated with courtship behavior (Dauwalder et al. 2002). Therefore, studying the correlation between the TO genes and the sex pheromone is important for the understanding of the function of this protein family. The mating ratio of male and female diamondback moths, that is, Plutella xylostella, is decreased in an avermectin-resistant strain, indicating that the mating behavior of P. xylostella is affected by the insecticide application and the resistance generated (Xu et al. 2010). Volatiles from host plants also contribute to the response of Spodoptera exigua to sex pheromones (Deng et al. 2004). The electroantennogram (EAG) responses of Choristoneura occidentalis and Orgyia pseudotsugata to sex pheromones and a mixture of sex pheromones and insecticides were significantly different. Following a treatment of sex pheromones, the EAG response is high, although the EAG response to the sex pheromone/insecticide mixture was significantly lower than that in the control group, indicating that the insecticides affected the response to the sex pheromone (Sower and Shorb 1985). To control the pest both short term and long term, the pheromone and the insecticide are sometimes used together (Trimble et al. 2001). Insecticides have been shown to affect the courtship and sex pheromone communication systems in insects (Dallaire et al. 2004, Wei and Du 2004, Clark and Haynes 1992, Yang and Du 2003). Changes in the sex pheromone communication system are correlated with insecticide resistance in moths (Xu et al. 2010, Elsayed et al. 2001). Sub-lethal doses of the insecticide malathion decrease the localization of females by sex pheromone (Zhou et al. 2005, Elsayed et al. 2001). In addition, it has been reported that insecticides could affect olfactory function even in vertebrate, such as in salmon (Moore and Waring 1996).
It has been reported that the TO gene is highly expressed in the antennae of Aedes aegypti (Bohbot and Vogt 2005), and previous studies have also shown that the TO gene is highly expressed in D. melanogaster and Phormia regina (Vanaphan et al. 2012, Fujikawa et al. 2006). Our recent work revealed that the TO genes in Nilaparvata lugens (N. lugens) are male-biased and regulated by JH signaling (Lin et al. 2017). However, the role of the TO family genes in the cross interaction between insecticides and the sex pheromone remains unclear. Here, we studied the potential role of the TO family genes by measuring their expression levels in the antennae of male and female S. litura adults and the changes in the expression of the TO genes in response to insecticides and the sex pheromone.
Materials and methods
Sequence Analysis
Eighteen S. litura TO gene sequences were obtained from previously reported transcriptome data (Feng et al. 2015), which were named TO1-18. All sequences were deposited in GenBank. The accession numbers are: SlituTO1 (MF196295), SlituTO2 (MF196296), SlituTO3 (MF196297), SlituTO4 (MF196298), SlituTO5 (MF196299), SlituTO6 (MF196300), SlituTO7 (MF196301), SlituTO8 (MF196302), SlituTO9 (MF196303), SlituTO10 (MF196304), SlituTO11 (MF196305), SlituTO12 (MF196306), SlituTO13 (MF196307), SlituTO14 (MF196308), SlituTO15 (MF196309), SlituTO16 (MF196310), SlituTO17 (MF196311), SlituTO18 (MF196312). A Blast search was performed in NCBI. Maximum Likelihood (ML) was used to construct a phylogenetic tree using previously identified 92 TO genes from eight species along with 18 SlituTO genes using MEGA6 (Hall 2013). The SlituTO genes were translated into amino acid sequences, and the sequences were aligned using ClustalW2 (Larkin et al. 2007). A WebLogo sequence alignment map was generated at http://weblogo.berkeley.edu/logo.cgi.
Biological Materials and Experimental Treatment
Male and female S. litura pupae were purchased from Jiyuan Baiyun Industrial Co., Ltd. (Henan, China). The male S. litura used in the insecticide treatment experiment and the sex pheromone treatment experiment were trapped using traps (SL02-SP, main components: Z9, Z11-14: Oac, Z9, Z12-14: OAc), which were a gift from Ningbo Newcomb Biotechnology Company (Ningbo, China). The trapped male S. litura adults were incubated with 10% sucrose for 24 h. The moths were divided into the control group, chlorpyrifos group, emamectin Benzoate group, fipronil group, and sex pheromone group. The control group was treated with 10% sucrose water; the insecticide treatment was performed by adding 1 ppm (Haynes 1988) of the insecticide in 10% sucrose water; and the sex pheromone group was treated with 10% sucrose water, the moths were placed in a plastic basket and PVC lure was placed outside the basket. The antennae of the surviving insects were cut 1 day and 2 days after the treatment. Three sets of biological replicates were established in each group.
RNA Extraction, cDNA Synthesis and qRT-PCR
The antennae were cut and ground in TRIzol (Takara, Dalian, China), extracted using chloroform, precipitated using isopropanol, washed with ethanol, and finally dissolved in DEPC. The PrimeScript RT-PCR Kit (Roche, Shanghai) was used for the reverse transcription with a total of 20 μL in the following three steps: 1) 1 μg of total RNA, 1 μL of OligdT, and RNA free Water to 13 μL were added in a 65 °C water bath for 10 min; 2) 4 μL of Buffer, 2 μL of dNTP, 0.5 μL of the inhibitor, and 0.5 μL of Transcriptase were added in a 55 °C water bath for 30 min; and 3) the solution was placed in an 85 °C water bath for 5 min. The SYBR FAST qPCR Master Mix2 (KAPA) was used for the qRT-PCR with a total volume of 20 μL as follows: 10 μL of 2 × KAPA SYBR FAST qPCR Master Mix 2 Universal, 0.4 μL each (10 μM), ≤ 20 ng of cDNA, 0.4 μL of 50 × ROX High, and PCR-grade water to 20 μL. The qRT-PCR reaction conditions were as follows: 95°C for 3 min, 95°C for 3 s, and 55°C for 20 s (40×). The primers are listed in Table 1. RPL10 was used as the reference gene (Lu et al. 2013).
Table 1.
Primers used in the qRT-PCR
| Gene | 5′ primer | 3′ primer |
|---|---|---|
| SlituTO1 | TGCCATCCTACATATCCTCA | GTCCTTGAAAGTGACCTTGA |
| SlituTO2 | GATGGAGAGAAACACTGGAG | GCTAATGATGGCCTTGACTA |
| SlituTO3 | GACTACATCATGAGAGGCAG | GTTGAAGAGGTTGGAGAAGT |
| SlituTO4 | ACAAGATCAACCCAGACAAG | CTATCCAATGTCCGTCCTTT |
| SlituTO5 | CGAGTACATGAAAGGAGGAG | GAACTGATGCGTTTCTAGGT |
| SlituTO6 | TATCGCTTCGTCATCCAAG | CCACTTCTTTCCAGTTTTCG |
| SlituTO7 | GGATGCTCAAGTCCTCATAG | AAGTCCTGTCTCCATTGAAC |
| SlituTO8 | CAACTGGCATTTCACTCATC | GTGCTTCTACATCTTCCACA |
| SlituTO9 | CAGAGCCAGCATGTCTAATA | TCTCTAAGTATGCCTCGACA |
| SlituTO10 | TGGTGGACTTCAAGTTGAG | ATGGGGATTTTAGAGAAGGC |
| SlituTO11 | GAACGGACCTGGATCTAAAG | CAATCTTTCACCACCCAGTA |
| SlituTO12 | GTGACGAAGCACCTACTTAT | CGATCTTCCAGTGTTTCTCT |
| SlituTO13 | ATACACCTTTGACTACGGTG | GCACTGCAATGAAGAACTTT |
| SlituTO14 | GCTACGGCCTAAGATGATAG | ACCTGTTCTTGTCTAGGTCT |
| SlituTO15 | TAAAACTCGACGGACAGTAC | AACACTAGGTCGGACTTTTC |
| SlituTO16 | CTGTCGATCTGGAGTTGAAA | ATCTGTACTTCATGAGCGAC |
| SlituTO17 | GTAGACGCTAGTTCACCAAA | AGAATATAGACCACCGTTGC |
| SlituTO18 | TCTCGTAATCACCCTGACTA | GAAATCGCCTTCCAGTTTTC |
| SlituRPL10 | GACTTGGGTAAGAAGAAG | GATGACATGGAATGGATG |
Data Analysis
The qRT-PCR data of the TO gene expression levels in the antennae of male and female S. litura adults were analyzed using the 2-ΔΔCt method (Livak and Schmittgen 2001). The TO gene expression in the S. litura female antennae was analyzed by SPSS20.0 (IBM-SPSS, Somers, NY) using Student's t-test. The effects of the insecticides and sex pheromones on the expression of the TO gene in male S. litura were analyzed using a single factor ANOVA analysis, and the LSD method was used for multiple comparisons. The figures were generated using OriginPro 9.1.
Heat Map
The heat map was generated with HemI 1.0 using the data of the effects of the insecticide and sex pheromone on the expression levels of the TO genes in male moths, and the hierarchical average linkage method was used as the clustering method.
Results
Sequence Analysis of the TO Family Genes in S. litura
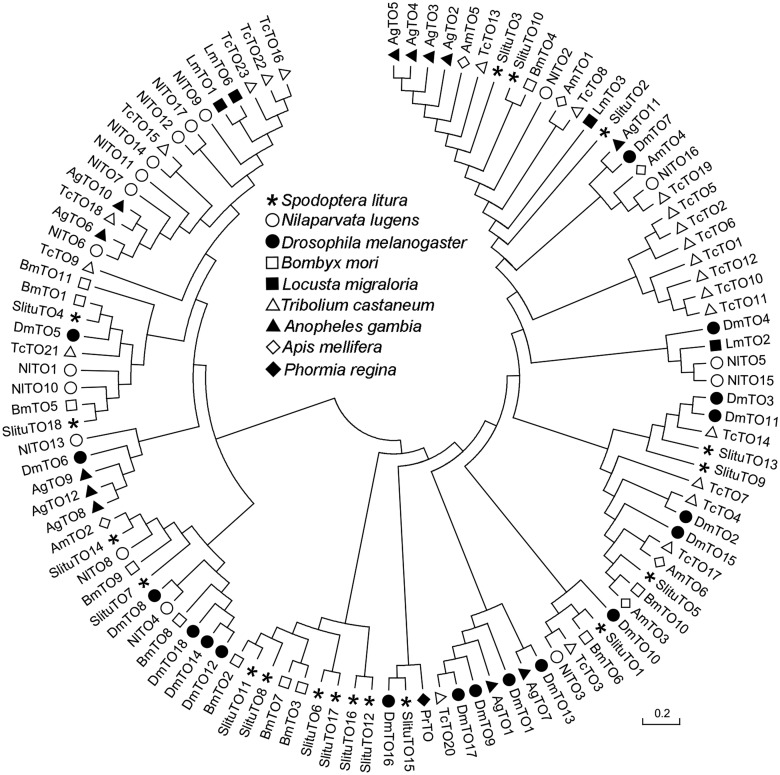
Eighteen TO family genes were identified by analyzing previously reported S. litura transcriptome data (Feng et al. 2015) and named SlituTO1–SlituTO18. We translated the 18 S. litura TO gene sequences into amino acids, and a phylogenetic tree was generated by comparing 92 TO genes from other eight species with the TO genes from S. litura (Fig. 1). SlituTO8, SlituTO11, SlituTO6, SlituTO17, SlituTO12, and SlituTO16 clustered together, and they are highly homologous intraspecies (Fig. 1). SlituTO1, SlituTO4, SlituTO8, SlituTO10, SlituTO11, Slitu12, and SlituTO18 have the highest homology with the TO genes from B. mori (Bm), which indicates that these TO genes are conserved, at least in Lepidoptera.
Fig. 1.
Phylogenetic analysis of the Takeout family proteins from S. litura and other species. Sl: Spodoptera litura; Nl: Nilaparvata lugens; Dm: Drosophila melanogaster; Bm: Bombyx mori; Lm: Locusta migraloria; Tc: Tribolium castaneum; Ag: Anopheles gambia; Am: Apis mellifera; Pr: Phormia regina.
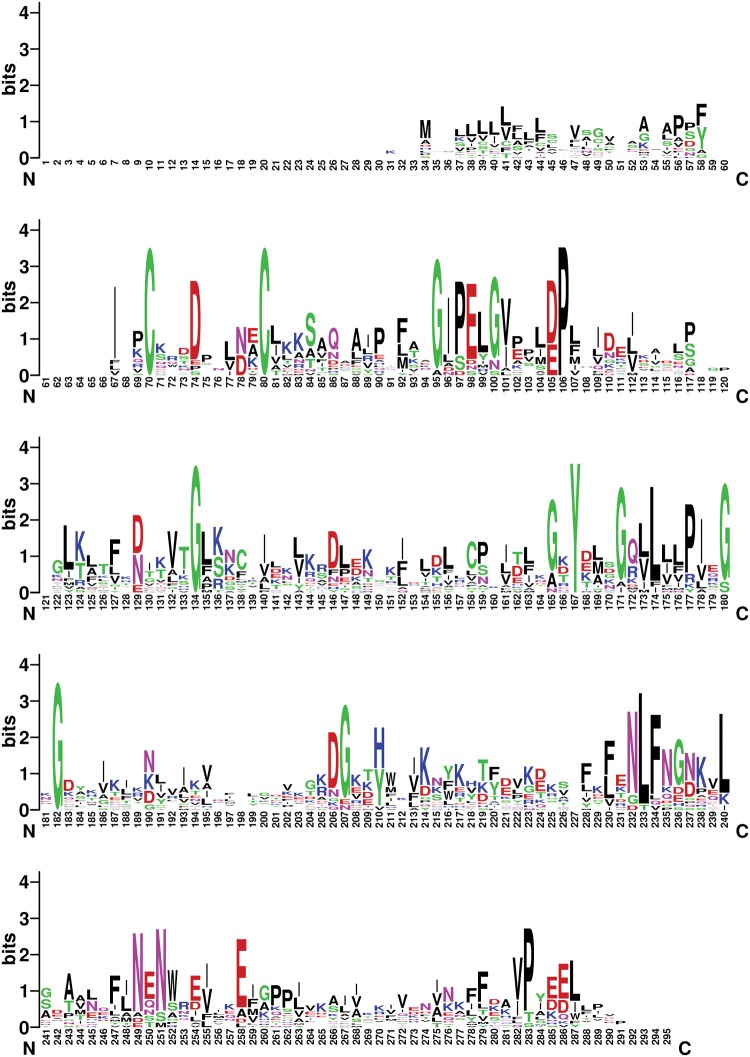
We also compared the TO genes using WebLogo (Fig. 2). There are two highly conserved Cys (100%) in the N-terminus; three highly conserved amino acids, that is, Proline (100%), Glycine (100%), Tyrosine (100%), in the middle; and a highly conserved Glycine (100%) in the C-terminus (Fig. 2).
Fig. 2.
WebLogo alignment of Takeout protein sequences. Alignment of 18 Takeout proteins from S. litura. The x-axis represents the amino acid position. The y-axis (bits) represents the relative proportion of the amino acid at one position. The height of the logo varied inversely with the variability at the position.
Differential Expression Levels of the TO Genes Between Male and Female S. litura
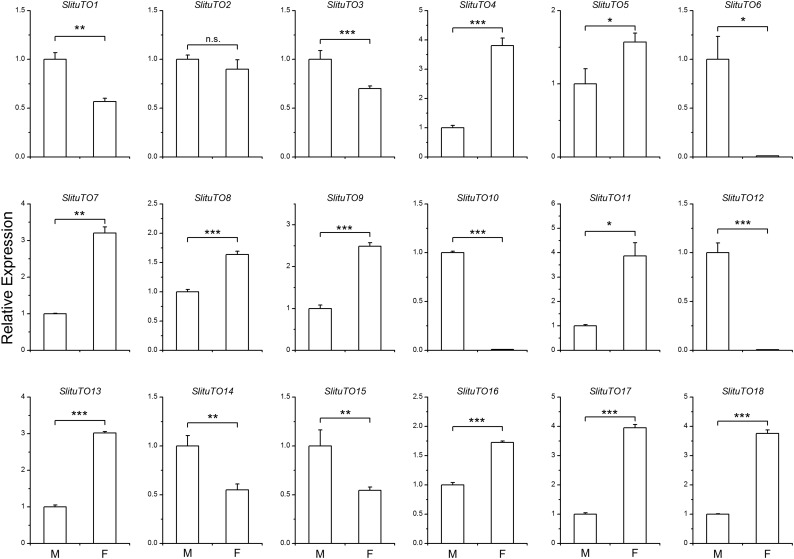
The expression levels of the TO genes in the antenna of the female and male adults are shown in Figure 3. The expression level of the genes in the females is normalized to that in the males. There was no significant difference in the expression of SlTO2 between the male and the female (Fig. 3). There were seven SlTO genes that were more highly expressed in the male than in the female, and three of these genes were the most highly expressed genes, that is, SlTO6 (94.2-fold), SlTO10 (115.3-fold), and SlTO12 (167.2-fold). Ten TO genes were more highly expressed in the female than in the male, and five of the TO genes, that is, SlTO4, SlTO7, SlTO11, SlTO17, and SlTO18, were more highly expressed by more than threefold (Fig. 3).
Fig. 3.
Difference in the expression levels of the Takeout genes between S. litura male and female adults. M: Male; F: Female; Student’s t-test, *: p < 0.05; **: p < 0.01; ***: p < 0.001.
Effects of the Insecticides and the Sex Pheromones on the Expression Levels of the TO Genes
We assessed the expression levels of 18 TO genes in male and female S. litura 1 day and 2 days after the insecticide treatment and the sex pheromone treatment.
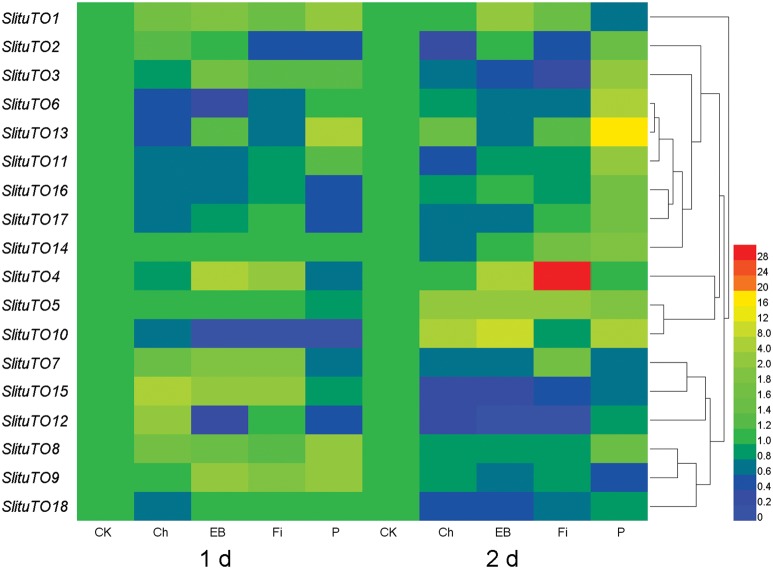
Most of the genes (11 of 18) are not changed or only have minor changes after the insecticide or sex pheromone treatment (≤ fourfold, Supp Table 2 [online only], Supp Fig. 1 [online only]). However, the remaining seven genes have relatively higher fold changes after the insecticide treatment or the sex pheromone treatment (> fourfold, Supp Table 2 [online only], Supp Fig. 1 [online only]). The expression level of SlTO6 was significantly decreased 1 day after the chlorpyrifos treatment, although the expression level of SlTO12 and
SlTO15 significantly increased. The expression levels of SlTO6, SlTO10, and SlTO12 were significantly decreased after a 1-day treatment with emamectin benzoate (Fig. 4, Supp Table 2 [online only], Supp Fig. 1 [online only]), although the expression levels of SlTO4, SlTO9, and SlTO15 significantly increased (Fig. 4, Supp Table 2 [online only], Supp Fig. 1 [online only]). The expression levels of SlTO2, SlTO6, and SlTO10 were significantly decreased 1 day after the treatment with fipronil, although the expression of SlTO15 significantly increased (Fig. 4, Supp Table 2 [online only], Supp Fig. 1 [online only]). The expression levels of SlTO2, SlTO7, SlTO10, SlTO12, and SlTO17 were significantly decreased 1 day after the sex pheromone treatment, although those of SlTO1, SlTO8, SlTO9, and SlTO13 significantly increased (Fig. 4, Supp Table 2 [online only], Supp Fig. 1 [online only]).
Fig. 4.
Heatmap showing the influence of three insecticides and the sex pheromone on the expression levels of 18 Takeout genes in S. litura male adults after a 1 d and 2 d treatment. 1 d: expression changes in TO genes in male S. litura after 1 d of insecticide or sex pheromone treatment; 2 d: expression changes in TO genes in male S. litura after two days of insecticide or sex pheromone treatment; CK: control; Ch: chlorpyrifos; EB: emamectin benzoate; Fi: fipronil; P: sex pheromone. Hierarchical average linkage was used as the clustering method.
The expression levels of SlTO2, SlTO11, SlTO12, SlTO15, and SlTO18 were significantly decreased after the 2-day treatment with chlorpyrifos (Fig. 4, Supp Table 2 [online only], Supp Fig. 1 [online only]), although the expression level of SlTO10 significantly increased (Fig. 4, Supp Table 2 [online only], Supp Fig. 1 [online only]). The expression levels of SlTO3, SlTO12, SlTO15, and SlTO18 significantly decreased after the 2-day treatment with emamectin benzoate (Fig. 4, Supp Table 2 [online only], Supp Fig. 1 [online only]), although the expression levels of SlTO1, SlTO4, SlTO5, and SlTO10 significantly increased (Fig. 4, Supp Table 2 [online only], Supp Fig. 1 [online only]). The expression of SlTO2, SlTO3, and SlTO12 significantly decreased after the 2-day treatment with fipronil, although the expression levels of SlTO4 (28.5-fold), SlTO5, and SlTO7 significantly increased (Fig. 4, Supp Table 2 [online only], Supp Fig. 1 [online only]). The expression levels of SlTO3, SlTO6, SlTO11, and SlTO13 significantly increased after the 2-day treatment with the sex pheromone, although the expression levels of SlTO1, SlTO7, SlTO9 significantly decreased (Fig. 4, Supp Table 2 [online only], Supp Fig. 1 [online only]).
Discussion
The TO proteins are highly conserved and contain N-terminal Cys that influence the disulphide bond formation (Dauwalder et al. 2002, Du et al. 2003, Fujikawa et al. 2006, Sarovblat et al. 2000, So et al. 2000, Larkin et al. 2007). Moreover, the TO proteins contain four highly conserved amino acids, two Glycines, one Proline, and one Tyrosine (Fig. 2). The role of the TO protein family remains unknown (Chamseddin et al. 2012, Saito et al. 2006, Fujikawa et al. 2006, So et al. 2000). The TO family genes in S. litura are closely related to those in B. mori, which indicates that the TO gene in the two Lepidoptera species had a high homology.
By searching previously reported studies, we analyzed the bias of the TO genes expressed in the male and female of species, including D. melanogaster, N. lugens, B. mori, and P. regina (Supp Table 1 [online only]). There are three types of TO genes as follows: male biased, female biased, and not sex biased. In S. litura, 10 TO genes are female biased, 7 TO genes are male biased, and 1 TO gene has no bias. However, in the other three species, most of the TO genes are actually male biased (Supp Table 1 [online only]). The expression profiles of the TO genes in D. melanogaster and N. lugens are male biased (Sarovblat et al. 2000, Lin et al. 2017, Dauwalder et al. 2002), whereas here, we found that the expression levels of the TO family genes in S. litura are different from those in D. melanogaster and N. lugens (Supp Table 1 [online only], Fig. 3). The expression levels of the TO family genes in female S. litura adults are higher than those in the male, suggesting that the sex bias of the TO family genes is different in different species. As shown in Supp Table 1 [online only], the sex bias of the TO family genes is similar in S. litura and B. mori, which is likely because these two species belong to Lepidoptera and is consistent with the phylogenetic analysis (Fig. 3). Interestingly, we found that three S. litura TO genes, that is, SlTO6, SlTO10, and SlTO12, are expressed only in the male; however, we have not identified TO genes that are expressed specifically in the female (Fig. 3). In fact, we found that the fold-change of all TO genes that were highly expressed in the female are less than fourfold.
The insecticide treatments showed that certain S. litura TO genes, such as SlTO4, SlTO10, and SlTO15, are highly induced by at least one insecticide with a fold-change > 4. SlTO3, SlTO4, SlTO6, SlTO10, SlTO12, SlTO13, and SlTO15 have a high degree of fold-change (Supp Table 2 [online only]). Altogether, SlTO6 and SlTO13 are mainly responsive to the sex pheromone treatment; SlTO3, SlTO4, SlTO12, and SlTO15 are mainly responsive to the insecticide treatment, and SlTO10 is responsive to both the insecticide and sex pheromone treatment with a high degree of fold-change (Supp Table 2 [online only]). Moreover, the expression changes in SlTO10 after the treatment with the insecticides were similar to those observed after the sex pheromone treatment.
We recently showed that the N. lugens TO genes are regulated by the JH signaling pathway (Lin et al. 2017), but the mechanism by which the TO family genes regulate physiological functions remains unclear. Moreover, an understanding of the role of the TO genes in the insecticide-induced gene expression and inhibition of the TO genes could help us understand the role of the TO family genes in vivo. Future works investigating the function of the TO genes in vivo are required.
Insecticides and sex pheromones are directly and indirectly linked via physiological and biochemical pathways. The application of sex pheromones decreases the pest population and achieves a long-term pest population decrease; it is also an important pest management tool for controlling several notorious insect pests (Witzgall et al. 2010, Hirano 1980). In addition, the application of insecticides can modify sex pheromone communication in insects, including natural enemies (Delpuech et al. 1999). Understanding the effect of insecticides on the application of sex pheromones is crucial for the efficient control of insect pests in the field because the interaction between insecticides and trapping males might be correlated; if the application of insecticides lowers the trapping efficiency of the sex pheromone lure, additional measures should be taken to increase the pest control efficiency, such as adjusting the recipe of the sex pheromone lure. In addition, understanding the potential interaction between insecticides and sex pheromones at the molecular level could also help us understand the molecular mechanisms underlying the sex pheromone signaling pathway and insecticide toxicology. Our work provided a basis for exploring this important issue in the future.
Supplementary Data
Supplementary data are available at Journal of Insect Science online.
Authors’ Contributions
X.L. and L.Z. designed the research study, analyzed the data, and wrote the paper. X.L., L.Z., and Y.J. performed the experiments.
Supplementary Material
Acknowledgments
We would like to thank Bo Wang for the collection of the insects. This work was supported by the Special Fund for Agro-scientific Research in the Public Interest in China (Grant 201203036).
References Cited
- Angioy A. M., Liscia A., Crnjar R., Porcu A., Cancedda A., Pietra P. 1983. Mechanism of JH influence on the function of labellar chemosensilla in Phormia: experimental suggestions. Bollet. Della Soc. Ital. Biol. Speriment. 59(10): 1453–1456. [PubMed] [Google Scholar]
- Bauer J., Antosh M., Chang C., Schorl C., Kolli S., Neretti N., Helfand S. L. 2010. Comparative transcriptional profiling identifies takeout as a gene that regulates life span. Aging. 2: 298–310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benito J., Hoxha V., Lama C., Lazareva A. A., Ferveur J. F., Hardin P. E., Dauwalder B. 2010. The circadian output gene takeout is regulated by Pdp1epsilon. Proc. Natl. Acad. Sci. U. S. A. 107: 2544–2549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bohbot J., Vogt R. G. 2005. Antennal expressed genes of the yellow fever mosquito (Aedes aegypti L.); characterization of odorant-binding protein 10 and takeout. Insect Biochem. Molec. Biol. 35: 961–979. [DOI] [PubMed] [Google Scholar]
- Chamseddin K. H., Khan S. Q., Nguyen M. L., Antosh M., Morris S. N., Kolli S., Neretti N., Helfand S. L., Bauer J. H. 2012. Takeout-dependent longevity is associated with altered Juvenile Hormone signaling. Mech. Ageing Dev. 133: 637–646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark D. C., Haynes K. F. 1992. Sublethal effects of cypermethrin on chemical communication, courtship, and oviposition in the cabbage looper (Lepidoptera: Noctuidae). J. Econ. Entomol. 85: 1771–1778. [Google Scholar]
- Dallaire R., Labrecque A., Marcotte M., Bauce E., Delisle J. 2004. The sublethal effects of tebufenozide on the precopulatory and copulatory activities of Choristoneura fumiferana and C. rosaceana. Entomol. Exp. Appl. 112: 169–181. [Google Scholar]
- Dauwalder B., Tsujimoto S., Moss J., Mattox W. 2002. The Drosophila takeout gene is regulated by the somatic sex-determination pathway and affects male courtship behavior. Genes Dev. 16: 2879–2892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delpuech J., Legallet B., Terrier O., Fouillet P. 1999. Modifications of the sex pheromonal communication of Trichogramma brassicae by a sublethal dose of deltamethrin. Chemosphere. 38: 729–739. [DOI] [PubMed] [Google Scholar]
- Deng J. Y., Wei H. Y., Huang Y. P., Du J. W. 2004. Enhancement of attraction to sex pheromones of Spodoptera exigua by volatile compounds produced by host plants. J. Chem. Ecol. 30: 2037–2045. [DOI] [PubMed] [Google Scholar]
- Du J., Hiruma K., Riddiford L. M. 2003. A novel gene in the takeout gene family is regulated by hormones and nutrients in Manduca larval epidermis. Insect Biochem. Molec. Biol. 33: 803–814. [DOI] [PubMed] [Google Scholar]
- Elsayed A. M., Fraser H. M., Trimble R. M. 2001. Modification of the sex-pheromone communication system associated with organophosphorus-insecticide resistance in the obliquebanded leafroller (Lepidoptera: Tortricidae). Can. Entomol. 133, 15. [Google Scholar]
- Feng B., Lin X., Zheng K., Qian K., Chang Y., Du Y. 2015. Transcriptome and expression profiling analysis link patterns of gene expression to antennal responses in Spodoptera litura. BMC Genomics. 16: 269.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujikawa K., Seno K., Ozaki M. 2006. A novel Takeout-like protein expressed in the taste and olfactory organs of the blowfly, Phormia regina. FEBS J. 273: 4311–4321. [DOI] [PubMed] [Google Scholar]
- Guo W., Wang X., Ma Z., Xue L., Han J., Yu D., Kang L. 2011. CSP and takeout genes modulate the switch between attraction and repulsion during behavioral phase change in the migratory locust. PLoS Genet. 7: 199–212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagai T., Cohen M., Bloch G. 2007. Genes encoding putative Takeout/juvenile hormone binding proteins in the honeybee (Apis mellifera) and modulation by age and juvenile hormone of the takeout-like gene GB19811. Insect Biochem. Molec. Biol. 37: 689–701. [DOI] [PubMed] [Google Scholar]
- Hall B. G. 2013. Building phylogenetic trees from molecular data with MEGA. Molec. Biol. Evol. 30: 1229.. [DOI] [PubMed] [Google Scholar]
- Hamiaux C., Stanley D., Greenwood D. R., Baker E. N., Newcomb R. D. 2009. Crystal structure of Epiphyas postvittana takeout 1 with bound ubiquinone supports a role as ligand carriers for takeout proteins in insects. J. Biol. Chem. 284: 3496–3503. [DOI] [PubMed] [Google Scholar]
- Haynes K. F. 1988. Sublethal effects of neurotoxic insecticides on insect behavior. Ann. Rev. Entomol. 33: 149.. [DOI] [PubMed] [Google Scholar]
- Hirano C. 1980. Capture efficiency of sex pheromone traps for males of Spodoptera litura. Jpn. J. Appl. Entomol. Zool. 24: 217–220. [Google Scholar]
- Justice R. W., Dimitratos S., Walter M. F., Woods D. F., Biessmann H. 2003. Sexual dimorphic expression of putative antennal carrier protein genes in the malaria vector Anopheles gambiae. Insect Molec. Biol. 12: 581–594. [DOI] [PubMed] [Google Scholar]
- Larkin M. A., Blackshields G., Brown N. P., Chenna R. M., Mcgettigan P. A., Mcwilliam H., Valentin F., Wallace I.M.W., Wilm A., Lopez R. 2007. Clustal W. Clustal X version. Bioinformatics. 23: 2947–2948. [DOI] [PubMed] [Google Scholar]
- Lin X., Zhang L., Jiang Y. 2017. Distinct roles of Met and interacting proteins on the expressions of takeout family genes in brown planthopper. Front. Physiol. 8: 100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin X., Zhang Q., Wu Z., Du Y. 2015. Identification and differential expression of a candidate sex pheromone receptor in natural populations of Spodoptera litura. PLoS ONE. 10(6): e0131407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Livak K. J., Schmittgen T. D. 2001. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT method. Methods. 25: 402–408. [DOI] [PubMed] [Google Scholar]
- Lu Y., Yuan M., Gao X., Kang T., Zhan S., Hu W., Li J. 2013. Identification and validation of reference genes for gene expression analysis using quantitative PCR in Spodoptera litura (Lepidoptera: Noctuidae). PLoS ONE. 8: e68059.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meagher R. L., Brambila J., Hung E. 2008. Monitoring for exotic spodoptera species (Lepidoptera: Noctuidae) in Florida. Florida Entomol. 91: 517–522. [Google Scholar]
- Meunier N., Belgacem Y. H., Martin J. R. 2007. Regulation of feeding behaviour and locomotor activity by takeout in Drosophila. J. Exp. Biol. 210: 1424–1434. [DOI] [PubMed] [Google Scholar]
- Moore A., Waring C. P. 1996. Sublethal effects of the pesticide Diazinon on olfactory function in mature male Atlantic salmon parr. J. Fish Biol. 48: 758–775. [Google Scholar]
- Rehan A., Freed S. 2014. Selection, mechanism, cross resistance and stability of spinosad resistance in Spodoptera litura (Fabricius) (Lepidoptera: Noctuidae). Crop Protect. 56: 10–15. [Google Scholar]
- Saito K., Su Z. H., Emi A., Mita K., Takeda M., Fujiwara Y. 2006. Cloning and expression analysis of takeout/JHBP family genes of silkworm, Bombyx mori. Insect Mol. Biol. 15: 245–251. [DOI] [PubMed] [Google Scholar]
- Sarovblat L., So W. V., Liu L., Rosbash M. 2000. The Drosophila takeout gene is a novel molecular link between circadian rhythms and feeding behavior. Cell. 101: 647–656. [DOI] [PubMed] [Google Scholar]
- Schwinghammer M. A., Zhou X., Kambhampati S., Bennett G. W., Scharf M. E. 2011. A novel gene from the takeout family involved in termite trail-following behavior. Gene. 474: 12–21. [DOI] [PubMed] [Google Scholar]
- So W. V., Sarov-Blat L., Kotarski C. K., Mcdonald M. J., Allada R., Rosbash M. 2000. Takeout, a novel Drosophila gene under circadian clock transcriptional regulation. Mol. Cell. Biol. 20: 6935–6944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sower L. L., Shorb M. D. 1985. Pesticides have little impact on attraction of three species of male moths to sex pheromone. J. Econ. Entomol. 78: 908–912. [Google Scholar]
- Trimble R. M., Pree D. J., Carter N. J. 2001. Integrated control of oriental fruit moth (Lepidoptera: Tortricidae) in peach orchards using insecticide and mating disruption. J. Econ. Entomol. 94: 476–485. [DOI] [PubMed] [Google Scholar]
- Vanaphan N., Dauwalder B., Zufall R. A. 2012. Diversification of takeout, a male-biased gene family in Drosophila. Gene. 491: 142–148. [DOI] [PubMed] [Google Scholar]
- Wei H., Du J. 2004. Sublethal effects of larval treatment with deltamethrin on moth sex pheromone communication system of the Asian corn borer, Ostrinia furnacalis. Pest. Biochem. Physiol. 80: 12–20. [Google Scholar]
- Witzgall P., Kirsch P., Cork A. 2010. Sex pheromones and their impact on pest management. J. Chem. Ecol. 36: 80–100. [DOI] [PubMed] [Google Scholar]
- Wojtasek H., Prestwich G. D. 1995. Key disulfide bonds in an insect hormone binding protein: cDNA cloning of a juvenile hormone binding protein of Heliothis virescens and ligand binding by native and mutant forms. Biochemistry. 34: 5234–5241. [DOI] [PubMed] [Google Scholar]
- Xu Z., Cao G. C., Dong S. L. 2010. Changes of sex pheromone communication systems associated with Tebufenozide and Abamectin resistance in Diamondback Moth, Plutella xylostella (L.). J. Chem. Ecol. 36: 526–534. [DOI] [PubMed] [Google Scholar]
- Yang Z. H., Du J. 2003. Effects of sublethal deltamethrin on the chemical communication system and PBAN activity of Asian corn borer, Ostrinia furnacalis (Güenee). J. Chem. Ecol. 29: 1611–1619. [DOI] [PubMed] [Google Scholar]
- Zhou H., Du J., Huang Y. 2005. Effects of sublethal doses of malathion on responses to sex pheromones by male Asian corn borer moths, Ostrinia furnacalis (Guenée). J. Chem. Ecol. 31: 1645–1656. [DOI] [PubMed] [Google Scholar]
- Zhu H., Casselman A., Reppert S. M. 2008. Chasing migration genes: a brain expressed sequence tag resource for summer and migratory monarch butterflies (Danaus plexippus). PLoS ONE. 3: e1345. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.