Abstract
Infectious complications, particularly viral infections, remain a significant cause of morbidity and mortality after allogeneic hematopoietic cell transplantation (alloHCT). Only a handful of studies in children have analyzed the risks for and impact of viremia on alloHCT-related outcomes. We conducted a retrospective study of 140 pediatric patients undergoing alloHCT to investigate the incidence of and risk factors for cytomegalovirus (CMV), adenovirus (ADV), and Epstein-Barr virus (EBV) viremia and viral disease after alloHCT. Furthermore, we assessed the impact of viremia on days of hospitalization and develop an algorithm for routine monitoring of viremia. Patients were monitored before alloHCT and then weekly for 180 days after alloHCT. Patients were considered to have viremia if CMV were > 600 copies/mL, EBV were > 1000 copies/mL, or ADV were > 1000 copies/mL on 2 consecutive PCRs. The overall incidences of viremia and viral disease in all patients from day 0 to +180 after alloHCT were 41.4% (n = 58) and 17% (n = 24), respectively. The overall survival for patients with viremia and viral disease was significantly lower compared with those without viremia (58% versus 74.2%, P = .03) and viral disease (48.2% versus 71.2%, P = .024). We identified that pretransplantation CMV risk status, pre-alloHCT viremia, and use of alemtuzumab were associated with the risk of post-alloHCT viremia. The average hospitalization days in patients with CMV risk (P = .011), viremia (P = .024), and viral disease (P = .002) were significantly higher. The algorithm developed from our data can potentially reduce viral PCR testing by 50% and is being studied prospectively at our center. Improved preventative treatment strategies for children at risk of viremia after alloHCT are needed.
Keywords: Viral infections, Pediatrics, Bone marrow transplantation
INTRODUCTION
Over the past 4 decades, allogeneic (allo) hematopoietic cell transplantation (HCT) has provided a curative treatment option for pediatric patients with both malignant and nonmalignant diseases [1,2]. In alloHCT recipients, advances in methods of donor selection, graft-versus-host disease (GVHD) management, and molecular monitoring for infectious organisms have resulted in improved overall survival (OS) [3–7]. Despite these advances, infectious complications, particularly viral infections, remain a significant cause of morbidity and mortality after alloHCT. Because of a period of prolonged immune suppression after alloHCT, patients undergoing alloHCT are at high risk for infection with cytomegalovirus (CMV), Epstein-Barr virus (EBV), and adenovirus (ADV) [8].
After alloHCT, early treatment with antiviral medications in a patient with rising viral copies has reduced the risk of morbidity and mortality associated with viral infections, most notably, with CMV [7,9,10]. Although pre-emptive treatment with i.v. ganciclovir has been shown to decrease the cumulative incidence of CMV disease [11,12], pre-emptive treatment of ADV and EBV is not well established [13]. There are currently no well-established guidelines for the frequency of viral monitoring in children after alloHCT. Only a handful of studies in children have analyzed the risks for and impact of viremia on alloHCT-related outcomes [8,14]. Pretransplantation CMV serostatus is an important risk factor for the development of CMV viremia. In patients who are seropositive before transplantation, the incidence of CMV viremia approaches 70% [15].
Ex vivo T cell depletion and T cell–depleting agents such as alemtuzumab or antithymocyte globulin (ATG) are used for the prevention of GVHD and graft rejection but carry an increased risk of viral infection because of the resultant delay in immune reconstitution [16,17].
We conducted a retrospective study of pediatric patients undergoing alloHCT to investigate the incidence of and risk factors for viremia and viral disease (CMV, ADV, and EBV) after transplantation. Furthermore, we assessed the impact of viremia on length of hospitalization and used the gathered data to develop an algorithm for routine monitoring of viremia for first 6 months in pediatric patients undergoing alloHCT.
METHODS
Patients
This was a retrospective study of 140 pediatric patients who received alloHCT for malignant and nonmalignant diseases at the Columbia University Medical Center. Patients underwent transplantation between 2008 and 2014. For this analysis, eligible patients were identified from a transplantation database and clinical data were collected from the electronic medical record. This study was approved by the institutional review board of the Columbia University Medical Center.
Conditioning Regimens
The myeloablative conditioning regimen consisted of either total body irradiation (12 Gray) plus 1 or 2 high-dose alkylators or busulfan (12.8 mg/kg-16 mg/kg) plus 1 high-dose alkylator. Reduced-toxicity regimens consisted of busulfan (12.8 mg/kg-16 mg/kg) or cyclophosphamide (200 mg/kg) plus fludarabine. Reduced-intensity regimens contained busulfan (6.4 mg/kg-8 mg/kg) or melphalan (140 mg/m2) plus fludarabine. Serotherapy consisted of alemtuzumab (54 mg/m2) or ATG (8 mg/kg) [3].
GVHD Prophylaxis and Grading
Acute GVHD (aGVHD) prophylaxis in the majority of patients consisted of tacrolimus and mycophenolate mofetil, as previously described [18,19]. Tacrolimus and/or mycophenolate mofetil were tapered if patients had ≤grade II aGVHD on day +30 for those with malignant diseases and on day +180 for those with nonmalignant diseases [18,19]. Grading of aGVHD was per established criteria by Glucksberg et al. [20].
Post-alloHCT Viral Prophylaxis
Patients at risk for CMV received acyclovir prophylaxis, which was continued until the following criteria were met: absolute neutrophil count (ANC) > .75 × 109/L × 2 days and < grade II mucositis. When these criteria were met, patients were transitioned to ganciclovir/foscarnet or valganciclovir daily until day +100, as previously published [21].
Viral Monitoring and Treatment of Viremia
In 2008, our center created a standard operating procedure for prospective quantitative PCR monitoring for CMV, EBV, and ADV. Patients were monitored at least once within 4 weeks before the start of conditioning regimen and then weekly for 180 days after alloHCT. CMV PCR was performed in house as per protocol established by Roche Diagnostic (Indianapolis, IN) and EBV and ADV PCR were performed by Viracor (Lee, Summit, MO). Patients with a positive CMV PCR after HCT were treated with induction ganciclovir for 2 weeks (or until PCR negative), followed by maintenance ganciclovir or foscarnet for at least 4 weeks. Foscarnet was administered in a few patients with severe neutropenia and a few patients with increasing CMV copy number on ganciclovir treatment. Foscarnet was started at 90 mg/kg twice a day and the dose was adjusted based on creatinine clearance. Patients with ADV viremia were treated with cidofovir (5 mg/kg/dose once a week). When the viral copy number started to decline by a log, cidofovir was administered every 2 weeks. The dose and duration of cidofovir were titrated based on renal functions and viral load. Those with EBV viremia received rituximab (375 mg/m2) for 2 to 4 weeks and were tapered off immune suppression as tolerated.
Definitions
CMV risk
Patients were considered to have a positive CMV risk status if either donor or recipient or both were CMV IgG positive before transplantation.
Viremia
Patients were considered to have viremia if CMV, EBV, ADV copies/mL were > 600, > 1000, and > 1000, respectively, on 2 consecutive PCRs. The cutoff for positivity for EBV and ADV were PCR levels above these thresholds of detection. This was similar to cutoffs used in published studies [16,22]. Post-transplantation viremia was defined as very early (day 0–14), early viremia (day 15–98), and late viremia (day 99–180). Resolution of viremia was defined as negative PCR testing for ≥ 2 weeks.
Viral disease
Definitions for CMV disease and ADV disease are those as previously described by Ljungman et al. [23–25]. A diagnosis of CMV/EBV/ADV disease required the presence of signs or symptoms of viral disease and radiological findings suggestive of viral infection along with 1 of the following: (1) detection of CMV/EBV/ADV in bronchoalveolar fluid, cerebrospinal fluid, or urine or tissue samples such as lung, gastrointestinal, liver, or lymph node biopsy, or (2) rising viral loads in patient unable to tolerate invasive procedures.
Hematopoietic recovery
Neutrophil engraftment was defined as the first day of 3 consecutive ANC greater than .5 × 109/L. Absolute lymphocyte count is the product of the white blood cell count (cells/L) and the fraction of lymphocytes. Primary graft failure was defined as failure to achieve a donor-derived ANC > .5 × 109/L by day +42. All patients who received stem cell boost because of impending graft failure after achieving neutrophil engraftment were considered to have secondary graft failure.
OS
OS analysis included patients who were alive with or without their original disease.
Statistical Analyses
Continuous variables were summarized as mean ± standard deviation and categorical variables were summarized as percentages. The comparisons between 2 groups were done by either t-test or Wilcoxon rank-sum test for continuous variables and by either chi-squared test or Fisher’s exact test for categorical variables. P values ≤ .05 were considered significant. Univariate and multivariate Cox proportional hazards regression analysis and Fine and Gray’s competing risks regression analysis were carried to examine risk factors associated viremia, viral disease, and OS. In univariate analysis for viremia, viral disease and OS, risk factors analyzed included age, sex, stem cell source, disease type, CMV risk status, conditioning regimen, use of alemtuzumab or rabbit-ATG (r-ATG), grade II to IV aGVHD, ANC, and absolute lymphocyte count at day +30. The covariates with a P value of ≤ .10 in the univariate analysis were included in the multivariate analysis. Statistical calculations were carried out in SAS 9.3 (Cary, NC).
RESULTS
A summary of the key demographics and clinical characteristics of the patient population is given in Table 1.
Table 1.
Patient Demographics and Transplantation Characteristics (n = 140)
| Characteristic | Value |
|---|---|
| Age at transplantation, mean ± SD (range), yr | 9.46 ± 6.09 (.25–22) |
| Female | 55 (39.3%) |
| Malignant disease | 69 (49.3%) |
| Matched related donor | 57 (40.7%) |
| Stem cell source | |
| Cord blood | 22 (15.7%) |
| Peripheral blood stem cells | 40 (28.6%) |
| Bone marrow | 78 (55.7%) |
| Conditioning regimen | |
| MAC | 58 (41.4%) |
| RTC | 53 (37.9%) |
| RIC | 29 (20.7%) |
| Serotherapy | |
| Alemtuzumab | 75 (53.6%) |
| r-ATG | 40 (28.6%) |
| Neither r-ATG nor alemtuzumab | 25 (17.9%) |
MAC indicates myeloablative conditioning; RTC, reduced-toxicity conditioning; RIC, reduced-intensity conditioning.
Data presented are n (%), unless otherwise indicated.
Kinetics and Clearance of Viremia
Of the 58 patients who developed viremia, the majority developed viremia before day 100 after transplantation; more specifically, only 3 patients had their first viral PCR become positive past day 100 (Table 2). All 3 cases of viremia were related EBV. Two patients who received matched sibling donor alloHCT for severe aplastic anemia, conditioned with r-ATG, developed post-transplantation lymphoproliferative disease (PTLD) and 1 patient had EBV viremia and secondary hemophagocytic lymphohistiocytosis. This patient had an EBV-related Hodgkin lymphoma secondary to an inducible interleukin-2 tyrosine kinase mutation.
Table 2.
Kinetics of Onset of Viremia Based on Use of Serotherapy and CMV Risk Status
| Variable | n | CMV | ADV | EBV | Total | |||
|---|---|---|---|---|---|---|---|---|
|
|
|
|
||||||
| Day 0–99 | Day 100–180 (New Onset) | Day 0–99 | Day 100–180 (New Onset) | Day 0–99 | Day 100–180 (New Onset) | |||
| Alemtuzumab | 75 | 23 | 0 | 10 | 0 | 4 | 1 | 38 |
| r-ATG | 40 | 6 | 0 | 3 | 0 | 6 | 2 | 17 |
| No serotherapy | 25 | 2 | 0 | 2 | 0 | 1 | 0 | 5 |
| CMV −/− | 55 | 0 | 0 | 5 | 0 | 8 | 2 | 15 |
The mean times to resolution of viremia seen in our patient population were 5.8 weeks (range, 1 to 19) for CMV, 2 weeks (range, 1 to 7) for EBV, and 6.8 weeks (range, 1 to 16) for ADV.
Pretransplantation Viremia
The incidence of viremia before alloHCT was 6.4% (CMV, n = 2; EBV, n = 5; ADV, n = 2). In these patients, the indications for alloHCT were primary or secondary refractory hemophagocytic lymphohistiocytosis (n = 7), relapsed Burkitt lymphoma (n = 1), and relapsed T cell acute lymphoblastic leukemia (n = 1). Of these 9 patients, 5 did not clear their viremia before their transplantation and were included in the viremia incidence in first 100 days.
Viremia
The overall incidence of viremia in all patients from day 0 to +180 after alloHCT was 41.4% (n = 58) (Table 3). CMV viremia was detected in 21.4% of patients (n = 30), ADV viremia in 11.4% (n = 16), and EBV viremia in 10% (n = 14). The median time to onset for CMV viremia was 33 days (range, 5 to 89 days). For ADV, the time to onset was 40 days (range, 8 to 96 days), and for EBV, the time to onset was 55 days (range, 20 to 180 days). The incidence of multiple viremia, defined as PCR positivity for ≥ 2 viruses, was 14.3% (n = 20). All patients who developed CMV viremia had a positive pretransplantation CMV risk status. The majority of viremia seen was in the early period (from day +14 to +98), where 36% of all patients in our cohort had a PCR positivity for at least 1 virus.
Table 3.
Incidence of Viremia, Viral Disease, and Survival Outcomes
| Characteristic | N = 140 | Alemtuzumab (n = 75) | No Alemtuzumab (n = 65) | P Value |
|---|---|---|---|---|
| Pretransplantation viremia | 9 (6.4%) | 5 (6.7%) | 4 (6.2%) | 1.00 |
| Pretransplantation CMV risk | 85 (60.7%) | 47 (62.7%) | 38 (58.5%) | .611 |
| Incidence of all viremia | 58 (41.4%) | 37 (49.3%) | 21 (32.3%) | .041 |
| CMV | 30 (21.4%) | 23 (30.7%) | 7 (10.8%) | .007 |
| EBV | 14 (10%) | 5 (6.7%) | 9 (13.9%) | .172 |
| ADV | 16 (11.4%) | 10 (13.3%) | 6 (9.2%) | .596 |
| Incidence of multiple viremia | 20 (14.3%) | 14 (18.7%) | 6 (9.2%) | .147 |
| Incidence of viral disease | 24 (17.1%) | 17 (22.7%) | 7 (10.8%) | .074 |
| 1-year OS | 77.6% (SE = 3.55%) | 82.6% (SE = 4.39%) | 72.1% (SE = 5.6%) | .070 |
The incidence of viremia in patients conditioned without serotherapy was 20% (n =5 of 25) and the incidence of viremia in patients receiving serotherapy was 48% (n = 55 of 115).
Viral Disease
Twenty-four of 140 patients developed viral disease (17%). The most common form of viral disease was CMV pneumonitis (8 of 24, 33%) followed by EBV PTLD (7 of 24, 29%) and ADV pneumonitis (4 of 24, 17%). Among patients with viral disease, 17 of 24 (71%) received alemtuzumab and 7 of 24 (29%) received r-ATG. The incidence of viral disease in patients who did not receive serotherapy was 0.
The progression of viremia to viral disease was noted in 36% (n = 11 of 30) patients with CMV (pneumonitis, n = 8; colitis, n = 2; encephalitis, n = 1), 31% (n = 5 of 16) patients with ADV (pneumonitis, n = 4; hemorrhagic cystitis, n = 1), and 57% (n = 8 of 14) with EBV (PTLD, n = 7 and pneumonitis, n = 1). In patients with ADV pneumonitis 75% (n = 3 of 4) died and 37% (n = 3 of 8) patients with CMV pneumonitis died. There was no significant difference in the pneumonia occurrence between patients with CMV and the patients with ADV (P = 1.00).
Alemtuzumab-Associated Viremia and Viral Disease
Seventy-five patients received alemtuzumab as part of their conditioning regimen and the overall incidence of viremia in those patients was 50%. Fourteen of these patients developed 1 or more viremia after transplantation. There were a total of 47 of 75 patients who were at risk of CMV before transplantation and received alemtuzumab. Among those 47 patients, 30 (63.8%) had viremia. The overall incidence of viral disease was 22.7%.
OS
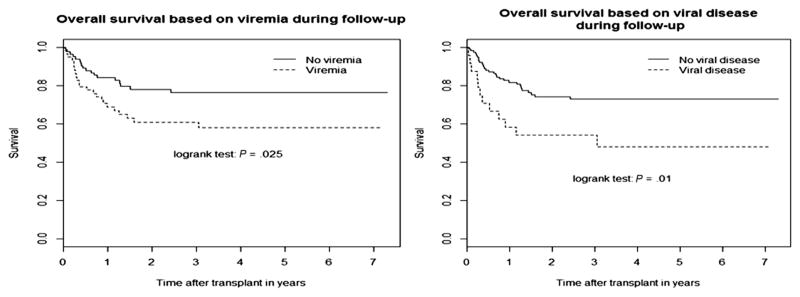
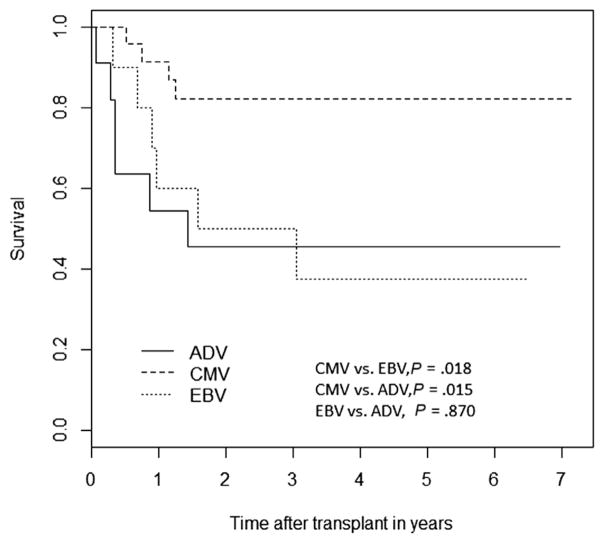
One-year OS in our cohort was 77.65% (SE = 3.55%). The OS for the entire study period for patients with viremia was significantly lower compared with the OS in those without viremia (58% [SE = 6.86%] versus 74.2% [SE = 5.25%], P =.03) (Figure 1). Similarly, the OS in those who developed viral disease was inferior to those who did not develop viral disease (48.2% [SE = 10.7%] versus 71.2% [SE = 4.59%], P = .024) (Figure 1). Poor OS was associated with pre-alloHCT viremia (P = .001) and development of viral disease (P = .04) on multivariate analysis (Table 4). The OS was higher for patients with CMV viremia than for those with ADV or EBV viremia (Figure 2).
Figure 1.
Overall survival in patients with viremia and viral disease.
Table 4.
Risk Factors for OS Based on Univariate and Multivariate Cox Proportional Regression Analysis
| Parameter | Univariate Analysis | Multivariate Analysis | ||||
|---|---|---|---|---|---|---|
|
|
|
|||||
| HR | 95% CI | P Value | HR | 95% CI | P Value | |
| Age | 1.006 | .956–1.058 | .821 | |||
| Gender | ||||||
| Female | 1 | |||||
| Male | .645 | .351–1.185 | .158 | |||
| Donor | ||||||
| Related | 1 | 1 | ||||
| Unrelated | 2.428 | 1.193–4.941 | .014 | 2.677 | 1.112–6.442 | .028 |
| Disease | ||||||
| Nonmalignant | 1 | 1 | ||||
| Malignant | 2.755 | 1.411–5.383 | .003 | 1.738 | .791–3.818 | .168 |
| Regimen | ||||||
| MAC | 1 | 1 | ||||
| RTC | .321 | .151–.682 | .003 | .409 | .144–1.161 | .093 |
| RIC | .390 | .161–.946 | .037 | .206 | .067–.635 | .006 |
| CMV risk | ||||||
| No | 1 | |||||
| Yes | 1.474 | .766–2.836 | .245 | |||
| Alemtuzumab | ||||||
| No | 1 | 1 | ||||
| Yes | .530 | .284–.989 | .046 | 1.309 | .385–4.457 | .666 |
| r-ATG | ||||||
| No | 1 | 1 | ||||
| Yes | 2.006 | 1.088–3.696 | .026 | 1.373 | .470–4.010 | .562 |
| ANC day 30 | 1.000 | 1.000 | .137 | |||
| ALC day 30 | .999 | .998 | .201 | |||
| Pre-alloHCT viremia | ||||||
| No | 1 | 1 | ||||
| Yes | 4.227 | 1.773–10.1 | .001 | 6.304 | 2.056–19.3 | .001 |
| aGVHD | ||||||
| No | 1 | 1 | ||||
| Yes | 1.865 | 1.012–3.438 | .046 | 1.080 | .566–2.061 | .817 |
| Viremia | ||||||
| No | 1 | 1 | ||||
| Yes | 3.297 | 1.319–8.241 | .011 | 2.108 | .788–5.642 | .138 |
| Viral disease | ||||||
| No | 1 | 1.174–4.484 | 1 | |||
| Yes | 2.294 | .015 | 2.248 | 1.040–4.858 | .039 | |
ALC indicates absolute lymphocyte count.
Figure 2.
Overall survival in patients with cytomegalovirus (CMV), Epstein-Barr virus (EBV), and adenovirus (ADV) viremia.
Risk Factors for Viremia and Viral Disease
All 3 viral infections were combined for univariate and multivariate Cox proportional regression analyses for risk factors associated with viremia (Table 5). On multivariate analysis, risk factors associated with viremia included pretransplantation CMV risk status (hazard ratio [HR], 2.005; 95% confidence interval [CI], 1.078 to 3.726; P =.02), patients with pre-alloHCT viremia (HR, 3.748; 95% CI, 1.733 to 8.107; P = .0008), and conditioning with alemtuzumab (HR, 1.693; 95% CI, .987 to 2.904; P = .05). The single significant risk factor associated with development of post-transplantation viral disease in multivariate analysis (HR, 7.442; 95% CI, 1.569 to 35.23; P = .01) was pre-alloHCT viremia.
Table 5.
Risk Factors for Viremia: Univariate and Multivariate Cox Proportional Regression Analysis
| Effect | Univariate Analysis | Multivariate Analysis | ||||
|---|---|---|---|---|---|---|
|
|
|
|||||
| HR | 95% CI | P Value | HR | 95% CI | P Value | |
| Age | .949 | .908–.991 | .017 | .962 | .923–1.003 | .07 |
| Gender | ||||||
| Female | 1 | |||||
| Male | .931 | .552–1.570 | .789 | |||
| Donor | ||||||
| Related | 1 | |||||
| Unrelated | 1.030 | .609–1.743 | .912 | |||
| Disease | ||||||
| Nonmalignant | 1 | |||||
| Malignant | .906 | .541–1.516 | .707 | |||
| Regimen | ||||||
| MAC | 1 | |||||
| RTC | 1.076 | .588–1.970 | .813 | |||
| RIC | 1.732 | .903–3.321 | .098 | |||
| CMV risk | ||||||
| No | 1 | 1 | ||||
| Yes | 2.538 | 1.389–4.636 | .003 | 2.005 | 1.078–3.726 | .02 |
| Alemtuzumab | ||||||
| No | 1 | 1 | ||||
| Yes | 1.708 | .999–2.920 | .050 | 1.693 | .987–2.904 | .05 |
| r-ATG | ||||||
| No | 1 | |||||
| Yes | .829 | .461–1.493 | .533 | |||
| Pre-alloHCT viremia | ||||||
| No | 1 | 1 | ||||
| Yes | 5.323 | 2.510–11.3 | <.0001 | 3.748 | 1.733–8.107 | .0008 |
| ANC day 30 | 1.000 | 1.000–1.000 | .800 | |||
| ALC day 30 | 1.000 | .999–1.001 | .804 | |||
| aGVHD | ||||||
| Yes | 1 | |||||
| No | .999 | .594–1.681 | .999 | |||
In the subset of patients who received alemtuzumab (n = 75), CMV risk status was significantly associated with post-transplantation overall viremia on multivariate analysis (point estimate, 2.851; 95% CI, 1.182 to 6.875; P = .002) (Table 6).
Table 6.
Risk Factors for Viremia in Patients Receiving Alemtuzumab
| Effect | Univariate Analysis | Multivariate Analysis | ||||
|---|---|---|---|---|---|---|
|
|
|
|||||
| HR | 95% CI | P Value | Point Estimate | 95% Wald Confidence Limits | P Value | |
| Age | .947 | .897–1.000 | .050 | .967 | .918–1.019 | .210 |
| Gender | ||||||
| Female | 1 | |||||
| Male | .753 | .394–1.439 | .391 | |||
| Donor | ||||||
| Related | 1 | |||||
| Unrelated | 1.087 | .564–2.096 | .803 | |||
| Disease | ||||||
| Nonmalignant | 1 | |||||
| Malignant | 1.229 | .617–2.448 | .558 | |||
| Regimen | ||||||
| MAC | 1 | 1 | ||||
| RTC | .365 | .134–.995 | .049 | .509 | .184–1.407 | .193 |
| RIC | .848 | .303–2.372 | .754 | .953 | .309–2.945 | .934 |
| CMV risk | ||||||
| No | 1 | 1 | ||||
| Yes | 3.416 | 1.495–7.801 | .004 | 2.851 | 1.182–6.875 | .020 |
| aGVHD | ||||||
| No | 1 | |||||
| Yes | 1.059 | .545–2.059 | .865 | |||
| ANC day 30 | 1.000 | 1.000–1.000 | .896 | |||
| ALC day 30 | 1.001 | .999–1.002 | .382 | |||
| Pre-alloHCT viremia | ||||||
| No | 1 | 1 | ||||
| Yes | 4.064 | 1.569–10.5 | .004 | 1.968 | .635–6.096 | .241 |
Multivariate analysis of risk factors for development of viral disease in patients receiving alemtuzumab revealed that graft failure (point estimate, 6.882; 95% CI,1.452 to 32.627; P =.015) and pre-alloHCT viremia (point estimate,19.7; 95% CI,1.443 to 268.991; P = .0254) were associated with higher risk of viral disease. Reduced-toxicity conditioning was associated lower risk of viral disease compared with myeloablative conditioning regimen (point estimate, .1; 95% CI, .017 to .7; P =.02).
Days of Hospitalization
As a marker of overall health care utilization, we examined the mean and median days of hospitalization from the day of alloHCT to day +180 (Table 7). The average hospitalizations in patients with CMV risk versus none were 74.56 versus 59.02 days (P = .011), viremia versus without viremia were 77.19 versus 62.28 days (P = .024), and viral disease versus without viral disease were 90.17 versus 63.97 days (P = .002). Patients receiving alemtuzumab also had longer hospitalization by 15 days in comparison to who did not receive alemtuzumab, which approached but did not reach statistical significance (P = .058).
Table 7.
Days of Hospitalization for First 180 Days after alloHCT
| Risk Factor | n | No. of Days in Hospital from Day 0–180 | P Value (t-test) |
|---|---|---|---|
| CMV risk | |||
| No CMV risk | 55 | 59.02 (28.58), 49 (16–136) | |
| CMV risk | 85 | 74.56 (42.96), 63 (8–180) | .011 |
| No viremia | 82 | 62.28 (38.11), 49 (16–176) | |
| Viremia | 58 | 77.19 (37.93), 72 (8–180) | .024 |
| No viral disease | 116 | 63.97 (36.76), 52 (16–180) | |
| Viral disease | 24 | 90.17 (40.74), 96 (8–162) | .002 |
| Patients with viremia | 58 | ||
| No viral disease | 34 | 68.03 (33.45), 57 (20–180) | |
| Viral disease | 24 | 90.17 (40.74), 96 (8–162) | .027 |
| No alemtuzumab | 65 | 61.82 (35.53), 49 (16–176) | |
| Alemtuzumab | 75 | 74.21 (40.45), 65 (8–180) | .058 |
Data presented are mean (SD), median (range).
DISCUSSION
Ours is the second-largest study of viremia monitoring in children after alloHCT. In this study, we were able to identify 3 pretransplantation risk factors (pretransplantation viremia, CMV risk status, and use of alemtuzumab) that should guide centers in terms of management of viremia ahead of time. We were able to generate a model for PCR monitoring and elaborated on the impact of viremia on clinical outcomes including health care utilization.
A large study of 291 pediatric patients published by Hirwarker et al. had similar results to ours [8]. Their incidence of viremia was similar to our cohort: CMV, 16%; ADV, 15%; and EBV, 11%. However, the threshold for starting pre-emptive treatment was much higher in their study: CMV PCR ≥ 10,000 copies/mL, EBV PCR ≥ 40,000 copies/mL, and ADV PCR ≥ 10,000 copies/mL compared with CMV PCR ≥ 600 copies/mL, EBV PCR ≥ 1000 copies/mL, and ADV PCR ≥ 1000 copies/mL. In a smaller study of 40 children, Schonberger et al. reported the relatively higher incidence of EBV (48%) and CMV (28%). This could be related to use of ATG.
Similar to Hirwarker et al., we noted poor OS in patients who developed viremia and viral disease [8]. Establishing causality of viremia/viral diseases and mortality is challenging. In these patients, viral PCRs may become negative after treatment with antiviral medications. However, renal, hepatic, and bone marrow injury associated with viral infections and antiviral medications can cause long-term morbidities that eventually can impact survival. One other potential modality that may further improve the survival would be to limit exposure to antiviral medications. Monitoring in vivo generation of viral-specific T cells may allow us to more accurately tailor our treatment approaches and may potentially decrease the need for prolonged antivirals [26].
One of the major challenges we face is in identifying patients who might benefit from prophylactic interventions aimed at mitigating the risk of viremia or viral disease [8,16,27]. We identified that CMV risk status, incidence of pretransplantation viremia, and use of alemtuzumab during conditioning were significantly associated with an increased viremia risk. Recent trials have demonstrated that maribavir and brincidofovir are not efficacious in CMV prevention after alloHCT [28,29]. Another potential strategy might be broad-spectrum antiviral cellular therapy. Viral cytotoxic T cells have also been studied as prophylactic and adjunctive treatment agents [30–32]. Perhaps patients with any of the 3 pretransplantation risk factors demonstrated in our study should have planned manufacturing of multivalent viral cytotoxic T cells, as this therapy may have the potential to reduce poor outcomes.
The majority of patients with pretransplantation viremia experienced a worse post-transplantation outcome, possibly due to the increased risk of development of post-alloHCT viral disease and/or its treatment and potentially also due to the poor prognosis of the primary disease. Hirsch et al. have recommended delaying transplantation in patients with CMV disease and, if possible, including secondary anti-CMV prophylaxis during transplantation [33]. Although these recommendations are aimed at addressing the risks associated with the development of post-transplantation viremia, the risks of delaying transplantation in patients with immunodeficiency, hemophagocytic disorders, and refractory malignancies must also be weighed. Patients analyzed for this study who had pretransplantation viremia were either refractory to antiviral therapies or were in critical condition at the time of transplantation and, therefore, were unable to have full resolution of viremia before proceeding to transplantation. Novel treatment strategies are critical for the management of these groups of patients.
Knowledge of health care utilization associated with viremia and viral disease is critical, as centers might be encouraged to invest more resources in novel strategies to reduce the incidence of viremia with the ultimate goal of improving outcomes. Our data reveals that days of hospitalization increases proportionally with CMV risk, viremia, and viral disease. A previous study has shown that pre-emptive screening and treatment for viruses is more cost-effective than treating viral disease when routine monitoring is not implemented [34]. In recent years, use of donor-derived or third-party viral-specific cytotoxic T lymphocytes have proven effective and safe treatment for viral infections [31,35]. The cost-benefit of viral PCR monitoring with pre-emptive antivirals versus the cost of treatment with prophylactic virus-specific cytotoxic T lymphocytes warrant further investigation.
Guidelines for monitoring viral PCRs after alloHCT are not well established. Routine viral PCR monitoring results increases health care utilization and iatrogenic blood loss. A single quantitative CMV PCR costs $110, with $95 per EBV PCR and $125 per ADV PCR. In the first 180 days after transplantation, a minimum of 27 weekly PCRs per virus are performed per patient. For each patient, this adds up to approximately $9000.
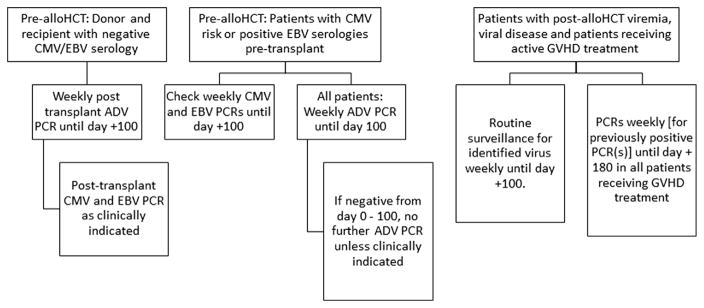
Using the results of this analysis (Table 2), we aimed to develop an evidence-based algorithm for viral PCR monitoring (Figure 3). Before alloHCT, all donors and recipients should have CMV and EBV IgG serological testing performed. In recipient/donor negative patients, PCR should be performed as clinically indicated. All other patients should have PCR testing for CMV, EBV, and ADV once before alloHCT.
Figure 3.
Algorithm for viral PCR monitoring in children after alloHCT.
A plan for after alloHCT from day 0 to day 100 is as follows: those at risk for CMV/EBV based on serological testing should have weekly PCRs performed. All patients should have weekly ADV PCRs performed. After day 100, no further routine surveillance is indicated for patients who were negative in the first 100 days. Further PCRs can be done as clinically indicated. Patients with viremia during the first 100 days and/or those receiving anti-GVHD treatments should only have routine weekly surveillance done for the positive virus in the first 100 days. Other PCRs can be done as clinically indicated.
We have implemented the algorithm discussed above for viral PCR monitoring to decrease the amount of unnecessary testing performed. We anticipate a 50% drop in viral PCR testing.
Limitations of our study include minimal immune reconstitution data, a small number of patients, and a heterogeneous spectrum of diagnoses and conditioning regimens. Despite various limitations, we were able to glean information that is clinically impactful, especially pre-alloHCT risk factors associated with post-alloHCT viremia.
In summary, post-transplantation viremia is associated with reduced OS and increased hospitalization. Improved treatment strategies for patients with viremia, such as multivalent antiviral cytotoxic T lymphocytes, should be studied for efficacy and feasibility. Additionally, in patients with low risk of post-transplantation viremia, routine viral monitoring can be tailored to each patient’s individual risk in an effort to reduce blood draws and decrease excess health care utilization.
Acknowledgments
Financial disclosure statement: There is nothing to disclose.
Conflict of interest statement: There are no reported conflicts.
References
- 1.Satwani P, Kahn J, Jin Z. Making strides and meeting challenges in pediatric allogeneic hematopoietic cell transplantation clinical trials in the United States: Past, present and future. Contemp Clin Trials. 2015;45:84–92. doi: 10.1016/j.cct.2015.06.011. [DOI] [PubMed] [Google Scholar]
- 2.Miano M, Labopin M, Hartmann O, et al. Haematopoietic stem cell transplantation trends in children over the last three decades: a survey by the paediatric diseases working party of the European Group for Blood and Marrow Transplantation. Bone Marrow Transplant. 2007;39:89–99. doi: 10.1038/sj.bmt.1705550. [DOI] [PubMed] [Google Scholar]
- 3.Barrell C, Dietzen D, Jin Z, et al. Reduced-intensity conditioning allogeneic stem cell transplantation in pediatric patients and subsequent supportive care. Oncol Nurs Forum. 2012;39:E451–458. doi: 10.1188/12.ONF.E451-E458. [DOI] [PubMed] [Google Scholar]
- 4.Gooley TA, Chien JW, Pergam SA, et al. Reduced mortality after allogeneic hematopoietic-cell transplantation. N Engl J Med. 2010;363:2091–2101. doi: 10.1056/NEJMoa1004383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Horan JT, Logan BR, Agovi-Johnson MA, et al. Reducing the risk for transplantation-related mortality after allogeneic hematopoietic cell transplantation: how much progress has been made? J Clin Oncol. 2011;29:805–813. doi: 10.1200/JCO.2010.32.5001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Shaw PJ, Kan F, Woo Ahn K, et al. Outcomes of pediatric bone marrow transplantation for leukemia and myelodysplasia using matched sibling, mismatched related, or matched unrelated donors. Blood. 2010;116:4007–4015. doi: 10.1182/blood-2010-01-261958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kline J, Pollyea DA, Stock W, et al. Pre-transplant ganciclovir and post transplant high-dose valacyclovir reduce CMV infections after alemtuzumab-based conditioning. Bone Marrow Transplant. 2006;37:307–310. doi: 10.1038/sj.bmt.1705249. [DOI] [PubMed] [Google Scholar]
- 8.Hiwarkar P, Gaspar HB, Gilmour K, et al. Impact of viral reactivations in the era of pre-emptive antiviral drug therapy following allogeneic haematopoietic SCT in paediatric recipients. Bone Marrow Transplant. 2013;48:803–808. doi: 10.1038/bmt.2012.221. [DOI] [PubMed] [Google Scholar]
- 9.Boeckh M, Ljungman P. How we treat cytomegalovirus in hematopoietic cell transplant recipients. Blood. 2009;113:5711–5719. doi: 10.1182/blood-2008-10-143560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ljungman P, Aschan J, Lewensohn-Fuchs I, et al. Results of different strategies for reducing cytomegalovirus-associated mortality in allogeneic stem cell transplant recipients. Transplantation. 1998;66:1330–1334. doi: 10.1097/00007890-199811270-00012. [DOI] [PubMed] [Google Scholar]
- 11.Nichols WG, Corey L, Gooley T, et al. Rising pp65 antigenemia during preemptive anticytomegalovirus therapy after allogeneic hematopoietic stem cell transplantation: risk factors, correlation with DNA load, and outcomes. Blood. 2001;97:867–874. doi: 10.1182/blood.v97.4.867. [DOI] [PubMed] [Google Scholar]
- 12.Boeckh M, Nichols WG, Papanicolaou G, et al. Cytomegalovirus in hematopoietic stem cell transplant recipients: current status, known challenges, and future strategies. Biol Blood Marrow Transplant. 2003;9:543–558. doi: 10.1016/s1083-8791(03)00287-8. [DOI] [PubMed] [Google Scholar]
- 13.Styczynski J, Gil L, Tridello G, et al. Response to rituximab-based therapy and risk factor analysis in Epstein-Barr Virus-related lymphoproliferative disorder after hematopoietic stem cell transplant in children and adults: a study from the Infectious Diseases Working Party of the European Group for Blood and Marrow Transplantation. Clin Infect Dis. 2013;57:794–802. doi: 10.1093/cid/cit391. [DOI] [PubMed] [Google Scholar]
- 14.Schonberger S, Meisel R, Adams O, et al. Prospective, comprehensive, and effective viral monitoring in children undergoing allogeneic hematopoietic stem cell transplantation. Biol Blood Marrow Transplant. 2010;16:1428–1435. doi: 10.1016/j.bbmt.2010.04.008. [DOI] [PubMed] [Google Scholar]
- 15.George B, Pati N, Gilroy N, et al. Pre-transplant cytomegalovirus (CMV) serostatus remains the most important determinant of CMV reactivation after allogeneic hematopoietic stem cell transplantation in the era of surveillance and preemptive therapy. Transpl Infect Dis. 2010;12:322–329. doi: 10.1111/j.1399-3062.2010.00504.x. [DOI] [PubMed] [Google Scholar]
- 16.Bordon V, Padalko E, Benoit Y, et al. Incidence, kinetics, and risk factors of Epstein-Barr virus viremia in pediatric patients after allogeneic stem cell transplantation. Pediatr Transplant. 2012;16:144–150. doi: 10.1111/j.1399-3046.2011.01634.x. [DOI] [PubMed] [Google Scholar]
- 17.Chakrabarti S, Mackinnon S, Chopra R, et al. High incidence of cytomegalovirus infection after nonmyeloablative stem cell transplantation: potential role of Campath-1H in delaying immune reconstitution. Blood. 2002;99:4357–4363. doi: 10.1182/blood.v99.12.4357. [DOI] [PubMed] [Google Scholar]
- 18.Bhatia M, Militano O, Jin Z, et al. An age-dependent pharmacokinetic study of intravenous and oral mycophenolate mofetil in combination with tacrolimus for GVHD prophylaxis in pediatric allogeneic stem cell transplantation recipients. Biol Blood Marrow Transplant. 2010;16:333–343. doi: 10.1016/j.bbmt.2009.10.007. [DOI] [PubMed] [Google Scholar]
- 19.Offer K, Kolb M, Jin Z, et al. Efficacy of tacrolimus/mycophenolate mofetil as acute graft-versus-host disease prophylaxis and the impact of subtherapeutic tacrolimus levels in children after matched sibling donor allogeneic hematopoietic cell transplantation. Biol Blood Marrow Transplant. 2015;21:496–502. doi: 10.1016/j.bbmt.2014.11.679. [DOI] [PubMed] [Google Scholar]
- 20.Glucksberg H, Storb R, Fefer A, et al. Clinical manifestations of graft-versus-host disease in human recipients of marrow from HL-A-matched sibling donors. Transplantation. 1974;18:295–304. doi: 10.1097/00007890-197410000-00001. [DOI] [PubMed] [Google Scholar]
- 21.Shereck EB, Cooney E, van de Ven C, et al. A pilot phase II study of alternate day ganciclovir and foscarnet in preventing cytomegalovirus (CMV) infections in at-risk pediatric and adolescent allogeneic stem cell transplant recipients. Pediatr Blood Cancer. 2007;49:306–312. doi: 10.1002/pbc.21043. [DOI] [PubMed] [Google Scholar]
- 22.Mynarek M, Ganzenmueller T, Mueller-Heine A, et al. Patient, virus, and treatment-related risk factors in pediatric adenovirus infection after stem cell transplantation: results of a routine monitoring program. Biol Blood Marrow Transplant. 2014;20:250–256. doi: 10.1016/j.bbmt.2013.11.009. [DOI] [PubMed] [Google Scholar]
- 23.Ljungman P, Griffiths P, Paya C. Definitions of cytomegalovirus infection and disease in transplant recipients. Clin Infect Dis. 2002;34:1094–1097. doi: 10.1086/339329. [DOI] [PubMed] [Google Scholar]
- 24.Ljungman P, Ribaud P, Eyrich M, et al. Cidofovir for adenovirus infections after allogeneic hematopoietic stem cell transplantation: a survey by the Infectious Diseases Working Party of the European Group for Blood and Marrow Transplantation. Bone Marrow Transplant. 2003;31:481–486. doi: 10.1038/sj.bmt.1703798. [DOI] [PubMed] [Google Scholar]
- 25.Matthes-Martin S, Feuchtinger T, Shaw PJ, et al. European guidelines for diagnosis and treatment of adenovirus infection in leukemia and stem cell transplantation: summary of ECIL-4 (2011) Transpl Infect Dis. 2012;14:555–563. doi: 10.1111/tid.12022. [DOI] [PubMed] [Google Scholar]
- 26.Tischer S, Dieks D, Sukdolak C, et al. Evaluation of suitable target antigens and immunoassays for high-accuracy immune monitoring of cytomegalovirus and Epstein-Barr virus-specific T cells as targets of interest in immunotherapeutic approaches. J Immunol Methods. 2014;408:101–113. doi: 10.1016/j.jim.2014.05.011. [DOI] [PubMed] [Google Scholar]
- 27.Olkinuora HA, Taskinen MH, Saarinen-Pihkala UM, Vettenranta KK. Multiple viral infections post-hematopoietic stem cell transplantation are linked to the appearance of chronic GVHD among pediatric recipients of allogeneic grafts. Pediatr Transplant. 2010;14:242–248. doi: 10.1111/j.1399-3046.2009.01226.x. [DOI] [PubMed] [Google Scholar]
- 28.Marty FM, Ljungman P, Papanicolaou GA, et al. Maribavir prophylaxis for prevention of cytomegalovirus disease in recipients of allogeneic stem-cell transplants: a phase 3, double-blind, placebo-controlled, randomised trial. Lancet Infect Dis. 2011;11:284–292. doi: 10.1016/S1473-3099(11)70024-X. [DOI] [PubMed] [Google Scholar]
- 29.Marty FM, Winston DJ, Rowley SD, et al. CMX001 to prevent cytomegalovirus disease in hematopoietic-cell transplantation. N Engl J Med. 2013;369:1227–1236. doi: 10.1056/NEJMoa1303688. [DOI] [PubMed] [Google Scholar]
- 30.Gerdemann U, Keirnan JM, Katari UL, et al. Rapidly generated multivirus-specific cytotoxic T lymphocytes for the prophylaxis and treatment of viral infections. Mol Ther. 2012;20:1622–1632. doi: 10.1038/mt.2012.130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Leen AM, Christin A, Myers GD, et al. Cytotoxic T lymphocyte therapy with donor T cells prevents and treats adenovirus and Epstein-Barr virus infections after haploidentical and matched unrelated stem cell transplantation. Blood. 2009;114:4283–4292. doi: 10.1182/blood-2009-07-232454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sili U, Leen AM, Vera JF, et al. Production of good manufacturing practice-grade cytotoxic T lymphocytes specific for Epstein-Barr virus, cytomegalovirus and adenovirus to prevent or treat viral infections post-allogeneic hematopoietic stem cell transplant. Cytotherapy. 2012;14:7–11. doi: 10.3109/14653249.2011.636963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hirsch HH, Lautenschlager I, Pinsky BA, et al. An international multi-center performance analysis of cytomegalovirus load tests. Clin Infect Dis. 2013;56:367–373. doi: 10.1093/cid/cis900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Evers PD. Pre-emptive virology screening in the pediatric hematopoietic stem cell transplant population: a cost effectiveness analysis. Hematol Oncol Stem Cell Ther. 2013;6:81–88. doi: 10.1016/j.hemonc.2013.08.003. [DOI] [PubMed] [Google Scholar]
- 35.Saglio F, Hanley PJ, Bollard CM. The time is now: moving toward virus-specific T cells after allogeneic hematopoietic stem cell transplantation as the standard of care. Cytotherapy. 2014;16:149–159. doi: 10.1016/j.jcyt.2013.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]