Abstract
Objective
To determine the long term effects of vorinostat on safety and virological parameters in HIV-infected individuals on suppressive antiretroviral therapy (ART).
Design
Prospective longitudinal observational extended follow up of 20 HIV-infected individuals on ART previously enrolled in a clinical trial of daily vorinostat 400 mg for 14 days. Extended follow up included visits at 6, 12, 18 and 24 months post enrolment in the initial clinical trial.
Methods
Cell-associated unspliced (CA-US) HIV RNA, total HIV DNA and plasma HIV RNA were quantified by PCR and, CD4+ and CD8+ T cells quantified by flow cytometry. Changes over time in each parameter were assessed using Wilcoxon matched pair signed-rank test and Generalised Estimating Equations for trend modelling.
Results
We recorded a total of 31 adverse events (26 grade 1 and 5 grade 2) in all study participants (n = 20). There were no significant changes in the number of CD4+ or CD8+ T cells or plasma HIV RNA over time. In 12 participants for whom baseline samples were available, there were no significant changes in total HIV DNA, CA-US HIV RNA, plasma RNA, or CD4 and CD8+ T cells at 6, 12, 18 or 24 months.
Conclusions
Extended follow up for 24 months did not reveal any long-term toxicity or changes in markers of HIV persistence or transcription in participants on ART who had received 14 days of vorinostat.
Introduction
Long lived latently infected resting CD4+ (rCD4+) T cells persist in HIV-infected individuals on suppressive antiretroviral therapy (ART) [1, 2]. These latently infected cells remain the major barrier to HIV cure. One strategy for eliminating these cells is activating HIV production to trigger immune- or viral-mediated lysis, often referred to as “shock and kill”. Histone deacetylase inhibitors (HDACi) have been investigated in clinical trials in HIV-infected individuals on ART and have been shown to increase viral transcription in total and resting CD4+ T cells in blood and rectal tissue [3–6]. However, the long term effects of these drugs in people living with HIV remain unknown.
We previously conducted a clinical trial of 400 mg of vorinostat once daily for 14 days in HIV-infected individuals on suppressive ART with pre-specified follow up for 84 days [3]. Vorinostat administration led to significant increases in cell-associated unspliced (CA-US) HIV RNA in CD4+ T cells during treatment and up to 70 days after completing vorinostat. However, there were no changes in the levels of total HIV DNA or plasma HIV RNA. In addition, we observed significant changes in host gene expression up to 70 days post-treatment [3]. We and others have also observed similar sustained increases in CA-US HIV RNA in HIV-infected participants on ART following the HDACi panobinostat [5] and with standard and high doses of another latency reversing agent (LRA) disulfiram [7, 8].
In this current study, we aimed to determine the long-term safety and changes in HIV transcription in HIV-infected participants on ART who had received 14 days of vorinostat. During 24 months of extended follow up, we found no evidence of long term toxicities and all markers of viral persistence, including CA-US HIV RNA, returned to baseline levels.
Patients and Methods
Patients
The original 20 participants from the vorinostat trial [3] were enrolled in a prospective extension study. Study visits were conducted at 6, 12, 18 and 24 months following initial enrolment in the clinical trial and included clinical assessment, collection of blood and storage of peripheral blood mononuclear cells (PBMCs) and plasma. The study was approved by the Human Research Ethics Committee of Alfred Hospital, Melbourne, Australia, and was conducted in accordance with the ethical principles laid out in the Declaration of Helsinki (1996) and the National Health and Medical Research Council (NHMRC) of Australia National Statement on Ethical Conduct in Human Research (2007).
Clinical and laboratory assessment
To assess long-term safety, we evaluated the clinical status of study participants at each study visit during extended follow up and recorded any incident adverse events. For each adverse event, we assessed severity and the causal association with previous vorinostat treatment.
Long-term changes in CD4+ T cell count, CD8+ T cell count and CD4/CD8 ratio were determined by flow cytometry. Changes in plasma HIV RNA was measured using a conventional clinical assay (Roche Cobas Taqman), with a lower limit of detection of 20 copies/mL.
Quantitative real-time PCR for CA-HIV RNA and DNA
To analyse the long-term effects on CA-US HIV RNA and HIV DNA, total CD4+ T cells were isolated from PBMCs and RNA and DNA were extracted using the Qiagen All-prep kit. CA-US HIV RNA and HIV DNA were quantified using real-time PCR as described previously [3]. Only participants with baseline PBMC were evaluated. Cellular DNA and RNA were extracted from all time points for each participant at the same time, frozen for subsequent analysis and then run on the same PCR plate.
Statistics
Pairwise comparisons of actual values and median fold changes from baseline were performed using Wilcoxon matched-pairs signed rank. In addition, we analysed the overall change from baseline using generalized estimating equation (GEE) statistics. P-values <0.05 were considered significant.
Results
All 20 individuals who enrolled in the original vorinostat study provided consent to prolonged follow up and completed all study visits up to 24 months. During follow up, we recorded a total of 31 adverse events (26 grade 1 and 5 grade 2). Of these, only one adverse clinical event was possibly related to previous vorinostat treatment (grade 1 acid reflux, which resolved to the level of severity present prior to vorinostat) (Table 1b). There were some minor changes to ART regimens over the time of the study in four participants (one for adverse events related to efavirenz, two for simplification and one for whom no reason was provided).
Table 1. Adverse events during extended 24 months follow up after daily vorinostat for 14 days.
Adverse events unrelated (a) and related (b) to vorinostat were reported during the follow up study.
| (a) Adverse events unrelated to vorinostat | |||||
|---|---|---|---|---|---|
| Adverse events | Gradea | ||||
| 1 | 2 | 3 | 4 | Total | |
|
| |||||
| Respiratory tract infection | 3 | 1 | 4 | ||
|
| |||||
| Sinusitis | 1 | 1 | |||
|
| |||||
| Cough | 4 | 4 | |||
|
| |||||
| Rhinorrhea | 2 | 2 | |||
|
| |||||
| Nasal congestion | 1 | 1 | |||
|
| |||||
| Rectal bleeding | 2 | 2 | |||
|
| |||||
| Anal lump | 1 | 1 | |||
|
| |||||
| Abdominal pain | 1 | 1 | |||
|
| |||||
| Shigellosis | 1 | 1 | |||
|
| |||||
| Sore throat | 1 | 1 | |||
|
| |||||
| Urethral discharge | 1 | 1 | |||
|
| |||||
| Rectal chlamydia | 1 | 1 | |||
|
| |||||
| Boil (posterior neck) | 1 | 1 | |||
|
| |||||
| Tympanomastoidectomy | 1 | 1 | |||
|
| |||||
| Paresthesia | 1 | 1 | |||
|
| |||||
| Bruised ribs | 1 | 1 | |||
|
| |||||
| Epididymitis/orchitis | 1 | 1 | |||
|
| |||||
| Rash | 1 | 1 | |||
|
| |||||
| Knee pain | 1 | 1 | |||
|
| |||||
| Sprained ankle | 1 | 1 | |||
|
| |||||
| Sore shoulder | 2 | 2 | |||
| (b) Adverse events possibly related to vorinostat | |||||
|---|---|---|---|---|---|
| Adverse events | Gradea | ||||
| 1 | 2 | 3 | 4 | Total | |
|
| |||||
| Acid reflux | 1 | 1 | |||
Grading according to National Cancer Institute Common Terminology Criteria for Adverse Events v 4.0.
In the 20 participants, the levels of CD4+ or CD8+ T cells did not change significantly during follow up (Supplementary Fig. 1a and 1b, respectively). The ratio of CD4+ to CD8+ T cells was significantly higher at 24 months compared to baseline (P = 0.012), although the median fold change was 1.1 (Interquartile range (IQR) 0.67–1.24) and there was no upward trend over the 24 month period (P = 0.206) (Supplementary Fig. 1c). Plasma HIV RNA remained undetectable (<20 copies/mL) throughout the extension study with the exception of three participants in whom plasma HIV RNA was detected at low levels at one time point for each participant (Supplementary Fig. 1d).
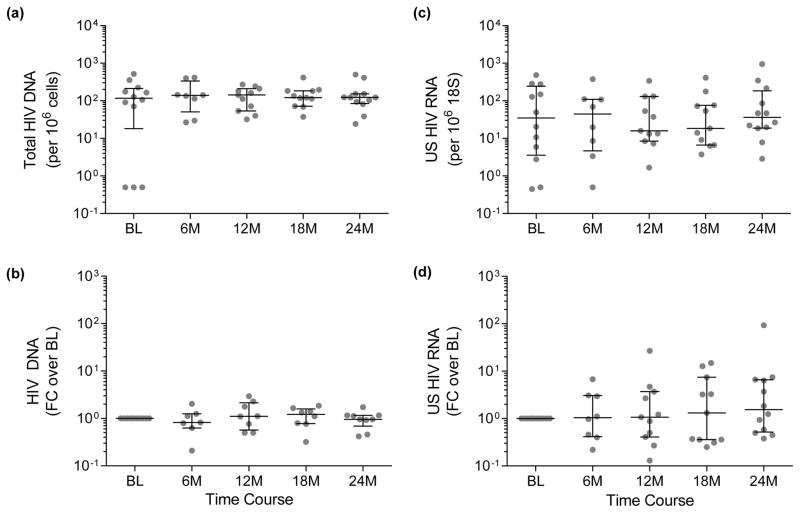
Of the original 20 individuals, 12 had baseline PBMCs available and these samples were used for the comparison with samples obtained during the extended follow up period. These participants were all male and their demographics (Supplementary Table S1) were not significantly different from the total cohort that we have previously described [3]. Compared to baseline, there were no significant differences in the absolute levels of total HIV DNA at any time point (Fig. 1a), or when analysed as fold changes relative to baseline (Fig. 1b). Trend analysis revealed no change in total HIV DNA in CD4+ T cells over the 24-month period (P = 0.723). Similarly, there were no changes in the levels (Fig. 1c) or fold change (Fig. 1d) of CA-US HIV RNA at each time point compared to baseline, and no change in trend across the 24 months (P = 0.518).
Fig. 1. Changes in total HIV DNA and CA-US HIV RNA over 24 months follow up after daily vorinostat for 14 days.
(a) Total HIV DNA was measured per million cells and (b) fold changes (FC) in DNA were calculated relative to baseline values. (c) CA-US HIV RNA was measured relative to 18S RNA and (d) fold changes were calculated relative to baseline. Median and IQR are shown for each time point. Comparisons of RNA or DNA to baseline were made using the Wilcoxon test. There were no significant changes in these parameters over time.
Discussion
In this observational follow up study of HIV-infected individuals on suppressive ART who had received vorinostat for 14 days, extended clinical follow up for 24 months did not reveal long-term adverse effects of vorinostat. We observed no changes in total HIV DNA, CA-US HIV RNA, or plasma HIV RNA compared to pre-vorinostat levels. Taken together, these data show that vorinostat treatment is not associated with long-term adverse effects or sustained increases in HIV transcription.
Understanding the long-term effects of HDACi on the host transcriptome, viral transcription and safety in HIV-infected individuals on ART is important, but to date has not been explored. We were the first to show that changes in host gene expression and CA-US HIV RNA were significantly increased from baseline 70 days after the last dose of vorinostat [3]. Short term follow up at 30 days following panobinostat [5] and 84 days following disulfiram [8], respectively, also demonstrated elevated CA-US HIV RNA that persisted after cessation of the LRA. In this study we demonstrate that by six months following vorinostat, CA-US HIV RNA returned to baseline and remained stable over extended follow up.
This follow up extension study was designed to measure CA-US HIV RNA at an initial time point of 6 months post vorinostat initiation. We therefore are unable to determine exactly when CA-US HIV RNA returned to baseline levels, which we assume was sometime between three and six months after vorinostat was initiated. Although the optimal dosing schedule for LRAs to achieve maximal efficacy in latency reversal and minimal effects on T-cell function [9] remains unclear, following HDACi including vorinostat, increases in CA-US HIV RNA occur quickly and in most studies the maximal increases were observed within the first 12 hours [3–6] and when repeated dosing was used, some studies demonstrated further increases in CA-US HIV RNA following additional doses [3, 6, 8]. Additional increases in CA-US HIV RNA with multiple dosing of vorinostat were not observed in all participants [10], and with repeated dosing of panobinostat, no further increases in CA-US HIV RNA were observed [5]. Here, our main goal was not to identify the optimal dosing for efficacy of latency reversal but rather to determine the long term safety of vorinostat and to ensure there were no long term changes in HIV expression.
In conclusion, we show that following 14 days of vorinostat in HIV-infected participants on ART, all changes in viral transcription returned to baseline by 6 months and there were no long term adverse effects up to 24 months post treatment. Given that HIV-infected individuals on ART have an excellent prognosis, careful attention to any long term adverse consequences of LRAs or other interventions aimed at achieving HIV remission is warranted. Here, we demonstrate no long term adverse events related to vorinostat.
Supplementary Material
Total (a) CD4+ and (b) CD8+ T cells were determined by flow cytometry and (c) CD4+/CD8+ ratios were calculated. (d) Plasma HIV RNA was measured using a commercial assay (Roche; lower limit of detection = 20 copies/mL). Pairwise comparisons to baseline were measured using the Wilcoxon test for each graph (n = 20). There were no significant changes in these parameters over time.
Acknowledgments
We acknowledge the participation and commitment of study participants, which made the study possible, the contribution of the Alfred Hospital Infectious Diseases Clinical Research Unit and helpful discussions with members of the Lewin-Cameron laboratory. We would also like to acknowledge Aaron Cogle and Jo Watson from the National Association of People Living with HIV Australia for their contributions as members of the protocol steering committee.
Funding source: This work was supported by an investigator initiated grant from Merck. Other sources of support included the National Health and Medical Research Council (NHMRC) of Australia and the National Institutes for Health U19 AI096109. SRL is an NHMRC Practitioner Fellow.
Footnotes
Author Contributions: Conceived and designed the experiments: SRL JHE TMM TR. Performed the experiments: TMM AR ST AD MH JR. Clinical assessment of participants: JM, JHE, MH. Analyzed the data: TS TMM TAR AR SRL. Wrote the manuscript: TMM SRL. Intellectual input: FW HMP DFJP AC. Approved the manuscript: all
Disclaimers: SRL’s institution has received funding for investigator initiated research grants, consultant activities and for participation in educational meetings from Merck, Viiv Healthcare, Gilead and Bristol Myers Squibb
References
- 1.Chun TW, Carruth L, Finzi D, Shen X, DiGiuseppe JA, Taylor H, et al. Quantification of latent tissue reservoirs and total body viral load in HIV-1 infection. Nature. 1997;387:183–188. doi: 10.1038/387183a0. [DOI] [PubMed] [Google Scholar]
- 2.Finzi D, Blankson J, Siliciano JD, Margolick JB, Chadwick K, Pierson T, et al. Latent infection of CD4+ T cells provides a mechanism for lifelong persistence of HIV-1, even in patients on effective combination therapy. Nat Med. 1999;5:512–517. doi: 10.1038/8394. [DOI] [PubMed] [Google Scholar]
- 3.Elliott JH, Wightman F, Solomon A, Ghneim K, Ahlers J, Cameron MJ, et al. Activation of HIV transcription with short-course vorinostat in HIV-infected patients on suppressive antiretroviral therapy. PLoS Pathog. 2014;10:e1004473. doi: 10.1371/journal.ppat.1004473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Archin NM, Liberty AL, Kashuba AD, Choudhary SK, Kuruc JD, Crooks AM, et al. Administration of vorinostat disrupts HIV-1 latency in patients on antiretroviral therapy. Nature. 2012;487:482–485. doi: 10.1038/nature11286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rasmussen TA, Tolstrup M, Brinkmann CR, Olesen R, Erikstrup C, Solomon A, et al. Panobinostat, a histone deacetylase inhibitor, for latent-virus reactivation in HIV-infected patients on suppressive antiretroviral therapy: a phase 1/2, single group, clinical trial. Lancet HIV. 2014;1:e13–21. doi: 10.1016/S2352-3018(14)70014-1. [DOI] [PubMed] [Google Scholar]
- 6.Sogaard OS, Graversen ME, Leth S, Olesen R, Brinkmann CR, Nissen SK, et al. The Depsipeptide Romidepsin Reverses HIV-1 Latency In Vivo. PLoS Pathog. 2015;11:e1005142. doi: 10.1371/journal.ppat.1005142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Spivak AM, Andrade A, Eisele E, Hoh R, Bacchetti P, Bumpus NN, et al. A pilot study assessing the safety and latency-reversing activity of disulfiram in HIV-1-infected adults on antiretroviral therapy. Clin Infect Dis. 2014;58:883–890. doi: 10.1093/cid/cit813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Elliott JH, McMahon JH, Chang CC, Lee SA, Hartogensis W, Bumpus N, et al. Short-term administration of disulfiram for reversal of latent HIV infection: a phase 2 dose-escalation study. Lancet HIV. 2015;2:e520–529. doi: 10.1016/S2352-3018(15)00226-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jones RB, Mueller S, O’Connor R, Rimpel K, Sloan DD, Karel D, et al. A Subset of Latency-Reversing Agents Expose HIV-Infected Resting CD4+ T-Cells to Recognition by Cytotoxic T-Lymphocytes. PLoS Pathog. 2016;12:e1005545. doi: 10.1371/journal.ppat.1005545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Archin NM, Bateson R, Tripathy MK, Crooks AM, Yang KH, Dahl NP, et al. HIV-1 expression within resting CD4+ T cells after multiple doses of vorinostat. J Infect Dis. 2014;210:728–735. doi: 10.1093/infdis/jiu155. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Total (a) CD4+ and (b) CD8+ T cells were determined by flow cytometry and (c) CD4+/CD8+ ratios were calculated. (d) Plasma HIV RNA was measured using a commercial assay (Roche; lower limit of detection = 20 copies/mL). Pairwise comparisons to baseline were measured using the Wilcoxon test for each graph (n = 20). There were no significant changes in these parameters over time.