Abstract
In pediatric and adolescent patients undergoing allogeneic hematopoietic cell transplantation, treatment-related toxicities remain a clinical challenge. A paucity of data investigates the risks for and survival impact of treatment-related toxicities in this population. Here the authors assess the relative toxicity of myeloablative, reduced-toxicity, and reduced-intensity conditioning regimens; identify patient-related predictors of post-transplant toxicities; and investigate the impact of early post-transplant toxicities on transplant-related mortality (TRM). In this retrospective study, 164 patients (aged 1 to 22 years) underwent allogeneic stem cell transplantation after busulfan-based conditioning for malignant and nonmalignant diseases between 2000 and 2014. The number of grades III to IV toxicities between days 0 and +30 was calculated for each patient. TRM was calculated to 2 years. Median patient age was 9 years, and median number of toxicities was 3 (range, 0 to 17). The 100-person day incidence of post-transplant toxicities in myeloablative conditioning was not different from the incidence in reduced-toxicity conditioning (13.88 versus 13.59, P = .812). Reduced intensity was less toxic than both myeloablative and reduced toxicity (13.75 versus 8.41, P <.001). Age ≥ 12 years (.276 with SE = .138, P =.045) and unrelated donor transplant (.318 with SE = 0.113, P = .005) were risk factors for ≥3 toxicities. Having ≥3 toxicities or a performance score < 90 conferred higher risk of TRM (P = .021). In pediatric and adolescent patients undergoing hematopoietic cell transplantation, reduced-toxicity conditioning was not significantly less toxic than myeloablative conditioning. Additionally, the number of post-transplant toxicities correlated with the risk of mortality. Further investigations to confirm our findings are warranted.
Keywords: Pediatric, Adolescent, Morbidity, Mortality, Predictors, Allogeneic
INTRODUCTION
Despite continued advances in the field of allogeneic hematopoietic cell transplantation (alloHCT), the success of transplant in pediatric and adolescent patients is hindered by persistently high rates of treatment-related toxicities (TRTs) and transplant-related mortality (TRM) [1]. Studies in adults have demonstrated identifiable predictors of poorer outcomes after alloHCT [2]. Broadly, these predictors fall into 3 main categories: patient-related, treatment-related, and post-transplant events or complications. In adult transplant recipients, patient age and performance status as well as pretransplant organ function have all been linked to post-transplant complications and TRM [3]. Treatment-related factors such as intensity of conditioning regimen, donor source, and degree of donor mismatch have also been linked to morbidity and mortality in adult transplant patients [4,5]. Finally, post-transplant events such as delayed neutrophil engraftment, acute graft-versus-host disease (GVHD), and opportunistic infections have also been associated with a higher incidence of serious post-transplant organ dysfunction and, by extension, poorer overall survival (OS) [6].
In the field of pediatric alloHCT there remains a paucity of data investigating patient- and treatment-related predictors of TRTs. With regard to conditioning regimens, there is little information about the relative incidence of toxicities between myeloablative conditioning (MAC), reduced-toxicity conditioning (RTC), and reduced-intensity conditioning (RIC) regimens. Additionally, only a handful of studies have investigated whether the extent of early post-transplant toxicities can be correlated with the risk of subsequent TRM in children and adolescents [7]. Here we report the results of a retrospective study aimed at identifying whether (1) the incidence of TRTs is actually lower in patients who receive RTC versus MAC regimens, (2) there are identifiable patient-related predictors associated with higher risk of TRTs, and (3) the number of early post-transplant TRTs is predictive of survival outcomes in children and adolescents undergoing alloHCT.
METHODS
Patients
We retrospectively evaluated 164 pediatric patients with malignant and nonmalignant diseases undergoing alloHCT following busulfan (Bu)-based conditioning regimens at the Morgan Stanley Children’s Hospital of New York-Presbyterian between 2000 and 2014. Data were gathered from the hospital’s electronic medical records between September 2014 and February 2015. The protocol for this investigation met regulatory requirements for approval by the Institutional Review Board at Columbia University Medical Center.
Toxicities
The incidence of TRTs occurring from post-transplant days 0 to +30 was evaluated for each patient. Toxicities of 8 organ systems (central nervous system/brain, heart, lungs, liver, kidney, gastrointestinal tract, mucosal, and bladder) and 8 metabolic/biochemical toxicities (glucose, sodium, potassium, calcium, magnesium, phosphate, albumin, and uric acid) were included for analysis. Each event was graded using the National Cancer Institute Common Terminology Criteria for Adverse Events, version 4.0, grading system [8]. For analysis, toxicities were grouped into 2 categories: metabolic and organ related.
Conditioning Regimens
Patients included in this analysis were conditioned on 1 of 3 Bu-based regimens, and those who received total body irradiation (TBI) were omitted. The 3 Bu-based regimens were as follows:
RIC: Bu (6.4 to 8 mg/kg), fludarabine (Flu; 180 mg/m2) ± rabbit antithymocyte globulin (ATG; 8 mg/kg)
RTC: Bu (12.8 to 16 mg/kg), Flu (180 mg/m2) ± alemtuzumab (54 mg/m2)
MAC: Bu (12.8 to 16 mg/kg), cyclophosphamide (120 to 200 mg/kg) ± ATG (8 mg/kg) or Bu (12.8 to 16 mg/m2) and melphalan (135 mg/m2) ± ATG (8 mg/kg)
Bu steady-state concentrations were maintained between 600 and 900 ng/mL in patients receiving RTC and MAC regimens. Steady-state levels were not analyzed for patients receiving RIC. Antimicrobial prophylaxis and supportive care measures were described previously [9].
Data Analysis and Statistical Methods
Three groups defined by conditioning regimen (MAC, RTC, or RIC) were formed. Within each of these groups, patient and transplant demographics were compared by chi-square test. Two-sided t-test or Wilcoxon rank sum test and analysis of variance or Kruskal-Wallis test were used to compare continuous variables. Continuous variables were summarized as mean ± standard deviation and median (minimum to maximum). Categorical variables were summarized as percentages.
The incidence of grades III to IV toxicities was based on the involvement of various organ systems or laboratory parameters and was calculated for each conditioning regimen. For a given patient, the maximum toxicity grade for each system (either organ or metabolic) in the first 30 days after transplant was included and analyzed as 1 episode. Toxicity episodes were calculated based on 100-person day incidence, which is 100 times to the ratio of the total number of toxicity episodes divided by the total number of follow-up days. Performance status was documented for each patient at the time of transplantation using Lansky or Karnofsky scales [10]. Poisson regression with robust standard errors for parameter estimates was used to examine risk factors for grades III to IV toxicities. Variables significant at .05 were used to build multivariate models.
For survival analyses, events were defined as disease progression or relapse, death without relapse, death from disease progression, or death from any cause. TRM was defined as death from any cause between days 0 and +30. After day +30, TRM was defined as nonrelapse mortality. The Kaplan-Meier method was used to estimate the 2-year OS, and the cumulative incidence function was used to estimate the 2-year TRM. The cause-specific hazard competing risk regression analysis was carried out to identify factors potentially related to TRM. Nonevent death was considered a competing event. P <.05 was considered to be significant, and multivariable analyses were carried out with factors significant at P = .05 on univariate analysis. Analyses were performed using SAS 9.3 (Cary, NC) and R program “cmprsk” package (www.r-project.org).
RESULTS
Patient Characteristics
One hundred sixty-four patients were included in this study. Median age at transplant was 9 years (range, 3 months to 22 years). Thirty-nine percent of patients had nonmalignant diseases, and 61% had malignant diseases. Forty-one percent of patients received MAC, 32% of patients received RTC, and approximately 27% of patients received RIC before transplant. In all patients, the median number of combined organ and metabolic toxicities between days 0 and +30 was 3 (range, 0 to 17). Additional patient and transplant demographics are outlined in Table 1.
Table 1.
Patient Characteristics and Transplant Demographics (N = 164)
| Characteristic | N | Percent |
|---|---|---|
| Male | 101 | 61.6 |
| Female | 63 | 38.4 |
| Age, yr | ||
| Mean (SD) | 9.38 (6.69) | |
| Median | 8.5 | |
| Range | .25–22 | |
| Disease type | ||
| Malignant | 100 | 61 |
| Nonmalignant | 64 | 39 |
| Conditioning regimen | ||
| MAC | 67 | 40.9 |
| RTC | 53 | 32.3 |
| RIC | 44 | 26.8 |
| Stem cell source | ||
| Peripheral blood stem cells | 44 | 26.8 |
| Bone marrow | 55 | 33.5 |
| Umbilical cord blood | 65 | 39.6 |
| Donor source | ||
| Related | 67 | 40.9 |
| Unrelated | 97 | 59.2 |
| HLA match | ||
| 6/6 HLA-matched sibling | 68 | 41.5 |
| 10/10 HLA-matched unrelated | 14 | 8.5 |
| 8–9/10 or 7/8 HLA-matched unrelated | 27 | 16.5 |
| 4–5/6 HLA-matched cord blood | 55 | 33.5 |
| Body mass index | ||
| Mean (SD) | 19.7 (5.11) | |
| Median | 18.3 | |
| Range | 13.4–47.3 | |
SD indicates standard deviation.
Survival Analyses
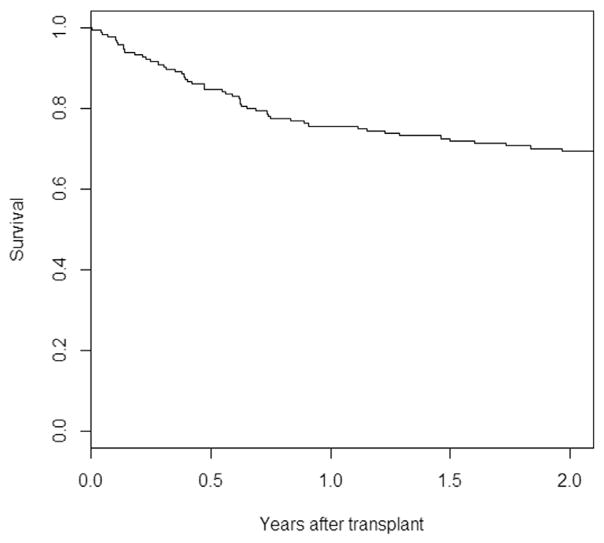
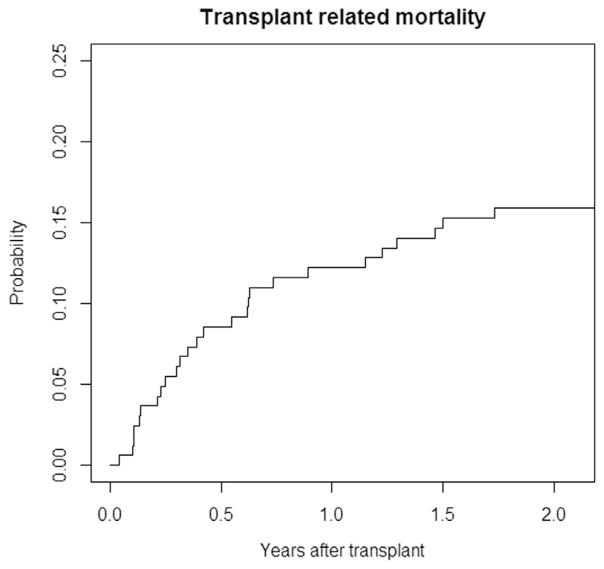
Mean follow-up was 5.16 ± 4.14 years. Rate of 2-year OS was 69.4% (95% confidence interval [CI], 62.6% to 76.8%) (Figure 1) and 2-year TRM was 15.9% (95% CI, .3% to 21.6%) (Figure 2).
Figure 1.
Rate of 2-year OS in the studied cohort was 69.4% (95% CI, 62.6% to 76.8%).
Figure 2.
Rate of 2-year TRM in the studied cohort was 15.9% (95% CI, .3% to 21.6%).
Risk Factors for Early Post-Transplant Toxicities
Conditioning regimens MAC versus RTC versus RIC
The relative incidence of grades III to IV metabolic and organ toxicity episodes in patients receiving MAC versus RTC versus RIC are shown in Table 2. A comparison of the toxicity rates between MAC and RTC revealed no difference in the 100-person daily incidence of grades III to IV toxicities between patients receiving these 2 types of conditioning regimens (13.88 versus 13.59, P =.812). In patients receiving RIC the total number of metabolic toxicity events was significantly lower than in patients receiving MAC regimens (4.09 versus 6.69, P = .001), as was the 100-person day incidence of organ toxicity events (5.08 versus 7.06, P =.013). Because analyses of the type and frequency of grades III to IV toxicities revealed no significant difference between MAC and RTC regimens, the 2 groups were subsequently combined into 1 group representing “myeloablative” regimens for further toxicity analyses. The incidence of early post-transplant toxicities was significantly lower in patients who received RIC than in those who received MAC (8.41 versus 13.75, P < .001).
Table 2.
Toxicity Events and Conditioning Regimens
| Grades III–IV Toxicity Episodes | Conditioning Regimen | P | Conditioning Regimen | P | ||
|---|---|---|---|---|---|---|
|
|
|
|||||
| MAC | RTC | MAC + RTC | RIC | |||
| Organ toxicities | 138 (6.87) | 116 (7.30) | .630 | 255 (7.06) | 67 (5.08) | .013 |
| Metabolic toxicities | 141 (7.01) | 100 (6.29) | .402 | 241 (6.69) | 54 (4.09) | .001 |
| Total toxicity episodes | 279 (13.88) | 216 (13.59) | .812 | 495 (13.75) | 111 (8.41) | <.001 |
Values are number of toxicity events, with 100-person day incidences in parentheses.
The relative incidence of grades III to IV metabolic and organ toxicity episodes was compared in patients receiving MAC versus RTC versus RIC. The incidence of hyperglycemia was higher in patients who received MAC than in those who received RIC (52% versus 30%, P = .022). Similarly, patients who received MAC had higher rates of hypokalemia (62% versus 36%, P = .005). There was no significant difference in the incidence of hyper- or hyponatremia, hyper- or hypocalcemia, or hyper- or hypomagnesemia among conditioning regimens.
The incidence of organ-related toxicities in the early post-transplant period was significantly higher in patients who received MAC. Compared with those who received RIC, these patients had higher rates of grades III to IV oral mucositis (33% versus 16%, P =.005) and abdominal pain (41.7% versus 18.2%, P = .006). Additionally, patients who received MAC had higher rates of hemorrhagic cystitis (9% versus 0%, P = .037) and intubation for respiratory failure (16% versus 0%, P = .022). There was no difference in the incidence of cardiac toxicities, neurologic events (seizure), or hepatotoxicity between RIC and MAC.
Patient- and transplant-related predictors of TRTs
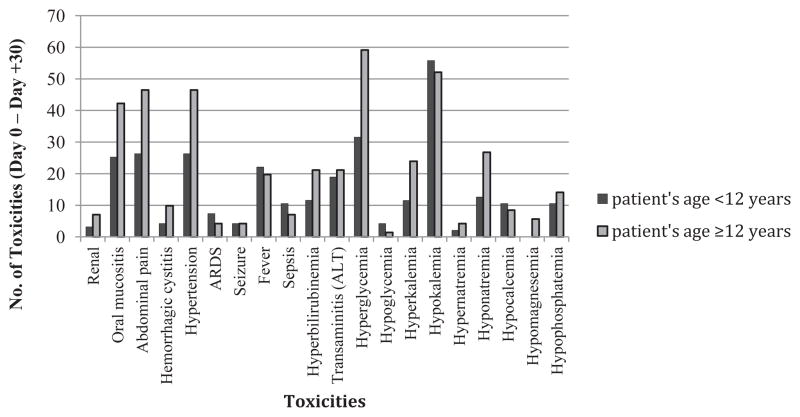
Univariate analyses of potential risk factors for early post-transplant TRTs are shown in Table 3. Multivariable analyses revealed that age ≥ 12 years (coefficient estimate, .276 [standard error, .138], P =.045), MAC versus RIC conditioning regimen (coefficient estimate, .474 [standard error, .132], P < .001), and unrelated donor source (coefficient estimate, .318 [standard error, .113], P = .005) were significant risk factors for having a higher number of grades III to IV toxicities between days 0 and +30. The relative incidence of biochemical and organ toxicities in patients older and younger than 12 years is shown in Figure 3. Low pretransplant performance status was not associated with higher risk for TRTs.
Table 3.
Univariate Analyses of Risk Factors Associated with Increased Risk of Grades III to IV Toxicities between Transplant Days 0 and +30
| Risk Factors | Poisson Regression | |
|---|---|---|
|
| ||
| Estimate (SE) | P | |
| Age | ||
| <12 yr | 0 | |
| ≥12 yr | .315 (.122) | .010 |
| Female | 0 | |
| Male | −.098 (.127) | .443 |
| Disease type | ||
| Nonmalignant | 0 | |
| Malignant | −.058 (.131) | .657 |
| Conditioning regimen | ||
| MAC | 0 | |
| RTC | −.022 (.143) | .880 |
| RIC | −.415 (.143) | .004 |
| Stem cell source | ||
| BM | 0 | |
| PBSC | −.023 (.154) | .882 |
| UCB | −.038 (.148) | .796 |
| Donor source | ||
| Related | −.289 (.118) | .014 |
| Unrelated | 0 | |
| HLA | ||
| Mismatch | 0 | |
| Match | 0 (.124) | 1 |
| CMV status (recipient) | ||
| Negative | 0 | |
| Positive | −.210 (.142) | .139 |
| BMI | .030 (.009) | .001 |
| Performance status | ||
| <90 | 0 | |
| ≥90 | −.280 (.172) | .103 |
SE indicates standard error; BM, bone marrow; PBSC, peripheral blood stem cells; UCB, umbilical cord blood; CMV, cytomegalovirus; BMI, body mass index.
Figure 3.
Patients who were 12 years and older had more frequent toxicity events than younger patients between transplant days 0 and +30. ARDS indicates acute respiratory distress syndrome; ALT, alanine transaminase.
Risk Factors for TRM
Univariate analysis demonstrated that the following patient- and treatment-related factors were significantly associated with increased risk of TRM: patient age ≥ 12 years (hazard ratio [HR], 2.263; 95% CI, 1.068 to 4.796; P = .033), cord blood source (HR, 3.758; 95% CI, 1.252 to 11.280; P =.018), unrelated donor (HR, 3.731; 95% CI, 1.423 to 9.783; P =.007), pretransplant performance status < 90 (HR, 2.605; 95% CI, 1.114 to 6.091; P =.027), and having ≥3 grades III to IV toxicities before day +30 (HR, 3.827; 95% CI, 1.459 to 10.038; P = .006). Multivariable models with the above factors significant at .05 revealed that having ≥3 grades III to IV toxicities in the first 30 days post-transplant was linked to a higher risk of TRM (HR, 3.297; 95% CI, 1.195 to 9.096; P = .021). Additionally, pretransplant performance status ≥ 90 was protective against TRM (HR, .394; 95% CI, .161 to .9; P = .042). Development of acute GVHD was not associated with an increased risk for TRM in our patient population (HR, 1.715; P = .149). When compared with RIC, MAC was not associated with an increased risk of TRM on multivariate analysis (HR, 2.44; P = .099) (Table 4).
Table 4.
Multivariable Analysis
| Parameter | HR | 95% CI | P |
|---|---|---|---|
| Age ≥ 12 | 1.886 | .715–4.975 | .200 |
| PBSC vs. BM | 1.126 | .308–4.113 | .858 |
| Cord vs. BM | 3.087 | .883–10.80 | .077 |
| Unrelated (vs. related) | 3.135 | .938–10.50 | .064 |
| Performance score ≥ 90 | .394 | .161–.966 | .042 |
| Grades III–IV toxicities ≥ 3 | 3.297 | 1.195–9.096 | .021 |
Risk factors for TRM: having ≥3 grades III–IV toxicities in the first 30 days post-transplant was a consistently statistically significant variable linked to a higher risk of TRM (HR, 3.297; 95% CI, 1.195–9.096; P = .021); pretransplant performance status ≥ 90 was protective against TRM (HR, .394; 95% CI, .161–.966; P = .0419).
Organ toxicities and TRM
For a given patient, the risk of TRM increased proportionally with increasing number of early post-transplant toxicities. Patients who had between 0 and 2 post-transplant organ toxicities (n = 115) had a 10.4% risk of TRM. Patients who had between 3 and 6 post-transplant organ toxicities (n = 44) had a 29.6% risk of TRM, and patients with >6 post-transplant organ toxicities (n = 5) had an 80% risk of TRM (P < .001).
Metabolic toxicities and TRM
Patients who had between 0 and 2 metabolic toxicities (n = 120) in the 30 days post-alloHCT had a 10% risk of TRM. Patients who had between 3 and 6 post-transplant metabolic toxicities (n = 42) had a 35.7% risk of TRM, and patients with >6 metabolic toxicities in the 30 days post-transplant (n = 2) had a 100% risk of TRM (P < .001).
DISCUSSION
This investigation identified 3 key findings. First, we identified that despite its name, RTC was not associated with a reduction in TRTs when compared with traditional MAC. Second, we identified that patient age ≥ 12 years and MAC regimens were associated with a higher incidence of TRTs in the early post-transplant period. Finally, we found that in pediatric and adolescent patients undergoing alloHCT, pretransplant performance status < 90 and/or having ≥3 post-transplant TRTs between days 0 and +30 were associated were higher risk of TRM.
Similar to prior studies in pediatric patients, we were unable to demonstrate a difference in incidence of grades III to IV toxicities between MAC and RTC regimens. One recent study published in 2015 by Ishida et al. [11] retrospectively compared the relative efficacy of alloHCT after MAC versus RTC in 136 pediatric patients with acute myeloid leukemia. The RTC regimen consisted of Flu + melphalan ± cytarabine or etoposide with low-dose TBI. The MAC regimen included TBI and/or Bu-based conditioning. Investigators found no significant difference in the rate of engraftment, early complications, or incidence of GVHD between the 2 groups [11]. Although we did not include engraftment rate in the analysis for this study, we did assess morbidity as a function of conditioning regimen and found no significant difference in the incidence of early post-transplant complications between the MAC and RTC cohorts.
Our analysis revealed that the risk of TRTs in the 30 days post-transplant was significantly lower in patients who received RIC regimens. In 2005, Shenoy et al. [12] reported the outcomes of 16 pediatric and adult patients with nonmalignant diseases undergoing alloHCT after RIC. The conditioning regimen used in this prospective study included alemtuzumab, Flu, and melphalan and was well tolerated overall with primarily infectious toxicities. Pulsipher et al. [13] reported the outcomes of a prospective investigation looking at 47 children with malignant diseases who underwent transplant after RIC (Bu/Flu/ATG). The aim of this study was to investigate whether RIC would be sufficient to allow for suitable engraftment in high-risk patients. The authors concluded that the use of RIC in pediatric patients unable to receive MAC resulted in low rates of TRM and high rates of engraftment [13].
Despite improvements in survival over the past decade, the outcomes of adolescent patients undergoing alloHCT remains inferior to the excellent survival rates achieved in the younger pediatric population [14]. Our analysis revealed that age ≥ 12 years was significantly associated with a higher risk of grades III to IV toxicities between days 0 and +30. Majhail et al. [15] reported results from a multicenter review comparing the outcomes of 900 children and 2708 adolescent and young adult patients with acute myeloid leukemia after transplant. Compared with children, adolescents had twice the relative risk of TRM (P < .01). Burke et al. [16,17] investigated the impact of adolescent age on post-transplant outcomes in patients with acute lymphoblastic leukemia. In these studies, patient age > 13 years was associated with a 2-fold increased risk TRM (P =.05). Medical literature suggests that adolescent patients have lower event-free survival and OS after alloHCT than their younger pediatric counterparts. These outcome differences may, in part, be related to higher rates of TRTs in the older age group [18,19]. In our study older age was a significant risk factor for ≥3 post-transplant toxicities; however, it was not significantly associated with TRM in multivariate models, which may in part be due to the small number of adolescent patients in our study cohort.
One of the most striking results of our study was the finding that having ≥3 grades III to IV toxicities in the 30 days post-transplantwas statistically linked to higher risk for TRM at 2 years. In 2002, Balduzzi et al. [7] published results of a prospective study in 636 pediatric patients undergoing alloHCT for acute leukemia in which they investigated the incidence and impact of early post-transplant toxicities. Investigators found that toxicity of any organ aside from the gastrointestinal tract or mucosa was positively correlated with increased early TRM. The findings of our retrospective investigation parallel those reported in this prospective study [20].
In their landmark publication, Sorror et al. [20] developed a hematopoietic cell transplantation comorbidity index (HCT-CI) for adult transplantation patients. This comorbidity index has been an effective tool used to risk stratify adult transplant patients to guide therapeutic decision-making. At present, 1 study has investigated the utility of the HCT-CI in the pediatric transplant population. In this retrospective study, Smith et al. [21] concluded that Sorror et al.’s 17-item pre-alloHCT adult comorbidity index was also an effective tool for risk-stratifying pediatric alloHCT patients. Our group is in the process of investigating the utility of the HCT-CI in the patient population described in this article. Although this study did not directly investigate the utility of the HCT-CI in our patient population, multivariable analyses did identify that pretransplant performance status is predictive of poorer outcomes, an observation that has been well-documented by Sorror et al. [22].
Low pretransplant performance status is a well-established risk factor for poorer outcomes in adults undergoing alloHCT. Sorror et al.’s HCT-CI included performance status as 1 of the pretransplant predictors in the comorbidity index. As part of their investigation into pretransplant comorbidities, Sorror et al. [20] identified that the Karnofsky performance status was an independent predictor of morbidity and mortality after alloHCT. Subsequent studies in adults have corroborated Sorror et al.’s findings by identifying that pretransplant performance status alone can be predictive of post-transplant outcomes [23].
This retrospective study is limited by a small sample size, which was not powered to compare the treatment efficacy or outcomes of RTC and MAC regimens. Prior studies have demonstrated that non-MAC is significantly associated with a lower risk of TRM; however, likely because of our sample size we were unable to corroborate this finding [24,25]. Additionally, small sample size and the complex course of alloHCT patients with multiple toxicities occurring over a short time period precluded us from directly linking specific TRTs with identifiable outcomes. A prospective study with well-defined a priori criterion might provide conclusive associations between TRTs and TRM. Small sample size may also have contributed to our finding that older adolescent age was associated with TRM on univariate but not on multivariate analysis. A unique strength of this study is that we deliberately omitted patients who received TBI, which is known to have significant TRTs. In so doing we were able to analyze a cohort of patients whose pretransplant conditioning was relatively homogeneous, despite their having a wide range of transplant indications. A small study sample likely precluded us from identifying the same risk factors for toxicities and TRM; however, our data collection is ongoing.
In summary, we identified no difference in the number of grades III to IV toxicities between MAC and RTC regimens and found that patients receiving RIC had significantly fewer early post-transplant toxicities than both MAC and RIC. Predictors of having ≥3 early post-transplant toxicities included age ≥ 12 years old, MAC, and unrelated donor source. This study also identified a significant correlation between the number of post-transplant toxicities and the risk of TRM in our pediatric and adolescent patient population. The finding that patients ≥ 12 years old experienced more post-transplant TRTs corroborates those of prior investigations, which identify adolescent age as a significant risk factor for poor outcomes after transplant. Significant predictors of TRM in our patient cohort were having ≥3 early post-transplant organ and/or metabolic toxicities and having a pretransplant performance status < 90.
To our knowledge this is the first study to report a direct correlation between the number of post-transplant toxicities and the likelihood of TRM in the pediatric and adolescent patient population. Further analyses are underway to assess whether identifiable, specific toxicity events in the early post-transplant period may have predisposed patients to a higher likelihood of TRM. The development of methods aimed at decreasing the incidence of serious toxicities and TRM in the pediatric and adolescent alloHCT population is imperative, and prospective studies to confirm our findings are warranted.
Acknowledgments
The authors thank all patients and families who were included in this study as well as all faculty and staff in the division of Pediatric Hematology, Oncology and Stem Cell Transplantation at Columbia University Medical Center. The authors also give special thanks to Dr. John Horan.
Financial disclosure: J.M.K. is supported in part by a fellowship from the National Cancer Institute (R25 CA094061).
Footnotes
Conflict of interest statement: There are conflicts of interest to report.
Authorship statement: N.A.M. and J.M.K. are co-first authors. All authors contributed equally to the completion of this work, and all are in agreement with the content as it is presented in this manuscript.
References
- 1.Horan JT, Logan BR, Agovi-Johnson MA, et al. Reducing the risk for transplantation-related mortality after allogeneic hematopoietic cell transplantation: how much progress has been made? J Clin Oncol. 2011;29:805–813. doi: 10.1200/JCO.2010.32.5001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bacigalupo A, Oneto R, Bruno B, et al. Early predictors of transplant-related mortality (TRM) after allogeneic bone marrow transplants (BMT): blood urea nitrogen (BUN) and bilirubin. Bone Marrow Transplant. 1999;24:653–659. doi: 10.1038/sj.bmt.1701953. [DOI] [PubMed] [Google Scholar]
- 3.Sorror ML, Maris MB, Storb R, et al. Hematopoietic cell transplantation (HCT)-specific comorbidity index: a new tool for risk assessment before allogeneic HCT. Blood. 2005;106:2912–2919. doi: 10.1182/blood-2005-05-2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ringden O, Erkers T, Aschan J, et al. A prospective randomized toxicity study to compare reduced-intensity and myeloablative conditioning in patients with myeloid leukaemia undergoing allogeneic haematopoietic stem cell transplantation. J Intern Med. 2013;274:153–162. doi: 10.1111/joim.12056. [DOI] [PubMed] [Google Scholar]
- 5.Bredeson CN, Zhang MJ, Agovi MA, et al. Outcomes following HSCT using fludarabine, busulfan, and thymoglobulin: a matched comparison to allogeneic transplants conditioned with busulfan and cyclophosphamide. Biol Blood Marrow Transplant. 2008;14:993–1003. doi: 10.1016/j.bbmt.2008.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gooley TA, Chien JW, Pergam SA, et al. Reduced mortality after allogeneic hematopoietic-cell transplantation. N Engl J Med. 2010;363:2091–2101. doi: 10.1056/NEJMoa1004383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Balduzzi A, Valsecchi MG, Silvestri D, et al. Transplant-related toxicity and mortality: an AIEOP prospective study in 636 pediatric patients transplanted for acute leukemia. Bone Marrow Transplant. 2002;29:93–100. doi: 10.1038/sj.bmt.1703337. [DOI] [PubMed] [Google Scholar]
- 8.National Cancer Institute. Common terminology criteria for adverse events (CTCAE) Bethesda, MD: U.S. Dept. of Health and Human Services, National Institutes of Health, National Cancer Institute; 2009. [Google Scholar]
- 9.Satwani P, Cooper N, Rao K, et al. Reduced intensity conditioning and allogeneic stem cell transplantation in childhood malignant and nonmalignant diseases. Bone Marrow Transplant. 2008;41:173–182. doi: 10.1038/sj.bmt.1705923. [DOI] [PubMed] [Google Scholar]
- 10.Johnson MJ, Bland JM, Davidson PM, et al. The relationship between two performance scales: New York Heart Association Classification and Karnofsky Performance Status Scale. J Pain Sympt Manage. 2014;47:652–658. doi: 10.1016/j.jpainsymman.2013.05.006. [DOI] [PubMed] [Google Scholar]
- 11.Ishida H, Adachi S, Hasegawa D, et al. Comparison of a fludarabine and melphalan combination-based reduced toxicity conditioning with myeloablative conditioning by radiation and/or busulfan in acute myeloid leukemia in Japanese children and adolescents. Pediatr Blood Cancer. 2015;62:883–889. doi: 10.1002/pbc.25389. [DOI] [PubMed] [Google Scholar]
- 12.Shenoy S, Grossman WJ, DiPersio J, et al. A novel reduced-intensity stem cell transplant regimen for nonmalignant disorders. Bone Marrow Transplant. 2005;35:345–352. doi: 10.1038/sj.bmt.1704795. [DOI] [PubMed] [Google Scholar]
- 13.Pulsipher MA, Boucher KM, Wall D, et al. Reduced-intensity allogeneic transplantation in pediatric patients ineligible for myeloablative therapy: results of the Pediatric Blood and Marrow Transplant Consortium Study ONC0313. Blood. 2009;114:1429–1436. doi: 10.1182/blood-2009-01-196303. [DOI] [PubMed] [Google Scholar]
- 14.Mertens AC, Yong J, Dietz AC, et al. Conditional survival in pediatric malignancies: analysis of data from the Childhood Cancer Survivor Study and the Surveillance, Epidemiology, and End Results Program. Cancer. 2015;121:1108–1117. doi: 10.1002/cncr.29170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Majhail NS, Brazauskas R, Hassebroek A, et al. Outcomes of allogeneic hematopoietic cell transplantation for adolescent and young adults compared with children and older adults with acute myeloid leukemia. Biol Blood Marrow Transplant. 2012;18:861–873. doi: 10.1016/j.bbmt.2011.10.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Burke MJ, Lindgen B, Verneris MR. Treatment of relapsed acute lymphoblastic leukemia: approaches used by pediatric oncologists and bone marrow transplant physicians. Pediatr Blood Cancer. 2012;58:840–845. doi: 10.1002/pbc.23269. [DOI] [PubMed] [Google Scholar]
- 17.Burke MJ, Gossai N, Cao Q, et al. Similar outcomes between adolescent/young adults and children with AML following allogeneic hematopoietic cell transplantation. Bone Marrow Transplant. 2014;49:174–178. doi: 10.1038/bmt.2013.171. [DOI] [PubMed] [Google Scholar]
- 18.Canner J, Alonzo TA, Franklin J, et al. Differences in outcomes of newly diagnosed acute myeloid leukemia for adolescent/young adult and younger patients: a report from the Children’s Oncology Group. Cancer. 2013;119:4162–4169. doi: 10.1002/cncr.28342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wongso D, Fuchs M, Plutschow A, et al. Treatment-related mortality in patients with advanced-stage Hodgkin lymphoma: an analysis of the German Hodgkin study group. J Clin Oncol. 2013;31:2819–2824. doi: 10.1200/JCO.2012.47.9774. [DOI] [PubMed] [Google Scholar]
- 20.Sorror M, Storer B, Sandmaier BM, et al. Hematopoietic cell transplantation-comorbidity index and Karnofsky performance status are independent predictors of morbidity and mortality after allogeneic nonmyeloablative hematopoietic cell transplantation. Cancer. 2008;112:1992–2001. doi: 10.1002/cncr.23375. [DOI] [PubMed] [Google Scholar]
- 21.Smith AR, Majhail NS, MacMillan ML, et al. Hematopoietic cell transplantation comorbidity index predicts transplantation outcomes in pediatric patients. Blood. 2011;117:2728–2734. doi: 10.1182/blood-2010-08-303263. [DOI] [PubMed] [Google Scholar]
- 22.Kahn J. High hematopoietic transplantation comorbidity index is not associated with increased transplant related mortality: review of a large cohort of pediatric patients undergoing allogeneic stem cell transplantation following busulfan-based regimens. Biol Blood Marrow Transpl. 2015;21:S98–S99. [Google Scholar]
- 23.Sorror ML, Logan BR, Zhu X, et al. Prospective validation of the predictive power of the hematopoietic cell transplantation comorbidity index: a Center for International Blood and Marrow Transplant Research Study. Biol Blood Marrow Transplant. 2015;21:1479–1487. doi: 10.1016/j.bbmt.2015.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Artz AS, Pollyea DA, Kocherginsky M, et al. Performance status and comorbidity predict transplant-related mortality after allogeneic hematopoietic cell transplantation. Biol Blood Marrow Transplant. 2006;12:954–964. doi: 10.1016/j.bbmt.2006.05.015. [DOI] [PubMed] [Google Scholar]
- 25.Diaconescu R, Flowers CR, Storer B, et al. Morbidity and mortality with nonmyeloablative compared with myeloablative conditioning before hematopoietic cell transplantation from HLA-matched related donors. Blood. 2004;104:1550–1558. doi: 10.1182/blood-2004-03-0804. [DOI] [PubMed] [Google Scholar]