Abstract
Myocardial infarction (MI) is an acute event characterized by myocardial necrosis. Thrombotic MI is caused by spontaneous atherosclerotic plaque disruption that results in a coronary thrombus; Non-thrombotic MI occurs secondary to oxygen supply-demand mismatch. We sought to characterize the differential metabolic perturbations associated with these subtypes utilizing a systems approach. Subjects presenting with thrombotic MI, non-thrombotic MI and stable coronary artery disease (CAD) were included. Whole blood was collected at two acute time-points and at a time-point representing the quiescent stable disease state. Plasma metabolites were analyzed by untargeted UPLC-MS/MS and GC-MS. A weighted network was constructed, and modules were determined from the resulting topology. To determine perturbed modules, an enrichment analysis for metabolites that demonstrated between-group differences in temporal change across the disease state transition was then conducted.
Biological Significance
We report evidence of metabolic perturbations of acute MI and determine perturbations specific to thrombotic MI. Specifically, a module characterized by elevated glucocorticoid steroid metabolites following acute MI showed greatest perturbation following thrombotic MI. Modules characterized by elevated pregnenolone metabolites, monoacylglycerols, and acylcarnitines were perturbed following acute MI. A module characterized by a decrease in plasma amino acids following thrombotic MI was differentially perturbed between MI subtypes.
Keywords: Metabolomics, Myocardial Infarction, Coronary Artery Disease, Systems Biology, Network Analysis
Subject terms: Metabolomics, Myocardial Infarction, Coronary Artery Disease, Systems Biology, Network Analysis
1. Introduction
Heart disease is the most prevalent cause of global mortality1. While heart disease is a ubiquitous disease with respect to prevalence, there is significant heterogeneity in outcomes2–4 and in the incidence and presentation of acute events such as acute myocardial infarction (AMI)5. Given this heterogeneity, elucidating the metabolic perturbations associated with the transition from a stable disease state such as stable coronary artery disease (sCAD), to an acute event such as AMI is of critical importance. While myocardial necrosis is a pathological characteristic common to all acute myocardial infarctions, there are multiple proximate causes of AMI6. A classification system based on etiology has been developed and includes 6 types6. Of interest is differentiating AMI caused by spontaneous atherosclerotic plaque disruption that results in a coronary thrombus (thrombotic MI), versus AMI caused by a deficit in oxygen supply secondary to other non-thrombotic causes such as vasospasm or stress cardiomyopathy. This distinction is important as the course of treatment differs between types and misclassification may result in negative outcomes such as iatrogenic bleeding7.
The pathophysiology of thrombotic MI can be conceptualized as a “perfect storm” in which a vulnerable atherosclerotic plaque ruptures or is disrupted in the presence of thrombogenic blood8. While plaque rupture or disruption is a prerequisite for coronary thrombosis, it is not sufficient. The insufficiency has been demonstrated by autopsy study of cases of sudden cardiac death in which evidence of a healing thrombus (as opposed to pathological) was found in 69% of cases9. This characterization of thrombotic MI suggests that a biological processes or metabolic response distinct from a plaque rupture or disruption may provide sufficient amplification for pathological thrombosis. Elucidation of such amplifying factors in thrombotic MI includes identifying metabolites that are differentially abundant at the time of thrombotic MI compared to a quiescent stable disease state. To demonstrate specificity for thrombotic MI, these factors must not be differentially abundant secondary to downstream ischemia/necrosis or medical and pharmacological interventions that individuals undergoing MI receive. Furthermore, to the authors knowledge, an examination of the metabolic perturbations following AMI differentiated by etiological subtype (thrombotic versus non-thrombotic) has not yet been conducted. We have thus executed such a study evaluating the plasma metabolome of subjects experiencing AMI differentiated by subtype at the acute event state and the quiescent stable disease state.
Metabolic phenotyping is well suited for studying disease state transitions as changes in metabolite concentrations are dependent on genetic factors, environmental influences, and gene-environment interactions10. Since blood plasma functions as a liquid carrier, plasma contains enzymes, lipoproteins, hormones, nutrients, metabolic waste products, and many other small molecules dissolved or in suspension11 it has the advantage of being a repository of metabolic changes from all tissues and therefore reflective of the state of the entire organism at the time of sampling. However, attributing the root cause of changes in metabolite concentration in plasma or serum across state transitions is not straightforward. Developing a mechanistic reconstruction of metabolic pathway impacts is complicated by the following: (1) plasma contains intermediates and products of multiple metabolic pathways, (2) the tissue source of metabolites in plasma may not be known, and (3) curated metabolic pathway models may not generalize to plasma when a preponderance of the model is localized to unobserved cellular compartments such as mitochondria. Consequently, a data-dependent network reconstruction of the plasma metabolome for determining related sets of metabolites was conducted. This is consistent with the function of metabolites as substrates, products, and regulatory factors within discrete biological processes.
In this study, we recruited three patient groups: (1) subjects presenting with acute thrombotic MI, (2) subjects presenting with acute non-thrombotic MI, and (3) subjects presenting with stable CAD undergoing cardiac catheterization. Plasma metabolites were extracted from whole blood collected from each subject during both the acute/procedural phase and at a follow-up time-point (approximately 3 months later) regarded as a subject’s quiescent stable disease phase. We used a data-dependent strategy to identify “modules” of related metabolites based on network topology. The methodology used for discovering metabolite modules belongs to a class of techniques known as “weighted network analysis”12 and was originally developed as a framework for analyzing gene co-expression networks13. After module discovery, we then sought to evaluate whether any of the modules were significantly associated with the transition from the stable disease state to the acute disease state and demonstrated phenotype specificity. We report multiple modules enriched for related metabolites that distinguish acute MI from stable CAD, a module enriched for metabolites differing between thrombotic MI and non-thrombotic MI, and a module enriched for differences across all three groups.
2. Methods
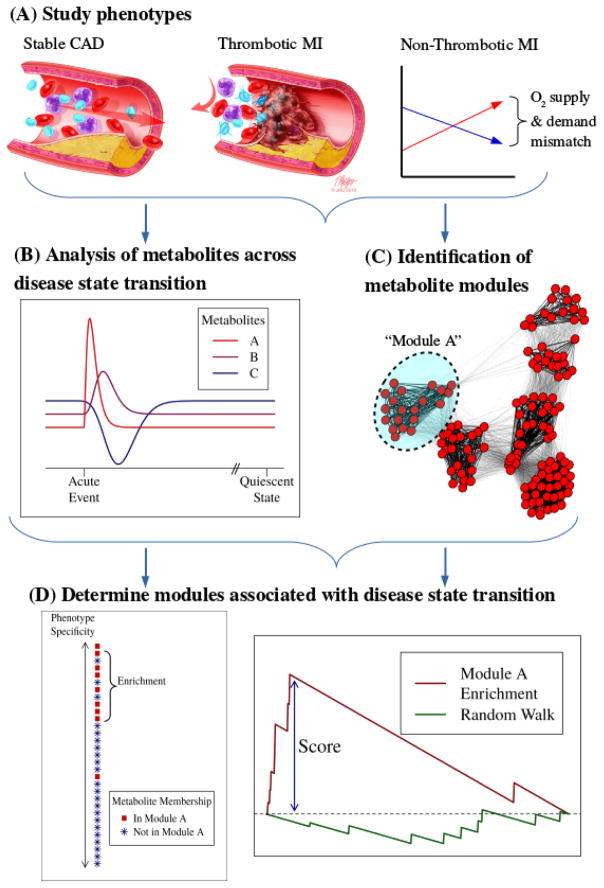
A graphical overview of the analytical approach utilized in this study is presented in the graphical abstract (Figure 1).
Figure 1.
Graphical Abstract: (A) Study phenotypes. Thrombotic MI subjects presented with a pathological thrombus that occluded blood flow in a coronary artery. Non-thrombotic MI subjects presented with myocardial infarction secondary to other causes—all involving an oxygen supply deficit. Stable coronary artery disease subjects were included to ensure that constitutive atherosclerotic disease factors were controlled for in examining thrombotic MI. (B) Metabolite abundances were evaluated across time, with sampling in the acute phase compared to a follow-up time-point representing the quiescent stable disease state approximately 3 months following MI. (C) Modules of related metabolites were determined by constructing a weighted network from the plasma metabolome and interrogating the network for clusters. (D) Modules were evaluated for enrichment of metabolites that demonstrated differential change across time (change that differed between study groups). Light blue arrows indicate chronology of analytical approach.
2.1. Study cohort
A patient cohort was recruited to allow for determining the unique plasma metabolomic signature of AMI differentiated by proximate cause14. A novel criteria was developed for discriminating between thrombotic and non-thrombotic MI patients as widely accepted guidelines for such discrimination were unavailable. This criteria was sufficiently stringent to minimize misclassification through the elimination of borderline/non-definitive cases. In addition to enrolling AMI patients, subjects with stable coronary artery disease presenting for an elective procedure requiring cardiac catheterization were enrolled. As thrombotic MI is defined with respect to a present characteristic (thrombosis) while non-thrombotic MI is defined by an absence of a characteristic, our cohort was suitable for demonstrating specificity for MI secondary to thrombosis with proper control. Specifically, the non-thrombotic MI group was utilized to control for the presence of metabolic changes associated with ischemia and myocardial necrosis. The stable CAD group was used to control for the presence of underlying constitutive atherosclerotic disease factors that would be present in thrombotic MI subjects but may not be present in non-thrombotic MI subjects. Both groups were utilized to control for metabolic changes associated with cardiac catheterization. Participants were recruited from two hospitals following approval by the University of Louisville Institutional Review Board. Participants were provided with written informed consent and the study was conducted in accordance with the ethical standards defined in the 1964 Helsinki declaration. A total of 11 thrombotic MI, 12 non-thrombotic MI, and 15 stable CAD subjects were eligible and enrolled in the study. Further details on the human subject cohort and enrollment criterion are provided in the Supplement (Supplementary Table 1).
2.2. Plasma metabolomics
Whole blood was collected from study subjects immediately prior to cardiac catheterization (denoted T0) and 6-hours post catheterization (denoted T6). These samples represented acute phase time-points for MI subjects. To represent a subject’s quiescent phase, whole blood was again collected at a follow-up time-point approximately 3 months after the cardiac catheterization procedure. Plasma samples were then prepared for identification and quantification of metabolite relative abundances by Metabolon, Inc (Research Triangle Park, NC). The metabolite extraction process was conducted using the Microlab STAR® system, an automated liquid handling workstation (Hamilton Company, Reno, NV). After adding a recovery standard, methanol was added to precipitate proteins and vigorous shaking was applied using a GenoGrinder 2000 (Glen Mills, Metuchen, NJ). The resulting extract containing metabolites was divided into five aliquots—one each for positive and negative ion mode ultra-performance liquid chromatography-tandem mass spectrometry (UPLC-MS/MS) with electrospray ionization, one for polar metabolite quantification (UPLC-MS/MS with negative ion mode electrospray ionization), and one for gas chromatography-mass spectrometry (GC-MS) analysis. UPLC-MS/MS analysis was conducted using a Waters ACQUITY UPLC (Milford, MA) and a Thermo Scientific (Waltham, MA) Q-Exactive high resolution/accurate mass spectrometer interfaced with a heated electrospray ionization (HESI-II) source and Orbitrap mass analyzer operated with a scanning range from 80–1000 m/z and 35,000 mass resolution. Separate Waters UPLC BEH C18 (2.1×100mm, 1.7 μm) columns were used for positive and negative ion optimized conditions using water and methanol containing 0.1% formic acid (positive ion optimization) or water and acetonitrile with 10mM ammonium formate (negative ion optimization). The aliquots for GC-MS analysis were dried under vacuum for a minimum of 18 hours and derivatized under dried nitrogen using bistrimethyl-silyltrifluoroacetamide. Each aliquot was then separated on a 5% diphenyl/95% dimethyl polysiloxane fused silica column (20 m × 0.18 mm ID; 0.18 um film thickness) with helium as the carrier gas and a temperature ramp from 60° to 340°C in a 17.5 min period. GC-MS analysis was then conducted utilizing a Thermo-Finnigan Trace DSQ fast-scanning single-quadrupole mass spectrometer with electron impact ionization. The scan range was from 50–750 m/z and had unit mass resolving power. Molecular identification was performed by matching on retention index, mass to charge ratio, and spectral data from Metabolon’s library of known standards and unknown molecules that were detected in other projects. Unless specified as “tentative identification” all metabolites were identified as Metabolomics Standards Initiative (MSI) level 1 or MSI level 2 (denoted with a *). After identification of peaks, molecular abundances were quantified using area-under-the-curve. If the molecular abundance of a biochemical could not be quantified for a sample, the minimum value of the remaining samples was imputed. Raw biochemical data was then scaled by dividing each abundance by the median value and log-transformed. The acute phase was represented by the average of T0 and T6 abundances to increase the stability of estimates and to reduce the effects of differential time from event onset (unknown) to catheterization.
2.3. Statistical analysis
2.3.1. Cohort characteristics
Cohort characteristics were summarized by study group. Relevant summary statistics (mean, standard deviation, median, and interquartile range) were produced for summarizing continuous characteristics by study group. For approximately normally distributed continuous characteristics, one-way analysis of variance (ANOVA) F -test p-values are reported for comparing distributions across study groups. For continuous characteristics that were not approximately normally distributed, Kruskal-Wallis test p-values are reported. Frequencies, percentages, and Fisher’s exact test p-values are reported for comparing categorical characteristics between study groups.
2.3.2. Weighted network analysis of plasma metabolites
To define groups of functionally related metabolites, henceforth referred to as modules, a weighted network analysis was conducted12. While directly observing the topology of a metabolic pathway in circulation is often not feasible (e.g. pathways that are localized to cellular compartments), a weighted network approach allows for discovering modules of metabolites that exhibit significant topological overlap and are thus more likely to be related via a common biological process or related via disease-associated regulation such as the response to ischemia. An imprecise conceptual definition of topological overlap is the degree to which two metabolites share a common set of neighbor metabolites with respect to a network constructed from adjacencies. Adjacency was determined by examining the strength of the abundance correlation between pairs of metabolites. A weighted network was constructed using the “Weighted Gene Co-Expression Network Analysis” (WGCNA) methodology pioneered by Zhang and Horvath13. While this methodology was originally formulated for examining gene co-expression networks, the methodology is sufficiently general for examining metabolite co-abundance. Our application of this methodology consisted of the following steps: (1) generating a scale-free weighted network from the acute-phase metabolite abundances averaged within-subject, (2) detection of modules from the weighted network using they Dynamic Hybrid Cutting algorithm15, (3) determination of univariate statistical significance for each metabolite—the change from the quiescent stable disease state to acute event was compared between groups, (4) determination of which metabolite modules were enriched for disease state-associated metabolite changes using the univariate significance determined in the previous step. For determining statistical significance, the Gene Set Enrichment Analysis algorithm (GSEA)16, 17 was modified to accommodate the test statistic employed. The GSEA algorithm returns a weighted Kolmogorov–Smirnov-like statistic that can be used to determine if a gene or metabolite list ranked by statistical significance is differentially abundant for phenotype correlations based on list position17. We have modified the algorithm for our task of determining significance-weighted enrichment of pairwise comparisons in metabolite abundances across time. As three statistical tests were conducted for each module, we report q-values for protecting the False Discovery Rate (FDR) rather than unadjusted p-values18. Further details of the construction of the weighted network, module discovery procedure, and determination of statistical significance are provided in the supplement.
3. Results
Descriptive statistics for the patient subject cohort are presented in Table 1. The prevalence of diabetes differed between study groups (p = 0.03) with 40.0% in the stable CAD group, 0.0% in the non-thrombotic MI group, and 18.2% in the thrombotic MI group. Likewise, the prevalence of dyslipidemia was significantly different between study groups (p = 0.003 with 86.7% in the stable CAD group, 33.3% in the non-thrombotic MI group, and 54.5% in the thrombotic MI group. History of atherosclerosis (MI, CAD, percutaneous coronary intervention [PCI], and coronary artery bypass graft [CABG]), baseline troponin, peak troponin, at least one vessel with >50% coronary stenosis indicator, and ST elevation on an electrocardiogram (EKG) at baseline each differed significantly between study groups; each of these characteristics were used to define study group phenotypes and therefore differences were an artifact of study group inclusion criteria. Baseline glucose and heart rate differed between study groups (p = 0.03 and p = 0.01, respectively). Finally, the proportion of subjects who underwent PCI at the time of enrollment differed between study groups (p < 0.0001) with 13.3% of the stable CAD subjects undergoing PCI compared to 0.0% of non-thrombotic MI subjects, and 100.0% of the thrombotic MI subjects.
Table 1.
Cohort Characteristics
| Variable | Thrombotic MI (N=11) | Non-Thrombotic MI (N=12) | Stable CAD (N=15) | p-value |
|---|---|---|---|---|
| Age (mean ± SD) yrs | 60.0 ± 17.7 | 56.3 ± 16.6 | 61.3 ± 8.9 | 0.66 |
| Males (%) | 63.6 | 41.7 | 53.3 | 0.17 |
| Caucasian Race (%) | 90.9 | 66.7 | 93.3 | 0.60 |
| Current Smoker (%) | 36.4 | 50.0 | 20.0 | 0.66 |
| History of Dyslipidemia (%) | 54.5 | 33.3 | 86.7 | 0.003 |
| History of Diabetes Mellitus (%) | 18.2 | 0.0 | 40.0 | 0.03 |
| History of Hypertension (%) | 63.6 | 75.0 | 93.3 | 0.35 |
| History of Atherosclerosis (%) (MI, CAD, PCI, CABG) | 27.3 | 33.3 | 100.0 | <0.0001 |
| History of Congestive Heart Failure (%) | 0.0 | 8.3 | 6.7 | 1.00 |
| History of Chronic Renal Failure (%) | 0.0 | 8.3 | 0.0 | 0.66 |
| History of Stroke (%) | 0.0 | 25.0 | 0.0 | 0.05 |
| HR at time of presentation (mean ± SD) | 80.6 ± 10.1 | 88.3 ± 27.5 | 65.9 ± 9.6 | 0.01 |
| SBP at time of presentation (mean ± SD) | 138.1 ± 30.6 | 124.0 ± 31.9 | 133.4 ± 16.5 | 0.43 |
| DBP at time of presentation (mean ± SD) | 84.2 ± 20.3 | 81.2 ± 25.1 | 70.3 ± 15.3 | 0.19 |
| MAP at time of presentation (mean ± SD) | 102.2 ± 22.7 | 95.4 ± 26.9 | 91.4 ± 14.3 | 0.45 |
| BMI at time of presentation (mean ± SD) | 29.3 ± 7.2 | 27.8 ± 6.7 | 33.0 ± 7.1 | 0.15 |
| Time (hours) from presentation to T0 (median ± IQR) | 1.4 ± 1.4 | 17.6 ± 9.8 | NA | <0.0001 |
| Time (hours) symptoms to T0 (median ± IQR) | 8.4 ± 26.1 | 20.2 ± 17.6 | NA | 0.19 |
| Baseline Troponin (median ± IQR) | 0.48 ± 5.64 | 1.64 ± 2.23 | 0.01 ± 0.00 | <0.0001 |
| Peak Troponin (median ± IQR) | 46.57 ± 71.00 | 2.06 ± 4.14 | 0.01 ± 0.00 | <0.0001 |
| Glucose at Baseline (median ± IQR) | 143.0 ± 71.0 | 103.5 ± 32.5 | 115.0 ± 44.0 | 0.03 |
| Creatinine at Baseline (median ± IQR) | 0.90 ± 0.42 | 0.80 ± 0.65 | 0.90 ± 0.24 | 0.68 |
| Platelets at Baseline (mean ± SD) | 189.4 ± 80.1 | 217.6 ± 64.9 | 236.4 ± 54.5 | 0.21 |
| ST Elevation on EKG at Baseline | 90.9 | 25.0 | 0.0 | <0.0001 |
| At least one vessel with >50% Coronary Stenosis on Enrollment Angiogram | 100.0 | 25.0 | 66.7 | 0.0004 |
| PCI at time of Enrollment | 100.0 | 0.0 | 13.3 | <0.0001 |
| Aspirin use at time of Enrollment (%) | 100.0 | 91.7 | 86.7 | 0.77 |
| P2Y12 Inhibitors use at Enrollment (%) | 63.6 | 50.0 | 60.0 | 0.84 |
Abbreviations used in table
MI: Myocardial Infarction; CAD: Coronary Artery Disease; PCI: percutaneous coronary intervention;
CABG: coronary artery bypass graft; HR: heart rate; SBP: systolic blood pressure;
DBP: diastolic blood pressure; MAP: mean arterial pressure; BMI: body mass index;
IQR: Inter-quartile range; EKG: electrocardiogram
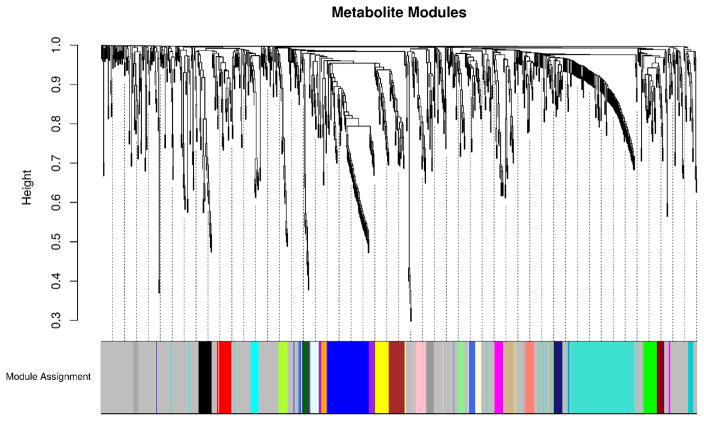
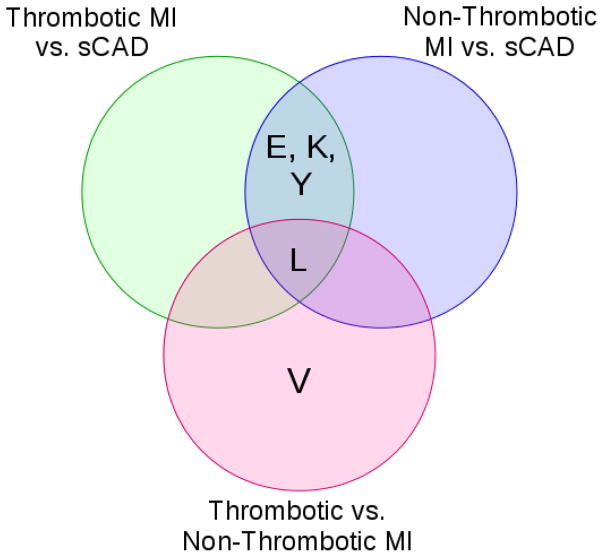
From the 1,032 metabolites quantified, 25 unique modules covering 504 plasma metabolites were detected from the weighted plasma metabolome network. The results of the module discovery process can be visualized in Figure 2. This dendrogram shows the degree of co-localization of metabolites within the weighted network and the propensity of metabolites in a cluster to have overlapping sets of neighbors. Clusters determined from analysis of the dendrogram by the Dynamic Hybrid Tree Cutting algorithm are shown as colored bands. The significance of each of these identified metabolite modules was determined by quantifying the enrichment of each module for quiescent to acute metabolite abundance changes that differed between study groups using a GSEA procedure. As an example, a module that was enriched for quiescent to acute abundance changes that differed between thrombotic and non-thrombotic MI subjects would indicate that this module contained significantly more metabolites that were differentially perturbed between MI subtypes than would be expected by chance alone. Supplemental Figure 1 shows the maximum enrichment score attained by each of the metabolite modules in the GSEA procedure for determining statistical significance. Of these modules, 5 modules comprised of 115 plasma metabolites differed significantly (q < 0.05 between two or more groups. These modules are characterized by biochemical composition in Table 2. As can be seen in Figure 3 (Venn diagram), 4/5 modules were enriched for differences between MI (thrombotic or non-thrombotic) and stable CAD, while 1 module was enriched for differences between all three groups, and 1 module was enriched for differences between thrombotic and non-thrombotic MI. Graphical summaries for each of these significant modules are presented as Supplemental Figure 2. These figures show the co-location and degree of shared neighbors (topological overlap) between the metabolites by a dendrogram while a heatmap shows the average metabolite abundances across time (from quiescent to acute) and by study group.
Figure 2. Metabolite module discovery.
Hierarchical clustering dendrogram of the 1,032 metabolites quantified by untargeted mass spectrometry. After constructing a weighted network, 25 modules were identified by the Dynamic Hybrid Tree Cutting Algorithm. Lower join heights indicate that metabolites in a cluster were co-located within the network and had overlapping sets of neighbors. 504 Individual metabolites were uniquely assigned to one module (shown as a colored panel) or remained unassigned (colored in light grey).
Table 2. Module enrichment analysis.
Significantly enriched modules are presented with the comparisons that were significantly enriched and characterized by the metabolites they contain.
| Module | Significant Comparison | Characterized by |
|---|---|---|
| E | MI (both) vs. sCAD | 1- & 2- Monoacylgylcerols |
| K | MI (both) vs. sCAD | Pregnenolone metabolites: Pregnenolone sulfate, Pregnanediol, Pregnanediol-3-glucuronide, 21-Hydroxypregnenolone disulfate |
| L | All | Cortisol, Corticosterone, C21-steroid metabolites |
| V | Thrombotic MI vs. Non-Thrombotic MI | Amino Acids, Gamma-glutamyl Amino Acids |
| Y | MI (both) vs. sCAD | Primarily un-identified compounds. Identified: Acetylcarnitine, Hydroxybutyrlcarnitine, 3-Methylglutarylcarnitine, Ethylmalonic acid, 3-Methyladipic acid |
Figure 3.
Venn diagram of modules with significant enrichment.
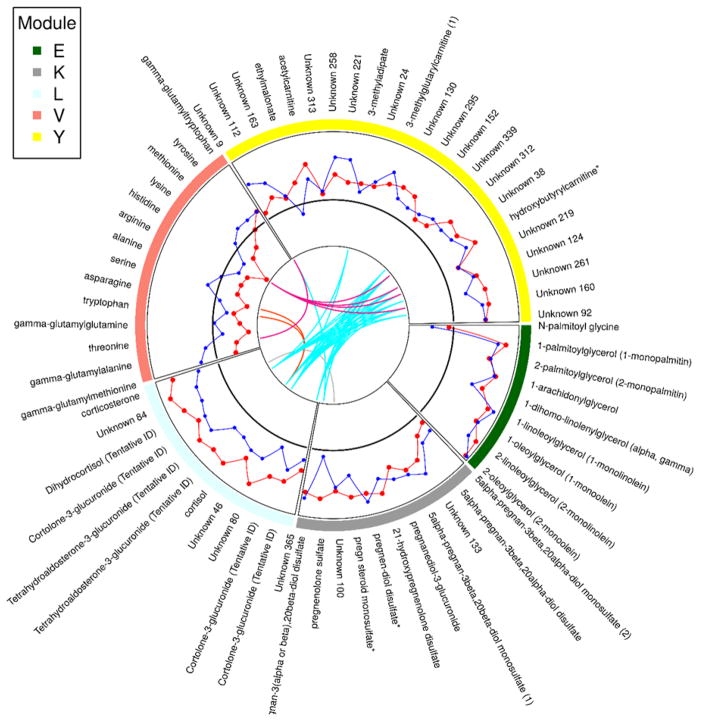
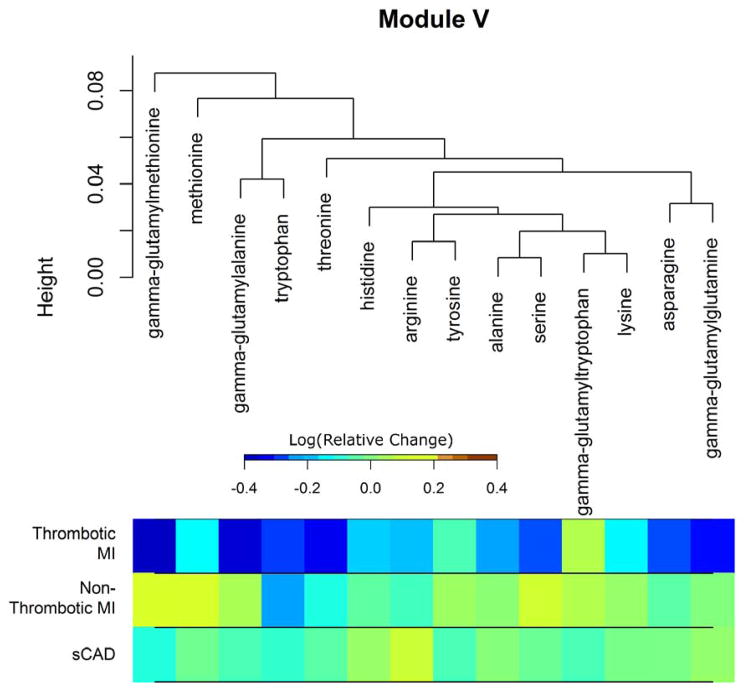
Figure 4 presents all the plasma metabolites that were members of significantly enriched modules as a circos plot. In this plot, the abundance change from the quiescent to acute state in stable CAD is provided as a reference line. The abundance changes across time are then presented for the acute MI groups in comparison. Pairwise topological overlap across modules is presented after thresholding. This indicates that some metabolites, notably those in the module characterized by glucocorticoids and C21 steroid metabolites, exhibit connections across modules. Module E, which differed between both acute MI groups and the stable CAD group, consisted of eight 1-monoacylglycerols and 2-monoacylgylcerols in addition to N-palmitoyl glycine. Each of the metabolites of Module E, with the exception of N-palmitoyl glycine, exhibited increased metabolite abundance following MI compared to the quiescent stable disease state. Specifically, these metabolites had acute to quiescent abundance ratios greater than 1 in the acute MI groups. Module K consisted of 9 pregnenelone metabolites and two unknowns. The metabolites of Module K generally exhibited increased metabolite relative abundances in the acute phase in acute MI but not stable CAD subjects. Pregnenolone sulfate was a singular exception which did not have an abundance ratio greater than 1 in the non-thrombotic MI group. Module Y, which also differed between both acute MI groups and the stable CAD group primarily consisted of unknown metabolites (18). The known metabolites consisted of ethylmalonate, 3-methyladipate, 3-methylglutarylcarnitine, acetylcarnitine, and hydroxybutyrylcarnitine. Module V was significantly enriched for differences between the acute MI groups. This module consisted of 10 amino acids and four gamma-glutamyl amino acids. Each of the amino acids/dipeptides in Module V demonstrated decreased abundance following MI in the thrombotic MI group, except for gamma-glutamyltryptophan. This decrease in plasma amino acids was not observed following non-thrombotic MI or in the stable CAD subjects. This module is presented in Figure 5.
Figure 4. Significantly enriched modules.
Circle plot showing the normalized relative change from the quiescent stable disease state to the acute event state for metabolites in the 5 modules enriched for differences across groups. Stable CAD values are used as a reference and are represented by a solid black line. Thrombotic MI values are represented by a red line while non-thrombotic MI are represented by a blue line. Colored lines that span the center of the circle represents pairwise metabolite topological overlap above a fixed threshold.
Figure 5.
Clustering dendrogram of metabolites in Module V. Remaining modules are depicted similarly in the Supplement. The dendrogram indicates proximity with respect to overlapping sets of neighbors between metabolites. Average log-transformed relative change from the quiescent stable disease state to the acute event state for each metabolite is shown below the dendrogram by study group.
One module, Module L, was enriched for pairwise temporal differences between each of the study groups in metabolite abundance change. Module L consisted of cortisol, dihydrocortisol, corticosterone, C21-steroid metabolites, and 3 unknowns. A systematic trend was noted with acute to quiescent abundance ratios being greatest in the thrombotic MI study group, relatively smaller ratios in the non-thrombotic MI group compared to the thrombotic MI study group, and relatively smaller ratios in the stable CAD group relative to the non-thrombotic MI group. Qualitatively, this indicates elevation of these metabolites following MI with greater elevation in thrombotic as opposed to non-thrombotic subjects with little change observed in stable CAD subjects.
4. Discussion
This study achieved two objectives. First, we identified groups of related metabolites in a medium-specific (blood plasma) and state-specific manner by interrogating the topology of a weighted network constructed from the plasma metabolome. Second, we utilized the identified modules to examine the differential metabolic perturbations associated with thrombotic MI and non-thrombotic MI.
Module Discovery and Enrichment Analysis
Given that metabolic processes are linked biochemical reactions, evaluating metabolic changes associated with a disease state transition requires defining sets of “related” metabolites. Once a set is defined, disease state changes may be evaluated using appropriate multivariate statistics or by conducting an enrichment analysis. In this analysis, we have used the network topology of an unbiased data-dependent weighted network to determine the relatedness of metabolites. This approach is predicated on the assumption that while the pairwise correlations between metabolites in a metabolic pathway may decrease dramatically with reaction distance, pairwise topological overlap should be preserved. Interestingly, many of the modules that we “discovered” exhibited a degree of structural homogeneity, which we submit evidences the robustness of this approach for determining relatedness. For example, all of the metabolites in Module V are amino acids or gamma-glutamyl amino acids and 8/9 metabolites in Module E are monoacylglycerols. Of the enriched modules, we found that the majority (4/5: Modules E, K, L, and Y) exhibited differences between thrombotic MI and stable CAD; and between non-thrombotic MI and stable CAD. Within these modules a consistent pattern emerged: individual metabolites were elevated following acute MI relative to stable CAD. Given that this qualitative pattern was similar in both MI groups in comparison to stable CAD, we hypothesize that the state changes observed in these modules are indicative of myocardial ischemia, necrosis, and/or reperfusion injury. Two out of five of the significantly enriched modules exhibited quiescent stable disease to acute event state changes that differed between the acute MI subgroups. We hypothesize that the changes observed within these modules may indicate plaque disruption and/or resultant thrombosis as opposed to the effect of ischemia, necrosis, or reperfusion.
Steroid Hormones
Two of the four modules we hypothesize to contain metabolites indicative of myocardial ischemia and necrosis were characterized by steroid hormone metabolites. Module K was characterized by pregnenolone metabolites such as pregnenolone sulfate, 21-hydroxypregnenolone disulfate, pregnenediol-3-glucuronine, and pregnen-diol disulfate (pregnanediol). The increase in pregnenolome metabolites may be due to stimulation of the hypothalamic-pituitary-adrenal axis following acute MI. Stimulation of this axis has been noted following acute MI with levels of both adrenocorticotropic hormone (ACTH)19 and copeptin20 increasing acutely. Changes in circulating levels of ACTH have been observed within hours of acute myocardial infarction19, 21. ACTH is a pituitary hormone that regulates steroid hormone biosynthesis that has both short-term (by increasing bioavailability of cholesterol) and long-term regulatory effects (by stimulating transcription of steroidogenic enzymes)22. The ACTH-stimulated increased bioavailability of cholesterol and steroidogenic enzymes may explain the increased abundance of pregnenolone metabolites following acute myocardial infarction. As pregnenolone is the precursor to mineralocorticoids, glucocorticoids, androgens, and estrogens23 and the synthesis of pregnenolone from cholesterol is the rate-limiting step in steroidogenesis22, the increased availability of pregnenolone may increase the flux of metabolites through other steroid hormone biosynthetic pathways.
Further evidence of increased flux through steroid hormone biosynthetic pathways for which pregnenolone is a precursor is exhibited in Module L. This module contained C21 corticosteroid metabolites (cortisol, corticosterone, dihydrocortisol [tentative ID], and tentatively identified aldosterone conjugates). This module was enriched for pairwise differences between all three study groups; both acute MI groups showed increases in these hormones following acute events, with significantly greater increases observed in thrombotic MI versus non-thrombotic MI. We posit two non-mutually exclusive mechanisms explaining this observation. First, corticosteroid elevation may be an acute response to thrombosis via platelet-activating factor (PAF). PAF is a phospholipid signaling molecule that acts through a G-protein coupled receptor and is implicated in thrombotic and inflammatory cascades24. PAF stimulates platelet activation and aggregation as well as participates in a pathway that results in leukocyte adherence to endothelial surfaces24. Interestingly, cross-regulation of PAF and glucocorticoids has been demonstrated in animal models. Adding PAF to the perfused adrenal glands of canines25 and guinea pigs26 has been shown to increase glucocorticoid output. Shimada, Hirose, Matsumoto and Aikawa26 have also shown that glucocorticoids suppress the production of PAF and have suggested that this negative-feedback mechanism might represent an adaptive host response. Further evidence of this was observed in a case-control study of patients who died within 30 days of acute MI versus those who survived. Reynolds, et al.27 observed significantly lower cortisol levels in patients who died—this effect was especially significant in the bottom quartile after adjusting for age and cardiac troponin concentration. Corticosteroid elevation as a response to PAF and related regulatory mechanisms would suggest that such elevation should be observed in thrombotic MI but not in non-thrombotic MI. However, our results show relative temporal elevation in non-thrombotic MI to an extent greater that that observed in stable CAD subjects but less than that observed in thrombotic MI subjects. This finding highlights the challenge in determining whether corticosteroid elevation following thrombotic MI is indicative of an amplified stress response in thrombotic MI relative to non-thrombotic MI or a response to thrombosis.
Monoacylglycerols and Lipase activity
Consistent with the observed increase in pregnenolone and corticosteroid hormone metabolites, increased ACTH following acute MI may explain the increased abundance of monoacylglycerols. All the monoacylglycerols in Module E demonstrated increased concentrations in plasma following thrombotic and non-thrombotic MI relative to the stable quiescent state. This pattern of elevation was not observed in stable CAD subjects. The concentration of fatty acids in blood is regulated by the enzyme hormone-sensitive lipase (HSL)23 which are stimulated by ACTH as well as catecholamines, beta adrenergic agonists, and glucagon via cyclic AMP dependent protein kinase (PKA)28. HSL catalyzes the hydrolysis of the C1 and C3 fatty acids from triacylglycerol which results in monoacylglycerols that can then be hydrolyzed by monoacylglycerol lipase29, 30. HSL also catalyzes the hydrolysis of other substrates including monoacylglycerol, diacylglycerol, and cholesteryl esters28. In addition to ACTH stimulated lipolysis resulting in increased plasma fatty acid and monoacylglycerol concentrations, cortisol has also been shown to stimulate lipolysis in a human study in which short-duration hypercortisolemia (excess cortisol) was induced31.
Plasma Amino Acids
The remaining module that was enriched for differences between the acute MI subgroups in quiescent to acute event metabolite changes was Module V. This module was characterized by amino acids and gamma-glutamyl amino acids; the trend observed in this module was for these metabolites to be depressed following thrombotic MI but not in non-thrombotic MI or in stable CAD subjects. A decrease in circulating amino acids coincident with increased levels corticosteroids following thrombotic myocardial infarction is a surprising finding. It has been established that protein catabolism increases following secretion of glucocorticoids31, 32. Hypercortisolemia induced catabolism of proteins has been shown to increase the flux of certain amino acids33. It is thus unlikely that a decrease in plasma amino acids following acute myocardial infarction results from the glucocorticoid response observed in thrombotic MI. Rather, glucocorticoid induced protein catabolism may mask a more precipitous general decrease in circulating amino acids. A decrease in plasma amino acids may indicate increased catabolism of amino acids to furnish adenosine triphosphate (ATP) for the ischemic heart. All of the amino acids in the module can be catabolized to produce ATP; nine out of ten can be catabolized to furnish precursors of gluconeogenesis, and four can be used as substrates for ketogenesis23. Under ischemic conditions, the heart must utilize metabolic substrates that do not require oxygen34; hence, the inability to oxidize fatty acids may lead to increased utilization of amino acids. If plasma amino acids decrease specifically as a result of increased catabolism to meet the energy needs of the ischemic heart, then this signal should also be found in non-thrombotic MI subjects. However, we did not observe this result. A decrease in plasma amino acid concentrations observed in thrombotic but not non-thrombotic MI subjects would be consistent with an increase in protein synthesis in activated platelets. Weyrich et al.35 provide a thorough review of evidence showing that platelets synthesize proteins, and that platelet activation results in signal dependent translation of factors central to thrombosis such as tissue factor (TF)36 and plasminogen activator inhibitor-1 (PAI-1)37. Given the important clinical consequence of thrombosis, this finding of diminished circulating amino acids following thrombotic MI deserves further exploration.
Limitations
The process utilized to define modules has the limitation that a substantial number of metabolites quantified were not assigned to a module. Metabolites may not have been assigned to a module either because they did not exhibit enough topological overlap with other metabolites or because a group of metabolites with a high degree of topological overlap was too small in number. Consequently, the temporal change in these metabolites from quiescent stable disease state to acute event were not evaluated. We justify not considering these metabolites as our objective was to understand how systems of metabolites reflect metabolic perturbations. Another limitation of this analysis was the small cohort size. A larger sample size would facilitate examining how other covariates such as gender, race, and other comorbidities mediate the metabolic changes associated with acute myocardial infarction. Our use of each subject as his/her own reference controls for many potential confounders (e.g. a subject’s gender and race does not change across study time-points) and increased statistical power relative to an unpaired design.
Conclusion
We successfully identified modules of topologically related metabolites and analyzed these modules to determine plasma metabolome perturbations that are associated with thrombotic and non-thrombotic MI. We observed evidence of hypothalamic-pituitary-adrenal axis activation following acute myocardial infarction and evidence that this activation may be greater in thrombotic as opposed to non-thrombotic MI. Further, a precipitous increase in monoacylglycerol abundance was observed in plasma following acute MI indicating an increase of triacylglycerol hydrolysis common to both types of acute MI. We report a novel finding that a decrease in plasma amino acids and gamma-glutamyl amino acids is associated with thrombotic but not non-thrombotic MI.
Supplementary Material
Acknowledgments
The authors would like to acknowledge the human participants who graciously agreed to take part in the study.
Funding Sources: This work was supported in part by a grant from the American Heart Association (11CRP7300003) and the National Institutes of Health (GM103492 and HL130174).
Footnotes
Compliance with ethical standards:
All authors declare that they have no conflicts of interest. All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. Informed consent was obtained from all individual participants included in the study.
Disclosures:
Sample measurements were made by Metabolon, Duram, North Carolina. Coronary aspiration material was evaluated at CVPath Institute, Inc., Gaithersburg, Maryland. Coronary angiography was evaluated at the Johns Hopkins Quantitative Angiographic Core Laboratory, Baltimore, Maryland. No author has any relationships with industry pertinent to this work.
Contributor Information
Patrick J. Trainor, Department of Medicine, Division of Cardiovascular Medicine, University of Louisville, Diabetes and Obesity Center, University of Louisville.
Bradford G. Hill, Department of Medicine, Division of Cardiovascular Medicine, University of Louisville, Diabetes and Obesity Center, University of Louisville.
Samantha M. Carlisle, Department of Pharmacology and Toxicology, University of Louisville.
Eric C. Rouchka, Department of Computer Engineering and Computer Science, University of Louisville.
Shesh N. Rai, Department of Bioinformatics and Biostatistics, University of Louisville.
Aruni Bhatnagar, Department of Medicine, Division of Cardiovascular Medicine, University of Louisville, Diabetes and Obesity Center, University of Louisville.
Andrew P. DeFilippis, Department of Medicine, Division of Cardiovascular Medicine, University of Louisville, Diabetes and Obesity Center, University of Louisville, KentuckyOne/Jewish Hospital, Johns Hopkins University.
References
- 1.Mozaffarian D, Benjamin EJ, Go AS, Arnett DK, Blaha MJ, Cushman M, et al. Heart Disease and Stroke Statistics-2016 Update: A Report From the American Heart Association. Circulation. 2016;133:e38–e360. doi: 10.1161/CIR.0000000000000350. [DOI] [PubMed] [Google Scholar]
- 2.Bhatt DL, Eagle KA, Ohman EM, Hirsch AT, Goto S, Mahoney EM, et al. Comparative determinants of 4-year cardiovascular event rates in stable outpatients at risk of or with atherothrombosis. JAMA. 2010;304:1350–7. doi: 10.1001/jama.2010.1322. [DOI] [PubMed] [Google Scholar]
- 3.Ducrocq G, Bhatt DL, Labreuche J, Corbalan R, Porath A, Gao R, et al. Geographic differences in outcomes in outpatients with established atherothrombotic disease: results from the REACH Registry. Eur J Prev Cardiol. 2014;21:1509–16. doi: 10.1177/2047487313501278. [DOI] [PubMed] [Google Scholar]
- 4.Elbez Y, Cheong AP, Fassa A-A, Cohen E, Reid CM, Babarskiene R, et al. Clinical outcomes in patients with stable coronary artery disease with vs. without a history of myocardial revascularization. European Heart Journal - Quality of Care and Clinical Outcomes. 2016;2:23–32. doi: 10.1093/ehjqcco/qcv017. [DOI] [PubMed] [Google Scholar]
- 5.Yusuf S, Hawken S, Ounpuu S, Dans T, Avezum A, Lanas F, et al. Effect of potentially modifiable risk factors associated with myocardial infarction in 52 countries (the INTERHEART study): case-control study. Lancet. 2004;364:937–52. doi: 10.1016/S0140-6736(04)17018-9. [DOI] [PubMed] [Google Scholar]
- 6.Thygesen K, Alpert JS, Jaffe AS, Simoons ML, Chaitman BR, White HD, et al. Third universal definition of myocardial infarction. J Am Coll Cardiol. 2012;60:1581–98. doi: 10.1016/j.jacc.2012.08.001. [DOI] [PubMed] [Google Scholar]
- 7.Amsterdam EA, Wenger NK, Brindis RG, Casey DE, Jr, Ganiats TG, Holmes DR, Jr, et al. 2014 AHA/ACC Guideline for the Management of Patients with Non-ST-Elevation Acute Coronary Syndromes: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol. 2014;64:e139–228. doi: 10.1016/j.jacc.2014.09.017. [DOI] [PubMed] [Google Scholar]
- 8.Montecucco F, Carbone F, Schindler TH. Pathophysiology of ST-segment elevation myocardial infarction: novel mechanisms and treatments. Eur Heart J. 2016;37:1268–83. doi: 10.1093/eurheartj/ehv592. [DOI] [PubMed] [Google Scholar]
- 9.Kramer MC, Rittersma SZ, de Winter RJ, Ladich ER, Fowler DR, Liang YH, et al. Relationship of thrombus healing to underlying plaque morphology in sudden coronary death. J Am Coll Cardiol. 2010;55:122–32. doi: 10.1016/j.jacc.2009.09.007. [DOI] [PubMed] [Google Scholar]
- 10.Nicholson JK, Holmes E, Kinross JM, Darzi AW, Takats Z, Lindon JC. Metabolic phenotyping in clinical and surgical environments. Nature. 2012;491:384–92. doi: 10.1038/nature11708. [DOI] [PubMed] [Google Scholar]
- 11.Psychogios N, Hau DD, Peng J, Guo AC, Mandal R, Bouatra S, et al. The human serum metabolome. PLoS One. 2011;6:e16957. doi: 10.1371/journal.pone.0016957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Horvath S. Weighted network analysis: application in genomics and systems biology. New York, NY: Springer; 2011. [Google Scholar]
- 13.Zhang B, Horvath S. A general framework for weighted gene co-expression network analysis. Stat Appl Genet Mol Biol. 2005;4:Article17. doi: 10.2202/1544-6115.1128. [DOI] [PubMed] [Google Scholar]
- 14.DeFilippis AP, Chernyavskiy I, Amraotkar AR, Trainor PJ, Kothari S, Ismail I, et al. Circulating levels of plasminogen and oxidized phospholipids bound to plasminogen distinguish between atherothrombotic and non-atherothrombotic myocardial infarction. J Thromb Thrombolysis. 2015 doi: 10.1007/s11239-015-1292-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Langfelder P, Zhang B, Horvath S. Defining clusters from a hierarchical cluster tree: the Dynamic Tree Cut package for R. Bioinformatics. 2008;24:719–20. doi: 10.1093/bioinformatics/btm563. [DOI] [PubMed] [Google Scholar]
- 16.Mootha VK, Lindgren CM, Eriksson KF, Subramanian A, Sihag S, Lehar J, et al. PGC-1alpha-responsive genes involved in oxidative phosphorylation are coordinately downregulated in human diabetes. Nat Genet. 2003;34:267–73. doi: 10.1038/ng1180. [DOI] [PubMed] [Google Scholar]
- 17.Subramanian A, Tamayo P, Mootha VK, Mukherjee S, Ebert BL, Gillette MA, et al. Gene set enrichment analysis: a knowledge-based approach for interpreting genome-wide expression profiles. Proc Natl Acad Sci U S A. 2005;102:15545–50. doi: 10.1073/pnas.0506580102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Storey JD. The positive false discovery rate: A Bayesian interpretation and the q-value. Annals of Statistics. 2003;31:2013–2035. [Google Scholar]
- 19.Paganelli F, Frachebois C, Velut JG, Boullu S, Sauze N, Rosso JP, et al. Hypothalamo-pituitary-adrenal axis in acute myocardial infarction treated by percutaneous transluminal coronary angioplasty: effect of time of presentation. J Endocrinol Invest. 2003;26:407–13. doi: 10.1007/BF03345195. [DOI] [PubMed] [Google Scholar]
- 20.Maisel A, Mueller C, Neath SX, Christenson RH, Morgenthaler NG, McCord J, et al. Copeptin helps in the early detection of patients with acute myocardial infarction: primary results of the CHOPIN trial (Copeptin Helps in the early detection Of Patients with acute myocardial INfarction) J Am Coll Cardiol. 2013;62:150–60. doi: 10.1016/j.jacc.2013.04.011. [DOI] [PubMed] [Google Scholar]
- 21.Donald RA, Crozier IG, Foy SG, Richards AM, Livesey JH, Ellis MJ, et al. Plasma corticotrophin releasing hormone, vasopressin, ACTH and cortisol responses to acute myocardial infarction. Clin Endocrinol (Oxf) 1994;40:499–504. doi: 10.1111/j.1365-2265.1994.tb02489.x. [DOI] [PubMed] [Google Scholar]
- 22.Miller WL, Auchus RJ. The molecular biology, biochemistry, and physiology of human steroidogenesis and its disorders. Endocr Rev. 2011;32:81–151. doi: 10.1210/er.2010-0013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Voet D, Voet JG, Pratt CW. Fundamentals of biochemistry: life at the molecular level. 4. Hoboken, NJ: Wiley; 2013. [Google Scholar]
- 24.Zimmerman GA, McIntyre TM, Prescott SM, Stafforini DM. The platelet-activating factor signaling system and its regulators in syndromes of inflammation and thrombosis. Crit Care Med. 2002;30:S294–301. doi: 10.1097/00003246-200205001-00020. [DOI] [PubMed] [Google Scholar]
- 25.Aikawa T, Hirose T, Matsumoto I, Morikawa T, Shimada T, Mine Y, et al. Effect of platelet-activating factor on cortisol and corticosterone secretion by perfused dog adrenal. Lipids. 1991;26:1108–11. doi: 10.1007/BF02536511. [DOI] [PubMed] [Google Scholar]
- 26.Shimada T, Hirose T, Matsumoto I, Aikawa T. Platelet-activating factor acts on cortisol secretion by perfused guinea-pig adrenals via calcium-/phospholipid-dependent mechanisms. J Endocrinol. 2005;184:381–91. doi: 10.1677/joe.1.05937. [DOI] [PubMed] [Google Scholar]
- 27.Reynolds RM, Walker BR, Haw S, Newby DE, Mackay DF, Cobbe SM, et al. Low serum cortisol predicts early death after acute myocardial infarction. Crit Care Med. 2010;38:973–5. doi: 10.1097/CCM.0b013e3181cdf6de. [DOI] [PubMed] [Google Scholar]
- 28.Kraemer FB, Shen WJ. Hormone-sensitive lipase: control of intracellular tri-(di-)acylglycerol and cholesteryl ester hydrolysis. J Lipid Res. 2002;43:1585–94. doi: 10.1194/jlr.r200009-jlr200. [DOI] [PubMed] [Google Scholar]
- 29.Ahmadian M, Duncan RE, Jaworski K, Sarkadi-Nagy E, Sul HS. Triacylglycerol metabolism in adipose tissue. Future Lipidol. 2007;2:229–237. doi: 10.2217/17460875.2.2.229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lieberman M, Marks AD, Peet A. Marks’ basic medical biochemistry: a clinical approach. 4. Philadelphia: Wolters Kluwer Health/Lippincott Williams & Wilkins; 2013. [Google Scholar]
- 31.Djurhuus CB, Gravholt CH, Nielsen S, Mengel A, Christiansen JS, Schmitz OE, et al. Effects of cortisol on lipolysis and regional interstitial glycerol levels in humans. Am J Physiol Endocrinol Metab. 2002;283:E172–7. doi: 10.1152/ajpendo.00544.2001. [DOI] [PubMed] [Google Scholar]
- 32.Christiansen JJ, Djurhuus CB, Gravholt CH, Iversen P, Christiansen JS, Schmitz O, et al. Effects of cortisol on carbohydrate, lipid, and protein metabolism: studies of acute cortisol withdrawal in adrenocortical failure. J Clin Endocrinol Metab. 2007;92:3553–9. doi: 10.1210/jc.2007-0445. [DOI] [PubMed] [Google Scholar]
- 33.Brillon DJ, Zheng B, Campbell RG, Matthews DE. Effect of cortisol on energy expenditure and amino acid metabolism in humans. Am J Physiol. 1995;268:E501–13. doi: 10.1152/ajpendo.1995.268.3.E501. [DOI] [PubMed] [Google Scholar]
- 34.Drake KJ, Sidorov VY, McGuinness OP, Wasserman DH, Wikswo JP. Amino acids as metabolic substrates during cardiac ischemia. Exp Biol Med (Maywood) 2012;237:1369–78. doi: 10.1258/ebm.2012.012025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Weyrich AS, Schwertz H, Kraiss LW, Zimmerman GA. Protein synthesis by platelets: historical and new perspectives. Journal of Thrombosis and Haemostasis. 2009;7:241–246. doi: 10.1111/j.1538-7836.2008.03211.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Panes O, Matus V, Saez CG, Quiroga T, Pereira J, Mezzano D. Human platelets synthesize and express functional tissue factor. Blood. 2007;109:5242–5250. doi: 10.1182/blood-2006-06-030619. [DOI] [PubMed] [Google Scholar]
- 37.Brogren H. Platelets synthesize large amounts of active plasminogen activator inhibitor 1. Blood. 2004;104:3943–3948. doi: 10.1182/blood-2004-04-1439. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.