To the Editor
Rhinoviruses (RV) infect up to 90% of school-age children with asthma during the month of September, and the severity of clinical illness varies from no symptoms to severe wheezing illnesses.(1) We previously reported that that detection by PCR of S. pneumoniae or M. catarrhalis in the upper airway is associated with RV-induced asthma exacerbations.(2) Based on those findings, we hypothesized that RV infection alters the upper airway microbiota, and that microbial changes correspond with infection severity. To test this hypothesis, we prospectively monitored respiratory symptoms in children with asthma during the peak fall RV season, obtained weekly nasal secretions, and concurrently analyzed these samples for RVs and airway bacteria.
Children included in this analysis were enrolled in a larger study to determine genetic correlates with severe RV illnesses (“RhinoGen”). Subjects collected nasal mucus samples on a weekly basis for five consecutive weeks during September (peak RV season). All samples were analyzed for common respiratory viruses and RV abundance (qPCR), and RV typing was determined.(3) Cold and asthma symptoms recorded in daily diaries were linked with RV infection data to identify infections that were either asymptomatic or associated with an asthma exacerbation. RV-B types caused 70% of asymptomatic RV infections, while exacerbations were only associated with RV-A or RV-C types. This study included 10 RhinoGen participants with asymptomatic infections and 7 participants with exacerbations of asthma (Table 1; Supplemental Figure 1; also see supplemental data for inclusion and exclusion criteria). Compared to the other children with asthma in the RhinoGen cohort, the children in the asymptomatic and the exacerbation groups had similar total IgE levels and rates of allergic sensitization (Supplemental Table 1). 16S rRNA gene sequencing was performed on bacterial DNA isolated from each sample and statistical analysis was performed to identify bacterial taxa associated with viral infection and RV-associated asthma exacerbations (see Online Repository).
Table I.
Paired samples for analysis: 34 samples (17 pairs). Within each pair, the first sample was RV negative, and a second sample obtained 1–3 weeks later was RV positive.
| Asymptomatic during RV infection | Moderate Asthma Exacerbation during RV infection | |
|---|---|---|
| RV-negative | 10 samples | 7 samples |
| RV-positive | 10 samples | 7 samples |
Within the 34 samples before and after RV infection, the dominant phyla detected were Firmicutes (50.3%); Proteobacteria (24.9%); Actinobacteria (17%); Bacteroidetes (4.3%); Fusobacteria (1.5%); and unclassified (1.1%). The most abundant genera were Dolosigranulum (12.2% total abundance); Streptococcus (11.3%); Staphylococcus (10.1%); Corynebacterium (9.7%); Moraxella (7.2%); unclassified OTU #1 (5.6%); unclassified OTU #2 (3.1%); Neisseria (3%); Gemella (2.2%); Rothia (1.9%); Actinomyces (1.6%); Haemophilus (1.4%); Acinetobacter (1.4%) and unclassified OTU #3 (1.1%). We then compared the RV-negative and RV-positive samples, and found a similar number of overall sequences before and after RV infection (p=0.95); and similar evenness and diversity. Furthermore, using principal component analysis (PCoA) of the Unifrac and Bray-Curtis distance matrices, there were no distinct clustering patterns between the two groups, suggesting that the overall community composition of the RV-negative and RV-positive samples were similar.
In the combined asymptomatic and exacerbation groups, RV infection was associated with several significant changes in specific genera in airway secretions (Supplemental Figure 2). RV infection was associated with increased abundance of Dolosigranulum (base mean=213; log2 fold change=0.60) and Moraxella (base mean=116; log2 fold change=0.79), and reduced abundance of unclassified OTU #1 (base mean=209; log2 fold change=2.54). These findings support our previous report based on PCR detection that RV infection increases Moraxella detection,(2) and indicate that RV infection also influences microbial community composition.
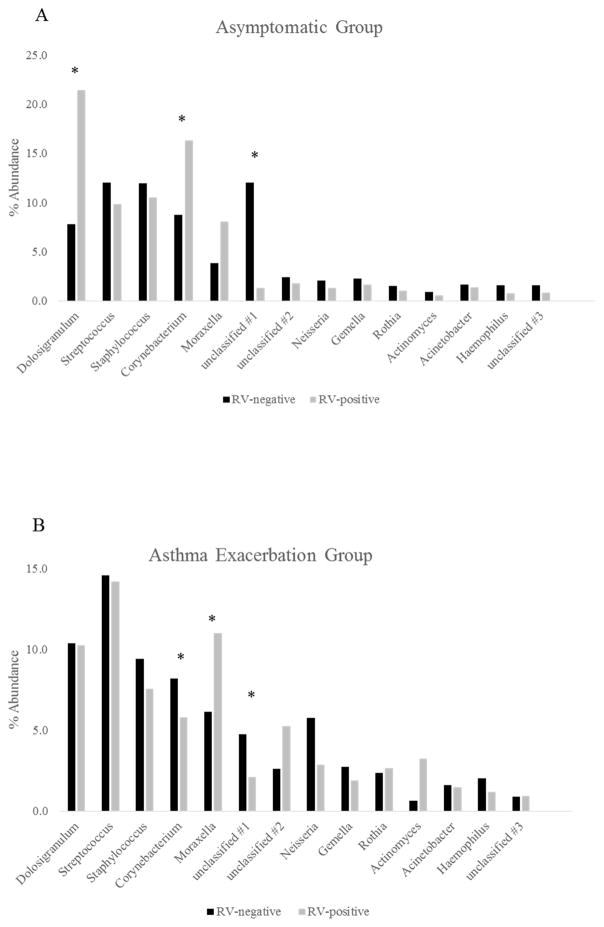
We next tested whether microbial changes during RV infection differed between asymptomatic RV infections and RV-associated asthma exacerbations (Figure 1 and Supplemental Figure 3). RV infection was associated with increased abundance of Moraxella in both groups (asymptomatic group: base mean=175; log2 fold change=1.04; and exacerbation group: base mean=158; log2 fold change=0.9), and reduced abundance of unclassified OTU#1 (asymptomatic group: base mean=269; log2 fold change=−3.6; and exacerbation group: base mean=123; log2 fold change=−1.12). Interestingly, RV associations with the abundance of some bacterial OTUs depended on the symptom group. Namely, within the asymptomatic group, RV infection was associated with increased abundance of Corynebacterium (base mean=196; log2 fold change=0.48), while in the exacerbation group the association was in the opposite direction (base mean=212; log2 fold change=−0.45). RV infection was also associated with increased abundance of Dolosigranulum in the asymptomatic group (base mean=175; log2 fold change=1.04).
Figure 1.
Relative abundance at the Genera level (OTUs >1%) between RV-negative and RV-positive samples in the asymptomatic group (A) and asthma exacerbation group (B).
Asymptomatic group: Dolosigranulum q-value=1.4x10−8; Corynebacterium q-value=1.5x10−111; Moraxella q-value=1.4x10−8; unclassified OTU#1 q-value=0.
Exacerbation group: Corynebacterium q-value=7.8x10−25; Moraxella q-value=9.8x10−47; unclassified OTU#1 q-value=5.9x10−50.
An association network constructed to link RV quantity with the presence of specific OTUs of bacteria demonstrated that as the quantity of RV increased, the abundance of Dolosigranulum and Corynebacterium decreased while the abundance of Haemophilus increased (Supplemental figure 4). Furthermore, there were both increases and decreases of OTUs belonging to Streptococcus and Moraxella, indicating that the amount of RV replication is related to the magnitude of composition changes in the microbiome.(4)
Asymptomatic RV infections were associated with a significant increase in the abundance of Dolosigranulum and Corynebacterium compared to pre-infection samples, and the quantities of these bacteria were inversely correlated with viral shedding. Dolosigranulum and Corynebacterium are commensal bacteria within the respiratory tract in children and commonly co-occur.(5) They both are negatively associated with S. pneumoniae abundance, have been associated with reduced airway symptoms and a lower risk of otitis media during infancy,(6) and are inversely related to episodes of wheeze during infancy.(7) Our findings extend these findings and suggest that microbial communities featuring abundant Corynebacterium and possibly Dolosigranulum may confer protection against symptoms during RV infection.
This study has a number of advantages, and some limitations. The prospective study design allowed us to obtain samples from the same subject prior to and during RV infection. Samples were obtained during the same season, eliminating seasonal influences on microbial composition. Our findings are based on samples obtained from the upper airway for practical reasons. RV infections begin in the upper respiratory tract and thus the microbial environment in the upper airway is likely to influence initiation of RV infection and downstream events. Therefore investigations of the upper airway may identify new strategies for prevention and/or treatment of RV-induced exacerbations. Our results should be interpreted with caution due to the small sample size, and the observational study design cannot distinguish causality among the observed associations between bacteria, viruses and symptoms.
In summary, RV infection is associated with changes in microbial composition of the upper airway. These changes differed between asymptomatic infection and exacerbation of asthma, and were related to RV quantity and possibly RV species.(8) While RV infection was generally related to increased abundance of Moraxella, a well-known airway pathogen; RV was related to increased commensal bacteria (Dolosigranulum and Corynebacterium) during asymptomatic infection. Finally, while bacterial pathogens such as Moraxella can contribute to respiratory symptoms, our findings suggest that other microbial communities may help to maintain normal airway physiology during RV infection and thereby moderate or prevent respiratory symptoms. Addressing these gaps in knowledge may lead to new preventive strategies for RV illnesses and virus-induced exacerbations of asthma.
Supplementary Material
Acknowledgments
This work was supported by the following grants: U19 AI070503-01 (RhinoGen); P01 HL070831 (Childhood Origins of Asthma); 1UL1RR025011 from the Clinical and Translational Science Award (CTSA) program of the National Center for Research Resources, National Institutes of Health; T32AI007635 (University of Wisconsin Allergy Research Training program); and 1K12HD068371-01A1 Indiana University School of Medicine (IUSM) Indiana Pediatric Scientist Award (IPSA) program through the Child Health Research Career Development Award (CHRCDA).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Olenec JP, Kim WK, Lee WM, Vang F, Pappas TE, Salazar LE, et al. Weekly monitoring of children with asthma for infections and illness during common cold seasons. The Journal of allergy and clinical immunology. 2010;125(5):1001–6. e1. doi: 10.1016/j.jaci.2010.01.059. Epub 2010/04/16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kloepfer KM, Lee WM, Pappas TE, Kang TJ, Vrtis RF, Evans MD, et al. Detection of pathogenic bacteria during rhinovirus infection is associated with increased respiratory symptoms and asthma exacerbations. The Journal of allergy and clinical immunology. 2014;133(5):1301–7. e3. doi: 10.1016/j.jaci.2014.02.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lee WM, Lemanske RF, Jr, Evans MD, Vang F, Pappas T, Gangnon R, et al. Human rhinovirus species and season of infection determine illness severity. Am J Respir Crit Care Med. 2012;186(9):886–91. doi: 10.1164/rccm.201202-0330OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Nakagome K, Bochkov YA, Ashraf S, Brockman-Schneider RA, Evans MD, Pasic TR, et al. Effects of rhinovirus species on viral replication and cytokine production. The Journal of allergy and clinical immunology. 2014;134(2):332–41. doi: 10.1016/j.jaci.2014.01.029. Epub 2014/03/19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bogaert D, Keijser B, Huse S, Rossen J, Veenhoven R, van Gils E, et al. Variability and diversity of nasopharyngeal microbiota in children: a metagenomic analysis. PloS one. 2011;6(2):e17035. doi: 10.1371/journal.pone.0017035. Epub 2011/03/10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Laufer AS, Metlay JP, Gent JF, Fennie KP, Kong Y, Pettigrew MM. Microbial communities of the upper respiratory tract and otitis media in children. mBio. 2011;2(1):e00245–10. doi: 10.1128/mBio.00245-10. Epub 2011/02/03. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Teo SM, Mok D, Pham K, Kusel M, Serralha M, Troy N, et al. The infant nasopharyngeal microbiome impacts severity of lower respiratory infection and risk of asthma development. Cell Host Microbe. 2015;17(5):704–15. doi: 10.1016/j.chom.2015.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cox DW, Bizzintino J, Ferrari G, Khoo SK, Zhang G, Whelan S, et al. Human rhinovirus species C infection in young children with acute wheeze is associated with increased acute respiratory hospital admissions. Am J Respir Crit Care Med. 2013;188(11):1358–64. doi: 10.1164/rccm.201303-0498OC. Epub 2013/09/03. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.