Abstract
INTRODUCTION
Information on anticipated survival time after dementia diagnosis among racially/ethnically diverse patients is needed to plan for care and evaluate disparities.
METHODS
Dementia-free healthcare members age>=64 years were followed (1/1/2000–12/31/2013) for dementia diagnosis and subsequent survival (n=23,032 Asian American; n=18,778 African American; n=21,000 Latino; n=4,543 American Indian/Alaska Native; n=206,490 White). Kaplan-Meier curves were estimated for survival after dementia diagnosis by race/ethnicity. We contrasted mortality patterns among people with vs. without dementia using Cox proportional hazards models.
RESULTS
After dementia diagnosis (n=59,494), Whites had shortest median survival (3.1 years), followed by American Indian/Alaska Natives (3.4 years), African Americans (3.7 years), Latinos (4.1 years), and Asian Americans (4.4 years). Longer post-diagnosis survival among racial/ethnic minorities compared with Whites persisted after adjustment for comorbidities. Racial/ethnic mortality inequalities among dementia patients mostly paralleled mortality inequalities among people without dementia.
DISCUSSION
Survival after dementia diagnosis differs by race/ethnicity, with shortest survival among Whites and longest among Asian Americans.
Keywords: Dementia, survival, mortality, race, ethnicity, disparities, cohort, epidemiology
1. Background
Understanding survival time after dementia diagnosis is important for patients, families, and public health planning. Little is known about possible racial/ethnic differences in survival after dementia diagnosis [1], but the limited evidence suggests longer post-diagnosis survival in some racial/ethnic minorities compared with Whites [2–4]. Racial/ethnic differences in post-diagnosis survival are plausible, given potential differences in care or support after diagnosis [5–7], the progression of the underlying neuropathological disease process [8, 9], prevalence of comorbidities at time of diagnosis, and timing of diagnosis with respect to the underlying neuropathological disease process [10].
Evaluating racial/ethnic differences in survival after dementia diagnosis requires a large, diverse cohort, but few samples have sufficient data on incident dementia cases. Racial/ethnic differences in post-diagnosis survival must be evaluated in the context of racial/ethnic patterns in survival among people without dementia. Such comparisons will demonstrate whether racial/ethnic differences in post-diagnosis survival reflect mortality differences in the general population of older adults [11] or relate specifically to dementia. The goal of the present study was to examine survival after dementia diagnosis in five racial/ethnic groups in a population with access to healthcare.
2. Methods
2.1 Setting
Kaiser Permanente Northern California (KPNC) is an integrated healthcare delivery system that provides comprehensive medical care to more than 3.7 million members (approximately 30% of the population in the geographic region), with 16% of members enrolled in Medicare and 8% of members enrolled in the California Medical Assistance Program (Medi-Cal) or another State subsidy for health insurance. KPNC members are generally representative of the overall population of the region, but underrepresent people at the very extreme tails of the income distribution [12–14]. Older adults (age ≥65) included in the KPNC membership are similar to the general population of Northern Californian older adults with respect to history of chronic conditions, including diabetes, hypertension, heart disease, and asthma, and lifestyle risk factors, including smoking, obesity, and sedentary behavior [14].
2.2 Study design
This is a cohort study of KPNC health plan members (n=372,925) who were enrolled in the health plan and age ≥60 years as of January 1, 1996, the year electronic medical records were introduced in the KPNC system. Figure A.1 describes the study flow. We implemented a four-year washout period from January 1, 1996 to December 31, 1999 to ensure identification of dementia cases from time of first diagnosis. During the washout period, n=20,075 people had a diagnosis of dementia; we considered these people to have prevalent dementia and excluded them from our analyses. At the end of the washout period, n=280,147 people remained (a) alive, (b) KPNC members, and (c) with no diagnosis of dementia. We excluded members missing race (n=5,682) and unknown or other sex (n=43). We also excluded members who identified as multi-racial (n=139) and Native Hawaiian or other Pacific Islander (n=440) due to small sample sizes for a final sample of n=273,843 members. These individuals were followed for incident diagnosis of dementia and subsequent mortality from January 1, 2000 until a lapse in health plan membership (defined as ≥90 days) or December 31, 2013, for a total study follow-up period of up to 14 years. We censored health plan members after a ≥90 day lapse in membership, following previous work in KPNC [15–17], to avoid the potential for interval censoring during which a dementia diagnosis might have occurred and not been recorded. The study was approved by the KPNC institutional review board, which waived the requirement for informed consent.
2.3 Measures
Race/ethnicity
Self-reported race/ethnicity was identified from KPNC membership databases and categorized into five racial/ethnic groups: Asian American, African American or Black, American Indian or Alaska Native, Latino or Hispanic, and non-Latino White. Chinese, Filipino, and Japanese members were the primary Asian American national origin groups represented; Mexican Americans were the primary Latino national origin group represented.
Dementia
Dementia diagnoses were identified from electronic medical records of inpatient and outpatient encounters between January 1, 2000 and December 31, 2013 based on International Classification of Diseases, Ninth Revision (ICD-9) diagnostic codes for Alzheimer’s disease (331.0), vascular dementia (290.4x), and nonspecific dementia (290.0, 290.1x, 290.2x, 290.3, 294.2x, 294.8). Identification of incident dementia using ICD-9 codes has been implemented successfully in other publications in this population [15–19]. Diagnosis of dementia in neurology, memory clinic, and neuropsychology departments in the KPNC system is typically based on information from medical history, physical examination, mental status examination, blood tests, functional ability, and neuroimaging. A similar battery of ICD-9 codes was reported to have a sensitivity of 77% and a specificity of 95% compared with a consensus diagnosis of dementia in a healthcare system in Seattle, Washington [20]. In Medicare claims data, this method of identifying cases had a sensitivity of 87% in a sample of Alzheimer’s disease patients who participated in the Consortium to Establish a Registry for Alzheimer’s Disease (CERAD) [21]. Because of the rarity and unique clinical courses of frontotemporal dementia (331.1x), dementia with Lewy bodies (331.82), and Parkinson’s dementia (332.0+294.1x), we did not include these codes in our definition of dementia. These three dementias in total had a cumulative incidence of <2% over the entire washout and follow-up period (January 1, 1996 to December 31, 2013).
Mortality
Following previous studies of KPNC health plan member survival, mortality and date of death were identified from the California Automated Mortality Linkage System, which captures deaths from the California State Mortality File, Social Security Death Records, and electronic medical records [15, 22].
Covariates
Age and sex were identified from KPNC health plan membership databases. We identified comorbidities at baseline (diagnoses occurring during the dementia washout period of January 1, 1996 to December 31, 1999), including diabetes, depression, hypertension, stroke (ischemic stroke, transient ischemic attack, and hemorrhagic stroke), and cardiovascular disease (myocardial infarction, heart failure, ischemic heart disease, and peripheral arterial disease) from inpatient and outpatient medical encounters using ICD-9 diagnostic codes (Table A.1). KPNC records do not include socioeconomic status.
2.4 Statistical Analysis
Among members with incident dementia, we used the Kaplan-Meier method to estimate survival times after dementia diagnosis, censoring the small fraction of the sample who left KPNC membership (overall and stratified by age at diagnosis: 64–69, 70–74, 75–79, 80–84, 80–89, 90+ years). We also estimated mortality rates after dementia diagnosis stratified by age at diagnosis, allowing individuals to transition from one age band to another as they aged. To assess whether post-diagnosis mortality differences mirrored the racial/ethnic differences in mortality rates that occur in the general population of older adults, rather than reflecting patterns unique to dementia, we used Cox proportional hazards models with age as the timescale for mortality with all cohort members (both people with and without dementia). In these models, dementia diagnosis was a time-varying binary predictor variable, set to zero at baseline for everyone in the sample and updated to one at the time of dementia diagnosis and all subsequent follow-up times for that individual. We included two-way multiplicative interaction terms between time-varying dementia and race/ethnicity, with White race as the reference category. Because of previously documented age trends in mortality patterns by race/ethnicity [11], we estimated these models separately for the following age bands: 64–69, 70–74, 75–79, 80–84, 80–89, and 90+ years, allowing individuals to transition from one age band to another as they aged. In Model 1, we adjusted for age (as timescale) and sex. To examine whether burden of comorbidities influenced associations between race and mortality, Model 2 additionally adjusted for baseline comorbidities: depression, diabetes, hypertension, stroke, and cardiovascular disease. Based on these models, we report hazard ratios (HRs) relating dementia and mortality by racial/ethnic group, HRs relating race/ethnicity and mortality separately for people with and people without dementia, and ratio of hazard ratios (RHRs), calculated as , to evaluate whether the relative impact of dementia on mortality differed by race/ethnicity [23]. An RHR=1 (HRminority=HRwhites; no multiplicative interaction) indicates no difference in the association between dementia and mortality by race/ethnicity. An RHR>1 (HRminority>HRwhites; positive multiplicative interaction) indicates a larger impact of dementia on mortality in the racial/ethnic minority group compared with Whites (suggests delayed diagnosis in racial/ethnic minority group compared with Whites). An RHR<1 (HRminority<HRwhites; negative multiplicative interaction) indicates a smaller impact of dementia on mortality in the racial/ethnic minority group compared with Whites (suggests earlier diagnosis in racial/ethnic minority group compared with Whites). We used SAS 9.4 for all data analyses.
3. Results
Cohort members were followed for up to 14 years, during which time 59,494 health plan members developed dementia. The number of people in each age group at time of dementia diagnosis is presented in supplemental Table A.2. Among people with incident dementia, 64.1% (n=38,159) died during follow-up, 10.8% (n=6,428) were censored due to a lapse in health plan membership, and 25.1% (n=14,907) were still alive and health plan members at the end of the study (Table A.3). Prevalence of baseline comorbidities was higher among people who developed dementia during the study (Table 1). Although prevalence of individual comorbidities varied across racial/ethnic groups, no single group stood out as having a higher burden of comorbidities overall.
Table 1.
Baseline characteristics of the sample by incidence of dementia and race/ethnicity: Kaiser Permanente Northern California, 2000–2013.
| Variable | Asian American | Latino | African American | American Indian/Alaska Native | White |
|---|---|---|---|---|---|
|
| |||||
| People who developed incident dementia | |||||
|
| |||||
| n | 3,847 | 4,371 | 4,942 | 1,224 | 45,110 |
| Age (years), mean (SD) | 75.1 (6.2) | 75.2 (6.4) | 75.5 (6.6) | 76.1 (6.2) | 77.1 (6.6) |
| Female, % | 57.7 | 57.1 | 59.1 | 58.1 | 60.0 |
| ≥1 heath care visit per year, % | 83.4 | 83.7 | 83.9 | 88.6 | 86.4 |
| Depression, % | 8.3 | 16.4 | 10.8 | 16.8 | 15.9 |
| Hypertension, % | 59.8 | 58.4 | 72.4 | 59.0 | 54.8 |
| Diabetes, % | 36.9 | 39.5 | 36.8 | 28.5 | 22.0 |
| Stroke, % | 9.7 | 11.6 | 12.6 | 13.2 | 11.9 |
| Cardiovascular disease*, % | 20.6 | 24.8 | 27.5 | 30.0 | 27.0 |
| Myocardial infarction, % | 2.6 | 2.7 | 1.9 | 3.4 | 2.8 |
| Heart failure, % | 6.5 | 8.3 | 10.8 | 9.6 | 9.2 |
| Ischemic heart disease, % | 15.8 | 18.4 | 17.5 | 22.6 | 19.5 |
| Peripheral arterial disease, % | 4.4 | 6.9 | 9.0 | 7.7 | 7.5 |
|
| |||||
| People who remained dementia-free | |||||
|
| |||||
| n | 19,185 | 16,629 | 13,836 | 3,319 | 161,380 |
| Age (years), mean (SD) | 71.0 (5.6) | 71.1 (5.5) | 71.6 (6.1) | 72.5 (6.0) | 73.0 (6.5) |
| Female, % | 52.2 | 51.2 | 53.4 | 52.9 | 53.5 |
| ≥1 heath care visit per year, % | 78.0 | 81.0 | 79.2 | 87.1 | 81.2 |
| Depression, % | 5.0 | 10.1 | 7.3 | 13.0 | 10.4 |
| Hypertension, % | 52.6 | 51.0 | 66.9 | 52.7 | 48.5 |
| Diabetes, % | 32.8 | 36.6 | 35.1 | 30.0 | 21.1 |
| Stroke, % | 5.5 | 6.2 | 8.0 | 8.1 | 7.5 |
| Cardiovascular disease*, % | 16.1 | 19.2 | 23.6 | 27.5 | 23.3 |
| Myocardial infarction, % | 2.0 | 2.5 | 2.4 | 3.1 | 2.8 |
| Heart failure, % | 4.9 | 6.2 | 9.8 | 8.6 | 8.1 |
| Ischemic heart disease, % | 12.9 | 15.0 | 15.8 | 20.6 | 17.2 |
| Peripheral arterial disease, % | 2.9 | 4.8 | 7.2 | 7.9 | 6.6 |
Cardiovascular disease = peripheral arterial disease, acute myocardial infarction, heart failure, or ischemic heart disease.
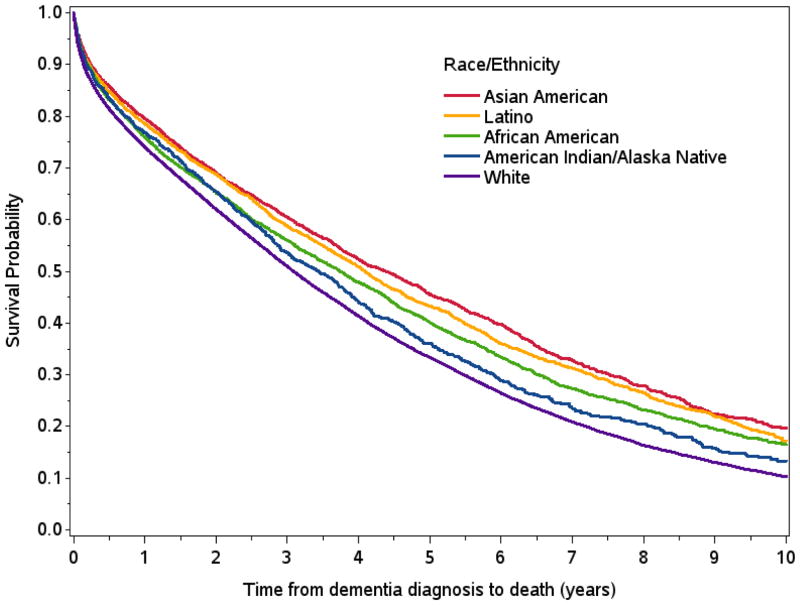
Mean age at dementia diagnosis was 83.4 years; 75.0% were ages 75–89 at diagnosis and 54.5% were ages 80–89 at diagnosis. Median (25th percentile–75th percentile) survival time after dementia diagnosis was shortest among Whites (3.1 years; 0.9–6.3). Relative to Whites, median survival time was 0.3 years longer for American Indians/Alaska Natives (3.4 years; 1.2–6.7), 0.6 years longer for African Americans (3.7 years; 1.1–7.6), 1.0 year longer for Latinos (4.1 years; 1.3–8.2), and 1.3 years longer for Asian Americans (4.4 years; 1.4–8.6) (Figure 1, Table 2). Post-diagnosis survival time decreased with age, and median post-diagnosis survival times were longest for Asian Americans and shortest for Whites in nearly every age group (Table 2). For example, median post-diagnosis survival time was 5.2 years among Whites and 6.7 years among Asian Americans diagnosed between ages 70–74 and 1.4 years among Whites and 1.9 years among Asian Americans diagnosed at ages 90+. Racial/ethnic patterns in age-stratified post-diagnosis mortality rates were consistent with survival time patterns described above, with highest mortality rates among Whites and lowest mortality rates among Asian Americans (Table 3).
Figure 1.
Kaplan-Meier survival curves from time of dementia diagnosis by race/ethnicity: Kaiser Permanente Northern California, 2000–2013.
Total study follow-up was up to 14 years; we present Kaplan-Meier curves for 10 years because less than 25% of people with incident dementia survived more than 10 years.
Table 2.
Median (25th percentile and 75th percentile) years of survival after dementia diagnosis by age at diagnosis: Kaiser Permanente Northern California, 2000–2013.
| 64–69 years (1.7%) | 70–74 years (8.2%) | 75–79 years (20.5%) | 80–84 years (29.3%) | 85–89 years (25.2%) | 90+ years (15.1%) | |
|---|---|---|---|---|---|---|
|
| ||||||
| Asian American | 7.8 (2.3, 14.0) | 6.7 (2.8, 12.0) | 5.5 (2.0, 10.6) | 4.8 (1.5, 8.1) | 3.1 (1.1, 6.0) | 1.9 (0.5, 4.1) |
| Latino | 6.8 (1.6, 13.4) | 6.6 (2.2, 11.5) | 5.3 (2.1, 9.4) | 4.3 (1.6, 8.0) | 3.0 (1.0, 5.8) | 1.7 (0.4, 3.4) |
| African American | 7.4 (2.3, 11.8) | 5.9 (1.9, 10.6) | 4.9 (1.7, 9.4) | 3.4 (1.1, 6.7) | 3.0 (0.8, 5.9) | 1.9 (0.5, 4.5) |
| American Indian/Alaska Native | - | 4.9 (2.4, 10.1) | 4.7 (2.6, 9.1) | 4.1 (1.6, 7.6) | 2.1 (0.8, 4.9) | 1.6 (0.4, 3.7) |
| White | 5.5 (2.0, 11.0) | 5.2 (1.9, 9.6) | 4.4 (1.8, 8.0) | 3.5 (1.2, 6.4) | 2.6 (0.7, 5.0) | 1.4 (0.3, 3.3) |
Percent of dementia cases occurring in each age group presented in column header. Survival estimates for American Indian/Alaska Native dementia patients diagnosed between ages 64–69 not included due to small number of dementia cases and deaths in this age group.
Table 3.
Age-specific mortality rates (95% confidence interval) per 100 person-years after dementia diagnosis: Kaiser Permanente Northern California, 2000–2013.
| 64–69 years | 70–74 years | 75–79 years | 80–84 years | 85–89 years | 90+ years | |
|---|---|---|---|---|---|---|
|
| ||||||
| Asian American | 12.2 (6.2, 18.1) | 10.1 (7.9, 12.3) | 12.7 (11.3, 14.1) | 13.8 (12.7, 15.0) | 18.8 (17.4, 20.3) | 30.8 (28.2, 33.4) |
| Latino | 17.3 (10.4, 24.2) | 11.1 (9.0, 13.1) | 12.4 (11.2, 13.6) | 15.4 (14.3, 16.5) | 19.9 (18.4, 21.4) | 34.4 (31.6, 37.1) |
| African American | 14.3 (8.2, 20.4) | 13.5 (11.5, 15.6) | 13.9 (12.6, 15.1) | 18.7 (17.4, 20.0) | 22.2 (20.6, 23.7) | 29.4 (27.2, 31.6) |
| American Indian/Alaska Native | - | 10.8 (6.7, 15.0) | 14.2 (11.5, 17.0) | 15.7 (13.4, 18.0) | 25.4 (22.3, 28.5) | 36.7 (32.0, 41.5) |
| White | 14.7 (12.2, 17.1) | 15.0 (14.0, 15.9) | 15.2 (14.7, 15.7) | 19.3 (18.9, 19.8) | 25.3 (24.8, 25.8) | 38.7 (37.9, 39.5) |
Mortality rates were calculated allowing individuals to transition from one age band to another as they aged. Mortality rate estimates for American Indian/Alaska Native dementia patients ages 64–69 not included due to small number of dementia cases and deaths in this age group.
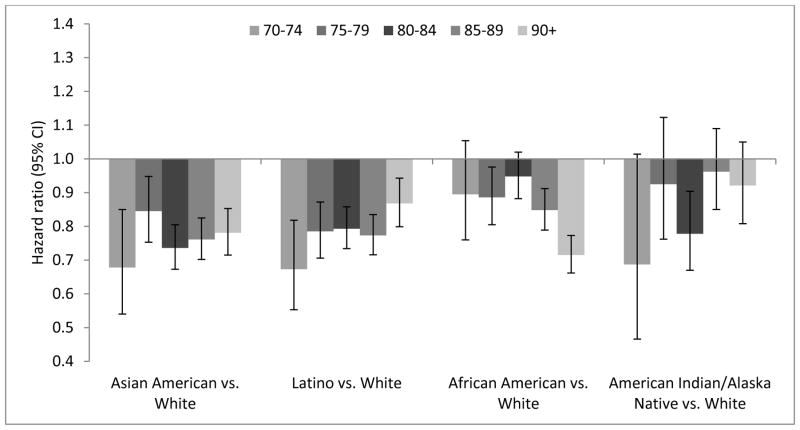
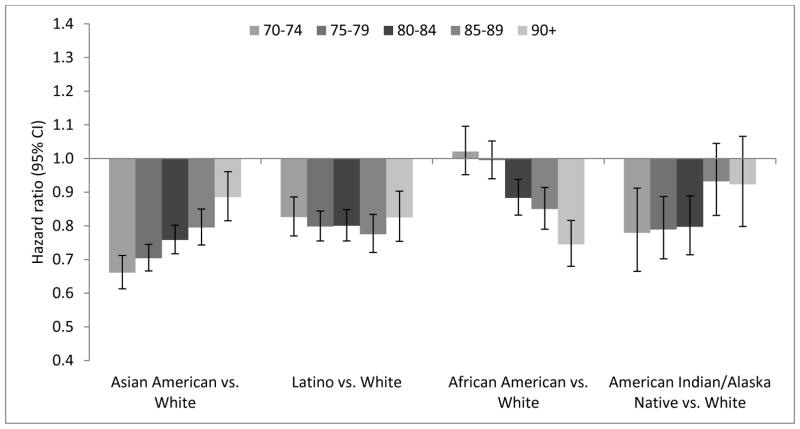
Dementia was associated with mortality across age groups in all racial/ethnic groups (Table A.4). Age-stratified hazard ratios (HRs) for mortality by race/ethnicity demonstrate that across age groups, White dementia patients tended to have higher mortality rates than other racial/ethnic groups (Figure 2, Table A.5). However, associations between race/ethnicity and mortality varied by age among people without dementia (Figure 3, Table A.5). African Americans ages <80 years without dementia had similar or higher hazard of mortality compared with Whites of the same age without dementia (e.g., mortality HR adjusted for comorbidities for African Americans vs. Whites ages 70–74 years=1.02; 95% CI: 0.95–1.10), but African Americans ages ≥80 years without dementia had lower hazard of mortality compared with Whites of the same age without dementia (e.g., mortality HR adjusted for comorbidities for African Americans vs. Whites ages ≥90 years=0.75; 95% CI: 0.68–0.82) (Figure 2, Table A.5).
Figure 2.
Hazard ratios (95% confidence intervals) relating race/ethnicity and mortality for people with incident dementia by age group from Cox proportional hazards models adjusted for comorbidities (Model 2): Kaiser Permanente Northern California, 2000–2013.
Model 2 is adjusted for age (as timescale), sex, and comorbidities (depression, diabetes, hypertension, stroke, cardiovascular disease). Hazard ratios relating race/ethnicity and mortality for people with incident dementia are not presented for ages 64–69 because estimates were imprecise due to the small number of incident dementia cases and deaths in this age group; age 70–74 is therefore the farthest left column shown for each racial/ethnic comparison. The hazard ratios and 95% confidence intervals are presented in Table A.4.
Figure 3.
Hazard ratios (95% confidence intervals) relating race/ethnicity and mortality for people without dementia, adjusted for comorbidities (Model 2): Kaiser Permanente Northern California, 2000–2013.
Model 2 is adjusted for age (as timescale), sex, and comorbidities (depression, diabetes, hypertension, stroke, cardiovascular disease). Hazard ratios relating race/ethnicity and mortality for people without dementia are not presented for ages 64–69 for consistency with Figure 2 (hazard ratios relating race/ethnicity and mortality for people with incident dementia); age 70–74 is therefore the farthest left column shown for each racial/ethnic comparison. The hazard ratios and 95% confidence intervals are presented in Table A.5.
The ratio of hazard ratios (RHR), comparing HRs relating race/ethnicity and mortality among people with dementia versus people without dementia were mostly close to 1.0 (Figure A.2, Table A.6). For example, the RHR adjusted for comorbidities for Asian Americans compared with Whites ages 80–84 years was 0.97 (95% CI: 0.87–1.08), indicating no difference in the relative impact of dementia on mortality among Asian Americans and Whites of the same age. For Asian Americans younger than 80 years, the RHRs were slightly greater than 1.0, but most of the confidence intervals included 1.0. For African Americans younger than 80 years, RHRs were less than 1.0. In other words, African Americans with dementia had lower mortality than Whites with dementia, whereas African Americans under age 80 without dementia had similar or higher mortality than their White counterparts.
4. Discussion
In nearly 60,000 incident dementia cases, we observed substantial differences in post-diagnosis survival by race/ethnicity, with lower mortality among Asian Americans, Latinos, African Americans, and American Indians/Alaska Natives compared with Whites in a setting with access to healthcare. These trends were evident across age groups and persisted after adjustment for comorbidities. Racial/ethnic mortality patterns among people with dementia were largely similar to patterns among people without dementia. In other words, dementia tended to have a similar relative impact on mortality across racial ethnic groups. A notable exception occurred among African Americans diagnosed with dementia before age 80, among whom dementia appeared to have a smaller relative impact on mortality than among Whites of the same age.
Racial/ethnic patterns in survival after dementia should be contextualized with racial/ethnic patterns among older adults without dementia. The mortality patterns among people without dementia in the present study are consistent with established patterns of lower mortality among racial/ethnic minorities compared with Whites in late-life, a phenomenon that is referred to as the mortality crossover [11]. Latinos and Asian Americans have lower overall mortality than Whites [24]. The mortality advantage among older African Americans and American Indians/Alaska Natives compared with Whites reflects a mortality reversal commonly observed in old age, often thought to reflect selective survival in these populations [11, 25]. By examining the relative impact of dementia on mortality across racial/ethnic groups, we found that longer survival after dementia diagnosis among racial/ethnic minorities largely mirrored mortality differences in the general population of older adults. Thus, higher mortality rates among older Whites compared with other racial/ethnic groups is not unique to dementia; the main factors driving differences in post-diagnosis survival are also likely present among older adults without dementia.
Although the simplest explanation is that the same factors that drive racial/ethnic mortality differences in people without dementia account for the similar racial/ethnic mortality differences in people with dementia, other possible explanations merit consideration. Longer survival after dementia diagnosis among racial/ethnic minority groups compared with Whites include differences in care or support after diagnosis [5–7], differences in the progression of the underlying neuropathological disease process [8, 9], prevalence of comorbidities at time of diagnosis beyond those accounted for by our measures, and timing of diagnosis with respect to the underlying neuropathological disease process [10]. Given that there are no effective disease-modifying treatments for dementia [26], it is unlikely that differences in treatment contribute to the observed trends. Differences in social support or type of supportive care may occur: prior studies have reported that Latino and African American dementia patients are less likely to be placed in nursing homes than White dementia patients [5–7], which could impact survival. Differences in the underlying etiology of dementia could contribute to racial/ethnic differences in post-diagnosis survival. For example, vascular pathology may play a greater role among Asian Americans[9] and African Americans [8], although this may lead to faster mortality rates than Alzheimer’s disease pathology [27]. Dementia dramatically increases mortality [1], so differences in timing of diagnosis with respect to the neuropathological disease process could also contribute to differences in post-diagnosis survival (i.e., a “lead-time bias” [28] with respect to the underlying neuropathological disease process) [10]. Differences in timing of diagnosis could be conceptualized as reflecting either bias or the influence of cognitive reserve [29], and could contribute to earlier or later diagnosis in racial/ethnic minority groups compared with Whites. [30, 31] For example, education and other social factors may protect against cognitive impairment in the presence of neuropathology and delay diagnosis, leading to faster post-diagnosis deterioration [3, 29]. Alternatively, cultural differences in perceptions of healthy aging, dementia, and care-seeking could delay diagnoses in some populations [10, 32–34] and shorten post-diagnosis survival.
Our study adds to evidence that African Americans tend to live longer after dementia diagnosis than Whites [2–4]. In contrast to our finding that survival after dementia diagnosis was similar for Latinos and Whites, previous studies have suggested longer post-diagnosis survival among Caribbean Latinos compared with Whites in the New York City-based Washington Heights-Inwood Community Aging Project (WHICAP) [4] and longer post-diagnosis survival in Latinos in a nationally-representative cohort study [3]. Mehta et al. reported longer survival of Latinos in the National Alzheimer’s Coordinating Center data set including patients seen at 30 U.S. memory clinics [2]. Mexican Americans are the largest Latino national origin group represented in this Northern California population, whereas previous studies had greater representation of Caribbean Latinos. This compositional difference may explain why Latinos and Whites had similar dementia incidence in Kaiser Permanente Northern California [17], but Latinos had higher dementia incidence rates than Whites in WHICAP [35]. To our knowledge, Mehta et al. is the only prior study comparing dementia survival for Whites with other racial/ethnic groups besides African Americans and Latinos; survival was similar for both American Indians/Alaska Natives and Asian Americans compared to Whites, although sample size was limited in these racial/ethnic groups [2].
Our finding that survival time after dementia diagnosis decreases with age at diagnosis is consistent with prior research [1, 36–38]. Estimates of median survival time after diagnosis in the present study are shorter than incident dementia cases identified in cohort studies with dementia ascertainment at pre-specified intervals or studies of prevalent dementia cases seen at memory clinics [2–4]. The longer survival time estimates in cohort or clinic enrollment studies are not surprising because these designs systematically omit patients with rapid disease progression due to selective dropout or death prior to the next scheduled study visit [37].
A major strength of this study is the racial/ethnic diversity and long follow up, allowing for identification of incident dementia cases and follow up for mortality after diagnosis. By following participants from the time of dementia diagnosis, we likely achieved better estimates of post-diagnosis survival time, and therefore estimates more relevant to patients and families, than is feasible with other study designs [37]. Furthermore, we were able to compare mortality patterns observed among dementia patients with mortality patterns among people of the same age and within the same healthcare system without dementia.
Without neuroimaging or neuropathology information to identify neuropathological burden at time of diagnosis or death in the present study, we could not examine dementia subtypes. Ongoing discussion and evolving diagnostic criteria call into question subtype characterizations based on medical records [39–42]. Future research with biomarkers to characterize neuropathological burden among people with dementia in diverse populations is needed. Our study relied on clinicians making diagnoses, which may underestimate the prevalence of dementia, and it is possible that there were secular trends in dementia diagnosis that our analyses did not capture. Another limitation is that some people were censored due to a lapse in health plan membership (annual censoring rate of 3.8% among people with dementia and 2.3% among people who remained dementia-free); thus, follow-up time was censored for these individuals at end of membership. However, censoring rates were similar across racial/ethnic groups. We were also unable to account for social determinants such as quantity or quality of education, which is associated with progression to dementia [3, 29], or important behaviors such as physical activity [43, 44]. Finally, although this is the largest and most diverse study to examine survival after dementia diagnosis, we combined diverse individuals within broad categories, such as Asian American or Latino, potentially missing important heterogeneity within these groups.
In summary, our study provides age-specific estimates of survival after dementia diagnosis for diverse racial/ethnic groups, documenting substantial differences in post-diagnosis survival. With the exception of African Americans diagnosed with dementia before age 80, the racial/ethnic differences in survival among people with dementia largely reflect mortality differences in the general population of older adults. Estimates of survival after dementia diagnosis provide important information for patients, their families, and public health planning. Although a diagnosis of dementia predicts substantially shortened life expectancy, most patients live for years after diagnosis. The social and financial costs of dementia overall are high; a recent study found that end-of-life care, including insurance costs, out-of-pocket expenses, and informal care, for Medicare patients with dementia far exceeded the costs for patients who died of other conditions [45]. The economic burden related to dementia care may exacerbate large racial/ethnic disparities in wealth [46]. Given the relatively long life expectancy of dementia patients, initiatives to promote quality of life after diagnosis, delay loss of independence, and reduce burden of care are critical.
Supplementary Material
Research in Context.
Systematic review: In a PubMed literature review, we identified no studies that have contrasted survival after diagnosis across the five major racial/ethnic groups in the United States in a usual care setting.
Interpretation: After initial dementia diagnosis, Whites had shortest median survival time, followed by American Indian/Alaska Natives, African Americans, Latinos, and Asian Americans. The racial/ethnic mortality patterns among people with dementia tended to parallel racial/ethnic mortality patterns among dementia-free older adults.
Future directions: Racial/ethnic differences in survival after dementia diagnosis may reflect diverse contributions of care, timing of diagnosis, or social and biological processes influencing disease progression. Better understanding of the mechanisms of these differences may improve outcomes for dementia patients and their families.
Acknowledgments
Funding for this study was supported in part by the University of California at San Francisco Center for Aging in Diverse Communities (P30-AG15272) under the Resource Centers for Minority Aging Research program of the National Institute on Aging, National Institutes of Health, a grant from the Kaiser Permanente Community Benefits Health Policy and Disparities Research Program, National Institute on Aging (K99AG053410, RF1A6052132), and National Institute Of Neurological Disorders And Stroke of the National Institutes of Health (U54NS081760). The funding organizations played no role in the design and conduct of the study; in the management, analysis, and interpretation of the data; or in the preparation, review, or approval of the manuscript.
Footnotes
Author contributions: ERM was responsible for study concept and design, analysis and interpretation of data, and drafting and revising of the manuscript. MMG was responsible for the study concept and design, interpretation of data, and revising of the manuscript. CPQ, JKJ, and EJPS were responsible for interpretation of the data and revising of the manuscript. RAW is the guarantor and was responsible for the study concept and design, interpretation of data, revising of the manuscript, and study supervision.
Disclaimer: The contents and views in this manuscript are those of the authors and should not be construed to represent the views of the National Institutes of Health or any of the sponsoring organizations and agencies of the US government.
References
- 1.Todd S, Barr S, Roberts M, Passmore AP. Survival in dementia and predictors of mortality: a review. International journal of geriatric psychiatry. 2013;28:1109–24. doi: 10.1002/gps.3946. [DOI] [PubMed] [Google Scholar]
- 2.Mehta KM, Yaffe K, Perez-Stable EJ, Stewart A, Barnes D, Kurland BF, et al. Race/ethnic differences in AD survival in US Alzheimer’s Disease Centers. Neurology. 2008;70:1163–70. doi: 10.1212/01.wnl.0000285287.99923.3c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Reuser M, Willekens FJ, Bonneux L. Higher education delays and shortens cognitive impairment: a multistate life table analysis of the US Health and Retirement Study. European journal of epidemiology. 2011;26:395–403. doi: 10.1007/s10654-011-9553-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Helzner EP, Scarmeas N, Cosentino S, Tang MX, Schupf N, Stern Y. Survival in Alzheimer disease: a multiethnic, population-based study of incident cases. Neurology. 2008;71:1489–95. doi: 10.1212/01.wnl.0000334278.11022.42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yaffe K, Fox P, Newcomer R, Sands L, Lindquist K, Dane K, et al. Patient and caregiver characteristics and nursing home placement in patients with dementia. JAMA : the journal of the American Medical Association. 2002;287:2090–7. doi: 10.1001/jama.287.16.2090. [DOI] [PubMed] [Google Scholar]
- 6.Mausbach BT, Coon DW, Depp C, Rabinowitz YG, Wilson-Arias E, Kraemer HC, et al. Ethnicity and time to institutionalization of dementia patients: a comparison of Latina and Caucasian female family caregivers. Journal of the American Geriatrics Society. 2004;52:1077–84. doi: 10.1111/j.1532-5415.2004.52306.x. [DOI] [PubMed] [Google Scholar]
- 7.Schulz R, Belle SH, Czaja SJ, McGinnis KA, Stevens A, Zhang S. Long-term care placement of dementia patients and caregiver health and well-being. JAMA : the journal of the American Medical Association. 2004;292:961–7. doi: 10.1001/jama.292.8.961. [DOI] [PubMed] [Google Scholar]
- 8.Barnes LL, Leurgans S, Aggarwal NT, Shah RC, Arvanitakis Z, James BD, et al. Mixed pathology is more likely in black than white decedents with Alzheimer dementia. Neurology. 2015;85:528–34. doi: 10.1212/WNL.0000000000001834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.White L, Petrovitch H, Ross GW, Masaki KH, Abbott RD, Teng EL, et al. Prevalence of dementia in older Japanese-American men in Hawaii: the Honolulu-Asia aging study. JAMA : the journal of the American Medical Association. 1996;276:955–60. [PubMed] [Google Scholar]
- 10.Barnes LL, Bennett DA. Alzheimer’s Disease In African Americans: Risk Factors And Challenges For The Future. Health Affairs. 2014;33:580–6. doi: 10.1377/hlthaff.2013.1353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hummer RA, Rogers RG, Masters RK, Saint Onge JM. International handbook of population aging. Springer; 2009. Mortality patterns in late life; pp. 521–42. [Google Scholar]
- 12.Gordon NP, Kaplan GA. Some evidence refuting the HMO “favorable selection” hypothesis: the case of Kaiser Permanente. Advances in health economics and health services research. 1991;12:19–39. [PubMed] [Google Scholar]
- 13.Krieger N. Overcoming the absence of socioeconomic data in medical records: validation and application of a census-based methodology. American journal of public health. 1992;82:703–10. doi: 10.2105/ajph.82.5.703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gordon NP. Similarity of the Kaiser Permanente Senior Member Population in Northern California to the Non-Kaiser Permanente Covered and General Population of Seniors in Northern California: Statistics from the 2009 California Health Interview Survey. Kaiser Permanente Northern California Division of Research; 2012. [Google Scholar]
- 15.Mayeda ER, Karter AJ, Huang ES, Moffet HH, Haan MN, Whitmer RA. Racial/ethnic differences in dementia risk among older type 2 diabetic patients: the diabetes and aging study. Diabetes care. 2014;37:1009–15. doi: 10.2337/dc13-0215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Exalto LG, Quesenberry CP, Barnes D, Kivipelto M, Biessels GJ, Whitmer RA. Midlife risk score for the prediction of dementia four decades later. Alzheimer’s & Dementia. 2014;10:562–70. doi: 10.1016/j.jalz.2013.05.1772. [DOI] [PubMed] [Google Scholar]
- 17.Mayeda ER, Glymour MM, Quesenberry CP, Whitmer RA. Inequalities in dementia incidence between six racial and ethnic groups over 14 years. Alzheimers & Dementia. 2016;12:216–24. doi: 10.1016/j.jalz.2015.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Whitmer RA, Karter AJ, Yaffe K, Quesenberry CP, Jr, Selby JV. Hypoglycemic episodes and risk of dementia in older patients with type 2 diabetes mellitus. JAMA : the journal of the American Medical Association. 2009;301:1565–72. doi: 10.1001/jama.2009.460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Katon W, Lyles CR, Parker MM, Karter AJ, Huang ES, Whitmer RA. Association of depression with increased risk of dementia in patients with type 2 diabetes: the Diabetes and Aging Study. Archives of general psychiatry. 2012;69:410–7. doi: 10.1001/archgenpsychiatry.2011.154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Katon WJ, Lin EH, Williams LH, Ciechanowski P, Heckbert SR, Ludman E, et al. Comorbid depression is associated with an increased risk of dementia diagnosis in patients with diabetes: a prospective cohort study. Journal of general internal medicine. 2010;25:423–9. doi: 10.1007/s11606-009-1248-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Taylor DH, Fillenbaum GG, Ezell ME. The accuracy of medicare claims data in identifying Alzheimer’s disease. Journal of clinical epidemiology. 2002;55:929–37. doi: 10.1016/s0895-4356(02)00452-3. [DOI] [PubMed] [Google Scholar]
- 22.Huang ES, Laiteerapong N, Liu JY, John PM, Moffet HH, Karter AJ. Rates of complications and mortality in older patients with diabetes mellitus: the diabetes and aging study. JAMA internal medicine. 2014;174:251–8. doi: 10.1001/jamainternmed.2013.12956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.VanderWeele TJ, Knol MJ. A tutorial on interaction. Epidemiologic Methods. 2014;3:33–72. [Google Scholar]
- 24.Miniño AM, Statistics NCfH. Death in the United States, 2007. US Department of Health and Human Services, Centers for Disease Control and Prevention, National Center for Health Statistics; 2009. [Google Scholar]
- 25.Jackson JS, Hudson D, Kershaw K, Mezuk B, Rafferty J, Tuttle KK. International handbook of adult mortality. Springer; 2011. Discrimination, chronic stress, and mortality among Black Americans: A life course framework; pp. 311–28. [Google Scholar]
- 26.Alzheimer’s Association. 2015 Alzheimer’s disease facts and figures. Alzheimer’s & Dementia. 2015;11:332–84. doi: 10.1016/j.jalz.2015.02.003. [DOI] [PubMed] [Google Scholar]
- 27.Fitzpatrick AL, Kuller LH, Lopez OL, Kawas CH, Jagust W. Survival following dementia onset: Alzheimer’s disease and vascular dementia. Journal of the neurological sciences. 2005;229:43–9. doi: 10.1016/j.jns.2004.11.022. [DOI] [PubMed] [Google Scholar]
- 28.Newman TB, Kohn MA. Evidence-based diagnosis. Cambridge University Press; 2009. [Google Scholar]
- 29.Stern Y. Cognitive reserve in ageing and Alzheimer’s disease. Lancet neurology. 2012;11:1006–12. doi: 10.1016/S1474-4422(12)70191-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Byrd DA, Sanchez D, Manly JJ. Neuropsychological test performance among Caribbean-born and US-born African American elderly: The role of age, education and reading level. Journal of Clinical and Experimental Neuropsychology. 2005;27:1056–69. doi: 10.1080/13803390490919353. [DOI] [PubMed] [Google Scholar]
- 31.Glymour MM, Manly JJ. Lifecourse social conditions and racial and ethnic patterns of cognitive aging. Neuropsychology review. 2008;18:223–54. doi: 10.1007/s11065-008-9064-z. [DOI] [PubMed] [Google Scholar]
- 32.Clark PC, Kutner NG, Goldstein FC, Peterson-Hazen S, Garner V, Zhang R, et al. Impediments to timely diagnosis of Alzheimer’s disease in African Americans. Journal of the American Geriatrics Society. 2005;53:2012–7. doi: 10.1111/j.1532-5415.2005.53569.x. [DOI] [PubMed] [Google Scholar]
- 33.Fitten LJ, Ortiz F, Pontón M. Frequency of Alzheimer’s disease and other dementias in a community outreach sample of Hispanics. Journal of the American Geriatrics Society. 2001;49:1301–8. doi: 10.1046/j.1532-5415.2001.49257.x. [DOI] [PubMed] [Google Scholar]
- 34.Romo RD, Wallhagen MI, Yourman L, Yeung CC, Eng C, Micco G, et al. Perceptions of successful aging among diverse elders with late-life disability. The Gerontologist. 2013;53:939–49. doi: 10.1093/geront/gns160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Tang M-X, Cross P, Andrews H, Jacobs D, Small S, Bell K, et al. Incidence of AD in African-Americans, Caribbean hispanics, and caucasians in northern Manhattan. Neurology. 2001;56:49–56. doi: 10.1212/wnl.56.1.49. [DOI] [PubMed] [Google Scholar]
- 36.Brookmeyer R, Corrada MM, Curriero FC, Kawas C. Survival following a diagnosis of Alzheimer disease. Archives of neurology. 2002;59:1764–7. doi: 10.1001/archneur.59.11.1764. [DOI] [PubMed] [Google Scholar]
- 37.Wolfson C, Wolfson DB, Asgharian M, M’Lan CE, Østbye T, Rockwood K, et al. A reevaluation of the duration of survival after the onset of dementia. New England Journal of Medicine. 2001;344:1111–6. doi: 10.1056/NEJM200104123441501. [DOI] [PubMed] [Google Scholar]
- 38.Ganguli M, Dodge HH, Shen C, Pandav RS, DeKosky ST. Alzheimer disease and mortality: a 15-year epidemiological study. Archives of neurology. 2005;62:779–84. doi: 10.1001/archneur.62.5.779. [DOI] [PubMed] [Google Scholar]
- 39.McKhann G, Drachman D, Folstein M, Katzman R, Price D, Stadlan EM. Clinical diagnosis of Alzheimer’s disease Report of the NINCDS-ADRDA Work Group* under the auspices of Department of Health and Human Services Task Force on Alzheimer’s Disease. Neurology. 1984;34:939. doi: 10.1212/wnl.34.7.939. [DOI] [PubMed] [Google Scholar]
- 40.McKhann GM, Knopman DS, Chertkow H, Hyman BT, Jack CR, Kawas CH, et al. The diagnosis of dementia due to Alzheimer’s disease: Recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimer’s & Dementia. 2011;7:263–9. doi: 10.1016/j.jalz.2011.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Román GC, Tatemichi TK, Erkinjuntti T, Cummings J, Masdeu J, Garcia Ja, et al. Vascular dementia Diagnostic criteria for research studies: Report of the NINDS-AIREN International Workshop*. Neurology. 1993;43:250. doi: 10.1212/wnl.43.2.250. [DOI] [PubMed] [Google Scholar]
- 42.Gorelick PB, Scuteri A, Black SE, DeCarli C, Greenberg SM, Iadecola C, et al. Vascular contributions to cognitive impairment and dementia a statement for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2011;42:2672–713. doi: 10.1161/STR.0b013e3182299496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Singh MAF, Gates N, Saigal N, Wilson GC, Meiklejohn J, Brodaty H, et al. The Study of Mental and Resistance Training (SMART) study—resistance training and/or cognitive training in mild cognitive impairment: a randomized, double-blind, double-sham controlled trial. Journal of the American Medical Directors Association. 2014;15:873–80. doi: 10.1016/j.jamda.2014.09.010. [DOI] [PubMed] [Google Scholar]
- 44.Langa KM, Levine DA. The diagnosis and management of mild cognitive impairment: a clinical review. JAMA : the journal of the American Medical Association. 2014;312:2551–61. doi: 10.1001/jama.2014.13806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kelley AS, McGarry K, Gorges R, Skinner JS. The Burden of Health Care Costs for Patients With Dementia in the Last 5 Years of Life. Annals of internal medicine. 2015;163:729–36. doi: 10.7326/M15-0381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Conley D. Being black, living in the red: Race, wealth, and social policy in America. Univ of California Press; 1999. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.