Abstract
Background
Aspirin-exacerbated respiratory disease (AERD) is characterized by tissue eosinophilia and mast cell activation, including abundant production of prostaglandin D2 (PGD2). Group 2 innate lymphoid cells (ILC2s), which promote tissue eosinophilia and mast cell responses, undergo chemotaxis and cytokine production in response to PGD2 but it is unknown whether ILC2s are active in AERD.
Objective
We sought to determine whether ILC2 numbers change in peripheral blood and nasal mucosa during cyclooxygenase-1 (COX-1) inhibitor-induced reactions in patients with AERD.
Methods
Blood and nasal scrapings were collected at baseline, during reaction, and after completion of ketorolac/aspirin challenge/desensitization in twelve patients with AERD. ILC2s and eosinophils were quantitated by flow cytometry. Urine was also collected and quantification of PGD2 metabolite and leukotriene E4 (LTE4) was done by ELISA. Baseline and NSAID-reaction clinical data was correlated with cell changes.
Results
ILC2s significantly increased in nasal mucosal samples and decreased in blood at the time of COX-1 inhibitor reactions in 12 patients with AERD. These changes were not observed in two patients without AERD. Further, eosinophils decreased in blood concurrently with significant increases in urinary PGD2 metabolite and LTE4. The magnitude of increases in nasal mucosal ILC2s positively correlated with maximum symptom score during the challenges. Further, the levels of blood ILC2s during the reaction correlated with the time for the reaction to resolve, possibly reflecting reaction severity.
Conclusions
ILC2s are recruited to the nasal mucosa during COX-1 inhibitor-induced reactions in patients with AERD, correlating with enhanced production of prostaglandins and leukotrienes.
Keywords: Group 2 innate lymphoid cells (ILC2s), Aspirin-exacerbated respiratory disease
Introduction
Aspirin-exacerbated respiratory disease (AERD) is clinically characterized by a relentless progression of nasal congestion, anosmia, formation of nasal polyps and, typically, asthma. Pathognomonic for AERD are naso-ocular and lower respiratory tract symptoms after ingestion of cyclooxygenase-1 (COX-1) inhibitors. AERD is present in about 7% of adult asthmatics and 14% of severe asthmatics (1) and may develop in patients without a prior history of atopy, asthma or rhinitis (2). The nasal polyps of AERD patients are intensely eosinophilic and contain activated mast cells (3, 4). The disease is characterized by release of large amounts of cysteinyl leukotrienes (CysLTs) and mast cells mediators, particularly prostaglandin D2 (PGD2), after COX-1 inhibitor ingestion (5, 6). Recently, thymic stromal lymphopoietin (TSLP) and IL-33 have also been implicated in AERD pathogenesis (7, 8). These two cytokines, in addition to PGD2 and CysLTs, are known to promote group 2 innate lymphoid cell (ILC2) activation (9-12) and their presence suggests that ILC2s may be active in AERD.
ILC2s are a recently described lymphocyte population that lack antigen specificity but are capable of producing large amounts of type 2 cytokines (IL-4, IL-5, IL-6, IL-9, and IL-13) and have been shown to promote type 2 inflammation (13). In humans, ILC2s are increased in affected tissues from patients with atopic dermatitis, allergic asthma, eosinophilic esophagitis, and allergic rhinitis (14-18). Importantly, ILC2s are increased in nasal polyps and levels correlate with the degree of eosinophilia (19). ILC2s are identified as lymphocytes that are lineage-negative (i.e. lacking the markers of B-, T-, or NK-cells) and express chemoattractant receptor-homologous molecule expressed on Th2 cells (CRTH2) and CD161 (3). CRTH2 is a G protein-coupled receptor that binds to prostaglandin D2 (PGD2) and PGD2 has been shown to promote ILC2 chemotaxis and cytokine production in vitro (10, 20). Thus, these suggest a potential role for ILC2s in AERD.
Our aim for this study was to determine ILC2 changes in the blood and nasal mucosa of AERD patients during COX-1 inhibitor challenges. To our knowledge there are no reports focused on a potential role for ILC2s in AERD NSAID-induced reactions. Importantly, we found that ILC2s were significantly increased in nasal scrapings and decreased in peripheral blood during the ketorolac-induced reactions in patients with AERD. These patients also had a corresponding decrease in blood eosinophils, indicating that these processes are happening simultaneously. Concomitantly, patients showed an increase in 11β-PGF2α, a stable metabolite of PGD2 (21), as well as the terminal cysteinyl leukotriene leukotriene E4 (LTE4). Overall, this study implicates ILC2s as an additional cell type apart from mast cells and eosinophils that may contribute to AERD pathogenesis.
Methods
AERD study subjects
Patients (n=14) undergoing aspirin desensitization for AERD at the Allergy Divisions of the University of California, San Diego (UCSD) and Scripps Clinic were recruited after institutional review board (IRB) approval at both institutions. Informed consent was obtained from all patients prior to starting the study. All patients were suspected of having a diagnosis of AERD due to history of nasal polyps, asthma, and compelling history of reactions to aspirin/NSAIDs within 2-3 hours. Demographic and clinical data was collected from retrospective chart review and included age, gender, ethnicity, asthma and rhinoconjunctivitis characteristics, nasal polyp surgical details and clinical response, aspirin/NSAID reaction characteristics, current medications and laboratory data. Symptom scores and spirometry were assessed at baseline. The modified AERD symptom scoring system included eight symptoms (nasal congestion, runny nose, sneezing, itchy nose, itchy/watery eyes, tight throat, wheezing and chest tightness) which the patients rate on a scale of 0-3 (range of possible scores are 0-24) (supplemental figure 2). SNOT-22 was recorded either that day or from a recent clinic visit.
Intranasal ketorolac and aspirin challenge/desensitizations
Intranasal ketorolac and aspirin challenge/desensitizations were done per standard protocol at each institution (Supplement figure 1, adapted from (22)). Reactions were identified as any upper and/or lower respiratory tract signs or symptoms consistent with conjunctivitis, rhinitis, laryngospasm, and bronchospasm or decrease in FEV1.
Nasal scrapings, blood draws, and urine samples
Nasal scraping was done using a Rhino-probe (Arlington Scientific, Springville, UT) and involved 1-3 passes over the inferior turbinate on one side. The nasal scrapings were transported in RPMI for processing for FACS analysis to detect ILC2s. Blood was collected in BD vacutainers (BD Biosciences, San Diego, CA) to quantitate levels of ILC2s and eosinophils. Samples, including nasal scraping, blood and urine, were collected at baseline. At the time of the first clinician-confirmed reaction, blood and a nasal scraping were again collected and urine was collected one hour later. A third set of samples was collected at the end of the desensitization prior to patient discharge. Some patients were not able to provide all three samples. Symptom scores (per the system noted above), FEV1, and medications required were recorded at each time point.
Identification of ILC2s and Eosinophils in peripheral blood and nasal scrapings
Peripheral blood mononuclear cells (PMBC) and granulocytes were separated with Histopaque 1119 and Histopaque 1077 (Sigma Aldrich, St. Louis, MO) per manufacturers protocol. Nasal scrapings were processed through a strainer and washed prior to staining. Cells were first incubated with Fc Receptor Blocking Reagent (Miltenyi Biotec, San Diego, CA). Granulocytes were then stained with PerCP-conjugated CD45, FITC-conjugated FcεR1α, and APC-conjugated CCR3 (Biolegend, San Diego, CA). Eosinophils were defined as the percentage of CD45+ granulocytes that were CCR3+ FcεR1α−. PBMCs and nasal scrapings were stained with PerCP-conjugated CD45 (Biolegend), FITC-conjugated lineage markers (CD235a, FcεR1α, TCR γ/δ, CD4 (Biolegend), and BD Lineage Cocktail 1 [CD3, CD14, CD16, CD19, CD20, CD56]) and PE-conjugated CRTH2 (Miltenyi Biotec). PBMCs were also stained for APC-conjugated CD161 (Biolegend). Blood ILC2s were defined as CD45+ lineage negative CD161+ CRTH2+ lymphocytes as percentage of all cells. Percent of ILC2s are reported as percent of enriched PBMCs. Nasal scraping ILC2s were defined as CD45+ lineage-negative CRTH2+ lymphocytes. Percent of ILC2s are reported as percent of total cells in the nasal scraping sample. Flow cytometry was performed using a Novocyte cytometer (Acea Biosciences, Inc, San Diego, CA). Data were further analyzed with FlowJo software (FlowJo LLC, Ashland, OR).
Urine 11β-PGF2α and LTE4
Urine samples were stored at −80C. PGD2 is metabolized to 11β-PGF2α, a stable, active metabolite, which is measurable in the urine (21). 11β-PGF2α levels were analyzed by ELISA per manufacturers protocol (Cayman Chemical, Ann Arbor, MI). Urinary LTE4 levels were analyzed by ELISA per manufacturers protocol (Cayman Chemical). Values were corrected for specific gravity as previously described (23).
Nasal Polyps
Nasal polyps were collected from 2 subjects undergoing endoscopic sinus surgery at UCSD who ultimately underwent desensitization for AERD and are included in that analysis. Tissue samples were transported in RPMI and processed for flow cytometry the same day. Nasal polyp tissue was digested with Collagenase Type 4 (Worthington) and DNase I (200 µg/mL) (Roche, Indianapolis, IN) and then filtered into a single cell suspension. ILC2s were identified per above. GATA3-positive cells were identified as CD45+ lineage-negative GATA3+ lymphocytes. The cells were initially stained for the cell surface CD45 and lineage markers per above. The cells then underwent fixation and permeabilization (eBioscience) and stained for PE-conjugated GATA-3 (eBioscience). Flow cytometry and analysis was done per above.
Statistical Analyses
Statistical analysis was performed with Graphpad Prism software (Graphpad Software Inc, La Jolla, CA). The paired t-test or Pearson’s correlation test were used, where indicated. A P value of less than .05 was considered statistically significant.
Results
AERD patient demographics and NSAID reaction characteristics
A total of 14 suspected AERD patients were enrolled in the study. Baseline patient characteristics are summarized in Table 1. At the time of desensitization, thirteen patients were on a leukotriene modifier drug (LMD) for a minimum of one week. One was not on a LMD due to adverse effects. Two patients (14%) were on daily oral corticosteroids. Eleven patients had typical COX-1 inhibitor-induced reactions including bronchoconstriction and/or naso-ocular symptoms during ketorolac challenge. Three patients did not have reactions to ketorolac or aspirin challenge. One of the three patients had a history of recurrent, aggressive nasal polyposis, severe asthma and was on omalizumab which could possibly avert COX-1 inhibitor-induced exacerbations (24), so was determined to nonetheless have AERD (a “silent reaction”). The two other patients had less consistent histories of reactions to COX-1 inhibitors, were not on omalizumab, and had no clinical response to aspirin. These two were determined not to have AERD and served as the controls. One of these control patients was the sole patient taking zileuton. “Reaction” samples for these three were taken after the final dose of ketorolac.
Table 1.
Patient characteristics
| Number of patients | 14 |
|---|---|
| Sex (male/female [no.]) | 5/9 |
| Median age (range [y]) | 54.5 (40-66) |
| Race (no.) | |
| Caucasian | 13 |
| African-American | 1 |
| Personal history of reaction to Aspirin/NSAID (%) | 100 |
| Leukotriene modifier drug use (%) | 92.8 |
| Montelukast (%) | 85.7 |
| Zileuton (%) | 7.1 |
| Asthma (%) | 92.8 |
| Baseline FEV1 % (range) | 82 (67-96) |
| Daily inhaled corticosteroid use (%) | 85.7 |
| Daily oral corticosteroid use (%) | 14.3 |
| Omalizumab treatment (%) | 21.4 |
| Personal history of sinus surgery (%) | 100 |
| Median number of sinus surgeries (range [no.]) | 4 (1-7) |
| Snot 22 Score (range) | 30 (0-73) |
| Nasal corticosteroid use (%) | 100 |
| Nasal budesonide rinse use (%) | 28.6 |
| Personal history of atopy (%) | 64.2 |
| Median total IgE IU/mL (range) | 52 (9-354) |
| Median blood eosinophils/mm3 (range) | 519 (100-1200) |
| Reaction | |
| Number of positive ketorolac challenges | 11 |
| Number of silent desensitizations | 1 |
| Number considered aspirin tolerant | 2 |
| Provocative Dose (no.) | |
| Ketorolac 2.53 mg | 1 |
| Ketorolac 5.05 mg | 6 |
| Ketorolac 7.58 mg | 4 |
| Median decrease in FEV1 % (range) | 9.45 (0-43.9) |
| Abdominal Symptoms (no.) | 4 |
| Treatment requiring corticosteroids (no.) | 6 |
Identification of ILC2s in Nasal Scrapings and Blood
Nasal polyp ILC2s have been previously reported as CD45+ lineage negative lymphocytes that express CRTH2 (3, 19). To confirm our ILC2 gating strategy for nasal scrapings, we first stained nasal polyps from two patients with AERD, along with additional staining for the master Th2 cytokine transcription factor GATA3, as proof of concept that ILC2s are indeed identifiable in these patients (Supplemental figure 3). Nasal scraping ILC2s were identified as CD45+ lineage-negative CRTH2+ lymphocytes (Figure 1A). We did not use CD161 for tissue ILC2 staining to keep consistency with what we had previously performed on nasal polyps (19). Blood samples were separated into PBMCs and granulocytes as described in the Methods.
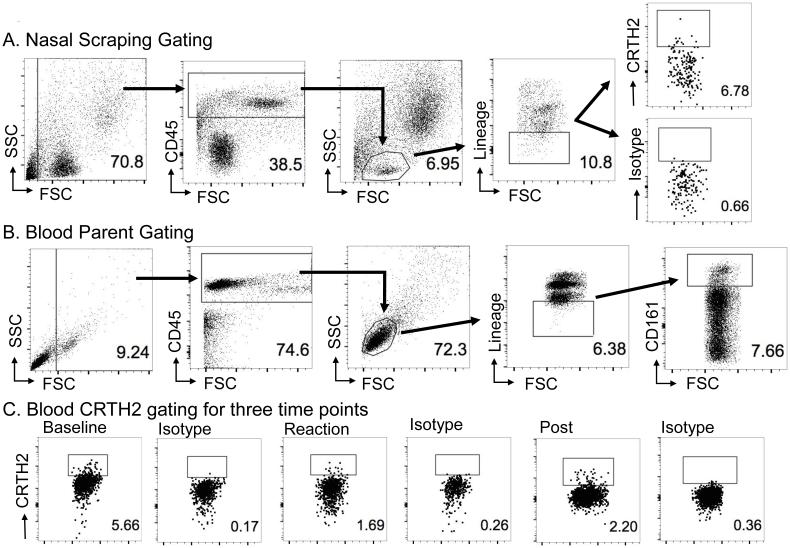
Figure 1. Gating strategy for nasal scrapings and blood ILC2s.
Nasal scraping gating strategy derived from polyp data (Supplement fig. 2). (A) Representative nasal sample ILC2 gating from post-desensitization sample in an AERD patient. ILC2s were identified as CD45+ lineage-negative lymphocytes that expressed CRTH2. (B) Representative parent gating for blood ILC2s. Blood ILC2s were identified as CD45+ lineage-negative lymphocytes that expressed both CD161+ and CRTH2+. (C) Representative data of blood CRTH2 plots of baseline, reaction, and post-desensitization samples along with their respective isotypes.
ILC2s were obtained from the enriched PBMCs and identified as CD45+ lineage-negative lymphocytes that expressed CD161 and CRTH2 (Figure 1B). Isotype controls were done for all CRTH2 gating. Overall, we were able to identify ILC2s in blood and nasal scrapings of patients with AERD.
ILC2s increase in nasal mucosa and decrease in blood from baseline during COX-1 inhibitor challenge
The percent of ILC2s of all cells in nasal scrapings increased significantly from the baseline to COX-1 inhibitor-induced reaction (P=0.001)(Fig 2A). There was also a subsequent significant decrease in nasal scraping ILC2s from reaction to post-aspirin desensitization samples (P=0.004). Further, blood ILC2s decreased significantly from baseline to reaction samples (P=0.031)(Figure 2B). Representative baseline, reaction, and post-desensitization blood ILC2 plots are shown in Figure 1C. The trend for blood ILC2s to increase from reaction to post-desensitization samples approached but did not reach significance (P=0.084). The changes in nasal scraping and blood ILC2s were not seen in two control patients (Fig 2C). The decrease in blood ILC2s occurred concurrently to a significant decrease in blood eosinophils from baseline to time of reaction (P=0.044)(Figure 2D). Due to low nasal scraping cell numbers, we were able to identify eosinophils in only two patients. These two samples showed a 1.5 and 7.9-fold increase in nasal scraping eosinophils from baseline to reaction (3.34% to 5.01% reaction and 0.34% to 2.68%, respectively).
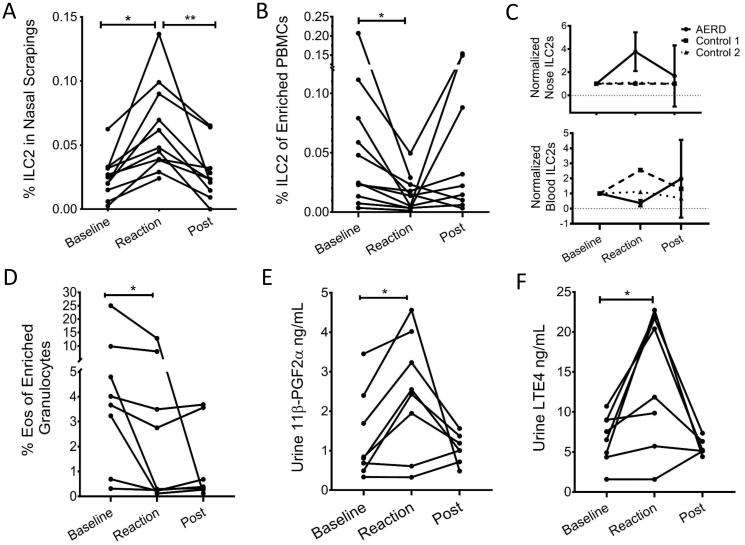
Figure 2. Changes in ILC2s, eosinophils and urinary 11β-PGF2α and LTE4 during aspirin desensitization.
(A) Change in percentage ILC2s as percentage of all cells in nasal scrapings before challenge, during reaction, and after desensitization complete (post). Change between baseline and reaction samples was significant (paired t-test, P=0.001) as well as between reaction and post desensitization samples (paired t-test, P=0.004). (B) Change in percentage of ILC2s from enriched PBMCs before challenge, during reaction, and after desensitization complete (post). Change between baseline and reaction samples was significant (paired t-test, P=0.031). (C) Normalized values for nasal scraping and blood ILC2s at the three times points. Solid line is for patients with AERD and dotted lines are for controls. (D) Eosinophils as percentage of enriched granulocytes. Difference between baseline and reaction samples was significant (paired t-test, P=0.044). (E) Change in urine PGD-2 metabolite 11β-PGF2α. Difference between baseline and reaction samples was significant (P=0.008). (F) Change in urine LTE4. Difference between baseline and reaction sample was significant (P=0.017).
As expected, there was an increase in the PGD2 metabolite 11β-PGF2α and LTE4 measured in the urine during the COX-1 inhibitor-induced reaction (11β-PGF2α P=0.008, LTE4 P=0.017, difference between baseline and reaction samples)(Figs 2D and 2E). The increases in urine 11β-PGF2α and LTE4 were observed at the same time as the increase in nasal scraping ILC2s and decrease in the blood ILC2s and eosinophils. Baseline nasal scraping ILC2s did not correlate with reaction levels of 11β-PGF2α (P=0.12) or LTE4 (0.16). The magnitude of increase in nasal scraping ILC2s did not correlate with the magnitude of increase in 11β-PGF2α (P=0.105) or LTE4 (P=0.470) or the decrease in blood ILC2s (P=0.713) or eosinophils (P=0.281). The baseline blood ILC2s negatively correlated with levels of reaction urine 11β-PGF2α (R2=0.559, P=0.033) and LTE4 (R2=0.506, P=0.048). The magnitude of decrease in blood ILC2s did not directly correlate with the magnitude of decrease in blood eosinophils (P=0.536) or increase in 11β-PGF2α (P=0.327) or LTE4 (P=0.064). Thus, ILC2s increase in the nasal mucosa and decrease in the blood during COX-1 inhibitor-induced reactions in patients with AERD.
Nasal ILC2s correlate with maximum symptom score
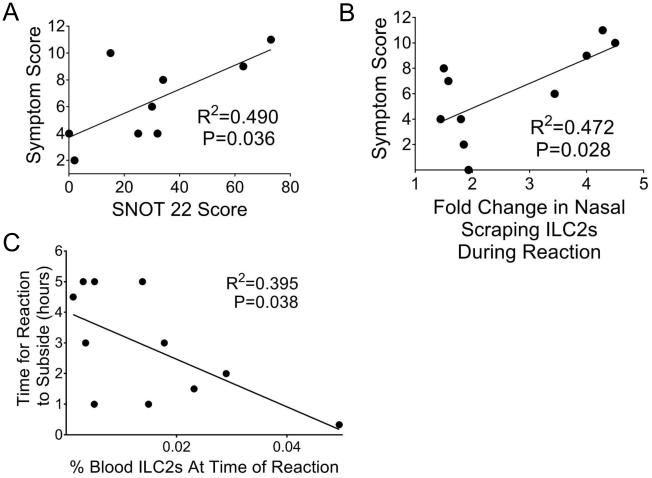
Symptom scores (evaluating upper and lower airway symptoms as noted in Methods) were assessed at regular intervals during the desensitization. Baseline SNOT-22 scores positively correlated with maximum symptom scores during desensitization (Pearson correlation coefficient, two-tailed R2= 0.490, P=0.036)(Figure 3A). The patient’s baseline nasal scraping ILC2 level was assigned a value of one and fold change from there was calculated. Interestingly, the fold change from baseline to reaction of nasal ILC2s positively correlated with the maximum symptom score (R2=0.472, P=0.028)(Fig 3B). The percentage of ILC2s in the blood negatively correlated with the maximum symptom score, although this fell short of significance (Pearson correlation coefficient, two-tailed R2=0.396, P=0.051). Baseline nasal scraping and blood ILC2s did not correlate with maximum symptom score (P=0.95 and P=0.21, respectively).
Figure 3. Change in ILC2s as related to reaction characteristics.
(A) Significant correlation was seen between maximum symptom score during reaction and baseline SNOT 22 score (Pearson correlation coefficient, two-tailed R2=0.490, P=0.036). (B) Significant correlation seen between maximum symptom score and fold increase in nasal scraping ILC2s during the reaction (Pearson correlation coefficient, R2=0.472, P=0.028). (C) Correlation between level of ILC2s in the blood and time for reaction to subside (Pearson correlation coefficient, two-tailed R2=0.395, P=0.038).
The level of ILC2s in the blood at the time of the reaction negatively correlated with the time it took for the reaction to subside (Pearson correlation coefficient, two-tailed R2=0.395, P=0.038)(Fig 3C). This may reflect that the more severe the reaction, the more ILC2s respond to stimuli to move out of the blood and into the tissues. Baseline nasal scraping and blood ILC2s did not correlate with time for reaction to subside (P=0.31 and 0.25, respectively). In those patients with symptoms on the final day of their desensitization (skin, GI, or respiratory) that required treatment, their average level of ILC2s in the blood was lower than those without symptoms (although this trend fell short of significance) (two-tailed unpaired t-test, 0.027% vs 0.11%, P=0.061). Neither post-desensitization blood ILC2 level nor eosinophils correlated with time since the reaction on day 1 or final dose of aspirin. The level of ILC2s on the last day did not differ if the patient received systemic corticosteroids during the reaction on day one. We did not find a correlation of blood ILC2 level with decrease in FEV1 (P=0.52). Reaction nasal scraping or blood ILC2s did not differ based on gender, age, atopic status, severity of asthma, use of nasal steroids, number of sinus surgeries or time since last surgery.
Discussion
Though intensive investigation has recently characterized ILC2s in asthma, allergic rhinitis, and nasal polyposis, there are no reports evaluating changes in ILC2s in AERD during NSAID reactions. Our novel findings demonstrate that ILC2s are recruited to the nasal mucosa during COX-1 inhibitor-induced reactions in AERD patients and the increase in nasal ILC2 levels from baseline correlates with reaction symptom severity. Concomitantly, ILC2s were reduced in peripheral blood along with eosinophils during NSAID reactions and correlated inversely with duration of the NSAID reaction. Further, levels of lipid mediators that could recruit and activate ILC2s in tissues including LTE4 and PGD2 metabolite were increased in the urine during reaction.
ILC2s are activated by PGD2 and are capable of producing large amounts of type 2 cytokines leading to tissue eosinophilia (10, 13, 20). Importantly, AERD is also associated with these characteristics and we postulated that ILC2s may be active in the disease. In this study, we found that after ketorolac challenge-induced reactions in patients with AERD, blood ILC2s decreased with a concurrent increase in nasal scraping ILC2s. These changes were contemporaneous with an increase in the stable PGD2 metabolite 11β-PGF2α and the terminal cysteinyl leukotriene LTE4. PGD2 and LTE4 are increased at baseline in patients with AERD and increase after COX-1 inhibitor ingestion (5, 25) and PGD2 has been shown to promote chemotaxis in ILC2s (10, 20). Notably, CysLTs have been shown to be chemotactic for Th2 cells (26) and eosinophils (27), and could be also be contributing to the ILC2 responses we observed. The level of blood ILC2s during the reactions negatively correlated with the time it took for the reactions to subside which could be due to ongoing ILC2 recruitment during more severe reactions but this hypothesis will require further study.
Changes in nasal mucosal ILC2s also correlated positively with severity of symptoms during reactions. Buchheitt et al. reported that increased nasal symptoms in AERD correlated with peak levels of a PGD2 metabolite which could promote ILC2 recruitment and activation (7). These data also indicate that subjective patient symptoms, particularly naso-ocular symptoms, can be correlated with objective laboratory measurements of reaction intensity. In our study, though nasal mucosal ILC2s correlated with reaction severity, we were unable to show a correlation between change in ILC2s and change in FEV1. There may be several reasons for this finding. First, all of our patients were taking a leukotriene modifier drug for at least one week which has been shown to reduce the decrease in FEV1 after aspirin challenge leading to more upper airway only reactions (28). Second, all patients were first challenged/desensitized to nasal ketorolac, which also limits lower airway reactions (22). Finally, nasal recruitment of ILC2s may not be completely representative of recruitment and activation of lung ILC2s during AERD reactions.
At the end of the clinical desensitization, we did not observe a consistent pattern of changes in blood ILC2 or eosinophil levels as values remained low in some patients while others increased back to pre-desensitization levels. The difference in dose of aspirin and duration of protocols could contribute to some of this variability, although subgroup analysis with small numbers of patients continued to show lack of consistent responses in post-desensitization ILC2 and eosinophil levels. These individual differences did not correlate with systemic steroid use, which could have an effect on ILC2 numbers (19). Those patients who had symptoms on the last day had a trend to lower number of ILC2s in their blood, although this fell short of significance. The differences in post desensitization levels may be due to ongoing mast cell activation, noted by continued symptoms, and recruitment of ILC2s into the tissue. Indeed, one mechanism by which aspirin desensitization may work is by preventing cells from homing into the tissues (29). PGD2 is increased in mast cells from patients with AERD (7) and high doses of aspirin are able to block both COX-1 and COX-2, which may allow for prevention of high amounts of PGD2. Cahill et al. showed that, PGD2 decreases after aspirin desensitization and blood eosinophils actually increase in the blood over time (29), presumably due to loss of the PGD2 gradient. Whether there is also an increase in blood ILC2s is not known.
Previous work has shown that AERD may be mediated by innate type 2 immunity. First, the potency of the COX-1 inhibition determines the severity of the reaction which indicates that it is not adaptive recognition of a specific drug structure that activates the immune system (30). Second, two danger signals released from epithelial cells, IL-33, an alarmin-like cytokine, and TSLP, an IL-7-like cytokine, have been shown to be upregulated in nasal polyps from patients with AERD and important drivers for COX-1 inhibitor-induced reactions (7, 8, 31). In a mouse model, blockage of IL-33 abrogated the change in airway resistance and cytokine release after aspirin-lysine challenge (8) which supports in vitro studies that showed IL-33 alone can induce human mast cell cytokine production (32). Dysregulation of the leukotriene pathway contributes to the pathogenesis of AERD (6) and IL-33 expression was found to be dependent on leukotrienes acting on epithelial cells. High levels of PGD2 are characteristic for AERD and Buchheitt et al. showed that TSLP was able to induce PGD2 generation in culture (7). Interestingly, IL-33 and TSLP strongly activate ILC2s to produce cytokines capable of driving eosinophilia independently of adaptive immunity (11, 12). ILC2s also express CyLT1R and generate cytokines in response to stimulation with CysLTs (9). Thus, though we have suggested that PGD2 may be a key mediator for ILC2 influx into the nose, it is possible that IL-33, TSLP, and/or CysLTs are also contributing.
The role of ILC2s after recruitment into tissue is not clear, though the cytokines produced by ILC2s may promote several feature of AERD including eosinophilia and airway hyperresponsiveness (33). Intensely eosinophilic mucosal tissues is one of the disease’s hallmarks and ILC2s produce abundant amounts IL-5, which is the major promoter of eosinophils (34). Further, IL-9 is also produced by ILC2s which contributes to mast cell accumulation and mucus production in animal asthma models (35). Interestingly, both mast cells and eosinophils are an important source of PGD2 (36) indicating a reciprocal relationship between ILC2s and these cell populations. Thus, ILC2s are a good candidate to support the prominent eosinophilic inflammation and mast cell activation seen in AERD, especially given the lack of requirement for specific antigen.
We were able to replicate earlier data which showed a decrease in blood eosinophils after aspirin challenge (6, 7). Eosinophils have been studied considerably in patients with AERD. Eosinophils, which express CRTH2, respond to PGD2 by undergoing chemotaxis and this can be blocked with a CRTH2 antagonist (37). Eosinophils counts decrease in the blood of patients with AERD after aspirin challenge (6, 7) presumably because they are migrating to the tissue in response to the high levels of PGD2 that are released during these reactions. Indeed, eosinophils have been shown to increase in nasal lavage after nasal lysine-aspirin challenge in patients with AERD (38), although in this previous study blood eosinophils were not measured to show whether a corresponding decrease occurs. ILC2s also express CRTH2 and undergo chemotaxis in response to PGD2 in vitro (10), and we hypothesized that they too may migrate in response to the high levels of PGD2 made during COX-1 inhibitor-provoked reactions.
We did not find a direct correlation between increases in ILC2s in the tissue and declines in peripheral blood ILC2s or eosinophil counts. This may be due to several factors. First, animal studies have shown that ILC2s are recruited from the bone marrow and the kinetics of replenishment in humans is currently unknown (13). Furthermore, there may be many more ILC2s in the blood versus the nasal mucosa and a small change in circulating ILC2s could lead to a much larger change in the nose. Thus, a direct inverse correlation may not have been detected due to disproportionate changes in both compartments (nose vs. blood), replenishment from bone marrow, and recruitment to other tissues (lung, GI tract, skin) during reactions.
Though our findings are novel in connecting ILC2s to AERD, there are several limitations of our investigations. First, we did not perform functional studies of nasal ILC2s due to very limited cell numbers. Second, CRTH2 was used to identify nasal scraping ILC2s which can be down-regulated in the presence of PGD2 (7, 10). However, CRTH2 has been shown to be the most specific marker for human ILC2s (39) and we have not reliably found other surface markers including IL-33R to be useful. Finally, all of the patients were taking leukotriene modifying drugs which have been shown to decrease the airway response seen after aspirin challenge (28). Naso-ocular symptoms are not typically blocked by these drugs, but it is possibly that these agents blunted the recruitment of ILC2s.
In summary, the AERD patients we studied demonstrated ILC2 increases in nasal scrapings and decreases in blood and during COX-1 inhibitor challenges, which also corresponded to increases in urinary 11β-PGF2α and severity of symptoms. Thus, ILC2s are recruited to the nasal mucosa in AERD and could contribute to pathogenesis through production of Th2 cytokines.
Supplementary Material
Key Messages.
ILC2s increased in nasal mucosa and decreased in blood during COX-1 inhibitor reactions in patients with AERD.
Eosinophils also decreased in blood and urinary PGD2 metabolite and LTE4 levels increased during AERD reactions.
Levels of nasal mucosa ILC2s correlated positively with symptom scores.
Capsule Summary.
Group 2 innate lymphoid cells (ILC2s) that promote eosinophil and mast cell responses were increased in the nasal mucosa and decreased in the blood during COX-1 inhibitor reactions in AERD. Nasal mucosal ILC2 levels correlated with symptom scores that reflected reaction severity.
Acknowledgements
Dr. Katherine Woessner, nursing staff at Scripps Clinic and University of Southern California, San Diego.
This study was supported by NIH AI114585, AI 70535, to T.A.D., NIH T32 AI 007469 to J.J.E., NIH AI 107779, AI 38425, AI 70535, AI 72115 to D.H.B., and Division of Rheumatology, Allergy and Immunology at UC San Diego.
Abbreviations
- AERD
aspirin-exacerbated respiratory disease
- COX-1
cyclooxygenase-1
- CysLTs
cysteinyl leukotrienes
- ELISA
enzyme-linked immunosorbent assay
- FACS
fluorescence-activated cell sorting
- FEV1
volume that is exhaled at the end of the first second of forced expiration
- IL-33
interleukin 33
- ILC2
type 2 innate lymphoid cell
- LTC4
leukotriene C4
- LTE4
leukotriene E4
- LMD
leukotriene modifier drug
- NSAID
nonsteroidal anti-inflammatory drug
- PBMC
peripheral blood mononuclear cells
- PGD2
prostaglandin D2
- 11β-PGF2α
11β prostaglandin F2α, a stable metabolite of PGD2
- RPMI
Roswell Park Memorial Institute medium
- SNOT-22
sino-nasal outcome test 22
- TSLP
thymic stromal lymphopoietin
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Rajan JP, Wineinger NE, Stevenson DD, White AA. Prevalence of aspirin-exacerbated respiratory disease among asthmatic patients: A meta-analysis of the literature. J Allergy Clin Immunol. 2015 Mar;135(3):676–81. doi: 10.1016/j.jaci.2014.08.020. e1. [DOI] [PubMed] [Google Scholar]
- 2.Szczeklik A, Nizankowska E, Duplaga M. Natural history of aspirin-induced asthma. AIANE Investigators. European Network on Aspirin-Induced Asthma. Eur Respir J. 2000 Sep;16(3):432–6. doi: 10.1034/j.1399-3003.2000.016003432.x. [DOI] [PubMed] [Google Scholar]
- 3.Mjosberg JM, Trifari S, Crellin NK, Peters CP, van Drunen CM, Piet B, et al. Human IL-25- and IL-33-responsive type 2 innate lymphoid cells are defined by expression of CRTH2 and CD161. Nat Immunol. 2011 Sep 11;12(11):1055–62. doi: 10.1038/ni.2104. [DOI] [PubMed] [Google Scholar]
- 4.Stevens WW, Schleimer RP, Kern RC. Chronic Rhinosinusitis with Nasal Polyps. J Allergy Clin Immunol Pract. 2016 Jul-Aug;4(4):565–72. doi: 10.1016/j.jaip.2016.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bochenek G, Nagraba K, Nizankowska E, Szczeklik A. A controlled study of 9alpha,11beta-PGF2 (a prostaglandin D2 metabolite) in plasma and urine of patients with bronchial asthma and healthy controls after aspirin challenge. J Allergy Clin Immunol. 2003 Apr;111(4):743–9. doi: 10.1067/mai.2003.1387. [DOI] [PubMed] [Google Scholar]
- 6.Sladek K, Szczeklik A. Cysteinyl leukotrienes overproduction and mast cell activation in aspirin-provoked bronchospasm in asthma. Eur Respir J. 1993 Mar;6(3):391–9. [PubMed] [Google Scholar]
- 7.Buchheit KM, Cahill KN, Katz HR, Murphy KC, Feng C, Lee-Sarwar K, et al. Thymic stromal lymphopoietin controls prostaglandin D2 generation in patients with aspirin-exacerbated respiratory disease. J Allergy Clin Immunol. 2016 May;137(5):1566–76. doi: 10.1016/j.jaci.2015.10.020. e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Liu T, Kanaoka Y, Barrett NA, Feng C, Garofalo D, Lai J, et al. Aspirin-Exacerbated Respiratory Disease Involves a Cysteinyl Leukotriene-Driven IL-33-Mediated Mast Cell Activation Pathway. J Immunol. 2015 Oct 15;195(8):3537–45. doi: 10.4049/jimmunol.1500905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Doherty TA, Khorram N, Lund S, Mehta AK, Croft M, Broide DH. Lung type 2 innate lymphoid cells express cysteinyl leukotriene receptor 1, which regulates TH2 cytokine production. J Allergy Clin Immunol. 2013 Jul;132(1):205–13. doi: 10.1016/j.jaci.2013.03.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Xue L, Salimi M, Panse I, Mjosberg JM, McKenzie AN, Spits H, et al. Prostaglandin D2 activates group 2 innate lymphoid cells through chemoattractant receptor-homologous molecule expressed on TH2 cells. J Allergy Clin Immunol. 2014 Apr;133(4):1184–94. doi: 10.1016/j.jaci.2013.10.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bartemes KR, Iijima K, Kobayashi T, Kephart GM, McKenzie AN, Kita H. IL-33-responsive lineage-CD25+ CD44(hi) lymphoid cells mediate innate type 2 immunity and allergic inflammation in the lungs. J Immunol. 2012 Feb 1;188(3):1503–13. doi: 10.4049/jimmunol.1102832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kim HY, Chang YJ, Subramanian S, Lee HH, Albacker LA, Matangkasombut P, et al. Innate lymphoid cells responding to IL-33 mediate airway hyperreactivity independently of adaptive immunity. J Allergy Clin Immunol. 2012 Jan;129(1):216–27. doi: 10.1016/j.jaci.2011.10.036. e1-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Doherty TA. At the bench: understanding group 2 innate lymphoid cells in disease. J Leukoc Biol. 2015 Mar;97(3):455–67. doi: 10.1189/jlb.5BT0814-374R. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kim BS, Siracusa MC, Saenz SA, Noti M, Monticelli LA, Sonnenberg GF, et al. TSLP elicits IL-33-independent innate lymphoid cell responses to promote skin inflammation. Sci Transl Med. 2013 Jan 30;5(170):170ra16. doi: 10.1126/scitranslmed.3005374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bartemes KR, Kephart GM, Fox SJ, Kita H. Enhanced innate type 2 immune response in peripheral blood from patients with asthma. J Allergy Clin Immunol. 2014 Sep;134(3):671–8. doi: 10.1016/j.jaci.2014.06.024. e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nagakumar P, Denney L, Fleming L, Bush A, Lloyd CM, Saglani S. Type 2 innate lymphoid cells in induced sputum from children with severe asthma. J Allergy Clin Immunol. 2016 Feb;137(2):624–6. doi: 10.1016/j.jaci.2015.06.038. e6. [DOI] [PubMed] [Google Scholar]
- 17.Doherty TA, Baum R, Newbury RO, Yang T, Dohil R, Aquino M, et al. Group 2 innate lymphocytes (ILC2) are enriched in active eosinophilic esophagitis. J Allergy Clin Immunol. 2015 Sep;136(3):792–4. doi: 10.1016/j.jaci.2015.05.048. e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lao-Araya M, Steveling E, Scadding GW, Durham SR, Shamji MH. Seasonal increases in peripheral innate lymphoid type 2 cells are inhibited by subcutaneous grass pollen immunotherapy. J Allergy Clin Immunol. 2014 Nov;134(5):1193–5. doi: 10.1016/j.jaci.2014.07.029. e4. [DOI] [PubMed] [Google Scholar]
- 19.Walford HH, Lund SJ, Baum RE, White AA, Bergeron CM, Husseman J, et al. Increased ILC2s in the eosinophilic nasal polyp endotype are associated with corticosteroid responsiveness. Clin Immunol. 2014 Nov;155(1):126–35. doi: 10.1016/j.clim.2014.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chang JE, Doherty TA, Baum R, Broide D. Prostaglandin D2 regulates human type 2 innate lymphoid cell chemotaxis. J Allergy Clin Immunol. 2014 Mar;133(3):899–901. doi: 10.1016/j.jaci.2013.09.020. e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Beasley RC, Featherstone RL, Church MK, Rafferty P, Varley JG, Harris A, et al. Effect of a thromboxane receptor antagonist on PGD2- and allergen-induced bronchoconstriction. J Appl Physiol (1985) 1989 Apr;66(4):1685–93. doi: 10.1152/jappl.1989.66.4.1685. [DOI] [PubMed] [Google Scholar]
- 22.Lee RU, White AA, Ding D, Dursun AB, Woessner KM, Simon RA, et al. Use of intranasal ketorolac and modified oral aspirin challenge for desensitization of aspirin-exacerbated respiratory disease. Ann Allergy Asthma Immunol. 2010 Aug;105(2):130–5. doi: 10.1016/j.anai.2010.05.020. [DOI] [PubMed] [Google Scholar]
- 23.Miller RC, Brindle E, Holman DJ, Shofer J, Klein NA, Soules MR, et al. Comparison of specific gravity and creatinine for normalizing urinary reproductive hormone concentrations. Clin Chem. 2004 May;50(5):924–32. doi: 10.1373/clinchem.2004.032292. [DOI] [PubMed] [Google Scholar]
- 24.Bergmann KC, Zuberbier T, Church MK. Omalizumab in the treatment of aspirin-exacerbated respiratory disease. J Allergy Clin Immunol Pract. 2015 May-Jun;3(3):459–60. doi: 10.1016/j.jaip.2015.01.012. [DOI] [PubMed] [Google Scholar]
- 25.Higashi N, Taniguchi M, Mita H, Yamaguchi H, Ono E, Akiyama K. Aspirin-intolerant asthma (AIA) assessment using the urinary biomarkers, leukotriene E4 (LTE4) and prostaglandin D2 (PGD2) metabolites. Allergol Int. 2012 Sep;61(3):393–403. doi: 10.2332/allergolint.11-RA-0403. [DOI] [PubMed] [Google Scholar]
- 26.Parmentier CN, Fuerst E, McDonald J, Bowen H, Lee TH, Pease JE, et al. Human T(H)2 cells respond to cysteinyl leukotrienes through selective expression of cysteinyl leukotriene receptor 1. J Allergy Clin Immunol. 2012 Apr;129(4):1136–42. doi: 10.1016/j.jaci.2012.01.057. [DOI] [PubMed] [Google Scholar]
- 27.Gauvreau GM, Parameswaran KN, Watson RM, O'Byrne PM. Inhaled leukotriene E(4), but not leukotriene D(4), increased airway inflammatory cells in subjects with atopic asthma. Am J Respir Crit Care Med. 2001 Oct 15;164(8):1495–500. doi: 10.1164/ajrccm.164.8.2102033. Pt 1. [DOI] [PubMed] [Google Scholar]
- 28.Stevenson DD, Simon RA, Mathison DA, Christiansen SC. Montelukast is only partially effective in inhibiting aspirin responses in aspirin-sensitive asthmatics. Ann Allergy Asthma Immunol. 2000 Dec;85(6):477–82. doi: 10.1016/S1081-1206(10)62575-6. Pt 1. [DOI] [PubMed] [Google Scholar]
- 29.Cahill KN, Bensko JC, Boyce JA, Laidlaw TM. Prostaglandin D(2): a dominant mediator of aspirin-exacerbated respiratory disease. J Allergy Clin Immunol. 2015 Jan;135(1):245–52. doi: 10.1016/j.jaci.2014.07.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Szczeklik A, Gryglewski RJ, Czerniawska-Mysik G, Zmuda A. Aspirin-induced asthma. Hypersensitivity to fenoprofen and ibuprofen in relation to their inhibitory action on prostaglandin generation by different microsomal enzymic preparations. J Allergy Clin Immunol. 1976 Jul;58(1):10–8. doi: 10.1016/0091-6749(76)90102-0. PT 1. [DOI] [PubMed] [Google Scholar]
- 31.Nagarkar DR, Poposki JA, Tan BK, Comeau MR, Peters AT, Hulse KE, et al. Thymic stromal lymphopoietin activity is increased in nasal polyps of patients with chronic rhinosinusitis. J Allergy Clin Immunol. 2013 Sep;132(3):593–600. doi: 10.1016/j.jaci.2013.04.005. e12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Allakhverdi Z, Smith DE, Comeau MR, Delespesse G. Cutting edge: The ST2 ligand IL-33 potently activates and drives maturation of human mast cells. J Immunol. 2007 Aug 15;179(4):2051–4. doi: 10.4049/jimmunol.179.4.2051. [DOI] [PubMed] [Google Scholar]
- 33.Chang YJ, Kim HY, Albacker LA, Baumgarth N, McKenzie AN, Smith DE, et al. Innate lymphoid cells mediate influenza-induced airway hyper-reactivity independently of adaptive immunity. Nat Immunol. 2011;12(7):631–8. doi: 10.1038/ni.2045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Holgate ST. Innate and adaptive immune responses in asthma. Nat Med. 2012 May;18(5):673–83. doi: 10.1038/nm.2731. [DOI] [PubMed] [Google Scholar]
- 35.Soroosh P, Doherty TA. Th9 and allergic disease. Immunology. 2009 Aug;127(4):450–8. doi: 10.1111/j.1365-2567.2009.03114.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Feng X, Ramsden MK, Negri J, Baker MG, Payne SC, Borish L, et al. Eosinophil production of prostaglandin D2 in patients with aspirin-exacerbated respiratory disease. J Allergy Clin Immunol. 2016 Jun 14; doi: 10.1016/j.jaci.2016.04.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hirai H, Tanaka K, Takano S, Ichimasa M, Nakamura M, Nagata K. Cutting edge: agonistic effect of indomethacin on a prostaglandin D2 receptor, CRTH2. J Immunol. 2002 Feb 1;168(3):981–5. doi: 10.4049/jimmunol.168.3.981. [DOI] [PubMed] [Google Scholar]
- 38.Kupczyk M, Kurmanowska Z, Kuprys-Lipinska I, Bochenska-Marciniak M, Kuna P. Mediators of inflammation in nasal lavage from aspirin intolerant patients after aspirin challenge. Respir Med. 2010 Oct;104(10):1404–9. doi: 10.1016/j.rmed.2010.04.017. [DOI] [PubMed] [Google Scholar]
- 39.Bal SM, Bernink JH, Nagasawa M, Groot J, Shikhagaie MM, Golebski K, et al. IL-1beta, IL-4 and IL-12 control the fate of group 2 innate lymphoid cells in human airway inflammation in the lungs. Nat Immunol. 2016 Jun;17(6):636–45. doi: 10.1038/ni.3444. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.