Abstract
About one-third of individuals with 22q11.2 deletion syndrome (22q11.2DS) develop schizophrenia. Notably, a full-blown psychotic disorder is usually preceded by subthreshold symptoms. Therefore, it is important to identify early signs of psychosis in this population, a task that is complicated by the intellectual disabilities typically seen in 22q11.2DS. We aimed to identify subthreshold psychotic symptoms that distinguish 22q11.2DS from other neurodevelopmental disorders. The study included two independent cohorts from Tel Aviv and Philadelphia. 22q11.2DS (N=171) and typically developing (TD; N=832) individuals were enrolled at both sites and further compared to two groups with intellectual disabilities: Williams syndrome (WS; N=21) in the Tel Aviv cohort and idiopathic developmental disabilities (IDD; N=129) in the Philadelphia cohort. Participants and their primary caregivers were interviewed with the Structured Interview for Prodromal Symptoms (SIPS) and psychopathologies were assessed using standardized tools; general cognitive abilities were assessed with the Computerized Neurocognitive Battery. Negative/disorganized subthreshold syndrome was significantly more common in the 22q11.2DS group than in the WS (OR=3.90, 95% CI=1.34–11.34) or IDD (OR=5.05, 95% CI=3.01–10.08) groups. The 22q11.2DS group had higher scores than the two intellectual disabilities groups on several SIPS negative items, including avolition and decreased expression of emotion. Overall, there were few significant correlations between level of cognitive deficits and severity of negative symptoms in 22q11.2DS and only in the Tel Aviv cohort. Our findings suggest that 22q11.2DS individuals at the age of risk for developing psychosis should be closely monitored for negative symptoms.
Keywords: Velocardiofacial syndrome, Williams syndrome, negative symptoms, Structured Interview for Prodromal Symptoms (SIPS), Computerized Neurocognitive Battery (CNB)
1. Introduction
The 22q11.2 deletion syndrome (22q11.2DS) is a common neurogenetic disorder with an estimated prevalence of ~1 per 3,000 to 1 per 6,000 live births (McDonald-McGinn et al., 2015). It is caused by a microdeletion in the long arm of the chromosome 22 and has a typical phenotype. Williams syndrome (WS) is a rare genetic disorder, occurring in ~1:20,000 live births (Stromme et al., 2002) and caused by a microdeletion in the long arm of chromosome 7 (7q11.23). Both syndromes have overlapping phenotypic manifestations, including cardiovascular anomalies, calcium dysregulation, cognitive deficits and high rates of psychiatric comorbidities (Campbell et al., 2009). As a group, individuals with neurodevelopmental disabilities (e.g., 22q11DS, WS, fragile X, or Turner syndrome) have higher rates of neuropsychiatric, cognitive and social-behavioral deficits including nonverbal learning disorder, visuospatial deficits, attention deficit/hyperactivity disorder (ADHD), anxiety disorders and affective disorders, compared to typically developing (TD) individuals (Schneider et al., 2014; Siegel and Smith, 2011; Weisman et al., 2015; Zarchi et al., 2014). Additionally, individuals with 22q11.2DS have a 30-fold increased risk of developing psychosis compared to TD individuals and individuals with other neurodevelopmental disabilities (10-fold) (Schneider et al., 2014). Therefore, 22q11.2DS is currently considered the strongest known genetic risk factor for schizophrenia.
Intense efforts are underway worldwide for early identification and treatment of psychosis in 22q11.2DS and the syndrome serves as a promising model for deciphering the pathways leading to schizophrenia. Previous studies have shown that, as is the case in non-deleted individuals, psychotic symptoms develop gradually in 22q11.2DS and that a full-blown psychosis is typically preceded by subthreshold symptoms (Gothelf et al., 2007; Gothelf et al., 2013; Tang et al., 2014b). Finally, studies suggest that the prevalence of subthreshold psychotic syndromes vary significantly in this population, ranging between 20% to 55% of the individuals assessed (Table 1).
Table 1.
Studies of subthreshold psychotic syndrome in 22q11.2 deletion syndrome (22q11.2DS).
| Age in years Age Range |
N | Definition of Prodromal Syndrome | Source of Information | Rates of Prodromal Symptoms | |
|---|---|---|---|---|---|
| Stoddard et al. 2010 | 15.1 (4.3) 12 – 22 |
20 | At least one positive symptom scored ≥3 on SIPS | Participants and caregivers interviewed together | 45% |
| Antshel et al. 2010 | 15.0 (1.9) 12.0–19.9 |
70 | At least one positive symptom scored ≥3 on SIPS | Participants and caregivers interviewed collaterally | 20% |
| Shapiro et al. 2011 | 17.5 (2.5) 14 – 22 |
23 | At least one positive symptom scored ≥3 on SIPS | Participants and caregivers interviewed collaterally | 56.5% |
| Schneider et al. 2012 | 15.4 (2.3) 11 – 20 |
47 | At least one positive symptom scored ≥3 or at least one negative symptom scored ≥3 on SIPS | Participants only | 83% negative 40.4% positive |
| Tang et al. 2014 | 15.2 (4.8) 8 – 25 |
157 | At least one positive symptom scored 3 – 5 or at least two negative or disorganized symptoms scored ≥3 on SIPS | Participants and caregivers interviewed collaterally | 44% positive 54% positive or negative |
Mean (SD); SIPS – The Structured Interview for Prodromal Symptoms.
The Structured Interview for Prodromal Symptoms (SIPS) is a well validated and commonly used tool for the assessment of subthreshold psychotic symptoms in individuals at high risk for schizophrenia (Miller et al., 2003a). It was designed for older adolescents and young adults with intelligence within the normal range (McGlashan et al., 2010). Although it is already being used in individuals with neurodevelopmental disabilities, its validity in 22q11.2DS has not been thoroughly tested. Specifically, the SIPS scores of individuals with 22q11.2DS have not been compared to individuals with intellectual disability or other neuro-genetic syndromes. Due to significant overlap between subthreshold psychotic symptoms and intellectual disabilities (e.g., problems with conceptual organization and poor ideational richness) and the difficulties of participants with developmental disabilities in understanding some questions (e.g., “Do you ever seem to live through events exactly as you have experienced them before?”, “Are you feeling emotionally flat?”), it is important to compare the rate of subthreshold psychotic symptoms between 22q11.2DS and IQ-matched control group.
Utilizing two independent cohorts in Tel Aviv (Israel) and Philadelphia (USA), we explored the following aims: Establish the utility of the SIPS in 22q11.2DS by testing its ability to detect differences in the rate of subthreshold psychotic symptoms and subthreshold syndromes between 22q11.2DS, WS and demographically matched TD controls (Tel Aviv); and between individuals with 22q11.2DS, individuals with idiopathic developmental disabilities (IDD), and demographically matched TD controls (Philadelphia). We hypothesized that individuals with 22q11.2DS would have higher rates of subthreshold psychotic symptoms and syndromes compared to WS and IDD and that the three clinical groups would have higher rates of subthreshold symptoms and syndromes compared to TD controls; We further aimed to test whether the differences in rates and severity of subthreshold psychotic symptoms between the clinical groups and within 22q11.2DS is affected by the overall cognitive deficits. Finally, we aimed to characterize the differences in psychiatric comorbidities between 22q11.2DS individuals with and without negative subthreshold psychotic syndrome. Based on previous studies in 22q11.2DS (Gothelf et al., 2007; Gothelf et al., 2013; Yi et al., 2015), we hypothesized that those with subthreshold psychotic syndrome would have higher rates of anxiety disorders and higher degree of social withdrawal and depressive symptoms, compared to those without subthreshold psychotic syndrome.
2. Materials and methods
2.1. Participants
The demographic characteristics of both cohorts are presented in Table 2.
Table 2.
Demographic characteristics and psychiatric medications in the Tel Aviv and the Philadelphia cohorts.
| Tel Aviv | Philadelphia | |||||||
|---|---|---|---|---|---|---|---|---|
|
| ||||||||
| 22q11.2DS (n = 52) | WS (n =21) | TD (n = 32) | Statistics (df=2, 102) | 22q11.2DS (n = 119) | IDD (n = 129) | TD (n = 800) | Statistics (df=2, 1047) | |
| Age in years | 20.97 (6.49) | 21.50 (6.12) | 20.05 (6.52) | F=0.361 p=0.70 |
16.51 (2.71) | 15.83 (2.43) | 16.29 (2.70) | F=2.276 P=0.10 |
| Age Range | 12.00–36.00 | 11.10 – 36.01 | 11.00 – 34.00 | 12.08 – 22.0 | 12.00 – 21.42 | 12.00– 21.83 | ||
| Sex (n, % male) | 26 (50.0) | 8 (38.0) | 12 (47.0) | χ2=0.85 P= 0.65 |
75 (63.0) | 78 (60.5) | 483 (60.4) | χ2=0.31 p=0.86 |
| Full scale IQ | 77.98 (11.4) | 65.95 (11.3) | 103.23 (7.6) | F=91.01 p<0.001 |
77.04 (12.83) | NA | NA | |
| IQ Range | 58–100 | 40–88 | 88–120 | 47 – 106 | NA | NA | ||
| Average parental education in years | 13.43 (2.3) | 14.05 (2.05) | 14.72 (2.2) | χ2=1.37 p=0.51 |
14.57 (2.04) | 14.11 (2.12) | 14.81 (2.36) | IDD > TD χ2=8.31 P = 0.16 |
| Ethnicity (n, %) | ||||||||
| Caucasian | 52 (100.0) | 21 (100.0) | 32 (100.0) | 103 (85.1) | 80 (63.0) | 528 (66.8) | ||
| African American | 0 (0.0) | 0 (0.0) | 0 (0.0) | 12 (9.9) | 34 (26.8) | 195 (24.7) | ||
| Other | 0 (0.0) | 0 (0.0) | 0 (0.0) | 6 (5.0) | 13 (10.2) | 67 (8.5) | ||
| Medication (current)* | ||||||||
| Antipsychotics | 1 (1.9) | 0 (0.0) | 0 (0.0) | 2 (1.7) | 7 (5.4) | 2 (0.25) | ||
| Mood stabilizers | 0 (0.0) | 1 (4.8) | 0 (0.0) | 5 (4.2) | 16 (12.4) | 1 (0.13) | ||
| Anti- depressants | 11 (21.2) | 2 (9.5) | 0 (0.0) | 12 (10.1) | 18 (14.0) | 12 (1.5) | ||
| Stimulants | 9 (17.3) | 2 (9.5) | 0 (0.0) | 7 (5.9) | 19 (14.7) | 18 (2.3) | ||
| Anxiolytics | 0 (0.0) | 0 (0.0) | 0 (0.0) | 2 (1.7) | 1 (0.8) | 1 (0.13) | ||
Mean (SD);
Several participants are on multiple medications.
Tel Aviv
Fifty-two participants with 22q11.2DS and 21 with WS were recruited from the Behavioral Neurogenetics Center at the Sheba Medical Center in Tel Aviv. The participants were referred from genetic clinics and through parents’ associations. The diagnosis of 22q11.2DS or WS was confirmed in all participants by fluorescent in situ hybridization (FISH) and by multiplex ligation probe amplification (MLPA) (Michaelovsky et al., 2012). TD controls were recruited through advertisements within the local community. They all had normal IQ and completed the SCL90 questionnaire (Derogatis, 1992) to rule out any major psychopathology. Age and sex distribution did not differ among the groups (Table 2).
The study was approved by the Sheba Medical Center Review Board. After providing a complete description of the nature of this study, informed consent was obtained from all participants and from the parents of minors.
Philadelphia
The Philadelphia cohort consisted of 119 participants with 22q11.2DS, 129 with IDD and 800 TD controls. Age and sex distribution did not differ among the groups (Table 2). 22q11.2DS individuals were recruited through the ‘22q and You Center’ at the Children’s Hospital of Philadelphia (CHOP) and online social networks as previously described (Gur et al., 2014b; Tang et al., 2014b). FISH and/or MLPA tests were performed for all participants to confirm the deleted region. The 129 IDD individuals are a part of the Philadelphia Neurodevelopmental Cohort (PNC) and had comorbid medical conditions with no known chromosomal anomalies. Organ systems affected were similar to those with 22q11DS as previously described (Gur et al., 2014b). The TD group included physically healthy youth, based on history provided at evaluation and review of electronic medical records as well as no psychiatric condition based on the clinical assessment detailed below. All procedures were approved by the Review Boards of the University of Pennsylvania and CHOP. Informed consent/assent was obtained from each participant and accompanying caregiver.
2.2. Clinical assessments
Tel Aviv
All of the participants were evaluated for the presence of subthreshold psychotic symptoms by a trained clinical psychologist using the Hebrew version of the Scale of Prodromal Symptoms (SOPS) of the SIPS v. 4 (Miller et al., 2003b). The SOPS is composed of 19 items, each representing a different possible subthreshold psychotic symptom, divided into four domains: positive, negative, disorganized and general symptoms (McGlashan et al., 2010).
In line with previous studies (See Table 1), three different definitions of subthreshold psychotic syndromes were used to compare between the study’s groups: 1. At least one positive symptom with a score of ≥3; 2. At least two negative/disorganized symptoms with a score of ≥3; and, 3. At least one positive symptom with a score of ≥3 or at least two negative/disorganized symptoms with a score of ≥3.
Individuals with 22q11.2DS and WS and their main caregivers were interviewed by trained psychiatrists using the Hebrew version of the Schedule for Affective Disorders and Schizophrenia for School-Aged Children, Present and Lifetime (K-SADS-PL) (Kaufman et al., 1997). Adult participants and their parents were interviewed using the Structured Clinical Interview for Axis I DSM-IV (SCID) (First et al., 1997). ADHD items from the K-SADS-PL were added to the SCID to evaluate the presence of ADHD in adults (Zarchi et al., 2014), and psychiatric diagnoses were established, when appropriate, according to the fifth edition of the Diagnostic and Statistical Manual of mental disorders (DSM-5) (Association, 2013). The 22q11.2DS and WS groups were also assessed for overall functioning with the Global Assessment Functioning scale (GAF) (Luborsky, 1962). Additional psychiatric symptoms were evaluated using the parental form of the Anxiety, Depression and Mood Scale (ADAMS), designed specifically for individuals with intellectual disabilities (Esbensen et al., 2003).
Philadelphia
All subjects underwent clinical evaluation of psychopathology including subthreshold psychotic symptoms. The measures applied in the PNC study to TD and IDD participants were based on epidemiologic version of the NIMH Genetic Epidemiology Research Branch Kiddie-SADS as previously detailed (Calkins et al., 2015; Calkins et al., 2014). Individuals with 22q11.2DS were further evaluated using the GAF scale for overall functioning, SCID modules C and D for differential diagnoses among mood and psychotic disorders, and a modified version of the K-SADS sections for psychosis, ADHD, mood disorders, generalized anxiety, separation anxiety, obsessive-compulsive and substance-related disorders (Tang et al., 2014a).
Among the IDD and TD groups, three screening tools to assess the psychosis spectrum were embedded within the psychopathology assessment as previously described. Positive subthreshold psychotic symptoms in the past year were assessed using the 12-item, assessor administered, PRIME Screen-Revised (PS-R) (Kobayashi et al., 2008; Miller et al., 2004); positive subthreshold psychotic symptoms (lifetime hallucinations and/or delusions) were assessed with the K-SADS psychosis screen; negative/disorganized symptoms were assessed using six assessor-rated SOPS items: N2, avolition; N3, expression of emotion; N4, experience of emotions and self; N6, occupational functioning; D3, trouble with focus and attention; and P5, disorganized communication, in line with recently published study (Gur et al., 2014a). IDD and TD participants were assessed for overall functioning using the Children’s Global Assessment Scale (CGAS) (Shaffer et al., 1983).
2.3. Neurocognitive assessment
All participants in the Tel Aviv and the Philadelphia cohorts underwent the Computerized Neurocognitive Battery (CNB) (Gur et al., 2012). A Global Neurocognitive Performance (GNP) efficiency score was calculated as the mean of five domains (executive function, episodic memory, complex cognition, social cognition and sensorimotor speed). The GNP efficiency scores were calculated by taking the arithmetic mean of the standardized (z-transformed) accuracy and speed scores. The z-scores for accuracy (number of correct responses) and speed (median time for correct responses) were based on the means and standard deviations of TD participants from each cohort (Yi et al., 2016). The Tel Aviv cohort participants were also evaluated for full scale IQ (FSIQ) using the age appropriate Wechsler test (Zarchi et al., 2014).
2.4. Statistical analysis
Data were analyzed using SPSS 20.0. Categorical variables were compared by Pearson’s χ2 for proportions analysis, and with the Fisher exact test when the χ2 test assumptions were not met. Continuous variables were checked for normality using the Shapiro-Wilk test. Most variables were skewed, therefore group means were compared using the non-parametric Kruskal–Wallis when comparing three groups, and the Mann–Whitney U test when two-group comparisons were done. Odds ratio (OR) with 95% confidence intervals (CI) were calculated in order to estimate the effect of variables. Spearman correlations were used to assess potential links between GNP scores and SOPS item scores. Weighted kappa intraclass correlation coefficients (ICC) were calculated for assessment of inter-sites reliability in SOPS items.
3. Results
3.1. Subthreshold psychotic syndrome in the study groups
Tel Aviv
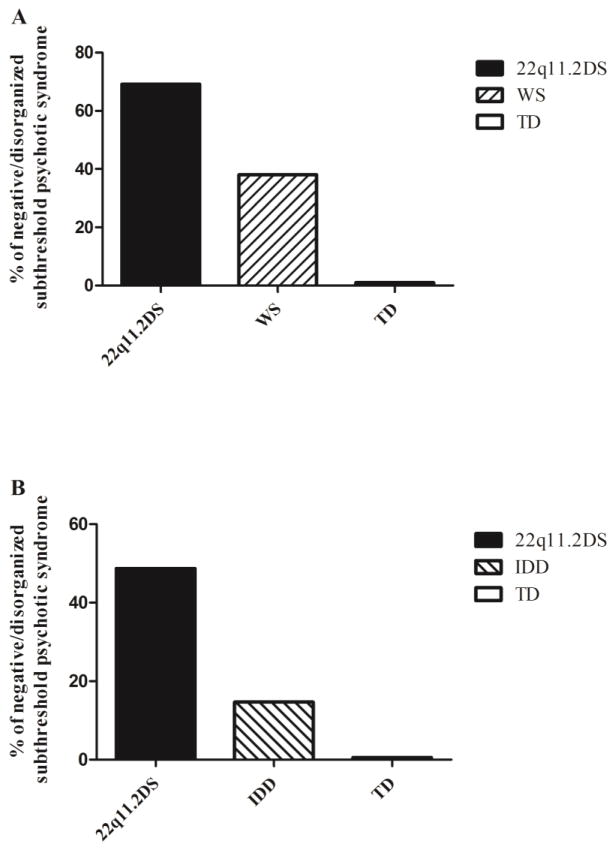
Individuals with 22q11.2DS and WS participants had significantly higher rates of subthreshold psychotic syndrome compared to TD controls based on all three definitions (all p values < 0.001, see Table 3a). None of the TD controls had SOPS scores within the subthreshold range. 22q11.2DS participants were more likely to have subthreshold psychotic symptoms compared with WS. Differences reached statistical significance only for the negative/disorganized subthreshold psychotic syndrome (OR=3.90, 95% CI=1.34–11.34, p=0.01; Table 3a and Figure 1a). We then analyzed the SOPS scores as continuous variables, summing the total positive, negative and disorganized symptom scores. N5- ideational richness was not included in the negative symptom score, as it is highly correlated with IQ. The overall between-group differences were driven by higher scores of 22q11.2DS and WS compared to controls on all three SOPS total sub-scores (Table 3a). In post-hoc pair-wise comparisons only the negative symptoms total scores were significantly higher in 22q11.2DS compared with WS (5.80±5.72 vs. 2.43±3.17, Z=−2.67, p<0.01; Table 3a). Further analysis showed that two negative symptoms were significantly higher in 22q11.2DS compared to WS: avolition (N2) and expression of emotion (N3). Social isolation or withdrawal (N1) emerged as marginally significant (Table 3a). Both 22q11.2DS and WS groups had significantly lower scores than TD on the GAF scale, but there were no significant differences in GAF scores between 22q11.2DS and WS (Table 3a).
Table 3a.
Comparison of rates of subthreshold psychotic syndromes and subthreshold psychotic symptom scores in 22q11.2 deletion syndrome (22q11.2DS) compared to Williams syndrome (WS) and typically developing (TD) controls in the Tel Aviv cohort.
| 22q11.2DS (n=52) | WS (n=21) | TD (n=32) | Statistics (df=2, 102) | Post-hoc | |
|---|---|---|---|---|---|
| Rates of subthreshold psychotic syndrome | |||||
| Positive symptoms (%) | 20 (38.5) | 7 (0.3) | 0 (0.0) | χ2=16.12 p<0.001 |
22q11.2DS, WS > TD |
| Negative/disorganized symptoms (%) | 36 (69.2) | 8 (38.1) | 0 (0.0) | χ2=40.33 p<0.001 |
22q11.2DS > WS > TD |
| Broad definition (%) | 38 (73.1) | 13 (61.9) | 0 (0.0) | χ2=44.22 p<0.001 |
22q11.2DS, WS > TD |
| SOPS scores | |||||
| Positive – total | 5.46 (4.86) | 5.71 (5.35) | 0.37 (1.19) | χ2=41.00 p<0.001 |
22q11.2DS, WS > TD |
| P1. Unusual thought content | 1.31 (1.39) | 0.86 (1.32) | 0.07 (0.25) | Z=20.64 p<0.001 |
22q11.2DS, WS > TD |
| P2. Suspiciousness | 1.40 (1.33) | 1.48 (1.50) | 0.09 (0.39) | Z=27.33 p<0.001 |
22q11.2DS, WS > TD |
| P3. Grandiosity | 0.73 (1.19) | 1.48 (1.40) | 0.13 (0.43) | Z=18.08 p<0.001 |
WS>22q11.2DS, TD |
| P4. Perceptual Abnormalities | 1.06 (1.27) | 1.10 (1.58) | 0.06 (0.25) | Z=19.01 p<0.001 |
22q11.2DS, WS > TD |
| P5. Conceptual disorganization | 0.96 (1.30) | 0.81 (0.98) | 0.00 (0.00) | Z=20.04 p<0.001 |
22q11.2DS, WS > TD |
| Negative – totala | 5.80 (5.72) | 2.43 (3.17) | 0.17 (0.75) | χ2=46.17 p<0.001 |
22q11.2DS, WS > TD |
| N1. Social isolation or withdrawal | 1.08 (1.71) | 0.57 (1.50) | 0.03 (0.18) | Z=14.74 p=0.001 |
22q11.2DS, WS > TD |
| N2. Avolition | 1.59 (1.63) | 0.52 (0.75) | 0.03 (0.2) | Z=29.19 p<0.001 |
22q11.2DS > WS > TD |
| N3. Expression of emotions | 0.96 (1.37) | 0.29 (0.72) | 0.00 (0.00) | Z=20.43 p<0.001 |
22q11.2DS > WS > TD |
| N4. Experience of emotions and self | 0.78 (1.28) | 0.29 (0.56) | 0.03 (0.18) | Z=12.14 p=0.002 |
22q11.2DS > TD |
| N5. Ideational richness | 3.10 (1.39) | 3.86 (1.62) | 0.00 (0.00) | Z=59.20 p<0.001 |
WS > 22q11.2DS > TD |
| N6. Occupational functioning | 1.39 (1.52) | 0.76 (1.14) | 0.07 (0.25) | Z=19.33 p<0.001 |
22q11.2DS > TD |
| Disorganized – total | 4.43 (3.22) | 3.62 (3.31) | 0.30 (0.70) | χ2=45.27 p<0.001 |
22q11.2DS, WS > TD |
| D1. Odd behavior of appearance | 0.49 (1.07) | 0.57 (1.08) | 0.0 (0.0) | Z=9.74 p=0.08 |
22q11.2DS, WS > TD |
| D2. Bizarre thinking | 0.37 (1.08) | 0.14 (0.66) | 0.1 (0.4) | Z=3.29 p=0.193 |
22q11.2DS, WS > TD |
| D3. Trouble with focus and attention | 2.67 (1.57) | 1.71 (1.42) | 0.2 (0.6) | Z=43.73 p<0.001 |
22q11.2DS > WS > TD |
| D4. Impairment in personal hygiene | 0.90 (1.42) | 1.19 (1.25) | 0.0 (0.0) | Z=20.87 p<0.001 |
22q11.2DS, WS > TD |
| GAF/CGAS | 73.92 (15.02) | 76.29 (12.83) | 98.13 (4.18) | F=57.33 p<0.001 |
TD > 22q11.2DS, WS |
Mean (SD); SOPS – Scale of Prodromal Symptoms; GAF – Global Assessment of Functioning; CGAS – Children’s Global Assessment Scale;
Total score of negative symptoms excluding the Ideational Richness item.
Fig 1.
Prevalence of negative/disorganized subthreshold psychotic syndrome in (A) 22q11.2 deletion syndrome (22q11.2DS) compared to Williams syndrome (WS) and typically developing (TD) controls in the Tel Aviv cohort, and in (B) 22q11.2DS compared to idiopathic developmental disabilities (IDD) and TD controls in the Philadelphia cohort.
p < .001 for both cohorts.
Philadelphia
Since the SIPS data of the Philadelphia cohort included only 6 items, all of them evaluating negative and disorganized symptoms (Gur et al., 2014a), only the negative/disorganized subthreshold psychotic syndrome was assessed in this cohort. In line with the results from the Tel Aviv cohort, both 22q11.2DS and IDD had significantly higher rates of subthreshold psychotic syndrome compared to TD controls (Table 3b; Figure 1b). Individuals with 22q11.2DS had higher rates of negative/disorganized subthreshold psychotic syndrome compared to IDD (OR=5.05, 95% CI=3.01–10.08, p<0.01). Further analysis revealed that the 22q11.2DS group scored significantly higher than the IDD group on all 6 SOPS items (Table 3b). Both 22q11.2DS and IDD groups had significantly lower scores than TD on the global functioning scale (GAF for 22q11.2DS, and CGAS for IDD and TD), but the two clinical groups (22q11.2DS and IDD) did not differ between themselves in this measure (Table 3b).
Table 3b.
Comparison of the rates of subthreshold psychotic syndrome and subthreshold psychotic symptom scores in 22q11.2 deletion syndrome (22q11.2DS), idiopathic developmental disabilities (IDD) and typically developing (TD) controls in the Philadelphia cohort.
| 22q11.2DS (n=119) | IDD (n=129) | TD (n=800) | Statistics (df=2, 1035) | Post-hoc | |
|---|---|---|---|---|---|
| Rates of subthreshold psychotic syndrome (%) | 58 (48.7) | 19 (14.7) | 0 (0.0) | χ2=373.26 p<0.001 |
22q11.2DS > IDD > TD |
| SOPS (total score) | 8.85 (5.47) | 4.16 (3.74) | 0.70 (1.09) | χ2=400.66 P<0.01 |
22q11.2DS > IDD > TD |
| P5. Conceptual disorganization | 1.32 (1.35) | 0.63 (0.94) | 0.05 (0.23) | Z=−4.17 p<0.001 |
22q11.2DS > IDD > TD |
| N2. Avolition | 1.97 (1.58) | 0.48 (1.02) | 0.03 (0.19) | Z=−8.08 p<0.001 |
22q11.2DS > IDD > TD |
| N3. Expression of emotions | 1.40 (1.46) | 0.79 (1.25) | 0.12 (0.43) | Z=−3.74 p<0.001 |
22q11.2DS > IDD > TD |
| N4. Experience of emotions and self | 0.57 (1.12) | 0.23 (0.68) | 0.06 (0.27) | Z=−2.37 p<0.001 |
22q11.2DS > IDD > TD |
| N6. Occupational functioning | 1.44 (1.44) | 0.51 (0.98) | 0.05 (0.25) | Z=−5.85 p<0.001 |
22q11.2DS > IDD > TD |
| D3. Trouble with focus and attention | 2.12 (1.42) | 1.59 (1.31) | 0.41 (0.71) | Z=−2.92 p<0.001 |
22q11.2DS > IDD > TD |
| GAF/CGAS | 60.64 (14.32) | 69.67 (21.22) | 82.68 (10.37) | F=64.46 P<0.001 |
TD > 22q11.2 |
Mean (SD); SOPS – Scale of Prodromal Symptoms; GAF – Global Assessment of Functioning; CGAS – Children’s Global Assessment Scale.
3.2 Association between level of cognitive deficits and SOPS scores
Tel Aviv
In the 22q11.2DS group, GNP efficiency score was found to significantly and negatively correlate with SOPS item D3 Trouble with Focus and Attention (r=-.54, p<.001) and with item N6, Occupational functioning (r=-.31, p=.03). No significant correlations were found between the other 4 SOPS items and GNP scores (Table 4). GNP score was significantly lower among 22q11.2DS participants with negative subthreshold syndrome vs. those without (−1.2±.51 vs. -.67±.37, p<.05).
Table 4.
Correlations between SOPS items scores and cognitive scores in 22q11.2 deletion syndrome (22q11.2DS).
| SOPS items | GNP scores | |||
|---|---|---|---|---|
|
| ||||
| Tel Aviv | Philadelphia | |||
|
|
|
|||
| R | P | R | P | |
| N2. Avolition | −0.26 | .06 | −0.02 | 0.80 |
| N3. Expression of emotions | −0.02 | .91 | 0.02 | 0.81 |
| N4. Experience of Emotions and Self | 0.02 | .90 | 0.14 | 0.12 |
| N6. Occupational functioning | −0.31 | .03 | −0.05 | 0.55 |
| D3. Trouble with focus and attention | −0.54 | .0001 | −0.09 | 0.31 |
| P5. Conceptual disorganization | −0.14 | .30 | −0.02 | 0.84 |
Philadelphia
In the 22q11.2DS group, GNP efficiency score was not associated with any of the SOPS item scores, all p’s>0.1, suggesting that differences in SOPS scores between these groups were not derived by the differences in cognitive ability (Table 4).
3.3 Psychiatric comorbidities in 22q11.2DS individuals with vs. without subthreshold psychotic syndrome
Tel Aviv
Compared to 22q11.2DS participants without subthreshold psychotic syndrome, 22q11.2DS participants with negative/disorganized subthreshold psychotic syndrome had significantly higher rates of ADHD (72.2% vs. 13.3%, p<0.01). Affected (with subthreshold psychotic symptoms) vs. non-affected 22q11.2DS subgroups did not differ in the rates of other psychiatric disorders including anxiety disorders, mood disorders and obsessive-compulsive disorder. Several differences were found between 22q11.2DS participants with subthreshold psychotic syndrome vs. those without on the dimensional measures of psychopathology; Compared to non-affected participants, those affected had higher ADAMS total scores (p < 0.05; Table 5), as a result of significantly higher scores on the manic/hyperactive behavior, depressed mood, and social avoidance. However, following Bonferroni corrections for multiple comparisons, only depressed mood remained statistically significant. On the GAF scale, the affected 22q11.2DS subgroup had significantly lower scores compared to the non-affected 22q11.2DS subgroup (p=0.01; Table 5).
Table 5.
Differences in the Anxiety, Depression and Mood Scale (ADAMS) and the Global Assessment of Functioning (GAF) scale between 22q11.2 deletion syndrome (22q11.2DS) individuals with subthreshold psychotic syndrome vs. without (in the Tel Aviv cohort).
| 22q11.2DS Prodromal (n=38) | Non-prodromal (n=14) | Statistics (df=1, 38) | |
|---|---|---|---|
| ADAMS | |||
| Total | 21.55 (15.53) | 9.36 (9.37) | Z=−2.41 p=0.016 |
| Manic/Hyperactive behavior | 4.72 (3.40) | 2.18 (2.44) | Z=−2.41 P=0.016 |
| Depressed mood | 5.13 (4.50) | 1.45 (1.81) | Z=−2.71 p=0.007 |
| Social avoidance | 5.48 (4.82) | 2.55 (3.64) | Z=−1.98 P=0.047 |
| General anxiety | 4.86 (4.05) | 2.37 (2.25) | Z=−1.88 p=0.060 |
| Compulsive behavior | 1.34 (1.71) | 0.81 (1.40) | Z=−1.22 p=0.223 |
| GAF | 71.08 (15.50) | 81.40 (11.54) | df=1, 48 Z=−2.35 p=0.019 |
Mean (SD); ADAMS – Anxiety, Depression and Mood Scale; GAF – Global Assessment of Functioning.
Philadelphia
Generalized anxiety disorder (GAD) was significantly higher in 22q11.2DS participants with subthreshold psychotic syndrome compared to those without (29.3% vs. 13.1%, p<0.05, respectively), but significance was lost following Bonferroni correction. On the GAF scale, the affected subgroup had significantly lower scores compared to the non-affected 22q11.2DS subgroup (p=0.02, Table 5).
4. Discussion
In this coordinated two-site study we used the SIPS to detect attenuated psychotic symptoms in individuals with 22q11.2DS. In addition to the 22q11.2DS group, SIPS was administered to two other groups diagnosed with developmental disability –WS in the Tel Aviv cohort and IDD in the Philadelphia cohort, as well as to healthy controls in both sites.
Negative symptoms (specifically, avolition and decreased expression of emotion) differentiated 22q11.2DS from both WS and IDD groups. In addition, in the Tel Aviv cohort, 22q11.2DS individuals with negative subthreshold psychotic syndrome vs. those without had higher rates of ADHD and affective symptoms, and lower scores on the GAF scale (in both cohorts). As lower functioning and severity of psychiatric symptoms has already been shown to predict the later onset of psychosis in 22q11.2DS (Gothelf et al., 2013; Schneider et al., 2014), the findings of the current analysis support the utility of negative symptoms as potential predictors of the likelihood to convert to psychosis in 22q11.2DS.
A limitation of previous studies was the lack of a control group of individuals with intellectual disabilities. Individuals with developmental disabilities are likely to score high on negative symptoms such as social isolation and expression of emotion and indeed in our study we found that all groups of developmental disabilities (22q11.2DS, WS and IDD) had higher scores on the negative symptoms scale than TD group. The 22q11.2DS group, however, also scored higher on five out of six negative SOPS items compared to WS group, even though the 22q11.2DS had significantly higher FSIQ than WS (Table 2). The only negative item that 22q11.2DS participants scored lower than WS was N5 (Ideational richness) as this item is strongly related to aspects of cognitive functioning reflected in IQ (e.g., abstraction). The 22q11.2DS participants also had significantly higher rates of negative subthreshold psychotic syndrome compared to WS. Similarly, the findings were replicated in the Philadelphia cohort, where 22q11.2DS individuals compared to individuals with IDD scored higher on all SOPS negative and disorganized items tested and also had higher rates of negative subthreshold psychotic syndrome. Taken together our findings suggest that there are higher rates of negative symptoms in 22q11.2DS beyond what is expected from their developmental disabilities.
As noted previously, there is a potential overlap between negative symptoms and cognitive deficits. We therefore examined correlations between overall cognitive level, as measured by GNP scores in both sites, and SOPS items. We found that none of the SOPS items correlated with GNP scores in the Philadelphia cohort. In the Tel Aviv cohort, none of the items that were significantly higher in 22q11.2DS compared to WS (i.e., N2- avolition and N3- expression of emotions) correlated with GNP scores. These findings further indicate that the high scores on negative SOPS items found in our 22q11.2DS are beyond their overall cognitive deficits and highlighting their potential role as indicators for future evolution of psychosis.
Studies in non–22q11.2DS schizophrenia have shown that negative symptoms predict the conversion to psychosis in high-risk population (Piskulic et al., 2012; Schneider et al., 2012). In the large multi-site North American Prodromal Longitudinal Study (NAPLS), moderate and severe attenuated negative symptoms were prevalent in individuals with high risk for psychosis and the severity and persistence of these symptoms were associated with higher rates of conversion to psychosis as assessed at follow-up visits 6- and 12-months post baseline (Piskulic et al., 2012).
In addition, we found significantly higher rates of ADHD and lower GAF scores as well as a tendency towards a higher rate of generalized anxiety disorder (GAD) in 22q11.2DS participants with subthreshold psychotic symptoms vs. those without. Both ADHD and anxiety disorders have been shown to be common in 22q11.2DS and, importantly, to significantly predict the likelihood to convert to psychosis in this population (Antshel et al., 2006; Feinstein et al., 2002; Gothelf et al., 2004). Taken together, our results suggest that negative symptoms are potentially important risk factors for the evolution of psychosis in 22q11.2DS.
A limitation of the current analysis might be related to its cross sectional rather than longitudinal design. As such, it is unable to identify predictive features of symptom progression over time. Another limitation is the fact that assessment protocols in Tel Aviv and Philadelphia were different in several aspects. At both centers, psychiatric diagnoses were made by interviewing the participants and their available caregivers using the K-SADS and for older adults in the Tel Aviv cohort, the SCID was also used. The GAF scores were similarly evaluated at both centers. Cognitive assessment, at both centers, was conducted using the CNB battery and in Tel Aviv participants were also assessed for FSIQ using the Wechsler test. Evaluation of subthreshold psychotic symptoms at both centers was conducted using the SOPS. While in Tel Aviv all SOPS items were rated, only six SOPS items were rated in the Philadelphia cohort in the IDD group, and therefore these six items were included in the comparison with 22q11.2DS. Of note, that the Tel Aviv team was trained by the PI in Philadelphia (REG) to achieve reliability in administering the SOPS. The negative subthreshold symptoms, the focus of our manuscript, that were more prominent in 22q11.2DS compared to WS were N2, avolition and N3 expression of emotion. These two negative symptoms were among the six SOPS items assessed in the Philadelphia cohort and thus we could replicate the findings from the Tel Aviv cohort in the comparison of 22q11.2DS to IDD in the Philadelphia cohort. Since there were some differences between the two cohorts- the control group with developmental disability (WS and IDD, at Tel Aviv and Philadelphia, respectively), age range (Philadelphia cohort were younger than Tel Aviv cohort) and number of SOPS items assessed- we did not merge the cohorts but analyzed the two cohort separately. Our results are consistent showing in both cohorts high level of negative subthreshold symptoms in 22q11.2DS and their association to psychiatric comorbidities and lower functioning.
5. Conclusions
Our findings suggest that negative subthreshold psychotic symptoms should be carefully evaluated and monitored in individuals with 22q11.2DS. The next step should be longitudinal studies, now underway, to test whether indeed pre-psychotic negative symptoms predict the onset of schizophrenia-spectrum disorders in 22q11.2DS population.
Acknowledgments
We kindly thank the individuals with 22q11.2DS and their families for participating in the study.
Role of funding source
The study reported in this publication was supported by the Binational Science Foundation, grant number 2011378, the National Institute of Mental Health of the National Institutes of Health under Award Numbers U01MH101722, RC2 MH089983 and MH089924, K08MH079364, and the Dowshen Program for Neuroscience. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Footnotes
Contributors
EMD, OW, JY and DG drafted the manuscript. Authors YG, MEC, DMM, BSE, EHZ, RCG, REG and DG were involved in the design of the study. Authors YG, JY, SXT undertook clinical data collection. EMD, YG, OW and DG undertook statistical analysis. EMD, YG, JY, MEC, SXT, RG, GZ, AW, RCG, REG and DG were involved in quality control. All contributors were involved in the final version of the manuscript.
Conflict of interests
The authors declare having no conflict of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Antshel KM, Fremont W, Roizen NJ, Shprintzen R, Higgins AM, Dhamoon A, Kates WR. ADHD, major depressive disorder, and simple phobias are prevalent psychiatric conditions in youth with velocardiofacial syndrome. Journal of the American Academy of Child & Adolescent Psychiatry. 2006;45(5):596–603. doi: 10.1097/01.chi.0000205703.25453.5a. [DOI] [PubMed] [Google Scholar]
- Association, A.P. Diagnostic and Statistical Manual of Mental Disorders (DSM-5®) American Psychiatric Pub; 2013. [Google Scholar]
- Calkins ME, Merikangas KR, Moore TM, Burstein M, Behr MA, Satterthwaite TD, Ruparel K, Wolf DH, Roalf DR, Mentch FD. The Philadelphia Neurodevelopmental Cohort: constructing a deep phenotyping collaborative. Journal of Child Psychology and Psychiatry. 2015;56(12):1356–1369. doi: 10.1111/jcpp.12416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calkins ME, Moore TM, Merikangas KR, Burstein M, Satterthwaite TD, Bilker WB, Ruparel K, Chiavacci R, Wolf DH, Mentch F. The psychosis spectrum in a young US community sample: findings from the Philadelphia Neurodevelopmental Cohort. World Psychiatry. 2014;13(3):296–305. doi: 10.1002/wps.20152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell LE, Stevens A, Daly E, Toal F, Azuma R, Karmiloff-Smith A, Murphy DG, Murphy KC. A comparative study of cognition and brain anatomy between two neurodevelopmental disorders: 22q11.2 deletion syndrome and Williams syndrome. Neuropsychologia. 2009;47(4):1034–1044. doi: 10.1016/j.neuropsychologia.2008.10.029. [DOI] [PubMed] [Google Scholar]
- Derogatis LR. SCL-90-R: Administration, Scoring of Procedures Manual-II for the R (evised) Version and Other Instruments of the Psychopathology Rating Scale Series. Clinical Psychometric Research Incorporated; 1992. [Google Scholar]
- Esbensen AJ, Rojahn J, Aman MG, Ruedrich S. Reliability and validity of an assessment instrument for anxiety, depression, and mood among individuals with mental retardation. J Autism Dev Disord. 2003;33(6):617–629. doi: 10.1023/b:jadd.0000005999.27178.55. [DOI] [PubMed] [Google Scholar]
- Feinstein C, Eliez S, Blasey C, Reiss AL. Psychiatric disorders and behavioral problems in children with velocardiofacial syndrome: usefulness as phenotypic indicators of schizophrenia risk. Biological psychiatry. 2002;51(4):312–318. doi: 10.1016/s0006-3223(01)01231-8. [DOI] [PubMed] [Google Scholar]
- First MB, Spitzer RL, Gibbon M, Williams JB. User’s guide for the Structured clinical interview for DSM-IV axis I disorders SCID-I: clinician version. American Psychiatric Pub; 1997. [Google Scholar]
- Gothelf D, Feinstein C, Thompson T, Gu E, Penniman L, Van Stone E, Kwon H, Eliez S, Reiss AL. Risk factors for the emergence of psychotic disorders in adolescents with 22q11.2 deletion syndrome. Am J Psychiatry. 2007;164(4):663–669. doi: 10.1176/ajp.2007.164.4.663. [DOI] [PubMed] [Google Scholar]
- Gothelf D, Presburger G, Levy D, Nahmani A, Burg M, Berant M, Blieden LC, Finkelstein Y, Frisch A, Apter A. Genetic, developmental, and physical factors associated with attention deficit hyperactivity disorder in patients with velocardiofacial syndrome. American Journal of Medical Genetics Part B: Neuropsychiatric Genetics. 2004;126(1):116–121. doi: 10.1002/ajmg.b.20144. [DOI] [PubMed] [Google Scholar]
- Gothelf D, Schneider M, Green T, Debbane M, Frisch A, Glaser B, Zilkha H, Schaer M, Weizman A, Eliez S. Risk factors and the evolution of psychosis in 22q11.2 deletion syndrome: a longitudinal 2-site study. J Am Acad Child Adolesc Psychiatry. 2013;52(11):1192–1203. e1193. doi: 10.1016/j.jaac.2013.08.008. [DOI] [PubMed] [Google Scholar]
- Gur RC, Calkins ME, Satterthwaite TD, Ruparel K, Bilker WB, Moore TM, Savitt AP, Hakonarson H, Gur RE. Neurocognitive growth charting in psychosis spectrum youths. JAMA psychiatry. 2014a;71(4):366–374. doi: 10.1001/jamapsychiatry.2013.4190. [DOI] [PubMed] [Google Scholar]
- Gur RC, Richard J, Calkins ME, Chiavacci R, Hansen JA, Bilker WB, Loughead J, Connolly JJ, Qiu H, Mentch FD, Abou-Sleiman PM, Hakonarson H, Gur RE. Age group and sex differences in performance on a computerized neurocognitive battery in children age 8–21. Neuropsychology. 2012;26(2):251–265. doi: 10.1037/a0026712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gur RE, Yi JJ, McDonald-McGinn DM, Tang SX, Calkins ME, Whinna D, Souders MC, Savitt A, Zackai EH, Moberg PJ, Emanuel BS, Gur RC. Neurocognitive development in 22q11.2 deletion syndrome: comparison with youth having developmental delay and medical comorbidities. Molecular psychiatry. 2014b;19(11):1205–1211. doi: 10.1038/mp.2013.189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaufman J, Birmaher B, Brent D, Rao U, Flynn C, Moreci P, Williamson D, Ryan N. Schedule for affective disorders and schizophrenia for school-age children-present and lifetime version (K-SADS-PL): initial reliability and validity data. Journal of the American Academy of Child & Adolescent Psychiatry. 1997;36(7):980–988. doi: 10.1097/00004583-199707000-00021. [DOI] [PubMed] [Google Scholar]
- Kobayashi H, Nemoto T, Koshikawa H, Osono Y, Yamazawa R, Murakami M, Kashima H, Mizuno M. A self-reported instrument for prodromal symptoms of psychosis: testing the clinical validity of the PRIME Screen—Revised (PS-R) in a Japanese population. Schizophrenia research. 2008;106(2):356–362. doi: 10.1016/j.schres.2008.08.018. [DOI] [PubMed] [Google Scholar]
- Luborsky L. Clinician’s judgments of mental health. Arch Gen Psychiatry. 1962;7:407–417. doi: 10.1001/archpsyc.1962.01720060019002. [DOI] [PubMed] [Google Scholar]
- McDonald-McGinn DM, Sullivan KE, Marino B, Philip N, Swillen A, Vorstman JAS, Zackai EH, Emanuel BS, Vermeesch JR, Morrow BE, Scambler PJ, Bassett AS. 22q11.2 deletion syndrome. Nature Reviews Disease Primers. 2015:15071. doi: 10.1038/nrdp.2015.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGlashan T, Walsh B, Woods S. The psychosis-risk syndrome: handbook for diagnosis and follow-up. Oxford University Press; 2010. [Google Scholar]
- Michaelovsky E, Frisch A, Carmel M, Patya M, Zarchi O, Green T, Basel-Vanagaite L, Weizman A, Gothelf D. Genotype-phenotype correlation in 22q11.2 deletion syndrome. BMC Med Genet. 2012;13:122. doi: 10.1186/1471-2350-13-122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller T, Cicchetti D, Markovich P, McGlashan T, Woods S. Schizophrenia Research. ELSEVIER SCIENCE BV; PO BOX 211, 1000 AE AMSTERDAM, NETHERLANDS: 2004. The SIPS screen: a brief self-report screen to detect the schizophrenia prodrome; pp. 78–78. [Google Scholar]
- Miller TJ, McGlashan TH, Rosen JL, Cadenhead K, Cannon T, Ventura J, McFarlane W, Perkins DO, Pearlson GD, Woods SW. Prodromal assessment with the structured interview for prodromal syndromes and the scale of prodromal symptoms: predictive validity, interrater reliability, and training to reliability. Schizophr Bull. 2003a;29(4):703–715. doi: 10.1093/oxfordjournals.schbul.a007040. [DOI] [PubMed] [Google Scholar]
- Miller TJ, McGlashan TH, Rosen JL, Cadenhead K, Ventura J, McFarlane W, Perkins DO, Pearlson GD, Woods SW. Prodromal assessment with the structured interview for prodromal syndromes and the scale of prodromal symptoms: predictive validity, interrater reliability, and training to reliability. Schizophrenia Bulletin. 2003b;29(4):703. doi: 10.1093/oxfordjournals.schbul.a007040. [DOI] [PubMed] [Google Scholar]
- Piskulic D, Addington J, Cadenhead KS, Cannon TD, Cornblatt BA, Heinssen R, Perkins DO, Seidman LJ, Tsuang MT, Walker EF. Negative symptoms in individuals at clinical high risk of psychosis. Psychiatry research. 2012;196(2):220–224. doi: 10.1016/j.psychres.2012.02.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider M, Debbane M, Bassett AS, Chow EW, Fung WL, van den Bree M, Owen M, Murphy KC, Niarchou M, Kates WR, Antshel KM, Fremont W, McDonald-McGinn DM, Gur RE, Zackai EH, Vorstman J, Duijff SN, Klaassen PW, Swillen A, Gothelf D, Green T, Weizman A, Van Amelsvoort T, Evers L, Boot E, Shashi V, Hooper SR, Bearden CE, Jalbrzikowski M, Armando M, Vicari S, Murphy DG, Ousley O, Campbell LE, Simon TJ, Eliez S. Psychiatric disorders from childhood to adulthood in 22q11.2 deletion syndrome: results from the International Consortium on Brain and Behavior in 22q11.2 Deletion Syndrome. Am J Psychiatry. 2014;171(6):627–639. doi: 10.1176/appi.ajp.2013.13070864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider M, Van der Linden M, Glaser B, Rizzi E, Dahoun SP, Hinard C, Bartoloni L, Antonarakis SE, Debbane M, Eliez S. Preliminary structure and predictive value of attenuated negative symptoms in 22q11.2 deletion syndrome. Psychiatry Res. 2012;196(2–3):277–284. doi: 10.1016/j.psychres.2011.08.017. [DOI] [PubMed] [Google Scholar]
- Shaffer D, Gould MS, Brasic J, Ambrosini P, Fisher P, Bird H, Aluwahlia S. A children’s global assessment scale (CGAS) Archives of General psychiatry. 1983;40(11):1228. doi: 10.1001/archpsyc.1983.01790100074010. [DOI] [PubMed] [Google Scholar]
- Siegel MS, Smith WE. Psychiatric features in children with genetic syndromes: toward functional phenotypes. Pediatric clinics of North America. 2011;58(4):833–864. doi: 10.1016/j.pcl.2011.06.010. [DOI] [PubMed] [Google Scholar]
- Stromme P, Bjornstad PG, Ramstad K. Prevalence estimation of Williams syndrome. J Child Neurol. 2002;17(4):269–271. doi: 10.1177/088307380201700406. [DOI] [PubMed] [Google Scholar]
- Tang SX, Yi JJ, Calkins ME, Whinna DA, Kohler CG, Souders MC, McDonald-McGinn DM, Zackai EH, Emanuel BS, Gur RC, Gur RE. Psychiatric disorders in 22q11.2 deletion syndrome are prevalent but undertreated. Psychol Med. 2014a;44(6):1267–1277. doi: 10.1017/S0033291713001669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang SX, Yi JJ, Moore TM, Calkins ME, Kohler CG, Whinna DA, Souders MC, Zackai EH, McDonald-McGinn DM, Emanuel BS, Bilker WB, Gur RC, Gur RE. Subthreshold psychotic symptoms in 22q11.2 deletion syndrome. J Am Acad Child Adolesc Psychiatry. 2014b;53(9):991–1000. e1002. doi: 10.1016/j.jaac.2014.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weisman O, Feldman R, Burg-Malki M, Keren M, Geva R, Diesendruck G, Gothelf D. Mother-Child Interaction as a Window to a Unique Social Phenotype in 22q11.2 Deletion Syndrome and in Williams Syndrome. J Autism Dev Disord. 2015;45(8):2567–2577. doi: 10.1007/s10803-015-2425-6. [DOI] [PubMed] [Google Scholar]
- Yi JJ, Calkins ME, Tang SX, Kohler CG, McDonald-McGinn DM, Zackai EH, Savitt AP, Bilker WB, Whinna DA, Souders MC, Emanuel BS, Gur RC, Gur RE. Impact of psychiatric comorbidity and cognitive deficit on function in 22q11.2 deletion syndrome. J Clin Psychiatry. 2015;76(10):e1262–1270. doi: 10.4088/JCP.14m09197. [DOI] [PubMed] [Google Scholar]
- Yi JJ, Weinberger R, Moore TM, Calkins ME, Guri Y, McDonald-McGinn DM, Zackai EH, Emanuel BS, Gur RE, Gothelf D, Gur RC. Performance on a computerized neurocognitive battery in 22q11.2 deletion syndrome: A comparison between US and Israeli cohorts. Brain and cognition. 2016;106:33–41. doi: 10.1016/j.bandc.2016.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zarchi O, Diamond A, Weinberger R, Abbott D, Carmel M, Frisch A, Michaelovsky E, Gruber R, Green T, Weizman A, Gothelf D. A comparative study of the neuropsychiatric and neurocognitive phenotype in two microdeletion syndromes: velocardiofacial (22q11.2 deletion) and Williams (7q11.23 deletion) syndromes. Eur Psychiatry. 2014;29(4):203–210. doi: 10.1016/j.eurpsy.2013.07.001. [DOI] [PubMed] [Google Scholar]