Abstract
Context
Evidence suggests that chemotherapy-induced neuropathy (CIN) is a significant problem for cancer survivors. However, a detailed phenotypic characterization of CIN in cancer survivors is not available.
Objectives
To evaluate between group differences in demographic and clinical characteristics, as well as in measures of sensation, function, and postural control, in a sample of cancer survivors who received a platinum and/or a taxane-based CTX regimen and did (n=426) and did not (n=197) develop CIN.
Methods
Survivors completed self-report questionnaires and underwent objective testing (i.e., light touch, pain sensation, cold sensation, vibration, muscle strength, grip strength, Purdue Pegboard test, Timed Get Up and Go Test, Fullerton Advanced Balance test). Parametric and non-parametric statistics were used to compare between group differences in study outcomes.
Results
Of the 426 survivors with CIN, 4.9% had CIN only in their upper extremities, 27.0% only in their lower extremities, and 68.1% in both their upper and lower extremities. Demographic and clinical characteristics associated with CIN included: older age, lower annual income, higher body mass index, a higher level of comorbidity, being born prematurely, receipt of a higher cumulative dose of chemotherapy, and a poorer functional status. Survivors with CIN had worse outcomes for all of the following objective measures: light touch, pain, temperature, vibration, upper and lower extremity function and balance.
Conclusions
This study is the first to provide a detailed phenotypic characterization of CIN in cancer survivors who received a platinum and/or a taxane compound. These data can serve as a benchmark for future studies of CIN in cancer survivors.
Keywords: chemotherapy-induced neuropathy, cancer, chemotherapy, pain, pain qualities, gait, balance, sensations, vibration
INTRODUCTION
More than 14 million cancer survivors are living in the United States; a number that is expected to double in the next decade.1 This increase in cancer survivors mandates that greater emphasis be placed on the assessment and management of persistent adverse effects associated with cancer treatment.2,3 Among these adverse events, chemotherapy-induced neuropathy (CIN) can be particularly severe and long-lasting.4,5 While CIN was described initially as a predominantly reversible condition that produced its effects within the peripheral nervous system, a growing body of evidence suggests that it persists long into survivorship6 and that the neurotoxic effects can occur within the central nervous system.7–9 Therefore, in this paper the abbreviation CIN was used instead of CIPN to describe this condition. Of note, in 2014, CIN was added to the National Comprehensive Cancer Center (NCCN) Clinical Practice Guideline for Survivorship2,10 and the American Society of Clinical Oncology published a guideline on the prevention and management of CIN in cancer survivors.3
While the exact prevalence of CIN in cancer survivors is unknown, findings from cross-sectional and cancer registry studies suggest that between 10% and 60% of survivors have CIN.11–15 Self-report data from one of these studies, collected using the Quality of Life Questionnaire – CIPN20 (CIPN20),15 found that the most common symptoms were tingling in the hands and feet (30%), numbness in the toes and feet (19%), tingling in hands or fingers (15%), and burning or shooting pain in the toes or feet (13%).16 Of note, survivors reported symptoms for up to 11 years following the completion of chemotherapy (CTX).16 Equally important, recent findings suggest that CIN has a negative impact on survivors’ functional status and quality of life (QOL).12,17–20 While all of these studies provide information on the extent and impact of CIN in cancer survivors, none have provided a detailed phenotypic description of this clinical condition.
Given the significant impact of CIN and the paucity of research on this condition in cancer survivors, we evaluated between group differences in demographic and clinical characteristics, as well as in measures of sensation, function, and postural control, in a sample of cancer survivors who received a platinum and/or a taxane-based CTX regimen and did (n=426) and did not (n=197) develop CIN.
METHODS
Survivors and Settings
Survivors were recruited from throughout the San Francisco Bay area using the following strategies: direct referral from clinicians; direct mailing to survivors who were identified through targeted searches of our medical center’s electronic health record; newspaper advertisements; emails to participants in the Dr. Susan Love Research Foundation’s Army of Women® Program; emails to support group members; postings on survivorship websites; postings on ClinicalTrials.gov; presentations at support group meetings; and snowball sampling through referrals from survivors. The National Coalition for Cancer Survivorship’s (NCCS) definition of cancer survivor was used in this study (i.e., NCCS considers a person a cancer survivor from the moment of diagnosis through the balance of life. Whether treatment is being received or has been completed anyone who has received a diagnosis is a survivor).21
Survivors with CIN met the following inclusion criteria: were ≥18 years of age; had received a platinum and/or a taxane compound; had completed their course of CTX ≥3 months prior to enrollment; had changes in sensation and/or pain in their feet and/or hands of ≥3 months duration following the completion of CTX; had a rating of ≥3 on a 0 to 10 numeric rating scale (NRS) for any one of the following sensations from the Pain Qualities Assessment Scale (i.e., numb, tender, shooting, sensitive, electrical, tingling radiating, throbbing, cramping, itchy, unpleasant)22; if they had pain associated with the CIN, had an average pain intensity score in their feet and/or hands of ≥3 on a 0 to 10 NRS; had a Karnofsky Performance Status (KPS) score of ≥50; and were able to read, write, and understand English.23
Survivors without CIN met the following inclusion criteria: were ≥18 years of age; had received a platinum and/or a taxane compound; had completed their course of CTX ≥3 months prior to enrollment; did not have persistent changes in sensation and/or pain in their hands or feet at the time of enrollment; had a KPS score of ≥50; and were able to read, write, and understand English.
Survivors with and without CIN were excluded if they had: peripheral vascular disease, vitamin B12 deficiency, thyroid dysfunction, HIV neuropathy, another painful condition that was difficult for them to distinguish from their CIN, a hereditary sensory or autonomic neuropathy,24 and/or a hereditary mitochondrial disorder.25 A detailed patient history was obtained to evaluate for the presence of these conditions. Of the 1450 survivors who were screened, 754 were enrolled, and 623 completed the self-report questionnaires and the study visit.
Study procedures
Survivors communicated their willingness to participate in our study by phone, email, or completion of an online screening questionnaire. Research nurses phoned survivors who expressed interest and determined their eligibility to participate. For survivors who met the study’s inclusion criteria, the research nurses obtained consent over the phone; sent and asked the survivors to complete the self-report questionnaires prior to their study visit; and scheduled the survivors’ study visit. During the study visit, the research nurse obtained written informed consent, reviewed the study questionnaires for completeness, and performed the objective measures.
Subjective Measures
Demographic and Clinical Characteristics
Survivors completed a demographic questionnaire that obtained information on age, gender, race/ethnicity, living arrangements, marital status, education, and employment. Survivors were interviewed to obtain information on their cancer diagnosis, previous and current cancer treatments, CTX regimens, prior hand and foot surgeries, and concurrent medications. Medical records were reviewed for detailed information on cancer diagnosis, previous cancer treatments, and CTX regimens.
The Karnofsky Performance Status (KPS) scale is widely used to evaluate functional status in cancer survivors and has well established validity and reliability.26 Survivors rated their functional status using the KPS scale that ranged from 30 (I feel severely disabled and need to be hospitalized) to 100 (I feel normal; I have no complaints or symptoms).26–28
The Self-Administered Comorbidity Questionnaire (SCQ) consists of 13 common medical conditions.29 Survivors were asked to indicate if they had the condition; if they received treatment for it (proxy for disease severity); and if it limited their activities (indication of functional limitations). The total SCQ score can range from 0 to 39. The SCQ has well-established validity and reliability and has been used in studies of survivors with a variety of chronic conditions.30,31
The Alcohol Use Disorders Identification Test (AUDIT) is a 10-item questionnaire that assesses alcohol consumption, alcohol dependence, and the consequences of alcohol abuse in the last 12 months. The AUDIT gives a total score that ranges from 0 to 40. Scores of ≥8 are defined as hazardous use and scores of ≥16 are defined as use of alcohol that is likely to be harmful to health.32,33 The AUDIT has well established validity and reliability.34–36
Pain questionnaires
For survivors with CIN, separate assessments were done for pain intensity and pain quality ratings for the hands and feet. A detailed history of the CIN was obtained using a pain questionnaire that was used in our previous37,38 and ongoing studies. This questionnaire obtained information on date of onset of pain, duration, location(s), aggravating and relieving factors, level of interference with function, as well as previous and current treatments and their effectiveness. Average and worst pain intensity over the past 24 hours was assessed using 0 (no pain) to 10 (worst pain imaginable) NRSs.39,40
The Pain Qualities Assessment Scale (PQAS) was used to assess the qualities associated with CIN.22,41 The 20-item PQAS is an adaptation of the Neuropathic Pain Scale developed by Galer and Jensen.22 Sixteen items evaluate the magnitude of the different pain quality descriptors (e.g., sharp, hot, aching, cold) measured with an NRS. Four items evaluate global and spatial qualities of pain. Three subscale scores were calculated (i.e., paroxysmal pain [shooting, sharp, electric, hot, radiating], surface pain [itchy, cold, numb, sensitive, tingling], deep pain [aching, heavy, dull, cramping, throbbing, tender]). The PQAS has well established validity and reliability in studies of various types of neuropathic pain.22,41–44
Objective Measures
Objective measures of sensation included: light touch, pain, cold, and vibration. Objective measures of motor function included: muscle strength, grip strength, manual dexterity, heel lift, and Achilles deep tendon reflex. Balance and postural control were evaluated using the Timed Get Up and Go test (TUG) and the Fullerton Advanced Balance (FAB) test. All of the objective measures were done in survivors with and without CIN.
Light touch sensation
Semmes-Weinstein monofilaments (SWM; North Coast Medical, Inc., Morgan Hill, CA) were used to test light touch sensation in the upper and lower extremities following the procedure of Bell-Krotoski.45 Each upper extremity was evaluated at 7 locations: the pad of the thumb, thumb webspace, tip of the index finger, tip of the little finger, midway up the base of the palm, one third up the anterior arm, and two thirds up the anterior arm. Each lower extremity was evaluated at 14 locations: pad of the great, 3rd, and 5th toes; plantar surface of the great, 3rd, and 5th toe at the metacarpophalangeal (MP) joint; plantar medial arch; base of the heel; dorsal surface of the MP joint of the great, 3rd, and 5th toes; dorsum medial navicular; midway along the anterior surface of the tibia, and the patella. These locations were tested in random order.
SWM sizes used for the upper extremities were: 3.61 (0.4 grams (g)), 4.31 (2 g), 4.56 (4 g), 5.07 (10 g), and 6.65 (300 g). SWM used for the lower extremities were: 4.31, 4.56, 5.07, and 6.65. Before testing began, survivors were familiarized with the filament being used and the expected sensation was demonstrated. Then with the survivors’ eyes closed, starting with the smallest, filaments were applied in ascending order at each site. The filaments were applied perpendicular to the skin and pressed until the filament bowed for 1.5 seconds. The survivor was asked to respond to the stimulus by stating the location of the light touch sensation. In contrast to Bell-Krotoski’s recommendation, two correct responses out of three rather than one out of three at each location were considered a positive response, as the survivor could make one correct response by guessing.46 At each location, once a positive response was identified, the next location was tested in random order. SWM measurements have good inter-and intrarater reliability and validity when calibrated and applied correctly.45,47,48 For each location, the smallest SWM that the survivor sensed was used in the statistical analyses.
Pain sensation
Pain sensation was tested using the Neurotip (MedExSupply Medical Supplies, NY) which has a spring mechanism that is calibrated to exert a force of 40 grams.49 Each upper and lower extremity was evaluated once in each of the same locations as the SWM measurements. Survivors were familiarized with the device and the expected sensations were demonstrated on skin in an unaffected region. Then with their eyes closed, the survivor was asked to indicate “sharp” or “dull” when the applicator stick was applied to each location in random order. At each site, pain response was coded as present or absent for use in the statistical analyses.
Cold sensation
Cold sensation was evaluated using the Tiptherm rod (Bailey Instruments; Trafford Park, United Kingdom). This pen like device consists of a polymer cylinder at one end and a cool metal cylinder at the other end. The device provides a constant temperature that is applied to the skin. After familiarizing the survivors with the two sensations, the research nurse applied each side of the device at each location stating “one” and “two”. The survivor was asked to indicate which application (i.e. “one” or “two”) felt colder. Each upper extremity was evaluated at four locations: pad of the index finger, pad of the little finger, dorsal metacarpal area of the hand, and the dorsal side of the wrist. Each lower extremity was evaluated at four locations: top of great toe at the 1st MP joint, pad of great toe, dorsum of the foot at the midpoint, and medial malleolus. Cold sensation sites were tested in random order up to three times and scored as impaired if the survivor had two incorrect responses.49 The Tiptherm has high specificity (100%) and sensitivity (97.3%) in diagnosing diabetic peripheral neuropathy.50
Vibration threshold
Vibration threshold was tested using a Biothesiometer (Bio-Medical Instrument Company; Newbury, OH). After familiarizing survivors with the sensation, vibration thresholds were tested at four sites in the upper extremities (i.e., dorsal interphalangeal (IP) joint of the thumb and index finger, ulnar prominence of the wrist, lateral epicondyle) and three sites in the lower extremities (i.e., dorsal IP joint of the great toe, medial malleolus, patella). Following the manufacturer’s instructions, the Biothesiometer was placed on the skin over the bone at each location. Beginning at zero, the amplitude of the vibration was increased until the survivor reported feeling vibration (i.e., vibration perception threshold). Then the amplitude was turned down to zero and the procedure was repeated, increasing the intensity more slowly as the initial value was approached. The survivor reported the perception of the sensation of vibration by saying, “NOW.” Each site was tested three times and the mean score was used in the statistical analyses.
Muscle strength
For each movement tested, muscle strength was assessed once in the upper (i.e., abduction of the little finger, opposition of the thumb and little finger, wrist extension) and lower (i.e., extension of the great toe, dorsiflexion of the foot, plantar flexion of the foot) extremities using the Medical Research Council Scale (i.e., 0 = no visible contraction to 5 = normal strength).51
Grip strength
Hand grip strength was assessed using a hand dynamometer (Smedley III Analgou Grip Tester, Creative Health Products, Ann Arbor, MI). Survivors stood upright with their weight evenly distributed on both feet. The grip size was adjusted for each individual survivor and both arms were extended downward. Survivors were coached to squeeze the hand dynamometer with as much force as possible. The survivor performed the test three times on each hand, alternating sides. Fifteen seconds separated each trial. The mean force in kilograms (kg) of the three trials was calculated for each hand.52
Pegboard test
Manual dexterity in the upper extremities was assessed using the Purdue Pegboard (Lafayette Instrument, Lafayette, IN). Survivors were seated at a desk and the Purdue Pegboard was placed in front of them. Each upper extremity was tested separately. Survivors were instructed to place as many pins in the column of the pegboard that corresponded to their right or left hand, until they were told to stop. The number of pins placed in 30 seconds was recorded. Each upper extremity was tested twice and a mean score for each extremity was calculated.53–55
Heel lift
Lower extremity strength was evaluated using the heel lift test.56,57 Survivors were instructed to stand on the floor with one leg using the wall for balance and lift their heel for a maximum of 15 seconds. Each lower extremity was tested once. The amount of time the survivor stood with the heel lifted was recorded.
Achilles tendon reflex
Achilles tendon reflex was tested with a Taylor Percussion Hammer (Prestige Medical, Los Angeles, CA). The response was coded as present or absent.
Timed get up and go test (TUG)
The TUG test is a timed test of a person’s ability to stand from an armed chair, walk 10 feet, turn, and return to a seated position.58 Survivors were instructed to walk as quickly as possible, without running. The time needed to perform the test was recorded.
Fullerton Advanced Balance (FAB) test
The FAB is a measure of balance that includes ten tasks: standing with feet together and eyes closed, reaching forward to retrieve a pencil held at shoulder height, turning 360° in a right then in a left direction, stepping up and over a 15.2 cm (6 in) bench, tandem walking, standing on one leg, standing on foam with eyes closed, 2-footed jumping for a distance, walking with head turns, and responding to an unexpected trunk perturbation.59,60 The FAB was chosen because the tasks challenge the sensory systems (i.e., visual, somatosensory, vestibular) used for postural control that may be more sensitive to balance problems in individuals with CIN, a primary sensory neuropathy. The quality of the performance of each task is scored using standardized ordinal scoring criteria. Total scores can range from 0 to 40. Higher scores indicate a better performance.
Reliability assessments of the study nurses
A neurologist and a physical therapist taught the research nurses how to perform all of the objective measures and determined their inter-rater reliability. Every 6 months, these reliability assessments were done. Measures were repeated until an inter-rater reliability of ≥0.80 was achieved among all of the research nurses.
Data Analysis
Data were analyzed using SPSS version 23.61 Descriptive statistics and frequency distributions were calculated for survivors’ demographic and clinical characteristics. Differences between the CIN and no CIN groups on demographic and clinical characteristics, as well as objective measures were evaluated using independent sample t-tests, Chi square analyses, and Mann Whitney U tests. A p-value of <0.05 was considered statistically significant.
RESULTS
Differences in Demographic and Clinical Characteristics
As shown in Table 1, for the majority of the demographic characteristics, no differences were found between the CIN and no CIN groups. However, survivors with CIN were older (60.90 (±10.52) versus 58.38 (±12.27), p=.013) and were more likely to have a lower annual household income (p=.031).
Table 1.
Differences in Demographic Characteristics Between Cancer Survivors With (n=426) and Without (n=197) Chemotherapy Induced Neuropathy (CIN)
| Characteristic | No CIN 31.6% (n=197) |
CIN 68.4% (n=426) |
Test, p-value |
|---|---|---|---|
| Mean (SD) | Mean (SD) | ||
| Age (years) | 58.38 (12.27) | 60.90 (10.52) | t=−2.48, .013 |
| Education (years) | 16.42 (2.61) | 16.37 (2.76) | t=0.20, .839 |
| % (n) | % (n) | ||
| Female | 80.7 (159) | 86.6 (368) | FE, .072 |
| Married/partnered | 63.2 (122) | 60.9 (252) | FE, .592 |
| Lives alone | 27.7 (54) | 29.2 (122) | FE, .774 |
| Employed | 49.2 (97) | 42.1 (179) | FE, .100 |
| Ethnicity | |||
| White | 82.2 (162) | 77.2 (329) | |
| Asian/Pacific Islander | 6.1 (12) | 7.0 (30) | Χ2 = 2.17, .539 |
| Black | 4.1 (8) | 5.2 (22) | |
| Hispanic/Mixed/Other | 7.6 (15) | 10.6 (45) | |
| Annual household income | |||
| <$30,000 | 14.8 (27) | 23.3 (92) | |
| $30,000 – $69,999 | 19.1 (35) | 21.0 (83) | U, .031 |
| $70,000 – $99,999 | 21.3 (39) | 16.2 (64) | |
| >$100,000 | 44.8 (82) | 39.5 (156) | |
| Child care responsibilities | 19.2 (37) | 13.3 (56) | FE, .069 |
| Adult care responsibilities | 3.8 (7) | 3.8 (15) | FE, 1.000 |
Abbreviations: FE = Fisher’s Exact test, U = Mann Whitney U test, SD = standard deviation
As shown in Table 2, compared to survivors without CIN, survivors with CIN had a higher body mass index (BMI; p<.001), a lower AUDIT score (p=.002), a higher number of comorbidities (p<.001), a higher SCQ score (p<.001), a lower number of previous cancer treatments (p=.018), and a lower KPS score (p<.001). In addition, a significantly higher percentage of survivors in the CIN group were born prematurely (6.6% versus 1.1%, p=.002); reported a previous injury to their hands (34.7% versus 23.7%, p=.008); and reported higher occurrence rates for osteoarthritis (30.3% versus 16.8%, p<.001), back pain (34.7% versus 23.9%, p=.007), kidney disease (2.3% versus 0.0%, p=.035), and liver disease (3.3% versus 0.0%, p=.007).
Table 2.
Differences in Clinical Characteristics Between Cancer Survivors With (n=426) and Without (n=197) Chemotherapy-Induced Neuropathy (CIN)
| Characteristic | No CIN (1) 31.6% (n=197) |
CIN (2) 68.4% (n=426) |
Test, p-value |
|---|---|---|---|
| Mean (SD) | Mean (SD) | ||
| Karnofsky Performance Status score | 91.20 (9.33) | 83.22 (10.21) | t=9.51, <.001 |
| Body mass index (kg/m2) | 24.85 (5.03) | 26.56 (5.55) | t=−3.63, <.001 |
| Number of comorbidities | 1.47 (1.35) | 2.02 (1.48) | t=−4.46, <.001 |
| Self-Administered Comorbidity Questionnaire score |
2.87 (2.96) | 4.20 (3.39) | t=−4.99, <.001 |
| Alcohol Use Disorders Identification Test score |
2.85 (2.50) | 2.24 (2.20) | t=3.05, .002 |
| Years since cancer diagnosis | 4.40 (4.82) | 4.82 (4.84) | t=−1.01, .311 |
| Number of prior cancer treatments | 3.32 (0.98) | 3.12 (0.97) | t=2.37, .018 |
| Number of current cancer treatments | 0.46 (0.60) | 0.41 (0.59) | t=0.86, .392 |
| Number of metastatic sites (out of 7) | 0.70 (0.75) | 0.75 (0.78) | t=−0.67, .503 |
| Number of metastatic sites without lymph node involvement |
0.16 (0.52) | 0.22 (0.57) | t=−1.29, .198 |
| % (n) | % (n) | ||
| Smoker (ever) | 33.0 (64) | 37.8 (160) | FE, .279 |
| Exercise on a regular basis (% yes) | 87.2 (170) | 85.9 (365) | FE, .707 |
| Born prematurely (% yes) | 1.1 (2) | 6.6 (26) | FE, .002 |
| Surgery on arms (% yes) | 17.9 (35) | 21.5 (91) | FE, .335 |
| Surgery on hands (% yes) | 8.2 (16) | 10.6 (45) | FE, .387 |
| Surgery on legs (% yes) | 21.8 (42) | 24.7 (103) | FE, .475 |
| Surgery on feet (% yes) | 15.0 (29) | 17.0 (71) | FE, .638 |
| Injury to arms (% yes) | 25.8 (50) | 26.3 (110) | FE, .992 |
| Injury to hands (% yes) | 23.7 (45) | 34.7 (143) | FE, .008 |
| Injury to legs (% yes) | 18.8 (36) | 22.4 (93) | FE, .338 |
| Injury to feet (% yes) | 26.7 (51) | 27.9 (115) | FE, .770 |
| Comorbid conditions (% yes) | |||
| Osteoarthritis | 16.8 (33) | 30.3 (129) | FE, <.001 |
| Back pain | 23.9 (47) | 34.7 (148) | FE, .007 |
| Depression | 17.3 (34) | 23.9 (102) | FE, .061 |
| High blood pressure | 19.8 (39) | 26.5 (113) | FE, .072 |
| Heart disease | 4.1 (8) | 7.5 (32) | FE, .115 |
| Diabetes | 4.6 (9) | 5.6 (24) | FE, .702 |
| Lung disease | 6.6 (13) | 4.7 (20) | FE, .339 |
| Anemia or blood disease | 4.6 (9) | 5.9 (25) | FE, .574 |
| Ulcer or stomach disease | 3.0 (6) | 3.8 (16) | FE, .817 |
| Kidney disease | 0.0 (0) | 2.3 (10) | FE, .035 |
| Liver disease | 0.0 (0) | 3.3 (14) | FE, .007 |
| Rheumatoid arthritis | 2.0 (4) | 2.8 (12) | FE, .786 |
| Pain not related to cancer | 51.8 (101) | 58.0 (246) | FE, .163 |
| Type of cancer | Χ2 = 17.41, .002 | ||
| Breast | 57.4 (113) | 54.9 (234) | |
| Colon | 4.6 (9) | 9.6 (41) | 1<2 |
| Lung | 5.6 (11) | 1.9 (8) | 1>2 |
| Ovarian | 4.6 (9) | 10.6 (45) | 1<2 |
| Other | 27.9 (55) | 23.0 (98) | |
| Any metastatic disease | 58.2 (114) | 60.2 (253) | FE, .660 |
| Chemotherapy regimen | Χ2 = 7.86, .020 | ||
| Only a platinum compound | 28.6 (56) | 22.3 (95) | |
| Only a taxane compound | 51.0 (100) | 46.9 (200) | 1<2 |
| Both a platinum and a taxane compound |
20.4 (40) | 30.8 (131) | |
| Dose of platinum compound for patients who received only a platinum (mg) |
778.94 (563.32) | 1323.95 (923.29) | U, <.001 |
| Dose of taxane compound for patients who received only a taxane (mg) |
1113.11 (517.80) | 1365.27 (1172.54) | U, .010 |
| Dose of drugs for patients who received both a platinum and a taxane compound |
|||
| Platinum dose (mg) | 2845.75 (1341.27) | 3224.51 (1469.18) | U, .072 |
| Taxane dose (mg) | 1223.01 (564.85) | 1625.18 (883.10) | U, .008 |
| Patients who had a dose reduction or delay due to neuropathy (% (n)) |
1.6 (3) | 13.8 (56) | FE, <.001 |
Abbreviations: FE = Fisher’s Exact test, kg = kilograms, m2 = meters squared, mg = milligrams, U = Mann Whitney U test, SD = standard deviation
In terms of cancer diagnosis, post hoc contrasts demonstrated that while no between group differences were found in the percentage of survivors with breast cancer, a higher percentage of survivors with CIN had a diagnosis of colon (p=.038) or ovarian cancer (p=.014) and a lower percentage had a diagnosis of lung cancer (p=.021). A higher percentage of survivors with CIN received a combination of a platinum and taxane CTX regimen (p=.007) and had received a dose reduction or delay in their CTX treatment due to CIN (p<.001). When doses of single agents were compared, patients with CIN received a higher cumulative dose of either a platinum (p<.001) or a taxane (p=.010) (Table 2).
Self-reported Pain Characteristics of Survivors with CIN
Table 3 summarizes pain intensity, pain interference, and pain quality scores for the upper and lower extremities for the survivors with CIN. Of the 426 survivors with CIN, 4.9% had CIN only in their upper extremities, 27.0% only in their lower extremities, and 68.1% in both their upper and lower extremities. The average duration of CIN was 3.62 (±4.08) years in the upper extremities and 3.94 (±4.24) years in the lower extremities. For the majority of the pain intensity, interference, and quality items, the scores were higher in the lower extremities compared to the upper extremities.
Table 3.
Pain Characteristics of the Cancer Survivors With Chemotherapy-Induced Neuropathy (CIN)
| Characteristic | Lower Extremity (n=405) |
Upper Extremity (n=311) |
|---|---|---|
| Mean (SD) | Mean (SD) | |
| Pain Characteristics | ||
| Duration of CIN (years) | 3.94 (4.24) | 3.62 (4.08) |
| Pain now | 3.60 (2.27) | 2.77 (2.06) |
| Average pain | 3.99 (2.11) | 3.11 (2.14) |
| Worst pain | 6.04 (2.54) | 4.65 (2.66) |
| Days per week in pain | 3.63 (3.03) | 3.60 (3.01) |
| Hours per day in pain | 14.94 (9.46) | 12.94 (9.84) |
| Pain Interference Scale | ||
| Balance Routine activities+ |
3.58 (3.04) | 2.54 (2.71) |
| Walking ability | 3.36 (3.02) | 0.45 (1.44) |
| Enjoyment of life | 2.86 (2.80) | 2.13 (2.68) |
| Normal work | 2.69 (2.82) | 2.74 (2.73) |
| Sleep | 2.74 (2.87) | 1.56 (2.40) |
| General activity | 2.65 (2.68) | 2.48 (2.68) |
| Mood | 2.38 (2.51) | 1.90 (2.32) |
| Relations with other people | 1.53 (2.28) | 0.78 (1.65) |
| Sexual activity | 0.95 (2.14) | 0.70 (1.96) |
| Mean interference score | 2.55 (2.21) | 1.72 (1.88) |
| Pain Qualities Assessment Scale Scores | ||
| Numb | 5.41 (3.04) | 3.86 (2.86) |
| Unpleasant | 4.49 (2.47) | 3.62 (2.50) |
| Tingling | 4.32 (3.02) | 3.18 (2.83) |
| Intense | 3.24 (2.49) | 2.61 (2.32) |
| Dull | 3.11 (2.75) | 2.37 (2.47) |
| Cramping | 2.86 (3.20) | 1.78 (2.63) |
| Electrical | 2.53 (3.06) | 1.82 (2.65) |
| Shooting | 2.47 (2.92) | 1.59 (2.48) |
| Sharp | 2.33 (2.87) | 1.38 (2.30) |
| Aching | 2.20 (2.72) | 1.81 (2.52) |
| Heavy | 2.08 (2.73) | 1.29 (2.29) |
| Cold | 2.06 (2.83) | 1.44 (2.36) |
| Radiating | 2.06 (2.74) | 1.27 (2.23) |
| Hot | 1.98 (2.69) | 1.01 (1.97) |
| Tender | 1.93 (2.50) | 1.58 (2.29) |
| Sensitive skin | 1.80 (2.32) | 1.34 (2.13) |
| Throbbing | 1.73 (2.59) | 1.30 (2.25) |
| Itchy | 1.04 (2.06) | 0.82 (1.91) |
| Intense – surface pain | 3.27 (2.71) | 2.92 (2.55) |
| Intense – deep pain | 3.29 (2.82) | 2.41 (2.65) |
Dressing, toileting, typing
Abbreviations: SD = standard deviation
Differences in Objective Measures of Sensation
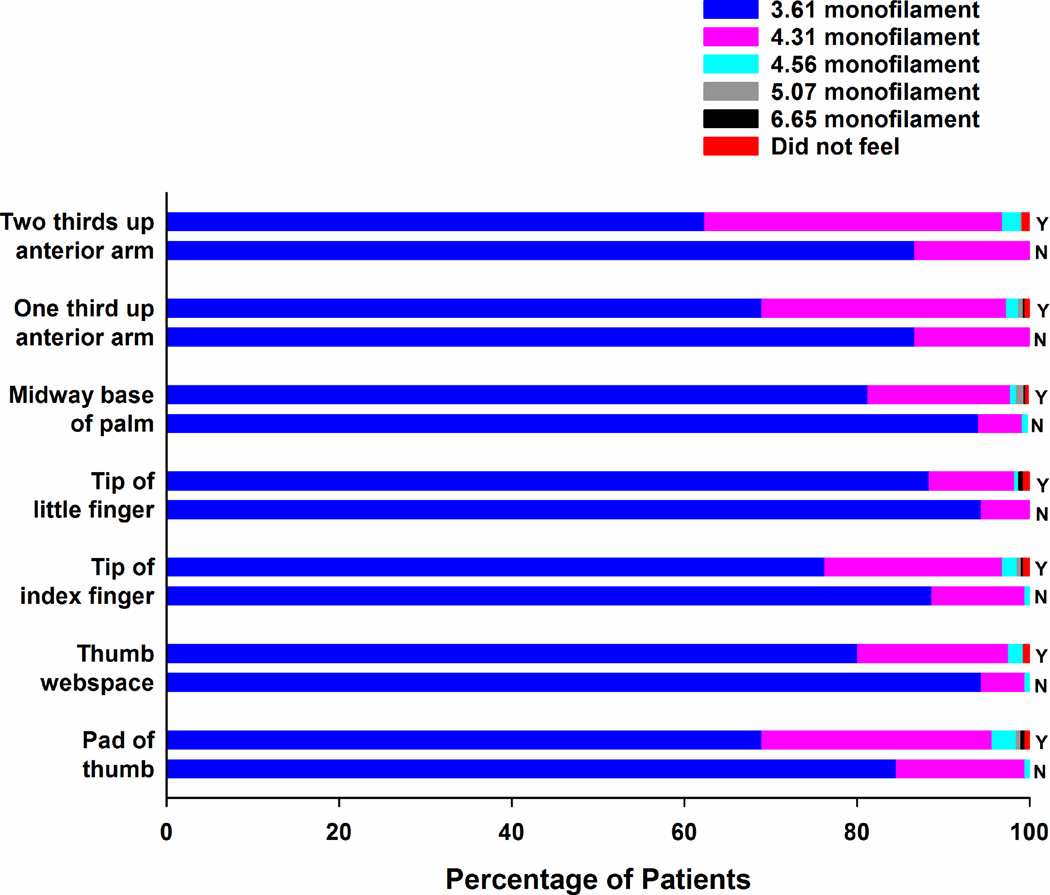
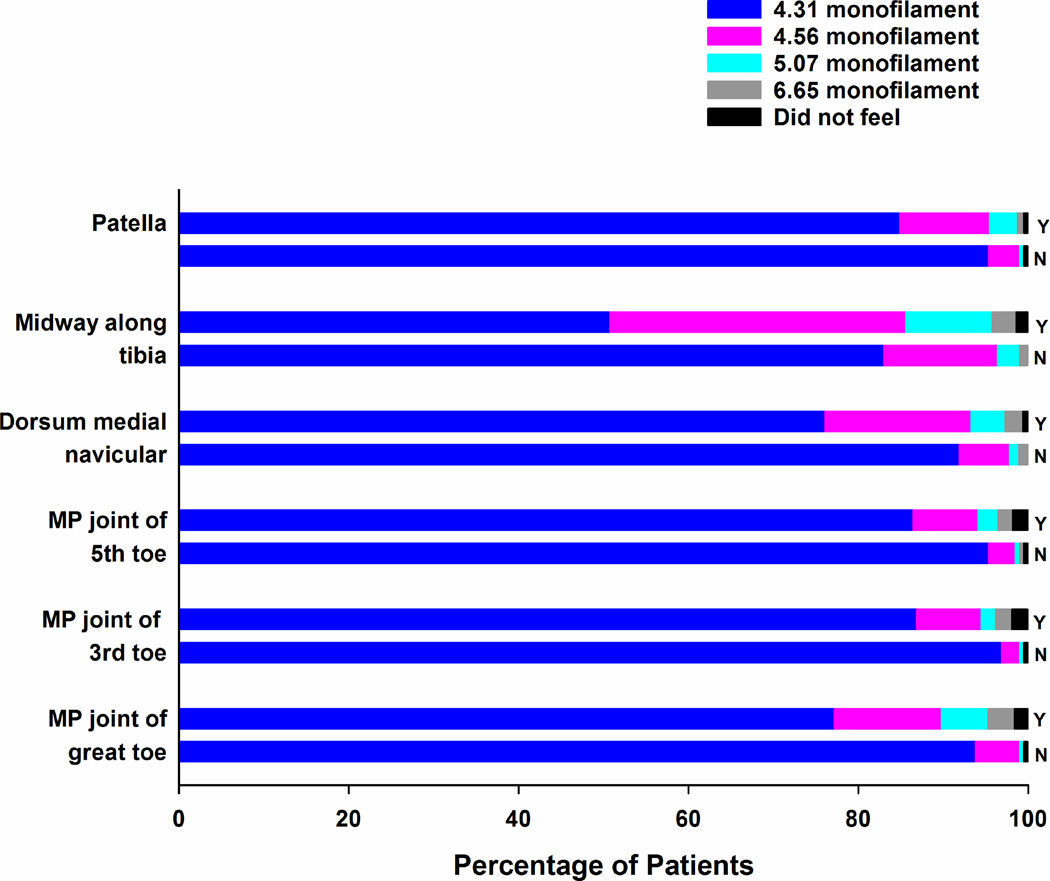
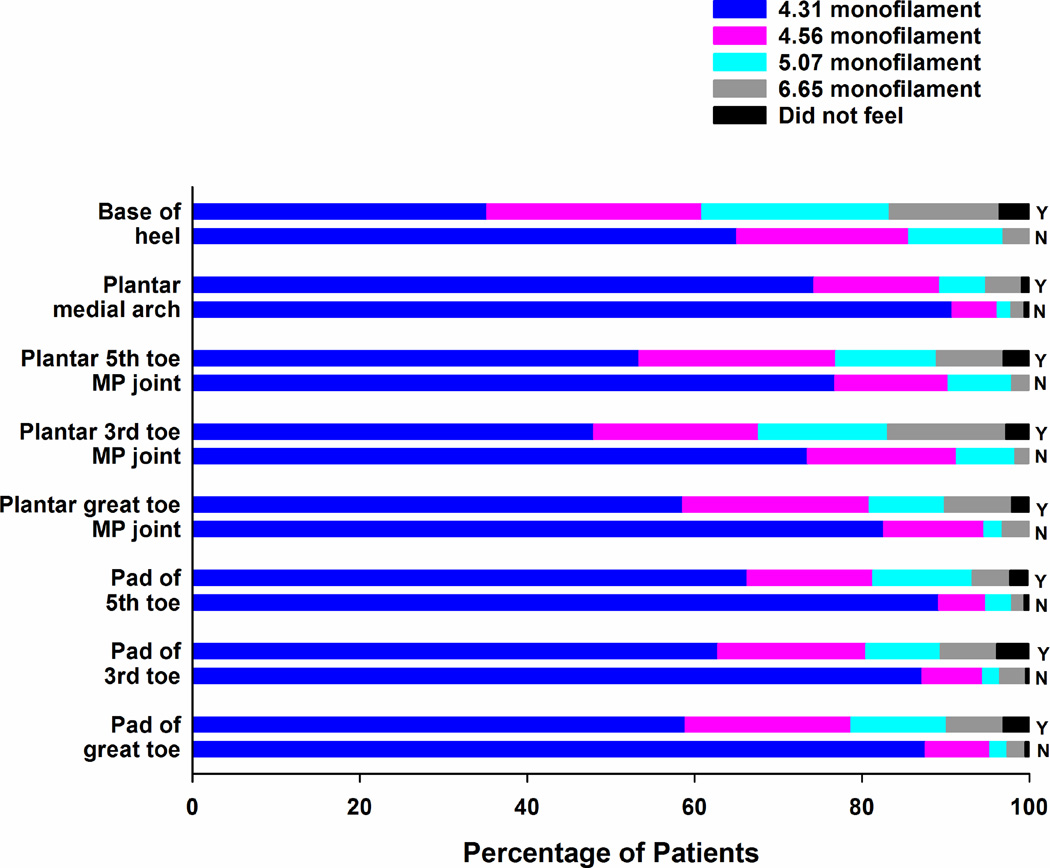
In terms of light touch sensation, for every site that was tested in the upper (Figure 1) and lower (Figures 2 and 3) extremities, the percentage of survivors with CIN who felt the smallest monofilament (i.e., 3.61 in the upper extremity, 4.31 in the lower extremity) was significantly lower (all, p<.001) and the mean monofilament score was significantly higher (all, p<.001; see Supplementary Table 1) compared to survivors without CIN. Except for one site in the lower extremities (i.e., dorsum medial navicular) and three sites in the upper extremities (i.e., midway up the base of the palm, one third and two thirds up the anterior arm), a significantly higher percentage of survivors with CIN did not feel pain at each of the sites tested using the Neurotip (see Table 4). In terms of cold sensation, a significantly higher percentage of survivors with CIN did not feel the Tiptherm device in all of the lower extremity sites tested, as well as in the pad of the index and little finger (see Table 5). For all of the sites tested in the upper and lower extremities, survivors with CIN had significantly higher vibration thresholds (Table 6).
Figure 1.
Percentages of patients with (Y) and without (N) chemotherapy-induced neuropathy who felt each of the Semmes Weinstein monofilaments at each of the sites tested in the upper extremities.
Figure 2.
Percentages of patients with (Y) and without (N) chemotherapy-induced neuropathy who felt each of the Semmes Weinstein monofilaments at each of the sites tested on the dorsal surface of the lower extremities.
Figure 3.
Percentages of patients with (Y) and without (N) chemotherapy-induced neuropathy who felt each of the Semmes Weinstein monofilaments at each of the sites tested on the plantar surface of the lower extremities.
Table 4.
Differences in the Percentage of Sites in the Dominant Lower and Upper Extremities That Did Not Feel Pain Between Cancer Survivors With (n=426) and Without (n=197) Chemotherapy Induced Neuropathy (CIN)
| Site | No CIN 31.6% (n=197) |
CIN 68.4% (n=426) |
p-value* |
|---|---|---|---|
| % (n) | % (n) | ||
| Lower Extremity Sites | |||
| Pad of great toe | 32.3 (63) | 59.0 (248) | <.001 |
| Pad of 3rd toe | 32.3 (63) | 58.7 (247) | <.001 |
| Pad of 5th toe | 22.1 (43) | 50.8 (214) | <.001 |
| Plantar great toe MP joint+ | 40.5 (75) | 69.1 (226) | <.001 |
| Plantar 3rd toe MP joint+ | 51.4 (95) | 82.9 (271) | <.001 |
| Plantar 5th toe MP joint+ | 45.9 (85) | 79.5 (260) | <.001 |
| Plantar medial arch+ | 18.4 (34) | 43.7 (143) | <.001 |
| Base of heel | 50.3 (98) | 79.7 (333) | <.001 |
| MP joint of great toe | 8.7 (17) | 20.4 (86) | <.001 |
| MP joint of 3rd toe | 4.6 (9) | 16.0 (67) | <.001 |
| MP joint of 5th toe | 5.1 (10) | 19.0 (80) | <.001 |
| Dorsum medial navicular+ | 5.9 (11) | 9.8 (32) | .140 |
| Midway along tibia | 11.3 (22) | 23.4 (98) | <.001 |
| Patella | 6.2 (12) | 14.8 (62) | .002 |
| Upper Extremity Sites | |||
| Pad of thumb | 22.6 (44) | 38.5 (162) | <.001 |
| Thumb webspace | 12.3 (24) | 19.2 (81) | .038 |
| Tip of index finger | 6.7 (13) | 24.0 (101) | <.001 |
| Tip of little finger | 4.1 (8) | 9.5 (40) | .023 |
| Midway base of palm | 4.1 (8) | 6.9 (29) | .205 |
| One third up anterior arm | 5.6 (11) | 6.4 (27) | .857 |
| Two thirds up anterior arm | 6.7 (13) | 10.7 (45) | .138 |
Fisher’s Exact test
Completed on 512 of 623 cancer survivors
Abbreviation: MP = metocarpophalangeal
Table 5.
Differences in the Percentage of Sites in the Dominant Lower and Upper Extremities That Did Not Feel Cold Between Cancer Survivors With (n=426) and Without (n=197) Chemotherapy Induced Neuropathy (CIN)
| Site | No CIN 31.6% (n=197) |
CIN 68.4% (n=426) |
p-value* |
|---|---|---|---|
| % (n) | % (n) | ||
| Lower Extremity Sites | |||
| Top of great toe at 1st MP joint | 47.7 (93) | 62.6 (265) | .001 |
| Pad of great toe | 64.6 (126) | 80.9 (342) | <.001 |
| Dorsum of foot midpoint | 24.6 (48) | 36.4 (154) | .004 |
| Medial malleolus | 33.8 (66) | 45.6 (193) | .007 |
| Upper Extremity Sites | |||
| Pad of index finger | 15.9 (31) | 29.6 (125) | <.001 |
| Pad of little finger | 28.7 (56) | 37.4 (158) | .037 |
| Dorsal metacarpal area of the hand | 7.7 (15) | 9.7 (41) | .455 |
| Wrist | 3.1 (6) | 5.7 (24) | .226 |
Fisher’s Exact test
Abbreviations: MP = metocarpophalangeal
Table 6.
Differences in the Vibration Thresholds, Grip Strength, Pegboard Test, Heel Lift, Balance, and Reflexes in the Dominant Extremities Between Cancer Survivors With (n=426) and Without (n=197) Chemotherapy Induced Neuropathy (CIN)
| Site | No CIN 31.6% (n=197) |
CIN 68.4% (n=426) |
t-test,* p-value | A % |
B % |
|---|---|---|---|---|---|
| Mean (SD) | Mean (SD) | ||||
| Vibration Thresholds (volts) | |||||
| Dorsal IP joint of great toe | 17.01 (10.64) | 25.14 (14.66) | −8.06, <.001 | 1.5 | 8.0 |
| Medial malleolus | 20.98 (11.23) | 28.10 (13.53) | −6.85, <.001 | 2.1 | 9.0 |
| Patella | 22.12 (10.80) | 27.26 (11.90) | −5.31, <.001 | 1.5 | 3.3 |
| Dorsal IP joint of thumb | 5.19 (2.26) | 6.92 (4.56) | −6.31, <.001 | 0.0 | 0.0 |
| Dorsal IP joint of index finger | 5.77 (3.10) | 7.44 (4.56) | −5.32, <.001 | 0.0 | 0.0 |
| Ulnar prominence of the wrist | 6.99 (2.81) | 9.18 (5.51) | −6.52, <.001 | 0.0 | 0.0 |
| Lateral epicondyle | 10.33 (4.31) | 12.71 (6.23) | −5.49, <.001 | 0.0 | 0.0 |
| Additional Tests | |||||
| Grip strength (kilograms) | 27.70 (8.01) | 25.39 (8.28) | 3.25, .001 | n/a | |
| Number of pins in 30 seconds | 14.63 (2.48) | 14.04 (2.26) | 2.92, .004 | n/a | |
| Heel lift (seconds) | 14.16 (2.98) | 12.63 (4.56) | 4.97, <.001 | n/a | |
| Timed get up and go test (seconds) | 6.57 (1.51) | 7.75 (2.49) | −7.27, <.001 | n/a | |
| Fullerton Advanced Balance test | 36.17 (4.77) | 33.25 (6.69) | 6.18, <.001 | n/a | |
| Achilles reflex (% (n) absent) | 54.2 (104) | 78.2 (326) | FE, <.001 | n/a | |
Independent sample t-test
Abbreviations: A = Percentage of cancer survivors without CIN who did not feel the maximum vibration threshold, B = Percentage of cancer survivors with CIN who did not feel the maximum vibration threshold, IP = interphalangeal, n/a = not applicable, SD = standard deviation
Differences in Measures of Motor Function
As shown in Table 6, the measures of upper extremity strength and dexterity were significantly worse in the survivors with CIN. In the lower extremities, survivors with CIN had a significantly shorter heel lift time and were more likely to have lost their Achilles reflex. Finally, as shown in Table 7, for every site tested in the upper and lower extremities, a higher percentage of survivors with CIN had decreases in muscle strength.
Table 7.
Differences in Muscle Strength Testing in the Dominant Lower and Upper Extremities Between Cancer Survivors With (n=426) and Without (n=197) Chemotherapy Induced Neuropathy (CIN)
| Location | Extremity | 0+ | 1+ | 2+ | 3+ | 4+ | 5+ | p-value* |
|---|---|---|---|---|---|---|---|---|
| % (n) | % (n) | % (n) | % (n) | % (n) | % (n) | |||
| Extension of great toe | No CIN | 0.0 (0) | 0.0 (0) | 0.0 (0) | 0.0 (0) | 5.1 (10) | 94.9 (185) | <.001 |
| CIN | 0.7 (3) | 0.9 (4) | 1.4 (6) | 2.8 (12) | 16.8 (71) | 77.3 (327) | ||
| Dorsiflexion of ankle | No CIN | 0.5 (1) | 0.0 (0) | 0.0 (0) | 0.0 (0) | 1.0 (2) | 98.5 (192) | .004 |
| CIN | 0.5 (2) | 0.0 (0) | 0.2 (1) | 1.2 (5) | 5.2 (22) | 92.9 (393) | ||
| Plantar flexion of ankle | No CIN | 0.5 (1) | 0.0 (0) | 0.0 (0) | 0.0 (0) | 2.6 (5) | 96.9 (188) | .010 |
| CIN | 0.5 (2) | 0.0 (0) | 0.2 (1) | 1.4 (6) | 6.6 (28) | 91.2 (385) | ||
| Abduction of the little finger | No CIN | 0.0 (0) | 0.0 (0) | 0.5 (1) | 0.5 (1) | 13.3 (26) | 85.6 (167) | <.001 |
| CIN | 0.0 (0) | 0.0 (0) | 1.7 (7) | 5.9 (25) | 23.6 (100) | 68.8 (291) | ||
| Opposition of thumb and little finger |
No CIN | 0.0 (0) | 0.0 (0) | 0.5 (1) | 1.0 (2) | 3.6 (7) | 94.9 (185) | <.001 |
| CIN | 0.2 (1) | 0.0 (0) | 1.2 (5) | 4.5 (19) | 15.1 (64) | 79.0 (334) | ||
| Wrist extension | No CIN | 0.0 (0) | 0.0 (0) | 0.0 (0) | 0.0 (0) | 2.1 (4) | 97.9 (191) | .001 |
| CIN | 0.0 (0) | 0.2 (1) | 0.7 (3) | 1.4 (6) | 7.3 (31) | 90.3 (381) |
Mann Whitney U test
Medical Research Council Scale: 0=no flicker of movement, 1=visible contraction without movement of the site, 2=movement of the site but not against gravity, 3=movement over the full range against gravity, 4=movement against gravity and resistance, 5=normal
Differences in Balance and Postural Control
As shown in Table 6, survivors with CIN had a significantly longer TUG test (p<.001) and a significantly lower FAB score (p<.001).
DISCUSSION
This study is the first to perform a detailed characterization of CIN in cancer survivors. In our study, enrollment was restricted to platinum and/or taxane-based regimens because these two classes of neurotoxic drugs are used to treat the most commonly occurring cancers. Of note, this study is the first to report on the occurrence rates for CIN in both the upper and lower extremities. While our findings confirm the clinical impression that most survivors have CIN in both their upper and lower extremities, 4.9% had CIN only in their upper extremities and 27.0% only in their lower extremities.
The higher percentage of patients with CIN in the lower extremities is expected given that this condition is characterized as a length dependent neuropathy.62,63 When the percentages of survivors, in our study, with only upper extremity CIN who had hand (14.3%) or arm (23.8%) surgery were compared to survivors with both upper and lower extremity CIN who had hand (9.7%) or arm (21.8%) surgery, no significant differences were found. However, compared to survivors with both upper and lower extremity CIN (34.4% and 25.1%, respectively), a significantly higher percentage of survivors with only upper extremity CIN reported injuries to their hands (57.9%, p=.048) and arms (47.6%, p=.038). Carpal tunnel syndrome was the most common condition reported by the survivors with only hand CIN. While no information is available on the onset of carpal tunnel syndrome in relationship to the initiation of CTX, this finding warrants further investigation given that 3.8% of the general population carpal tunnel syndrome.64
Pain characteristics of patients with CIN
In terms of the pain characteristics listed in Table 2, it is difficult to compare our findings with previous reports because the majority of the studies of CIN in cancer survivors focused on the determination of occurrence rates using the National Cancer Institute Common Toxicity Criteria65 or the occurrence rates for common symptoms on the CIPN20 scale.11,16,19,66 In the one study that reported severity scores,17 survivors were asked to rate the severity of numbness and tingling as a single item. In terms of pain severity, while no studies have established cutpoints for CIN, in our study, survivors’ worst pain scores were in the moderate to severe range for both the upper and lower extremities.67 In terms of the interference items that were adapted from the Brief Pain Inventory,68,69 our scores were in the mild to moderate range. When compared with other studies, our survivors’ interference scores were similar to scores reported by oncology patients experiencing taxane-induced arthralgias and myalgias,70 but lower than scores reported by oncology patients with bone metastasis67 or oncology patients with a variety of neuropathic pain conditions.71 In order to better assess interference with function in the upper extremities, additional items need to be developed for the BPI.
While the PQAS was developed for and used in studies of neuropathic pain,41,42,72–75 our study is the first to use it to evaluate the qualities associated with CIN. Consistent with previous descriptions of CIN (for reviews see6,62,76), numb, followed by unpleasant and tingling, were the three qualities with the highest severity ratings in both the upper and lower extremities. The scores for numb and tingling in the survivors with CIN were higher than those reported by patients with low back pain and osteoarthritis, but lower than scores reported by patients with carpal tunnel syndrome.74 Differences in these pain quality scores may reflect different underlying mechanisms for these chronic pain conditions.
Differences in demographic and clinical characteristics
Little information is available on the demographic and clinical characteristics that increase an individual’s risk for the development of CIN. Data from two recent reviews62,77 and a study from the Southwest Oncology Group78 found that older age, African race, prior treatment with a neurotoxic agent, a history of smoking, diabetes mellitus, autoimmune disease, folate/vitamin B12 deficiency, decreased creatinine clearance, neuropathy prior to the initiation of CTX, and sensory changes during CTX were associated with the development of CIN. However, in the registry studies of CIN in cancer survivors,11,12,16 risk factors for CIN were not evaluated.
While no between group differences were found in the years since cancer diagnosis and the presence or extent of metastatic disease, a number of demographic and clinical characteristics provide new insights into potential risk factors for CIN. In terms of demographic characteristics, consistent with the risk factors for CIN noted above, as well as risk factors associated with painful diabetic neuropathy,79–82 survivors with CIN were older and had a lower annual household income.
An extremely important finding from our study, that is consistent with recent reports in diabetic neuropathy,79,80 is the association between comorbidity and CIN. The SCQ score assesses not only the number but the impact of comorbidities on an individual. It should be noted that the difference in SCQ scores between the survivors with and without CIN represents not only a statistically significant but a clinically meaningful difference (Cohen’s d = .40).83 In terms of specific co-morbidities, a higher percentage of survivors with CIN reported the occurrence of osteoarthritis and back pain. Both of these conditions are reported to have a neuropathic component which may suggest a predisposition for neuropathic pain.84–88
A novel finding in our study is that a higher percentage of survivors with CIN were born prematurely. This finding may be partially explained by the growing body of preclinical and clinical evidence that suggests that the extensive exposure to painful stimuli and stress that premature infants experience results in prolonged and irreversible changes to the peripheral and central nervous systems (for reviews see89–91). These changes may place an individual who was born prematurely at higher risk for CIN. Given the small number of survivors who were born prematurely, this finding warrants confirmation in future studies. However, if replicated it may be an important screening question prior to the initiation of CTX given that 18% of infants worldwide and 11.4% of infants in the United States are born prematurely.92
In terms of previous injuries or surgeries to the upper and lower extremities, the only significant finding was that a higher percentage of patients with CIN reported an injury to their hands. This finding is consistent with our previous work that demonstrated that a higher percentage of women who reported pain in their breast prior to surgery experienced persistent pain following the procedure.93,94 Of note, a higher number of breast biopsies was associated with the occurrence of preoperative breast pain in these patients.38,95 Taken together, these findings suggest that previous injury can influence the development of CIN.
Consistent with previous reports in patients with colon cancer who received oxaliplatin96 and patients with breast cancer who received docetaxel,97 as well as patients with fibromyalgia98 and diabetes mellitus,99 patients with CIN had a higher BMI. As expected, and consistent with recent studies,78,100 patients with CIN were more likely to have received a combination of a platinum and a taxane compound and had received higher cumulative doses of CTX. This finding may be related to the receipt of two different classes of CTX drugs that have both overlapping (e.g., mitochondrial dysfunction) and differential (e.g. formation of platinum-DNA adducts, taxanes’ interference with the stabilization of microtubules (for reviews see63,101) mechanisms for CIN. However, patients with CIN reported a slightly lower number of previous cancer treatments. This finding may relate to the diverse types of cancers included in this study because some cancers may have been detected at an earlier stage and/or different cancers require fewer types of treatments.
Differences in sensations
In terms of differences in the effect of neurotoxic CTX on sensory modalities (i.e., light touch (Figures 1 to 3 and Supplemental Table 1), pain (Table 4), temperature (Table 5), vibration (Table 6)), the overall findings are in the expected direction including more severe changes in patients with CIN and more severe changes associated with distal as compared to proximal sites. However, this study is the first to provide a detailed characterization of changes in sensation at multiple sites in both the upper and lower extremities. In terms of an evaluation of light touch sensation, while no recommendation exists for the size of the monofilament to use to test the upper extremities, most clinicians use a 5.07 monofilament to evaluate for diabetic neuropathy. Our findings suggest that a finer monofilament should be used to assess survivors for changes in light touch sensation.
Vibration thresholds are known to vary by age. In one study that evaluated non-diabetic patients (n=662, mean age 66.1, range 55 to 85 years),102 the mean vibration thresholds in the upper extremities ranged from 7.9 volts (±2.0 for patients ≤70 years of age) to 9.4 volts (±3.7 for patients >70 years of age). For the lower extremities, the range was 15.3 volts (±7.1 for patients ≤70 years of age) to 20.5 volts (±9.8 for patients >70 years of age). Given that, in our study, the mean age of the survivors with CIN was 60.9, the increases in vibration thresholds in their lower extremity sites represent clinically meaningful changes. Of note, in a study of patients with diabetes,103 a vibration threshold of >25 volts was associated with an 8-fold increase in the likelihood of developing a diabetic foot ulcer.
Differences in function and postural control
Grip strength and the Pegboard test were used to evaluate for differences in upper extremity strength and dexterity (Table 6). In the only study identified that compared patients who were CTX naïve (n=20), to patients who received CTX (n=9),104 the mean grip strengths were 33.5 kg (±8.5) and 30.1 kg (±11.4), respectively. For both groups of patients in our study, the mean grip strengths were lower which may be related to differences in the mean age of the patients and the fact that survivors without CIN did receive CTX.
In the manual provided by the manufacturer of the Purdue Pegboard test, healthy individuals were able to insert 17.95 pins in 30 seconds using their dominant hand with clinically meaningful decrements of −1 (15.91) to −2 (13.88) standard deviations.105 While no studies were identified that used this test of upper extremity function in survivors with CIN, our findings suggest that both groups have some clinically meaningful decrements which may not be related to CIN. A potential factor that could influence performance on the Pegboard test is age.54,106 Given that the survivors with CIN were older than the survivors without CIN and they had slightly worse performance on the Pegboard test, the influence of age and other demographic (e.g., gender, handedness) and clinical characteristics on tests of hand function warrant consideration in future studies.
A growing body of evidence suggests that patients with CIN are at increased risk for falling.18,20,107–109 In terms of the effects of CIN on lower extremity function, in the one study that compared 20 breast cancer patients who had taxane-induced CIN to 20 healthy controls, TUG scores in the breast cancer patients (6.69 ± 0.99) were worse than in the healthy controls (5.85 ± 0.86).108 While the survivors with CIN in the current study had worse TUG scores than survivors without CIN, both groups of survivors in the current study had worse TUG scores than the patients with breast cancer who had taxane-induced CIN.108 However, the TUG scores of the cancer survivors in the current study are lower than 13.5 which is associated with a higher risk for falls.110
Similarly, the FAB scores of our cancer survivors are almost identical to those reported by Wampler and colleagues for the patients with breast cancer who completed taxane treatment.108 In addition, the mean FAB scores in our study were above the clinically meaningful cutoff score of ≤25 that is associated with a higher risk of falls.59 The findings for muscle testing confirm previous reports that CIN is primarily a sensory neuropathy.
Limitations
A number of limitations warrant consideration. Given the cross-sectional nature of our study, the associations identified between demographic and clinical characteristics warrant confirmation as predictors of CIN in a prospective, longitudinal study. Second, survivors were not evaluated using quantitative sensory testing. Rather, subjective and objective measures were used that could be easily implemented in clinical practice. While only survivors who received a platinum and/or a taxane compound were included in our study, other CTX agents induce CIN (for reviews see63,101). Therefore, our findings may not generalize to survivors who received other types of neurotoxic CTX.
Conclusions
Despite these limitations, our study is the largest study of CIN in cancer survivors. The detailed characterization of changes in sensation, function, and postural control serves as benchmark data for future studies of neurotoxic CTX in cancer survivors and for clinical trials of pharmacologic and nonpharmacologic interventions. In addition, many of the differences in demographic and clinical characteristics, as well as in the objective measures, between survivors with and without CIN, are similar to those found in other forms of peripheral neuropathy (e.g., diabetic neuropathy, anti-retroviral therapy).
Supplementary Material
Acknowledgments
This study was funded by the National Cancer Institute (NCI, CA151692). Dr. Miaskowski is supported by a grant from the American Cancer Society and NCI (CA168960). This project was supported by the National Center for Advancing Translational Sciences, National Institutes of Health, through UCSF-CTSI Grant Number UL1 TR000004. Its contents are solely the responsibility of the authors and do not necessarily represent the official views of the NIH. Recruitment was facilitated by Dr. Susan Love Research Foundation’s Army of Women® Program.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.DeSantis CE, Lin CC, Mariotto AB, et al. Cancer treatment and survivorship statistics, 2014. CA Cancer J Clin. 2014;64:252–271. doi: 10.3322/caac.21235. [DOI] [PubMed] [Google Scholar]
- 2.Denlinger CS, Ligibel JA, Are M, et al. Survivorship: screening for cancer and treatment effects, version 2.2014. J Natl Compr Canc Netw. 2014;12:1526–1531. doi: 10.6004/jnccn.2014.0152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hershman DL, Lacchetti C, Dworkin RH, et al. Prevention and management of chemotherapy-induced peripheral neuropathy in survivors of adult cancers: American Society of Clinical Oncology clinical practice guideline. J Clin Oncol. 2014;32:1941–1967. doi: 10.1200/JCO.2013.54.0914. [DOI] [PubMed] [Google Scholar]
- 4.Pachman DR, Barton DL, Swetz KM, et al. Troublesome symptoms in cancer survivors: fatigue, insomnia, neuropathy, and pain. J Clin Oncol. 2012;30:3687–3696. doi: 10.1200/JCO.2012.41.7238. [DOI] [PubMed] [Google Scholar]
- 5.Bennion AE, Molassiotis A. Qualitative research into the symptom experiences of adult cancer patients after treatments: a systematic review and meta-synthesis. Support Care Cancer. 2013;21:9–25. doi: 10.1007/s00520-012-1573-x. [DOI] [PubMed] [Google Scholar]
- 6.Cavaletti G, Alberti P, Marmiroli P. Chemotherapy-induced peripheral neurotoxicity in cancer survivors: an underdiagnosed clinical entity? Am Soc Clin Oncol Educ Book. 2015;35:e553–e560. doi: 10.14694/EdBook_AM.2015.35.e553. [DOI] [PubMed] [Google Scholar]
- 7.Boland EG, Selvarajah D, Hunter M, et al. Central pain processing in chronic chemotherapy-induced peripheral neuropathy: a functional magnetic resonance imaging study. PLoS One. 2014;9:e96474. doi: 10.1371/journal.pone.0096474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dougherty PM. Is chemotherapy-induced peripheral neuropathy more than just a peripheral nervous system disorder? Anesthesiology. 2016;124:992–993. doi: 10.1097/ALN.0000000000001085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Huang ZZ, Li D, Ou-Yang HD, et al. Cerebrospinal fluid axaliplatin contributes to the acute pain induced by systemic administration of oxaliplatin. Anesthesiology. 2016;124:1109–1121. doi: 10.1097/ALN.0000000000001084. [DOI] [PubMed] [Google Scholar]
- 10.Kvale E, Urba SG. NCCN guidelines for survivorship expanded to address two common conditions. J Natl Compr Canc Netw. 2014;12:825–827. doi: 10.6004/jnccn.2014.0199. [DOI] [PubMed] [Google Scholar]
- 11.Mols F, Beijers T, Lemmens V, et al. Chemotherapy-induced neuropathy and its association with quality of life among 2- to 11-year colorectal cancer survivors: results from the population-based PROFILES registry. J Clin Oncol. 2013;31:2699–2707. doi: 10.1200/JCO.2013.49.1514. [DOI] [PubMed] [Google Scholar]
- 12.Mols F, Beijers T, Vreugdenhil G, et al. Chemotherapy-induced peripheral neuropathy and its association with quality of life: a systematic review. Support Care Cancer. 2014;22:2261–2269. doi: 10.1007/s00520-014-2255-7. [DOI] [PubMed] [Google Scholar]
- 13.Bao T, Basal C, Seluzicki C, et al. Long-term chemotherapy-induced peripheral neuropathy among breast cancer survivors: prevalence, risk factors, and fall risk. Breast Cancer Res Treat. 2016;159:327–333. doi: 10.1007/s10549-016-3939-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Brown MR, Ramirez JD, Farquhar-Smith P. Pain in cancer survivors. Br J Pain. 2014;8:139–153. doi: 10.1177/2049463714542605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Postma TJ, Aaronson NK, Heimans JJ, et al. The development of an EORTC quality of life questionnaire to assess chemotherapy-induced peripheral neuropathy: the QLQ-CIPN20. Eur J Cancer. 2005;41:1135–1139. doi: 10.1016/j.ejca.2005.02.012. [DOI] [PubMed] [Google Scholar]
- 16.Beijers AJ, Mols F, Tjan-Heijnen VC, et al. Peripheral neuropathy in colorectal cancer survivors: the influence of oxaliplatin administration. Results from the population-based PROFILES registry. Acta Oncol. 2015;54:463–469. doi: 10.3109/0284186X.2014.980912. [DOI] [PubMed] [Google Scholar]
- 17.Tofthagen C, Donovan KA, Morgan MA, et al. Oxaliplatin-induced peripheral neuropathy's effects on health-related quality of life of colorectal cancer survivors. Support Care Cancer. 2013;21:3307–3313. doi: 10.1007/s00520-013-1905-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tofthagen C, Visovsky C, Berry DL. Strength and balance training for adults with peripheral neuropathy and high risk of fall: current evidence and implications for future research. Oncol Nurs Forum. 2012;39:E416–E424. doi: 10.1188/12.ONF.E416-E424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ezendam NP, Pijlman B, Bhugwandass C, et al. Chemotherapy-induced peripheral neuropathy and its impact on health-related quality of life among ovarian cancer survivors: results from the population-based PROFILES registry. Gynecol Oncol. 2014;135:510–517. doi: 10.1016/j.ygyno.2014.09.016. [DOI] [PubMed] [Google Scholar]
- 20.Gewandter JS, Fan L, Magnuson A, et al. Falls and functional impairments in cancer survivors with chemotherapy-induced peripheral neuropathy (CIPN): a University of Rochester CCOP study. Support Care Cancer. 2013;21:2059–2066. doi: 10.1007/s00520-013-1766-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Clark EJ. Teamwork - The Cancer Patient's Guide to Talking With Your Doctor. 5th. Silver Springs, MD: Natonal Coalition for Cancer Survivorship; 2011. [Google Scholar]
- 22.Galer BS, Jensen MP. Development and preliminary validation of a pain measure specific to neuropathic pain: the Neuropathic Pain Scale. Neurology 197. 48:332–338. doi: 10.1212/wnl.48.2.332. [DOI] [PubMed] [Google Scholar]
- 23.Watson CP, Evans RJ. The postmastectomy pain syndrome and topical capsaicin: a randomized trial. Pain. 1992;51:375–379. doi: 10.1016/0304-3959(92)90223-X. [DOI] [PubMed] [Google Scholar]
- 24.Rotthier A, Baets J, Vriendt ED, et al. Genes for hereditary sensory and autonomic neuropathies: a genotype-phenotype correlation. Brain. 2009;132:2699–2711. doi: 10.1093/brain/awp198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.McFarland R, Turnbull DM. Batteries not included: diagnosis and management of mitochondrial disease. J Intern Med. 2009;265:210–228. doi: 10.1111/j.1365-2796.2008.02066.x. [DOI] [PubMed] [Google Scholar]
- 26.Karnofsky D, Abelmann WH, Craver LV, et al. The use of nitrogen mustards in the palliative treatment of carcinoma. Cancer. 1948;1:634–656. [Google Scholar]
- 27.Karnofsky D. Performance scale. New York: Plenum Press; 1977. [Google Scholar]
- 28.Schnadig ID, Fromme EK, Loprinzi CL, et al. Patient-physician disagreement regarding performance status is associated with worse survivorship in patients with advanced cancer. Cancer. 2008;113:2205–2214. doi: 10.1002/cncr.23856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sangha O, Stucki G, Liang MH, et al. The Self-Administered Comorbidity Questionnaire: a new method to assess comorbidity for clinical and health services research. Arthritis Rheum. 2003;49:156–163. doi: 10.1002/art.10993. [DOI] [PubMed] [Google Scholar]
- 30.Brunner F, Bachmann LM, Weber U, et al. Complex regional pain syndrome 1--the Swiss cohort study. BMC Musculoskelet Disord. 2008;9:92. doi: 10.1186/1471-2474-9-92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cieza A, Geyh S, Chatterji S, et al. Identification of candidate categories of the International Classification of Functioning Disability and Health (ICF) for a Generic ICF Core Set based on regression modelling. BMC Med Res Methodol. 2006;6:36. doi: 10.1186/1471-2288-6-36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Babor TF, Higgins-Biddle JC, Saunders JB, et al. AUDIT: The Alcohol Use Disorders Identification Test: Guidelines for Use in Primary Care. Geneva, Switzerland: World Health Organization; 2001. [Google Scholar]
- 33.Babor TF, de la Fuente JR, Saunders J, et al. AUDIT: The Alcohol Use Disorders Identification Test: Guidelines for Use in Primary Care. Geneva, Switzerland: World Health Organization; 1992. [Google Scholar]
- 34.Berks J, McCormick R. Screening for alcohol misuse in elderly primary care patients: a systematic literature review. Int Psychogeriatr. 2008;20:1090–1103. doi: 10.1017/S1041610208007497. [DOI] [PubMed] [Google Scholar]
- 35.Berner MM, Kriston L, Bentele M, et al. The alcohol use disorders identification test for detecting at-risk drinking: a systematic review and meta-analysis. J Stud Alcohol Drugs. 2007;68:461–473. doi: 10.15288/jsad.2007.68.461. [DOI] [PubMed] [Google Scholar]
- 36.Reinert DF, Allen JP. The alcohol use disorders identification test: an update of research findings. Alcohol Clin Exp Res. 2007;31:185–199. doi: 10.1111/j.1530-0277.2006.00295.x. [DOI] [PubMed] [Google Scholar]
- 37.Posternak V, Miaskowski C. Differences in demographic, clinical, and symptom characteristics and quality of life outcomes among oncology patients with different pain experiences. Pain. 2016;157:892–900. doi: 10.1097/j.pain.0000000000000456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Langford DJ, Schmidt B, Levine JD, et al. Preoperative breast pain predicts persistent breast pain and disability after breast cancer surgery. J Pain Symptom Manage. 2015;49:981–994. doi: 10.1016/j.jpainsymman.2014.11.292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Downie WW, Leatham PA, Rhind VM, et al. Studies with pain rating scales. Ann Rheum Dis. 1978;37:378–381. doi: 10.1136/ard.37.4.378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lundeberg T, Lund I, Dahlin L, et al. Reliability and responsiveness of three different pain assessments. J Rehabil Med. 2001;33:279–283. doi: 10.1080/165019701753236473. [DOI] [PubMed] [Google Scholar]
- 41.Jensen MP, Gammaitoni AR, Olaleye DO, et al. The pain quality assessment scale: assessment of pain quality in carpal tunnel syndrome. J Pain. 2006;7:823–832. doi: 10.1016/j.jpain.2006.04.003. [DOI] [PubMed] [Google Scholar]
- 42.Jensen MP, Dworkin RH, Gammaitoni AR, et al. Assessment of pain quality in chronic neuropathic and nociceptive pain clinical trials with the Neuropathic Pain Scale. J Pain. 2005;6:98–106. doi: 10.1016/j.jpain.2004.11.002. [DOI] [PubMed] [Google Scholar]
- 43.Jensen MP, Friedman M, Bonzo D, et al. The validity of the neuropathic pain scale for assessing diabetic neuropathic pain in a clinical trial. Clinical Journal of Pain. 2006;22:97–103. doi: 10.1097/01.ajp.0000173018.64741.62. [DOI] [PubMed] [Google Scholar]
- 44.Jensen TS, Baron R. Translation of symptoms and signs into mechanisms in neuropathic pain. Pain. 2003;102:1–8. doi: 10.1016/s0304-3959(03)00006-x. [DOI] [PubMed] [Google Scholar]
- 45.Bell-Krotoski JA. Sensibility testing with the Semmes-Weinstein monofilaments. 5th. St Louis: Mosby, Inc.; 2002. pp. 194–212. [Google Scholar]
- 46.Birke JA, Sims DS. Plantar sensory threshold in the ulcerative foot. Lepr Rev. 1986;57:261–267. doi: 10.5935/0305-7518.19860028. [DOI] [PubMed] [Google Scholar]
- 47.Birke JA, Brandsma JW, Schreuders TA, et al. Sensory testing with monofilaments in Hansen's disease and normal control subjects. Int J Lepr Other Mycobact Dis. 2000;68:291–298. [PubMed] [Google Scholar]
- 48.Massy-Westropp N. The effects of normal human variability and hand activity on sensory testing with the full Semmes-Weinstein monofilaments kit. J Hand Ther. 2002;15:48–52. doi: 10.1053/hanthe.2002.v15.01548. [DOI] [PubMed] [Google Scholar]
- 49.Papanas N, Ziegler D. New diagnostic tests for diabetic distal symmetric polyneuropathy. J Diabetes Complications. 2011;25:44–51. doi: 10.1016/j.jdiacomp.2009.09.006. [DOI] [PubMed] [Google Scholar]
- 50.Viswanathan V, Snehalatha C, Seena R, et al. Early recognition of diabetic neuropathy: evaluation of a simple outpatient procedure using thermal perception. Postgrad Med J. 2002;78:541–542. doi: 10.1136/pmj.78.923.541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Medical, Research, Council: Aids to examination of the peripheral nervous system. London, England: Her Majesty's Stationary Office; 1976. [Google Scholar]
- 52.Schmidt RT, Toews JV. Grip strength as measured by the Jamar dynamometer. Arch Phys Med Rehabil. 1970;51:321–327. [PubMed] [Google Scholar]
- 53.Buddenberg LA, Davis C. Test-retest reliability of the Purdue Pegboard Test. Am J Occup Ther. 2000;54:555–558. doi: 10.5014/ajot.54.5.555. [DOI] [PubMed] [Google Scholar]
- 54.Desrosiers J, Hebert R, Bravo G, et al. The Purdue Pegboard Test: normative data for people aged 60 and over. Disabil Rehabil. 1995;17:217–224. doi: 10.3109/09638289509166638. [DOI] [PubMed] [Google Scholar]
- 55.Reddon JR, Gill DM, Gauk SE, et al. Purdue Pegboard: test-retest estimates. Percept Mot Skills. 1988;66:503–506. doi: 10.2466/pms.1988.66.2.503. [DOI] [PubMed] [Google Scholar]
- 56.Hebert-Losier K, Newsham-West RJ, Schneiders AG, et al. Raising the standards of the calf-raise test: a systematic review. J Sci Med Sport. 2009;12:594–602. doi: 10.1016/j.jsams.2008.12.628. [DOI] [PubMed] [Google Scholar]
- 57.Hebert-Losier K, Schneiders AG, Newsham-West RJ, et al. Scientific bases and clinical utilisation of the calf-raise test. Phys Ther Sport. 2009;10:142–149. doi: 10.1016/j.ptsp.2009.07.001. [DOI] [PubMed] [Google Scholar]
- 58.Mathias S, Nayak US, Isaacs B. Balance in elderly patients: the "get-up and go" test. Arch Phys Med Rehabil. 1986;67:387–389. [PubMed] [Google Scholar]
- 59.Hernandez D, Rose DJ. Predicting which older adults will or will not fall using the Fullerton Advanced Balance scale. Arch Phys Med Rehabil. 2008;89:2309–2315. doi: 10.1016/j.apmr.2008.05.020. [DOI] [PubMed] [Google Scholar]
- 60.Rose DJ, Lucchese N, Wiersma LD. Development of a multidimensional balance scale for use with functionally independent older adults. Arch Phys Med Rehabil. 2006;87:1478–1485. doi: 10.1016/j.apmr.2006.07.263. [DOI] [PubMed] [Google Scholar]
- 61.SPSS: IBM SPSS for Windows (Version 23) Chicago: Illinois, SPSS, Inc.; 2015. [Google Scholar]
- 62.Brewer JR, Morrison G, Dolan ME, et al. Chemotherapy-induced peripheral neuropathy: Current status and progress. Gynecol Oncol. 2016;140:176–183. doi: 10.1016/j.ygyno.2015.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Taillibert S, Le Rhun E, Chamberlain MC. Chemotherapy-Related Neurotoxicity. Curr Neurol Neurosci Rep. 2016;16:81. doi: 10.1007/s11910-016-0686-x. [DOI] [PubMed] [Google Scholar]
- 64.Aboonq MS. Pathophysiology of carpal tunnel syndrome. Neurosciences. 2015;20:4–9. [PMC free article] [PubMed] [Google Scholar]
- 65.Padman S, Lee J, Kumar R, et al. Late effects of oxaliplatin-induced peripheral neuropathy (LEON)--cross-sectional cohort study of patients with colorectal cancer surviving at least 2 years. Support Care Cancer. 2015;23:861–869. doi: 10.1007/s00520-014-2423-9. [DOI] [PubMed] [Google Scholar]
- 66.Mols F, Beijers AJ, Vreugdenhil G, et al. Chemotherapy-induced peripheral neuropathy, physical activity and health-related quality of life among colorectal cancer survivors from the PROFILES registry. J Cancer Surviv. 2015;9:512–522. doi: 10.1007/s11764-015-0427-1. [DOI] [PubMed] [Google Scholar]
- 67.Paul SM, Zelman DC, Smith M, et al. Categorizing the severity of cancer pain: further exploration of the establishment of cutpoints. Pain. 2005;113:37–44. doi: 10.1016/j.pain.2004.09.014. [DOI] [PubMed] [Google Scholar]
- 68.Daut RL, Cleeland CS. The prevalence and severity of pain in cancer. Cancer. 1982;50:1913–1918. doi: 10.1002/1097-0142(19821101)50:9<1913::aid-cncr2820500944>3.0.co;2-r. [DOI] [PubMed] [Google Scholar]
- 69.Daut RL, Cleeland CS, Flanery RC. Development of the Wisconsin Brief Pain Questionnaire to assess pain in cancer and other diseases. Pain. 1983;17:197–210. doi: 10.1016/0304-3959(83)90143-4. [DOI] [PubMed] [Google Scholar]
- 70.Chiu N, Zhang L, Gallo-Hershberg D, et al. Which pain intensity scale from the Brief Pain Inventory correlates most highly with functional interference scores in patients experiencing taxane-induced arthralgia and myalgia? Support Care Cancer. 2016;24:2979–2988. doi: 10.1007/s00520-016-3106-5. [DOI] [PubMed] [Google Scholar]
- 71.Lee SC, Park KS, Moon JY, et al. An exploratory study on the effectiveness of "Calmare therapy" in patients with cancer-related neuropathic pain: A pilot study. Eur J Oncol Nurs. 2016;21:1–7. doi: 10.1016/j.ejon.2015.12.001. [DOI] [PubMed] [Google Scholar]
- 72.Jensen MP, Chiang YK, Wu J. Assessment of pain quality in a clinical trial of gabapentin extended release for postherpetic neuralgia. Clin J Pain. 2009;25:286–292. doi: 10.1097/AJP.0b013e318192bf87. [DOI] [PubMed] [Google Scholar]
- 73.Jensen MP, Gammaitoni AR, Bolognese JA, et al. The pain quality response profile of pregabalin in the treatment of neuropathic pain. Clin J Pain. 2012;28:683–686. doi: 10.1097/AJP.0b013e31823f9e64. [DOI] [PubMed] [Google Scholar]
- 74.Victor TW, Jensen MP, Gammaitoni AR, et al. The dimensions of pain quality: factor analysis of the Pain Quality Assessment Scale. Clin J Pain. 2008;24:550–555. doi: 10.1097/AJP.0b013e31816b1058. [DOI] [PubMed] [Google Scholar]
- 75.Waterman C, Victor TW, Jensen MP, et al. The assessment of pain quality: an item response theory analysis. J Pain. 2010;11:273–279. doi: 10.1016/j.jpain.2009.07.014. [DOI] [PubMed] [Google Scholar]
- 76.Travis LB, Fossa SD, Sesso HD, et al. Chemotherapy-induced peripheral neurotoxicity and ototoxicity: new paradigms for translational genomics. J Natl Cancer Inst. 2014;106 doi: 10.1093/jnci/dju044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Seretny M, Currie GL, Sena ES, et al. Incidence, prevalence, and predictors of chemotherapy-induced peripheral neuropathy: A systematic review and meta-analysis. Pain. 2014;155:2461–2470. doi: 10.1016/j.pain.2014.09.020. [DOI] [PubMed] [Google Scholar]
- 78.Hershman DL, Till C, Wright JD, et al. Comorbidities and risk of chemotherapy-induced peripheral neuropathy among participants 65 years or older in Southwest Oncology Group Clinical Trials. J Clin Oncol. 2016;34:3014–3022. doi: 10.1200/JCO.2015.66.2346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.DuBrava S, Mardekian J, Sadosky A, et al. Using random forest models to identify correlates of a diabetic peripheral neuropathy diagnosis from electronic health record data. Pain Med. 2016 doi: 10.1093/pm/pnw096. [DOI] [PubMed] [Google Scholar]
- 80.Sadosky A, Mardekian J, Parsons B, et al. Healthcare utilization and costs in diabetes relative to the clinical spectrum of painful diabetic peripheral neuropathy. J Diabetes Complications. 2015;29:212–217. doi: 10.1016/j.jdiacomp.2014.10.013. [DOI] [PubMed] [Google Scholar]
- 81.Anderson SG, Malipatil NS, Roberts H, et al. Socioeconomic deprivation independently predicts symptomatic painful diabetic neuropathy in type 1 diabetes. Prim Care Diabetes. 2014;8:65–69. doi: 10.1016/j.pcd.2013.08.004. [DOI] [PubMed] [Google Scholar]
- 82.Anderson SG, Narayanan RP, Malipatil NS, et al. Socioeconomic deprivation independently predicts painful diabetic neuropathy in type 2 diabetes. Exp Clin Endocrinol Diabetes. 2015;123:423–427. doi: 10.1055/s-0035-1549966. [DOI] [PubMed] [Google Scholar]
- 83.Sloan JA. Assessing the minimally clinically significant difference: scientific considerations, challenges and solutions. COPD. 2005;2:57–62. doi: 10.1081/copd-200053374. [DOI] [PubMed] [Google Scholar]
- 84.Havelin J, Imbert I, Cormier J, et al. Central sensitization and neuropathic features of ongoing pain in a rat model of advanced osteoarthritis. J Pain. 2016;17:374–382. doi: 10.1016/j.jpain.2015.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Konstantinou K, Dunn KM, Ogollah R, et al. Characteristics of patients with low back and leg pain seeking treatment in primary care: baseline results from the ATLAS cohort study. BMC Musculoskelet Disord. 2015;16:332. doi: 10.1186/s12891-015-0787-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Van Ginckel A, Bennell KL, Campbell PK, et al. Location of knee pain in medial knee osteoarthritis: patterns and associations with self-reported clinical symptoms. Osteoarthritis Cartilage. 2016;24:1135–1142. doi: 10.1016/j.joca.2016.01.986. [DOI] [PubMed] [Google Scholar]
- 87.Rahman W, Dickenson AH. Emerging targets and therapeutic approaches for the treatment of osteoarthritis pain. Curr Opin Support Palliat Care. 2015;9:124–130. doi: 10.1097/SPC.0000000000000125. [DOI] [PubMed] [Google Scholar]
- 88.Orita S, Yamashita T, Ohtori S, et al. Prevalence and location of neuropathic pain in lumbar spinal disorders: Analysis of 1,804 consecutive patients with primary lower back pain. Spine. 2016;41:1224–1231. doi: 10.1097/BRS.0000000000001553. [DOI] [PubMed] [Google Scholar]
- 89.Beggs S. Long-Term Consequences of Neonatal Injury. Can J Psychiatry. 2015;60:176–180. doi: 10.1177/070674371506000404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Fitzgerald M, McKelvey R. Nerve injury and neuropathic pain - A question of age. Exp Neurol. 2016;275:296–302. doi: 10.1016/j.expneurol.2015.07.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Victoria NC, Murphy AZ. The long-term impact of early life pain on adult responses to anxiety and stress: Historical perspectives and empirical evidence. Exp Neurol. 2016;275:261–273. doi: 10.1016/j.expneurol.2015.07.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Martin JA, Hamilton BE, Osterman MJ, et al. Births: final data for 2013. Natl Vital Stat Rep. 2015;64:1–65. [PubMed] [Google Scholar]
- 93.Miaskowski C, Cooper B, Paul SM, et al. Identification of patient subgroups and risk factors for persistent breast pain following breast cancer surgery. J Pain. 2012;13:1172–1187. doi: 10.1016/j.jpain.2012.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Miaskowski C, Paul SM, Cooper B, et al. Identification of patient subgroups and risk factors for persistent arm/shoulder pain following breast cancer surgery. Eur J Oncol Nurs. 2014;18:242–253. doi: 10.1016/j.ejon.2013.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.McCann B, Miaskowski C, Koetters T, et al. Associations between pro- and anti-inflammatory cytokine genes and breast pain in women prior to breast cancer surgery. J Pain. 2012;13:425–437. doi: 10.1016/j.jpain.2011.02.358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Ottaiano A, Nappi A, Tafuto S, et al. Diabetes and body mass index are associated with neuropathy and prognosis in colon cancer patients treated with capecitabine and oxaliplatin adjuvant chemotherapy. Oncology. 2016;90:36–42. doi: 10.1159/000442527. [DOI] [PubMed] [Google Scholar]
- 97.Eckhoff L, Feddersen S, Knoop AS, et al. Docetaxel-induced neuropathy: a pharmacogenetic case-control study of 150 women with early-stage breast cancer. Acta Oncol. 2015;54:530–537. doi: 10.3109/0284186X.2014.969846. [DOI] [PubMed] [Google Scholar]
- 98.Gauffin J, Hankama T, Kautiainen H, et al. Neuropathic pain and use of PainDETECT in patients with fibromyalgia: a cohort study. BMC Neurol. 2013;13:21. doi: 10.1186/1471-2377-13-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Spallone V, Morganti R, D'Amato C, et al. Clinical correlates of painful diabetic neuropathy and relationship of neuropathic pain with sensorimotor and autonomic nerve function. Eur J Pain. 2011;15:153–160. doi: 10.1016/j.ejpain.2010.06.011. [DOI] [PubMed] [Google Scholar]
- 100.Ozcelik M, Korkmaz T, Odabas H, et al. Comparison of efficacy and safety of three different chemotherapy regimens delivered with concomitant radiotherapy in inoperable stage III non-small cell lung cancer patients. Tumour Biol. 2016;37:8901–8907. doi: 10.1007/s13277-015-4776-1. [DOI] [PubMed] [Google Scholar]
- 101.Addington J, Freimer M. Chemotherapy-induced peripheral neuropathy: an update on the current understanding. F1000Res. 2016;5 doi: 10.12688/f1000research.8053.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Duke J, McEvoy M, Sibbritt D, et al. Vibrotactile threshold measurement for detecting peripheral neuropathy: defining variability and a normal range for clinical and research use. Diabetologia. 2007;50:2305–2312. doi: 10.1007/s00125-007-0813-y. [DOI] [PubMed] [Google Scholar]
- 103.Young MJ, Breddy JL, Veves A, et al. The prediction of diabetic neuropathic foot ulceration using vibration perception thresholds. A prospective study. Diabetes Care. 1994;17:557–560. doi: 10.2337/diacare.17.6.557. [DOI] [PubMed] [Google Scholar]
- 104.Griffith KA, Couture DJ, Zhu S, et al. Evaluation of chemotherapy-induced peripheral neuropathy using current perception threshold and clinical evaluations. Support Care Cancer. 2014;22:1161–1169. doi: 10.1007/s00520-013-2068-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Purdue, Pegboard, Test. Lafayette, IN: Lafayette Instrument; 2002. [Google Scholar]
- 106.Haward BM, Griffin MJ. Repeatability of grip strength and dexterity tests and the effects of age and gender. Int Arch Occup Environ Health. 2002;75:111–119. doi: 10.1007/s004200100285. [DOI] [PubMed] [Google Scholar]
- 107.Tofthagen C, Overcash J, Kip K. Falls in persons with chemotherapy-induced peripheral neuropathy. Support Care Cancer. 2012;20:583–589. doi: 10.1007/s00520-011-1127-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Wampler MA, Topp KS, Miaskowski C, et al. Quantitative and clinical description of postural instability in women with breast cancer treated with taxane chemotherapy. Arch Phys Med Rehabil. 2007;88:1002–1008. doi: 10.1016/j.apmr.2007.05.007. [DOI] [PubMed] [Google Scholar]
- 109.Kolb NA, Smith AG, Singleton JR, et al. The association of chemotherapy-induced peripheral neuropathy symptoms and the risk of falling. JAMA Neurol. 2016;73:860–866. doi: 10.1001/jamaneurol.2016.0383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Beauchet O, Fantino B, Allali G, et al. Timed Up and Go test and risk of falls in older adults: a systematic review. J Nutr Health Aging. 2011;15:933–938. doi: 10.1007/s12603-011-0062-0. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.