Abstract
BACKGROUND
Genetic loci for Alzheimer disease (AD) have been identified in whites of European ancestry, but the genetic architecture of AD among other populations is less understood.
METHODS
We conducted a transethnic genome-wide association study (GWAS) for late-onset AD in Stage 1 sample including whites of European Ancestry, African Americans, Japanese, and Israeli-Arabs assembled by the Alzheimer’s Disease Genetics Consortium (ADGC). Suggestive results from Stage 1 from novel loci were followed up using summarized results in the International Genomics Alzheimer’s Project (IGAP) GWAS dataset.
RESULTS
Genome-wide significant (GWS) associations in SNP-based tests (P<5×10−8) were identified for SNPs in PFDN1/HBEGF, USP6NL/ECHDC3, and BZRAP1-AS1, and for the interaction of the APOE ε4 allele with NFIC SNP. We also obtained GWS evidence (P<2.7×10−6) for gene-based association in the total sample with a novel locus, TPBG (P=1.8×10−6).
DISCUSSION
Our findings highlight the value of transethnic studies for identifying novel AD susceptibility loci.
Keywords: transethnic, Alzheimer disease, genome-wide association, APOE interaction
1. Background
Alzheimer disease (AD) is the most prevalent neurodegenerative disease in persons aged 65 years and older and the sixth leading cause of death in the United States [1]. Total healthcare payments in 2014 for people aged 65 years and older with dementia are estimated at $214 billion [1]. By the middle of the century, the number of Americans with AD is projected at 13.8 million with one new case developing every 33 seconds or almost one million new cases per year. The global burden of AD or dementia in 2015 is more daunting with new cases of dementia in every 3 seconds, and the estimated worldwide costs of dementia are about $818 billion, rising to $2 trillion by 2030 [2]. The number of people living with dementia in 2015 is estimated to be 9.4 million in the Americas, 10.5 million in Europe, 4.0 million in Africa, and 22.9 million in Asia [2]. This is a tremendous global epidemic in elderly persons regardless of ethnic background.
AD with onset age after 65 years is highly heritable with an estimated 74% of the liability explained by genetic factors [3]. A major genetic risk factor for AD is APOE genotype [4] which accounts for approximately 35% of the genetic variance [5]. The three common APOE alleles (ε2, ε3 and ε4) are determined by combinations of polymorphic amino acid residues at Arg112 (rs429358) and Cys158 (rs7412) [6]. Among non-Hispanic whites of European Ancestry (EA), ε4 heterozygotes have a 2.5 to 3.0 fold increased risk and ε4 homozygotes have a 10-12 fold increased risk, compared to persons with the ε3/ε3 genotype [4]. The ε2 allele is protective [7] such that carriers of this allele have a 40% reduction in AD risk compared to ε3/ε3 individuals [4]. The effect of APOE genotype to AD risk is highly variable in other populations. The ε4 frequency is lower in Asians [8] and associated with higher AD risk among Japanese compared to EAs [9]. In contrast, the effect of ε4 on AD risk is much less in African Americans among whom the ε4 frequency is about 50% higher than in EAs [10]. It is noteworthy that the ε4 allele is virtually absent among Arabs living in northern Israeli community where the prevalence of dementia is roughly double than in EA populations [11].
More than 20 loci have been robustly associated with AD [12] and are enriched in immune response, regulation of endocytosis, cholesterol transport, and protein ubiquitination pathways [13]. A recent genome-wide association study (GWAS) identified significant association of AD with multiple single nucleotide polymorphisms (SNPs) in the MAPT-KANSL1 region among EAs lacking an APOE ε4 allele [14]. Genetic studies in other populations have increased our understanding of the genetic architecture of AD. For example, the effect of the APOE ε4 allele is much greater in Japanese and substantially weaker in African American and some Hispanic groups, due in part to varying frequencies of this allele across populations [4]. Three loci (SORL1, ABCA7, and ACE) whose association with AD attained genome-wide significance in EAs [12] were found to have larger effects on AD risk in African Americans, (ABCA7) [15], Japanese (SORL1) [9], and Israeli-Arabs (ACE) [16]. Some loci including PLXNA4 [17] and SORL1 [18] demonstrate allelic heterogeneity among genetically diverse populations. In the current study, we leveraged genetic diversity across ethnic groups to increase discovery of additional AD risk loci by combining GWAS results obtained from samples of EAs, African Americans, Japanese and Israeli-Arabs.
2. Methods
2.1. Subjects, Genotyping, and Data Processing
Details of subject recruitment and genotyping for individual case-control and family-based datasets, genotype imputation, quality control, population substructure, and statistical methods for association analyses were reported previously for Alzheimer’s Disease Genetics Consortium (ADGC) datasets containing EAs [5], African Americans (AA) [15], Japanese (JPN) [9], and Israeli-Arab (IA) [11]. Characteristics of the 33,269 ADGC subjects (26,320 EA, 4,983 AA, 1,845 JPN, and 115 IA) used for discovery in Stage 1 were shown in Supplementary Table 1. Summarized results archived in the NIA Genetics of Alzheimer's Disease Data Storage Site (https://www.niagads.org/) that are from a previous GWAS of EAs conducted by the International Genomics Alzheimer’s Project (IGAP) including 5,813 AD cases and 20,474 controls after excluding the ADGC datasets [12] were used in Stage 2 follow-up analyses (Supplementary Table 1).
2.2. Genome-wide Association Analysis in Stage 1
2.2.1. Design and Power Considerations
The primary analysis was a single GWAS including all discovery datasets. Analyses were performed separately for each dataset and the results were pooled sequentially, first within ethnic groups and then across ethnic groups. The minimum detectable genotype relative risk for EAs range from 1.16 for MAF=0.5 to 1.73 for MAF=0.01. The corresponding ranges for AAs and JPN are 1.40-2.69 and 1.74-3.78, respectively. GRRs of <5 are not detectable with 80% power in the small IA sample. However, the goal of this study was not for novel discovery within ethnic groups but rather in the total transethnic sample. Prompted by findings of previous studies [14], we also conducted separate GWAS in subgroups of subjects who have or lack an APOE ε4 allele. We also applied a complementary approach for assessing a differential effect of association by APOE genotype by evaluating association of AD with an interaction of SNP and ε4 status.
2.2.2. SNP-based association
Within each dataset, genome-wide association analyses were conducted using more than 7 million imputed SNPs in the total sample, as well as in subgroups of subjects with and without the APOE ε4 allele, using regression models including age, sex, the first three PCs. An additive effect of a SNP was included in the model as a quantitative estimate between 0 and 2 representing the probability score of the effect allele to incorporate the uncertainty of the imputation estimates. Models were evaluated using a logistic generalized linear model in case-control datasets and a logistic generalized estimating equation in family-based datasets. We also evaluated models including a term for the interaction of the SNP dosage with the APOE ε4 status and models among subgroups stratified by APOE ε4 status. Results for each model across datasets were combined by meta-analysis separately within each ethnic group using a fixed-effects, inverse-variance weighted meta-analysis in the METAL program [19]. SNPs with a minor allele frequency ≥1% and imputation quality≥0.4 that were available in at least 50% of the datasets were included in the meta-analysis. The meta-analysis P-value for association was estimated by the summarized test statistic, after applying genomic control within each individual study. Meta-analysis was also conducted using Han and Eskin’s modified random effects model (RE-HE) that is optimized to detect associations under effect heterogeneity, as implemented in METASOFT [20]. This model has similar power to the fixed effects model when heterogeneity is modest, e.g., when the standard deviation of the different ethnicities log odds ratios is ≤0.5 times the mean log odds ratio, but has better power than the fixed effects model for substantial heterogeneity. Thus, we do not expect the RE-HE model to produce substantially different results from the fixed effects model unless substantial heterogeneity among ethnicities exists.
2.2.3. Gene-based association
We conducted genome-wide gene-based tests using ethnic-specific association results from SNP-based tests. Intragenic SNPs and SNPs within 30 kilobases (kb) of transcription start and stop sites were included in each gene-based test. We used the GATES [21] method, which computes a gene-based P-value using SNP-based p values and SNP-SNP correlations by penalizing lack of association in correlated SNPs. Ethnic-specific gene-based results for EA, AA, JPN, and IA groups were combined using the sample-size weighted Z-score method in METAL assuming the same direction of effect.
2.3. Follow-Up Association Analysis
In Stage 2, we attempted to replicate Stage 1 top-ranked SNP-based (P<10−5) results and validate gene-based (P<10−4) results from each ethnic subgroup. Previously known AD genes were evaluated in Stage 2 only when both SNP-based and gene-based P values met threshold criteria for follow up. These analyses incorporated summarized results for the Stage 2 ADGC datasets and previously reported results for IGAP datasets excluding those from the ADGC that are described in Supplementary Table 1. The genome-wide significance threshold was set at P<5×10−8 for individual SNPs and P<2×10−6 for gene-based tests in the Stage 1+2 analyses.
3. Results
3.1. Findings with Individual SNPs
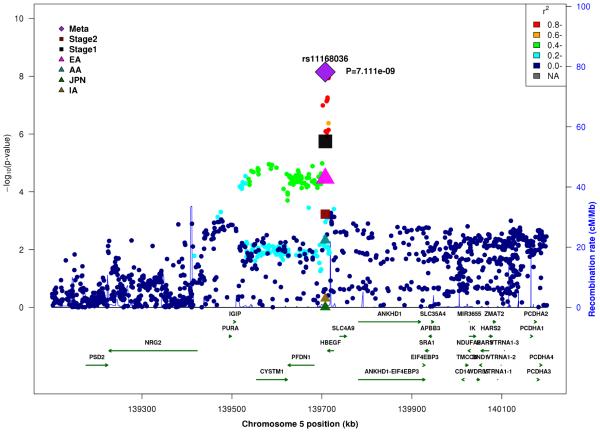
There was little evidence for genomic inflation in SNP-based GWA results in the total sample with main effect (λ=1.02) and interaction effect of a SNP with APOE ε4 status on AD risk (λ=1.02), as well as in APOE ε4+ subjects (λ=0.99) and APOE ε4− subjects (λ=0.99) (Supplementary Fig. 1). In the total sample, we confirmed genome-wide significant (GWS) association (p<5×10−8) with SNPs in several previously implicated AD loci including CR1, BIN1, PTK2B, MS4A2/MS4A6A, PICALM (Supplementary Table 2, Supplementary Fig. 2). GWS association was also observed with SNPs in NFIC and PRKCE through interaction with APOE (Supplementary Fig. 2B) and with SNPs between USP6N and ECHDC3 among subjects lacking APOE ε4 (Supplementary Fig. 2D). Top-ranked SNPs in EA for PICALM, SORL1, and ABCA7 had strong support for association in Japanese, whereas the top-ranked SNPs in CR1, BIN1, and EPHA1 were consistently associated in EA and AA (Supplementary Table 2). In contrast, the effect direction was significantly opposite in EA versus AA for NME8, ABCA7, and CASS4 SNPs (Supplementary Table 2). A total of 35 SNPs from nine novel loci met criteria for follow up in Stage 2 (Supplementary Table 3). Extensive evaluation of SNPs from the APOE region across the different ethnic groups demonstrated that only the APOE ε2 SNP (rs7412) remained genome-wide significant among APOE ε4− subjects (Supplementary Table 4), confirming our prior observation that APOE accounts for all association signals in this region [22]. SNPs in other loci showed suggestive evidence for association (P<10−6) in EAs or AAs (Supplementary Table 5), but these results were much less significant in the transethnic meta-analyses. Analysis of models including an interaction term for each SNP with APOE ε4 status identified a GWS significant interaction (interaction: P = 1.5×10−8) for NFIC SNP rs9749589 (Table 1). This SNP appeared protective in ε4+ subjects (OR=0.83, P=6.4×10−6) but slightly increased risk of AD in ε4− subjects (OR=1.11, P=6.0×10−3) (Supplementary Table 6).
Table 1.
Genome-wide significant results from individual SNP and SNP*APOE-ε4 interaction tests (P<5×10−8) in transethnic meta-analysis.
| SNP | CH | Locus | EfA | EAF | Stage 1 | Stage 2 | Stages 1 + 2 | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| EA | AA | JPN | IA | OR (95% CI) |
P | OR (95% CI) |
P | OR (95% CI) | P | ||||||
| SNP | |||||||||||||||
| rs11168036 | 5 |
PFDN1/ HBEGF |
T | 0.5 | 0.5 | 0.5 | 0.6 | 1.08 (1.04-1.13) |
1.8×10−6 | 1.08 (1.04-1.13) |
6.0×10−4 | 1.08 (1.06-1.10) |
7.1×10−9 | ||
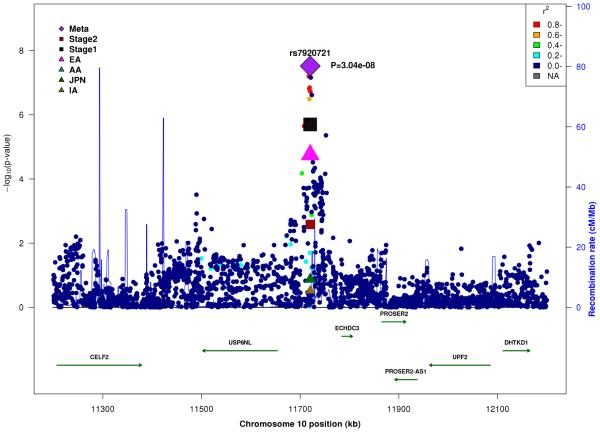
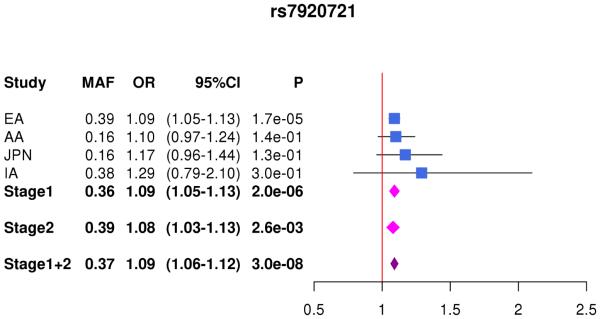
| rs7920721 | 10 |
USP6NL/ ECHDC3 |
G | 0.4 | 0.2 | 0.2 | 0.4 | 1.09 (1.05-1.14) |
2.0×10−6 | 1.07 (1.03-1.12) |
2.6×10−3 | 1.08 (1.04-1.13) |
3.0×10−8 | ||
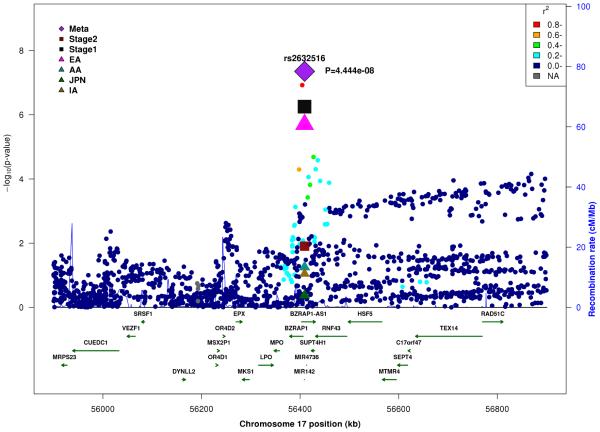
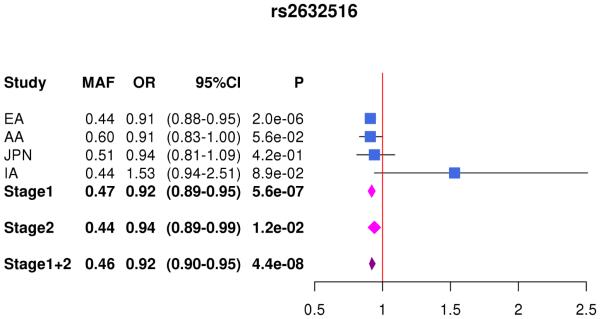
| rs2632516 | 17 | BZRAP1-AS1 | C | 0.4 | 0.6 | 0.5 | 0.4 | 0.91 (0.88-0.95) |
5.6×10−7 | 0.94 (0.89-1.00) |
0.01 | 0.92 (0.91-0.94) |
4.4×10−8 | ||
| Interaction † | |||||||||||||||
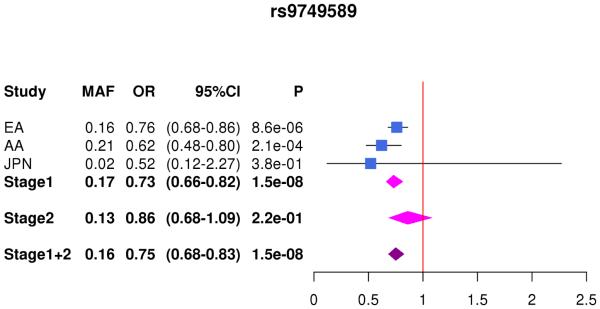
| rs9749589*ε4 | 19 | NFIC | A | 0.16 | 0.2 | 0.02 | na | 0.73 (0.66-0.81) |
1.5×10−8 | 0.86 (0.68-1.09) |
0.22 | 0.76 (0.69-0.83) |
1.5×10−8 | ||
| rs9749589 | 1.17 (1.04-1.20) |
2.5×10−3 | 1.04 (0.92-1.19) |
0.50 | 1.10 (1.03-1.16) |
3.3×10−3 | |||||||||
CH = chromosome; EfA = effect allele; EAF = effect allele frequency; EA = European Ancestry; AA = African American; JPN = Japanese; IA = Israeli-Arab; OR = odds ratio; CI = confidence interval; P = p-value;
results for interaction term (NFIC rs9749589 * APOE ε4) and main effect of rs9749589
In the combined Stage 1+2 sample, GWS association was observed with SNPs in several previously established AD loci (CR1, BIN1, PTK2B, MS4A4A, and PICALM) (Supplementary Fig. 3, Supplementary Fig. 4). Follow-up of the 35 SNPs from novel loci in Stage 2 revealed nominally significant associations for nine SNPs in PFDN1/HBEGF, USP6NL/ECHDC3, and BZRAP1-AS1 (Supplementary Table 6). In the combined Stage 1+2 sample, GWS association was attained with two intergenic SNPs between PFDN1 and HBEGF (best SNP: rs11168036, P=7×10−9), six intergenic SNPs between USP6NL and ECHDC3 (best SNP: rs7920721, P=3×10−8), BZRAP1-AS1 SNP rs2632516 (P=4×10−8) (Table 1, Fig. 1, Supplementary Table 6). Analyses of models that conditioned on the top SNP at the PFDN1/HBEGF, USP6NL/ECHDC3, and BZRAP1-AS1 loci confirmed a single association signal in each region (Supplementary Fig. 5). The significant interaction between NFIC SNP rs9749589 and ε4 status in Stage 1 was not significant in Stage 2 (P=0.2), however the magnitude and direction of effect was the same and the interaction P value in the total sample was not diminished (Table 1, Fig. 1). These GWS associations, except for rs7920721, were supported by evidence in multiple ethnic groups (Fig. 2). Further evaluation of the Stage 1+2 findings revealed that the association with the USP6NL/ECHDC3 SNPs was exclusive to subjects lacking APOE ε4 (e.g., rs7920721: ε4+, P=0.07, OR=1.05; ε4−, P=2.7×10−9, OR=1.14; interaction P = 0.01) and comparable in terms of effect size and direction in the non-European ancestry groups (Supplementary Table 6 and Supplementary Fig. 6). All GWS findings were similar using the METASOFT Han and Eskin modified random effects model (Supplementary Table 7).
Figure 1.
Regional association plots in the combined stage 1 and stage 2 sample including main effects at (A) PFDN1/HBEGF, (B) USP6NL/ECHDC3, (C) BZRAP1-AS1, and (D) SNP*APOE ε4 interaction near NFIC.
Figure 2.
Forest plots for by ethnicity and stage for (A) rs11168036 atPFDN1/HBEGF, (B) rs7920721 at USP6NL/ECHDC3, (C) rs2632516 at BZRAP1-AS1, (D) NFIC rs9749589*APOE ε4 interaction.
3.2. Gene-Based Test Findings
In Stage 1 analyses, there was strong evidence of association (gene-based P<10−4) with previously established loci and novel loci in the total sample (Supplementary Fig. 7 and Supplementary Table 8), but only seven known genes (CR1, BIN1, PTK2B, CLU, MS4A4A, PICALM, and ABCA7) and one novel one (TPBG) were GWS (P<2.7×10−6) in the combined Stage 1+2 sample (Table 2, Supplementary Table 8). Both EAs and AAs contributed to the association with TPBG. No additional genes were identified as GWS in interaction models or APOE genotype subgroups.
Table 2.
Genome-wide significant results (p<2.7×10−6) from gene-based tests in Stage 1+2.
| Gene | CH | Ethnic Specific P value in Stage1 |
Stage 1 | Stage 2 | Stage 1+2 | |||
|---|---|---|---|---|---|---|---|---|
| EA | AA | JPN | IA | |||||
| CR1 | 1 | 3.4×10−9 | 0.84 | 0.35 | 0.10 | 4.8×10−9 | 6.4×10−5 | 1.4×10−12 |
| BIN1 | 2 | 1.4×10−14 | 0.08 | 0.33 | 0.80 | 3.7×10−15 | 2.5×10−9 | 8.8×10−23 |
| TPBG | 6 | 2.2×10−3 | 3.5×10−3 | 0.29 | 0.70 | 6.8×10−5 | 8.2×10−3 | 1.8×10−6 |
| PTK2B | 8 | 4.7×10−6 | 0.26 | 0.49 | 0.81 | 2.2×10−6 | 1.9×10−3 | 7.6×10−8 |
| CLU | 8 | 7.0×10−6 | 0.11 | 0.59 | 0.10 | 1.1×10−6 | 1.1×10−9 | 1.4×10−12 |
| MS4A4A | 11 | 5.4×10−13 | 0.18 | 0.03 | 0.95 | 3.6×10−14 | 0.04 | 6.1×10−14 |
| PICALM | 11 | 1.9×10−8 | 0.71 | 1.8×10−3 | 0.93 | 1.6×10−9 | 3.6×10−3 | 2.7×10−11 |
| ABCA7 | 19 | 2.3×10−4 | 1.6×10−3 | 0.07 | 0.02 | 6.6×10−7 | 4.9×10−3 | 1.1×10−8 |
CH = chromosome; EA = European Ancestry; AA = African American; JPN = Japanese; IA = Israeli Arab; P = gene-based p value
4. Discussion
In this large transethnic genetic study of AD, we identified robust associations with several novel loci at the individual SNP level (PFDN1/HBEGF, USP6NL/ECHDC3, BZRAP1-AS1 and NFIC) and gene level (TPBG) in a sample of AD subjects and cognitively normal elders in cohorts containing whites of European ancestry, African Americans, Japanese, and Israeli-Arabs. Most of these findings are supported by evidence in more than one ethnic group (Fig. 2 and Table 2). Previous GWAS using the EA discovery cohorts in this study did not detect genome-wide significant association with any of these loci, although there was suggestive evidence of association (P>10-7) for the top SNPs in the PFDN1/HBEGF and USP6NL/ECHDC3 regions in EAs [12, 14]. The other novel genes identified in this study were not previously reported to be associated with AD in any ethnic groups. The association with SNPs in the USP6NL/ECHDC3 region was specific to persons lacking the APOE ε4 allele. Our study also showed that associations for several genes that have previously been robustly implicated in AD in Caucasians of European descent (CR1, BIN1, PTK2B, MS4A4A, and PICALM) were evident in other populations even at the SNP level.
HBEGF, heparin EGF like growth factor, has roles in wound healing, cardiac hypertrophy, and heart development [23]. Although the biological role for this gene in AD is not obvious, an HBEGF knock-out mouse that does not express HBEGF in cortex and hippocampus has psychiatric and cognitive dysfunctions that accompany down-regulated NMDA receptors [24]. Another study showed that rats exposed to the pesticide cypermethrin had a reduction of HBEGF expression leading to upregulation of GSK3b-dependent Aβ and phosphorylated tau [25].
A recent GWAS demonstrated pleiotrophic effects of SNPs in the USP6NL/ECHDC3 (including rs7920721) and BZRAP1-AS1 loci for AD and plasma C-reactive protein and lipid levels [26]. The pleiotropy at USP6NL/ECHDC3 may be related to the association finding at this locus among persons lacking the APOE ε4 allele. USP6NL, ubiquitin specific peptidase 6 N-terminal like, has a role in the EGF receptor (EGFR) signaling pathway by acting as a GTPase-activating protein and inhibiting internalization of EGFR [27]. Insight for a role of USP6NL may be gained from information about USP6 which regulates ubiquitylation and trafficking of cargo protein by clathrin-independent endocytosis [28]. There is a growing body of evidence from studies in humans and mice supporting a role for clathrin-mediated endocytosis in AD [29-31] In addition, the association of the phosphatidylinositol binding clathrin assembly protein (PICALM) gene to AD is well established [12].
ECHDC3, enoyl CoA hydratase domain containing 3, is involved in fatty acid biosynthesis in mitochondria and its expression is increased in patients with acute myocardial infarction [32]. It has been observed that ECHD3 expression is altered in brains from persons with AD compared to controls [26]. Although rs7920721 is closer to ECHDC3 than USP6NL, it is located on USP6NL side of a recombination hotspot between these two genes (Fig. 1B). Therefore, we cannot rule out either of these genes, or even one not adjacent to rs7920721, as explaining the association signal in this region.
BZRAP1, benzodiazepine-associated protein 1 (renamed as TSPO associated protein 1, TSPOAP1), is a subunit of the benzodiazepine receptor complex in mitochondria and a marker of neuroinflammation [34]. A recent prospective cohort study of 8,240 individuals aged 65 years and older showed an increased risk of dementia with use of long half-life benzodiazepines [35], a drug often prescribed for treatment of anxiety. A TSPO ligand (Ro5-4864) has been shown to reverse β-amyloid accumulation and behavioral impairment in 3xTgAD mice [36]. A recent PET imaging study demonstrated that the change over time of TSPO binding to radioligand 11C-PBR28 is correlated with progression of AD [37].
The relationship of AD to the other novel loci identified in this study is less clear. PFDN1, a prefoldin subunit, is upregulated in colorectal cancer [33]. NFIC is a CCAAT-binding transcription factor. A study comparing brain gene expression profiles between HIV seropositive individuals with cognitive impairment and AD cases identified NFIC as having significant high co-expression connectivity in white matter [38]. Trophoblast glycoprotein (TPBG), also known as 5T4, regulates development of the olfactory bulb GABAgenic interneurons and its overexpression in newborns is associated with abnormal dendrites [39].
Our study highlights the benefit of combining results obtained from genetically diverse populations. The transethnic approach applied here identified three novel loci (BZRAP1-AS1, NFIC, and TPBG) and GWS association for the first time with two other loci (PFDN1/HBEGF and USP6NL/ECHDC3) noting that the size of the discovery sample in this study was less than 45% of the one included in a previous GWAS that contained more than 74,000 EA subjects. The improved power in our smaller sample can be ascribed to allele frequency differences and allelic heterogeneity among the ethnic groups. As an example highlighting the importance of these differences, the top SNPs from BZRAP1-AS1 and NFIC had different minor allele frequencies across ethnic groups, but the effect sizes were similar and association signals were greater in fixed effect meta-analysis. In addition, gene-based tests, which consider association patterns with all SNPs in the locus, identified TPBG. Importantly, the most significant SNPs in these two regions differed among the ethnic groups. Gene-based tests also indicated potential allelic heterogeneity among ethnic groups for previously established AD genes including TREM2 and ABCA7. The novel GWS SNP associations were robust in analyses allowing for heterogeneity across different ethnic groups, and the P-values for the RE-HE approach were slightly larger than for the fixed effect model, suggesting that the effect size heterogeneity across the groups is modest.
Our study also revealed that the effect direction for several SNPs vary across ethnic groups. For example, the top-ranked SNPs in NME8, ABCA7, and CASS4 (Supplementary Table 2) were nominally significant in EAs and AAs, but the referent allele was associated with increased risk in one group and decreased risk in the other. One explanation for these differences is that the SNPs are tagging different functional variants across groups. This idea is consistent with our findings from gene-based tests showing that the constellation of variants contributing to the association with some genes was different across ethnic groups. Alternatively, when examining a large number of variants it is expected that a few will show nominal significance in opposite directions among groups.
There are several limitations associated with our study. The sample size imbalance between the EAs and the other populations weakened the opportunity to identify association patterns that may be unique to the non-EA groups. The small size of the non-EA groups also reduced power to detect novel gene associations if the functional variants (and the SNPs that tag them) differ among ethnic groups. An additional weakness is the lack of replication samples for the non-EA populations. Despite these limitations, our study highlights the importance of investigating the genetic architecture for AD in ethnically diverse populations.
Our findings warrant further replication in independent samples, deep sequencing and bioinformatics studies to identify the potentially functional variants, and experimental validation. We expect that additional novel gene discoveries will emerge in future transethnic studies including larger samples from non-European ancestry populations.
Supplementary Material
Research in Context.
Systematic review: We reviewed previously published genome-wide association studies (GWAS) for late onset Alzheimer disease (AD) including reports for non-white populations. Few GWAS have been conducted in populations of non-white European ancestry.
Interpretation: Transethnic meta-analysis of GWAS results for whites of European Ancestry, African Americans, Japanese, and Israeli-Arabs identified novel genome-wide significant associations with SNPs in PFDN1/HBEGF, USP6NL/ECHDC3, and BZRAP1-AS1, and with TPBG using a gene-based test. These findings further our understanding of the genetic basis of AD and provide insight about mechanisms leading to AD.
Future directions: These results should be confirmed in independent samples including subjects from the same ethnic populations and tested in populations of other genetic backgrounds. DNA sequencing studies are needed to identify the functional variants in these genes and their biological roles in AD should be determined experimentally.
ACKNOWLEDGEMENTS
We thank Drs. D. Stephen Snyder and Marilyn Miller from NIA who are ex-officio ADGC members.
Funding Support: The National Institutes of Health, National Institute on Aging (NIH-NIA) supported this work through the following grants: ADGC, U01 AG032984, RC2 AG036528; NACC, U01 AG016976; NCRAD, U24 AG021886; NIA LOAD, U24 AG026395, U24 AG026390; MIRAGE: R01 AG025259; Banner Sun Health Research Institute P30 AG019610; Boston University, P30 AG013846, U01 AG10483, R01 CA129769, R01 MH080295, R01 AG017173, R01 AG33193, R01 AG048927; Columbia University, P50 AG008702, R37 AG015473; Duke University, P30 AG028377, AG05128; Emory University, AG025688; Group Health Research Institute, UO1 AG06781, UO1 HG004610; U01 HG006375; Indiana University, P30 AG10133; Johns Hopkins University, P50 AG005146, R01 AG020688; Massachusetts General Hospital, P50 AG005134; Mayo Clinic, P50 AG016574; Mount Sinai School of Medicine, P50 AG005138, P01 AG002219; New York University, P30 AG08051, MO1RR00096, and UL1 RR029893; Northwestern University, P30 AG013854; Oregon Health & Science University, P30 AG008017, R01 AG026916; Rush University, P30 AG010161, R01 AG019085, R01 AG15819, R01 AG17917, R01 AG30146; TGen, R01 NS059873; University of Alabama at Birmingham, P50 AG016582, UL1RR02777; University of Arizona, R01 AG031581; University of California, Davis, P30 AG010129; University of California, Irvine, P50 AG016573, P50, P50 AG016575, P50 AG016576, P50 AG016577; University of California, Los Angeles, P50 AG016570; University of California, San Diego, P50 AG005131; University of California, San Francisco, P50 AG023501, P01 AG019724; University of Kentucky, P30 AG028383; University of Michigan, P50 AG008671; University of Pennsylvania, P30 AG010124; University of Pittsburgh, P50 AG005133, AG030653; University of Southern California, P50 AG005142; University of Texas Southwestern, P30 AG012300; University of Miami, R01 AG027944, AG010491, AG027944, AG021547, AG019757; University of Washington, P50 AG005136; Vanderbilt University, R01 AG019085; and Washington University, P50 AG005681, P01 AG03991. The Kathleen Price Bryan Brain Bank at Duke University Medical Center is funded by NINDS grant # NS39764, NIMH MH60451 and by Glaxo Smith Kline. Genotyping of the TGEN2 cohort was supported by Kronos Science. The TGen series was also funded by NIA grant AG034504 to AJM, The Banner Alzheimer’s Foundation, The Johnnie B. Byrd Sr. Alzheimer’s Institute, the Medical Research Council, and the state of Arizona and also includes samples from the following sites: Newcastle Brain Tissue Resource (funding via the Medical Research Council, local NHS trusts and Newcastle University), MRC London Brain Bank for Neurodegenerative Diseases (funding via the Medical Research Council),SouthWest Dementia Brain Bank (funding via numerous sources including the Higher Education Funding Council for England (HEFCE), Alzheimer’s Research Trust (ART), BRACE as well as North Bristol NHS Trust Research and Innovation Department and DeNDRoN), The Netherlands Brain Bank (funding via numerous sources including Stichting MS Research, Brain Net Europe, Hersenstichting Nederland Breinbrekend Werk, International Parkinson Fonds, Internationale Stiching Alzheimer Onderzoek), Institut de Neuropatologia, Servei Anatomia Patologica, Universitat de Barcelona. Marcelle Morrison-Bogorad, PhD., Tony Phelps, PhD and Walter Kukull PhD are thanked for helping to co-ordinate this collection. ADNI Funding for ADNI is through the Northern California Institute for Research and Education by grants from Abbott, AstraZeneca AB, Bayer Schering Pharma AG, Bristol-Myers Squibb, Eisai Global Clinical Development, Elan Corporation, Genentech, GE Healthcare, GlaxoSmithKline, Innogenetics, Johnson and Johnson, Eli Lilly and Co., Medpace, Inc., Merck and Co., Inc., Novartis AG, Pfizer Inc, F. Homan-La Roche, Schering-Plough, Synarc, Inc., Alzheimer’s Association, Alzheimer’s Drug Discovery Foundation, the Dana Foundation, and by the National Institute of Biomedical Imaging and Bioengineering and NIA grants U01 AG024904, RC2 AG036535, K01 AG030514. Support was also from the Alzheimer’s Association (LAF, IIRG-08-89720; MP-V, IIRG-05-14147) and the US Department of Veterans Affairs Administration, Office of Research and Development, Biomedical Laboratory Research Program. P.S.G.-H. is supported by the Wellcome Trust, Howard Hughes Medical Institute, and Canadian Institute of Health.
Alzheimer Disease Genetics Consortium (ADGC) Members
Perrie M. Adams, PhD; Marilyn S. Albert, PhD; Roger L. Albin, MD; Liana G. Apostolova, MD; Steven E.Arnold, MD; Sanjay Asthana, MD; Craig S. Atwood, PhD; Michjael M. Barmada, PhD; Lisa L. Barnes, PhD; Thomas G. Beach, MD PhD; James T. Becker, PhD; Eileen H. Bigio, MD; Thomas D. Bird, MD; Deborah Blacker, MD; Bradley F. Boeve, MD; James D. Bowen, MD; Adam Boxer, MD PhD, James R. Burke, MD PhD; Nigel J. Cairns, PhD FRCPath; Chuanhai Cao, PhD; Chris S. Carlson; PhD; Cynthia M. Carlsson, MD; Regina M. Carney, MD; Minerva M. Carrasquillo, PhD; Steven L. Carroll, MD PhD; Helena C. Chui, MD; David G. Clark, MD; Jason Corneveaux, BS; David H. Cribbs, PhD; Elizabeth A. Crocco, MD; Carlos Cruchaga, PhD; Philip L. De Jager, MD PhD; Charles DeCarli, MD; Steven T. DeKosky, MD; F. Yesim Demirci, MD; Malcolm Dick, PhD; Dennis W. Dickson, MD; Rachelle S. Doody, MD PhD; Ranjan Duara, MD; Nilufer Ertekin-Taner, MD PhD; Kelley M. Faber, MS; Thomas J. Fairchild, PhD; Kenneth B. Fallon, MD; Martin R. Farlow, MD; Steven Ferris, PhD; Matthew P. Frosch, MD PhD; Douglas R. Galasko, MD; Marla Gearing, PhD; Daniel H. Geschwind, MD PhD; Bernardino Ghetti, MD; John R. Gilbert PhD; Jonathan D. Glass, MD; Neill R. Graff-Radford, MD; Robert C. Green, MD MPH; John H. Growdon, MD; Hakon Hakonarson, MD PhD; Ronald L. Hamilton, MD; John Hardy, PhD; Lindy E. Harrell, MD PhD; Elizabeth Head, PhD; Lawrence S. Honig, MD PhD; Ryan M. Huebinger, PhD, Matthew J. Huentelman, PhD; Christine M. Hulette, MD; Bradley T. Hyman, MD PhD; Gail P. Jarvik, MD PhD; Gregory A. Jicha, MD PhD; Lee-Way Jin, MD PhD; Anna Karydas, BA; John S.K. Kauwe, PhD; Jeffrey A. Kaye, MD; Ronald Kim, MD; Edward H. Koo, MD; Neil W. Kowall, MD; Joel H. Kramer, PsyD; Frank M. LaFerla, PhD; James J. Lah, MD PhD; James B. Leverenz, MD; Allan I. Levey, MD PhD; Ge Li, MD PhD; Andrew P. Lieberman, MD PhD; Chiao-Feng Lin, PhD; Oscar L. Lopez, MD; Constantine G. Lyketsos, MD MHS; Wendy J. Mack, PhD; Daniel C. Marson, JD PhD; Frank Martiniuk, PhD; Deborah C. Mash, PhD; Eliezer Masliah, MD; Wayne C. McCormick, MD MPH; Susan M. McCurry, PhD; Andrew N. McDavid, BA; Ann C. McKee, MD; Marsel Mesulam, MD; Bruce L. Miller, MD; Carol A. Miller, MD; Joshua W. Miller, PhD; John C. Morris, MD; Shubhabrata Mukherjee, PhD; Jill R. Murrell, PhD, Amanda J. Myers, PhD; Sid O’Bryant, PhD; John M. Olichney, MD; Vernon S. Pankratz, PhD; Joseph E. Parisi, MD; Amanda Partch, MS; Henry L. Paulson, MD PhD; William Perry, MPH; Elaine Peskind, MD; Ronald C. Petersen, MD PhD; Aimee Pierce, MD; Wayne W. Poon, PhD; Huntington Potter, PhD; Joseph F. Quinn, MD; Ashok Raj, MD; Murray Raskind, MD; Barry Reisberg, MD; Joan S. Reisch, PhD; Christiane Reitz, MD PhD; John M. Ringman; MD; Erik D. Roberson, MD PhD; Ekaterina Rogaeva, PhD; Howard J. Rosen, MD; Roger N. Rosenberg, MD; Donald R. Royall, MD; Mark A. Sager, MD; Mary Sano, PhD; Andrew J. Saykin, PsyD; Julie A. Schneider, MD; Lon S. Schneider, MD; William W. Seeley, MD; Amanda G. Smith, MD; Joshua A. Sonnen, MD; Salvatore Spina, MD; Robert A. Stern, PhD; Rudolph E. Tanzi, PhD; Tricia A. Thornton-Wells, PhD; John Q. Trojanowski, MD PhD; Juan C. Troncoso, MD; Debby W. Tsuang, MD; Vivianna M. Van Deerlin, MD PhD; Linda J. Van Eldik, PhD; Badri N. Vardarajan, Ph.D.; Harry V. Vinters, MD; Jean Paul Vonsattel, MD; Sandra Weintraub, PhD; Kathleen A. Welsh-Bohmer, PhD; Jennifer Williamson, MS; Sarah Wishnek, MPH; Randall L. Woltjer, MD PhD; Clinton B. Wright, MD MS; Chuang-Kuo Wu, MD PhD; Chang-En Yu, PhD; Lei Yu, PhD; Xiaoling Zhang, PhD
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflicts of Interest: The authors of this paper have no conflicts of interest to report.
Web Resources:
NIA Genetics of Alzheimer's Disease Data Storage Site: https://www.niagads.org/
5. References
- [1].Association As 2014 Alzheimer's disease facts and figures. Alzheimers Dement. 2014;10:e47–92. doi: 10.1016/j.jalz.2014.02.001. [DOI] [PubMed] [Google Scholar]
- [2].International AsD World Alzheimer Report 2015: The Global Impact of Dementia. Alzheimer's Disease International (ADI) 2015 [Google Scholar]
- [3].Gatz M, Pedersen NL, Berg S, Johansson B, Johansson K, Mortimer JA, et al. Heritability for Alzheimer's disease: the study of dementia in Swedish twins. J Gerontol A Biol Sci Med Sci. 1997;52:M117–25. doi: 10.1093/gerona/52a.2.m117. [DOI] [PubMed] [Google Scholar]
- [4].Farrer LA, Cupples LA, Haines JL, Hyman B, Kukull WA, Mayeux R, et al. Effects of age, sex, and ethnicity on the association between apolipoprotein E genotype and Alzheimer disease. A meta-analysis. APOE and Alzheimer Disease Meta Analysis Consortium. Jama. 1997;278:1349–56. [PubMed] [Google Scholar]
- [5].Naj AC, Jun G, Beecham GW, Wang LS, Vardarajan BN, Buros J, et al. Common variants at MS4A4/MS4A6E, CD2AP, CD33 and EPHA1 are associated with late-onset Alzheimer's disease. Nat Genet. 2011;43:436–41. doi: 10.1038/ng.801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Yu L, Boyle PA, Leurgans S, Schneider JA, Bennett DA. Disentangling the effects of age and APOE on neuropathology and late life cognitive decline. Neurobiol Aging. 35:819–26. doi: 10.1016/j.neurobiolaging.2013.10.074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Corder EH, Saunders AM, Risch NJ, Strittmatter WJ, Schmechel DE, Gaskell PC, Jr., et al. Protective effect of apolipoprotein E type 2 allele for late onset Alzheimer disease. Nat Genet. 1994;7:180–4. doi: 10.1038/ng0694-180. [DOI] [PubMed] [Google Scholar]
- [8].Singh PP, Singh M, Mastana SS. APOE distribution in world populations with new data from India and the UK. Ann Hum Biol. 2006;33:279–308. doi: 10.1080/03014460600594513. [DOI] [PubMed] [Google Scholar]
- [9].Miyashita A, Koike A, Jun G, Wang LS, Takahashi S, Matsubara E, et al. SORL1 is genetically associated with late-onset Alzheimer's disease in Japanese, Koreans and Caucasians. PLoS One. 2013;8:e58618. doi: 10.1371/journal.pone.0058618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Graff-Radford NR, Green RC, Go RC, Hutton ML, Edeki T, Bachman D, et al. Association between apolipoprotein E genotype and Alzheimer disease in African American subjects. Arch Neurol. 2002;59:594–600. doi: 10.1001/archneur.59.4.594. [DOI] [PubMed] [Google Scholar]
- [11].Sherva R, Baldwin CT, Inzelberg R, Vardarajan B, Cupples LA, Lunetta K, et al. Identification of novel candidate genes for Alzheimer's disease by autozygosity mapping using genome wide SNP data. J Alzheimers Dis. 2011;23:349–59. doi: 10.3233/JAD-2010-100714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Lambert JC, Ibrahim-Verbaas CA, Harold D, Naj AC, Sims R, Bellenguez C, et al. Meta-analysis of 74,046 individuals identifies 11 new susceptibility loci for Alzheimer's disease. Nat Genet. 2013;45:1452–8. doi: 10.1038/ng.2802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].IGAP Convergent genetic and expression data implicate immunity in Alzheimer's disease. Alzheimers Dement. 2015;11:658–71. doi: 10.1016/j.jalz.2014.05.1757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Jun G, Ibrahim-Verbaas CA, Vronskaya M, Lambert JC, Chung J, Naj AC, et al. A novel Alzheimer disease locus located near the gene encoding tau protein. Mol Psychiatry. 2016;21:108–17. doi: 10.1038/mp.2015.23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Reitz C, Jun G, Naj A, Rajbhandary R, Vardarajan BN, Wang LS, et al. Variants in the ATP-binding cassette transporter (ABCA7), apolipoprotein E 4,and the risk of late-onset Alzheimer disease in African Americans. JAMA. 2013;309:1483–92. doi: 10.1001/jama.2013.2973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Meng Y, Baldwin CT, Bowirrat A, Waraska K, Inzelberg R, Friedland RP, et al. Association of polymorphisms in the Angiotensin-converting enzyme gene with Alzheimer disease in an Israeli Arab community. Am J Hum Genet. 2006;78:871–7. doi: 10.1086/503687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Jun G, Asai H, Zeldich E, Drapeau E, Chen C, Chung J, et al. PLXNA4 is associated with Alzheimer disease and modulates tau phosphorylation. Ann Neurol. 2014;76:379–92. doi: 10.1002/ana.24219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Rogaeva E, Meng Y, Lee JH, Gu Y, Kawarai T, Zou F, et al. The neuronal sortilin-related receptor SORL1 is genetically associated with Alzheimer disease. Nat Genet. 2007;39:168–77. doi: 10.1038/ng1943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Willer CJ, Li Y, Abecasis GR. METAL: fast and efficient meta-analysis of genomewide association scans. Bioinformatics. 2010;26:2190–1. doi: 10.1093/bioinformatics/btq340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Han B, Eskin E. Random-effects model aimed at discovering associations in meta-analysis of genome-wide association studies. Am J Hum Genet. 2011;88:586–98. doi: 10.1016/j.ajhg.2011.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Li MX, Gui HS, Kwan JS, Sham PC. GATES: a rapid and powerful gene-based association test using extended Simes procedure. Am J Hum Genet. 2011;88:283–93. doi: 10.1016/j.ajhg.2011.01.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Jun G, Vardarajan BN, Buros J, Yu CE, Hawk MV, Dombroski BA, et al. Comprehensive Search for Alzheimer Disease Susceptibility Loci in the APOE Region. Arch Neurol. 2012:1–10. doi: 10.1001/archneurol.2012.2052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Nanba D, Higashiyama S. Dual intracellular signaling by proteolytic cleavage of membrane-anchored heparin-binding EGF-like growth factor. Cytokine Growth Factor Rev. 2004;15:13–9. doi: 10.1016/j.cytogfr.2003.10.002. [DOI] [PubMed] [Google Scholar]
- [24].Sasaki K, Omotuyi OI, Ueda M, Shinohara K, Ueda H. NMDA receptor agonists reverse impaired psychomotor and cognitive functions associated with hippocampal Hbegf-deficiency in mice. Mol Brain. 2015;8:83. doi: 10.1186/s13041-015-0176-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Maurya SK, Mishra J, Abbas S, Bandyopadhyay S. Cypermethrin Stimulates GSK3beta-Dependent Abeta and p-tau Proteins and Cognitive Loss in Young Rats: Reduced HB-EGF Signaling and Downstream Neuroinflammation as Critical Regulators. Mol Neurobiol. 2016;53:968–82. doi: 10.1007/s12035-014-9061-6. [DOI] [PubMed] [Google Scholar]
- [26].Desikan RS, Schork AJ, Wang Y, Thompson WK, Dehghan A, Ridker PM, et al. Polygenic Overlap Between C-Reactive Protein, Plasma Lipids, and Alzheimer Disease. Circulation. 2015;131:2061–9. doi: 10.1161/CIRCULATIONAHA.115.015489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Martinu L, Santiago-Walker A, Qi H, Chou MM. Endocytosis of epidermal growth factor receptor regulated by Grb2-mediated recruitment of the Rab5 GTPase-activating protein RN-tre. J Biol Chem. 2002;277:50996–1002. doi: 10.1074/jbc.M204869200. [DOI] [PubMed] [Google Scholar]
- [28].Funakoshi Y, Chou MM, Kanaho Y, Donaldson JG. TRE17/USP6 regulates ubiquitylation and trafficking of cargo proteins that enter cells by clathrin-independent endocytosis. J Cell Sci. 2014;127:4750–61. doi: 10.1242/jcs.156786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Logue MW, Schu M, Vardarajan BN, Farrell J, Lunetta KL, Jun G, et al. Search for age-related macular degeneration risk variants in Alzheimer disease genes and pathways. Neurobiol Aging. 2014;35:1510. doi: 10.1016/j.neurobiolaging.2013.12.007. e7-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Thomas RS, Lelos MJ, Good MA, Kidd EJ. Clathrin-mediated endocytic proteins are upregulated in the cortex of the Tg2576 mouse model of Alzheimer's disease-like amyloid pathology. Biochem Biophys Res Commun. 2011;415:656–61. doi: 10.1016/j.bbrc.2011.10.131. [DOI] [PubMed] [Google Scholar]
- [31].Xiao Q, Gil SC, Yan P, Wang Y, Han S, Gonzales E, et al. Role of phosphatidylinositol clathrin assembly lymphoid-myeloid leukemia (PICALM) in intracellular amyloid precursor protein (APP) processing and amyloid plaque pathogenesis. J Biol Chem. 2012;287:21279–89. doi: 10.1074/jbc.M111.338376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Eicher JD, Wakabayashi Y, Vitseva O, Esa N, Yang Y, Zhu J, et al. Characterization of the platelet transcriptome by RNA sequencing in patients with acute myocardial infarction. Platelets. 2016;27:230–9. doi: 10.3109/09537104.2015.1083543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Wang P, Zhao J, Yang X, Guan S, Feng H, Han D, et al. PFDN1, an indicator for colorectal cancer prognosis, enhances tumor cell proliferation and motility through cytoskeletal reorganization. Med Oncol. 2015;32:264. doi: 10.1007/s12032-015-0710-z. [DOI] [PubMed] [Google Scholar]
- [34].Galiegue S, Jbilo O, Combes T, Bribes E, Carayon P, Le Fur G, et al. Cloning and characterization of PRAX-1. A new protein that specifically interacts with the peripheral benzodiazepine receptor. J Biol Chem. 1999;274:2938–52. doi: 10.1074/jbc.274.5.2938. [DOI] [PubMed] [Google Scholar]
- [35].Shash D, Kurth T, Bertrand M, Dufouil C, Barberger-Gateau P, Berr C, et al. Benzodiazepine, psychotropic medication, and dementia: A population-based cohort study. Alzheimers Dement. 2016;12:604–13. doi: 10.1016/j.jalz.2015.10.006. [DOI] [PubMed] [Google Scholar]
- [36].Barron AM, Garcia-Segura LM, Caruso D, Jayaraman A, Lee JW, Melcangi RC, et al. Ligand for translocator protein reverses pathology in a mouse model of Alzheimer's disease. J Neurosci. 2013;33:8891–7. doi: 10.1523/JNEUROSCI.1350-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Kreisl WC, Lyoo CH, Liow JS, Wei M, Snow J, Page E, et al. (11)C-PBR28 binding to translocator protein increases with progression of Alzheimer's disease. Neurobiol Aging. 2016;44:53–61. doi: 10.1016/j.neurobiolaging.2016.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Levine AJ, Miller JA, Shapshak P, Gelman B, Singer EJ, Hinkin CH, et al. Systems analysis of human brain gene expression: mechanisms for HIV-associated neurocognitive impairment and common pathways with Alzheimer's disease. BMC Med Genomics. 2013;6:4. doi: 10.1186/1755-8794-6-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Yoshihara S, Takahashi H, Nishimura N, Naritsuka H, Shirao T, Hirai H, et al. 5T4 glycoprotein regulates the sensory input-dependent development of a specific subtype of newborn interneurons in the mouse olfactory bulb. J Neurosci. 2012;32:2217–26. doi: 10.1523/JNEUROSCI.5907-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.