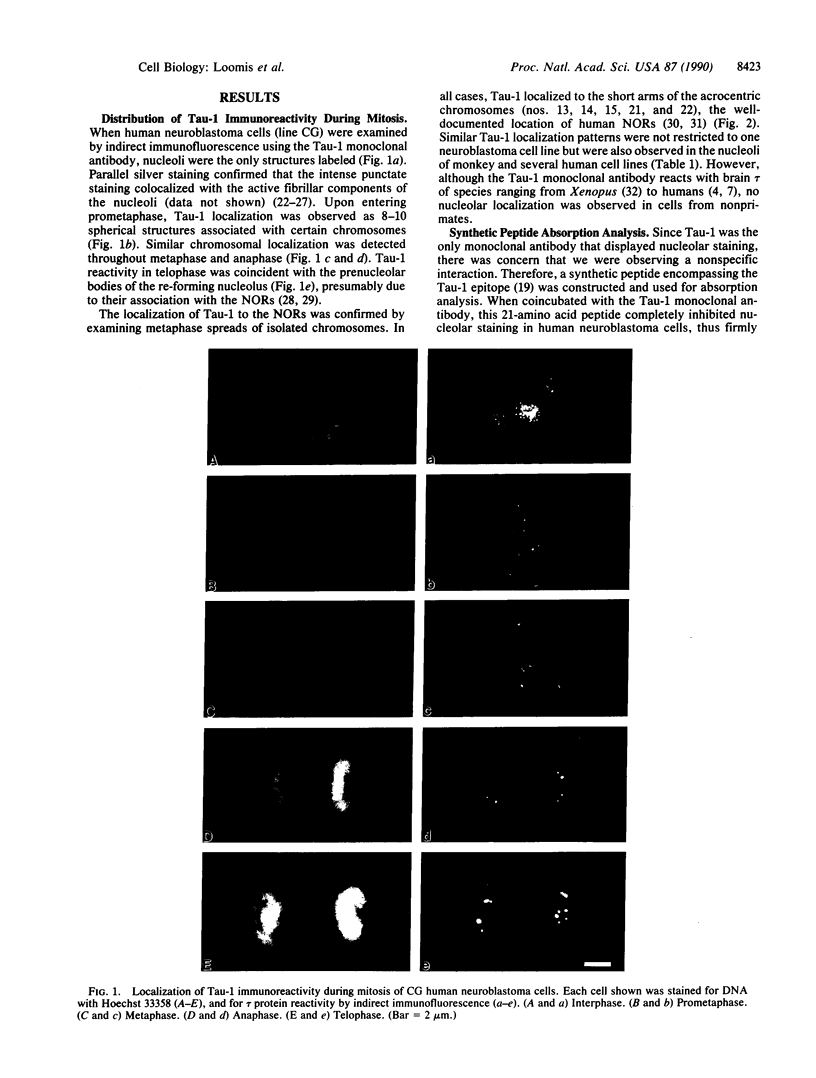
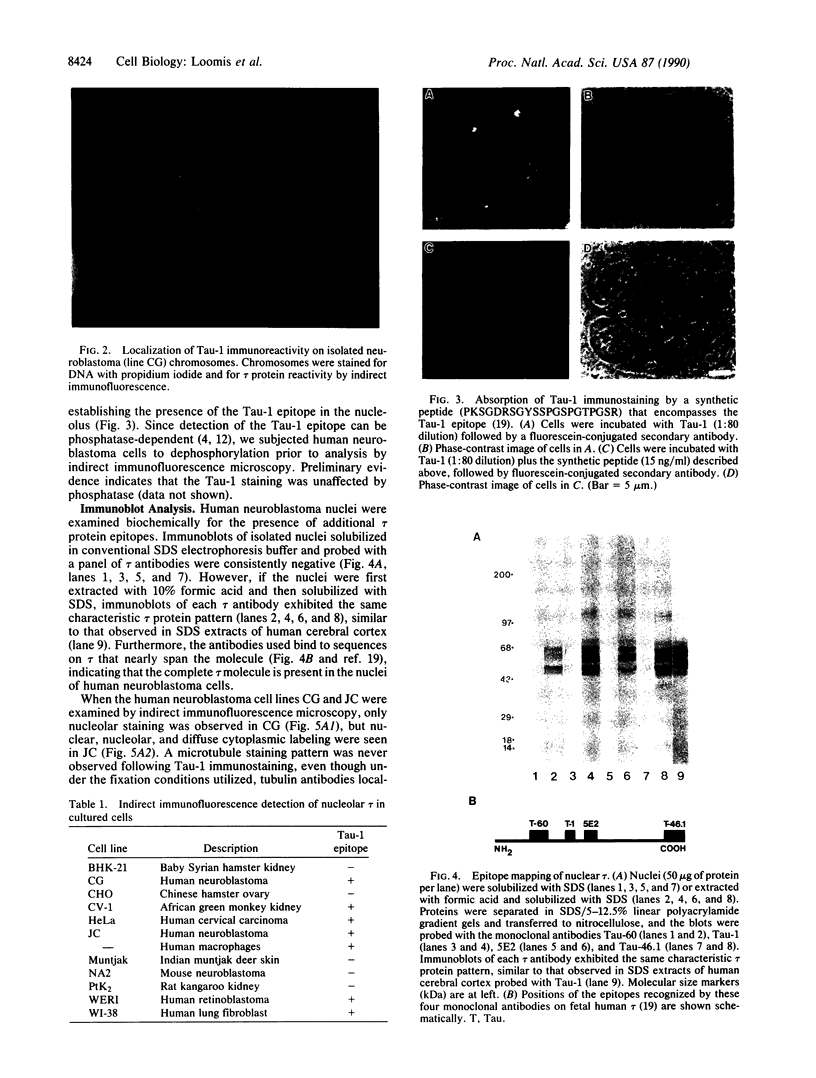
Abstract
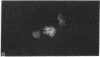
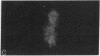
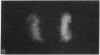
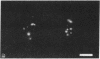
The tau proteins have been reported only in association with microtubules and with ribosomes in situ, in the normal central nervous system. In addition, tau has been shown to be an integral component of paired helical filaments, the principal constituent of the neurofibrillary tangles found in brains of patients with Alzheimer disease and of most aged individuals with Down syndrome (trisomy 21). We report here the localization of the well-characterized Tau-1 monoclonal antibody to the nucleolar organizer regions of the acrocentric chromosomes and to their interphase counterpart, the fibrillar component of the nucleolus, in human neuroblastoma cells. Similar localization to the nucleolar organizer regions was also observed in other human cell lines and in one monkey kidney cell line but was not seen in non-primate species. Immunochemically, we further demonstrate the existence of the entire tau molecule in the isolated nuclei of neuroblastoma cells. Nuclear tau proteins, like the tau proteins of the paired helical filaments, cannot be extracted in standard SDS-containing electrophoresis sample buffer but require pretreatment with formic acid prior to immunoblot analysis. This work indicates that tau may function in processes not directly associated with microtubules and that highly insoluble complexes of tau may also play a role in normal cellular physiology.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Binder L. I., Frankfurter A., Rebhun L. I. The distribution of tau in the mammalian central nervous system. J Cell Biol. 1985 Oct;101(4):1371–1378. doi: 10.1083/jcb.101.4.1371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bourgeois C. A., Hernandez-Verdun D., Hubert J., Bouteille M. Silver staining of NORs in electron microscopy. Exp Cell Res. 1979 Oct 15;123(2):449–452. doi: 10.1016/0014-4827(79)90498-1. [DOI] [PubMed] [Google Scholar]
- Brinkley B. R., Zinkowski R. P., Mollon W. L., Davis F. M., Pisegna M. A., Pershouse M., Rao P. N. Movement and segregation of kinetochores experimentally detached from mammalian chromosomes. Nature. 1988 Nov 17;336(6196):251–254. doi: 10.1038/336251a0. [DOI] [PubMed] [Google Scholar]
- Connolly J. A., Kalnins V. I., Cleveland D. W., Kirschner M. W. Immunoflourescent staining of cytoplasmic and spindle microtubules in mouse fibroblasts with antibody to tau protein. Proc Natl Acad Sci U S A. 1977 Jun;74(6):2437–2440. doi: 10.1073/pnas.74.6.2437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans R. M., Hollenberg S. M. Zinc fingers: gilt by association. Cell. 1988 Jan 15;52(1):1–3. doi: 10.1016/0092-8674(88)90522-3. [DOI] [PubMed] [Google Scholar]
- Galloway P. G., Perry G., Kosik K. S., Gambetti P. Hirano bodies contain tau protein. Brain Res. 1987 Feb 17;403(2):337–340. doi: 10.1016/0006-8993(87)90071-0. [DOI] [PubMed] [Google Scholar]
- Grundke-Iqbal I., Iqbal K., Quinlan M., Tung Y. C., Zaidi M. S., Wisniewski H. M. Microtubule-associated protein tau. A component of Alzheimer paired helical filaments. J Biol Chem. 1986 May 5;261(13):6084–6089. [PubMed] [Google Scholar]
- Grundke-Iqbal I., Iqbal K., Tung Y. C., Quinlan M., Wisniewski H. M., Binder L. I. Abnormal phosphorylation of the microtubule-associated protein tau (tau) in Alzheimer cytoskeletal pathology. Proc Natl Acad Sci U S A. 1986 Jul;83(13):4913–4917. doi: 10.1073/pnas.83.13.4913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henderson A. S., Warburton D., Atwood K. C. Location of ribosomal DNA in the human chromosome complement. Proc Natl Acad Sci U S A. 1972 Nov;69(11):3394–3398. doi: 10.1073/pnas.69.11.3394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hernandez-Verdun D., Hubert J., Bourgeois C., Bouteille M. Identification ultrastructurale de l'organisateur nucléolaire par la technique à l'argent. C R Acad Sci Hebd Seances Acad Sci D. 1978 Dec;287(16):1421–1423. [PubMed] [Google Scholar]
- Himmler A., Drechsel D., Kirschner M. W., Martin D. W., Jr Tau consists of a set of proteins with repeated C-terminal microtubule-binding domains and variable N-terminal domains. Mol Cell Biol. 1989 Apr;9(4):1381–1388. doi: 10.1128/mcb.9.4.1381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Himmler A. Structure of the bovine tau gene: alternatively spliced transcripts generate a protein family. Mol Cell Biol. 1989 Apr;9(4):1389–1396. doi: 10.1128/mcb.9.4.1389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ihara Y., Nukina N., Miura R., Ogawara M. Phosphorylated tau protein is integrated into paired helical filaments in Alzheimer's disease. J Biochem. 1986 Jun;99(6):1807–1810. doi: 10.1093/oxfordjournals.jbchem.a135662. [DOI] [PubMed] [Google Scholar]
- Iqbal K., Zaidi T., Thompson C. H., Merz P. A., Wisniewski H. M. Alzheimer paired helical filaments: bulk isolation, solubility, and protein composition. Acta Neuropathol. 1984;62(3):167–177. doi: 10.1007/BF00691849. [DOI] [PubMed] [Google Scholar]
- KIDD M. Paired helical filaments in electron microscopy of Alzheimer's disease. Nature. 1963 Jan 12;197:192–193. doi: 10.1038/197192b0. [DOI] [PubMed] [Google Scholar]
- Kosik K. S., Joachim C. L., Selkoe D. J. Microtubule-associated protein tau (tau) is a major antigenic component of paired helical filaments in Alzheimer disease. Proc Natl Acad Sci U S A. 1986 Jun;83(11):4044–4048. doi: 10.1073/pnas.83.11.4044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kosik K. S., Orecchio L. D., Binder L., Trojanowski J. Q., Lee V. M., Lee G. Epitopes that span the tau molecule are shared with paired helical filaments. Neuron. 1988 Nov;1(9):817–825. doi: 10.1016/0896-6273(88)90129-8. [DOI] [PubMed] [Google Scholar]
- Kowall N. W., Kosik K. S. Axonal disruption and aberrant localization of tau protein characterize the neuropil pathology of Alzheimer's disease. Ann Neurol. 1987 Nov;22(5):639–643. doi: 10.1002/ana.410220514. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lee G., Cowan N., Kirschner M. The primary structure and heterogeneity of tau protein from mouse brain. Science. 1988 Jan 15;239(4837):285–288. doi: 10.1126/science.3122323. [DOI] [PubMed] [Google Scholar]
- Lepoint A., Goessens G. Nucleologenesis in Ehrlich tumour cells. Exp Cell Res. 1978 Nov;117(1):89–94. doi: 10.1016/0014-4827(78)90430-5. [DOI] [PubMed] [Google Scholar]
- Miller D. A., Dev V. G., Tantravahi R., Miller O. J. Suppression of human nucleolus organizer activity in mouse-human somatic hybrid cells. Exp Cell Res. 1976 Sep;101(2):235–243. doi: 10.1016/0014-4827(76)90373-6. [DOI] [PubMed] [Google Scholar]
- Mitchison T. J., Kirschner M. W. Properties of the kinetochore in vitro. I. Microtubule nucleation and tubulin binding. J Cell Biol. 1985 Sep;101(3):755–765. doi: 10.1083/jcb.101.3.755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papasozomenos S. C., Binder L. I. Phosphorylation determines two distinct species of Tau in the central nervous system. Cell Motil Cytoskeleton. 1987;8(3):210–226. doi: 10.1002/cm.970080303. [DOI] [PubMed] [Google Scholar]
- Ploton D., Thiry M., Menager M., Lepoint A., Adnet J. J., Goessens G. Behaviour of nucleolus during mitosis. A comparative ultrastructural study of various cancerous cell lines using the Ag-NOR staining procedure. Chromosoma. 1987;95(2):95–107. doi: 10.1007/BF00332182. [DOI] [PubMed] [Google Scholar]
- TERRY R. D. THE FINE STRUCTURE OF NEUROFIBRILLARY TANGLES IN ALZHEIMER'S DISEASE. J Neuropathol Exp Neurol. 1963 Oct;22:629–642. doi: 10.1097/00005072-196310000-00005. [DOI] [PubMed] [Google Scholar]
- The nucleolar organizer regions. Biol Cell. 1983;49(3):191–202. [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Viereck C., Tucker R. P., Binder L. I., Matus A. Phylogenetic conservation of brain microtubule-associated proteins MAP2 and tau. Neuroscience. 1988 Sep;26(3):893–904. doi: 10.1016/0306-4522(88)90107-8. [DOI] [PubMed] [Google Scholar]
- Weingarten M. D., Lockwood A. H., Hwo S. Y., Kirschner M. W. A protein factor essential for microtubule assembly. Proc Natl Acad Sci U S A. 1975 May;72(5):1858–1862. doi: 10.1073/pnas.72.5.1858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wisniewski K. E., Wisniewski H. M., Wen G. Y. Occurrence of neuropathological changes and dementia of Alzheimer's disease in Down's syndrome. Ann Neurol. 1985 Mar;17(3):278–282. doi: 10.1002/ana.410170310. [DOI] [PubMed] [Google Scholar]
- Wood J. G., Mirra S. S., Pollock N. J., Binder L. I. Neurofibrillary tangles of Alzheimer disease share antigenic determinants with the axonal microtubule-associated protein tau (tau) Proc Natl Acad Sci U S A. 1986 Jun;83(11):4040–4043. doi: 10.1073/pnas.83.11.4040. [DOI] [PMC free article] [PubMed] [Google Scholar]