Abstract
IMPORTANCE
Topical application of azithromycin suppresses expression of proinflammatory mediators while restoring transforming growth factor β1 (TGF-β1) levels as evaluated by eyelid margin and conjunctival impression cytology.
OBJECTIVE
To explore the effects of azithromycin therapy on expression of proinflammatory and anti-inflammatory mediators in meibomian gland disease (MGD).
DESIGN, SETTING, AND PARTICIPANTS
Case-control study performed in a clinic setting from August 17, 2010, to December 31, 2010. Sixteen patients with posterior blepharitis and conjunctival inflammation due to MGD were treated with azithromycin, 1%, drops for 4 weeks. Impression cytology of the lower eyelid margin and tarsal conjunctiva to measure cytokine expression by quantitative real-time polymerase chain reaction as well as tear collection to measure matrix metalloproteinase 9 (MMP-9) activity were performed once in 8 asymptomatic healthy control participants and 5 times in the 16 symptomatic patients (every 2 weeks for 8 weeks), before, during, and after azithromycin treatment.
EXPOSURE
Azithromycin, 1%, drops for 4 weeks.
MAIN OUTCOMES AND MEASURES
Cytokine expression in the eyelid margin and conjunctiva, and MMP-9 activity in tears.
RESULTS
Compared with a 1-time measurement of 8 healthy participants, among 16 symptomatic patients, the mean (SD; 95% CI) fold change of expression of proinflammatory mediators interleukin 1β (IL-1β), IL-8, and MMP-9 increased to 13.26 (4.33; 11.14–15.38; P < .001), 9.38 (3.37; 7.73–11.03; P < .001), and 13.49 (4.92; 11.08–15.90; P < .001), respectively, in conjunctival cells and to 11.75 (3.96; 9.81–13.69; P < .001), 9.31 (3.28; 7.70–10.92; P < .001), and 11.52 (3.50; 9.81–13.24; P < .001), respectively, in the eyelid margin of patients with MGD. In contrast, the mean (SD; 96% CI) fold change of expression of TGF-β1 messenger RNA (mRNA) decreased to 0.58 (0.25; 0.46–0.70; P = .02) and 0.63 (0.14; 0.56–0.70; P = .02) in conjunctival and eyelid margin cells, respectively, of patients with MGD. Azithromycin, 1%, caused a change in the expression pattern of these mediators toward normal levels during 4 weeks of treatment. Levels of IL-1β, IL-8, and MMP-9 mRNA remained suppressed, although they rebounded toward pretreatment values 4 weeks after azithromycin withdrawal. Expression of TGF-β1 increased during treatment and remained at levels similar to the healthy controls after drug withdrawal. Change in tear MMP-9 activity was similar to the pattern of MMP-9 transcripts.
CONCLUSIONS AND RELEVANCE
While the study did not control for potential confounding factors over time independent of the intervention that may have contributed to the results, topical azithromycin suppressed expression of proinflammatory mediators and increased expression of TGF-β1 to normal levels. Increased TGF-β1 expression may contribute to the anti-inflammatory activity of azithromycin in MGD.
Blepharitis is one of the most common ocular surface disorders. The prevalence of this condition has ranged from 12% to 47% in previously reported surveys.1,2 Blepharitis can be broadly categorized into anterior and posterior, with the former involving an infectious and inflammatory condition of the external lamella of the eyelids and eyelashes, and the latter having atrophy and/or obstruction of the meibomian glands that is often accompanied by tear dysfunction and eyelid and conjunctival inflammation.3 Posterior blepharitis or meibomian gland disease (MGD) is the most prevalent type of blepharitis.
Although posterior blepharitis and meibomian gland dysfunction can develop in inflammatory conditions such as rosacea or atopic dermatitis, the etiology of most cases of posterior blepharitis remains to be determined.4 Inflammation induced by bacteria or microbial products has been implicated in the pathogenesis. Clinical improvement in symptoms and signs of meibomian disease has been observed following treatment with oral tetracyclines (doxycycline, minocycline) or topical azithromycin.4–7
Tetracycline and azithromycin antibiotics have also been shown to have anti-inflammatory properties.8,9 A decrease in the level of the inflammatory protease matrix metalloproteinase 9 (MMP-9) in tears has been reported after 1 month of oral doxycycline therapy for MGD.10 Azithromycin was previously reported to inhibit production of inflammatory cytokines (interleukin 1β [IL-1β], IL-8) and MMPs (MMP-1, MMP-3, and MMP-9) by cultured human corneal epithelial cells stimulated with toll-like receptor agonists.11 However, to our knowledge, the effects of topically applied azithromycin on expression of inflammatory mediators in the eyelid and conjunctiva of patients with MGD have not been evaluated. Using impression cytology to obtain cells from the surface of the eyelid margin and conjunctiva, this study characterized the gene expression profiles of proinflammatory and anti-inflammatory mediators in samples from patients with MGD and further evaluated the anti-inflammatory properties of topical azithromycin application on the expression of these mediators in the eyelid margin and conjunctiva as well as its effects on tear MMP-9 activity.
Methods
Patients and Healthy Participants
After receiving written informed consent, 16 patients with untreated MGD, diagnosed by a single ophthalmologist (S.C.P.) at Alkek Eye Center, Houston, Texas, and 8 asymptomatic healthy individuals were recruited for study. Criteria for diagnosis of MGD included plugging of at least 4 of the central 10 meibomian glands on the lower eyelid, at least grade 2 (moderate) eyelid margin injection, and at least grade 1 (mild) erythema of the inferior bulbar and palpebral conjunctiva. Injection and erythema were graded on a scale of 0 to 4 using standardized photographic standards, with 4 being the most severe. Individuals were excluded if they were using any therapy other than warm eyelid compresses and massage. The healthy controls had no signs of MGD and no conjunctival inflammation. Patients with MGD received topical treatment with a US Food and Drug Administration–approved azithromycin, 1%, ophthalmic suspension, 1 drop twice a day for 2 days followed by 1 drop per day in the evening for 28 days in both eyes. Impression cytology on the lower eyelid margin and inferior bulbar conjunctiva was performed once for healthy controls and 5 times for patients with MGD treated with azithromycin (before the treatment, after weeks 2 and 4 of the treatment, and weeks 2 and 4 after cessation of azithromycin). Participants did not use any other treatment throughout the study period. The study was conducted between August 17, 2010, and December 31, 2010. The clinical protocol was approved by the Baylor College of Medicine Institutional Review Board and adhered to the tenets of the Declaration of Helsinki and the Association for Research in Vision and Ophthalmology statement on human subjects.
Eyelid Margin and Conjunctival Impression Cytology
After administration of topical anesthesia with proparacaine hydrochloride, 0.5%, a 2 × 6-mm or 3 × 4-mm sterile cellulose acetate filter membrane (Supor 450 Gridded; Pall Life Sciences) was placed on the center of the lower eyelid margin or on the inferior bulbar conjunctiva, respectively, and the surface of the membrane was firmly rubbed over its entire length 3 times with blunt tying forceps. The membrane was then removed with the forceps, immediately placed into a tube containing 350 μL of lysis buffer (Qiagen), and stored at −80°C until total RNA was extracted.
RNA Extraction, Reverse Transcription, and Quantitative Real-Time Polymerase Chain Reaction
Total RNA was extracted with the RNeasy Micro Kit (Qiagen) according to the manufacturer’s instructions, measured with a NanoDrop spectrophotometer (ND-1000; Thermo Scientific), and stored at −80°C. First-strand complementary DNA (cDNA) was synthesized by reverse transcription from 100 ng of total RNA using Ready-To-Go You-Prime First-Strand Beads (GE Healthcare) as previously described.12,13 Quantitative real-time polymerase chain reaction was performed using a Mx3005P system (Stratagene) with a 20-μL reaction volume containing 5 μL of cDNA, 1 μL of gene expression assay mix, and 10 μL of TaqMan gene expression master mix (Applied Biosystems by Life Technologies). TaqMan gene expression assays were used to evaluate glyceraldehyde-3-phosphate de-hydrogenase (Assay ID Hs99999905_m1), IL-1β (Assay ID Hs00174097_m1), IL-8 (Assay ID Hs00174103_m1), MMP-9 (Assay ID Hs00234579_m1), and transforming growth factor β1 (TGF-β1; Assay ID Hs99999918_m1). The thermocycler parameters were 50°C for 2 minutes and 95°C for 10 minutes, followed by 40 cycles of 95°C for 15 seconds and 60°C for 1 minute. A nontemplate control was included to evaluate DNA contamination. The results were analyzed by the comparative threshold cycle method and normalized by glyceraldehyde-3-phosphate dehydrogenase as an internal control.14
Tear MMP-9 Activity Assay
Total MMP-9 enzyme activity was measured with an MMP-9 activity assay kit (GE Biosciences) as previously reported.15 One microliter of unstimulated tears was collected with a glass capillary tube from the central inferior tear meniscus of the left eye and was eluted into a tube containing 9 μL of buffer (phosphate-buffered saline with 0.1% bovine serum albumin). In brief, 100 μL of each pro–MMP-9 standard (0.125-4 ng/mL), tears (10 μL of diluted tears and 90 μL of assay buffer), and assay buffer (for blanks) were incubated at 4°C overnight in wells of a microtiter plate precoated with anti–MMP-9 mouse monoclonal capture antibody. Plates were washed 3 times with 0.01M sodium phosphate buffer (pH 7.0) containing 0.05% Tween 20. To measure total MMP-9 activity, bound pro–MMP-9 was activated with 50 μL of 1mM p-aminophenylmercuric acetate in assay buffer at 37°C for 1.5 hours. Detection reagent (50 μL) was added to each well and samples were incubated at 37°C for 6 hours. Active MMP-9 was detected through its ability to activate a modified prodetection enzyme that subsequently cleaved its chromogenic peptide substrate. Absorbance was read at 405 nm in a microplate reader (Versamax; Molecular Devices). The activity of MMP-9 in a sample was determined by interpolation from a standard curve. Tear sample absorbance readings were multiplied by a dilution factor of 100.
Statistical Analysis
One-way analysis of variance was used to make comparisons among 3 or more groups, followed by Dunnett post hoc test, and t test was used to compare differences between 2 groups. GraphPad Prism version 6 statistical software (GraphPad Software Inc) was used for statistical analysis. P < .05 was considered statistically significant.
Results
Gene Expression Profiles of Proinflammatory and Anti-inflammatory Mediators by Conjunctival and Eyelid Margin Cells in Patients With MGD
Impression cytology was performed in 16 patients with MGD (mean age, 54 years; range, 34–80 years) and 8 healthy control participants with no signs of MGD (mean age, 28 years; range, 25–27 years). The mean age of the MGD group was significantly older than the control group (P = .002). Participants tolerated impression cytology of the eyelid margin and conjunctiva well with minimal or no reported irritation and no adverse effects. As evaluated by quantitative real-time polymerase chain reaction, the mean (SD) messenger RNA (mRNA) expression of inflammatory cytokine IL-1β, chemokine IL-8, and MMP-9 increased to 13.26 (4.33) fold (95% CI, 11.14–15.38; P < .001), 9.38 (3.37) fold (95% CI, 7.73–11.03; P < .001), and 13.49 (4.92) fold (95% CI, 11.08–15.90; P < .001), respectively, in conjunctival cells from patients with MGD compared with the mRNA levels found in specimens from healthy controls (Figure 1A). Expression of TGF-β1 was lower (mean [SD], 0.58 [0.25] fold; 95% CI, 0.46–0.70; P = .02) in conjunctival cells of patients with MGD compared with healthy controls. Similar to the conjunctival cells, mean (SD) expression of IL-1β, IL-8, and MMP-9 transcripts in eyelid margin cells increased to 11.75 (3.96) fold (95% CI, 9.81–13.69; P < .001), 9.31 (3.28) fold (95% CI, 7.70–10.92; P < .001), and 11.52 (3.50) fold (95% CI, 9.81–13.24; P < .001), respectively, while the mean (SD) level of TGF-β1 decreased to 0.63 (0.14) fold (95% CI, 0.56–0.70; P = .02) compared with healthy controls (Figure 1B).
Figure 1. Cytokine Expression Profiles in Meibomian Gland Disease (MGD) by Quantitative Real-Time Polymerase Chain Reaction.
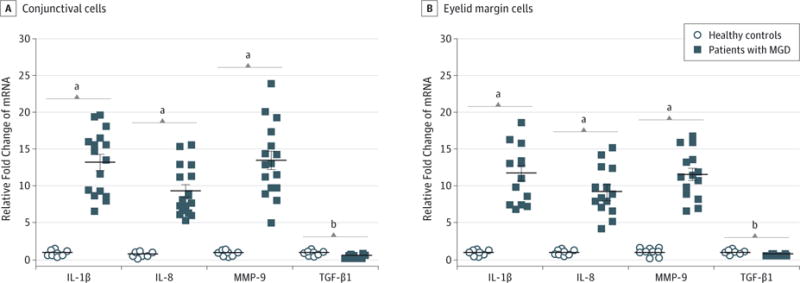
Increased expression of proinflammatory mediators interleukin 1β (IL-1β), IL-8, and matrix metalloproteinase 9 (MMP-9) and decreased transforming growth factor β1 (TGF-β1) messenger RNA (mRNA) by conjunctival (A) and eyelid margin (B) cells in patients with MGD compared with healthy controls. Horizontal lines indicate mean; error bars, standard deviation.
aP < .001 vs healthy controls.
bP < .05 vs healthy controls.
Azithromycin Inhibited Expression of Proinflammatory Mediators and Increased Expression of TGF-β1 in Patients With MGD
Patients with MGD were topically treated with azithromycin, 1%, for 4 weeks. The proinflammatory profiles were evaluated every 2 weeks during treatment and for 1 month after treatment and were compared with levels obtained from a 1-time measurement of healthy participants. As shown in Figure 2A, the mean (SD) expression of IL-1β, IL-8, and MMP-9 transcripts in conjunctival cells decreased to 2.81 (1.58) fold (95% CI, 2.04–3.58; P < .001), 4.20 (2.55) fold (95% CI, 2.95–5.45; P = .002), and 3.90 (2.20) fold (95% CI, 2.82–4.98; P < .001), respectively, after 2 weeks of azithromycin treatment, while the mean (SD) expression of TGF-β1 increased to slightly higher than the levels in healthy controls (1.27 [0.44] fold; 95% CI, 1.05–1.49; P = .002) compared with pretreatment levels. After 4 weeks of azithromycin treatment, expression of IL-1β, IL-8, and MMP-9 decreased further or remained lower (IL-1β: mean [SD], 2.88 [2.64] fold; 95% CI, 1.59–4.17; P < .001; IL-8: mean [SD], 2.82 [2.20] fold; 95% CI, 1.74–3.90; P < .001; and MMP-9: mean [SD], 4.44 [2.81] fold; 95% CI, 3.06–5.82; P < .001), while TGF-β1 mRNA levels remained elevated in the normal range (mean [SD], 1.16 [0.52] fold; 95% CI, 0.91–1.41; P = .001).
Figure 2. Effects of Azithromycin on Cytokine Profiles in Meibomian Gland Disease (MGD).
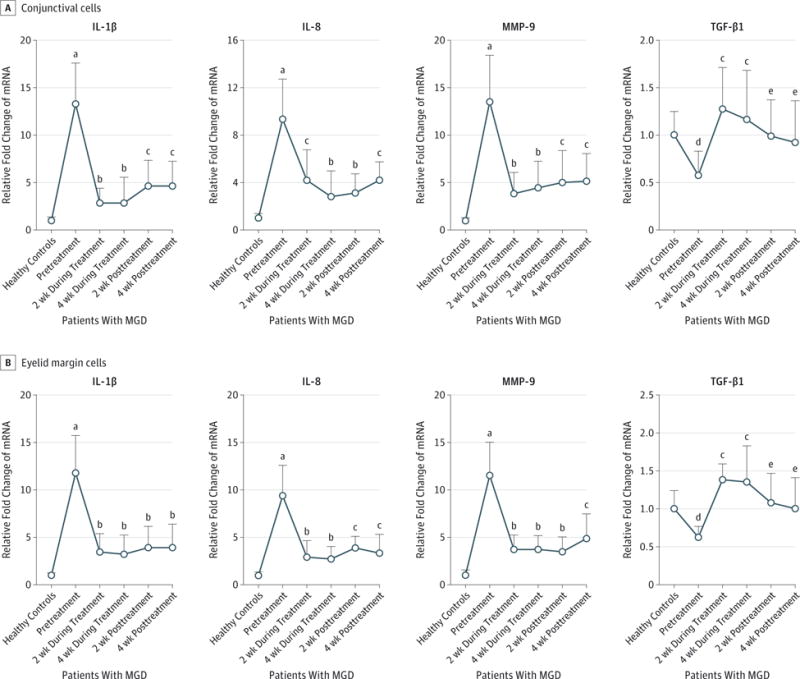
Messenger RNA (mRNA) levels of proinflammatory mediators interleukin 1β (IL-1β), IL-8, and matrix metalloproteinase 9 (MMP-9) and transforming growth factor β1 (TGF-β1) in conjunctival (A) and eyelid margin (B) cells, measured once in healthy controls and 5 times (every 2 weeks before, during, and after azithromycin treatment) in patients with MGD. Data points indicate mean; error bars, standard deviation.
aP < .001 vs healthy controls.
bP < .001 vs pretreatment levels.
cP < .01 vs pretreatment levels.
dP < .05 vs healthy controls.
eP < .05 vs pretreatment levels.
We reevaluated the gene expression profiles 2 and 4 weeks after cessation of azithromycin. Two weeks after stopping azithromycin, the mean (SD) expression of IL-1β, IL-8, and MMP-9 in conjunctival cells slightly or moderately rebounded to 4.61 (2.74) fold (95% CI, 3.27–5.95; P = .001), 3.10 (1.61) fold (95% CI, 2.31–3.89; P < .001), and 5.05 (3.31) fold (95% CI, 3.43–6.67; P = .002), respectively, from their nadir after 4 weeks of azithromycin treatment, but levels of these mediators were still lower than the pretreatment levels. Four weeks after azithromycin withdrawal, expression of IL-1β, IL-8, and MMP-9 remained lower than pretreatment levels. At 2 and 4 weeks after the drug was stopped, the mean (SD) TGF-β1 mRNA decreased slightly to 0.99 (0.38) fold (95% CI, 0.80–1.18; P = .01) and 0.92 (0.44) fold (95% CI, 0.70–1.14; P = .01), respectively, but remained higher than pretreatment levels.
As shown in Figure 2B, the expression patterns of IL-1β, IL-8, MMP-9, and TGF-β1 in eyelid margin cells showed a response to azithromycin similar to that in the conjunctival cells. The levels of IL-1β, IL-8, and MMP-9 decreased after azithromycin treatment for 2 and 4 weeks, and they remained lower after the drug was stopped. After azithromycin treatment, levels of TGF-β1 transcripts increased to slightly higher than those for the healthy controls and remained near the normal levels for 4 weeks after the drug was stopped. It should be noted that the study did not control for potential confounding factors over time independent of the intervention with azithromycin that may have contributed to the changes in expression of these inflammatory mediators in the conjunctival and eyelid margin cells.
Azithromycin Suppressed Tear MMP-9 Activity in Patients With MGD
To further investigate the anti-inflammatory activity of azithromycin, we evaluated MMP-9 activity in tears collected at 5 times from patients with MGD, before, during, and after azithromycin treatment. Compared with healthy controls, tear MMP-9 activity was 10-fold higher in tears obtained from patients with MGD. The mean (SD) tear MMP-9 activity decreased from 30.1 (12.6) ng/mL (95% CI, 23.93–36.27) to 9.4 (4.8) ng/mL (95% CI, 7.05–11.75; P < .001) after azithromycin treatment for 2 weeks and 6.1 (3.9) ng/mL (95% CI, 4.19–8.01; P < .001) after azithromycin treatment for 4 weeks. Interestingly, tear MMP-9 activity remained 70% lower than pretreatment levels for up to 4 weeks after cessation of azithromycin (Figure 3). As noted for the polymerase chain reaction results, the study did not control for potential confounding factors over time independent of the intervention with azithromycin that may have contributed to the changes in MMP-9 activity in tears.
Figure 3. Tear Matrix Metalloproteinase 9 (MMP-9) Activity.
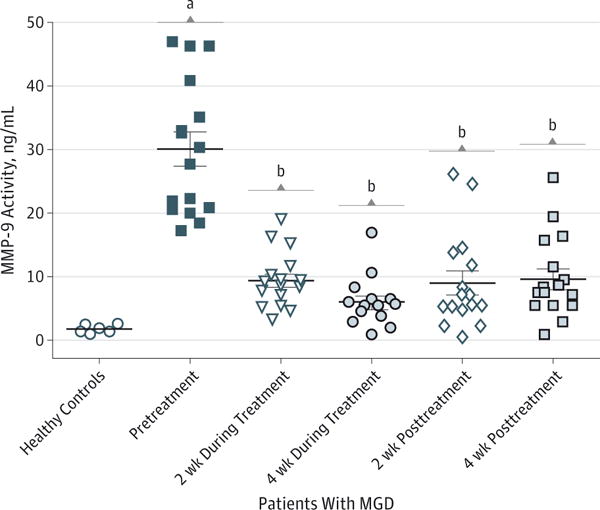
Azithromycin suppressed MMP-9 activity in tear samples from patients with meibomian gland disease (MGD) at 5 times (every 2 weeks before, during, and after azithromycin treatment). A healthy control group without MGD was included for comparison. Horizontal lines indicate mean; error bars, standard deviation.
aP < .001 vs healthy controls.
bP < .001 vs pretreatment levels.
Discussion
Meibomian gland disease is one of the most common ocular surface diseases. It can be associated with rosacea dermatitis and can cause conjunctival or corneal inflammation and tear instability.4 It is typically chronic, and the MGD workshop proposed treatment recommendations that included use of anti-inflammatory therapy.16 Our study revealed an interesting gene expression profile in the conjunctival and eyelid margin cells with elevated expression of proinflammatory mediators IL-1β, IL-8, and MMP-9 and reduced expression of anti-inflammatory cytokine TGF-β1 in patients with MGD compared with a younger healthy control group with no signs of MGD. We further demonstrated that the topical application of an azithromycin, 1%, ophthalmic solution altered this pattern by reversing the expression of these proinflammatory and anti-inflammatory mediators in patients with MGD. These findings suggest that the altered expression of these proinflammatory and anti-inflammatory mediators may be involved with the eyelid and ocular surface inflammation that develops in MGD. To our knowledge, the effects of azithromycin on expression of these mediators have not been previously evaluated in patients with MGD.
Azithromycin is a macrolide antibiotic used to treat or prevent certain bacterial infections such as otitis media,17 tonsillitis, pharyngitis,18,19 sinusitis, pneumonia,20,21 bronchitis,22 periodontitis,23 urethritis,24 and chlamydial infection.25,26 Azithromycin has also been used topically to treat ocular surface infections including bacterial conjunctivitis.27,28 In addition to its antibiotic effects, azithromycin has been shown to have a variety of anti-inflammatory effects, particularly in the context of microbial infections. Our previous study showed that azithromycin suppressed zymosan-induced mRNA expression and protein production of proinflammatory cytokines (tumor necrosis factor α and IL-1β), chemokines(IL-6 and RANTES), and MMPs (MMP-1, MMP-3, and MMP-9) by human corneal epithelial cells and suggested the potential for using azithromycin to treat ocular surface inflammation.11 Clinical trials have reported that azithromycin improved the signs and symptoms of blepharitis, including tear break-up time, corneal staining, conjunctival staining, Schirmer scores with anesthetic, meibomian gland score, and patients’ symptom scores.4,7,29,30
Impression cytology is an easy and economical noninvasive method to harvest cells from the ocular surface. Therefore, impression cytology has been widely used not only to facilitate the diagnosis of ocular surface disorders, including keratoconjunctivitis sicca, ocular surface squamous neoplasia, and ocular surface infections, but also to improve the understanding of pathogenesis of ocular surface diseases.31–34 Impression cytology has been successfully used to observe changes in cellular morphology and identify inflammatory markers.35,36 Some studies have also evaluated the expression of proinflammatory mediators in conjunctival impression cytology specimens.15,37 To our knowledge, this is the first study to assess the efficacy of topical azithromycin in blepharitis by evaluating the gene expression profiles of inflammatory mediators using eyelid margin and conjunctival impression cytology.
In this study, we performed impression cytology to obtain eyelid margin and conjunctival cells at 5 sequential visits for comparison of mRNA expression of inflammatory-associated mediators in patients with MGD before, during, and after topical azithromycin treatment compared with levels obtained from a 1-time measurement in healthy controls. The procedure was well tolerated with no adverse events. Interestingly, we observed that expression levels of major proinflammatory mediators IL-1β, IL-8, and MMP-9 were much higher in patients with MGD blepharoconjunctivitis than in healthy controls (P < .001). The elevated levels of those mediators gradually decreased (P < .001) during 4 weeks of azithromycin treatment. Expression of IL-1β, IL-8, and MMP-9 remained suppressed at the 4-week follow-up after drug withdrawal, although there was a slight rebound from their lowest point after 4 weeks of azithromycin treatment. The tear MMP-9 activity assay further confirmed higher MMP-9 activity in patients with MGD and the suppressive effects of topical azithromycin on this activity. These findings suggest that patients with MGD-associated eyelid and conjunctival inflammation may need intermittent pulse therapy with a topical anti-inflammatory agent such as azithromycin.
Surprisingly, we found that TGF-β1 expression was much lower in patients with MGD than in healthy controls and that it increased in the eyelid margin and conjunctiva during treatment with azithromycin. The TGF-β1 expression was still higher than in healthy controls 4 weeks after azithromycin withdrawal. Transforming growth factor β1 is a polypeptide member of the TGF-β cytokine superfamily. It was first identified in human platelets with a potential role in wound healing.38 Many types of cells secrete TGF-β1,39 including human corneal and conjunctival epithelia as well as lacrimal gland acinar cells.40,41 The pivotal function of TGF-β in the immune system is to maintain tolerance via the regulation of lymphocyte proliferation, differentiation, and survival. It controls the initiation and resolution of inflammatory responses through the regulation of chemotaxis, activation, and survival of lymphocytes.42 The anti-inflammatory role of TGF-β1 has been recognized in different cell types43–45 by inhibiting proinflammatory cytokines including tumor necrosis factor α, IL-1, and interferon γ.39,46,47 In this study, we observed that TGF-β1 expression was lower in patients with MGD than in healthy controls but increased after treatment with azithromycin, suggesting that TGF-β1 may contribute to the clinical improvement that has been observed. Transforming growth factor β1 also has the potential to induce fibrosis, but this adverse effect has not been reported to occur with topical azithromycin therapy.
There are several limitations of this study. First, changes in levels of inflammatory mediators in the conjunctiva and eyelid margin are relative to a single measurement in a healthy control group. Second, there was no control group to determine whether there were confounding factors during the study period that could have influenced the expression of these inflammatory mediators in the conjunctival and eyelid margin cells independent of the treatment with azithromycin. A randomized clinical trial would be required to confirm the efficacy of azithromycin for treatment of the ocular surface inflammation in MGD.
Conclusions
Our findings demonstrate that impression cytology is a simple and noninvasive technique to sample eyelid margin and conjunctival cells in ocular surface diseases. Topical application of azithromycin suppresses the expression of proinflammatory mediators IL-1β, IL-8, and MMP-9 while increasing expression of TGF-β1.
At a Glance.
This study explores the effects of topical azithromycin therapy on gene expression profiles of proinflammatory and anti-inflammatory mediators in the eyelid margin and conjunctiva of patients with meibomian gland disease.
Topical azithromycin suppressed messenger RNA expression of proinflammatory mediators interleukin 1β (IL-1β), IL-8, and matrix metalloproteinase 9 and increased expression of transforming growth factor β1.
Increased levels of transforming growth factor β1 may contribute to the anti-inflammatory activity of azithromycin in ocular surface inflammatory diseases.
Acknowledgments
Funding/Support: This work was supported in part by grant EY011915 from the National Eye Institute (Dr Pflugfelder) and by Inspire, Inc, Research to Prevent Blindness, the Oshman Foundation, and the William Stamps Farish Fund.
Role of the Funder/Sponsor: The funders had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication.
Footnotes
Author Contributions: Drs L. Zhang and Su contributed equally to this work. Drs Li and Pflugfelder had full access to all of the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis.
Study concept and design: L. Zhang, Li, Pflugfelder.
Acquisition, analysis, or interpretation of data: L. Zhang, Su, Z. Zhang, Lin, Li.
Drafting of the manuscript: L. Zhang, Su, Z. Zhang, Li, Pflugfelder.
Critical revision of the manuscript for important intellectual content: Lin, Li.
Statistical analysis: All authors.
Obtained funding: Pflugfelder.
Administrative, technical, or material support: Lin.
Study supervision: Z. Zhang, Li.
Conflict of Interest Disclosures: All authors have completed and submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest and none were reported.
References
- 1.Hom MM, Martinson JR, Knapp LL, Paugh JR. Prevalence of meibomian gland dysfunction. Optom Vis Sci. 1990;67(9):710–712. doi: 10.1097/00006324-199009000-00010. [DOI] [PubMed] [Google Scholar]
- 2.Lemp MA, Nichols KK. Blepharitis in the United States 2009: a survey-based perspective on prevalence and treatment. Ocul Surf. 2009;7(2 suppl):S1–S14. doi: 10.1016/s1542-0124(12)70620-1. [DOI] [PubMed] [Google Scholar]
- 3.Foulks GN, Bron AJ. Meibomian gland dysfunction: a clinical scheme for description, diagnosis, classification, and grading. Ocul Surf. 2003;1(3):107–126. doi: 10.1016/s1542-0124(12)70139-8. [DOI] [PubMed] [Google Scholar]
- 4.Pflugfelder SC, Karpecki PM, Perez VL. Treatment of blepharitis: recent clinical trials. Ocul Surf. 2014;12(4):273–284. doi: 10.1016/j.jtos.2014.05.005. [DOI] [PubMed] [Google Scholar]
- 5.Donaldson KE, Karp CL, Dunbar MT. Evaluation and treatment of children with ocular rosacea. Cornea. 2007;26(1):42–46. doi: 10.1097/ICO.0b013e31802e3a54. [DOI] [PubMed] [Google Scholar]
- 6.Gilbard JP. Dry eye, blepharitis and chronic eye irritation: divide and conquer. J Ophthalmic Nurs Technol. 1999;18(3):109–115. [PubMed] [Google Scholar]
- 7.Opitz DL, Tyler KF. Efficacy of azithromycin 1% ophthalmic solution for treatment of ocular surface disease from posterior blepharitis. Clin Exp Optom. 2011;94(2):200–206. doi: 10.1111/j.1444-0938.2010.00540.x. [DOI] [PubMed] [Google Scholar]
- 8.Scaglione F, Rossoni G. Comparative anti-inflammatory effects of roxithromycin, azithromycin and clarithromycin. J Antimicrob Chemother. 1998;41(suppl B):47–50. doi: 10.1093/jac/41.suppl_2.47. [DOI] [PubMed] [Google Scholar]
- 9.Kim HS, Luo L, Pflugfelder SC, Li D-Q. Doxycycline inhibits TGF-beta1-induced MMP-9 via Smad and MAPK pathways in human corneal epithelial cells. Invest Ophthalmol Vis Sci. 2005;46(3):840–848. doi: 10.1167/iovs.04-0929. [DOI] [PubMed] [Google Scholar]
- 10.Smith VA, Khan-Lim D, Anderson L, Cook SD, Dick AD. Does orally administered doxycycline reach the tear film? Br J Ophthalmol. 2008;92(6):856–859. doi: 10.1136/bjo.2007.125989. [DOI] [PubMed] [Google Scholar]
- 11.Li DQ, Zhou N, Zhang L, Ma P, Pflugfelder SC. Suppressive effects of azithromycin on zymosan-induced production of proinflammatory mediators by human corneal epithelial cells. Invest Ophthalmol Vis Sci. 2010;51(11):5623–5629. doi: 10.1167/iovs.09-4992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Luo L, Li D-Q, Doshi A, Farley W, Corrales RM, Pflugfelder SC. Experimental dry eye stimulates production of inflammatory cytokines and MMP-9 and activates MAPK signaling pathways on the ocular surface. Invest Ophthalmol Vis Sci. 2004;45(12):4293–4301. doi: 10.1167/iovs.03-1145. [DOI] [PubMed] [Google Scholar]
- 13.Yoon KC, De Paiva CS, Qi H, et al. Expression of Th-1 chemokines and chemokine receptors on the ocular surface of C57BL/6 mice: effects of desiccating stress. Invest Ophthalmol Vis Sci. 2007;48(6):2561–2569. doi: 10.1167/iovs.07-0002. [DOI] [PubMed] [Google Scholar]
- 14.Ma P, Bian F, Wang Z, et al. Human corneal epithelium-derived thymic stromal lymphopoietin links the innate and adaptive immune responses via TLRs and Th2 cytokines. Invest Ophthalmol Vis Sci. 2009;50(6):2702–2709. doi: 10.1167/iovs.08-3074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chotikavanich S, de Paiva CS, Li Q, et al. Production and activity of matrix metalloproteinase-9 on the ocular surface increase in dysfunctional tear syndrome. Invest Ophthalmol Vis Sci. 2009;50(7):3203–3209. doi: 10.1167/iovs.08-2476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Schaumberg DA, Nichols JJ, Papas EB, Tong L, Uchino M, Nichols KK. The international workshop on meibomian gland dysfunction: report of the subcommittee on the epidemiology of, and associated risk factors for, MGD. Invest Ophthalmol Vis Sci. 2011;52(4):1994–2005. doi: 10.1167/iovs.10-6997e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Liu P, Fang AF, LaBadie RR, Crownover PH, Arguedas AG. Comparison of azithromycin pharmacokinetics following single oral doses of extended-release and immediate-release formulations in children with acute otitis media. Antimicrob Agents Chemother. 2011;55(11):5022–5026. doi: 10.1128/AAC.00692-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jorgensen DM. Single-dose extended-release oral azithromycin vs 3-day azithromycin for the treatment of group A beta-haemolytic streptococcal pharyngitis/tonsillitis in adults and adolescents: a double-blind, double-dummy study. Clin Microbiol Infect. 2009;15(12):1103–1110. doi: 10.1111/j.1469-0691.2009.02718.x. [DOI] [PubMed] [Google Scholar]
- 19.Cohen R. Defining the optimum treatment regimen for azithromycin in acute tonsillopharyngitis. Pediatr Infect Dis J. 2004;23(2 suppl):S129–S134. doi: 10.1097/01.inf.0000112527.33870.0d. [DOI] [PubMed] [Google Scholar]
- 20.Luxameechanporn T, Blair C, Kirtsreesakul V, Thompson K, Naclerio RM. The effect of treatment with moxifloxacin or azithromycin on acute bacterial rhinosinusitis in mice. Int J Infect Dis. 2006;10(5):401–406. doi: 10.1016/j.ijid.2005.07.007. [DOI] [PubMed] [Google Scholar]
- 21.Swainston Harrison T, Keam SJ. Azithromycin extended release: a review of its use in the treatment of acute bacterial sinusitis and community-acquired pneumonia in the US. Drugs. 2007;67(5):773–792. doi: 10.2165/00003495-200767050-00010. [DOI] [PubMed] [Google Scholar]
- 22.Kopjar B. Azithromycin is effective in patients with chronic bronchitis. J Antimicrob Chemother. 2002;50(3):433–434. doi: 10.1093/jac/dkf114. [DOI] [PubMed] [Google Scholar]
- 23.Wang PL. Roles of oral bacteria in cardiovascular diseases: from molecular mechanisms to clinical cases: treatment of periodontal disease regarded as biofilm infection: systemic administration of azithromycin. J Pharmacol Sci. 2010;113(2):126–133. doi: 10.1254/jphs.09r25fm. [DOI] [PubMed] [Google Scholar]
- 24.Mena LA, Mroczkowski TF, Nsuami M, Martin DH. A randomized comparison of azithromycin and doxycycline for the treatment of Mycoplasma genitalium-positive urethritis in men. Clin Infect Dis. 2009;48(12):1649–1654. doi: 10.1086/599033. [DOI] [PubMed] [Google Scholar]
- 25.Srivastava P, Bhengraj AR, Jha HC, et al. Differing effects of azithromycin and doxycycline on cytokines in cells from Chlamydia trachomatis-infected women. DNA Cell Biol. 2012;31(3):392–401. doi: 10.1089/dna.2011.1333. [DOI] [PubMed] [Google Scholar]
- 26.Mishra MK, Kotta K, Hali M, et al. PAMAM dendrimer-azithromycin conjugate nanodevices for the treatment of Chlamydia trachomatis infections. Nanomedicine. 2011;7(6):935–944. doi: 10.1016/j.nano.2011.04.008. [DOI] [PubMed] [Google Scholar]
- 27.Bremond-Gignac D, Mariani-Kurkdjian P, Beresniak A, et al. Efficacy and safety of azithromycin 1.5% eye drops for purulent bacterial conjunctivitis in pediatric patients. Pediatr Infect Dis J. 2010;29(3):222–226. doi: 10.1097/INF.0b013e3181b99fa2. [DOI] [PubMed] [Google Scholar]
- 28.Abelson MB, Heller W, Shapiro AM, Si E, Hsu P, Bowman LM, AzaSite Clinical Study Group Clinical cure of bacterial conjunctivitis with azithromycin 1%: vehicle-controlled, double-masked clinical trial. Am J Ophthalmol. 2008;145(6):959–965. doi: 10.1016/j.ajo.2008.01.019. [DOI] [PubMed] [Google Scholar]
- 29.Igami TZ, Holzchuh R, Osaki TH, Santo RM, Kara-Jose N, Hida RY. Oral azithromycin for treatment of posterior blepharitis. Cornea. 2011;30(10):1145–1149. doi: 10.1097/ICO.0b013e318207fc42. [DOI] [PubMed] [Google Scholar]
- 30.Veldman P, Colby K. Current evidence for topical azithromycin 1% ophthalmic solution in the treatment of blepharitis and blepharitis-associated ocular dryness. Int Ophthalmol Clin. 2011;51(4):43–52. doi: 10.1097/IIO.0b013e31822d6af1. [DOI] [PubMed] [Google Scholar]
- 31.Singh R, Joseph A, Umapathy T, Tint NL, Dua HS. Impression cytology of the ocular surface. Br J Ophthalmol. 2005;89(12):1655–1659. doi: 10.1136/bjo.2005.073916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.McKelvie P. Ocular surface impression cytology. Adv Anat Pathol. 2003;10(6):328–337. doi: 10.1097/00125480-200311000-00003. [DOI] [PubMed] [Google Scholar]
- 33.Nolan GR, Hirst LW, Bancroft BJ. The cytomorphology of ocular surface squamous neoplasia by using impression cytology. Cancer. 2001;93(1):60–67. [PubMed] [Google Scholar]
- 34.Thiel MA, Bossart W, Bernauer W. Improved impression cytology techniques for the immunopathological diagnosis of superficial viral infections. Br J Ophthalmol. 1997;81(11):984–988. doi: 10.1136/bjo.81.11.984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Pflugfelder SC, De Paiva CS, Villarreal AL, Stern ME. Effects of sequential artificial tear and cyclosporine emulsion therapy on conjunctival goblet cell density and transforming growth factor-beta2 production. Cornea. 2008;27(1):64–69. doi: 10.1097/ICO.0b013e318158f6dc. [DOI] [PubMed] [Google Scholar]
- 36.Sheppard JD, Jr, Singh R, McClellan AJ, et al. Long-term supplementation with n-6 and n-3 PUFAs improves moderate-to-severe keratoconjunctivitis sicca: a randomized double-blind clinical trial. Cornea. 2013;32(10):1297–1304. doi: 10.1097/ICO.0b013e318299549c. [DOI] [PubMed] [Google Scholar]
- 37.Mohammed I, Abedin A, Tsintzas K, et al. Increased expression of hepcidin and toll-like receptors 8 and 10 in viral keratitis. Cornea. 2011;30(8):899–904. doi: 10.1097/ICO.0b013e31820126e5. [DOI] [PubMed] [Google Scholar]
- 38.Assoian RK, Komoriya A, Meyers CA, Miller DM, Sporn MB. Transforming growth factor-beta in human platelets: identification of a major storage site, purification, and characterization. J Biol Chem. 1983;258(11):7155–7160. [PubMed] [Google Scholar]
- 39.Letterio JJ, Roberts AB. Regulation of immune responses by TGF-beta. Annu Rev Immunol. 1998;16:137–161. doi: 10.1146/annurev.immunol.16.1.137. [DOI] [PubMed] [Google Scholar]
- 40.Gupta A, Monroy D, Ji Z, Yoshino K, Huang A, Pflugfelder SC. Transforming growth factor beta-1 and beta-2 in human tear fluid. Curr Eye Res. 1996;15(6):605–614. doi: 10.3109/02713689609008900. [DOI] [PubMed] [Google Scholar]
- 41.Yoshino K, Garg R, Monroy D, Ji Z, Pflugfelder SC. Production and secretion of transforming growth factor beta (TGF-beta) by the human lacrimal gland. Curr Eye Res. 1996;15(6):615–624. doi: 10.3109/02713689609008901. [DOI] [PubMed] [Google Scholar]
- 42.Li MO, Wan YY, Sanjabi S, Robertson AK, Flavell RA. Transforming growth factor-beta regulation of immune responses. Annu Rev Immunol. 2006;24:99–146. doi: 10.1146/annurev.immunol.24.021605.090737. [DOI] [PubMed] [Google Scholar]
- 43.Bongiovanni L, Müller EJ, Della Salda L. Survivin in skin pathologies. Exp Dermatol. 2011;20(6):457–463. doi: 10.1111/j.1600-0625.2011.01273.x. [DOI] [PubMed] [Google Scholar]
- 44.Xiao YQ, Malcolm K, Worthen GS, et al. Cross-talk between ERK and p38 MAPK mediates selective suppression of pro-inflammatory cytokines by transforming growth factor-beta. J Biol Chem. 2002;277(17):14884–14893. doi: 10.1074/jbc.M111718200. [DOI] [PubMed] [Google Scholar]
- 45.Incorvaia C, Frati F, Puccinelli P, et al. Effects of sublingual immunotherapy on allergic inflammation. Inflamm Allergy Drug Targets. 2008;7(3):167–172. doi: 10.2174/187152808785748191. [DOI] [PubMed] [Google Scholar]
- 46.Wahl SM, Hunt DA, Wong HL, et al. Transforming growth factor-beta is a potent immunosuppressive agent that inhibits IL-1-dependent lymphocyte proliferation. J Immunol. 1988;140(9):3026–3032. [PubMed] [Google Scholar]
- 47.Tiemessen MM, Kunzmann S, Schmidt-Weber CB, et al. Transforming growth factor-beta inhibits human antigen-specific CD4+ T cell proliferation without modulating the cytokine response. Int Immunol. 2003;15(12):1495–1504. doi: 10.1093/intimm/dxg147. [DOI] [PubMed] [Google Scholar]


