Abstract
Background
Although in vitro studies and investigations in animal models and small clinical populations have suggested that ceramides may represent an intermediate link between over-nutrition and certain pathological mechanisms underlying cardiovascular disease (CVD), no prospective studies have investigated the association between plasma ceramides and risk of CVD.
Methods
The study population consisted of 980 participants from the PREDIMED trial, including 230 incident cases of CVD and 787 randomly selected participants at baseline (including 37 overlapping cases), followed for up to 7.4 years. Participants were randomized to a Mediterranean diet (MedDiet) supplemented with extra-virgin olive oil, a MedDiet supplemented with nuts, or a control diet. Plasma ceramide concentrations were measured on a liquid chromatography tandem mass spectrometry metabolomics platform. The primary outcome was a composite of non-fatal acute myocardial infarction, non-fatal stroke, or cardiovascular death. Hazard Ratios (HRs) were estimated with weighted Cox regression models, using Barlow weights to account for the case-cohort design.
Results
The multivariable HRs [95% confidence interval (CI)] comparing the extreme quartiles of plasma concentrations of C16:0, C22:0, C24:0 and C24:1 ceramides were 2.39 (1.49–3.83, P trend <0.001), 1.91 (1.21–3.01, P trend =0.003), 1.97 (1.21–3.01, P trend =0.004), and 1.73 (1.09–2.74, P trend =0.011), respectively. The ceramide score, calculated as a weighted sum of concentrations of four ceramides, was associated with a 2.18-fold higher risk of CVD across extreme quartiles (HR =2.18, 95% CI, 1.36–3.49, P trend <0.001). The association between baseline ceramide score and incident CVD varied significantly by treatment groups (P interaction =0.010). Participants with a higher ceramide score and assigned to either of the two active intervention arms of the trial showed similar CVD risk to those with a lower ceramide score, whereas participants with a higher ceramide score and assigned to the control arm presented significantly higher CVD risk. Changes in ceramide concentration were not significantly different between MedDiet and control groups during the first year of follow-up.
Conclusions
Our study documented a novel positive association between baseline plasma ceramide concentrations and incident CVD. In addition, a Mediterranean dietary intervention may mitigate potential deleterious effects of elevated plasma ceramide concentrations on CVD.
Clinical Trial Registration
Controlled-Trials.com number, ISRCTN35739639. http://www.isrctn.com/ISRCTN35739639
Keywords: Ceramide, Mediterranean diet, cardiovascular disease, coronary heart disease, stroke
Journal Subject Terms: Biomarkers, Lipids and Cholesterol, Cardiovascular Disease, Diet and Nutrition, Epidemiology, Primary Prevention
Introduction
Ceramides are members of the sphingolipid family and precursors of complex sphingolipids. Since the early 1990s, studies in cultured cells and animal models have shown that the aberrant accumulation of ceramides may lead to the activation of several signaling and putative targets that may impair normal cellular function, including insulin action 1. Meanwhile, this evidence has also linked excess de novo ceramide biosynthesis to cellular stress stimuli, especially to the exposure to saturated free fatty acids 1–3. Ceramide and its metabolites have thus been proposed as an intermediate link between over-nutrition and certain underlying abnormalities driving cardio-metabolic disease risk, including insulin resistance and low-grade inflammation 2–4. However, existing evidence relating ceramides to health outcomes comes mostly from in vitro experiments and animal studies, and it is mainly based on intermediate outcomes of cardiovascular risk. No studies have prospectively investigated the association between ceramides and the incidence of hard cardiovascular disease (CVD) endpoints, e.g., coronary heart disease (CHD) and stroke, in a primary prevention setting. Recently, Laaksonen et al. reported divergent associations of distinct plasma ceramides with CVD death and proposed the ratio of two ceramides as the strongest predictor of CVD death among patients with stable CHD using a case-control study design 5.
Modification of overall dietary patterns, compared to individual dietary factors, has long been proposed as a more effective and actionable target for CVD prevention and intervention 6. Recently, the first randomized controlled trial targeting overall dietary patterns for the primary prevention of CVD, the PREvencion con DIeta MEDiterranea (PREDIMED) trial 7, found that the Mediterranean diet (MedDiet) enriched with extra-virgin olive oil or nuts significantly reduced CVD events by approximately 30% compared to the control diet 8. Based on strong and consistent evidence on hard CVD endpoints from the PREDIMED trial 8 and prospective cohort studies 9–12, the 2015–2020 Dietary Guidelines for Americans 13 and the American Heart Association (AHA) 14 both recommend the MedDiet for CVD prevention. However, the biological mechanisms underlying cardio-protective effects of the MedDiet are not completely understood.
Advances in metabolite profiling technology (metabolomics), especially liquid chromatography tandem mass spectrometry (LC-MS) techniques, provide powerful tools to decipher the biological mechanisms of disease. Several structurally different ceramides are among the lipid metabolites profiled by current metabolomics platforms. Recent evidence from two small short-term intervention studies found that ceramide concentration could be transiently decreased by adopting a healthy dietary pattern 15 and changes in primary dietary sources of fat 16. The MedDiet might also exert its effect through decreasing ceramide concentration. However, it is still largely unknown whether ceramide concentration responds to long-term dietary intervention in a large population. In the present study based on the PREDIMED trial, we hypothesized that 1) plasma ceramide concentrations at baseline were associated with incident clinical events of CVD, 2) the association between baseline plasma ceramide concentrations and incident CVD was modified by the MedDiet interventions, and 3) participants in MedDiet intervention groups showed more favorable changes in plasma ceramide concentration compared to those in the control group during the first year of follow-up.
Methods
Study design and population
This study was nested in the PREDIMED randomized trial, but adopted a case-cohort design 17,18 by including all the available incident CVD cases diagnosed during follow-up and randomly sampling 10% of the enrolled participants at baseline in the PREDIMED trial. The case-cohort design preserves random intervention assignments and maintains the causal integrity of the randomized design of the trial. The PREDIMED trial (www.predimed.es) was conducted from 2003 through 2010 in 11 centers in Spain to assess the effects of the MedDiet on the primary prevention of CVD. At baseline, this trial enrolled 7,447 participants aged 55–80 years with high cardiovascular risk but initially free from diagnosed CVD, including CHD (angina, myocardial infarction, coronary revascularization procedures or existence of abnormal Q waves in the electrocardiogram), stroke (ischemic or hemorrhagic, including transient ischemic attacks), and symptomatic peripheral artery disease at baseline. Participants were randomly assigned to a MedDiet supplemented with extra-virgin olive oil (MedDiet+EVOO), a MedDiet supplemented with nuts (MedDiet+nuts), or a control diet consisting of advice to reduce the intake of all types of fat. During a mean follow-up time of 4.8 years (maximum follow-up: 7.4 years), 288 incident CVD events occurred. The protocol was approved by the Institutional Review Boards at all study locations and all participants provided written informed consent. Detailed information about the PREDIMED trial can be found elsewhere7,8. The study population consisted of 980 participants with available EDTA plasma samples, including 230 incident cases of CVD and 787 randomly selected participants at baseline (sub-cohort). The sub-cohort included 37 overlapping cases of CVD. We excluded 2 participants with undetectable plasma ceramide concentrations.
Study samples and metabolomics profiling
All analyses used fasting (fasting for ≥8 hours) plasma EDTA samples collected at baseline and year 1. All samples were processed at each recruiting center no later than 2 hours after collection and stored in −80°C freezers. Samples from cases and sub-cohort participants were randomly distributed before being shipped to the Broad Institute in Boston, MA, for metabolomics assays. LC-MS techniques were used to quantitatively profile ceramides in plasma samples. Plasma ceramide metabolites were measured concurrently with other lipid metabolites on the same platform and were identified on the basis of total acyl carbon content and degrees of saturation. Details of the LC-MS platform can be found elsewhere 19–25. Internal standard peak areas were monitored for quality control and to ensure system performance throughout analyses. Pooled plasma reference samples were also inserted every 20 samples as an additional quality control.
Ascertainment of CVD outcomes
The primary outcome was a composite of non-fatal acute myocardial infarction (AMI), non-fatal stroke, or cardiovascular death. Information on outcomes was collected from continuous contact with participants and primary health care physicians, annual follow-up visits, yearly ad-hoc reviews of medical charts, and annual consultation of the National Death Index. Study physicians who were blinded to the intervention assignment collected information on primary outcomes. The Clinical End-Point Committee, also blinded to the intervention assignment, adjudicated these events according to published criteria 26–31.
Measurements of covariates
Medical conditions, family history of disease, and risk factors were collected through a questionnaire during the first screening visit. At baseline and during annual visits, participants completed a 14-item questionnaire in a personal interview with a registered dietitian to assess their adherence to the MedDiet 32. At baseline and then annually, trained personnel measured participants’ body weight, height, waist circumference, and blood pressure according to the study protocol. Body mass index (BMI) was calculated as weight in kilograms divided by height in meters squared. Participants’ triglyceride (TG), total cholesterol, low-density lipoprotein-cholesterol (LDL), high-density lipoprotein-cholesterol (HDL), and fasting blood glucose levels were measured using fasting plasma samples at baseline.
Statistical analysis
We transformed ceramide concentrations to the natural logarithm scale to render the distributions approximately Gaussian as well as to stabilize the variance. We categorized all the participants into quartiles of the ceramide concentration based on the distribution in the sub-cohort. Person-years of follow-up were calculated from baseline to the earliest CVD event, loss to follow-up, or the end of follow-up.
Weighted proportional hazards Cox regression models stratified on intervention group assignment (MedDiet+EVOO, MedDiet+nuts, and control) were applied to estimate hazard ratios (HRs) and their 95% confidence intervals (CIs) of CVD comparing participants in each quartile to the lowest quartile. We used the weighting scheme suggested by Barlow et al 33,34 to account for the over-representation of cases. To quantify a linear trend, we assigned the median value of ceramide concentration within each quartile and modeled this variable continuously. We also calculated HRs and 95% CIs of CVD associated with a 1-standard deviation (SD) increment in the transformed concentrations of ceramides. All multivariable models were simultaneously adjusted for age, sex, BMI, family history of premature CHD, smoking status, histories of hypertension, dyslipidemia, and diabetes. We adjusted P-values of the multivariable-adjusted associations between1-SD increment in ceramide concentration and CVD risk using Benjamini–Hochberg procedure to account for the number of other plasma lipid metabolites (n=196) measured concurrently with ceramides on the same sub-platform. We calculated a baseline ceramide score as the weighted sum of concentrations of four different ceramides and modeled the ceramide score as a main exposure variable in the Cox model in the same fashion as individual ceramides. The weight for each ceramide was the regression coefficient for a 1-SD increment in the ceramide concentration estimated from the multivariable Cox regression model 19. We also performed secondary analyses on the associations of ceramide concentrations with AMI and stroke separately. Because Laaksonen et al. 5 suggested that ratios of ceramides could be stronger predictors of cardiovascular risk than individual ceramides, we also examined these ratios in relation to CVD in a secondary analysis. To test the robustness of our findings, in secondary analyses, the multivariable model was adjusted for the ratio of high-density lipoprotein cholesterol to low-density lipoprotein cholesterol (HDL/LDL) and for triglycerides (TG) as continuous variables, as well as adjusted for non-HDL cholesterol as a surrogate for atherogenic lipoproteins, instead of adjusting for dyslipidemia as a dichotomous variable. In addition, we performed several secondary analyses to further adjust for other metabolites putatively associated with CVD that were targeted on the current metabolomics platform. We first additionally included other sphingolipid metabolites, including sphingomyelins and sphingosine 35,36, in the multivariable-adjusted models. Second, we further adjusted for three branched-chain amino acids, i.e., valine, leucine and isoleucine, because they have been identified as strong predictors of insulin resistance and cardio-metabolic risk in our previous study 37 and recent publications 19,38–41. We further explored the association between the ceramide score and incident CVD risk in subgroups defined by several dichotomous risk factors at baseline, including sex (male, female), age (≤65 years, >65 years), BMI categories (≤30.0, >30.0 kg/m2), smoking status (never smoking, current/ever smoking), family history of premature CHD (yes, no), hypertension (yes, no), dyslipidemia (yes, no), and diabetes (yes, no), leisure time physical activity (≤median, >median metabolic equivalent tasks min/day), and alcohol consumption (0, 0.1–4.9, ≥5 g/day). The interactions between these stratification variables and the ceramide score were tested by adding multiplicative terms into the multivariable Cox models; the likelihood ratio test was used for testing statistical significance of the interaction term.
In a secondary analysis, we evaluated the added predictive ability of ceramides by comparing the c-statistics between one model including conventional risk factors of CVD, i.e., age, sex, systolic blood pressure, total and high-density lipoprotein cholesterol, current smoking and diabetes, and the other model including ceramide score in addition to the conventional risk factors, as well as estimating the net reclassification improvement (RNI) 42 for the 7-year risk of CVD.
To examine whether the association between plasma ceramide concentration and incident CVD varied by intervention group, we first categorized participants into joint subgroups defined by intervention group assignment and whether their ceramide score was above/equal to or below the median value in the sub-cohort. Second, we constructed adjusted cumulative incidence curves for the joint subgroups by using Langholz et al.’s method for case-cohort design 43 and included all the aforementioned covariates in the model. Third, we calculated the multivariable adjusted HRs for CVD from Cox regression models for each joint subgroup using participants with a low ceramide score (below the median) and in the intervention groups as a reference group. Lastly, we added a multiplicative term between intervention assignment and the ceramide score into the multivariable Cox models stratified on intervention assignment to test for interaction. To compare the temporal changes in ceramide concentrations between intervention and control groups, we employed linear mixed model to account for the within-individual repeated measurements and restricted this analysis to the random sub-cohort. We also explored the associations between baseline characteristics and 1-year changes in ceramide concentration in the sub-cohort using general linear models that simultaneously included intervention assignments and baseline characteristics. All analyses were performed using SAS software, version 9.4 (SAS Institute, North Carolina), at a two-tailed α of 0.05.
Results
Baseline characteristics
The median follow-up of the analytic population was 4.5 years. The baseline characteristics of the sub-cohort were very similar to that of the full-cohort in the PREDIMED trial, 8 except for a slightly higher proportion of participants with a family history of premature CHD (Table 1). At baseline, participants with a higher ceramide score had higher levels of total cholesterol, LDL, triglycerides, and diastolic blood pressure.
Table 1.
Baseline characteristics of study participants.
| Sub-cohort * (n=787) | Cases (n=230) | Quartiles of the ceramide score ‡ | P trend § | ||||
|---|---|---|---|---|---|---|---|
|
| |||||||
| Q1 (n=195) | Q2 (n=197) | Q3 (n=197) | Q4 (n=198) | ||||
| Intervention group, % | |||||||
| Control | 234 (29.7) | 83 (36.1) | 67 (34.4) | 63 (32.0) | 57 (28.9) | 47 (23.7) | 0.117 |
| Mediterranean diet + EVOO | 291 (37.0) | 82 (35.7) | 62 (31.8) | 71 (36.0) | 78 (39.6) | 80 (40.4) | |
| Mediterranean diet + Nuts | 262 (33.3) | 65 (28.3) | 66 (33.8) | 63 (32.0) | 62 (31.5) | 71 (35.9) | |
| Women, % | 450 (57.2) | 91 (39.6) | 93 (47.7) | 101 (51.3) | 132 (67.0) | 124 (62.6) | <.001 |
| Family history of premature CHD, % | 196 (24.9) | 44 (19.1) | 56 (28.7) | 56 (28.4) | 43 (21.8) | 41 (20.7) | 0.030 |
| Smoking, % | |||||||
| Never | 491 (62.4) | 104 (45.2) | 119 (61.0) | 107 (54.3) | 135 (68.5) | 130 (65.7) | 0.063 |
| Current | 96 (12.2) | 46 (20.0) | 22 (11.3) | 27 (13.7) | 24 (12.2) | 23 (11.6) | |
| Former | 200 (25.4) | 80 (34.8) | 54 (27.7) | 63 (32.0) | 38 (19.3) | 45 (22.7) | |
| Baseline prevalent disease, % | |||||||
| Hypertension | 659 (83.7) | 190 (82.6) | 161 (82.6) | 167 (84.8) | 159 (80.7) | 172 (86.9) | 0.423 |
| Dyslipidemia | 579 (73.6) | 134 (58.3) | 134 (68.7) | 145 (73.6) | 146 (74.1) | 154 (77.8) | 0.047 |
| Diabetes | 372 (47.3) | 149 (64.8) | 105 (53.8) | 81 (41.1) | 93 (47.2) | 93 (47.0) | 0.312 |
| Age (years) | 67.2±5.9 | 69.5±6.5 | 67.3±5.8 | 67.5±6.0 | 67.1±6.0 | 66.9±6.0 | 0.457 |
| Body mass index (kg/m2) | 29.8±3.6 | 29.6±3.7 | 30.1±3.8 | 29.6±3.4 | 29.5±3.7 | 29.8±3.6 | 0.559 |
| Adherence to Mediterranean diet † | 8.8±1.9 | 8.4±1.8 | 8.9±2.0 | 9.0±1.7 | 8.5±2.0 | 8.8±1.8 | 0.202 |
| Fasting glucose (mg/dL) | 121.9±41.0 | 136.2±48.9 | 123.2±40.4 | 115.7±31.4 | 121.7±43.8 | 126.7±46.3 | 0.375 |
| Total cholesterol (mg/dl) | 210.3±37.1 | 212.1±35.7 | 186.6±32.4 | 201.7±30.7 | 215.9±30.7 | 236.2±35.4 | <.001 |
| HDL cholesterol (mg/dL) | 54.0±15.4 | 51.9±16.4 | 53.5±15.9 | 53.7±16.5 | 53.0±11.8 | 55.5±16.7 | 0.114 |
| LDL cholesterol (mg/dL) | 130.8±33.4 | 131.4±33.4 | 112.8±29.9 | 124.9±28.5 | 135.8±29.4 | 149.5±34.4 | <.001 |
| Triglyceride (mg/dL) | 135.0±79.3 | 151.5±83.4 | 114.6±81.9 | 122.7±56.9 | 133±55.0 | 168.6±102.2 | <.001 |
| Systolic blood pressure (mmHg) | 147.3±20.3 | 154.9±23.1 | 146.2±18.3 | 147.8±20.8 | 147.2±19.8 | 148.1±22.1 | 0.424 |
| Diastolic blood pressure (mmHg) | 82.0±10.5 | 83.0±11.6 | 80.3±10.4 | 82.0±10.2 | 81.5±10.5 | 84.2±10.7 | <.001 |
Abbreviations: EVOO, extra-virgin olive oil; CHD, coronary heart disease; HDL, high-density lipoprotein; LDL, low-density lipoprotein;
The sub-cohort also included 37 cases.
Adherence to the Mediterranean diet was assessed by a 14-item dietary screener.
Quartiles were calculated based on the distribution of the ceramide score (weighted sum of four ceramides) in the sub-cohort.
To quantify a linear trend, we assigned the median within each quartile and modeled this scored trend variable continuously; Wald test was used for calculating P for trend. Logistic model was used for categorical variable and general linear model was used for continuous variable.
Plasma ceramide concentrations and CVD
The current metabolomics platform identified four different ceramides, including C16:0 (Number of carbon atoms: Number of double bonds), C22:0, C24:0, and C24:1 ceramides. Ceramide C24:0 had the highest relative concentration, while ceramide C16:0 had the lowest relative concentration (Supplemental table 1). We observed moderate and positive correlations in plasma concentrations among the four ceramides, ranging from 0.49 to 0.63, except for a high correlation of 0.90 between ceramides C22:0 and C24:0 (Supplemental table 2). All the ceramides were positively associated with incident CVD risk; the positive associations only differed in magnitude across different ceramide species and became slightly stronger after multivariable adjustment (Table 2). The multivariable HRs comparing the extreme quartiles of plasma concentrations of C16:0, C22:0, C24:0 and C24:1 ceramides were 2.39 (95% CI, 1.49–3.83, P for trend <0.001), 1.91 (95% CI, 1.21–3.01, P for trend =0.003), 1.97 (95% CI, 1.21–3.01, P for trend =0.004), and 1.73 (95% CI, 1.09–2.74, P for trend =0.011), respectively. The P-values for the associations between a 1-SD increment in ceramide concentration and CVD risk after multiple comparison adjustment were 0.007, 0.045, 0.050 and 0.050 for ceramides C16:0, C22:0, C24:0 and C24:1, respectively. The ceramide score was associated with a 2.18-fold higher risk of CVD across quartiles (HR =2.18, 95% CI, 1.36–3.49, P for trend <0.001). The HR associated with a 1-SD increment in the ceramide score was 1.41 (95% CI, 1.17, 1.68). The associations of ceramides and the ceramide score with CVD risk barely changed after further adjustment for sphingomyelins, sphingosine, and branched-chain amino acids and they were slightly attenuated in models adjusting for HDL/LDL ratio and TG and for non-HDL cholesterol as continuous variables (Supplemental Table 3). Secondary analyses on stroke and AMI yielded similar associations between plasma ceramides and the specific CVD outcomes, compared to the main analysis of the composite CVD outcome (Supplemental Table 4). The associations between ceramide score and CVD risk were generally consistent across different risk strata subgroups defined by gender, age, BMI, smoking status, family history of CHD, baseline histories of diabetes, dyslipidemia, hypertension, alcohol consumption and leisure time physical activity (Supplemental Table 5). The addition of the ceramide score into the model with conventional risk factors of CVD improved the c-statistics from 0.70 (95% CI, 0.66–0.73) to 0.71 (95% CI, 0.67–0.74); P =0.064 for the difference between the two c-statistics. Although this was only a marginal improvement, comparing the 7-year CVD risk predicted by the two models yielded an NRI of 0.22 (95% CI, 0.04–0.45, P =0.037). The HR associated with 1-SD increment in the ratio between ceramide C16:0 and C24:0 was 1.24 (95% CI, 1.05–1.46, P =0.010). However, other two ceramide ratios (C22:0/C24:0 and C24:1/C24:0) were not significantly associated with the incidence of CVD (Supplemental table 6).
Table 2.
Associations of baseline plasma ceramide concentrations and the ceramide score with cardiovascular disease.
| Quartiles of ceramide species concentration * | P trend | HR per 1 SD increment † | P value | ||||
|---|---|---|---|---|---|---|---|
|
| |||||||
| Q1 | Q2 | Q3 | Q4 | ||||
| Ceramide (16:0) | |||||||
| Cases | 38 | 57 | 57 | 78 | |||
| MV1 ‡ | Ref. | 1.60 (1.00, 2.54) | 1.67 (1.04, 2.67) | 2.20 (1.40, 3.46) | <.001 | 1.43 (1.20, 1.70) | <.001 |
| MV2 § | Ref. | 1.72 (1.05, 2.81) | 1.87 (1.14, 3.07) | 2.39 (1.49, 3.83) | <.001 | 1.42 (1.19, 1.69) | <.001 |
| Ceramide (22:0) | |||||||
| Cases | 53 | 43 | 62 | 72 | |||
| MV1 | Ref. | 0.95 (0.60, 1.50) | 1.29 (0.83, 1.99) | 1.89 (1.22, 2.93) | 0.002 | 1.33 (1.12, 1.58) | 0.001 |
| MV2 | Ref. | 0.88 (0.54, 1.43) | 1.28 (0.81, 2.02) | 1.91 (1.21, 3.01) | 0.003 | 1.32 (1.10, 1.57) | 0.002 |
| Ceramide (24:0) | |||||||
| Cases | 48 | 56 | 59 | 67 | |||
| MV1 | Ref. | 1.26 (0.80, 1.97) | 1.40 (0.89, 2.18) | 1.88 (1.20, 2.95) | 0.006 | 1.29 (1.09, 1.53) | 0.003 |
| MV2 | Ref. | 1.20 (0.75, 1.94) | 1.51 (0.94, 2.42) | 1.97 (1.21, 3.20) | 0.004 | 1.32 (1.10, 1.57) | 0.002 |
| Ceramide (24:1) | |||||||
| Cases | 44 | 50 | 59 | 77 | |||
| MV1 | Ref. | 1.07 (0.67, 1.70) | 1.31 (0.82, 2.08) | 1.53 (0.98, 2.37) | 0.037 | 1.22 (1.04, 1.43) | 0.015 |
| MV2 | Ref. | 1.16 (0.72, 1.89) | 1.44 (0.88, 2.36) | 1.73 (1.09, 2.74) | 0.011 | 1.27 (1.08, 1.49) | 0.004 |
| Ceramide score | |||||||
| Cases | 45 | 51 | 59 | 75 | |||
| MV1 | Ref. | 1.14 (0.72, 1.81) | 1.53 (0.97, 2.41) | 2.04 (1.30, 3.18) | <.001 | 1.40 (1.17, 1.66) | <.001 |
| MV2 | Ref. | 1.25 (0.77, 2.03) | 1.68 (1.05, 2.69) | 2.18 (1.36, 3.49) | <.001 | 1.41 (1.17, 1.68) | <.001 |
Abbreviations: MV, multivariable model
Quartiles were calculated based on the distribution of the ceramide concentrations in the sub-cohort.
A logarithmic transformation was applied to the raw value.
Model 1 stratified on intervention group and simultaneously adjusted for age (continuous) and sex (male, female).
Model 2 additionally adjusted for body mass index (kg/m2, continuous), family history of premature coronary heart disease (yes, no), smoking status (current, never, former), histories of hypertension, dyslipidemia, and diabetes (all yes, no).
Interactions between plasma ceramide concentrations and the MedDiet interventions
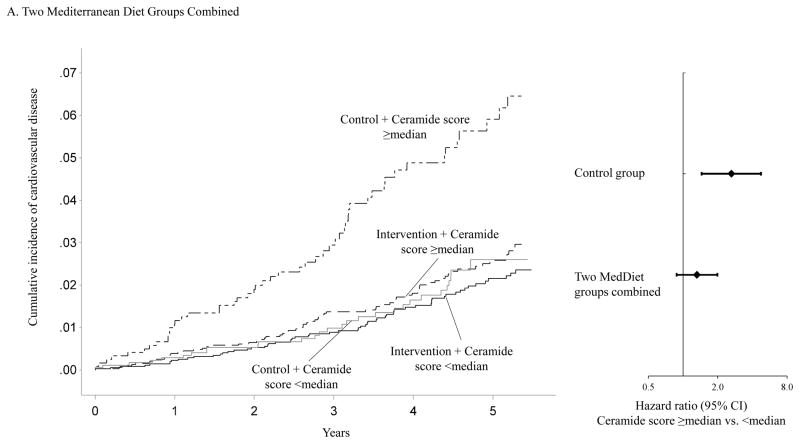
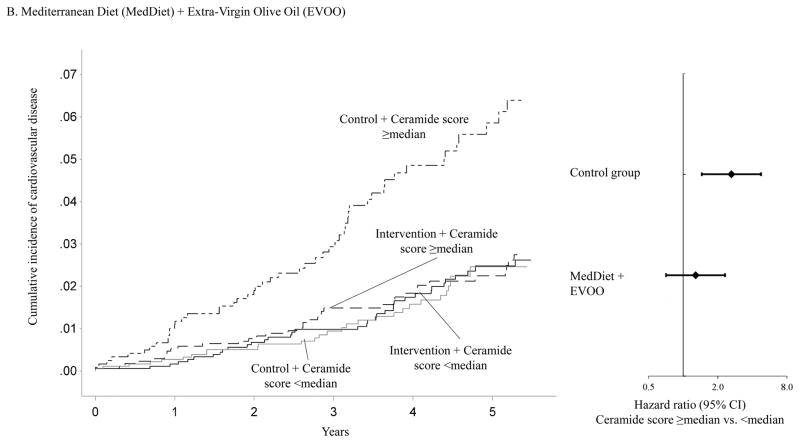
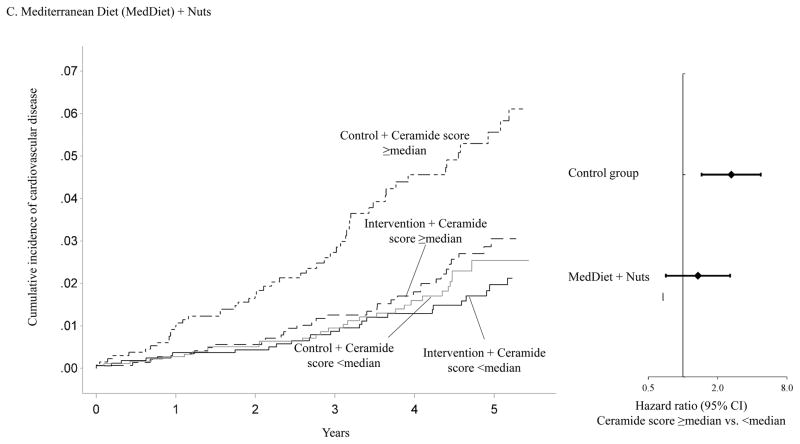
Figure 1 shows that the association between baseline ceramide score and the incidence of CVD clinical events varied significantly by intervention group assignment. Using the median ceramide score as the cut-point, participants with a higher ceramide score and randomized to either of the two active arms of the trial showed similar incidence of CVD to those with a lower ceramide score. However, the cumulative incidence curve for participants with a higher ceramide score and randomized to the control group diverged from those in other subgroups soon after the initiation of this trial. Compared to participants with a lower ceramide score and randomized to either of the two active intervention arms of the trial, the HRs were 2.76 (95% CI, 1.72–4.44) for participants with a higher ceramide score and randomized to the control group, 1.07 (95% CI, 0.64–1.78) for those with a lower ceramide score and randomized to the control group, and 1.26 (95% CI, 0.84–1.87) for those with a higher ceramide score and randomized to either of the two active intervention arms (P for interaction =0.010, Supplemental table 7). The association between the baseline ceramide score and incident CVD also varied significantly when the two intervention groups were examined separately (Figure 1). The interaction between the MedDiet+EVOO intervention and the ceramide score was more pronounced (P for interaction =0.009) than that between the MedDiet+nuts intervention and the ceramide score (P for interaction =0.053).
Figure 1.
Adjusted cumulative incidence curves in joint subgroups defined by ceramide score and intervention group assignment.
Panel A: Adjusted cumulative incidence curves in following joint subgroups: participants with a ceramide score ≥median and randomized to either of the two Mediterranean diet intervention arms, participants with a ceramide score <median and randomized to either of the two Mediterranean diet intervention arms, participants with a ceramide score ≥median and randomized to the control arm, and participants with a ceramide score <median and randomized to the control arm.
Panel B: Adjusted cumulative incidence curves in following joint subgroups: participants with a ceramide score ≥median and randomized to the intervention arm with Mediterranean diet + extra-virgin olive oil, participants with a ceramide score <median and randomized to the intervention arm with Mediterranean diet + extra-virgin olive oil, participants with a ceramide score ≥median and randomized to the control arm, and participants with a ceramide score <median and randomized to the control arm.
Panel C: Adjusted cumulative incidence curves in following joint subgroups: participants with a ceramide score ≥median and randomized to the intervention arm with Mediterranean diet + nuts, participants with a ceramide score <median and randomized to the intervention arm with Mediterranean diet + nuts, participants with a ceramide score ≥median and randomized to the control arm, and participants with a ceramide score <median and randomized to the control arm.
Cumulative incidence curves were adjusted for age (continuous) and sex (male, female), body mass index (kg/m2, continuous), family history of premature coronary heart disease (yes, no), smoking status (current, never, former), histories of hypertension, dyslipidemia, and diabetes (all yes, no)
Changes in ceramide concentration
One-year changes in ceramide concentration were not significantly different between participants in either of the two intervention groups and those in the control group (Supplemental table 8). We observed similar trends in ceramide concentrations when comparing each MedDiet group to the control group. In addition, none of the baseline characteristics were significantly associated with 1-year changes in ceramide concentration (Supplemental table 9).
Discussion
In this prospective case-cohort study within the PREDIMED trial, we observed that plasma ceramide concentrations were independently associated with elevated risk of the composite CVD outcome defined as non-fatal AMI, non-fatal stroke, or cardiovascular death, which was the primary end-point of the PREDIMED trial. The positive association was consistent for two major components of the composite CVD endpoint, namely AMI and stroke, and across different subgroups defined by baseline risk characteristics. In addition, the association between plasma ceramides and CVD risk varied significantly across intervention groups, suggesting that the MedDiet may have the potential to mitigate the detrimental effect associated with elevated baseline plasma ceramide concentrations on CVD risk.
This study, to our knowledge, is the first prospective study in a clinical trial setting to investigate the association between plasma ceramide concentrations and hard CVD endpoints. Previously, in vitro and in vivo animal studies have provided substantial evidence relating ceramide accumulation to multiple mechanisms underlying pathogenesis of CVD. However, human data are still sparse and limited by their small sample size and cross-sectional study design. The role of ceramides in the development of insulin resistance has been intensively studied in the past two decades. Earlier studies using cultured cells and animal models suggested that endogenous ceramides antagonized insulin-stimulated glucose uptake and anabolism 44,45 by blocking activation of Akt/PKB, a serine/threonine kinase that is obligate for insulin and growth-factor activation of anabolism and cell survival 46–51. Human studies reported increased ceramide concentration in obese insulin-resistant participants 52 and a negative correlation between muscle and plasma ceramide and insulin sensitivity 53–55. Interestingly, our data did not support cross-sectional associations between ceramide concentrations and several baseline characteristics related to insulin resistance, e.g., BMI, prevalence of diabetes, and fasting glucose. It is possible that these associations were diluted among this study population given that all our participants were selected because they were high-risk subjects and most of them might have already developed insulin resistance at the time of the enrollment. Beyond insulin resistance, limited human studies have observed positive correlations between plasma ceramide concentrations and inflammatory makers, e.g., interleukin-6 56 and TNF-α, 57 suggesting a relationship between excess ceramides and inflammation. Several lines of evidence in rodent models suggest that pharmacological inhibition of ceramide biosynthesis wards off atherogenesis.58,59 Ceramides and other sphingolipids may contribute to plaque erosion and therefore induce thrombosis 3. Of note, these studies on plaque formation 58,59 also found that inhibition of ceramide biosynthesis caused a reduction of circulating total cholesterol and LDL, which is consistent with our cross-sectional observations on ceramide concentrations and blood lipid profiles. Elevated ceramides were also implicated in cardiomyopathy. For example, Park, et al. observed that inhibition of a rate-limiting enzyme in ceramide biosynthesis (serine palmitoyltransferase [SPT]) improved systolic function and prolonged survival rates in a mouse model 60. Laaksonen et al. observed an elevated risk of CVD death in CHD patients with higher plasma concentration of three ceramide species (C16:0, C18:0 and C24:1) but a non-significantly lower risk of CVD death in those with higher concentration of ceramide C24:0 5. However, we found that all four ceramides were positively associated with the incidence of CVD. Further, the ratios of ceramides did not show stronger associations with CVD risk than individual ceramides or the summary ceramide score. The evidence regarding whether distinct ceramide metabolites were divergently associated with insulin resistance was also inconsistent 61–63. Further studies are warranted to investigate the potential different biological effects of ceramides with different acyl-chain length.
We observed that the detrimental effect of higher ceramide concentrations on CVD risk was modified by the MedDiet intervention. The potential mechanisms for the MedDiet’s modulatory effects on the ceramide pathway are two-fold. First, consumption of key components of the MedDiet may directly influence ceramide biosynthesis. Studies using cultured myotubes and animal models found that exposure to saturated free fatty acids (FFAs), especially long-chain saturated FFAs, promoted ceramide formation 64,65, while unsaturated FFAs prevented the excess ceramide accumulation stimulated by saturated FFAs 66, and therefore postulated the rate-limiting SPT was specific to the composition of circulating FFAs 3. The PREDIMED trial was effective in modifying intervention groups’ dietary patterns, 8 which were characterized by a high intake of virgin olive oil, fruit, nuts, vegetables, and cereals; a moderate intake of fish and poultry; a low intake of dairy products, red meat, processed meats, and sweets; and wine in moderation, consumed with meals 67. In conjunction with two supplemental foods, extra-virgin olive oil and nuts, the interventions might have changed circulating FFA composition through modifying dietary fat intake pattern, i.e., decreasing saturated fat intake and increasing monounsaturated and polyunsaturated fat intakes, and modulating de novo lipogenesis upon improvement in dietary carbohydrate quality. It is worth noting that our analysis did not find that the MedDiet was associated with favorable changes in ceramide concentrations during the first year of follow-up. However, we cannot rule out that MedDiet may directly mitigate aberrant ceramide accumulation in longer follow-up. Secondly, the MedDiet intervention could suppress deleterious effects following excess ceramide accumulation. Previous studies have suggested that the benefits of the Mediterranean dietary pattern on CVD could be mediated through several mechanisms, including the reduction of low-grade inflammation 68–72, enhanced endothelial function 70,73,74, lower oxidative stress 75,76, and lower levels of oxidized low-density lipoprotein (LDL) 77 and atherogenic lipoproteins 78.
Our results should be interpreted in the context of several limitations. First, participants of this project were mostly European Caucasians, which might limit the generalizability of our findings to other populations. Secondly, participants were recruited based on their high CVD risk. Therefore, our findings might not be applicable in populations with low CVD risk. Third, after adjusting for multiple comparisons, several of the p values were of borderline statistical significance. However, the consistency of these results across the plasma ceramides supports the robustness of these observations. Finally, even though we carefully adjusted for many potential confounders, residual confounding cannot be ruled out.
Our study possesses several major strengths. First, this study was built on a large, successful randomized controlled trial of hard clinical CVD endpoints, which provided a unique and powerful setting to address our research questions, because of its well-characterized study population, high compliance to the interventions, and low rates of drop-out. Secondly, the case-cohort design preserved the randomized design of this intervention trial and maintained the causal integrity of a randomized exposure status.
In summary, our study documented for the first time a strong positive association between plasma ceramide concentrations and incident CVD risk by using a prospective design nested in a well-known randomized trial. In addition, the traditional MedDiet intervention showed the potential to mitigate deleterious effects on CVD risk related to elevated plasma ceramide concentrations. Further studies are warranted to replicate these results in other populations and investigate potential mechanisms.
Supplementary Material
Clinical Perspective.
What’s new?
Our study documented for the first time an association between baseline plasma ceramide concentration and a composite cardiovascular disease outcome defined as non-fatal acute myocardial infarction, non-fatal stroke, or cardiovascular death by using a prospective design nested in a well-known primary prevention trial, the PREDIMED trial.
The traditional Mediterranean diet enriched with extra-virgin olive oil or nuts showed the potential to mitigate the deleterious effects of elevated plasma ceramide concentration on cardiovascular disease risk.
What are the clinical implications?
Our findings shed light on the biological mechanisms underlying cardio-protective effects of the Mediterranean diet, further strengthening the evidence base of recommending the Mediterranean diet for cardiovascular disease prevention.
The present results suggested that plasma ceramides measured by the liquid chromatography tandem mass spectrometry techniques had the potential to serve as markers of future cardiovascular disease risk in clinical practice.
Acknowledgments
We are very grateful to all the participants for their enthusiastic collaboration, the PREDIMED personnel for their excellent assistance, and the personnel of all affiliated primary care centers.
Sources of Funding
This study was supported by research grant R01 HL118264 from the National Institutes of Health. The Prevención con Dieta Mediterránea (PREDIMED) trial was supported by the official funding agency for biomedical research of the Spanish government, the Instituto de Salud Carlos III, through grants provided to research networks specifically developed for the trial [grant RTIC G03/140 (to Ramón Estruch); grant RTIC RD 06/0045 (to Miguel A. Martínez-González)] and through the Centro de Investigación Biomédica en Red de Fisiopatología de la Obesidad y Nutrición and by grants from Centro Nacional de Investigaciones Cardiovasculares (grant CNIC 06/2007), the Fondo de Investigación Sanitaria–Fondo Europeo de Desarrollo Regional (grants PI04–2239, PI 05/2584, CP06/00100, PI07/0240, PI07/1138, PI07/0954, PI 07/0473, PI10/01407, PI10/02658, PI11/01647, P11/02505, and PI13/00462), the Ministerio de Ciencia e Innovación (grants AGL-2009–13906-C02 and AGL2010–22319-C03), the Fundación Mapfre 2010, Consejería de Salud de la Junta de Andalucía (grant PI0105/2007), the Public Health Division of the Department of Health of the Autonomous Government of Catalonia, Generalitat Valenciana (grants ACOMP06109, GVA-COMP2010–181, GVACOMP2011–151, CS2010-AP-111, and CS2011-AP-042), and the Regional Government of Navarra (grant P27/2011). Dr. Dong D. Wang was supported by a postdoctoral fellowship granted by the American Heart Association (16POST31100031).
Footnotes
Conflict of Interest Disclosures
None
References
- 1.Chavez Jose A, Summers Scott A. A Ceramide-Centric View of Insulin Resistance. Cell Metab. 2012;15:585–594. doi: 10.1016/j.cmet.2012.04.002. [DOI] [PubMed] [Google Scholar]
- 2.Summers SA. Ceramides in insulin resistance and lipotoxicity. Prog Lipid Res. 2006;45:42–72. doi: 10.1016/j.plipres.2005.11.002. [DOI] [PubMed] [Google Scholar]
- 3.Holland WL, Summers SA. Sphingolipids, insulin resistance, and metabolic disease: new insights from in vivo manipulation of sphingolipid metabolism. Endocr Rev. 2008;29:381–402. doi: 10.1210/er.2007-0025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Summers Scott A. The ART of Lowering Ceramides. Cell Metab. 2015;22:195–196. doi: 10.1016/j.cmet.2015.07.019. [DOI] [PubMed] [Google Scholar]
- 5.Laaksonen R, Ekroos K, Sysi-Aho M, Hilvo M, Vihervaara T, Kauhanen D, Suoniemi M, Hurme R, Marz W, Scharnagl H, Stojakovic T, Vlachopoulou E, Lokki ML, Nieminen MS, Klingenberg R, Matter CM, Hornemann T, Juni P, Rodondi N, Raber L, Windecker S, Gencer B, Pedersen ER, Tell GS, Nygard O, Mach F, Sinisalo J, Luscher TF. Plasma ceramides predict cardiovascular death in patients with stable coronary artery disease and acute coronary syndromes beyond LDL-cholesterol. Eur Heart J. 2016;37:1967–1976. doi: 10.1093/eurheartj/ehw148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hu FB. Dietary pattern analysis: a new direction in nutritional epidemiology. Curr Opin Lipidol. 2002;13:3–9. doi: 10.1097/00041433-200202000-00002. [DOI] [PubMed] [Google Scholar]
- 7.Martínez-González MÁ, Corella D, Salas-Salvadó J, Ros E, Covas MI, Fiol M, Wärnberg J, Arós F, Ruíz-Gutiérrez V, Lamuela-Raventós RM. Cohort profile: design and methods of the PREDIMED study. Int J Epidemiol. 2012;41:377–385. doi: 10.1093/ije/dyq250. [DOI] [PubMed] [Google Scholar]
- 8.Estruch R, Ros E, Salas-Salvadó J, Covas M-I, Corella D, Arós F, Gómez-Gracia E, Ruiz-Gutiérrez V, Fiol M, Lapetra J. Primary prevention of cardiovascular disease with a Mediterranean diet. N Engl J Med. 2013;368:1279–1290. doi: 10.1056/NEJMc1806491. [DOI] [PubMed] [Google Scholar]
- 9.Martínez-González MA, García-López M, Bes-Rastrollo M, Toledo E, Martínez-Lapiscina EH, Delgado-Rodriguez M, Vazquez Z, Benito S, Beunza JJ. Mediterranean diet and the incidence of cardiovascular disease: a Spanish cohort. Nutr Metab Cardiovasc Dis. 2011;21:237–244. doi: 10.1016/j.numecd.2009.10.005. [DOI] [PubMed] [Google Scholar]
- 10.Knoops KT, de Groot LC, Kromhout D, Perrin A-E, Moreiras-Varela O, Menotti A, Van Staveren WA. Mediterranean diet, lifestyle factors, and 10-year mortality in elderly European men and women: the HALE project. JAMA. 2004;292:1433–1439. doi: 10.1001/jama.292.12.1433. [DOI] [PubMed] [Google Scholar]
- 11.Fung TT, Rexrode KM, Mantzoros CS, Manson JE, Willett WC, Hu FB. Mediterranean diet and incidence of and mortality from coronary heart disease and stroke in women. Circulation. 2009;119:1093–1100. doi: 10.1161/CIRCULATIONAHA.108.816736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sofi F, Cesari F, Abbate R, Gensini GF, Casini A. Adherence to Mediterranean diet and health status: meta-analysis. BMJ. 2008;337:a1344. doi: 10.1136/bmj.a1344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.U.S. Department of Health and Human Services and U.S. Department of Agriculture. 2015 – 2020 Dietary Guidelines for Americans. (8) 2015 Available at http://health.gov/dietaryguidelines/2015/guidelines.
- 14.Eckel RH, Jakicic JM, Ard JD, Hubbard VS, de Jesus JM, Lee I-M, Lichtenstein AH, Loria CM, Millen BE, Miller NH, Nonas CA, Sacks FM, Smith SC, Svetkey LP, Wadden TW, Yanovski SZ. 2013 AHA/ACC Guideline on Lifestyle Management to Reduce Cardiovascular Risk: A Report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. Circulation. 2014;129:S76–S99. doi: 10.1161/01.cir.0000437740.48606.d1. [DOI] [PubMed] [Google Scholar]
- 15.Lankinen M, Schwab U, Kolehmainen M, Paananen J, Nygren H, Seppanen-Laakso T, Poutanen K, Hyotylainen T, Riserus U, Savolainen MJ, Hukkanen J, Brader L, Marklund M, Rosqvist F, Hermansen K, Cloetens L, Onning G, Thorsdottir I, Gunnarsdottir I, Akesson B, Dragsted LO, Uusitupa M, Oresic M. A Healthy Nordic Diet Alters the Plasma Lipidomic Profile in Adults with Features of Metabolic Syndrome in a Multicenter Randomized Dietary Intervention. J Nutr. 2016;146:662–672. doi: 10.3945/jn.115.220459. [DOI] [PubMed] [Google Scholar]
- 16.Meikle PJ, Barlow CK, Mellett NA, Mundra PA, Bonham MP, Larsen A, Cameron-Smith D, Sinclair A, Nestel PJ, Wong G. Postprandial Plasma Phospholipids in Men Are Influenced by the Source of Dietary Fat. J Nutr. 2015;145:2012–2018. doi: 10.3945/jn.115.210104. [DOI] [PubMed] [Google Scholar]
- 17.Langholz B, Thomas DC. Nested case-control and case-cohort methods of sampling from a cohort: a critical comparison. Am J Epidemiol. 1990;131:169–176. doi: 10.1093/oxfordjournals.aje.a115471. [DOI] [PubMed] [Google Scholar]
- 18.Prentice RL. A case-cohort design for epidemiologic cohort studies and disease prevention trials. Biometrika. 1986;73:1–11. [Google Scholar]
- 19.Wang TJ, Larson MG, Vasan RS, Cheng S, Rhee EP, McCabe E, Lewis GD, Fox CS, Jacques PF, Fernandez C, O’Donnell CJ, Carr SA, Mootha VK, Florez JC, Souza A, Melander O, Clish CB, Gerszten RE. Metabolite profiles and the risk of developing diabetes. Nat Med. 2011;17:448–453. doi: 10.1038/nm.2307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rhee EP, Cheng S, Larson MG, Walford GA, Lewis GD, McCabe E, Yang E, Farrell L, Fox CS, O’Donnell CJ, Carr SA, Vasan RS, Florez JC, Clish CB, Wang TJ, Gerszten RE. Lipid profiling identifies a triacylglycerol signature of insulin resistance and improves diabetes prediction in humans. J Clin Invest. 2011;121:1402–1411. doi: 10.1172/JCI44442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lewis GD, Farrell L, Wood MJ, Martinovic M, Arany Z, Rowe GC, Souza A, Cheng S, McCabe EL, Yang E, Shi X, Deo R, Roth FP, Asnani A, Rhee EP, Systrom DM, Semigran MJ, Vasan RS, Carr SA, Wang TJ, Sabatine MS, Clish CB, Gerszten RE. Metabolic signatures of exercise in human plasma. Sci Transl Med. 2010;2:33ra37. doi: 10.1126/scitranslmed.3001006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gohil VM, Sheth SA, Nilsson R, Wojtovich AP, Lee JH, Perocchi F, Chen W, Clish CB, Ayata C, Brookes PS, Mootha VK. Nutrient-sensitized screening for drugs that shift energy metabolism from mitochondrial respiration to glycolysis. Nat Biotechnol. 2010;28:249–255. doi: 10.1038/nbt.1606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rhee EP, Souza A, Farrell L, Pollak MR, Lewis GD, Steele DJ, Thadhani R, Clish CB, Greka A, Gerszten RE. Metabolite profiling identifies markers of uremia. J Am Soc Nephrol. 2010;21:1041–1051. doi: 10.1681/ASN.2009111132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Shaham O, Slate NG, Goldberger O, Xu Q, Ramanathan A, Souza AL, Clish CB, Sims KB, Mootha VK. A plasma signature of human mitochondrial disease revealed through metabolic profiling of spent media from cultured muscle cells. Proc Natl Acad Sci U S A. 2010;107:1571–1575. doi: 10.1073/pnas.0906039107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zhong L, D’Urso A, Toiber D, Sebastian C, Henry RE, Vadysirisack DD, Guimaraes A, Marinelli B, Wikstrom JD, Nir T, Clish CB, Vaitheesvaran B, Iliopoulos O, Kurland I, Dor Y, Weissleder R, Shirihai OS, Ellisen LW, Espinosa JM, Mostoslavsky R. The histone deacetylase Sirt6 regulates glucose homeostasis via Hif1alpha. Cell. 2010;140:280–293. doi: 10.1016/j.cell.2009.12.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Alpert JS, Thygesen K, Antman E, Bassand JP. Myocardial infarction redefined--a consensus document of The Joint European Society of Cardiology/American College of Cardiology Committee for the redefinition of myocardial infarction. J Am Coll Cardiol. 2000;36:959–969. doi: 10.1016/s0735-1097(00)00804-4. [DOI] [PubMed] [Google Scholar]
- 27.Guidelines for management of ischaemic stroke and transient ischaemic attack 2008. Cerebrovasc Dis. 2008;25:457–507. doi: 10.1159/000131083. [DOI] [PubMed] [Google Scholar]
- 28.Morgenstern LB, Hemphill JC, 3rd, Anderson C, Becker K, Broderick JP, Connolly ES, Jr, Greenberg SM, Huang JN, MacDonald RL, Messe SR, Mitchell PH, Selim M, Tamargo RJ. Guidelines for the management of spontaneous intracerebral hemorrhage: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2010;41:2108–2129. doi: 10.1161/STR.0b013e3181ec611b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kidwell CS, Chalela JA, Saver JL, Starkman S, Hill MD, Demchuk AM, Butman JA, Patronas N, Alger JR, Latour LL, Luby ML, Baird AE, Leary MC, Tremwel M, Ovbiagele B, Fredieu A, Suzuki S, Villablanca JP, Davis S, Dunn B, Todd JW, Ezzeddine MA, Haymore J, Lynch JK, Davis L, Warach S. Comparison of MRI and CT for detection of acute intracerebral hemorrhage. JAMA. 2004;292:1823–1830. doi: 10.1001/jama.292.15.1823. [DOI] [PubMed] [Google Scholar]
- 30.Zipes DP, Camm AJ, Borggrefe M, Buxton AE, Chaitman B, Fromer M, Gregoratos G, Klein G, Moss AJ, Myerburg RJ, Priori SG, Quinones MA, Roden DM, Silka MJ, Tracy C, Smith SC, Jr, Jacobs AK, Adams CD, Antman EM, Anderson JL, Hunt SA, Halperin JL, Nishimura R, Ornato JP, Page RL, Riegel B, Blanc JJ, Budaj A, Dean V, Deckers JW, Despres C, Dickstein K, Lekakis J, McGregor K, Metra M, Morais J, Osterspey A, Tamargo JL, Zamorano JL. ACC/AHA/ESC 2006 guidelines for management of patients with ventricular arrhythmias and the prevention of sudden cardiac death: a report of the American College of Cardiology/American Heart Association Task Force and the European Society of Cardiology Committee for Practice Guidelines (Writing Committee to Develop Guidelines for Management of Patients With Ventricular Arrhythmias and the Prevention of Sudden Cardiac Death) J Am Coll Cardiol. 2006;48:e247–346. doi: 10.1016/j.jacc.2006.07.010. [DOI] [PubMed] [Google Scholar]
- 31.Maradit-Kremers H, Nicola PJ, Crowson CS, Ballman KV, Gabriel SE. Cardiovascular death in rheumatoid arthritis: a population-based study. Arthritis Rheum. 2005;52:722–732. doi: 10.1002/art.20878. [DOI] [PubMed] [Google Scholar]
- 32.Schröder H, Fitó M, Estruch R, Martínez-González MA, Corella D, Salas-Salvadó J, Lamuela-Raventós R, Ros E, Salaverría I, Fiol M. A short screener is valid for assessing Mediterranean diet adherence among older Spanish men and women. J Nutr. 2011;141:1140–1145. doi: 10.3945/jn.110.135566. [DOI] [PubMed] [Google Scholar]
- 33.Barlow WE. Robust variance estimation for the case-cohort design. Biometrics. 1994;50:1064–1072. [PubMed] [Google Scholar]
- 34.Barlow WE, Ichikawa L, Rosner D, Izumi S. Analysis of case-cohort designs. J Clin Epidemiol. 1999;52:1165–1172. doi: 10.1016/s0895-4356(99)00102-x. [DOI] [PubMed] [Google Scholar]
- 35.Ganna A, Salihovic S, Sundström J, Broeckling CD, Hedman ÅK, Magnusson PKE, Pedersen NL, Larsson A, Siegbahn A, Zilmer M, Prenni J, Ärnlöv J, Lind L, Fall T, Ingelsson E. Large-scale Metabolomic Profiling Identifies Novel Biomarkers for Incident Coronary Heart Disease. PLoS Genet. 2014;10:e1004801. doi: 10.1371/journal.pgen.1004801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Stegemann C, Pechlaner R, Willeit P, Langley SR, Mangino M, Mayr U, Menni C, Moayyeri A, Santer P, Rungger G, Spector TD, Willeit J, Kiechl S, Mayr M. Lipidomics profiling and risk of cardiovascular disease in the prospective population-based Bruneck study. Circulation. 2014;129:1821–1831. doi: 10.1161/CIRCULATIONAHA.113.002500. [DOI] [PubMed] [Google Scholar]
- 37.Ruiz-Canela M, Toledo E, Clish CB, Hruby A, Liang L, Salas-Salvadó J, Razquin C, Corella D, Estruch Rn, Ros E, Fitó M, Gómez-Gracia E, Arós F, Fiol M, Lapetra J, Serra-Majem L, Martínez-González MA, Hu FB. Plasma Branched-Chain Amino Acids and Incident Cardiovascular Disease in the PREDIMED Trial. Clin Chem. 2016;62:582–592. doi: 10.1373/clinchem.2015.251710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Rhee EP, Gerszten RE. Metabolomics and Cardiovascular Biomarker Discovery. Clin Chem. 2011;58:139–147. doi: 10.1373/clinchem.2011.169573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Shah SH, Kraus WE, Newgard CB. Metabolomic profiling for the identification of novel biomarkers and mechanisms related to common cardiovascular diseases: form and function. Circulation. 2012;126:1110–1120. doi: 10.1161/CIRCULATIONAHA.111.060368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Shah SH, Sun JL, Stevens RD, Bain JR, Muehlbauer MJ, Pieper KS, Haynes C, Hauser ER, Kraus WE, Granger CB, Newgard CB, Califf RM, Newby LK. Baseline metabolomic profiles predict cardiovascular events in patients at risk for coronary artery disease. Am Heart J. 2012;163:844–850. e841. doi: 10.1016/j.ahj.2012.02.005. [DOI] [PubMed] [Google Scholar]
- 41.Shah SH, Bain JR, Muehlbauer MJ, Stevens RD, Crosslin DR, Haynes C, Dungan J, Newby LK, Hauser ER, Ginsburg GS, Newgard CB, Kraus WE. Association of a peripheral blood metabolic profile with coronary artery disease and risk of subsequent cardiovascular events. Circ Cardiovasc Genet. 2010;3:207–214. doi: 10.1161/CIRCGENETICS.109.852814. [DOI] [PubMed] [Google Scholar]
- 42.Pencina MJ, D’Agostino RB, Vasan RS. Evaluating the added predictive ability of a new marker: from area under the ROC curve to reclassification and beyond. Stat Med. 2008;27:157–172. doi: 10.1002/sim.2929. [DOI] [PubMed] [Google Scholar]
- 43.Langholz B, Jiao J. Computational methods for case-cohort studies. Comput Stat Data Anal. 2007;51:3737–3748. [Google Scholar]
- 44.Hajduch E, Balendran A, Batty I, Litherland G, Blair A, Downes C, Hundal H. Ceramide impairs the insulin-dependent membrane recruitment of protein kinase B leading to a loss in downstream signalling in L6 skeletal muscle cells. Diabetologia. 2001;44:173–183. doi: 10.1007/s001250051596. [DOI] [PubMed] [Google Scholar]
- 45.Summers SA, Garza LA, Zhou H, Birnbaum MJ. Regulation of insulin-stimulated glucose transporter GLUT4 translocation and Akt kinase activity by ceramide. Mol Cell Biol. 1998;18:5457–5464. doi: 10.1128/mcb.18.9.5457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.STRATFORD S, Dewald D, Summers S. Ceramide dissociates 3′-phosphoinositide production from pleckstrin homology domain translocation. Biochem J. 2001;354:359–368. doi: 10.1042/0264-6021:3540359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Chavez JA, Knotts TA, Wang L-P, Li G, Dobrowsky RT, Florant GL, Summers SA. A role for ceramide, but not diacylglycerol, in the antagonism of insulin signal transduction by saturated fatty acids. J Biol Chem. 2003;278:10297–10303. doi: 10.1074/jbc.M212307200. [DOI] [PubMed] [Google Scholar]
- 48.Powell D, Turban S, Gray A, Hajduch E, Hundal H. Intracellular ceramide synthesis and protein kinase Czeta activation play an essential role in palmitate-induced insulin resistance in rat L6 skeletal muscle cells. Biochem J. 2004;382:619–629. doi: 10.1042/BJ20040139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Powell DJ, Hajduch E, Kular G, Hundal HS. Ceramide disables 3-phosphoinositide binding to the pleckstrin homology domain of protein kinase B (PKB)/Akt by a PKCζ-dependent mechanism. Mol Cell Biol. 2003;23:7794–7808. doi: 10.1128/MCB.23.21.7794-7808.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Yang G, Badeanlou L, Bielawski J, Roberts AJ, Hannun YA, Samad F. Central role of ceramide biosynthesis in body weight regulation, energy metabolism, and the metabolic syndrome. Am J Physiol Endocrinol Metab. 2009;297:E211–E224. doi: 10.1152/ajpendo.91014.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Holland WL, Brozinick JT, Wang L-P, Hawkins ED, Sargent KM, Liu Y, Narra K, Hoehn KL, Knotts TA, Siesky A. Inhibition of ceramide synthesis ameliorates glucocorticoid-, saturated-fat-, and obesity-induced insulin resistance. Cell Metabolism. 2007;5:167–179. doi: 10.1016/j.cmet.2007.01.002. [DOI] [PubMed] [Google Scholar]
- 52.Adams JM, Pratipanawatr T, Berria R, Wang E, DeFronzo RA, Sullards MC, Mandarino LJ. Ceramide content is increased in skeletal muscle from obese insulin-resistant humans. Diabetes. 2004;53:25–31. doi: 10.2337/diabetes.53.1.25. [DOI] [PubMed] [Google Scholar]
- 53.Amati F, Dubé JJ, Alvarez-Carnero E, Edreira MM, Chomentowski P, Coen PM, Switzer GE, Bickel PE, Stefanovic-Racic M, Toledo FG. Skeletal Muscle Triglycerides, Diacylglycerols, and Ceramides in Insulin Resistance Another Paradox in Endurance-Trained Athletes? Diabetes. 2011;60:2588–2597. doi: 10.2337/db10-1221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Dubé JJ, Amati F, Stefanovic-Racic M, Toledo FG, Sauers SE, Goodpaster BH. Exercise-induced alterations in intramyocellular lipids and insulin resistance: the athlete’s paradox revisited. Am J Physiol Endocrinol Metab. 2008;294:E882–E888. doi: 10.1152/ajpendo.00769.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Straczkowski M, Kowalska I, Nikolajuk A, Dzienis-Straczkowska S, Kinalska I, Baranowski M, Zendzian-Piotrowska M, Brzezinska Z, Gorski J. Relationship between insulin sensitivity and sphingomyelin signaling pathway in human skeletal muscle. Diabetes. 2004;53:1215–1221. doi: 10.2337/diabetes.53.5.1215. [DOI] [PubMed] [Google Scholar]
- 56.de Mello V, Lankinen M, Schwab U, Kolehmainen M, Lehto S, Seppänen-Laakso T, Orešič M, Pulkkinen L, Uusitupa M, Erkkilä A. Link between plasma ceramides, inflammation and insulin resistance: association with serum IL-6 concentration in patients with coronary heart disease. Diabetologia. 2009;52:2612–2615. doi: 10.1007/s00125-009-1482-9. [DOI] [PubMed] [Google Scholar]
- 57.Haus JM, Kashyap SR, Kasumov T, Zhang R, Kelly KR, DeFronzo RA, Kirwan JP. Plasma ceramides are elevated in obese subjects with type 2 diabetes and correlate with the severity of insulin resistance. Diabetes. 2009;58:337–343. doi: 10.2337/db08-1228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Park T-S, Panek RL, Mueller SB, Hanselman JC, Rosebury WS, Robertson AW, Kindt EK, Homan R, Karathanasis SK, Rekhter MD. Inhibition of sphingomyelin synthesis reduces atherogenesis in apolipoprotein E–knockout mice. Circulation. 2004;110:3465–3471. doi: 10.1161/01.CIR.0000148370.60535.22. [DOI] [PubMed] [Google Scholar]
- 59.Hojjati MR, Li Z, Zhou H, Tang S, Huan C, Ooi E, Lu S, Jiang X-C. Effect of myriocin on plasma sphingolipid metabolism and atherosclerosis in apoE-deficient mice. J Biol Chem. 2005;280:10284–10289. doi: 10.1074/jbc.M412348200. [DOI] [PubMed] [Google Scholar]
- 60.Park T-S, Hu Y, Noh H-L, Drosatos K, Okajima K, Buchanan J, Tuinei J, Homma S, Jiang X-C, Abel ED. Ceramide is a cardiotoxin in lipotoxic cardiomyopathy. J Lipid Res. 2008;49:2101–2112. doi: 10.1194/jlr.M800147-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Haus JM, Kashyap SR, Kasumov T, Zhang R, Kelly KR, Defronzo RA, Kirwan JP. Plasma ceramides are elevated in obese subjects with type 2 diabetes and correlate with the severity of insulin resistance. Diabetes. 2009;58:337–343. doi: 10.2337/db08-1228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Raichur S, Wang ST, Chan PW, Li Y, Ching J, Chaurasia B, Dogra S, Ohman MK, Takeda K, Sugii S, Pewzner-Jung Y, Futerman AH, Summers SA. Cers2 haploinsufficiency inhibits beta-oxidation and confers susceptibility to diet-induced steatohepatitis and insulin resistance. Cell Metab. 2014;20:687–695. doi: 10.1016/j.cmet.2014.09.015. [DOI] [PubMed] [Google Scholar]
- 63.Turpin SM, Nicholls HT, Willmes DM, Mourier A, Brodesser S, Wunderlich CM, Mauer J, Xu E, Hammerschmidt P, Bronneke HS, Trifunovic A, LoSasso G, Wunderlich FT, Kornfeld JW, Bluher M, Kronke M, Bruning JC. Obesity-induced cers6-dependent c16:0 ceramide production promotes weight gain and glucose intolerance. Cell Metab. 2014;20:678–686. doi: 10.1016/j.cmet.2014.08.002. [DOI] [PubMed] [Google Scholar]
- 64.Chavez JA, Summers SA. Characterizing the effects of saturated fatty acids on insulin signaling and ceramide and diacylglycerol accumulation in 3T3-L1 adipocytes and C2C12 myotubes. Arch Biochem Biophys. 2003;419:101–109. doi: 10.1016/j.abb.2003.08.020. [DOI] [PubMed] [Google Scholar]
- 65.Schmitz-Peiffer C, Craig DL, Biden TJ. Ceramide generation is sufficient to account for the inhibition of the insulin-stimulated PKB pathway in C2C12 skeletal muscle cells pretreated with palmitate. J Biol Chem. 1999;274:24202–24210. doi: 10.1074/jbc.274.34.24202. [DOI] [PubMed] [Google Scholar]
- 66.Lu Z-H, Mu Y-M, Wang B-A, Li X-L, Lu J-M, Li J-Y, Pan C-Y, Yanase T, Nawata H. Saturated free fatty acids, palmitic acid and stearic acid, induce apoptosis by stimulation of ceramide generation in rat testicular Leydig cell. Biochem Biophys Res Commun. 2003;303:1002–1007. doi: 10.1016/s0006-291x(03)00449-2. [DOI] [PubMed] [Google Scholar]
- 67.Willett WC, Sacks F, Trichopoulou A, Drescher G, Ferro-Luzzi A, Helsing E, Trichopoulos D. Mediterranean diet pyramid: a cultural model for healthy eating. Am J Clin Nutr. 1995;61:1402S–1406S. doi: 10.1093/ajcn/61.6.1402S. [DOI] [PubMed] [Google Scholar]
- 68.Chrysohoou C, Panagiotakos DB, Pitsavos C, Das UN, Stefanadis C. Adherence to the Mediterranean diet attenuates inflammation and coagulation process in healthy adults: The ATTICA Study. J Am Coll Cardiol. 2004;44:152–158. doi: 10.1016/j.jacc.2004.03.039. [DOI] [PubMed] [Google Scholar]
- 69.Urpi-Sarda M, Casas R, Chiva-Blanch G, Romero-Mamani ES, Valderas-Martinez P, Salas-Salvado J, Covas MI, Toledo E, Andres-Lacueva C, Llorach R, Garcia-Arellano A, Bullo M, Ruiz-Gutierrez V, Lamuela-Raventos RM, Estruch R. The mediterranean diet pattern and its main components are associated with lower plasma concentrations of tumor necrosis factor receptor 60 in patients at high risk for cardiovascular disease. J Nutr. 2012;142:1019–1025. doi: 10.3945/jn.111.148726. [DOI] [PubMed] [Google Scholar]
- 70.Esposito K, Marfella R, Ciotola M, Di Palo C, Giugliano F, Giugliano G, D’Armiento M, D’Andrea F, Giugliano D. Effect of a mediterranean-style diet on endothelial dysfunction and markers of vascular inflammation in the metabolic syndrome: a randomized trial. JAMA. 2004;292:1440–1446. doi: 10.1001/jama.292.12.1440. [DOI] [PubMed] [Google Scholar]
- 71.Mena MP, Sacanella E, Vazquez-Agell M, Morales M, Fito M, Escoda R, Serrano-Martinez M, Salas-Salvado J, Benages N, Casas R, Lamuela-Raventos RM, Masanes F, Ros E, Estruch R. Inhibition of circulating immune cell activation: a molecular antiinflammatory effect of the Mediterranean diet. Am J Clin Nutr. 2009;89:248–256. doi: 10.3945/ajcn.2008.26094. [DOI] [PubMed] [Google Scholar]
- 72.Kastorini CM, Milionis HJ, Esposito K, Giugliano D, Goudevenos JA, Panagiotakos DB. The effect of Mediterranean diet on metabolic syndrome and its components: a meta-analysis of 50 studies and 534,906 individuals. J Am Coll Cardiol. 2011;57:1299–1313. doi: 10.1016/j.jacc.2010.09.073. [DOI] [PubMed] [Google Scholar]
- 73.Ruano J, Lopez-Miranda J, Fuentes F, Moreno JA, Bellido C, Perez-Martinez P, Lozano A, Gomez P, Jimenez Y, Perez Jimenez F. Phenolic content of virgin olive oil improves ischemic reactive hyperemia in hypercholesterolemic patients. J Am Coll Cardiol. 2005;46:1864–1868. doi: 10.1016/j.jacc.2005.06.078. [DOI] [PubMed] [Google Scholar]
- 74.Fuentes F, Lopez-Miranda J, Perez-Martinez P, Jimenez Y, Marin C, Gomez P, Fernandez JM, Caballero J, Delgado-Lista J, Perez-Jimenez F. Chronic effects of a high-fat diet enriched with virgin olive oil and a low-fat diet enriched with alpha-linolenic acid on postprandial endothelial function in healthy men. Br J Nutr. 2008;100:159–165. doi: 10.1017/S0007114508888708. [DOI] [PubMed] [Google Scholar]
- 75.Dai J, Jones DP, Goldberg J, Ziegler TR, Bostick RM, Wilson PW, Manatunga AK, Shallenberger L, Jones L, Vaccarino V. Association between adherence to the Mediterranean diet and oxidative stress. Am J Clin Nutr. 2008;88:1364–1370. doi: 10.3945/ajcn.2008.26528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Chrysohoou C, Skoumas J, Pitsavos C, Masoura C, Siasos G, Galiatsatos N, Psaltopoulou T, Mylonakis C, Margazas A, Kyvelou S, Mamatas S, Panagiotakos D, Stefanadis C. Long-term adherence to the Mediterranean diet reduces the prevalence of hyperuricaemia in elderly individuals, without known cardiovascular disease: the Ikaria study. Maturitas. 2011;70:58–64. doi: 10.1016/j.maturitas.2011.06.003. [DOI] [PubMed] [Google Scholar]
- 77.Fito M, Guxens M, Corella D, Saez G, Estruch R, de la Torre R, Frances F, Cabezas C, Lopez-Sabater Mdel C, Marrugat J, Garcia-Arellano A, Aros F, Ruiz-Gutierrez V, Ros E, Salas-Salvado J, Fiol M, Sola R, Covas MI. Effect of a traditional Mediterranean diet on lipoprotein oxidation: a randomized controlled trial. Arch Intern Med. 2007;167:1195–1203. doi: 10.1001/archinte.167.11.1195. [DOI] [PubMed] [Google Scholar]
- 78.Jones JL, Comperatore M, Barona J, Calle MC, Andersen C, McIntosh M, Najm W, Lerman RH, Fernandez ML. A Mediterranean-style, low-glycemic-load diet decreases atherogenic lipoproteins and reduces lipoprotein (a) and oxidized low-density lipoprotein in women with metabolic syndrome. Metabolism. 2012;61:366–372. doi: 10.1016/j.metabol.2011.07.013. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.