Abstract
Data about primary gastric adenosquamous carcinoma (ASC) was limited due to rare incidence. Thus, the present study aims to investigate clinicopathological features and prognosis of gastric ASC. Cases of gastric ASC were obtained from our center and from case reports and series extracted from Medline. Clinicopathological features and prognosis of gastric ASC were analyzed and compared with gastric adenocarcinoma (AC) in our center. The commonest location was lower third (45.0%), followed by upper (26.2%) and middle third (24.2%). The median tumor size was 6 cm (0.8–17). The commonest differentiation status was well for both AC (44.4%) and SCC components (40.9%). Half of tumors (52.7%) were stage T4 and most patients (86.2%) suffered from lymph node metastasis (LNM). Tumor depth and TNM stage were risk factors for overall survival (OS) (both P < 0.05). The distribution of age, tumor size, tumor location, tumor depth, LNM and TNM stage were significantly different between gastric ASC and AC (all P < 0.05). The OS of gastric ASC was significantly worse than AC (P < 0.001), especially in stage III disease (P < 0.001). Gastric ASC differ significantly from AC with respect to clinicopathological features. The prognosis of gastric ASC was worse than AC.
Introduction
Gastric adenocarcinoma (AC) is the most common type of primary gastric cancer, whereas gastric primary adenosquamous carcinoma (ASC) is extremely rare. It accounted for less than 1% of all gastric cancers1. Gastric ASC is characterized by coexisting of two components (AC and ASC) within the same tumor2. Due to the rare incidence, gastric ASC was described in case reports and case series with small number of patients, study on gastric ASC with large series cases was lacking. Up to date, a variety of issues about gastric ASC remains unclear, including histogenesis, clinicopathological characteristics, optimal treatment strategies, and prognosis, etc. Thus, the present study aims to investigate the clinicopathological features and prognosis of gastric ASC based on a large series of cases.
Results
Clinicopathological characteristics of gastric ASC
The clinicopathological characteristics were summarized in Table 1. There were 121 male (73.3%) and 44 female (26.7%) patients. The median age was 63 years (range 26–88 years). Thirty-two patients (25.4%) accompanied with distant metastasis at the time of diagnosis. The commonest location was lower third (45.0%), followed by upper (26.2%) and middle third (24.2%). The median tumor size was 6 cm (range 0.8–17 cm). The commonest differentiation status for AC components was well differentiation (44.4%), followed by poorly (38.9%) and moderately differentiation (16.7%). The commonest differentiation status for SCC was well differentiation (40.9%), followed by moderately (34.1%) and poorly differentiation (25.0%). One hundred and twenty-three patients (78.9%) received complete resection, 25 patients (16.0%) received palliative resection, and 8 patients (5.1%) did not receive surgery. The distribution of T stage was 3.0% for T1, 16.8% for T2, 27.5% for T3 and 52.7% for T4. Most of the patients (86.2%) suffered from LNM. With respect to the components in metastatic LNs, AC was found in 58.7% of cases, SCC was found in 19.6% of cases, and both AC and SCC was found in 21.7% of cases.
Table 1.
Clinicopathological features of gastric primary ASC.
| Characteristics | Gastric ASC (n = 167) | Percentage |
|---|---|---|
| Age | ∑ = 154 | |
| ≤60 | 65 | 42.2% |
| >60 | 89 | 57.8% |
| Gender | ∑ = 165 | |
| Male | 121 | 73.3% |
| Female | 44 | 26.7% |
| Distant metastasis | ∑ = 126 | |
| Yes | 32 | 25.4% |
| No | 94 | 74.6% |
| Tumor location | ∑ = 149 | |
| Upper third | 39 | 26.2% |
| Middle third | 36 | 24.2% |
| Lower third | 67 | 45.0% |
| Two thirds or more | 7 | 4.6% |
| Tumor size (cm) | ∑ = 140 | |
| ≤5 | 58 | 41.4% |
| >5 | 82 | 58.6% |
| Differentiation status | AC ∑ = 18 | SCC ∑ = 44 |
| Well differentiated | 8 | 18 |
| Moderately differentiated | 3 | 15 |
| Poorly differentiated | 7 | 11 |
| Surgical resection | ∑ = 156 | |
| Complete resection | 123 | 78.9% |
| Incomplete resection | 25 | 16.0% |
| No surgery | 8 | 5.1% |
| Tumor depth | ∑ = 131 | |
| T1 | 4 | 3.0% |
| T2 | 22 | 16.8% |
| T3 | 36 | 27.5% |
| T4 | 69 | 52.7% |
| Lymph node metastasis | ∑ = 109 | |
| N0 | 15 | 13.8% |
| N1 | 36 | 33.0% |
| N2 | 25 | 22.9% |
| N3 | 33 | 30.3% |
| Metastatic components in lymph node | ∑ = 46 | |
| A | 27 | 58.7% |
| S | 9 | 19.6% |
| A & S | 10 | 21.7% |
| Adjuvant therapy | ∑ = 131 | |
| Yes | 42 | 32.1% |
| No | 89 | 67.9% |
Prognosis of gastric ASC
One hundred and nine patients with R0 resection and follow up data were selected for survival analysis. The median follow-up time was 33 months (range 5–118 months). The 1, 3 and 5-year OS was 58.1%, 32.4% and 26.4%, respectively. Prognostic predictors for patients were analyzed by univariate analysis (Table 2). The results showed that only tumor depth (P < 0.001) and TNM stage (P = 0.006) were prognostic risk factors. The OS stratified by tumor depth and TNM stage were shown in Fig. 1.
Table 2.
Risk factors for OS of gastric ASC patients according to univariate analysis (n = 109).
| Prognostic factors | β | Hazard ratio (95% CI) | P value |
|---|---|---|---|
| Age | −0.103 | 0.903 (0.546–1.492) | 0.689 |
| Gender | −0.473 | 0.623 (0.331–1.173) | 0.143 |
| Tumor location | −0.091 | 0.913 (0.689–1.208) | 0.523 |
| Tumor size | 0.372 | 1.451 (0.879–2.396) | 0.146 |
| Differentiation status | −0.657 | 0.519 (0.172–1.559) | 0.242 |
| Tumor depth | 0.490 | 1.633 (1.264–2.109) | <0.001 |
| Lymph node metastasis | 0.010 | 1.010 (0.488–2.088) | 0.979 |
| TNM stage | 0.907 | 2.477 (1.301–4.718) | 0.006 |
Figure 1.
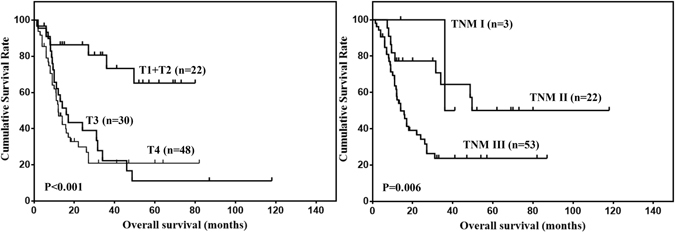
OS of gastric ASC stratified by tumor depth and TNM stage.
Comparison of clinicopathological characteristics and prognosis between gastric ASC and AC
The clinicopathological characteristics of 109 gastric ASC patients were compared with 3280 gastric AC patients in our center (Table 3). The results showed that the distribution of age, tumor size, tumor location, tumor depth, LNM and TNM stage were significantly different between gastric ASC and AC (all P < 0.05). Then, the prognosis of gastric ASC and AC were compared. The OS of gastric ASC was significantly worse than that of gastric AC (Fig. 2, Table 4, P < 0.001). Further, the OS of gastric ASC and AC with stage II/III disease were compared. The results showed that the prognosis of stage II gastric ASC was comparable to that of stage II gastric AC (Fig. 3, P = 0.102), and the prognosis of stage III gastric ASC was worse than that of stage III gastric AC (Fig. 3, P < 0.001).
Table 3.
Comparison of selected clinicopathological parameters between gastric AC and ASC patients underwent R0 resection.
| Characteristics | AC(n = 3280) | ASC(n = 109) | P value |
|---|---|---|---|
| Age | |||
| ≤60 | 1945 | 39 | <0.001 |
| >60 | 1335 | 62 | |
| Gender | |||
| Male | 2546 | 83 | 0.297 |
| Female | 734 | 30 | |
| Tumor location | |||
| Upper third | 1012 | 32 | 0.036 |
| Middle third | 541 | 28 | |
| Lower third | 1459 | 44 | |
| Two thirds or more | 268 | 4 | |
| Tumor size (cm) | |||
| ≤5 | 2266 | 41 | <0.001 |
| >5 | 1014 | 61 | |
| Tumor depth | |||
| T1 | 612 | 2 | <0.001 |
| T2 | 515 | 20 | |
| T3 | 1211 | 30 | |
| T4 | 942 | 48 | |
| Lymph node metastasis | |||
| N0 | 1178 | 13 | 0.006 |
| N1 | 639 | 21 | |
| N2 | 560 | 17 | |
| N3 | 903 | 27 | |
| TNM stage | |||
| I | 815 | 3 | <0.001 |
| II | 973 | 22 | |
| III | 1492 | 53 | |
Figure 2.
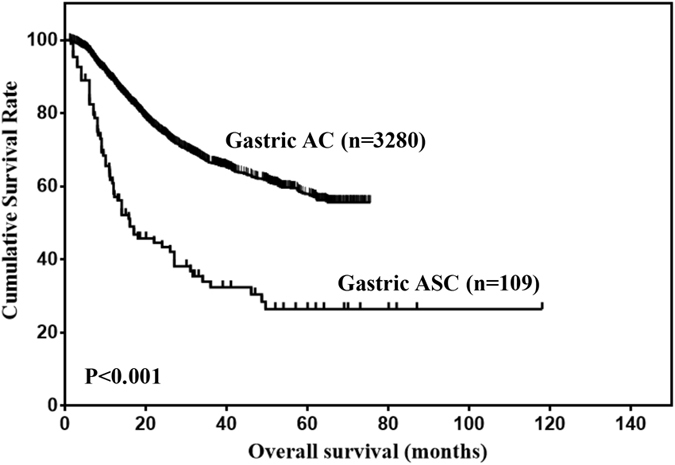
Comparison of OS between gastric ASC and AC for the entire cohort.
Table 4.
Comparative overall survival analysis of gastric AC and ASC using univariate and multivariate analysis.
| Overall survival | Gastric AC | Gastric ASC | Univariate analysis | Multivariate analysis | ||||
|---|---|---|---|---|---|---|---|---|
| n = 3280 | n = 109 | β | HR (95% CI) | P | β | HR (95% CI) | P | |
| −1.094 | 0.335(0.263–0.427) | <0.001 | −0.539 | 0.583(0.425–0.801) | 0.001 | |||
| 1-year | 89.0% | 58.1% | ||||||
| 3-year | 66.5% | 32.4% | ||||||
| 5-year | 57.9% | 26.4% | ||||||
Figure 3.
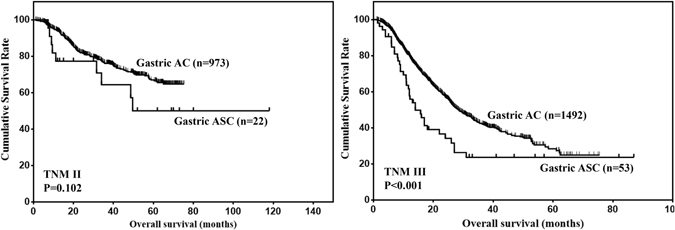
Comparison of OS between gastric ASC and AC in stage II/III patients.
Discussion
Gastric ASC is an extremely rare entity and account for less than 1% of all gastric malignancies1. Thus, the clinicopathological features and prognosis of gastric ASC was unclear. In the present study, we found that gastric ASC differ significantly from gastric AC with respect to clinicopathological features, and the prognosis of gastric ASC was worse than that of gastric AC.
Up to date, there was only one study containing a relatively large number of gastric ASC patients3. The clinicopathological features of 120 cases of gastric ASC was reviewed in this study. In their series, the male to female ratio was 2.3:1, and the mean age was 58.4 years. The most common location was lower third, followed by middle third and upper third. However, the prognosis of gastric ASC was not analyzed. In our present study, the male to female ratio was 2.8:1, and the patient age ranged from 26 to 88 years (mean: 61.3 years, median: 63 years). The most common location was lower third, followed by upper third and middle third. The results in our study was inconsistent with the previous reports.
It is well accepted that the diagnosis of gastric ASC was confirmed by the coexisting of AC and SCC components, with SCC accounting for at least 25% of tumors1. However, Faria et al. proposed that tumors should be located outside the cardia, without esophageal invasion and without ASC in any other organs4. The histogenesis of gastric ASC was still under debate. Several hypotheses have been proposed:5 (1) squamous metaplastic transformation of AC, (2) oncogenic transformation of ectopic squamous epithelium, (3) oncogenic transformation of metaplastic squamous cells, (4) collision of concurrent AC and SCC, (5) differentiation of stem cells toward both glandular and squamous cells. The first hypothesis was supported by many researchers based on accumulating evidence. Firstly, most of the SCC components were located in deeper layer, in contrast to the AC components being located in the mucosal layer6. Secondly, an obvious transition area exists between AC and SCC components1. Thirdly, the positive expression of CEA was found in SCC components1. Fourthly, identical levels of p53 gene was found in both components6, 7.
Gastric ASC was an extremely aggressive tumor. Most of them are found in an advanced stage at the time of diagnosis4, 8, 9. In our present study, 25.4% of patients accompanied by distant metastasis. Among them, the most common location for distant metastasis was liver, followed by peritoneal dissemination. Half of the tumors (52.7%) were stage T4 and the incidence of LNM was 86.2%. These findings were all consistent with previous reports1, 2, 10.
Both AC and SCC components have the potential for distant metastasis. Lee et al. reported that AC components were found in 10 of 14 cases, SCC component was found in 1 patient, and both components were found in 3 patients7. Chen et al. also analyzed the metastatic LNs and revealed that AC was the major component in 6 cases, and SCC was the major component in one case10. A study containing 12 cases of gastric ASC with LNM also found that 8 cases had AC components, 2 cases had SCC components and 2 cases had both components1. In our present study, for patients with LNM, AC was found in 58.7% of cases, SCC was found in 19.6% of cases, and both AC and SCC was found in 21.7% of cases. Thus, AC may be the predominant component for LNM. However, Mori et al. reported that both components existed in almost all the metastatic lesions in 9 patients at autopsy11. In all, the incidence of different components in the metastatic LNs needs further investigation based on larger sample size.
Radical resection remains the optimal treatment for local disease without distant metastasis. However, no standard adjuvant therapy strategies for gastric ASC has been established. Chemotherapy has been reported to be effective for gastric ASC12. However, there is no consensus on the optimal strategy of chemotherapy. Radiotherapy could also be used as one of the adjuvant treatment, as the SCC components in gastric ASC was sensitive to radiotherapy1.
The prognosis of gastric ASC was considered to be worse than typical gastric AC, although the biological behavior was mainly determined by the AC components13. Quan et al. reported that the median overall survival time was 12 months, and 87.5% of patients survived for less than 24 months after diagnosis2. Chen et al. reported that the median overall survival time was 22 months, and 3-year overall survival rate was 15.4%1. However, these data were based on small number of patients, and not all patients received radical resection. In our present study, the median overall survival time was 17 months for gastric ASC received R0 resection, and the 1, 3 and 5-year overall survival rate was 58.1%, 32.4% and 26.4%, respectively. Further, we compared the overall survival of gastric ASC with gastric AC patients. We found that the prognosis of gastric ASC was significantly poorer than that of gastric AC patients.
There are several limitations in our present study. First, the sample size was not large enough. Thus, the results of our present study should be explained cautiously. Second, the completeness of data is limited due to data acquisition. Third, data about differentiation status of both AC and SCC components in the primary tumor was limited. Thus, the association between the differentiation status of both components and the prognosis of patients could not be evaluated. Fourth, the association between clinicopathological features and components in the metastatic lymph nodes could not be analyzed due to the limited data. Fifth, data about the components in the recurrent and metastatic lesions was lacking. The influence of components on the prognosis of patients was unclear. Sixth, the constituent ratio of AC and SCC components was varied among primary tumors. The prognostic value of constituent ratio on the prognosis of gastric ASC was unclear. Seventh, the association between the components in the metastatic lymph nodes and components in the recurrent and distant metastatic lesions was unclear. The last, the disease free survival and disease specific survival could not be evaluated due to the data acquisition.
In conclusion, the majority of tumors were located in the lower third, well differentiation, stage T4 and stage N+. Tumor depth and TNM stage were risk factors for overall survival. Gastric ASC differ significantly from gastric AC with respect to clinicopathological features. The prognosis of gastric ASC was worse than gastric AC.
Methods
Gastric AC and ASC cases were from our center and literature. From September 2008 to March 2015, 21 cases of gastric ASC and 3280 cases of gastric AC received radical resection in our center. Literature search of Medline was performed for articles in English published from 1965 through 2015. Medline search resulted in 43 case reports and studies1–43 including 146 cases of gastric ASC. Finally, a total of 167 gastric ASC patients was identified (Fig. 4). This study was approved by the Ethics Committee of Xijing Hospital, all procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. Informed consent was obtained from all individual participants included in the study.
Figure 4.
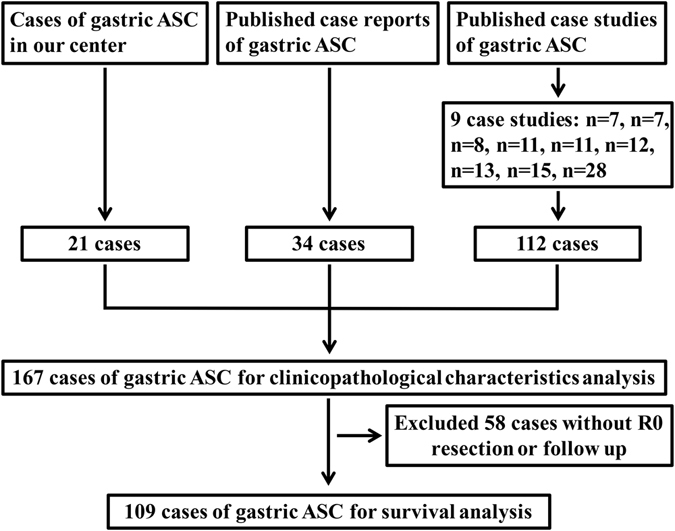
Flowchart of patient selection.
Data including gender, age, distant metastasis, tumor location, tumor size, differentiation status, surgical intervention, tumor depth, LNM, adjuvant therapy and survival data were extracted from case reports and studies of recorded from our center. Completeness of data is limited due to the type of data acquisition. Patients in our center were followed up till November 2015 by enhanced chest and abdominal CT and gastroscopy every 3 months.
Data were processed using SPSS 22.0 for Windows (SPSS Inc., Chicago, IL, USA). Discrete variables were analyzed using Chi-square test or Fisher’s exact test. Significant prognostic predictors for patients identified by univariate analysis were further assessed by multivariate analysis using the Cox’s proportional hazards regression model. OS was shown by Kaplan-Meier method. The P value was considered to be statistically significant at 5% level.
Acknowledgements
This study was supported in part by grants from the National Natural Scientific Foundation of China [No. 31100643, 31570907, 81300301, 81572306, 81502403, XJZT12Z03].
Author Contributions
F.F., Z.G.Z. and Q.J.P. conceived the study and drafted the manuscript. X.G.H., W.F. and W.Q. collected the data. G.M. and L.X. performed statistical analysis. Z.H.W. designed and supervised the study. All authors read and approved the final manuscript.
Competing Interests
The authors declare that they have no competing interests.
Footnotes
Fan Feng, Gaozan Zheng and Jingpeng Qi contributed equally to this work.
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Chen H, et al. Clinicopathological characteristics, diagnosis, treatment, and outcomes of primary gastric adenosquamous carcinoma. World J Surg Oncol. 2015;13:136. doi: 10.1186/s12957-015-0554-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Quan J, Zhang R, Liang H, Li F, Liu H. The clinicopathologic and prognostic analysis of adenosquamous and squamous cell carcinoma of the stomach. Am Surg. 2013;79:E206–8. [PubMed] [Google Scholar]
- 3.Ajoodhea H, et al. Fever as a first manifestation of advanced gastric adenosquamous carcinoma: a case report. World J Gastroenterol. 2014;20:10193–201. doi: 10.3748/wjg.v20.i29.10193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Faria GR, et al. Primary gastric adenosquamous carcinoma in a Caucasian woman: a case report. J Med Case Rep. 2010;4:351. doi: 10.1186/1752-1947-4-351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Shirahige A, et al. Fatal submucosal invasive gastric adenosquamous carcinoma detected at surveillance after gastric endoscopic submucosal dissection. World J Gastroenterol. 2015;21:4385–90. doi: 10.3748/wjg.v21.i14.4385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Saito S, et al. A clinicopathological and immunohistochemical study of gastric cancer with squamous cell carcinoma components: a clinically aggressive tumor. J Dig Dis. 2012;13:407–13. doi: 10.1111/j.1751-2980.2012.00610.x. [DOI] [PubMed] [Google Scholar]
- 7.Lee WA, Woo DK, Kim YI, Kim WH. p53, p16 and RB expression in adenosquamous and squamous cell carcinomas of the stomach. Pathol Res Pract. 1999;195:747–52. doi: 10.1016/S0344-0338(99)80116-2. [DOI] [PubMed] [Google Scholar]
- 8.Ebi M, et al. A patient with gastric adenosquamous carcinoma with intraperitoneal free cancer cells who remained recurrence-free with postoperative S-1 chemotherapy. Intern Med. 2012;51:3125–9. doi: 10.2169/internalmedicine.51.8402. [DOI] [PubMed] [Google Scholar]
- 9.Kim YS, et al. Clinicopathological features and differences of p53 and Ki-67 expression in adenosquamous and squamous cell carcinomas of the stomach. Korean J Gastroenterol. 2006;47:425–31. [PubMed] [Google Scholar]
- 10.Chen YY, et al. Adenosquamous carcinoma of the stomach and review of the literature. Pathol Oncol Res. 2015;21:547–51. doi: 10.1007/s12253-014-9890-7. [DOI] [PubMed] [Google Scholar]
- 11.Mori M, Iwashita A, Enjoji M. Adenosquamous carcinoma of the stomach. A clinicopathologic analysis of 28 cases. Cancer. 1986;57:333–9. doi: 10.1002/1097-0142(19860115)57:2<333::AID-CNCR2820570224>3.0.CO;2-U. [DOI] [PubMed] [Google Scholar]
- 12.Kadowaki S, Yatabe Y, Nitta S, Ito Y, Muro K. Durable response of human epidermal growth factor receptor-2-positive gastric adenosquamous carcinoma to trastuzumab-based chemotherapy. Case Rep Oncol. 2014;7:210–6. doi: 10.1159/000362088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bansal RK, Sharma P, Kaur R, Arora A. Primary gastric adenosquamous carcinoma in an Indian male. Indian J Pathol Microbiol. 2013;56:416–8. doi: 10.4103/0255-0857.118883. [DOI] [PubMed] [Google Scholar]
- 14.Kimura Y, et al. Case of early adenosquamous carcinoma of the stomach. Fukuoka Igaku Zasshi. 2013;104:315–20. [PubMed] [Google Scholar]
- 15.Saito H, et al. A case of gastric adenosquamous carcinoma producing granulocyte-colony stimulating factor. Gan To Kagaku Ryoho. 2013;40:799–802. [PubMed] [Google Scholar]
- 16.Fukuda Y, et al. Successful management of liver metastasis from gastric adenosquamous carcinoma with adjuvant chemotherapy and radiofrequency ablation. Nihon Shokakibyo Gakkai Zasshi. 2012;109:606–14. [PubMed] [Google Scholar]
- 17.Watanabe K, et al. Different sensitivity to chemotherapy between adenocarcinoma and adenosquamous carcinoma in a case of synchronous multicentric gastric cancer. Nihon Shokakibyo Gakkai Zasshi. 2012;109:408–17. [PubMed] [Google Scholar]
- 18.Ishiguro A, et al. A case of gastric adenosquamous carcinoma successfully treated with second-line chemotherapy (CPT-11 and CDDP) Gan To Kagaku Ryoho. 2010;37:1579–82. [PubMed] [Google Scholar]
- 19.Tohma T, et al. Weekly paclitaxel therapy is effective for gastric adenosquamous carcinoma: a case report. Hepatogastroenterology. 2009;56:568–70. [PubMed] [Google Scholar]
- 20.Ikeda E, et al. A case of adenosquamous gastric carcinoma successfully treated with TS-1, low-dose CDDP and docetaxel as neoadjuvant chemotherapy. Gan To Kagaku Ryoho. 2007;34:423–6. [PubMed] [Google Scholar]
- 21.Nomura M, et al. A case of gastric adenosquamous carcinoma with abdominal paraaortic lymph node metastases successfully treated by TS-1 plus CDDP neoadjuvant chemotherapy. Gan To Kagaku Ryoho. 2006;33:99–103. [PubMed] [Google Scholar]
- 22.Endo K, et al. Gastric adenosquamous carcinoma producing granulocyte-colony stimulating factor. Gastric Cancer. 2005;8:173–7. doi: 10.1007/s10120-005-0330-y. [DOI] [PubMed] [Google Scholar]
- 23.Blazquez S, et al. Adenosquamous gastric carcinoma in Caucasian patient. Rev Esp Enferm Dig. 2005;97:211–2. doi: 10.4321/S1130-01082005000300009. [DOI] [PubMed] [Google Scholar]
- 24.Mori E, et al. Adenosquamous carcinoma of the remnant stomach: report of a case. Surg Today. 2000;30:643–6. doi: 10.1007/s005950070105. [DOI] [PubMed] [Google Scholar]
- 25.Manna ED, Seixas AA, de Araújo RP, Ferro MC. Primary adenosquamous carcinoma of the stomach] Rev Assoc Med Bras (1992) 1998;44:152–4. doi: 10.1590/S0104-42301998000200016. [DOI] [PubMed] [Google Scholar]
- 26.Yoshida K, et al. Early gastric cancer of adenosquamous carcinoma type: report of a case and review of literature. Jpn J Clin Oncol. 1996;26:252–7. doi: 10.1093/oxfordjournals.jjco.a023224. [DOI] [PubMed] [Google Scholar]
- 27.Toyota N, Minagi S, Takeuchi T, Sadamitsu N. Adenosquamous carcinoma of the stomach associated with separate early gastric cancer (type IIc) J Gastroenterol. 1996;31:105–8. doi: 10.1007/BF01211195. [DOI] [PubMed] [Google Scholar]
- 28.Cabello RM, et al. Adenosquamous carcinoma of the stomach. Rev Esp Enferm Dig. 1994;86:757–60. [PubMed] [Google Scholar]
- 29.Tenma K, et al. A case report of adenosquamous cell carcinoma of the stomach responding well to combination chemotherapy. Gan To Kagaku Ryoho. 1993;20:647–50. [PubMed] [Google Scholar]
- 30.Kawabe K, Nakanuma Y, Terada T, Nakamura Y. Adenosquamous carcinoma of the stomach presenting “giant gastric folds”. Gastroenterol Jpn. 1990;25:739–45. [PubMed] [Google Scholar]
- 31.Tsukino H, et al. A case of primary adenosquamous carcinoma of the stomach showing Borrmann 4 type. Rinsho Hoshasen. 1990;35:649–52. [PubMed] [Google Scholar]
- 32.Yamamoto K, Ohnishi A, Noda S, Umezaki H, Yamamoto T. An autopsy case of carcinomatous sensory neuropathy associated with gastric adenosquamous carcinoma. Rinsho Shinkeigaku. 1989;29:493–6. [PubMed] [Google Scholar]
- 33.Shigematsu T, et al. A primary gastric adenosquamous carcinoma with remarkable lymphatic metastasis diagnosed by the stomach and lymph node biopsy. Gan No Rinsho. 1989;35:421–6. [PubMed] [Google Scholar]
- 34.Mori M, Fukuda T, Enjoji M. Enjoji, Adenosquamous carcinoma of the stomach. Histogenetic and ultrastructural studies. Gastroenterology. 1987;92:1078–82. doi: 10.1016/0016-5085(87)90986-3. [DOI] [PubMed] [Google Scholar]
- 35.Horikawa M, et al. A case of early primary adenosquamous carcinoma of the stomach. Gan No Rinsho. 1987;33:305–10. [PubMed] [Google Scholar]
- 36.Masuda T, et al. A case of primary adenosquamous carcinoma of the stomach preoperatively diagnosed by endoscopic biopsy. Gan No Rinsho. 1985;31:212–6. [PubMed] [Google Scholar]
- 37.Matsumoto K, et al. Primary adenosquamous carcinoma of the stomach–a case report. Gan No Rinsho. 1984;30:1726–31. [PubMed] [Google Scholar]
- 38.Sato N, Wada K, Kobayashi K, Hirai H, Yagi M. A case of primary adenosquamous carcinoma of the stomach associated with gastric polyposis] Gan No Rinsho. 1984;30:292–5. [PubMed] [Google Scholar]
- 39.Mingazzini PL, Barsotti P, Malchiodi Albedi F. Adenosquamous carcinoma of the stomach: histological, histochemical and ultrastructural observations. Histopathology. 1983;7:433–43. doi: 10.1111/j.1365-2559.1983.tb02256.x. [DOI] [PubMed] [Google Scholar]
- 40.Aoki Y, Tabuse K, Wada M, Katsumi M, Uda H. Primary adenosquamous carcinoma of the stomach: experience of 11 cases and its clinical analysis. Gastroenterol Jpn. 1978;13:140–5. doi: 10.1007/BF02773859. [DOI] [PubMed] [Google Scholar]
- 41.Straus R, Heschel S, Fortmann DJ. Primary adenosquamous carcinoma of the stomach. A case report and review. Cancer. 1969;24:985–95. doi: 10.1002/1097-0142(196911)24:5<985::AID-CNCR2820240518>3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]
- 42.Boswell JT, Helwig EB. Squamous cell carcinoma and adenoacanthoma of the stomach. A clinicopathologic study. Cancer. 1965;18:181–92. doi: 10.1002/1097-0142(196502)18:2<181::AID-CNCR2820180209>3.0.CO;2-3. [DOI] [PubMed] [Google Scholar]
- 43.Barussaud ML, Meurette G, Cassagnau E, Dupasc B, Le Borgne J. Mixed adenocarcinoma and squamous cell carcinoma arising in a gastric duplication cyst. Gastroenterol Clin Biol. 2008;32:188–91. doi: 10.1016/j.gcb.2008.01.014. [DOI] [PubMed] [Google Scholar]


