Abstract
The human pathogen Staphylococcus aureus expresses a set of transcriptional factors and small RNAs (sRNAs) to adapt to environmental variations. Recent harmonization of staphylococcal sRNA data allowed us to search for novel sRNAs using DETR’PROK, a computational pipeline for identifying sRNA in prokaryotes. We performed RNA-Seq on Newman strain and identified a set of 48 sRNA candidates. To avoid bioinformatic artefacts, we applied a series of cut-offs and tested experimentally each selected intergenic region. This narrowed the field to 24 expressed sRNAs, of which 21 were new and designated with Srn identifiers. Further examination of these loci revealed that one exhibited an unusual condensed sRNA cluster of about 650 nucleotides. We determined the transcriptional start sites within this region and demonstrated the presence of three contiguous sRNA genes (srn_9342, srn_9344 and srn_9345) expressed from the positive strand, and two others (srn_9343 and srn_9346) transcribed from the opposite one. Using comparative genomics, we showed that genetic organization of the srn_9342-9346 locus is specific to Newman and that its expression is growth-phase dependent and subjected to nutrient deprivation and oxidative stress. Finally, we demonstrated that srn_9343 encodes a secreted peptide that could belong to a novel S. aureus toxin-antitoxin system.
Introduction
Staphylococcus aureus is an opportunistic pathogen responsible for a large spectrum of human and animal infections1. The bacterium finely modulates gene expression to efficiently adapt its growth and physiology to the local environment. Besides global transcriptional regulators, small RNAs (sRNAs, also cited as regulatory RNAs when a regulatory function was demonstrated) have emerged as key players in a wide range of biological processes, from central metabolism to virulence and antibiotic resistance2–4. In S. aureus, sRNAs, are typically 50- to 500-nucleotides (nt) long. Some of them regulate mRNA expression and/or stability without the need of Hfq chaperone5, 6. sRNAs with regulatory functions were first discovered by Mizuno in E. coli 7 and then by Novick in S. aureus 8. They can modulate target mRNA expression by cis- or trans-acting mechanisms2. Cis-encoded sRNAs are transcribed from the opposite strand of an mRNA or another sRNA. Accordingly, they display perfect complementarity with their target sequences, although their ability to bind other RNAs cannot be excluded. Trans-encoded sRNAs are transcribed apart from their targets, and usually display only partial complementarity with them.
Over the last decade, several studies have focused on the identification of S. aureus sRNAs via bioinformatics, next-generation sequencing (NGS), and other experimental approaches9–21. This has resulted in the publication of hundreds of RNA sequences, but their functions are mostly unknown. Most of these studies were done using methicillin-resistant strain N31511, 18. In others, newly discovered sRNAs were described based on their genomic location in that particular strain9, 12, 13, 16. S. aureus sRNA identification has been hampered by the lack of naming consensus and the absence of dedicated annotation files. Recently, we collected all of the sequences published so far, proposed a simple sRNA gene identifier (srn) to avoid redundancies, and provided annotation files in the Gene File Format (.gff) to the SRD (Staphylococcus regulatory RNA Database)22. In this study, the sRNA content of four strains was compared, and their predicted locations suggested that sRNA content is strain-dependent. It therefore seems that global analysis devoted to the identification of novel sRNA is still useful for many bacteria, including S. aureus. This was recently confirmed by studies conducted on multilocus sequence typing 8 (ST8) strains, with new sRNAs described in USA300 and NCTC832515, 21. Here, we combined bioinformatics and experimental procedures to identify and characterize novel sRNAs expressed by the S. aureus strain Newman23. This is a methicillin-susceptible clinical isolate often used to study staphylococcal diseases in animal models23, and it belongs to the common ST8 clonal lineage. However, Newman has never before been used to discover new staphylococcal sRNAs, and only three previous studies have focused on the role of already identified sRNAs in Newman. The first described the Srn3580_SprA type I toxin-antitoxin system24; the second focused on the anti-virulent role of Srn_3610_SprC25; and the third highlighted the functional role of Srn3820_SprX1 in Newman pathogenicity26.
In this study, we performed deep RNA sequencing on Newman wild-type and its isogenic mutant Δsrn_3610_sprc. We combined the RNA-Seq data with the initial sRNA annotation from the SRD22, then used the DETR’PROK pipeline27 to identify 48 putative novel transcribed intergenic regions (IGRs). Bioinformatic and experimental analysis of the 48 loci led to the certain characterization of more than 20 novel sRNAs expressed from either the core or accessory genome. Further study of these sRNAs along with RACE-mapping of primary transcripts revealed that Newman has an unusual condensed cluster of five sRNAs. These are all located within 650 nucleotides, and their expression is growth-phase dependent and subject to nutrient starvation and oxidative stress. In the cluster, two RNA pairs are expressed as sense/cis-antisense sRNA, with one pair presenting toxin-antitoxin module features, since one of the sRNA was demonstrated to encode and express a 33 amino acid long secreted peptide. This work provides evidence for the existence of at least 21 new S. aureus sRNAs, and highlights the unprecedented organization of an sRNA cluster of unknown function. Our approach could be extended to wider experimental conditions on the most represented STs to characterize the full repertoire of staphylococcal sRNA (pan-RNome).
Results
Bioinformatic screening identifies 48 novel sRNA candidates in Staphylococcus aureus Newman
So far, more than 500 sRNAs are compiled under unique Srn identifiers in the SRD22. The majority were identified using high-throughput screening, with sixty experimentally confirmed22. Our goal here was to search for novel sRNAs by combining RNA-Seq, bioinformatics, and experimental assessments, thus reducing the false discovery rate inherent to this type of approach. We examined S. aureus Newman, a methicillin-susceptible clinical isolate23, 28, 29 which has never before been used for sRNA discovery. Whereas previous studies were based on the use of various growth conditions11, 15, 21, we compared wild-type Newman to an sRNA mutant strain, as sRNAs can modulate the expression of various targets including transcription factors (MgrA, Rot, SarT)3. These transcription factors could, in turn, control sRNA expression. Therefore, we used a strain deleted for Srn_3610_SprC sRNA to see whether that deletion allowed the identification of more sRNAs. Srn_3610_SprC is an sRNA that belongs to the SarA regulon and attenuates virulence25, 30. Indeed, in the absence of Srn_3610_SprC, S. aureus phagocytosis by macrophages increases, allowing for an internal proliferation of the bacterium and its subsequent release and dissemination into the organism. During the post-exponential phase, we extracted total RNA from isogenic and mutant strains, and prepared RNA-Seq libraries. We then used DETR’PROK27, a pipeline recently developed to identify sRNAs in prokaryotes. DETR’PROK assembled, in the non-annotated regions, overlapping reads into clusters representing sRNA candidates. Consequently, the workflow was designed and set to preferentially search for RNAs transcribed in an independent manner rather than mRNA leaders (see Methods for the parameters used). To avoid reexamining previously reported sRNAs, we combined the SRD gene annotation for srns 22 with gene-coding annotations in GFF format that were downloaded from NCBI. Among the six biological replicates (three wild-type Newman and three Newman Δsrn_3610_sprc transcriptomes), there were 18 to 27 novel sRNA candidates per replicate, resulting in a total of 48 independent IGRs that were not from mRNA UTRs (Table 1). Of these, 11 were detected by the framework only in the wild-type strain, while six clusters were only in the mutant (File S1). To verify whether these clusters were specific to the knockout or parental strain, or simply due to limitations in the DETR’PROK clustering process, we annotated the 48 IGRs in our GFF annotation file and used the HTSeq/DESeq pipeline31, 32. This pipeline count reads within annotation, normalizes data, and calculates differential expression levels between strains. Running this process on Newman and Δsrn_3610_sprc revealed that there were no significant (p < 0.05) transcript level variations for any of the IGRs. Consequently, it indicated that none of these 48 clusters were under the control of Srn_3610_SprC. This result can be explained by recent work showing this sRNA to be directly repressed by SarA which therefore significantly lower expression of Srn_3610_SprC in wild-type under normal growth condition30.
Table 1.
RNA-Seq identification of 48 transcribed IGRs within the Staphylococcus aureus Newman strain.
| IGR | Location | Size (nt) | Strand | Number of replicates | HTSeq mean count | FPKM | Repeated sequences |
|---|---|---|---|---|---|---|---|
| 1 | 108914 | 267 | − | 1/6 | 29 | 23 | Unique |
| 2 | 109544 | 539 | − | 3/6 | 33 | 14 | Unique |
| 3 | 115311 | 297 | − | 6/6 | 3603 | 2320 | Multiple (21) |
| 4 | 138924 | 274 | − | 1/6 | 15 | 13 | Unique |
| 5 | 363783 | 407 | + | 5/6 | 30 | 17 | Unique |
| 6 | 363785 | 304 | − | 6/6 | 20 | 14 | Unique |
| 7 | 493064 | 305 | + | 1/6 | 41 | 25 | Unique |
| 8 | 787855 | 483 | − | 3/6 | 14 | 5 | Unique |
| 9 | 819355 | 324 | + | 2/6 | 21 | 13 | Multiple (26) |
| 10 | 819365 | 324 | − | 2/6 | 13 | 7 | Multiple (26) |
| 11 | 824790 | 554 | − | 6/6 | 32 | 11 | Multiple (73) |
| 12 | 824832 | 450 | + | 6/6 | 1442 | 642 | Multiple (71) |
| 13 | 846032 | 175 | − | 1/6 | 7 | 10 | Unique |
| 14 | 897930 | 466 | + | 5/6 | 840 | 348 | Unique |
| 15 | 1005976 | 363 | + | 1/6 | 4 | 2 | Unique |
| 16 | 1050586 | 474 | − | 4/6 | 5044 | 2534 | Unique |
| 17 | 1079951 | 348 | − | 5/6 | 2448 | 1536 | Unique |
| 18 | 1102344 | 234 | + | 3/6 | 15 | 11 | Unique |
| 19 | 1103623 | 461 | − | 2/6 | 3511 | 1502 | Copy 1 |
| 20 | 1141544 | 424 | + | 5/6 | 110315 | 55478 | Unique |
| 21 | 1175020 | 490 | − | 4/6 | 294 | 136 | Unique |
| 22 | 1175024 | 480 | + | 4/6 | 23 | 9 | Unique |
| 23 | 1211256 | 286 | + | 1/6 | 11 | 8 | Multiple (21) |
| 24 | 1332002 | 154 | − | 1/6 | 10 | 14 | Unique |
| 25 | 1344201 | 624 | + | 3/6 | 15 | 4 | Unique |
| 26 | 1346470 | 479 | + | 4/6 | 29 | 14 | Unique |
| 27 | 1350072 | 600 | + | 3/6 | 170 | 57 | Unique |
| 28 | 1419610 | 342 | + | 1/6 | 12 | 4 | Unique |
| 29 | 1462664 | 341 | + | 4/6 | 86 | 47 | Unique |
| 30 | 1462677 | 229 | − | 2/6 | 22 | 18 | Unique |
| 31 | 1471642 | 205 | − | 1/6 | 28 | 29 | Unique |
| 32 | 1526641 | 270 | − | 1/6 | 10 | 5 | Multiple (4) |
| 33 | 1764262 | 147 | + | 6/6 | 1 | 0 | Multiple (14) |
| 34 | 1930943 | 264 | + | 1/6 | 38 | 20 | Unique |
| 35 | 1963532 | 241 | − | 4/6 | 13 | 5 | Unique |
| 36 | 1979939 | 388 | + | 3/6 | 102 | 49 | Unique |
| 37 | 2017461 | 520 | + | 5/6 | 1750 | 689 | Copy 2 |
| 38 | 2018908 | 364 | + | 6/6 | 146395 | 81475 | Unique |
| 39 | 2018949 | 1127 | − | 4/6 | 557 | 77 | Unique |
| 40 | 2019372 | 279 | + | 6/6 | 3692 | 2685 | Unique |
| 41 | 2083121 | 419 | − | 3/6 | 226 | 92 | Unique |
| 42 | 2345975 | 119 | + | 1/6 | 9 | 20 | Multiple (2) |
| 43 | 2436048 | 299 | − | 6/6 | 9 | 6 | Multiple (18) |
| 44 | 2456865 | 465 | − | 2/6 | 13 | 5 | Multiple (14) |
| 45 | 2548150 | 280 | − | 2/6 | 12 | 12 | Unique |
| 46 | 2548156 | 265 | + | 2/6 | 684 | 664 | Unique |
| 47 | 2605426 | 783 | − | 5/6 | 486 | 98 | Unique |
| 48 | 2839262 | 171 | − | 1/6 | 220 | 280 | Unique |
IGR, intergenic region; nt, nucleotides; FPKM, fragments per kilobase of exon per million reads mapped. When a sequence is repeated over the genome (at least 60% of the sequence of the candidate) the number of repetitions appears in parentheses.
Bioinformatic and manual curation of IGRs narrows the field to 17 sRNA candidates
Previous studies showed that erroneous annotation of repeated sequences or UTRs as sRNAs is likely to occur, and therefore that it may be challenging to identify novel sRNAs11, 14, 22. To address this issue, each sequence extracted from DETR’PROK’s output was systematically submitted to BLAST to determine whether the candidate sequence appeared elsewhere in the Newman genome. From the 48 IGRs listed in Table 1, 35 were found to be unique whereas the others were retrieved at multiple locations in Newman (from 2 copies to more than 70). To reduce this set to the most relevant ones (the unrepeated and truly expressed ones), we applied several cutoffs. First, IGRs identified 10 or more times in the Newman genome were discarded to avoid characterization of repeated sequences. Second, we removed all IGRs with a mean HTSeq count (number of paired-end fragments)31 lower than 20. Third, FPKM (fragments per kilobase of exon per million reads mapped) normalization33 was done to take into account not only the sequencing depths, but also the lengths of the transcribed regions. We eliminated all the clusters with an average FPKM of less than 9. The combination of these cutoffs led to the removal of 23 clusters (File S2). We then continued curation by using the Artemis genome browser34. This led to the elimination of 8 additional IGRs associated with UTRs, or misannotated genes that mostly corresponded to IGRs identified in few replicates (File S2). Reducing the number of candidates was essential to limit the spread of false sRNAs and so we could focus on the most accurate ones. We were thus able to make a new list of 17 IGRs (Table 2), 11 of which were located in the Newman accessory genome23. Interestingly, two of them (IGR_1103623 and IGR_2017461) located respectively in φNM1 and φNM2 are almost identical, with more than 98% sequence identity over 380 nt (Table 1, copies 1 and 2). Five candidates were located directly downstream or upstream of an already known sRNA. Altogether, our results strongly suggested the presence of additional sRNAs expressed in Newman strain.
Table 2.
Bioinformatic curation narrows the list of possible new sRNAs found in Staphylococcus aureus Newman strain to 17 candidates.
| IGR | Location | Core or accessory genome | Gene upstream | Gene downstream | GC % |
|---|---|---|---|---|---|
| 5 | 363783 | φNM4 | srn_3820.2/+ | NWMN_0314/− | 35.8 |
| 6 | 363785 | φNM4 | srn_3820.2/+ | NWMN_0314/− | 38 |
| 14 | 897930 | core | NWMN_0810/+ | NWMN_0811/+ | 28.7 |
| 16 | 1050586 | core | srn_2330/− | NWMN_0946/+ | 28.4 |
| 17 | 1079951 | core | NWMN_0973/+ | NWMN_0974/+ | 29.5 |
| 19 | 1103623 | φNM2 | NWMN_0995/+ | NWMN_0996/+ | 30.5 |
| 20 | 1141544 | φNM2 | Phage lysin amidase/+ | isdB/− | 42.1 |
| 21 | 1175020 | vsaγ | NWMN_1071/− | NWMN_1072/− | 23.2 |
| 22 | 1175024 | vsaγ | NWMN_1071/− | NWMN_1072/− | 23.1 |
| 27 | 1350072 | core | NWMN_1224/+ | NWMN_1225/+ | 28.3 |
| 36 | 1979939 | φNM1 | NWMN_1768/− | srn_9350/+ | 27.8 |
| 37 | 2017461 | φNM1 | NWMN_1810/− | NWMN_1811/− | 29.6 |
| 38 | 2018908 | φNM1 | NWMN_1811/− | NWMN_1812/+ | 38.9 |
| 40 | 2019372 | φNM1 | NWMN_1811/− | NWMN_1812/+ | 32.5 |
| 41 | 2083121 | φNM3 | NWMN_1870/− | srn_3770/+ | 25.5 |
| 47 | 2605426 | core | NWMN_2367/− | NWMN_2368/− | 34.8 |
| 48 | 2839262 | core | lip/− | hisIE/− | 27.3 |
The locations listed are based on coordinates in the Staphylococcus aureus Newman strain. Core and accessory candidates were determined based on the genome sequence published by Baba et al.23.
Experimental validation of 17 novel sRNAs expressed from core and accessory genomes
Using RNA-Seq data and after curation, the number of sRNA candidates was reduced considerably, from 48 to 17 IGRs. To avoid false positives, we needed to validate their expression by other means. Transfer-messenger RNA (tmRNA) was used as an internal control for northern blot analysis monitoring the expression of the 17 IGRs during growth in brain-heart infusion (BHI) medium at four time points (Fig. S1). In this way, the expression of 11 sRNAs was confirmed (Fig. 1A–K), corresponding to the regions with the highest HTSeq counts in the RNA-Seq dataset (Table 1). As six of the 17 new sRNAs were not detected by northern blot analysis, RT-PCR was conducted, and this confirmed that these IGRs were in fact transcribed (Fig. 1L). IGR copies 1103623 and 2017461, which share 98% identity, were detected by northern blots using probes directed against the sequence not conserved between the two sRNAs, indicating that they are both transcribed in Newman (Fig. 1C,F). The relative quantification of sRNAs revealed that the transcript levels expressed from IGRs 1050586, 1141544, and 2019372 all varied as a function of the growth phase (Figs 1A,D,H and S2). When compared with the sRNAs extracted in the middle of exponential phase (OD600nm = 0.5), a 2- to 4-fold increase in RNA levels was observed at an OD600nm of 6, followed by a decrease (although for IGR_1141544 this was moderate) (Fig. S2). The transcript length of each RNA was estimated using tmRNA (358 nt) combined with a prestain marker for small RNA (Fig. 1A–K). For the most part, the calculated lengths were close to those estimated by DETR’PROK and/or by visualization using Artemis software. However, significant differences were observed for a few sRNAs. For instance, IGR_1141544 (Fig. 1D) exhibited a strong northern blot signal for an approximately 150-nt long transcript with the presence of higher bands of weaker intensities. Similarly, northern blot revealed two RNA fragments for IGR_2839262 (Fig. 1K). To assess whether the presence of additional bands was due to RNase enzymatic cleavages, contribution of RNase Y and RNase III35 to a potential processing of IGR_2839262 was tested and rejected (Fig. S3). This suggests (i) that one or more other RNases (RNase J1 or J2, RNase R, etc.) may process the RNA; (ii) that cleavage may not be RNase-dependent; or (iii) that the multiple bands result from distinct transcriptional units.
Figure 1.
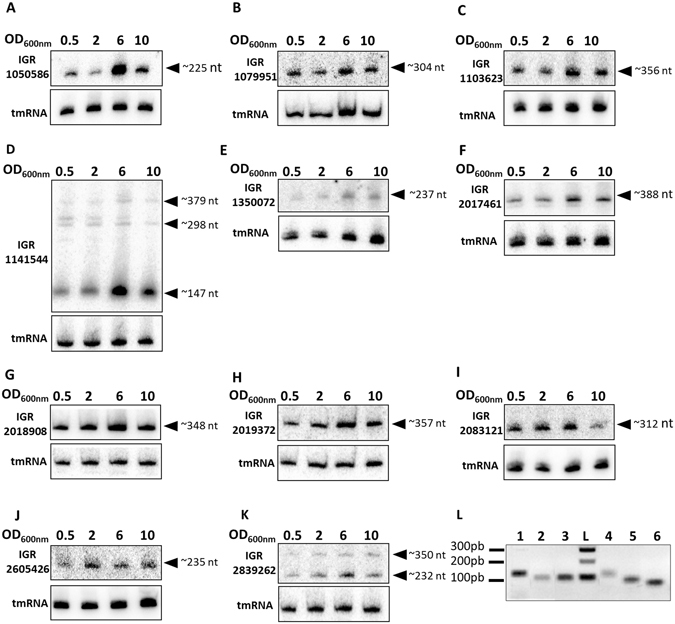
Experimental validation of 17 novel RNAs expressed from Staphylococcus aureus Newman strain. Northern blots (A–K) were performed on RNA extracted from cells collected at an OD600nm of 0.5, 2, 6, and 10. tmRNA, transfer-messenger RNA used as an internal control; nt, nucleotides. (L) RT-PCR was performed on RNA extracted from cells collected at an OD600nm of 6. Lanes 1 to 6 show IGR_ 1979939, IGR_ 363783, IGR_ 363785, IGR_ 1175020, IGR_ 1175024, and IGR_ 897930, from left to right. The data illustrate one representative experiment among three independent biological replicates. The gels presented here were cropped for clarity purpose.
To further examine the existence and conservation of these 17 sRNAs, we monitored the expression of the most-expressed transcripts (Fig. 1A–K, Table 1) in several S. aureus reference strains. To do that, we ran a BLAST query to look for homologous IGR sequences in the S. aureus N315, HG003, USA300, and UAMS1 genomes36–39. Among those that northern blots showed as being expressed, only IGRs 1050586, 1079951, 2083121, and 2605426 were retrieved in each of the four genomes, and their transcription was confirmed by northern blots (Fig. S4). Interestingly, IGR_2083121 was reported to belong to the accessory genome (φNM3) of Newman23. Taken together, our data confirmed the existence of at least 17 novel sRNAs located either in the core or accessory genome of Newman, including four sRNAs conserved among various S. aureus strains.
Screening for cis-antisense transcription specifically targeting the novel sRNAs raised the number of sRNAs to 22
Studies aiming at identifying novel sRNAs have reported antisense transcription of both coding sequences and sRNA genes14, 40. Two sense-antisense pairs have been identified24, 41 among the sRNAs expressed from S. aureus pathogenicity islands18. Here, we noted that there are sense-antisense pair characteristics in four of the experimentally confirmed RNAs (identified by RT-PCR in IGRs 363783, 363785, 1175020, and 1175024). Two by two, they share the same loci but are transcribed from opposite strands. This made us wonder whether the remaining 13 transcripts were associated with transcription located on the opposite strand and undetected by DETR’PROK due to the average library size and the sequencing mode (2 × 100 bp). To investigate this, and because so far the S. aureus RNAs transcribed from the opposite strand of a known sRNA are usually smaller than 100 nt14, 22, we performed a new RNA-Seq. In this one, we made and purified cDNAs with a minimal length of about 70 bp and generated single reads of 50 nt (sequenced using MiSeq), while the size of the smallest fragments generated for HiSeq sequencing was larger than 100 bp. We then used Artemis to specifically look for reads mapping on the opposite strand of these 13 RNAs, finding five additional read clusters (Table 3). Interestingly, we identified two of these (named as2 and as3) on the opposite strand of sRNAs expressed from IGR_1141544. Probes and primers were designed to verify whether transcripts could be detected by northern blots and RT-PCR. Even though no bands could be observed by northern blot using various DNA probes, all transcripts were individually recovered by RT-PCR (using specific primers for reverse transcription). This suggested low expression levels of these RNAs under our experimental conditions (Fig. 2). Altogether, this increased the novel sRNA count to 22, and highlights the importance of combining several sequencing protocols and detection methods for thorough and exhaustive sRNA identification.
Table 3.
Transcription detected on the opposite strand of the novel RNA transcripts determined by RNA-Seq.
| RNAs | Coordinates* | Targeted IGR | Strand | Length |
|---|---|---|---|---|
| as1 | 1080216–1080299 | 1079951 | + | 83 |
| as2 | 1141494–1141607 | 1141544 | − | 113 |
| as3 | 1141665–1141817 | 1141544 | − | 152 |
| as4 | 2019015–2019244 | 2018908 | − | 229 |
| as5 | 2019390–2019490 | 2019372 | − | 100 |
*The coordinates were determined using Artemis.
Figure 2.
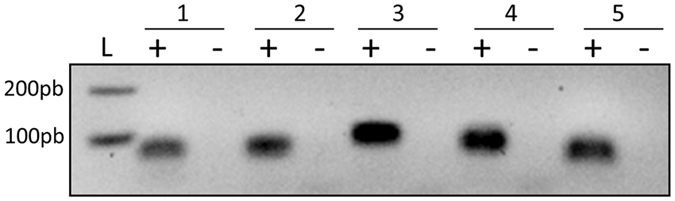
Antisense transcription detected on the opposite strand of some newly identified RNAs through MiSeq RNA sequencing. RT-PCR was performed on RNA extracted from cells collected at an OD600nm of 6. Lanes 1 to 5 show IGR_1080208, IGR_1141547, IGR_1141775IGR, IGR_2019414 and 2019159, from left to right after cDNA synthesis (+) or after DNase treatment (−). The gel presented here was cropped for clarity purpose.
Newman strain contains an unusual condensed sRNA cluster composed of five transcriptional start sites
In IGR_1141544, comparison of the transcript lengths determined by northern blots with the sRNA lengths either inferred from DETR’PROK or after Artemis visualization of single ends revealed major differences. While DETR’PROK predicted a single transcript of 424 nt (Table 1) and Artemis suggested a 650-nt one (Fig. 3A), we identified an sRNA about 150-nt long by northern blot, with increased expression levels during growth (Figs S1 and S2). Additional focus using an RNAseY mutant did not evidenced any maturation of a longer transcript (Fig. S3). To try to explain the differences between the predictions and our experimental results, we further investigated this particular sRNA-rich locus located within φNM2. Thorough inspection of RNA-Seq reads specifically mapping this region revealed that transcription of this RNA should start within the CDS 3′ end of NWMN_1039-1, which encodes the φNM2 autolysin amidase domain (Fig. 3A). Based on the heterogeneous depth and on the location of paired-end reads, read mapping profiles suggested the presence of several transcripts (Fig. S5). We therefore designed probes for northern blots targeting different loci (Fig. 3A, P1 to P3). This revealed the presence of several RNAs transcribed from the positive strand in this nearly 700-nt region. Interestingly, northern blot conducted with probe P1 resulted in the detection of two bands of around 130 and 260 nt (Fig. 3B). This suggested the presence of either two distinct transcription start sites (TSSs) or a single TSS with two transcription terminators. Sequence analysis using ARNold42 supported the second hypothesis, revealing two Rho-independent terminators: one corresponding to the NWMN_1039-1 3′ end, and another located further downstream. Conversely, probes P2 and P3 identified single transcripts of around 140 and 310 nt (Fig. 3B). Through RT-qPCR, we showed that levels of all three RNA (P1 to P3) were increased at OD600nm 6 and 10 (Fig. 3C), confirming the previously obtained northern blot results (Figs 1 and S2). Likewise, RT-qPCR conducted to monitor RNA levels of as2 and as3 (the MiSeq-identified RNAs; see Table 3) revealed a similar expression profile (Fig. 3C). This indicated that the entire locus responds to changes of growth phase and maybe also to nutrient starvation or cell density. Additionally, the differences observed in the CTs obtained by RT-qPCR confirmed that as2 and as3 were weakly expressed compared to the RNAs transcribed from the positive strand. We then used RACE-mapping on both strands to identify the 5′ and 3′ ends of the different RNA molecules expressed from this tiny region (Table 4). This confirmed the presence of the four transcripts detected by northern blot (Fig. 3B). Furthermore, it proved that the two transcripts detected with P1 share the same 5′ end, while the two 3′ends correspond to the position of predicted Rho-independent terminators and should therefore be considered as a single transcription unit. Because this genomic locus has a very compacted structure, we checked to see whether these novel RNAs were transcribed from distinct TSSs or were the result of the maturation of a long transcript. From total RNA, we degraded mono-phosphorylated RNAs (RNAs issued from post-transcription 5′ processing) using Terminator 5′-Phosphate-Dependent Exonuclease (TEX) followed by polyphosphatase treatment, to enrich the ligation of the TSS RNA. This confirmed that the three positive-strand RNAs are independent transcription units (Table 4). On the opposite strand, RACE-mapping similarly confirmed the presence of two RNAs of 138 and 170 nt, as2 and as3 (Table 4), as found in the previous MiSeq experiments (see above). Therefore, among the 22 previously identified sRNA, we were able to prove the existence of three sRNAs (instead of one) expressed from the positive strand of IGR_1141544, giving us a new total of 24 sRNAs expressed in Newman.
Figure 3.
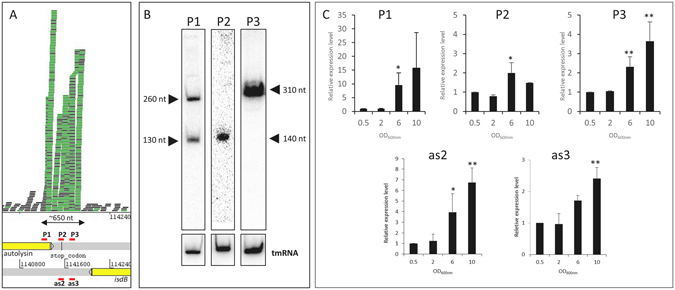
Characterization of an sRNA transcription hotspot in Staphylococcus aureus Newman. (A) RNA mapping profile of IGR_11415444 visualized with Artemis. P1, P2, P3, as2, and as3 correspond to the position of probes and/or primers for northern blot and RT-qPCR experiments. (B) Identification of multiple transcripts expressed from the positive strand of the Newman genome. Total RNA were extracted from cells collected at an OD600nm of 6 and tmRNA was used as an internal loading control. (C) Relative expression levels of the IGR_1141544 locus as a function of growth phase. The relative cDNA level was determined using HU as an internal control and OD600nm of 0.5 as a calibrator. The data shown are the means of three independent experiments. A student t-test was performed to determine differences with condition at OD600nm of 0.5 (*p < 0.05, **p < 0.01, ***p < 0.001). The gel presented here was cropped for clarity purpose.
Table 4.
RACE-mapping characterization of sRNAs transcribed from IGR_1141544.
| Target or probe | Coordinates | Strand | Length |
|---|---|---|---|
| P1 | 1141253–1141502 | + | 249 |
| P1 | 1141253–1141398 | + | 145 |
| P2 | 1141525–1141656 | + | 131 |
| P3 | 1141676–1141990 | + | 314 |
| as2 | 1141515–1141653 | − | 138 |
| as3 | 1141677–1141847 | − | 170 |
Extensive sRNA end-mapping and assignment of srn identifiers
We previously compiled staphylococcal sRNAs into the SRD database under simple srn identifiers to harmonize the sRNA repertoire22. To generate names for these 24 newly discovered sRNAs, we first determined the 5′ and 3′ ends of the transcripts by RACE-mapping (Table 5). Then, we provided an srn identifier for each RNA (Table 5) based on its location on the genome of five reference strains (N315, Newman, JDK6008, NCTC8325, and USA300) as provided in SRD22. The nucleotide sequences defined by RACE confirmed all of the previously estimated lengths, and provided additional proof that the two sRNA copies, hereby renamed Srn_9650 (IGR_1103623) and Srn_9660 (IGR_2017461), were transcribed. We also used TEX followed by polyphosphatase treatment to identify a TSS for srn_3765 (IGR_2083121) at position 2083472 (a 276-nt transcript), while an RNA fragment of only 204 nt was detected without TEX treatment, suggesting maturation of that particular RNA at position 72 (not detected by northern blot, Fig. 1I). TEX-RACE-mapping of Srn_5075 (IGR 2839262) only revealed a 220-nt fragment, indicating that the upper band detected by northern blot is non-specific (Figs 1K and S3). Using the accurate srn coordinates, we compared the 24 new sRNA sequences with sRNAs recently identified in USA300 and HG00115, 21 and for which the srn annotation was not yet available. This showed that, among the 24, only three of them (srn_2335, srn_9345, and srn_4635) were already identified as transcribed in those two recent studies (Table 5). All the 24 transcripts identified in this report fit in the sRNA class, as the longest is shorter than 400 nt. As studies on sRNAs showed that some can contain ORFs2, 11, 14, 21, 22, we used ORFfinder to search for their presence within each mapped sequence. This unveiled that 13 sRNAs contained a small ORF (Table 5). We then used them to BLASTP to assess whether they were automatically annotated as potential CDS within S. aureus taxon. This revealed that, among the 13 sRNAs that contained an ORF, 10 were predicted to encode a hypothetical small protein or peptide in at least one S. aureus strain although transcription of these putative genes (and therefore translation) was not demonstrated. We also used the Uniprot database to search for sequence homology with known proteins or domains. Eight of them did not match any referenced proteins or peptides, while the two remaining shared partial homologies with larger proteins. Together, these data indicate that 14 of the identified transcripts identified are bona fide non-coding sRNAs, and that the features of the remaining 10, make them good candidates for sRNA with potential dual-functions. Overall, it shows that the S. aureus sRNA content has not been fully uncovered yet.
Table 5.
Coordinates of 24 novel transcripts determined by RACE-mapping in Staphylococcus aureus Newman and their assigned Srn identifiers.
| Identifiers | Other names | Genomic coordinates | Strand | Length | ORF | Potential CDS | Homologies with known proteins or peptides |
|---|---|---|---|---|---|---|---|
| srn_0795 | IGR_363783 | 363827–364198a | + | 371 | YES | YES | Partial with lipase |
| srn_9640 | IGR_363785 | 363785–364009 | − | 265 | NO | NO | None |
| srn_2058 | IGR_897930 | 897979–898218 | + | 239 | YES | NO | None |
| srn_2335 | IGR_1050586; tsr18 b | 1050865–1051075 | − | 210 | YES | YES | Partial with SufA |
| srn_2347 | IGR_1079951 | 1079989–1080300 | − | 311 | YES | YES | None |
| srn_2348 | as1 | 1080214–1080281 | + | 67 | NO | NO | None |
| srn_9650 | IGR_1103623 | 1103685–1104067 | − | 382 | YES | YES | None |
| srn_9342 | IGR_1141544; P1 c | 1141253–1141502 | + | 249 | NO | NO | None |
| srn_9343 | as2 c | 1141515–1141653 | − | 138 | YES | YES | None |
| srn_9344 | IGR_1141544; P2 c | 1141525–1141656 | + | 131 | NO | NO | None |
| srn_9345 | IGR_1141544; S808 d, P3 c | 1141676–1141990 | + | 314 | NO | NO | None |
| srn_9346 | as3 c | 1141677–1141847 | − | 170 | NO | NO | None |
| srn_2467 | IGR_1175020 | 1175201–1175405a | − | 204 | NO | NO | None |
| srn_2468 | IGR_1175024 | 1175217–1175422 | + | 205 | NO | NO | None |
| srn_9348 | IGR_1350072 | 1350145–1350345 | + | 200 | YES | YES | None |
| srn_9349 | IGR_1979939 | 1980012–1980322 | + | 310 | YES | NO | None |
| srn_9660 | IGR_2017461 | 2017588–2017972 | + | 384 | YES | YES | None |
| srn_9670 | IGR_2018908 | 2018982–2019366 | + | 384 | YES | NO | None |
| srn_9671 | as4 | 2019068–2019159 | − | 91 | NO | NO | None |
| srn_9680 | IGR_2019372 | 2019373–2019729 | + | 356 | YES | YES | None |
| srn_9681 | as5 | 2019391–2019458 | − | 67 | NO | NO | None |
| srn_3765 | IGR_2083121 | 2083196–2083472 | − | 276 | YES | YES | None |
| srn_4635 | IGR_2605426; S1077 d | 2605636–2605853 | − | 217 | YES | YES | None |
| srn_5075 | IGR_2839262 | 2839213–2839434 | − | 221 | NO | NO | None |
anot mapped by RACE; bdescribed by Carroll et al.21; cdescribed in Fig. 3, Table 3, and Table 4; ddescribed by Mader et al.15. ORF of 30 codons or more were identified using ORFinder. Potential CDS were searched using BLAST within the non-redundant database. Homologies with known proteins were searched using Uniprot database. Homologies were described as partial when the coverage and length of the amino acid sequence did not exceed 60% of the homologous protein sequence.
Conservation of newly discovered sRNAs in Staphylococcus aureus
The assignment of srn identifiers followed with their alignment showed that some sRNAs (mostly those starting with srn_9XXX) were not detected in the four SRD reference strains (N315, NCTC8325, JKD6008, and USA300). Therefore, to estimate the conservation levels of the 24 novel sRNAs, we aligned their sequences to assess their presence and absence in 22 S. aureus strains that belong to well-characterized clonal complexes (Fig. 4). This revealed that 7 of them (srn_2058, srn_2335, srn_2347, srn_2348, srn_3765, srn_4635, and srn_5075) are conserved among several S. aureus strains and have high nucleotide sequence identities (Fig. S6). The analysis of the presence and absence of the 24 sRNAs showed similar sRNA content in Newman, JH1, and JH9. However, Newman is the sole strain that has both copies srn_9650 and srn_9660 within its genome, with srn_9650 unique to Newman and srn_9660 also encoded in CA-347, an MRSA USA600 strain isolated in 2005 from bloodstream infection. Interestingly, only JH1 and JH9 have the five sRNAs identified in Newman srn_9342-9346 cluster (IGR_1141544), while other strains contain none or just two or three of these sRNA genes (Fig. 4). MSSA476 contains the srn_9343/srn_9344 genes, but srn_9342 is absent for the first 156 nt and located downstream of another autolysin amidase domain. Collectively, these results show that some of the srn genes we identified here are well-conserved within S. aureus, while others are strain- or lineage-specific.
Figure 4.
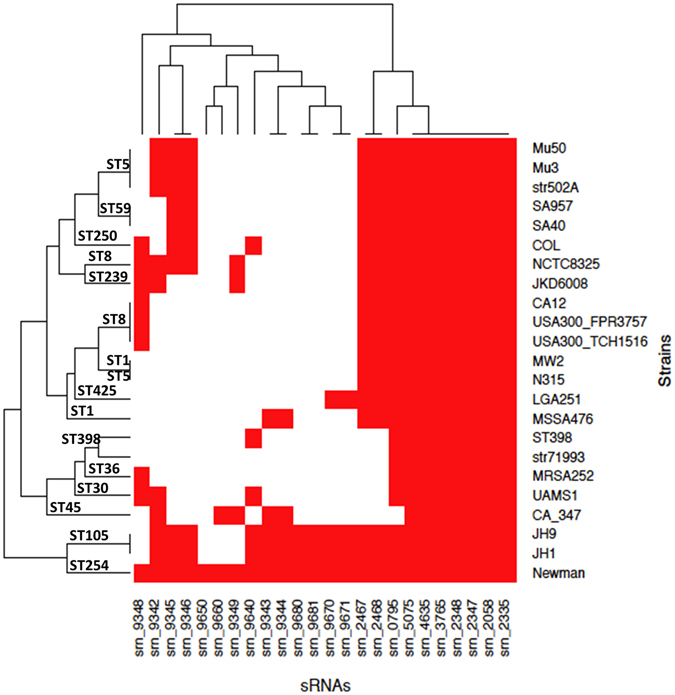
Comparative analysis of the presence and absence of novel srns in Staphylococcus strains. A heat map was generated based on the presence (red) and absence (white) of srns in strains belonging to various clonal complexes. The data were clustered based on srns and genome sequence identities.
Genetic organization of the srn_9342-9346 locus is unique to Newman strain
The analysis of these new sRNAs in various S. aureus genomes showed significant differences in the distribution of the five sRNAs expressed from the srn_9342-9346 locus. Therefore, we compared the genetic organization of this srn_9342-9346 locus in Newman, NCTC8325, CA-347, Mu50, and JH1 strains, all from different lineages (Fig. 4) and representative of the genomic distribution in S. aureus of these five sRNAs in S. aureus. This revealed that Newman is the only strain that has this highly compacted srn gene cluster (Fig. 5). In Newman, the srn set is located at the 3′ end of pathogenicity island φNM2, and between an autolysin amidase domain and isdB, a gene encoding a hemoglobin receptor required for heme iron utilization43. NCTC8325 is characterized by the presence of srn_9342 (also located at the 3′end of an autolysin amidase domain) and srn_9345/srn_9346, whereas a three-CDS insertion occurred where Newman has srn_9343/srn_9344. In CA-347, srn_9342-srn_9344 organization is conserved, but the last two sRNA genes are lacking. Interestingly, Mu50 and JH1 have two copies of srn_9342 (Fig. 5). In Mu50, the first copy is retrieved as a single srn upstream of fhuD (and therefore dissociated from an autolysin), and an organization similar to that of NCTC8325 is also present elsewhere in the genome. In JH1, three loci contain sRNA genes of Newman’s srn_9342-srn_9346 locus, with srn_9342 again found in two copies (the first at the 3′ end of an autolysin amidase gene). The srn_9345/srn_9346 gene cluster displays an organization that resembles that of Mu50. In a third genomic environment, srn_9342 and srn_9343/srn_9344 are located next to another autolysin amidase domain, upstream from an HtrA-like protein coding-gene. Therefore, the comparative analysis showed that the genetic organization of the accessory srn_9342-9346 sRNA genes is specific to Newman, and that differences in the nearby genetic environment may induce differences in sRNA expression patterns.
Figure 5.
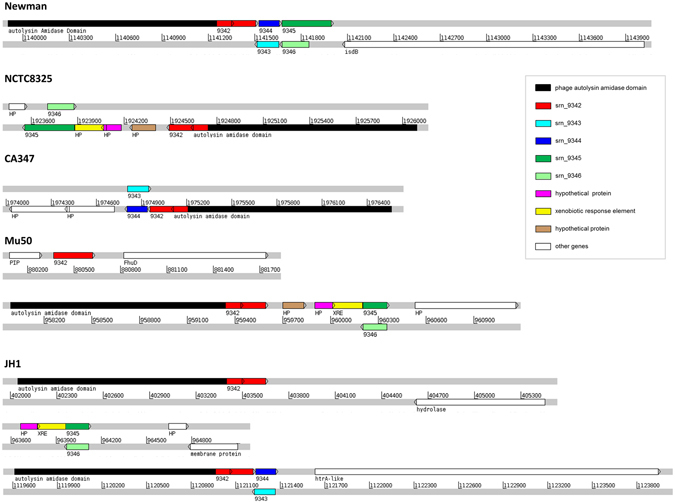
Comparative genomics of the genetic organization of the srn_9342–9346 cluster. The genetic organization of the srn9342–9346 locus from Staphylococcus aureus Newman strain was compared with that of the NCTC8325, CA347, Mu50, and JH1 strains.
Srn_9343 encodes a peptide secreted in the extracellular medium
Analysis of the distribution of srn_9343/srn_9344 revealed that their presence is less conserved than that of the other genes in the locus. While no ORF was identified on the positive strand of the srn_9342-9346 locus, analysis of the minus strand revealed a small ORF within the srn_9343 sequence (Table 5). It contains a start codon located downstream of a putative RBS and therefore potentially code for a 33-amino acid peptide. This putative peptide sequence was predicted and annotated as hypothetical protein in NCBI database only in three S. aureus strains and three phages (Fig. 6A), with a predicted molecular weight of 3.9 kDa. To assess whether it is produced by S. aureus, we cloned srn_9343 under the control of its own promoter by including up to 250 nt upstream in translational fusion with a 3xFLAG (adding a 2.9-kDa epitope at its C-terminus). Western blot analysis did not reveal any translation of the fusion peptide in cell extracts or in the Newman supernatant (Fig. S7), indicating a potential down-regulation of its expression under these conditions. Therefore, to avoid any regulatory effects due to srn_9342-9346 locus expression, we used a Pveg constitutive promoter44 and transformed N315, chosen because its genome includes none of these five srns (Fig. 4). Although Srn_9343 peptide expression was not detected in cell extracts, it was seen in the N315 supernatant after culture in BHI broth (Fig. 6B), indicating that the peptide is produced at the expected size (Fig. 6C) and secreted. Analysis of the amino acid sequence revealed that it does not share significant similarities with phenol-soluble modulin45, 46, but presents a hydrophobic C-terminus. This C-terminus domain is similar to a RelE addiction-module toxin domain in Streptococcus mutans (E-value 0.004) and Peptostreptococcaceae bacterium (E-value 5. 10−5). Altogether, the presence of transcription on both strands, as for Srn_3580_SprA24, and similarity with the RelE toxin suggests that srn_9343/9344 could belong to a putative toxin-antitoxin system.
Figure 6.
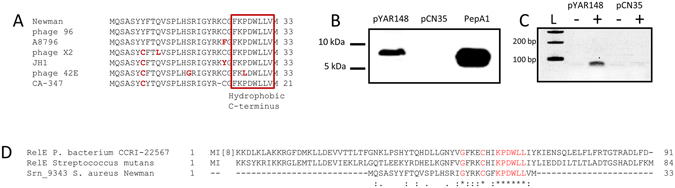
srn_9343 encodes a peptide expressed in Staphylococcus aureus that is secreted extracellularly. (A) Sequence alignment of the Newman peptide sequence with the S. aureus annotated peptides. (B) Western blot analysis of Srn_9343 flagged-peptide expression from pYAR148 (Table 6) under the pVeg promoter. This is compared to empty vector (pCN35) and to PepA1 from Sayed et al.66. (C) RT-PCR analysis of the expression of srn_9343 under the Pveg promoter. (D) Sequence alignment of Srn_9343 with RelE toxins. The experimental data shown here are representative of three independent experiments. The dot and gel presented here were cropped for clarity purpose.
Expression of novel S. aureus Srns under various stress conditions
Many sRNAs respond to specific environmental modifications and help regulate dedicated physiological networks12, 13, 47, 48. Therefore, the expression of the 11 srns detected by northern blot was assessed under various conditions, including some that mimic the changes encountered by S. aureus during host infection. Cells were grown in TSB until reaching the exponential phase of growth (OD600nm = 2), then subjected to stresses related to pH (acidification and alkalization), temperature (heat and cold shocks), oxidation, osmotic levels, and nutrient availability. After either 30 or 60 minutes of stress, the sRNA levels were monitored by northern blot and compared with those produced under unstressed conditions (Fig. 7). Among all of the tested transcripts, Srn_2347 was the only sRNA which had an unwavering transcript level no matter the stress applied. After 30 minutes of oxidative stress, the RNA levels decreased 3- to 10-fold in six Srns (Srn_5075, 9348, 9650, 9660, 9670, and 9680, a group which includes the two sRNA copies). This was also observed after one hour in all of them, except Srn_9348 (Fig. 7). Conversely, Srn_4635 was temporarily overexpressed under oxidative stress (about 3-fold after 30 minutes), while Srn_2335 and Srn_9344 were both overexpressed after one hour in the presence of 10 mM H2O2. Likewise, Srn_2335 and 4635 transcript levels increased by more than 2-fold after 30 minutes in alkaline conditions (pH 9.5), and remained high in Srn_2335 after one hour of stress. Nutrient deprivation (NZM) in both Srn_3765 and Srn_9348 led to decreased RNA levels after both 30 minutes and 1 hour. Similarly, Srn_9680 levels were around 3-fold lower after one hour in NZM medium. Cold shock (18 °C) in Srn_3765 led to increased RNA levels (3- and 4-fold after 30 minutes and one hour, respectively), whereas Srn_2335 level was multiplied by six after 1 hour. Finally, heat shock (42 °C) led only to an induction of Srn_5075. Interestingly, the two copies (Srn_9650 and Srn_9660) as well as Srn_9670 and Srn_9680 (located near Srn_9660) respond to the same stresses, suggesting similar regulatory mechanisms.
Figure 7.
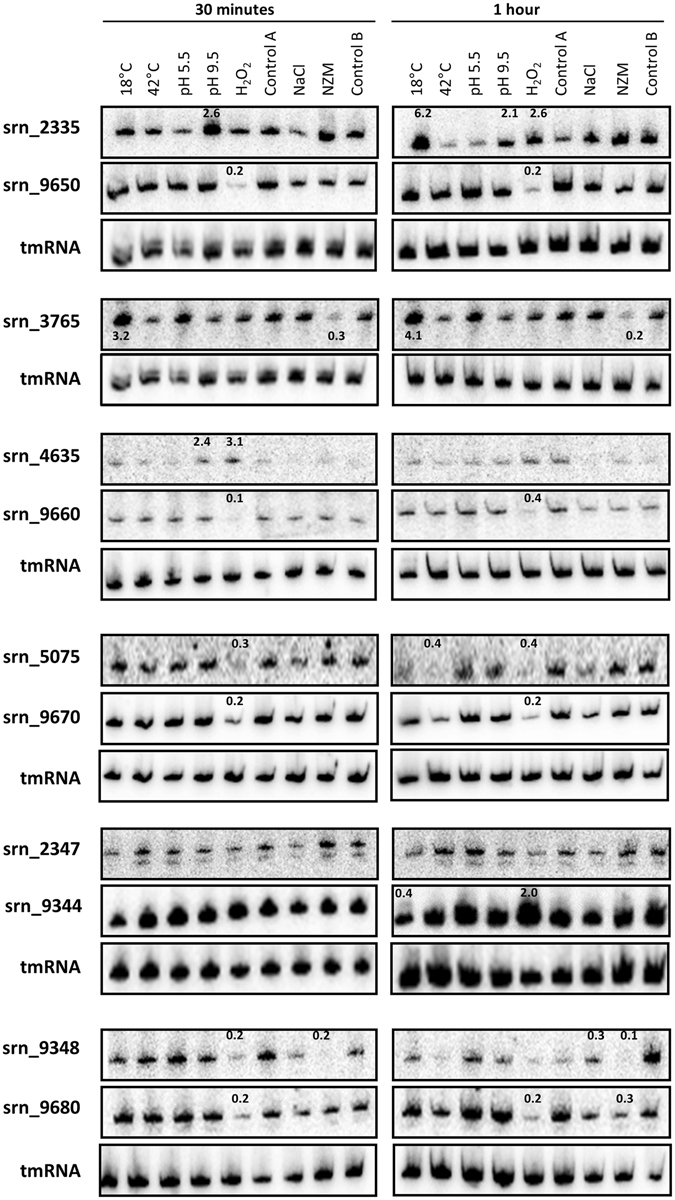
Expression of srns monitored under various stress. Staphylococcus aureus Newman strain was cultured in TSB broth to an OD600nm of 2 (exponential growth phase). Stress was induced by the addition of H2O2, HCl, or NaOH, or by changing the temperature to 18 °C or 42 °C. Control A corresponds to cells maintained in TSB under normal conditions. In parallel, other cultures were centrifuged at 4500 rpm for 8 min at RT and pellets resuspended in TSB supplemented with 1 M NaCl (mimicking osmotic stress), NZM medium (emulating a stringent response), or fresh TSB (control B). The relative expression levels are indicated where appropriate, and were calculated after quantification of the dots using control A or B as calibrators. For all experiments, tmRNA was used as an internal loading control. The gels presented here were cropped for clarity purpose.
Going further, to verify whether the whole srn_9342-9346 locus responds to stress similarly to srn_9344 (which is sensitive to oxidative stress and cold shock), we investigated the expression levels of these srns. We used RT-qPCR rather than northern blot as we found that Srn_9343 and Srn_9346 are expressed at low levels in RNA extracts (Fig. 2). After H2O2 exposure and nutrient starvation, data analysis revealed differences in the sRNA levels (Fig. 8). In NZM, Srn_9342 RNA levels doubled after 30 minutes and 1 hour, while Srn_9345 induction was observed later, after 1 hour of stress (Fig. 8A). Conversely, Srn_9344 RNA levels decreased slightly. Under oxidative pressure, Srn_9342 RNA levels dropped by around three-fold after 30 minutes, then recovered after 1 hour (Fig. 8B). The levels of the other sRNAs remained nearly identical, although Srn_9344 increased by 7-fold after 1 hour. Together, these data show that transcript levels of 15 novel Srns vary under specific stresses (see summary in File S3). This suggests that their expression is tightly regulated and that they must be involved in distinct physiological pathways. In addition, RT-qPCR analysis of differential expression of the srn_9342-9346 locus (Fig. 8) showed that the RNA levels of its components can be influenced by nutrient deprivation and oxidative stress.
Figure 8.
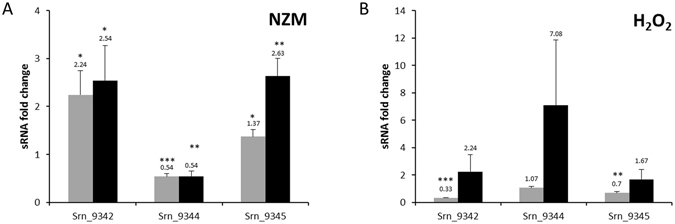
Differential expression of the srn_9342-9346 locus. sRNA expression levels (A) after nutrient starvation in NZM medium and (B) under oxidative stress (10 mM H2O2). The fold changes were calculated after 30 minutes (grey bars) and 1 hour (black bars) of stress using the TSB condition as a control. HU (for NZM) or 5 S cDNA levels were used as internal controls. Data presented are the mean of three independent experiments, all performed in duplicate. A student t-test was performed to determine differences with unstressed condition (*p < 0.05, **p < 0.01, ***p < 0.001).
Discussion
The last decade has led to the discovery of most S. aureus sRNAs, although their functions remain unknown in most cases22. This exponential rise in the identification of sRNAs expressed by this major pathogen was enabled by the wide availability and affordability of next-generation sequencing technologies. So far, studies devoted to the detection of novel sRNAs have focused on a few strains, and the results were recently compiled into a dedicated database22. Additionally, GenBank files that include sRNAs are available for three S. aureus strains21. It was necessary to clarify the count of already published sRNAs, so simple identifiers were provided for all of them in addition to their gene coordinates. This mandatory step allows for RNA-Seq studies of the whole transcriptome (mRNA, tRNA, rRNA and sRNA) and facilitates the identification of new sRNAs. Recent publications have demonstrated that knowledge of the full repertoire, the pan-sRNome, has not yet been achieved15, 21, as it varies significantly according to the strain22. S. aureus epidemiology indicates that clonal complex 8 lineages are widely distributed worldwide and dominate human carriage and infections49. We therefore continued our efforts to extend the S. aureus pan-sRNome by studying the Newman clonal complex 8 strain. This strain has been fully sequenced23 and used successfully in animal models of staphylococcal infections, but its sRNA content is quite under-investigated.
We began by using the DETR’PROK framework27 to search for unannotated transcribed regions within the Newman IGRs. This resulted in the identification of 48 loci for which RNA-Seq data allowed read clustering. Although DETR’PROK identified sRNA content differences between the Newman and the Newman Δsrn_3610_sprc strains, differential expression analysis revealed no statistical differences between the two. Previous studies underlined the high probability of identifying repeated sequences, or 5′ or 3′ UTRs11, 14, 22. To avoid this issue, we assigned specific parameters to DETR’PROK, used several cut-offs, systematically used BLAST, and viewed the read mappings to determine whether sRNA candidates seemed to be independent transcriptional units. In addition, misannotated genes were removed during a final manual cull (see File S2) to avoid the identification of putative artefacts. Although we cannot exclude the removal of some true sRNAs, by being safe the combination of these measures narrowed down the candidate set to 17 IGRs, and these were subsequently confirmed by northern blot and/or RT-PCR. None of them were previously reported in the SRD22 or BSRD databases50 devoted respectively to Staphylococcus and bacterial sRNA.
A careful study of the 17 transcribed IGRs began with MiSeq, which led to the discovery of five additional sRNAs. This was completed with RACE-mapping, which characterized the 24 independent transcriptional units or primary transcripts. According to Rfam and Riboswitch finder51, 52, the 24 novel sRNAs do not present the sequence and structural characteristics of riboswitches. An in-depth study of IGR_1141544 revealed the presence of three TSSs on the plus strand, and two sRNAs transcribed from the minus strand at the same locus as Srn_9344 and Srn_9345. Such transcripts are common in bacteria with the discovery of natural antisense transcription40, 53, 54. However, we found that this locus’ organization is specific to the Newman strain and unusual for sRNAs, and on the positive strand the transcription starts within the 3′ region of an autolysin amidase CDS in the 3′ region. Interestingly, no ORFs were found on the positive strand along this approximately 700-nt transcribed region. RACE-mapping of Srn_9342 surprisingly indicated that two terminators are used, generating two distinct 3′ ends. Its transcription starts within the autolysin coding sequence, mostly leaked through a first rho-independent terminator (which is from autolysin), and when it reaches the next rho-independent terminator the transcription stops. Analysis of the minus strand revealed the presence of an ORF within srn_9343 along with an RBS upstream the predicted AUG initiation codon. Using 3xFLAG, we showed that the Srn_9343 peptide is produced and secreted. However, failure to express this peptide in Newman suggests the existence of a specific regulatory mechanism that shuts down srn_9343 expression and translation. Looking outside the Staphylococcus genus, this peptide sequence presents similarities to the bacterial RelE toxin. RelE is a part of a type II toxin-antitoxin module55 in which the toxin RelE acts as a ribosome-dependent endoribonuclease activated in diverse cellular processes, including nutrient starvation and persistence56. The antitoxin encoded by RelB counteracts RelE’s toxic activity by modifying the structure of the RelE catalytic domain57. During amino acid starvation, RelE inhibits translation by cleaving within the mRNA ribosomal A-site. Interestingly, our expression analysis conducted in NZM-poor medium showed that Srn_9344 slightly decreased, while RNA levels of Srn_9342, Srn_9345, and Srn_9346 increased. However, no RelB homologues could be identified next to Srn_9343 or elsewhere in the Newman genome, suggesting that Srn_9343 may not be a part of a type II toxin-antitoxin system in S. aureus. The architecture of the srn_9342-9346 locus is unusual in S. aureus. The presence of an sRNA (Srn_9344) transcribed from the opposite strand of the peptide-coding Srn_9343, and at the same locus, suggests that Srn_9344 could function as a cis-antisense. We hypothesize that this pair may function as a type I toxin-antitoxin system. Apart from S. aureus, another unusual sRNA cluster was recently identified in the Enterococcus faecalis clinical isolate V583, where a chromosomal locus containing two toxin-antitoxin modules (type I and II) with cis-antisense transcription was discovered58.
Our search for ORFs within the 24 sRNAs mapped by RACE revealed that some of them may encode peptides or small proteins, although none of them are homologous to phenol-soluble modulins45, 46 or any peptide/proteins with a known function. Many published sRNAs were described as having coding capacities11, 14, 21, 22 and there are accumulating evidences for the existence of RNAs with dual functions in prokaryotes and eukaryotes59, 60. In S. aureus, this is the case for multifunctional regulatory RNAIII, that besides monitoring the expression of a large set of mRNA targets in its RNA form2, 3, encodes the delta-hemolysin. This raises the question of whether the term “noncoding RNA” sometimes used by the RNA community is still relevant and may suggest that some of the sRNAs described here may harbor dual-functions. Recent work in S. aureus showed that Srn_4340 (Teg23), which contains an ORF, is probably a small non-coding RNA rather than a small coding sequence, since no translation was seen at all21. In that study, the authors emphasized that current automatic annotation methods can lead to genome overannotation, therefore complicating the identification and study of new sRNA. It therefore appears that the distinction between the mRNA and sRNA world (apart from comparing RNA lengths) is probably more complicated and intertwined than expected, and that thorough experimental assessments of potential coding capacities and regulatory functions may be necessary.
The assignment of Srn identifiers as per a procedure established in SRD22 allowed us to investigate the distribution of the 24 srns within several S. aureus strains. This search revealed that seven of them were conserved among the strains we analyzed. Aligning these sRNA sequences revealed a high level of conservation, with only few SNPs. Such high conservation is usually observed for transcribed regions and is significantly decreased in true IGRs. One of the open questions is whether there are still additional sRNAs to discover in S. aureus. Based on the work we present here and on other recent studies21, all performed on different strains in the same clonal complex (CC 8), we can reasonably suppose that the annotation of the pan-sRNome is far from being complete. Therefore, the study of other clonal complexes and using various experimental conditions should help in the identification of novel sRNAs which could be specific to clonal complexes or conserved within the species and therefore belong to the sRNA core. Investigations could be extended to the study of strains deleted for transcription factors, since it was recently shown that sRNAs could belong to regulons in S. aureus 30. Indeed, studies of the expression of our novel sRNAs under various stress conditions revealed that they respond to few specific stresses, and that their expression is therefore tightly monitored. Interestingly, our data indicated that many of these novel sRNAs are downregulated under oxidative stress. This can be opposed to the observation made in N315 during the identification of the Teg sRNAs11, implying that each sRNA responds to its own set of physico-chemical modifications.
In conclusion, including the 24 novel sRNA coordinates in the SRD will enable subsequent RNA-Seq studies using various experimental conditions to monitor their physiological role. Global transcriptome approaches are necessary for an exhaustive characterization of the S. aureus RNome, and this step is essential before trying to understand its overarching role and function within the bacterium and especially during infection. Our work shows that the search for novel sRNAs in well-studied bacteria is valuable, and that this can be facilitated through setting conditions that mimic the in vivo environment, by using different RNA-Seq approaches, or by studying strains deleted for major transcription factors to start characterizing the complex connections between sRNAs and proteins in this major human pathogen.
Methods
Bacterial strains, growth, and stress conditions
The S. aureus strains used in this study are listed in Table 6. S. aureus was grown in Brain Heart Infusion broth (BHI, Oxoid) or in Tryptone Soya Broth (TSB, Oxoid) under agitation at 37 °C (except where stated). Growth was monitored by measuring the OD600nm at different time points. For the analysis of RNA levels under different stresses, S. aureus was cultured in TSB broth up to an OD600nm of 2 (exponential phase of growth). Stresses were induced by the addition of 10 mM H2O2 (oxidative stress), HCl (to lower the pH to 5.5), NaOH (to increase the pH to 9.5), or by temperature shifts to 18 °C or 42 °C (cold and heat shocks). In addition, 60 ml of culture was centrifuged at 4500 rpm for 8 min at room temperature and the pellet resuspended in TSB supplemented with 1 M NaCl (osmotic stress), NZM medium (stringent response), or fresh TSB as a control.
Table 6.
Bacterial strains and plasmids used in this study.
| Strains and plasmids | Characteristics | Reference | Accession number |
|---|---|---|---|
| S. aureus strains | |||
| Newman | Methicillin-sensible S. aureus strain | 23 | AP009351 |
| N315 | Methicillin-resistant S. aureus strain | 62 | BA000018 |
| HG003 | Derivative strain of NCTC8325 | 63 | JPPU00000000 |
| USA300 FPR3757 | Community-acquired methicillin-resistant clone | 38 | CP000255 |
| UAMS-1 | Oxacillin-susceptible clinical isolate | 64 | JTK00000000 |
| Newman-217 | Newman Δrny::ermC | 35 | |
| Newman-217 pCG296 | Newman Δrny::ermC with pCG296 for complementation | 35 | |
| Newman Δsrn_3610_sprC | Newman strain deleted for sRNA Srn_3610_SprC | 25 | |
| RN4220 | Type I restriction-modification-deficient strain | 65 | |
| E. coli strain | Srn_9343 under its promoter (100 nt) and with 3′ 3xFLAG | This study | |
| XL1 Blue | |||
| Plasmids | |||
| pYAR139 | |||
| pYAR140 | Srn_9343 under its promoter (250 nt) and with 3′ 3xFLAG | This study | |
| pYAR144 | Srn_9343 under its promoter (42 nt) and with 3′ 3xFLAG | This study | |
| pYAR146 | Srn_9343 under its promoter (70 nt) and with 3′ 3xFLAG | This study | |
| pYAR148 | Srn_9343 under Pveg promoter and with 3′ 3xFLAG | This study | |
Total RNA extraction
Cells were harvested by centrifugation at 4500 rpm for 10 min and pellets washed with 500 µL of cold lysis buffer (20 mM sodium acetate, 1 mM EDTA, 0.5% SDS, pH 5.5). Cell pellets were broken using acid-washed glass beads (Sigma) in the presence of phenol (pH 4) in an FP120 FastPrep cell disruptor (MP Biomedicals) for 30 sec at a power setting of 6.5. Lysates were centrifuged at 16,000 g for 5 min at 4 °C. Total RNA were extracted with phenol/chloroform and precipitated overnight with sodium acetate.
cDNA library construction and Illumina RNA sequencing
Overnight cultures of S. aureus were diluted in fresh BHI broth to an OD600nm of 0.1 then cultured at 160 rpm for 5 hr at 37 °C. Total RNA were extracted as described above and treated with Amplification Grade DNase I (Invitrogen) to remove genomic contaminations. The absence of DNA was checked by qPCR in an Applied Biosystems 7500 instrument and RNA integrity verified on a Bioanalyzer (Agilent). Ribosomal RNAs were depleted using a Ribo-Zero Magnetic Kit (Epicentre) according to the manufacturer’s recommendations. Stranded cDNA libraries were prepared using the NEBNext Ultra Directional RNA Library Prep Kit for Illumina (New England Biolabs). The concentration, quality, and purity of the libraries were determined using a BioAnalyzer, a Qubit fluorometer (Invitrogen), and a Nanodrop spectrophotometer (Thermo Scientific). Libraries were sequenced on either an Illumina MiSeq instrument (single-end, 50 cycles) or an Illumina HiSeq 1500 system (rapid run, 200 cycles, paired-end), as per the manufacturer’s instructions.
Reads mapping, analysis, and visualization
The Newman strain genome sequence and annotation file (in GFF format) was obtained from NCBI (ftp://ftp.ncbi.nlm.nih.gov/genomes/Bacteria/Staphylococcus_aureus_Newman_uid58839/). All of the SRD srns described for Newman strain22 were added to this GFF. Quality control of RNA-Seq reads and read mappings was performed as previously described22. SAM files were filtered on bitwise flag values61 to select properly paired reads. Fragments sorted in SAM files were counted by HTSeq count31 for stranded library with the union mode and differential expression calculated using DESeq32. BAM files were visualized using the Artemis browser34.
Identification of novel RNA candidates by RNA-Seq
We used the DETR’PROK27 workflow of 43 steps to identify novel RNAs in S. aureus Newman. Briefly, the pipeline clustered overlapping RNA-Seq reads from BWA alignments to identify novel transcripts in intergenic regions using a GFF file that combined 2614 CDS; 56 tRNAs; 16 rRNAs; RNaseP RNA; 4.5 S RNA; tmRNA; and 504 SRD-listed srns. We set DETR’PROK to identify and retain clusters of more than 12 overlapping reads that were located at least 25 nt apart from existing annotations and had a length over 50 nt. DETR’PROK then produced a list of candidates in a GFF annotation file.
Northern blot analysis and RACE-mapping
Northern blots were performed as previously described48. Briefly, 15 µg of total RNA were loaded and separated in 7% polyacrylamide/8 M urea gels. To estimate the actual length of transcripts, a DynaMarker® Prestain Marker for Small RNA Plus was loaded into each gel. RNAs were probed with γ32P 5′ endlabeled oligonucleotides (File S4) and detected using a Typhoon FLA 9500 scanner (GE Healthcare). RACE-mapping was performed as previously described41 with some modifications (see below), using the primers listed in File S4. To distinguish the transcription start sites from RNA maturation of transcripts, the RNA were treated with Terminator 5′-Phosphate-Dependent Exonuclease (Epicentre) to degrade 5′monophosphate RNAs, then with polyphosphatase (Epicentre) to remove pyrophosphate from TSS RNA. Primary transcript enriched-RNAs were then ligated as previously described41.
Peptide cloning and western blots
srn_9343 was cloned in pCN35 under its own promoter or under Pveg promoter44 and in fusion with a 3xFLAG (GACTACAAGGACCACGACGGTGACTACAAGGACCAC GACATCGACTACAAGGACGACGACGACAAGTGA) using the primers listed in File S4. Recombinant plasmids (Table 6) were transformed in S. aureus as previously described30. S. aureus was cultured in BHI broth at 37 °C and under agitation until reaching an OD600nm of 6. Cells were harvested by centrifugation, and cellular protein extracts were prepared with protease inhibitors as previously described41. Proteins from the supernatant were precipitated with TCA and then with acetone as previously described41. For western blots, samples were separated on 16% Tricine-SDS-PAGE and transferred onto Hybond-P PVDF membranes (Amersham). After overnight blocking at 4 °C, membranes were incubated with ANTI-FLAG horseradish peroxidase-conjugated antibodies (HRP) (Sigma). The membranes were revealed using an ECL Plus Western Blotting Detection Kit (Amersham) and scanned with an ImageQuant LAS 4000 imager.
RT-qPCR
Total RNA extraction was performed as described above42. RNA samples (1 µg of total RNA) were treated with 1 unit of Amplification Grade DNase I, and the absence of DNA contamination was checked by qPCR using an Applied Biosystems 7500 system. cDNA were synthesized from total RNA (250 ng) using a High-Capacity cDNA Archive Kit (Applied Biosystems) and specific primers to discriminate the strand transcribed. Real-time PCR was done using a QuantiTect SYBR Green PCR Kit (Qiagen) in an Applied Biosystems 7500 real-time PCR system. Data were analyzed using the comparative critical threshold (ΔΔCT) method as previously described: the target RNA amounts were compared with HU or 5 S RNA, which served as internal controls25. The primers used for cDNA synthesis and qPCR are listed in File S4.
Data Deposition
The RNA-Seq data generated during this study (by MiSeq and Hiseq) was submitted to the GEO repository under accession number GSE89487. Additionally, reads obtained from the work of Sassi et al.22, previously registered with GEO under accession number GSE64026 were used to enhance the number of replicates.
Electronic supplementary material
Acknowledgements
J.B. and T.M. are recipients of a fellowship from the Direction Générale pour l’Armement and the Conseil Régional de Bretagne. M.S. was supported in part by the region Bretagne grants SAD SARS 8254 and SARS_2 9181 (to Y.A.). Y.A. was supported by a Marie Curie International Incoming Fellowship (project 621959—SarHyb) within the 7th European Community Framework Programme. Other source of funding: Institut National de la Santé et de la Recherche Médicale; University of Rennes 1 and The French National Research Agency (sRNA-FIT to B.F.). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript. We are very grateful to the “plate-forme Génomique Santé” Biogenouest Génomique Biosit core facility for their technical assistance and to the CNRS-UPMC ABiMS bioinformatics platform (http://abims.sb-roscoff.fr) for providing computational resources and support. Y.A. thanks Philippe Bouloc for suggesting the use of DETR’PROK, Christiane Wolz for sharing Δrnase strains, and Astrid Rouillon for reading the manuscript.
Author Contributions
J.B. performed experiments, analyzed data, prepared figures and edited the manuscript. G.P. performed experiments, analyzed data and edited the manuscript. M.S. analyzed data, prepared figures and edited the manuscript. T.M. performed experiments and edited the manuscript. Y.A. designed research, supervised research, analyzed data, prepared figures and wrote the manuscript. B.F. supervised research, analyzed data and edited the manuscript.
Competing Interests
The authors declare that they have no competing interests.
Footnotes
Electronic supplementary material
Supplementary information accompanies this paper at doi:10.1038/s41598-017-04786-3
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Tong SY, Davis JS, Eichenberger E, Holland TL, Fowler VG., Jr. Staphylococcus aureus infections: epidemiology, pathophysiology, clinical manifestations, and management. Clinical microbiology reviews. 2015;28:603–661. doi: 10.1128/CMR.00134-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Caldelari I, Chao Y, Romby P, Vogel J. RNA-mediated regulation in pathogenic bacteria. Cold Spring Harb Perspect Med. 2013;3:a010298. doi: 10.1101/cshperspect.a010298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Fechter P, Caldelari I, Lioliou E, Romby P. Novel aspects of RNA regulation in Staphylococcus aureus. FEBS Lett. 2014;588:2523–2529. doi: 10.1016/j.febslet.2014.05.037. [DOI] [PubMed] [Google Scholar]
- 4.Wagner EG, Romby P. Small RNAs in bacteria and archaea: who they are, what they do, and how they do it. Adv Genet. 2015;90:133–208. doi: 10.1016/bs.adgen.2015.05.001. [DOI] [PubMed] [Google Scholar]
- 5.Jousselin A, Metzinger L, Felden B. On the facultative requirement of the bacterial RNA chaperone, Hfq. Trends Microbiol. 2009;17:399–405. doi: 10.1016/j.tim.2009.06.003. [DOI] [PubMed] [Google Scholar]
- 6.Storz G, Vogel J, Wassarman KM. Regulation by small RNAs in bacteria: expanding frontiers. Mol Cell. 2011;43:880–891. doi: 10.1016/j.molcel.2011.08.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mizuno T, Chou MY, Inouye M. A unique mechanism regulating gene expression: translational inhibition by a complementary RNA transcript (micRNA) Proc Natl Acad Sci USA. 1984;81:1966–1970. doi: 10.1073/pnas.81.7.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Novick, R. P., Iordanescu, S., Projan, S. J., Kornblum, J. & Edelman, I. pT181 plasmid replication is regulated by a countertranscript-driven transcriptional attenuator. Cell59, 395–404, doi:0092-8674(89)90300-0 (1989). [DOI] [PubMed]
- 9.Abu-Qatouseh LF, et al. Identification of differentially expressed small non-protein-coding RNAs in Staphylococcus aureus displaying both the normal and the small-colony variant phenotype. J Mol Med (Berl) 2010;88:565–575. doi: 10.1007/s00109-010-0597-2. [DOI] [PubMed] [Google Scholar]
- 10.Anderson, K. L. et al. Characterization of the Staphylococcus aureus heat shock, cold shock, stringent, and SOS responses and their effects on log-phase mRNA turnover. J Bacteriol188, 6739–6756, doi:188/19/6739 (2006). [DOI] [PMC free article] [PubMed]
- 11.Beaume M, et al. Cartography of methicillin-resistant S. aureus transcripts: detection, orientation and temporal expression during growth phase and stress conditions. PLoS One. 2010;5:e10725. doi: 10.1371/journal.pone.0010725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bohn C, et al. Experimental discovery of small RNAs in Staphylococcus aureus reveals a riboregulator of central metabolism. Nucleic Acids Res. 2010;38:6620–6636. doi: 10.1093/nar/gkq462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Geissmann T, et al. A search for small noncoding RNAs in Staphylococcus aureus reveals a conserved sequence motif for regulation. Nucleic Acids Res. 2009;37:7239–7257. doi: 10.1093/nar/gkp668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Howden BP, et al. Analysis of the small RNA transcriptional response in multidrug-resistant Staphylococcus aureus after antimicrobial exposure. Antimicrob Agents Chemother. 2013;57:3864–3874. doi: 10.1128/AAC.00263-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mader U, et al. Staphylococcus aureus Transcriptome Architecture: From Laboratory to Infection-Mimicking Conditions. PLoS genetics. 2016;12:e1005962. doi: 10.1371/journal.pgen.1005962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Marchais A, Bohn C, Bouloc P, Gautheret D. RsaOG, a new staphylococcal family of highly transcribed non-coding RNA. RNA biology. 2010;7:116–119. doi: 10.4161/rna.7.2.10925. [DOI] [PubMed] [Google Scholar]
- 17.Morrison JM, et al. Characterization of SSR42, a novel virulence factor regulatory RNA that contributes to the pathogenesis of a Staphylococcus aureus USA300 representative. J Bacteriol. 2012;194:2924–2938. doi: 10.1128/JB.06708-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pichon, C. & Felden, B. Small RNA genes expressed from Staphylococcus aureus genomic and pathogenicity islands with specific expression among pathogenic strains. Proc Natl Acad Sci USA102, 14249–14254, doi:0503838102 (2005). [DOI] [PMC free article] [PubMed]
- 19.Roberts, C. et al. Characterizing the effect of the Staphylococcus aureus virulence factor regulator, SarA, on log-phase mRNA half-lives. J Bacteriol188, 2593–2603, doi:188/7/2593 (2006). [DOI] [PMC free article] [PubMed]
- 20.Xue T, Zhang X, Sun H, Sun B. ArtR, a novel sRNA of Staphylococcus aureus, regulates alpha-toxin expression by targeting the 5′ UTR of sarT mRNA. Med Microbiol Immunol. 2014;203:1–12. doi: 10.1007/s00430-013-0307-0. [DOI] [PubMed] [Google Scholar]
- 21.Carroll, R. K. et al. Genome-wide Annotation, Identification, and Global Transcriptomic Analysis of Regulatory or Small RNA Gene Expression in Staphylococcus aureus. MBio7, doi:10.1128/mBio.01990-15 (2015). [DOI] [PMC free article] [PubMed]
- 22.Sassi M, et al. SRD: a Staphylococcus regulatory RNA database. RNA. 2015;21:1005–1017. doi: 10.1261/rna.049346.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Baba, T., Bae, T., Schneewind, O., Takeuchi, F. & Hiramatsu, K. Genome sequence of Staphylococcus aureus strain Newman and comparative analysis of staphylococcal genomes: polymorphism and evolution of two major pathogenicity islands. J Bacteriol190, 300–310, doi:JB.01000-07 (2008). [DOI] [PMC free article] [PubMed]
- 24.Sayed N, Jousselin A, Felden B. A cis-antisense RNA acts in trans in Staphylococcus aureus to control translation of a human cytolytic peptide. Nat Struct Mol Biol. 2012;19:105–112. doi: 10.1038/nsmb.2193. [DOI] [PubMed] [Google Scholar]
- 25.Le Pabic H, Germain-Amiot N, Bordeau V, Felden B. A bacterial regulatory RNA attenuates virulence, spread and human host cell phagocytosis. Nucleic acids research. 2015;43:9232–9248. doi: 10.1093/nar/gkv783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kathirvel, M., Buchad, H. & Nair, M. Enhancement of the pathogenicity of Staphylococcus aureus strain Newman by a small noncoding RNA SprX1. Medical microbiology and immunology, doi:10.1007/s00430-016-0467-9 (2016). [DOI] [PubMed]
- 27.Toffano-Nioche C, et al. Detection of non-coding RNA in bacteria and archaea using the DETR’PROK Galaxy pipeline. Methods. 2013;63:60–65. doi: 10.1016/j.ymeth.2013.06.003. [DOI] [PubMed] [Google Scholar]
- 28.Bae T, et al. Staphylococcus aureus virulence genes identified by bursa aurealis mutagenesis and nematode killing. Proceedings of the National Academy of Sciences of the United States of America. 2004;101:12312–12317. doi: 10.1073/pnas.0404728101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Duthie ES, Lorenz LL. Staphylococcal coagulase; mode of action and antigenicity. J Gen Microbiol. 1952;6:95–107. doi: 10.1099/00221287-6-1-2-95. [DOI] [PubMed] [Google Scholar]
- 30.Mauro, T., Rouillon, A. & Felden, B. Insights into the regulation of small RNA expression: SarA represses the expression of two sRNAs in Staphylococcus aureus. Nucleic acids research, doi:10.1093/nar/gkw777 (2016). [DOI] [PMC free article] [PubMed]
- 31.Anders, S., Pyl, P. T. & Huber, W. HTSeq - A Python framework to work with high-throughput sequencing data. Bioinformatics, doi:btu638 (2014). [DOI] [PMC free article] [PubMed]
- 32.Anders S, Huber W. Differential expression analysis for sequence count data. Genome Biol. 2010;11:R106. doi: 10.1186/gb-2010-11-10-r106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mortazavi A, Williams BA, McCue K, Schaeffer L, Wold B. Mapping and quantifying mammalian transcriptomes by RNA-Seq. Nature methods. 2008;5:621–628. doi: 10.1038/nmeth.1226. [DOI] [PubMed] [Google Scholar]
- 34.Carver T, Harris SR, Berriman M, Parkhill J, McQuillan JA. Artemis: an integrated platform for visualization and analysis of high-throughput sequence-based experimental data. Bioinformatics. 2012;28:464–469. doi: 10.1093/bioinformatics/btr703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Marincola G, et al. RNase Y of Staphylococcus aureus and its role in the activation of virulence genes. Molecular microbiology. 2012;85:817–832. doi: 10.1111/j.1365-2958.2012.08144.x. [DOI] [PubMed] [Google Scholar]
- 36.Sassi, M., Felden, B. & Augagneur, Y. Draft Genome Sequence of Staphylococcus aureus subsp. aureus Strain HG003, an NCTC8325 Derivative. Genome Announc2, doi:10.1128/genomeA.00855-14 (2014). [DOI] [PMC free article] [PubMed]
- 37.Sassi, M., Sharma, D., Brinsmade, S. R., Felden, B. & Augagneur, Y. Genome Sequence of the Clinical Isolate Staphylococcus aureus subsp. aureus Strain UAMS-1. Genome Announc3, doi:10.1128/genomeA.01584-14 (2015). [DOI] [PMC free article] [PubMed]
- 38.Diep BA, et al. Complete genome sequence of USA300, an epidemic clone of community-acquired meticillin-resistant Staphylococcus aureus. Lancet. 2006;367:731–739. doi: 10.1016/S0140-6736(06)68231-7. [DOI] [PubMed] [Google Scholar]
- 39.Kuroda, M. et al. Whole genome sequencing of meticillin-resistant Staphylococcus aureus. Lancet357, 1225–1240, doi:S0140673600044032 (2001). [DOI] [PubMed]
- 40.Lasa I, et al. Genome-wide antisense transcription drives mRNA processing in bacteria. Proc Natl Acad Sci USA. 2011;108:20172–20177. doi: 10.1073/pnas.1113521108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Pinel-Marie ML, Brielle R, Felden B. Dual toxic-peptide-coding Staphylococcus aureus RNA under antisense regulation targets host cells and bacterial rivals unequally. Cell Reports. 2014;7:424–435. doi: 10.1016/j.celrep.2014.03.012. [DOI] [PubMed] [Google Scholar]
- 42.Gautheret D, Lambert A. Direct RNA motif definition and identification from multiple sequence alignments using secondary structure profiles. J Mol Biol. 2001;313:1003–1011. doi: 10.1006/jmbi.2001.5102. [DOI] [PubMed] [Google Scholar]
- 43.Torres VJ, Pishchany G, Humayun M, Schneewind O, Skaar EP. Staphylococcus aureus IsdB is a hemoglobin receptor required for heme iron utilization. Journal of bacteriology. 2006;188:8421–8429. doi: 10.1128/JB.01335-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Biswas I, Jha JK, Fromm N. Shuttle expression plasmids for genetic studies in Streptococcus mutans. Microbiology. 2008;154:2275–2282. doi: 10.1099/mic.0.2008/019265-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Li S, et al. Phenol-soluble modulins: novel virulence-associated peptides of staphylococci. Future Microbiol. 2014;9:203–216. doi: 10.2217/fmb.13.153. [DOI] [PubMed] [Google Scholar]
- 46.Peschel A, Otto M. Phenol-soluble modulins and staphylococcal infection. Nature reviews. Microbiology. 2013;11:667–673. doi: 10.1038/nrmicro3110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Song J, et al. The expression of small regulatory RNAs in clinical samples reflects the different life styles of Staphylococcus aureus in colonization vs. infection. PLoS One. 2012;7:e37294. doi: 10.1371/journal.pone.0037294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Eyraud, A., Tattevin, P., Chabelskaya, S. & Felden, B. A small RNA controls a protein regulator involved in antibiotic resistance in Staphylococcus aureus. Nucleic Acids Res, doi:gku149 (2014). [DOI] [PMC free article] [PubMed]
- 49.Aanensen, D. M. et al. Whole-Genome Sequencing for Routine Pathogen Surveillance in Public Health: a Population Snapshot of Invasive Staphylococcus aureus in Europe. MBio7, doi:10.1128/mBio.00444-16 (2016). [DOI] [PMC free article] [PubMed]
- 50.Li L, et al. BSRD: a repository for bacterial small regulatory RNA. Nucleic Acids Res. 2013;41:D233–238. doi: 10.1093/nar/gks1264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Nawrocki EP, et al. Rfam 12.0: updates to the RNA families database. Nucleic acids research. 2015;43:D130–137. doi: 10.1093/nar/gku1063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Bengert P, Dandekar T. Riboswitch finder–a tool for identification of riboswitch RNAs. Nucleic acids research. 2004;32:W154–159. doi: 10.1093/nar/gkh352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Lacatena RM, Cesareni G. Base pairing of RNA I with its complementary sequence in the primer precursor inhibits ColE1 replication. Nature. 1981;294:623–626. doi: 10.1038/294623a0. [DOI] [PubMed] [Google Scholar]
- 54.Wight M, Werner A. The functions of natural antisense transcripts. Essays Biochem. 2013;54:91–101. doi: 10.1042/bse0540091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Overgaard M, Borch J, Gerdes K. RelB and RelE of Escherichia coli form a tight complex that represses transcription via the ribbon-helix-helix motif in RelB. Journal of molecular biology. 2009;394:183–196. doi: 10.1016/j.jmb.2009.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Griffin MA, Davis JH, Strobel SA. Bacterial toxin RelE: a highly efficient ribonuclease with exquisite substrate specificity using atypical catalytic residues. Biochemistry. 2013;52:8633–8642. doi: 10.1021/bi401325c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Li GY, Zhang Y, Inouye M, Ikura M. Inhibitory mechanism of Escherichia coli RelE-RelB toxin-antitoxin module involves a helix displacement near an mRNA interferase active site. The Journal of biological chemistry. 2009;284:14628–14636. doi: 10.1074/jbc.M809656200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Wessner F, et al. Regulatory crosstalk between type I and type II toxin-antitoxin systems in the human pathogen Enterococcus faecalis. RNA biology. 2015;12:1099–1108. doi: 10.1080/15476286.2015.1084465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Kumari P, Sampath K. cncRNAs: Bi-functional RNAs with protein coding and non-coding functions. Semin Cell Dev Biol. 2015;47–48:40–51. doi: 10.1016/j.semcdb.2015.10.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Vanderpool CK, Balasubramanian D, Lloyd CR. Dual-function RNA regulators in bacteria. Biochimie. 2011;93:1943–1949. doi: 10.1016/j.biochi.2011.07.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Li H, et al. The Sequence Alignment/Map format and SAMtools. Bioinformatics. 2009;25:2078–2079. doi: 10.1093/bioinformatics/btp352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Kuwahara-Arai K, Kondo N, Hori S, Tateda-Suzuki E, Hiramatsu K. Suppression of methicillin resistance in a mecA-containing pre-methicillin-resistant Staphylococcus aureus strain is caused by the mecI-mediated repression of PBP 2′ production. Antimicrobial agents and chemotherapy. 1996;40:2680–2685. doi: 10.1128/aac.40.12.2680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Herbert S, et al. Repair of global regulators in Staphylococcus aureus 8325 and comparative analysis with other clinical isolates. Infect Immun. 2010;78:2877–2889. doi: 10.1128/IAI.00088-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Gillaspy, A. F. et al. In Gram-positive pathogens (eds V. Fischetti et al.) 381–412 (ASM press, 2006).
- 65.Kreiswirth BN, et al. The toxic shock syndrome exotoxin structural gene is not detectably transmitted by a prophage. Nature. 1983;305:709–712. doi: 10.1038/305709a0. [DOI] [PubMed] [Google Scholar]
- 66.Sayed N, Nonin-Lecomte S, Rety S, Felden B. Functional and structural insights of a Staphylococcus aureus apoptotic-like membrane peptide from a toxin-antitoxin module. J Biol Chem. 2012;287:43454–43463. doi: 10.1074/jbc.M112.402693. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


