Abstract
Genetic associations for keratoconus could be useful for understanding disease pathogenesis and discovering biomarkers for early detection of the disease. We conducted a systematic review and meta-analysis to summarize all reported genetic associations for the disease. We searched in the MEDLINE, Embase, Web of Science, and HuGENET databases for genetic studies of keratoconus published from 1950 to June 2016. The summary odds ratio and 95% confidence intervals of all polymorphisms were estimated using the random-effect model. Among 639 reports that were retrieved, 24 fulfilled required criteria as eligible studies for meta-analysis, involving a total of 53 polymorphisms in 28 genes/loci. Results of our meta-analysis lead to the prioritization of 8 single-nucleotide polymorphisms (SNPs) in 6 genes/loci for keratoconus in Whites. Of them 5 genes/loci were originally detected in genome-wide association studies, including FOXO1 (rs2721051, P = 5.6 × 10−11), RXRA-COL5A1 (rs1536482, P = 2.5 × 10−9), FNDC3B (rs4894535, P = 1.4 × 10−8), IMMP2L (rs757219, P = 6.1 × 10−7; rs214884, P = 2.3 × 10−5), and BANP-ZNF469 (rs9938149, P = 1.3 × 10−5). The gene COL4A4 (rs2229813, P = 1.3 × 10−12; rs2228557, P = 4.5 × 10−7) was identified in previous candidate gene studies. We also found SNPs in 10 genes/loci that had a summary P value < 0.05. Sensitivity analysis indicated that the results were robust. Replication studies and understanding the roles of these genes in keratoconus are warranted.
Introduction
Keratoconus is a noninflammatory degenerative disorder that results in bulging and distortion of the corneal surface, leading to irregular astigmatism and progressive myopia. In advanced cases, corneal scarring and even corneal blindness can occur. Keratoconus has an incidence of approximately 1 in 2,000 individuals with a prevalence varying from 8.8 to 2300 per 100,0001, 2. It is a leading indication for corneal transplantation in many countries, especially in Australia, Middle East and Africa3. Management of keratoconus varies from conservative visual correction by spectacles or contact lenses for mild disease, to surgical interventions such as collagen cross-linking, intracorneal rings and keratoplasty for advanced disease. The onset of keratoconus is insidious and the progression is irreversible. Therefore, early diagnosis of keratoconus and its progression is needed. However, the variable risk of keratoconus progression poses a challenge to the personalized management for patients4. Knowing the risk factors for keratoconus would thus be helpful for early detection and monitoring the progression of the disease.
Keratoconus is a multifactorial disease resulting from the interaction of environmental, behavioural and genetic factors. Major environmental and behavioural factors include contact lens wear5 and chronic eye rubbing6. The genetic aetiology is evidenced by the bilaterality, familial aggregation7–9, monozygotic twins concordant of the disease10, its association with other genetic diseases such as Down syndrome11 and Leber’s congenital amaurosis12, and the ethnic difference in the prevalence and incidences13. Genetic associations for keratoconus will provide insight into disease mechanisms and help identify biomarkers for early detection of keratoconus onset and monitoring its progression. Of note, about 14% of the patients with keratoconus have a family history9. So far, however, the difference in the genetic basis of familial and sporadic keratoconus is unclear. Since the family history does not affect disease severity, the pooling of all cases in genetic studies is deemed reasonable14.
So far, 6 chromosomal loci have been identified for isolated keratoconus by linkage analysis, namely 2p24 15, 3p14-q13 16, 5q14.3-q21.1 2, 13q32 18, 16q22.3-q23.1 19, and 20q12 20. However, no disease-causing mutation has been identified from these loci. Besides, genome-wide association studies (GWAS) and candidate gene association studies have reported over 150 polymorphisms in more than 60 genes/loci for keratoconus. Among them, 7 genes/loci were identified by GWAS, including the HGF 21, LOX 22, FOXO1 and FNDC3B genes23, and the 3p26, 2q21.3 and 19q13.3 loci24. However, most of these associations were inconsistent across different study cohorts, making the roles of the genes/loci inconclusive.
In this study, we conducted a systematic review and meta-analysis to summarize the genetic association evidence for all variants in genes that were previously reported for keratoconus, and evaluated potential trans-ethnic heterogeneities. We first presented the association results from selected original studies/cohorts in the forest plots and then provided a prioritized list of studies and genes variants for further analysis. For SNPs that have been meta-analyzed in prior studies, our study provides an update of the summary association results by including new studies.
Results
Selection of studies
We retrieved a total of 978 records published between 1950 and 1 June 2016 from MEDLINE, Embase, Web of Science, and HuGENET for review. After removing 339 duplicated records we evaluated 639 citations and selected 36 articles for full-text assessment. Among them, 2 were reviews25, 26 and 32 were molecular genetic studies, including 2 GWAS23, 24 and 30 candidate gene association studies. A total of 64 genes/loci and 156 variants have been identified from the full-text review (Supplementary Table 1; Fig. 1). In the meta-analysis, we excluded 12 of the 36 articles because 7 of them were about gene variants that were not tested in additional independent studies27–33, 2 reported insufficient genotype data for meta-analysis34, 35, 2 were reviews25, 26, and 1 was an animal study36. We did not receive genotype data after contacting some of the authors34, 35. Finally, 24 studies were included for meta-analysis, involving a total of 53 SNPs in 28 genes/loci (Fig. 1)21–24, 32, 37–54. Among these 24 studies, 20 were candidate gene studies conducted in different populations, including Whites39, 41, 43, 44, 47, 49–52, 55, Arabic37, 38, 40, Chinese45, 56, Korean53, 54, Japanese48, Indian46, and Turkish42. The total sample sizes from these candidate gene studies were 3,037 patients with keratoconus and 9,928 controls. The 2 GWAS included 2,333 keratoconus patients and 16,655 controls of Caucasian origin (Table 1)23, 24.
Figure 1.
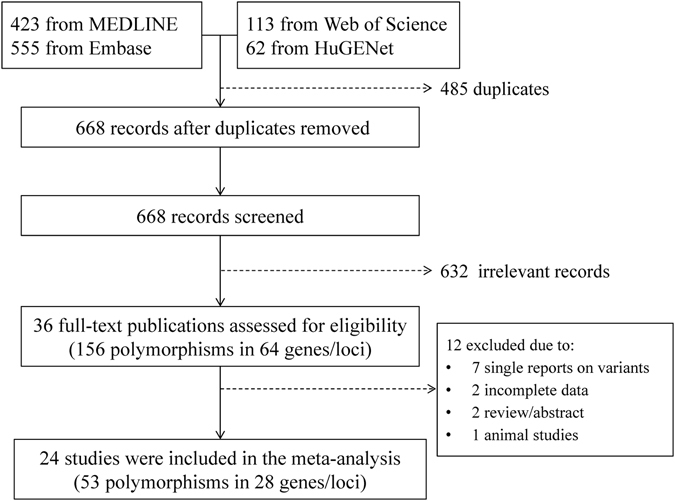
Flow diagram of study selection process.
Table 1.
Characteristics of eligible studies for the meta-analysis.
| No. | First author (year) | Country | Ethnicity | Study design | Age | Sex (% Female) | Sample size | Gene and locus | Test for HWE | |||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Case | Control | Case | Control | Case | Control | |||||||
| 1 | Abu-Amero, K. K.40 | Saudi Arabia | Arabic | CG | 28 ± 7 | n.r. | 0.54 | n.r. | 108 | 300 | BANP-ZNF469, and 6 loci | In HWE |
| 2 | Dudakova, L.39 | Czech | Whites | CG | 37 ± 13 | 40 ± 14 | 0.35 | 0.41 | 165 | 193 | HGF and LOX | In HWE |
| 3 | Hao, X. D.56 | China | Chinese | CG | 21 ± 6 | 27 ± 11 | 0.14 | 0.24 | 210 | 191 | HGF, LOX, and 6 loci | In HWE |
| 4 | Hasanian-Langroudi, F.38 | Iran | Arabic | CG | 30 ± 13 | 30 ± 16 | 0.50 | 0.56 | 112 | 150 | LOX | In HWE |
| 5 | Saravani, R.37 | Iran | Arabic | CG | 30 ± 13 | 30 ± 16 | 0.50 | 0.56 | 112 | 150 | COL4A4 | In HWE |
| 6 | Kokolakis, N. S.43 | Greece | Whites | CG | 33 ± 14 | 43 ± 16 | 0.38 | 0.44 | 45 | 78 | COL4A3 and COL4A4 | In HWE |
| 7 | Karolak, J. A.44 | Poland | Whites | CG | 22–67 | 13–83 | 0.33 | 0.52 | 42 | 50 | VSX1 | n.r. |
| 8 | Sahebjada, S.41 | Australia | Whites | CG | 38 ± 16 | 53 ± 15 | 0.41 | 0.61 | 157 | 673 | HGF | In HWE |
| 9 | Palamar, M.42 | Turkey | Turkish | CG | 25 ± 5 | 34 ± 12 | 0.54 | 0.51 | 121 | 121 | IL1B & IL1RN | In HWE |
| 10 | Bae, H. A.49* | Australia | Whites | CG | 43 ± 15 | 70 ± 10 | 0.45 | 0.43 | 524 | 2,761 | 12p13.3 and 11 loci | In HWE |
| 11 | Li, X.55 | USA-1 | Whites | C | 44 ± 13 | 72 ± 5 | 0.45 | 0.61 | 222 | 3,324 | COL5A1 | n.r. |
| USA-2 | Whites | CG | 43 ± 16 | 45 ± 14 | 0.32 | 0.48 | 304 | 518 | COL5A1 | n.r. | ||
| 12 | Sahebjada, S.47 | Australia | Whites | CG | 38 ± 16 | 53 ± 15 | 0.41 | 0.61 | 157 | 673 | BANP-ZNF469 and 4 loci | In HWE |
| 13 | Mikami, T.48 | Japan | Japanese | CG | 34 ± 10 | 33 ± 10 | 0.24 | 0.25 | 169 | 390 | IL1A and IL1B | In HWE |
| 14 | Verma, A.46 | India | Indian | CG | 23 ± 6 | 25 ± 9 | 0.41 | 0.75 | 117 | 108 | VSX1 | n.r. |
| 15 | Lu, Y.23 | Australia | Whites | GWAS | n.r. | n.r. | n.r. | n.r. | 652 | 2,761 | BANP-ZNF469 and 4 loci | n.r. |
| USA | Whites | CG | n.r. | n.r. | n.r. | n.r. | 222 | 3,324 | BANP-ZNF469 and 4 loci | n.r. | ||
| 16 | Wang, Y.45 | China | Chinese | CG | 21 ± 6 | 22 ± 5 | 0.36 | 0.53 | 97 | 101 | COL4A3 and 4 loci | In HWE |
| 17 | Bykhovskaya, Y.22 | USA-1 | Whites | CG | 44 ± 13 | 72 ± 5 | 0.45 | 0.61 | 222 | 3,324 | LOX | n.r. |
| USA-2 | Whites | CG | 43 ± 16 | 45 ± 14 | 0.32 | 0.48 | 304 | 518 | LOX | n.r. | ||
| 18 | Li, X.24 | USA-1 | Whites | GWAS | 44 ± 13 | 72 ± 5 | 0.45 | 0.61 | 222 | 3,324 | 12p13.3 and 11 loci | In HWE |
| USA-2 | Whites | CG | 43 ± 16 | 45 ± 14 | 0.32 | 0.48 | 304 | 518 | 12p13.3 and 11 loci | In HWE | ||
| 19 | Burdon, K. P.21 | Australia | Whites | CG | 48 ± 16 | 77 ± 9 | 0.53 | 0.29 | 97 | 216 | HGF | n.r. |
| Australia | Whites | CG | 43 ± 15 | 73 ± 11 | 0.39 | 0.10 | 96 | 72 | HGF | n.r. | ||
| Australia | Whites | CG | 41 ± 15 | 72 ± 9 | 0.39 | 0.50 | 215 | 112 | HGF | n.r. | ||
| USA-1 | Whites | CG | 44 ± 13 | 72 ± 5 | 0.45 | 0.61 | 222 | 3,324 | HGF | n.r. | ||
| USA-2 | Whites | CG | 43 ± 16 | 45 ± 14 | 0.32 | 0.48 | 304 | 518 | HGF | n.r. | ||
| 20 | Stabuc-Silih, M.50 | Slovenia | Whites | CG | 39 ± 10 | n.r. | 0.38 | n.r. | 113 | 100 | COL4A3 and COL4A4 | n.r. |
| 21 | Stabuc-Silih, M.51 | Slovenia | Whites | CG | 39 ± 10 | n.r. | 0.38 | n.r. | 113 | 100 | VSX1 | n.r. |
| 22 | Stabuc-Silih, M.52 | Slovenia | Whites | CG | 39 ± 8 | 37 ± 10 | 0.38 | 0.36 | 104 | 157 | COL4A3 and COL4A4 | In HWE |
| 23 | Kim, S. H.54 | Korea | Korean | CG | 18–33 | n.r. | n.r. | n.r. | 100 | 100 | IL1A and 2 loci | In HWE |
| 24 | Mok, J. W.53 | Korea | Korean | CG | n.r. | n.r. | n.r. | n.r. | 249 | 208 | VSX1 | In HWE |
*A small number of forme fruste Keratoconus was not excluded.
BANP-ZNF469 = BTG3 associated nuclear protein-zinc finger protein 469; COL4A3 = collagen, type IV, alpha 3; COL4A4 = collagen, type IV, alpha 4; COL5A1 = collagen, type V, alpha 1; HGF = hepatocyte growth factor; IL1A = interleukin 1, alpha; IL1B = interleukin 1, beta; IL1RN = interleukin 1 receptor antagonist; LOX = lysyl oxidase; VSX1 = visual system homeobox 1.
CG = candidate gene association study; GWAS = genome-wide association study; KCN = keratoconus; HWE = Hardy Weinberg equilibrium; PCs = principle components; n.r. = not reported.
Genes reported in keratoconus GWAS
We first meta-analyzed the SNPs that were reported in the four keratoconus GWAS23, 24 and additional independent studies based on the GWAS21, 22, 39–41, 47, 49, 55, 56. A total of 27 SNPs in 22 genes/loci were involved. Among them, 16 SNPs in 14 genes/loci showed a summary P value < 0.05 (Table 2). Of note, 3 SNPs in 3 respective genes/loci reached genome-wide significance, including FOXO1 rs2721051 (P = 5.6 × 10−11, I2 = 0), RXRA-COL5A1 rs1536482 (P = 2.5 × 10−9, I2 = 0), and FNDC3B rs4894535 (P = 1.4 × 10−8, I2 = 0) (Table 2 and Fig. 2). The P values for the remaining 13 significantly-associated SNPs ranged from 6.1 × 10−7 (IMMP2L rs757219) to 0.035 (19p12 rs8111998) (Table 2).
Table 2.
Allelic associations of gene variations with keratoconus using cohorts from both GWAS and subsequent replication studies.
| No. | Gene/locus | SNP | No. of cohorts | Ethnicity | Associated allele vs. Reference allele | Pooled sample size | Outcome* | Heterogeneity | Egger’s test (P) | |||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Case | Control | P | OR (95% CI) | P (Q) | I2 (%) | |||||||
| 1 | FOXO1 | rs2721051 | 5 | Multiple ancestries† | C vs. T | 1,345 | 7,246 | 5.6 × 10−11 | 0.65 (0.57–0.74) | 0.491 | 0 | 0.35 |
| 2 | RXRA-COL5A1 | rs1536482 | 4 | Whites | G vs. A | 1333 | 7276 | 2.5 × 10−9 | 0.77 (0.70–0.84) | 0.819 | 0 | 0.89 |
| 3 | FNDC3B | rs4894535 | 4 | Multiple ancestries† | T vs. C | 1,182 | 6,563 | 1.4 × 10−8 | 1.39 (1.24–1.55) | 0.628 | 0 | 0.76 |
| 4 | IMMP2L | rs757219 | 3 | Whites | C vs. T | 1,052 | 6,604 | 6.1 × 10−7 | 1.45 (1.25–1.67) | 0.266 | 26 | 0.61 |
| rs214884 | 3 | Whites | G vs. A | 1,051 | 6,603 | 2.3 × 10−5 | 1.56 (1.27–1.91) | 0.157 | 46 | 0.89 | ||
| 5 | BANP-ZNF469 | rs9938149 | 5 | Multiple ancestries† | C vs. A | 1,346 | 7,248 | 1.3 × 10−5 | 0.79 (0.70–0.88) | 0.422 | 12 | 0.77 |
| 6 | KCND3 | rs4839200 | 2 | Whites | A vs. G | 745 | 6,084 | 3.9 × 10−4 | 1.63 (1.25–2.14) | 0.068 | 70 | n.a. |
| 7 | RAB3GAP1 | rs4954218 | 3 | Whites | G vs. T | 1049 | 6604 | 8.2 × 10−4 | 0.64 (0.50–0.83) | 0.021 | 75 | 0.19 |
| 8 | UBXD2 | rs6430585 | 3 | Whites | A vs. C | 1049 | 6604 | 1.1 × 10−3 | 1.36 (1.13–1.64) | 0.065 | 63 | 0.62 |
| 9 | 13q33.3 | rs1328089 | 2 | Whites | C vs. T | 747 | 6,086 | 1.7 × 10−3 | 1.38 (1.13–1.68) | 0.109 | 61 | n.a. |
| rs1328083 | 3 | Whites | G vs. T | 1,050 | 6,604 | 3.0 × 10−2 | 1.38 (1.03–1.84) | 0.008 | 82 | 0.88 | ||
| 10 | MPDZ-NFIB | rs1324183 | 5 | Multiple ancestries† | C vs. A | 1,349 | 7,250 | 5.5 × 10−3 | 0.76 (0.63–0.92) | 0.034 | 67 | 0.75 |
| 11 | COL5A1 | rs7044529 | 6 | Multiple ancestries† | C vs. T | 1,652 | 7,766 | 7.0 × 10−3 | 0.84 (0.74–0.95) | 0.432 | 18 | 0.051 |
| 12 | LOX | rs10519694 | 3 | Whites | T vs. C | 692 | 6,599 | 0.018 | 0.76 (0.61–0.95) | 0.138 | 50 | 0.74 |
| rs2956540 | 4 | Multiple ancestries† | G vs. C | 901 | 6,788 | 0.28 | 0.83 (0.59–1.16) | <0.001 | 87 | 0.35 | ||
| 13 | HGF | rs3735520 | 6 | Multiple ancestries† | T vs. C | 1,311 | 4,545 | 0.027 | 1.25 (1.03–1.51) | 0.002 | 72 | 0.60 |
| rs1014091 | 2 | Whites | A vs. G | 362 | 480 | 0.41 | 0.70 (0.30–1.64) | 0.005 | 87 | n.a. | ||
| rs2286194 | 2 | Whites | A vs. T | 354 | 960 | 0.70 | 0.85 (0.39–1.89) | 0.001 | 91 | n.a. | ||
| 14 | 19p12 | rs8111998 | 3 | Whites | T vs. C | 1,049 | 6,603 | 0.035 | 1.48 (1.03–2.13) | 0.018 | 75 | 0.74 |
| 15 | PPP3CA | rs2659546 | 3 | Whites | A vs. G | 1,050 | 6,602 | 0.06 | 1.46 (0.99–2.15) | 0.014 | 75 | 0.77 |
| 16 | 3q26.2 | rs6792542 | 3 | Whites | C vs. A | 1,051 | 6,603 | 0.15 | 1.22 (0.93–1.61) | 0.001 | 84 | 0.48 |
| 17 | BHLHB2 | rs6442925 | 3 | Whites | T vs. C | 1,050 | 6,603 | 0.21 | 1.28 (0.87–1.88) | <0.001 | 89 | 0.93 |
| 18 | KIF26B | rs12407427 | 2 | Whites | T vs. C | 747 | 6,085 | 0.28 | 1.34 (0.79–2.30) | 0.001 | 92 | n.a. |
| 19 | BIRC8 | rs1428642 | 3 | Whites | A vs. G | 1,050 | 6,602 | 0.29 | 0.84 (0.61–1.16) | <0.001 | 90 | 0.45 |
| 20 | LRRN1 | rs3749350 | 3 | Whites | T vs. G | 1,052 | 6,603 | 0.32 | 1.24 (0.81–1.88) | <0.001 | 89 | 0.64 |
| 21 | 12p13.3 | rs1978238 | 2 | Whites | C vs. A | 746 | 6,086 | 0.36 | 0.81 (0.52–1.27) | <0.001 | 92 | n.a. |
| 22 | COL4A3 | rs7606754 | 2 | Multiple ancestries† | A vs. G | 760 | 3,061 | 0.42 | 1.10 (0.87–1.38) | 0.155 | 50 | n.a. |
*A random-effects model was used.
†Multiple ancestries included 2 or more ethnic groups from Whites and Asian (Arabic, Chinese, Korean, Japanese, or Indian).
CI = confidence interval; OR = odds ration; SNP = single nucleotide polymorphism; n.a. = not applicable; No. = number.
Figure 2.
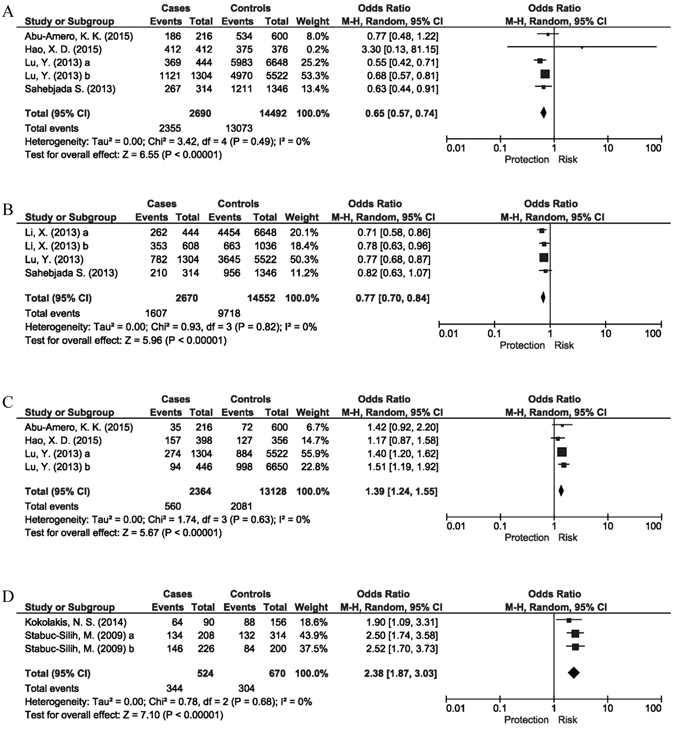
Meta-analysis of the 5 SNPs in 4 genes/loci showed genome-wide significance. Of the 4 genes/loci, 3 were detected in genome-wide association studies, including (A) FOXO1 (rs2721051, P = 5.6 × 10−11, I2 = 0), (B) RXRA-COL5A1 (rs1536482, P = 2.5 × 10−9, I2 = 0) and (C) FNDC3B (rs4894535, P = 1.4 × 10−8, I2 = 0). The (D) COL4A4 (rs2229813, P = 1.3 × 10−12, I2 = 0) gene was identified by candidate gene analysis.
We then performed meta-analysis only using the candidate gene studies, including those based on the GWAS findings or other hypotheses. One SNP from GWAS was significantly associated with keratoconus, i.e., RXRA-COL5A1 rs1536482 (P = 1.5 × 10−5, I2 = 0), while FOXO1 rs2721051 (P = 9.4 × 10−3, I2 = 0), BANP-ZNF469 rs9938149 (P = 0.017, I2 = 27%), COL4A4 (rs2228557, P = 0.020, I2 = 70%) and COL4A3 (c.2685 A > C, P = 0.032, I2 = 0) were nominally significant (Table 3). One SNP, FNDC3B rs4894535, reached a genome-wide significance in the overall population but did not show a significant association in the pooled Chinese and Arabic samples (P = 0.078, I2 = 0; Table 3). The other 4 genes/loci that have been reported in GWAS (i.e., MPDZ-NFIB, COL5A1, LOX and HGF) were also insignificant (P > 0.050; Table 3).
Table 3.
Allelic associations of gene variations with keratoconus based on purely candidate gene studies.
| No. | Gene/locus | SNP | No. of cohorts | Ethnicity | Associated allele vs. Reference allele | Pooled sample size | Outcome* | Heterogeneity | Egger’s test (P) | |||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Case | Control | P | OR (95% CI) | P (Q) | I2 (%) | |||||||
| 1 | RXRA-COL5A1 | rs1536482 | 3 | Whites | G vs. A | 681 | 4,515 | 1.5 × 10−5 | 0.76 (0.67–0.86) | 0.64 | 0 | 0.44 |
| 2 | FOXO1 | rs2721051 | 3 | Multiple ancestries† | C vs. T | 471 | 1,162 | 9.4 × 10−3 | 0.69 (0.52–0.91) | 0.51 | 0 | 0.28 |
| 3 | BANP-ZNF469 | rs9938149 | 3 | Multiple ancestries† | C vs. A | 472 | 1,164 | 0.017 | 0.75 (0.59–0.95) | 0.31 | 27 | 0.40 |
| 4 | COL4A4 | rs2228557 | 4 | Multiple ancestries† | T vs. C | 359 | 437 | 0.020 | 0.63 (0.43–0.93) | 0.021 | 70 | 0.41 |
| rs2229813 | 5 | Multiple ancestries† | G vs. A | 471 | 588 | 0.18 | 1.46 (0.84–2.55) | 1.2 × 10−8 | 90 | 0.67 | ||
| rs1800516 | 2 | Whites | C vs. G | 217 | 257 | 0.62 | 0.84 (0.42–1.66) | 0.90 | 0 | n.a. | ||
| rs2228555 | 3 | Multiple ancestries† | G vs. A | 329 | 407 | 0.74 | 1.04 (0.84–1.28) | 0.96 | 0 | 0.95 | ||
| rs2229814 | 3 | Multiple ancestries† | T vs. C | 315 | 358 | 0.78 | 1.03 (0.83–1.28) | 0.86 | 0 | 0.72 | ||
| rs56247709 | 2 | Whites | A vs. T | 217 | 257 | 1.00 | 1.00 (0.51–1.95) | 0.99 | 0 | n.a. | ||
| 5 | COL4A3 | c.2685 A > C | 2 | Whites | C vs. A | 217 | 258 | 0.032 | 1.36 (1.03–1.79) | 0.98 | 0 | n.a. |
| rs55703767 | 4 | Multiple ancestries† | T vs. G | 360 | 436 | 0.14 | 0.29 (0.06–1.48) | 5.1 × 10−16 | 96 | 0.18 | ||
| rs34019152 | 3 | Multiple ancestries† | A vs. G | 314 | 357 | 0.27 | 0.80 (0.53–1.19) | 0.95 | 0 | 0.74 | ||
| rs28381984 | 3 | Multiple ancestries† | T vs. C | 314 | 359 | 0.27 | 0.89 (0.71–1.10) | 0.92 | 0 | 0.74 | ||
| rs11677877 | 3 | Multiple ancestries† | G vs. A | 315 | 357 | 0.58 | 0.90 (0.62–1.30) | 0.77 | 0 | 0.49 | ||
| rs13424243 | 3 | Multiple ancestries† | C vs. G | 314 | 358 | 0.67 | 0.89 (0.51–1.54) | 0.51 | 0 | 0.30 | ||
| rs6436669 | 3 | Multiple ancestries† | G vs. A | 314 | 359 | 0.92 | 1.02 (0.75–1.38) | 0.94 | 0 | 0.73 | ||
| rs10178458 | 3 | Multiple ancestries† | T vs. C | 313 | 358 | 0.97 | 0.99 (0.73–1.34) | 0.93 | 0 | 0.70 | ||
| 6 | FNDC3B | rs4894535 | 2 | Chinese and Arabic | T vs. C | 307 | 477 | 0.078 | 1.25 (0.98–1.60) | 0.48 | 0 | n.a. |
| 7 | VSX1 | rs12480307 | 3 | Multiple ancestries† | G vs. A | 256 | 259 | 0.14 | 1.34 (0.91–1.98) | 0.30 | 0 | 0.23 |
| rs8123716 | 2 | Multiple ancestries† | A vs. C | 139 | 152 | 0.27 | 1.58 (0.70–3.57) | 0.34 | 0 | n.a. | ||
| rs74315433 | 2 | Multiple ancestries† | T vs. G | 139 | 151 | 0.48 | 1.76 (0.36–8.55) | 0.26 | 23 | n.a. | ||
| rs56157240 | 2 | Chinese and Indian | T vs. A | 214 | 209 | 0.53 | 1.80 (0.28–11.40) | 0.075 | 69 | n.a. | ||
| rs6138482 | 5 | Multiple ancestries† | A vs. G | 614 | 555 | 0.70 | 1.05 (0.83–1.32) | 0.20 | 36 | 0.060 | ||
| 8 | COL5A1 | rs7044529 | 5 | Multiple ancestries† | C vs. T | 1,001 | 5,005 | 0.17 | 0.90 (0.78–1.04) | 0.80 | 0 | 0.34 |
| 9 | MPDZ-NFIB | rs1324183 | 3 | Multiple ancestries† | C vs. A | 474 | 1,164 | 0.18 | 0.75 (0.5–1.14) | 7.3 × 10−3 | 81 | 0.051 |
| 10 | IL1A | rs2071376 | 3 | Korean, Chinese, and Japanese | A vs. C | 366 | 590 | 0.33 | 1.15 (0.87–1.52) | 0.16 | 43 | 0.89 |
| 11 | IL1B | rs16944 | 4 | Multiple ancestries† | T vs. C | 487 | 711 | 0.52 | 0.91 (0.69–1.21) | 0.047 | 63 | 0.51 |
| rs1143627 | 3 | Korean, Chinese, and Japanese | C vs. T | 366 | 591 | 0.53 | 0.87 (0.58–1.33) | 0.017 | 77 | 0.58 | ||
| 12 | IL1RN | rs2234663 | 2 | Multiple ancestries† | 1 vs. Non-1‡ | 221 | 221 | 0.93 | 0.98 (0.69–1.4) | 0.58 | 0 | n.a. |
| rs2234663 | 2 | Multiple ancestries† | 2 vs. Non-2‡ | 221 | 221 | 0.65 | 1.16 (0.61–2.18) | 0.15 | 51 | n.a. | ||
| rs2234663 | 2 | Multiple ancestries† | 3 vs. Non-3‡ | 221 | 221 | 0.84 | 0.92 (0.4–2.13) | 0.47 | 0 | n.a. | ||
| rs2234663 | 2 | Multiple ancestries† | 4 vs. Non-4‡ | 221 | 221 | 0.53 | 0.62 (0.14–2.75) | 0.47 | 0 | n.a. | ||
| 13 | HGF | rs3735520 | 2 | Multiple ancestries† | T vs. C | 375 | 382 | 0.57 | 1.14 (0.72–1.81) | 0.025 | 80 | n.a. |
| 14 | LOX | rs2288393 | 2 | Multiple ancestries† | C vs. G | 276 | 342 | 0.72 | 1.11 (0.63–1.95) | 0.057 | 72 | n.a. |
| rs1800449 | 2 | Multiple ancestries† | C vs. T | 277 | 343 | 0.84 | 0.92 (0.42–2.04) | 4.0 × 10−3 | 88 | n.a. | ||
| rs2956540 | 2 | Multiple ancestries† | G vs. C | 375 | 383 | 0.97 | 0.99 (0.47–2.10) | 6.8 × 10−4 | 91 | n.a. | ||
*A random-effects model was used.
†Multiple ancestries included 2 or more ethnic groups from Whites and Asian (Arabic, Chinese, Korean, Japanese, or Indian).
‡ IL1RN rs2234663 were designated as IL1RN∗1 [4 repeats, 410 base pairs (bp)], IL1RN∗2 (2 repeats, 240 bp), IL1RN∗3 (5 repeats, 500 bp), IL1RN∗4 (3 repeats, 325 bp), and IL1RN∗5 (6 repeats, 595 bp).
CI = confidence interval; OR = odds ration; SNP = single nucleotide polymorphism; n.a. = not applicable; No. = number.
Stratification analysis
To reduce the potential impact of trans-ethnical heterogeneity to the overall genetic association, we grouped the study cohorts into Whites and others (including Chinese, Korean, Japanese, Indian and Arabic). The 5 SNPs that were identified from GWAS showed a robust or nominal significance in Whites: FOXO1 rs2721051 (P = 1.5 × 10−9, I2 = 11%), MPDZ-NFIB rs1324183 (P = 1.8 × 10−4, I2 = 49%), BANP-ZNF469 rs9938149 (P = 2.6 × 10−4, I2 = 42%), COL5A1 rs7044529 (P = 9.9 × 10−4, I2 = 12%) and HGF rs3735520 (P = 3.6 × 10−3, I2 = 66%; Supplementary Table 2). Moreover, 2 SNPs in the COL4A4 gene identified by candidate gene studies were strongly associated with keratoconus in Whites, namely rs2229813 (P = 1.3 × 10−12, odds ratio (OR) = 2.38; I2 = 0) and rs2228557 (P = 4.5 × 10−7, OR = 0.54; I2 = 0) (Supplementary Table 2 and Fig. 2). In contrast, SNP rs2229813 showed a nominal association with keratoconus in combined Chinese and Arabic samples (P = 0.047, I2 = 16%). The odds ratio was notably toward an opposite direction (OR = 0.74; Supplementary Table 2). Moreover, most of aforementioned significant SNPs in Whites were not significant in the Chinese and Arabic samples, including FOXO1 rs2721051 (P = 0.31; I2 = 0), BANP-ZNF469 rs9938149 (P = 0.32; I2 = 0), MPDZ-NFIB rs1324183 (P = 0.63; I2 = 82%), and COL5A1 rs7044529 (P = 0.95; I2 = 0) (Supplementary Table 2), indicating ethnic diversities.
In this study, we were not able to evaluate the potential difference in the genetic basis of familial and sporadic cases as the data from familial cases were limited.
Assessment of potential biases and sensitivity analysis
For quality assessment every study was awarded a star for each of the items, i.e., case definition, ethnicity, and ascertainment of genotype (Supplementary Table 3) according to the Newcastle Ottawa Scale (NOS) system. All the 24 studies were awarded 5 or more stars out of a maximum of 8. Regarding Hardy-Weinberg Equilibrium (HWE), the control groups in 3 study cohorts showed deviation from HWE when tested for FOXO1 (rs2721051)56, COL4A3 (rs10178458 and rs55703767)52, COL4A4 (rs2229813, rs2228555, and rs2229814)52, and VSX1 (rs12480307)45. Therefore, in the sensitivity analysis we first excluded all the cohorts with HWE deviation and recalculated the summary ORs for the 7 SNPs in 4 genes. The associations were not altered (Supplementary Table 4). Furthermore, we omitted each study one at a time sequentially and recalculated the summary outcomes. The significance or insignificance of the summary outcomes was not altered in the sensitivity analysis (data not shown). We did not detect significant small study effects (e.g. publication bias) according to the shapes of funnel plots (Supplementary Figure 1) and the P values from the Egger’s tests, except for COL4A3 (rs55703767), LOX (rs2956540) and VSX1 (rs6138482) in the subgroup analysis by ethnicity (Supplementary Table 1).
Discussion
In this study, we meta-analyzed a total of 53 SNPs in 28 genes/loci for their genetic associations with keratoconus. We identified 8 SNPs in 6 genes/loci that were associated with keratoconus, i.e., FOXO1 rs2721051, FNDC3B rs4894535 and BANP-ZNF469 rs9938149 for the overall combined cohorts, and RXRA-COL5A1 rs1536482, IMMP2L rs757219 and rs214884, and COL4A4 rs2229813 and rs2228557 for Whites. Also, we found nominally significant associations in another 10 genes/loci, including KCND3, RAB3GAP1, UBXD2, MPDZ-NFIB, COL5A1, LOX, HGF, COL4A3, 13q33.3, and 19p12. In contrast, SNPs in 10 genes/loci that were reportedly associated with keratoconus were insignificant in our meta-analysis, including BHLHB2, BIRC8, IL1A, IL1B, KIF26B, LRRN1, PPP3CA, VSX1, 12p13.3 and 3q26.2.
Among the 6 significant genes/loci for keratoconus, 5 were originally identified by GWAS, including FOXO1, FNDC3B, BANP-ZNF469, RXRA-COL5A1, and IMMP2L. In our meta-analysis involving data from the GWAS and independent replication studies, 3 genes/loci (i.e., FOXO1, FNDC3B, BANP-ZNF469) showed consistent effects with low heterogeneity across different study cohorts. Three of them, FOXO1 rs2721051, FNDC3B rs4894535 and BANP-ZNF469 rs9938149, have been tested in both Whites and Asian populations. However, none of them showed a significant association in Chinese32 or Arabs40. Of note, FOXO1 rs2721051 was rare in Chinese with a minor allele frequency of 0.1%32. The lack of significant association in Asians could be due to the small sample size. In this meta-analysis, we also identified a SNP rs2229813 in the COL4A4 gene that showed a summary P value of genome-wide significant in Whites (P = 1.3 × 10−12; OR = 2.38). This gene was identified only in candidate gene studies37, 43, 45, 50, 52. Interestingly the summary P value in the pooled non-Caucasian samples was nominally significant (P = 0.047), but the OR was toward the opposite direction (OR = 0.74). This may suggest trans-ethnic diversities in the genetic components of keratoconus. In the COL4A4 gene, another SNP rs2228557, which was proposed in candidate gene studies, showed a significant summary P value (P = 4.5 × 10−7) in Whites, suggesting COL4A4 could be a genuine susceptibility gene for keratoconus in Whites. However, rs2228557 has only been tested in a Chinese population showing an insignificant association with an opposite OR (1.09)45. Therefore, whether COL4A4 is a biomarker with differential effects on keratoconus among different ethnic groups has yet to be confirmed. Interestingly, these 2 SNPs (i.e., rs2229813 and rs2228557) have not been reported in the published GWAS papers. In GWAS, only SNPs with P values surpassing a certain threshold would have been subjected to replication. Therefore, it would be intriguing to check the COL4A4 SNPs in the GWAS data and assess their association with keratoconus.
Although we were not able to evaluate the potential difference in the genetic basis of familial and sporadic cases, we found 2 familial cohorts being tested for different genes/loci22, 24, 50, 51, 55. In 3 studies22, 24, 55, the authors tested the associations of a few genes/loci (e.g. LOX and COL5A1) with keratoconus in a familial cohort using a generalized estimating equation accounting for familial correlations. Some of the significant SNPs identified in unrelated cases also showed significant association with keratoconus in the familial cohort. In another 2 studies50, 51, the authors reported a mutation, “627 + 23 G > A”, in VSX1 that was segregated in cases in several families. However, the mutation did not show significant association with keratoconus in the analysis using all the cases50. The results from the 2 cohorts indicated that the genetic association profiles of sporadic and familial keratoconus could be different.
Results of the present meta-analysis have led to a list of genes and loci associated with keratoconus that can be considered for functional investigations. Further biological investigation on these genes may throw light on new disease mechanisms for keratoconus. For example, FOXO1, RXRA and FNDC3B are the 3 genes that showed genome-wide significant association with keratoconus. FOXO1 is a member of the Forkhead Box (Fox) transcription factor family. Proteins from this family contain a conserved forkhead domain, which is a 110 amino acid DNA-binding domain. Fox proteins are known to be important regulators of the cellular oxidative stress57. For example, Fox proteins regulate the expressions of anti-oxidative enzymes such as superoxide dismutase and thioredoxin reductase58, 59. Moreover, reduced FOXO1 expression has been reported to induce apoptosis in human trabecular meshwork cells in response to oxidative stress60. It has been shown that increased oxidative damage to trabecular meshwork cells results in elevation of intraocular pressure and changing the anterior chamber angle, which would lead to corneal thinning61. We also found association of keratoconus with IMMP2L, a mitochondrial inner membrane protease. Mutation in IMMP2L also accumulates oxidative stress62. Therefore, FOXO1 and IMMP2L might regulate the oxidative stress in the anterior chamber, which affects the intraocular pressure and the corneal thickness. FOXO1 has also been linked to adipocyte differentiation63, which is affected by the gene FNDC3B 64. In this study, FNDC3B is another keratoconus associated gene. The link between adipogenesis and keratoconus is currently unclear. However, FNDC3B was associated with elevated intraocular pressure in a GWAS study65. Hence, FNDC3B may influence the intraocular pressure, the anterior chamber angle and the corneal thickness. Another keratoconus gene is RXRA, which encodes a nuclear retinoic acid receptor protein. There are two classes of nuclear retinoic acid receptors: RXR and RAR, which bind to each other to form RXR/RAR heterodimers66. Null mice of both RXRA and RXRA/RAR showed abnormal embryonic eye morphologies, including thickening of corneal stroma and absence of anterior chamber66. These results suggest a potential role of RXRA and retinoic acid signaling in the ocular development. However, the link among retinoic acid signalling, ocular development, and the abnormal corneal in keratoconus remains to be explored.
It is noteworthy that all of the identified SNPs in the 16 genes/loci are located in intronic, inter-genic, or in 3′- or 5′-untranslated regions. One SNP in HGF, rs3735520 (c.−1652C > T), was reported to modulate the severity of interstitial lung disease in patients with systemic sclerosis by altering the transcriptional efficiency of the HGF gene67. Whether they are in linkage disequilibrium with other coding variants in the relevant genes remained to be elucidated by sequencing analyses.
Although the mechanisms underlying the significant associations of the 16 identified genes/loci with keratoconus are largely unknown, it might be useful for understanding their pathogenic effects by referring to disease pathways identified for other conditions that share the same genes/loci. Eleven genes have been implicated in other diseases, including: COL5A1 for Ehlers-Danlos syndrome68; COL4A3 and COL4A4 for Alport syndrome69; HGF for non-syndromic hearing loss70; IMMP2L for Gilles de la Tourette syndrome71; KCND3 for spinocerebellar ataxia72; LOX for thoracic aortic aneurysms and dissections73; MPDZ for leber congenital amaurosis and retinitis pigmentosa74; RAB3GAP1 for Warburg Micro syndrome and Martsolf syndrome75; and ZNF469 for Brittle cornea syndrome76. The other 6 of the 16 identified genes, namely FOXO1, RXRA, FNDC3B, BANP, UBXD2, and NFIB of the MPDZ-NFIB locus, have not been directly linked to other human diseases.
In this study, we have identified and evaluated the genetic associations for keratoconus by conducting thorough assessments of the existing evidence. We have taken multiple measures to control for potential biases, including subgroup analysis, sensitivity analysis, and Egger’s test. However, this study has some limitations. First, our results could be more applicable to Whites, therefore most of the significant findings should be replicated in other populations with sufficient statistical power, such as the Asian populations. Second, the sample sizes in most of the candidate gene studies were small, especially in Asian populations. We observed lack of associations of almost all SNPs when summarizing the data from Asian cohorts. Therefore, larger cohorts are needed for further validation. Third, although we employed funnel plots and Egger’s tests to identify publication bias, there could still be remaining publication bias due to the reduced power when testing small number of studies in a meta-analysis. Moreover, the COL4A4 variants might not reach the genome-wide significance in the reported GWAS. The non-availability of the data for these variants could be a potential source of publication bias.
In conclusion, we have prioritized 8 SNPs in 6 genes/loci as significant genetic markers for keratoconus in Whites, including FOXO1 rs2721051, RXRA-COL5A1 rs1536482, FNDC3B rs4894535, IMMP2L rs757219 and rs214884, and BANP-ZNF469 rs9938149, and COL4A4 rs2229813 and rs2228557. We also identified 10 genes/loci with suggestive evidence of association with keratoconus. This study has thus provided an up-to-date list of candidate genetic markers for further investigations of their biological roles in keratoconus. More studies are warranted to confirm the reported genetic associations in different populations.
Methods
Searching methods for identifying studies
We searched for relevant records in the MEDLINE, Embase, Web of Science, and HuGENET databases via the Ovid platform. We used the Boolean logic to connect the free terms and controlled vocabularies (i.e. Medical Subject Heading terms): (“polymorphism(s)” OR “mutation” OR “genotype(s)” OR “genetic(s)” OR “gene(s)” OR “allele(s)” OR “DNA”) AND (“keratoconus”) (Supplementary Table 5). We also manually scanned the reference lists of the potentially eligible research articles, reviews and meta-analyses from the initial screening to ensure inclusion of all relevant publications. We did not use language filter in the literature search. The last search was performed on June 1, 2016.
Eligibility criteria
We set the following criteria for eligible studies for meta-analysis: (1) original case-control studies that evaluated the association of gene polymorphisms with keratoconus; (2) the study subjects were unrelated and recruited from explicitly defined populations; and (3) allele or genotype counts or frequencies in both case and control groups were reported or calculable; or odds ratio and 95% confidence intervals (CI) and/or standard error (SE) were reported. We excluded animal studies, case reports, reviews, abstracts, conference proceedings, and editorials.
Study selection, data collection and risk of bias assessment
Two reviewers (S.S.R. and S.T.U.M.) independently screened all the titles and abstracts of identified studies. Disagreements were resolved via discussions with a senior investigator (L.J.C.). After identifying potentially eligible articles, the 4 reviewers (S.S.R., S.T.U.M., X.T.Y., and L.M.) extracted the data separately and cross-validated the data. Consensus was reached via thorough discussion among all the reviewers. In this study, we used ‘Whites’ to represent individuals/populations whose ancestral origins are in the continent of Europe. We designed a customized datasheet for data extraction, which included the first author, year of publication, country of study, ethnicity, definition of case and control, sample sizes in the case and control groups, genes/loci, polymorphisms, allelic and genotypic counts and frequencies, ORs and 95% CIs or SEs of the polymorphisms and corresponding genetic models, and results of the test for HWE in the control group. First, we extracted all the polymorphisms and genes/loci reported in the potentially eligible studies searchable by the end of our search date. For GWAS, we extracted all the variants that were shown to be tested in replication cohorts in the result section and supplementary tables21–24. For candidate gene study, we extracted all the reported variants. No significance threshold for the genetic association has been applied during the data extraction. We also checked for potentially duplicated cohorts among the studies via comparing research groups and description of study populations. In the studies that had reported 2 or more independent cohorts, we extracted the data of each cohort separately. Second, we selected those polymorphisms that could be meta-analyzed. Third, the missing allele/genotype counts were calculated using the allele frequencies and sample sizes, assuming no deviation from HWE unless reported otherwise77. If only the OR and 95% CI were reported, we estimated the SE following the methods described in our previous papers77, 78. We attempted to contact the authors for additional information if necessary. If the HWE result was not reported, we tested it using the extracted data in the control group by the Chi-square test. Moreover, we used the NOS system (accessed via http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp) to evaluate the quality of the case-control studies (Supplementary Appendix 1)79, 80. We assigned one star to a study if it met one requirement in the NOS from 3 dimensions (i.e., selection, comparability and exposure). The maximum number of stars that a study could obtain was 8. A study of <5 stars in overall or earned no star in any one of the items (i.e., case definition, ethnicity, or ascertainment of genotype) was considered as of suboptimal quality and having high risk in introducing bias81.
Data analysis
We conducted meta-analysis for the SNPs that had been reported in 2 or more study cohorts from at least 2 separated reports. The genetic association was assessed using the allelic (a vs. A) model, where “a” and “A” represented the associated and the reference alleles, respectively. We evaluated the inter-cohort heterogeneity using the I 2 82. An I 2 value of lower than 25%, between 25% and 50%, and greater than 50% indicated low, moderate, and high heterogeneity, respectively. However, to obtain more conservative results we calculated the summary OR and 95% CI for each SNP only using the random-effect model, in which the weighted effect of a SNP was estimated by inverse variance (IV) and τ2 from the DerSimonian-Laird estimator83, regardless of the Q statistics and I 2. Of note, to assess the replication results of SNPs identified in the GWAS on keratoconus23, 24, we first combined the data from both the GWAS and replication studies, and then removed the data from the initial GWAS. Subgroup analysis by ethnicity was then conducted in Whites and Asian populations (i.e., populations of Asian ancestries including 2 or more ethnic groups from Arabic, Chinese, Korean, Japanese, or Indian populations). We adopted the funnel plots and Egger’s test to assess potential biases (e.g. publication bias)84, 85. A P value of <0.05 in the Egger’s test indicated statistically significant bias. We also conducted the sensitivity analysis to confirm the associations by sequentially omitting each of the included studies one at a time and recalculated the summary outcomes. We then omitted the studies that deviated from HWE (PChi-squre ≤ 0.05), or of suboptimal quality. A finding is more likely to be true when the result is stable in the sensitivity analysis.
Customized analytical scripts based on the metafor package in the R software (v3.0.0, http://cran.r-project.org/) were generated for the meta-analysis.
As a strategy to account for multiple testing, we used the Bonferroni corrected alpha as the cut-off value for confirming the genetic associations. To calculate the adjusted alpha value, we divided 0.05 by the number of SNPs tested (N = 53) and also by the maximum number of different tests a SNP could be done (N = 7). The adjusted significant threshold was therefore 1.35 × 10−4. The P values > 1.35 × 10−4 and ≤ 0.05 were considered nominally significant. We consider a P value < 5 × 10−8 as genome-wide significance.
Electronic supplementary material
Acknowledgements
We express our gratitude to all participants in this study. This study was supported in part by a Direct Grant from the Chinese University of Hong Kong, Hong Kong (4054116).
Author Contributions
Rong S.S., Vishal J. and Chen L.J. conceived and designed this study. Rong S.S., Ma S.T.U., Yu X.T., Ma L. and Wang Y.M. participated in the data acquisition. Rong S.S., Ma S.T.U., Yu X.T., Pang C.P., Vishal J. and Chen L.J. involved in the data analysis and interpretation. Rong S.S., Ma S.T.U. and Chu W.K. drafted the manuscript. Rong S.S., Chen L.J., Vishal J., Pang C.P., Chan T. and Young A.L. contributed to the critical revision of the manuscript for important intellectual content. Chen L.J., Vishal J. and Pang C.P. supervised this study.
Competing Interests
The authors declare that they have no competing interests.
Footnotes
Electronic supplementary material
Supplementary information accompanies this paper at doi:10.1038/s41598-017-04393-2
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Vishal Jhanji, Email: vishaljhanji@cuhk.edu.hk.
Li Jia Chen, Email: lijia_chen@cuhk.edu.hk.
References
- 1.Rabinowitz YS. Keratoconus. Surv Ophthalmol. 1998;42:297–319. doi: 10.1016/S0039-6257(97)00119-7. [DOI] [PubMed] [Google Scholar]
- 2.Jonas JB, Nangia V, Matin A, Kulkarni M, Bhojwani K. Prevalence and associations of keratoconus in rural maharashtra in central India: the central India eye and medical study. Am J Ophthalmol. 2009;148:760–765. doi: 10.1016/j.ajo.2009.06.024. [DOI] [PubMed] [Google Scholar]
- 3.Matthaei, M. et al. Changing Indications in Penetrating Keratoplasty: A Systematic Review of 34 Years of Global Reporting. Transplantation (2016). [DOI] [PubMed]
- 4.McGhee CN, Kim BZ, Wilson PJ. Contemporary Treatment Paradigms in Keratoconus. Cornea. 2015;34(Suppl 10):S16–23. doi: 10.1097/ICO.0000000000000504. [DOI] [PubMed] [Google Scholar]
- 5.Barr JT, et al. Estimation of the incidence and factors predictive of corneal scarring in the Collaborative Longitudinal Evaluation of Keratoconus (CLEK) Study. Cornea. 2006;25:16–25. doi: 10.1097/01.ico.0000164831.87593.08. [DOI] [PubMed] [Google Scholar]
- 6.Bawazeer AM, Hodge WG, Lorimer B. Atopy and keratoconus: a multivariate analysis. Br J Ophthalmol. 2000;84:834–836. doi: 10.1136/bjo.84.8.834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Karimian F, Aramesh S, Rabei HM, Javadi MA, Rafati N. Topographic evaluation of relatives of patients with keratoconus. Cornea. 2008;27:874–878. doi: 10.1097/ICO.0b013e31816f5edc. [DOI] [PubMed] [Google Scholar]
- 8.Kaya V, Utine CA, Altunsoy M, Oral D, Yilmaz OF. Evaluation of corneal topography with Orbscan II in first-degree relatives of patients with keratoconus. Cornea. 2008;27:531–534. doi: 10.1097/ICO.0b013e318165d110. [DOI] [PubMed] [Google Scholar]
- 9.Zadnik K, et al. Baseline findings in the Collaborative Longitudinal Evaluation of Keratoconus (CLEK) Study. Invest Ophthalmol Vis Sci. 1998;39:2537–2546. [PubMed] [Google Scholar]
- 10.Bechara SJ, Waring GO, 3rd, Insler MS. Keratoconus in two pairs of identical twins. Cornea. 1996;15:90–93. doi: 10.1097/00003226-199601000-00016. [DOI] [PubMed] [Google Scholar]
- 11.Cullen JF, Butler HG. Mongolism (Down’s Syndrome) and Keratoconus. Br J Ophthalmol. 1963;47:321–330. doi: 10.1136/bjo.47.6.321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Elder MJ. Leber congenital amaurosis and its association with keratoconus and keratoglobus. J Pediatr Ophthalmol Strabismus. 1994;31:38–40. doi: 10.3928/0191-3913-19940101-08. [DOI] [PubMed] [Google Scholar]
- 13.Georgiou T, Funnell CL, Cassels-Brown A, O’Conor R. Influence of ethnic origin on the incidence of keratoconus and associated atopic disease in Asians and white patients. Eye (Lond) 2004;18:379–383. doi: 10.1038/sj.eye.6700652. [DOI] [PubMed] [Google Scholar]
- 14.Szczotka-Flynn L, et al. Disease severity and family history in keratoconus. Br J Ophthalmol. 2008;92:1108–1111. doi: 10.1136/bjo.2007.130294. [DOI] [PubMed] [Google Scholar]
- 15.Hutchings H, et al. Identification of a new locus for isolated familial keratoconus at 2p24. J Med Genet. 2005;42:88–94. doi: 10.1136/jmg.2004.022103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Brancati F, et al. A locus for autosomal dominant keratoconus maps to human chromosome 3p14-q13. J Med Genet. 2004;41:188–192. doi: 10.1136/jmg.2003.012872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tang YG, et al. Genomewide linkage scan in a multigeneration Caucasian pedigree identifies a novel locus for keratoconus on chromosome 5q14.3-q21.1. Genet Med. 2005;7:397–405. doi: 10.1097/01.GIM.0000170772.41860.54. [DOI] [PubMed] [Google Scholar]
- 18.Gajecka M, et al. Localization of a gene for keratoconus to a 5.6-Mb interval on 13q32. Invest Ophthalmol Vis Sci. 2009;50:1531–1539. doi: 10.1167/iovs.08-2173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tyynismaa H, et al. A locus for autosomal dominant keratoconus: linkage to 16q22.3-q23.1 in Finnish families. Invest Ophthalmol Vis Sci. 2002;43:3160–3164. [PubMed] [Google Scholar]
- 20.Fullerton J, et al. Identity-by-descent approach to gene localisation in eight individuals affected by keratoconus from north-west Tasmania, Australia. Hum Genet. 2002;110:462–470. doi: 10.1007/s00439-002-0705-7. [DOI] [PubMed] [Google Scholar]
- 21.Burdon KP, et al. Association of polymorphisms in the hepatocyte growth factor gene promoter with keratoconus. Invest Ophthalmol Vis Sci. 2011;52:8514–8519. doi: 10.1167/iovs.11-8261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bykhovskaya Y, et al. Variation in the lysyl oxidase (LOX) gene is associated with keratoconus in family-based and case-control studies. Invest Ophthalmol Vis Sci. 2012;53:4152–4157. doi: 10.1167/iovs.11-9268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lu Y, et al. Genome-wide association analyses identify multiple loci associated with central corneal thickness and keratoconus. Nat Genet. 2013;45:155–163. doi: 10.1038/ng.2506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Li X, et al. A genome-wide association study identifies a potential novel gene locus for keratoconus, one of the commonest causes for corneal transplantation in developed countries. Hum Mol Genet. 2012;21:421–429. doi: 10.1093/hmg/ddr460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zhang J, Zhang L, Hong J, Wu D, Xu J. Association of Common Variants in LOX with Keratoconus: A Meta-Analysis. PLoS ONE [Electronic Resource] 2015;10:e0145815. doi: 10.1371/journal.pone.0145815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gordon-Shaag A, Millodot M, Shneor E, Liu Y. The genetic and environmental factors for keratoconus. Biomed Res Int. 2015;2015:795738. doi: 10.1155/2015/795738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Moschos MM, Droutsas K, Sioziou A, Dettoraki M, Gazouli M. Mutational Analysis of Pre-miR-184 and hsa-mir-568 in Greek Patients With Sporadic Keratoconus. Cornea. 2016;35:631–633. doi: 10.1097/ICO.0000000000000769. [DOI] [PubMed] [Google Scholar]
- 28.Wojcik KA, et al. Polymorphism of the APEX nuclease 1 gene in keratoconus and Fuchs endothelial corneal dystrophy. Cell Mol Biol Lett. 2015;20:48–65. doi: 10.1515/cmble-2015-0001. [DOI] [PubMed] [Google Scholar]
- 29.Synowiec E, et al. Polymorphism of the LIG3 gene in keratoconus and Fuchs endothelial corneal dystrophy. Cell Mol Biol (Noisy-le-grand) 2015;61:56–63. [PubMed] [Google Scholar]
- 30.Synowiec E, et al. Lack of association between polymorphisms of the DNA base excision repair genes MUTYH and hOGG1 and keratoconus in a Polish subpopulation. Arch Med Sci. 2015;11:1101–1110. doi: 10.5114/aoms.2015.54867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Moschos MM, et al. Polymorphism Analysis of VSX1 and SOD1 Genes in Greek Patients with Keratoconus. Ophthalmic Genet. 2015;36:213–217. doi: 10.3109/13816810.2013.843712. [DOI] [PubMed] [Google Scholar]
- 32.Hao XD, Chen P, Wang Y, Li SX, Xie LX. Mitochondrial DNA copy number, but not haplogroup is associated with keratoconus in Han Chinese population. Exp Eye Res. 2015;132:59–63. doi: 10.1016/j.exer.2015.01.016. [DOI] [PubMed] [Google Scholar]
- 33.Cuellar-Partida G, et al. WNT10A exonic variant increases the risk of keratoconus by decreasing corneal thickness. Hum Mol Genet. 2015;24:5060–5068. doi: 10.1093/hmg/ddv211. [DOI] [PubMed] [Google Scholar]
- 34.Al-Muammar AM, Kalantan H, Azad TA, Sultan T, Abu-Amero KK. Analysis of the SOD1 Gene in Keratoconus Patients from Saudi Arabia. Ophthalmic Genet. 2015;36:373–375. doi: 10.3109/13816810.2014.889173. [DOI] [PubMed] [Google Scholar]
- 35.Abu-Amero KK, et al. Screening of the Seed Region of MIR184 in Keratoconus Patients from Saudi Arabia. Biomed Res Int. 2015;2015:604508. doi: 10.1155/2015/604508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Koehn DR, Meyer KJ, Anderson MG. Genetic Evidence for Differential Regulation of Corneal Epithelial and Stromal Thickness. Invest Ophthalmol Vis Sci. 2015;56:5599–5607. doi: 10.1167/iovs.15-17179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Saravani R, et al. Evaluation of Possible Relationship Between COL4A4 Gene Polymorphisms and Risk of Keratoconus. Cornea. 2015;34:318–322. doi: 10.1097/ICO.0000000000000356. [DOI] [PubMed] [Google Scholar]
- 38.Hasanian-Langroudi F, Saravani R, Validad MH, Bahari G, Yari D. Association of Lysyl oxidase (LOX) Polymorphisms with the Risk of Keratoconus in an Iranian Population. Ophthalmic Genet. 2015;36:309–314. doi: 10.3109/13816810.2014.881507. [DOI] [PubMed] [Google Scholar]
- 39.Dudakova L, et al. Validation of rs2956540:G > C and rs3735520:G > A association with keratoconus in a population of European descent. Eur. J Hum Genet. 2015;23:1581–1583. doi: 10.1038/ejhg.2015.28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Abu-Amero KK, et al. Case-control association between CCT-associated variants and keratoconus in a Saudi Arabian population. J Negat Results Biomed. 2015;14:10. doi: 10.1186/s12952-015-0029-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sahebjada S, et al. Association of the hepatocyte growth factor gene with keratoconus in an Australian population. PLoS One. 2014;9:e84067. doi: 10.1371/journal.pone.0084067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Palamar M, et al. Relationship between IL1beta-511C > T and ILRN VNTR polymorphisms and keratoconus. Cornea. 2014;33:145–147. doi: 10.1097/ICO.0000000000000027. [DOI] [PubMed] [Google Scholar]
- 43.Kokolakis NS, et al. Polymorphism analysis of COL4A3 and COL4A4 genes in Greek patients with keratoconus. Ophthalmic Genet. 2014;35:226–228. doi: 10.3109/13816810.2014.946055. [DOI] [PubMed] [Google Scholar]
- 44.Karolak JA, Polakowski P, Szaflik J, Szaflik JP, Gajecka M. Molecular Screening of Keratoconus Susceptibility Sequence Variants in VSX1, TGFBI, DOCK9, STK24, and IPO5 Genes in Polish Patients and Novel TGFBI Variant Identification. Ophthalmic Genet. 2014;37:37–43. doi: 10.3109/13816810.2014.926375. [DOI] [PubMed] [Google Scholar]
- 45.Wang Y, et al. Common single nucleotide polymorphisms and keratoconus in the Han Chinese population. Ophthalmic Genet. 2013;34:160–166. doi: 10.3109/13816810.2012.743569. [DOI] [PubMed] [Google Scholar]
- 46.Verma A, Das M, Srinivasan M, Prajna NV, Sundaresan P. Investigation of VSX1 sequence variants in South Indian patients with sporadic cases of keratoconus. BMC Res Notes. 2013;6:103. doi: 10.1186/1756-0500-6-103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Sahebjada S, et al. Evaluating the association between keratoconus and the corneal thickness genes in an independent Australian population. Invest Ophthalmol Vis Sci. 2013;54:8224–8228. doi: 10.1167/iovs.13-12982. [DOI] [PubMed] [Google Scholar]
- 48.Mikami T, et al. Interleukin 1 beta promoter polymorphism is associated with keratoconus in a Japanese population. Mol Vis. 2013;19:845–851. [PMC free article] [PubMed] [Google Scholar]
- 49.Bae, H. A. et al. Replication and meta-analysis of candidate loci identified variation at RAB3GAP1 associated with keratoconus. Invest Ophthalmol Vis Sci54, (2013). [DOI] [PMC free article] [PubMed]
- 50.Stabuc-Silih M, Strazisar M, Ravnik-Glavac M, Hawlina M, Glavac D. Genetics and clinical characteristics of keratoconus. Acta Dermatovenerol Alp Pannonica Adriat. 2010;19:3–10. [PubMed] [Google Scholar]
- 51.Stabuc-Silih M, Strazisar M, Hawlina M, Glavac D. Absence of pathogenic mutations in VSX1 and SOD1 genes in patients with keratoconus. Cornea. 2010;29:172–176. doi: 10.1097/ICO.0b013e3181aebf7a. [DOI] [PubMed] [Google Scholar]
- 52.Stabuc-Silih M, Ravnik-Glavac M, Glavac D, Hawlina M, Strazisar M. Polymorphisms in COL4A3 and COL4A4 genes associated with keratoconus. Mol Vis. 2009;15:2848–2860. [PMC free article] [PubMed] [Google Scholar]
- 53.Mok JW, Baek SJ, Joo CK. VSX1 gene variants are associated with keratoconus in unrelated Korean patients. J Hum Genet. 2008;53:842–849. doi: 10.1007/s10038-008-0319-6. [DOI] [PubMed] [Google Scholar]
- 54.Kim SH, Mok JW, Kim HS, Joo CK. Association of −31T > C and −511 C > T polymorphisms in the interleukin 1 beta (IL1B) promoter in Korean keratoconus patients. Mol Vis. 2008;14:2109–2116. [PMC free article] [PubMed] [Google Scholar]
- 55.Li X, et al. Genetic association of COL5A1 variants in keratoconus patients suggests a complex connection between corneal thinning and keratoconus. Invest Ophthalmol Vis Sci. 2013;54:2696–2704. doi: 10.1167/iovs.13-11601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Hao XD, Chen P, Chen ZL, Li SX, Wang Y. Evaluating the Association between Keratoconus and Reported Genetic Loci in a Han Chinese Population. Ophthalmic Genet. 2015;36:132–136. doi: 10.3109/13816810.2015.1005317. [DOI] [PubMed] [Google Scholar]
- 57.Klotz LO, et al. Redox regulation of FoxO transcription factors. Redox Biol. 2015;6:51–72. doi: 10.1016/j.redox.2015.06.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Kops GJ, et al. Forkhead transcription factor FOXO3a protects quiescent cells from oxidative stress. Nature. 2002;419:316–321. doi: 10.1038/nature01036. [DOI] [PubMed] [Google Scholar]
- 59.Olmos Y, et al. SirT1 regulation of antioxidant genes is dependent on the formation of a FoxO3a/PGC-1alpha complex. Antioxid Redox Signal. 2013;19:1507–1521. doi: 10.1089/ars.2012.4713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Berry FB, et al. FOXC1 is required for cell viability and resistance to oxidative stress in the eye through the transcriptional regulation of FOXO1A. Hum Mol Genet. 2008;17:490–505. doi: 10.1093/hmg/ddm326. [DOI] [PubMed] [Google Scholar]
- 61.Siegfried, C. J., Shui, Y. B., Bai, F. & Beebe, D. C. Central corneal thickness correlates with oxygen levels in the human anterior chamber angle. Am J Ophthalmol159, 457-462 e451, (2015). [DOI] [PMC free article] [PubMed]
- 62.Lu B, et al. A mutation in the inner mitochondrial membrane peptidase 2-like gene (Immp2l) affects mitochondrial function and impairs fertility in mice. Biol Reprod. 2008;78:601–610. doi: 10.1095/biolreprod.107.065987. [DOI] [PubMed] [Google Scholar]
- 63.Nakae J, et al. The forkhead transcription factor Foxo1 regulates adipocyte differentiation. Dev Cell. 2003;4:119–129. doi: 10.1016/S1534-5807(02)00401-X. [DOI] [PubMed] [Google Scholar]
- 64.Kishimoto K, Kato A, Osada S, Nishizuka M, Imagawa M. Fad104, a positive regulator of adipogenesis, negatively regulates osteoblast differentiation. Biochem Biophys Res Commun. 2010;397:187–191. doi: 10.1016/j.bbrc.2010.05.077. [DOI] [PubMed] [Google Scholar]
- 65.Hysi PG, et al. Genome-wide analysis of multi-ancestry cohorts identifies new loci influencing intraocular pressure and susceptibility to glaucoma. Nat Genet. 2014;46:1126–1130. doi: 10.1038/ng.3087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Kastner P, et al. Genetic analysis of RXR alpha developmental function: convergence of RXR and RAR signaling pathways in heart and eye morphogenesis. Cell. 1994;78:987–1003. doi: 10.1016/0092-8674(94)90274-7. [DOI] [PubMed] [Google Scholar]
- 67.Hoshino K, Satoh T, Kawaguchi Y, Kuwana M. Association of hepatocyte growth factor promoter polymorphism with severity of interstitial lung disease in Japanese patients with systemic sclerosis. Arthritis Rheum. 2011;63:2465–2472. doi: 10.1002/art.30415. [DOI] [PubMed] [Google Scholar]
- 68.Symoens S, et al. COL5A1 signal peptide mutations interfere with protein secretion and cause classic Ehlers-Danlos syndrome. Hum Mutat. 2009;30:E395–403. doi: 10.1002/humu.20887. [DOI] [PubMed] [Google Scholar]
- 69.Fallerini C, et al. Unbiased next generation sequencing analysis confirms the existence of autosomal dominant Alport syndrome in a relevant fraction of cases. Clin Genet. 2014;86:252–257. doi: 10.1111/cge.12258. [DOI] [PubMed] [Google Scholar]
- 70.Schultz JM, et al. Noncoding mutations of HGF are associated with nonsyndromic hearing loss, DFNB39. Am J Hum Genet. 2009;85:25–39. doi: 10.1016/j.ajhg.2009.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Patel C, et al. Translocation breakpoint at 7q31 associated with tics: further evidence for IMMP2L as a candidate gene for Tourette syndrome. Eur J Hum Genet. 2011;19:634–639. doi: 10.1038/ejhg.2010.238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Duarri A, et al. Mutations in potassium channel kcnd3 cause spinocerebellar ataxia type 19. Ann Neurol. 2012;72:870–880. doi: 10.1002/ana.23700. [DOI] [PubMed] [Google Scholar]
- 73.Guo DC, et al. LOX Mutations Predispose to Thoracic Aortic Aneurysms and Dissections. Circ Res. 2016;118:928–934. doi: 10.1161/CIRCRESAHA.115.307130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Ali M, et al. Mpdz null allele in an avian model of retinal degeneration and mutations in human leber congenital amaurosis and retinitis pigmentosa. Invest Ophthalmol Vis Sci. 2011;52:7432–7440. doi: 10.1167/iovs.11-7872. [DOI] [PubMed] [Google Scholar]
- 75.Handley MT, et al. Mutation spectrum in RAB3GAP1, RAB3GAP2, and RAB18 and genotype-phenotype correlations in warburg micro syndrome and Martsolf syndrome. Hum Mutat. 2013;34:686–696. doi: 10.1002/humu.22296. [DOI] [PubMed] [Google Scholar]
- 76.Rohrbach M, et al. ZNF469 frequently mutated in the brittle cornea syndrome (BCS) is a single exon gene possibly regulating the expression of several extracellular matrix components. Mol Genet Metab. 2013;109:289–295. doi: 10.1016/j.ymgme.2013.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Rong SS, et al. Genetic Associations of Primary Angle-Closure Disease: A Systematic Review and Meta-analysis. Ophthalmology. 2016;123:1211–1221. doi: 10.1016/j.ophtha.2015.12.027. [DOI] [PubMed] [Google Scholar]
- 78.Rong SS, Peng Y, Liang YB, Cao D, Jhanji V. Does cigarette smoking alter the risk of pterygium? A systematic review and meta-analysis. Invest Ophthalmol Vis Sci. 2014;55:6235–6243. doi: 10.1167/iovs.14-15046. [DOI] [PubMed] [Google Scholar]
- 79.Kmet, L. M., Lee, R. C. & Cook, L. S. Standard quality assessment criteria for evaluating primary research papers from a variety of fields. (Alberta Heritage Foundation for Medical Research, 2004).
- 80.Khan, K. S., Riet, G. t., Popay, J., Nixon, J. & Kleijnen, J. In Undertaking systematic reviews of research effectiveness CDC’s guidance for those carrying out or commissioning reviews 20 (Centre of Reviews and Dissemination UoY, 2001).
- 81.McPheeters, M. L. et al. Closing the quality gap: revisiting the state of the science (vol. 3: quality improvement interventions to address health disparities). Evid Rep Technol Assess (Full Rep), 1-475, (2012). [PMC free article] [PubMed]
- 82.Higgins JP, Thompson SG. Quantifying heterogeneity in a meta-analysis. Stat Med. 2002;21:1539–1558. doi: 10.1002/sim.1186. [DOI] [PubMed] [Google Scholar]
- 83.DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials. 1986;7:177–188. doi: 10.1016/0197-2456(86)90046-2. [DOI] [PubMed] [Google Scholar]
- 84.Higgins J. P. T. & S, G. Cochrane Handbook for Systematic Reviews of Interventions, www.cochrane-handbook.org (2011).
- 85.Sterne JA, Gavaghan D, Egger M. Publication and related bias in meta-analysis: power of statistical tests and prevalence in the literature. J Clin Epidemiol. 2000;53:1119–1129. doi: 10.1016/S0895-4356(00)00242-0. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


