Abstract
Among children with asthma, black children are two to four times as likely to have an emergency department (ED) visit and die from asthma, respectively, compared to white children in the United States. Despite the availability of evidence-based asthma management guidelines, minority children are less likely than white children to receive or use effective options for asthma care. The CHICAGO Plan is a three-arm multi-center randomized pragmatic trial of children 5 to 11 years old presenting to the ED with uncontrolled asthma that compares: (1) an ED-focused intervention to improve the quality of care on discharge to home, (2) the same ED-focused intervention together with a home-based community health worker (CHW)-led intervention, and (3) enhanced usual care. All children receive spacers for the metered dose inhaler and teaching about its use. The Patient-Reported Outcomes Measurement Information System (PROMIS) Asthma Impact Scale and Satisfaction with Participation in Social Roles at 6 months are the primary outcomes in children and in caregivers, respectively. Other patient-reported outcomes and indicators of healthcare utilization are assessed as secondary outcomes. Innovative features of the CHICAGO Plan include early and continuous engagement of children, caregivers, the Chicago Department of Public Health, and other stakeholders to inform the design and implementation of the study and a shared research infrastructure to coordinate study activities. The objective of this report is to describe the development of the CHICAGO Plan, including the methods and rationale for engaging stakeholders, the shared research infrastructure, and other features of the pragmatic clinical trial design.
Keywords: pragmatic clinical trial, health disparities, uncontrolled asthma, stakeholder engagement, quality of asthma care, community health worker
1. INTRODUCTION
Asthma is among the most common chronic health conditions and reasons for missed school days, and disproportionately affects low-income and minority children in the United States (U.S.).(1,2) Asthma is more common in children whose family income is below the federal poverty line, and twice as common in black compared to white children.(3,4) Emergency department (ED) visits for asthma are an indicator for uncontrolled asthma, and individuals with frequent asthma-related ED visits are at increased risk for poor outcomes, including death.(1) Among children with asthma, black children are 2.6 and 4.1 times more likely to have an ED visit and die from asthma, respectively, compared to white children.(4) Despite the availability of effective treatment options and evidence-based asthma guidelines, minority children are less likely than white children to be prescribed or use effective options for asthma care.(5–7) Chicago is the third most populous city in the U.S. and an epicenter for asthma-related health inequities affecting children.(8,9) In 2012, representatives from government agencies, non-profits, community organizations, clinicians, administrators, and researchers from multiple healthcare systems in Chicago participated in the Chicago Emergency Department Asthma Summit to identify opportunities to more effectively address uncontrolled asthma. Among the topics that generated the strongest interest was the need for an effective, multi-stakeholder coordinated strategy to improve the quality of asthma care, self-management skills in the home, and health outcomes among minority children and their caregivers who present to the ED with uncontrolled asthma.
The summit led to the creation of the Coordinated Healthcare Interventions for Childhood Asthma Gaps in Outcomes (CHICAGO) Plan, which was funded as part of a targeted research initiative to improve adherence to the national asthma guidelines by the Patient-Centered Outcomes Research Institute (PCORI) Asthma Disparities program (Treatment Options for African Americans and Hispanics/Latinos with Uncontrolled Asthma contract; AS# 1307-05420).(10,11) The CHICAGO Plan is a three-arm randomized clinical effectiveness (pragmatic) trial that compares: 1) an ED-focused intervention to improve the quality of care on discharge to home, 2) the same ED-focused intervention together with a home-based community health worker (CHW)-led intervention at the child’s home, and 3) enhanced usual care. Innovative features of the CHICAGO plan include early and continuous engagement of children, caregivers, clinicians and other stakeholders to inform the design and implementation of the study and a shared research infrastructure to support and coordinate study activities at multiple healthcare systems. The CHICAGO Plan is the only project funded by the targeted research initiative within the PCORI Asthma Disparities program that enrolls children in the ED as they receive care for uncontrolled asthma.
The objective of this report is to describe the development of the CHICAGO Plan, including the methods and rationale for engaging stakeholders, the study population, the intervention and comparator groups, primary and secondary outcomes, organization of the study team, the shared research infrastructure, and other features of the ongoing study.
2. DESIGN & METHODS
2.1 Study overview
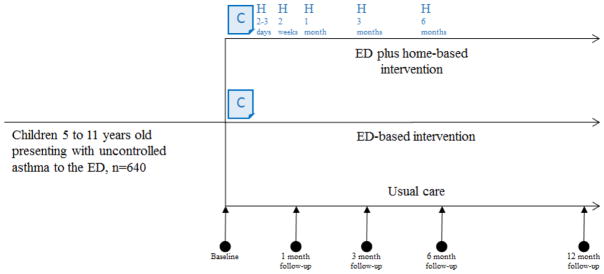
The study employs a pragmatic trial design using randomization and concurrent control groups to provide a rigorous evidence base that is applicable to routine clinical practice (i.e., evidence of effectiveness).(12) Qualitative interviews and observations of stakeholders were conducted to ensure that the study design was tailored to their expressed needs and feasible to employ within operating healthcare systems. The resulting CHICAGO Plan is a stakeholder-supported three-arm randomized clinical trial (Figure 1).
Figure 1. CHICAGO Plan study schema.
640 children age 5 to 11 years presenting with uncontrolled asthma to the emergency department (ED) will be randomized to one of three groups to evaluate ED-based interventions vs. ED plus home-based interventions to promote asthma self-management skills vs. enhanced usual care. All children, including those in enhanced usual care, receive education in the ED about the appropriate use of MDI devices and two MDI spacers free-of-charge. The ED-based intervention consists of a paper-based decision support and communication tool (“C”, CAPE or Chicago Action Plan after Emergency department discharge). The five CHW-led home visits (“H”) take place over 6 months (2–3 days, 2 weeks, 1 month, 3 months, and 6 months after ED discharge). The assessment of outcomes will be performed at baseline (in-person on the day of ED discharge); 1 month (via phone), 3 months (via phone), and 6 months (in-person) after ED discharge. Participants enrolled in the first half of the 15-month recruitment period are invited to participate in a 12-month follow-up phone assessment to assess the durability of effects after the end of the 6-month intervention in the ED plus home-based intervention group.
2.2 Population
Eligibility criteria are designed to be clinically relevant and feasible to implement in an ED setting: 1) child is 5–11 years of age (a population in whom a diagnosis of asthma is generally reliable, and in whom exacerbations are common); 2) child is presenting to the ED, urgent care center, or observation unit at a participating clinical center (see 2.5 Settings); 3) child is treated with at least 1 dose of an inhaled or nebulized short-acting bronchodilator (quick-relief medication); 4) child is treated with a systemic corticosteroid; 5) child and caregiver approached at least 1 hour after receipt of the first dose of quick-relief medication or systemic corticosteroids, whichever occurred first to permit sufficient time for the treating clinicians to make an initial assessment of the diagnosis and treatment plan, and for the child to benefit from initial asthma management; 6) diagnosis of asthma exacerbation by treating clinician; 7) treating ED clinician indicates the patients is likely to be discharged to home; and 8) caregiver reports that English or Spanish is the preferred language at home. Children were excluded if they met any of the following criteria: 1) caregiver declines to provide informed consent, or the child declines to provide assent; 2) child is discharged to a location other than home (e.g., another healthcare facility); 3) child or another member of the child’s primary household is a current or previous participant in the CHICAGO Plan; 4) child is enrolled in another study involving a health-related intervention; 5) a CHW is already visiting the home as part of another program; 6) child is expected to move out of Chicago within the next 6 months; or 7) child does not reside in Chicago.
2.3 Interventions (active comparators) and comparator conditions
All participants receive asthma care per their ED clinicians. In addition, the CHICAGO Plan ED Coordinator, a member of the study team, provides all participants two metered dose inhaler (MDI) spacers free-of-charge and uses teach-to-goal methodology (repeated rounds of education and evaluation until the child achieves mastery) to educate the child and the caregiver about appropriate inhaler technique for a MDI.(13,14) Patient education regarding the MDI device was selected because it is commonly used for quick-relief medications and is also the device for many inhaled controller medications. Stakeholders identified the need for all participants to benefit as a central aspect of the study design to ensure adequate support of the CHICAGO Plan by clinical staff and caregivers. Children are then randomly assigned to either of two active comparators or enhanced usual care, stratified by clinical center and race (child is black or not-black, as reported by caregiver).
2.3.1 ED-focused intervention to improve the quality of care on ED discharge (ED only intervention)
There is limited evidence to support interventions directed at clinicians to increase adherence to asthma guidelines in the ED, such as through the use of electronic health record-based audit and feedback, clinical pharmacy support, or pay-for-performance.(15) A previous single-center study in Canada that enrolled 219 children (62% white, 13% black, 9% Asian, 16% other) presenting with uncontrolled asthma to the ED demonstrated that a paper-based decision support and communication tool, compared to usual care, improved the quality of care (e.g., children were more likely to receive a prescription for inhaled corticosteroids on ED discharge and more likely to use inhaled corticosteroids after ED discharge) and the increased proportion of children with well-controlled asthma at 4 weeks after ED discharge.(16)
On the basis of this promising evidence, we developed a paper-based decision support and communication tool as the ED intervention (CHICAGO Action Plan after Emergency department discharge, CAPE).(17,18) The CAPE is intended to support guideline recommended asthma care on ED discharge (a course of systemic corticosteroids; daily inhaled corticosteroids or other controller; as needed quick-relief inhaled medication; assess and teach appropriate inhaler technique; arrange a post-discharge follow-up appointment; and avoid known asthma triggers) and to support appropriate asthma self-management in the home. The CAPE tool uses simplified language, visual learning, and options for individualization to also facilitate communication about discharge instructions between clinicians and the child and caregiver. The design of a culturally tailored and literacy-appropriate communication tool occurred over three phases: 1) define design requirements; 2) prototype and refine the communication tool; and 3) evaluate stakeholder preferences for the new communication tool vs. documents currently in use. The resulting communication tools incorporated health literacy and information design principles tailored to the study population (e.g., maximum Flesch-Kincaid 6th reading grade level; reduced word count, sentence length, text blocks, and medical jargon; consistent use of typographic hierarchy and underlying grid; and key information presented in illustration and callouts; use of terms understood by the target population). We used a co-design process with the CHWs to ensure tools fit the context of use, continued the CAPE visual and verbal language, and provided specific educational supports for CHICAGO CHWs to deliver consistent instruction.
ED clinicians (physicians and nurses) from each of the Clinical centers actively collaborated in designing the ED interventions and were therefore supportive of the ED-only interventions. The ED Coordinators complete the CAPE tool in the ED after consulting with the child’s clinicians and reviewing the discharge instructions in the electronic health records. The ED Coordinators refer to the CAPE tool to deliver the ED intervention using the teach-back method to improve and confirm the children’s and caregivers’ understanding of discharge instructions. Because ED clinicians are required to document their asthma instructions in the electronic health records, they were reluctant to also complete the CAPE tool (duplicate documentation).
2.3.2 ED-focused intervention together with CHW-led interventions at home (ED-plus-home interventions)
CHWs, also known as “lay health workers,” are community members who serve as connectors between healthcare consumers and needed services (e.g., arranging transportation for healthcare; scheduling an appointment).(19) Multi-component home-based interventions (e.g., self-management skills training to use medications appropriately, to avoid environmental triggers, and to recognize when to seek medical attention) conducted by CHWs improve clinical outcomes in children and caregiver quality of life.(20,21) The effectiveness of CHW-led home-based interventions in children enrolled from the ED, however, has not been tested and is the focus of the ED-plus-home intervention of the CHICAGO Plan.
Participants randomly allocated to this active comparator receive the same ED-only intervention described in 2.3.1, but are also offered a CHW who conducts up to five home visits over 6 months to assist children and their caregivers to 1) implement the ED discharge instructions, 2) update the asthma treatment plan with input from the patient’s ambulatory clinician, 3) develop a plan to manage asthma during school hours (e.g., access to quick-relief medications, action plan in case of respiratory difficulty), and 4) develop a specific and feasible plan to reduce environmental triggers (e.g., environmental tobacco smoke, roach, mice) at home. We selected five CHW-led home visits, based on previous studies which generally use four to six home visits.(22–24) Home visits take 60 to 90 minutes, and occur approximately 2–3 days, 2 weeks, 1 month, 3 month, and 6 months after ED discharge (Table 1).
Table 1.
Competencies reviewed at each home visit
| Home visits | |||||||
|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | |||
| Time since ED discharge | 2–3 days | 2 weeks | 1 month | 3 months | 6 months | ||
| Competency | Estimated time to teach compentency | ||||||
| 1 | Introductions and explanation of CHICAGO Plan, role of CHW | 10 minutes | Priority | Optional | Optional | Optional | Optional |
| 2 | Asthma basics: what is asthma, how does it affect the airways | 5 minutes | Priority | Priority | Optional | Optional | Optional |
| 3 | Symptom recognition and understanding of controlled vs. uncontrolled asthma; when to seek care | 10 minutes | Priority | Priority | Optional | Optional | Optional |
| 4 | Education using teach-back about appropriate use of medications, including MDI devices | 15 minutes | Priority | Priority | Priority | Priority | Priority |
| 5 | Identification of major triggers (cigarette smoke, cockroach, mouse) in home, remediation strategies, trigger avoidance strategies | 30 minutes | Optional | Priority | Priority | Priority | Priority |
| 6 | Identification of other triggers (mold, dust mite, pets, pollen, food, strong odors, cold/flu, extremes in weather, exercise, emotions) in home and strategies to avoid them | 30 minutes | Optional | Optional | Optional | Optional | Optional |
| 7 | Review and confirm understanding of the CAPE tool or updated asthma action plan | 10 minutes | Priority | Priority | Priority | Priority | Priority |
| 8 | Education about 504 plan and how to submit paperwork to schools | 5 minutes | Optional | Priority | Priority | Priority | Priority |
| 9 | Develop behavior change plan and assess progress since last visit | 10 minutes | Priority | Priority | Priority | Priority | Priority |
| 10 | Other topics (e.g., allergy testing, referrals, peak flow meter) | 5 minutes | Optional | Optional | Optional | Optional | Optional |
| Estimated time to complete each visit | 60–90 minutes | 60–90 minutes | 60–90 minutes | 60–90 minutes | 60–90 minutes | ||
There are 5 home visits conducted by community health workers (CHWs) for children randomly allocated to the ED plus home-based intervention group. Each visit is estimated to require 60 to 90 minutes to teach competencies. The CHW focuses on the “Priority” topics at each home visit. Based on caregiver and the child’s interest, CHWs may review fewer or additional “Optional” topics. The 504 plan refers to Section 504 of the Rehabilitation Act of 1973, which is an anti-discrimination civil rights statute that requires that the needs of students with disabilities be met as adequately as the needs of the non-disabled. In the context of the CHICAGO Plan, the 504 plan is used to ensure that the child with asthma receives appropriate accommodations while in school (e.g., ability to self-administer asthma medications while in school).
Environmental tobacco smoke was a focus of trigger avoidance because exposure to it is prevalent in this population, increases morbidity due to asthma, and is actionable.(25–27) Emphasis was placed on pest allergens because inner city homes have high levels of exposure to both cockroach and mouse allergen, and both of these are common sensitizers and associated with significant morbidity including respiratory symptoms and healthcare utilization. (28–32) Supplies to reduce or eliminate these triggers (cockroach gel and traps, rodent traps, steel wool, caulk, airtight food storage containers) are provided free-of-charge to children randomly assigned to ED-plus-home intervention. Participants in this intervention group also receive an asthma-friendly cleaning kit with irritant-free cleaning supplies (spray bottle, scratch-free sponge, baking soda, white vinegar, and liquid cleansers) and instructions for use. When time allows, assistance is given to develop specific strategies to reduce other possible asthma triggers identified during the home visits (e.g., mold, dust mites, pets, irritants and strong odors, cold or flu, extremes in weather, exercise, and emotions).
CHWs are trained to promote the child’s and caregiver’s self-efficacy for asthma self-management using interpretation, modeling, and performance mastery.(33–35) An example for symptom recognition and control is described here. Interpretation begins with knowledge of symptoms, followed by monitoring changes in those symptoms (self-monitoring), interpreting causes of symptoms, and then developing the problem solving and decision making skills necessary to adopt appropriate behaviors (e.g., use controller medication daily). The CHW teaches the caregiver (e.g., mother) to monitor how often the child takes the medication over a one-week baseline. If the child has daily asthma symptoms and is taking an asthma controller medication less frequently than prescribed (e.g., used twice per week, prescribed twice per day), the CHW encourages the mother to set a goal of increasing the frequency of controller use. If the child is already taking the controller medication as prescribed, the CHW may ask the child to demonstrate their inhaler technique and, if necessary, provides teaching to improve inhaler technique. The CHW also asks the child and caregiver about and records observed environmental triggers (e.g., smoking at home) to determine whether appropriate avoidance strategies have been implemented, or whether there is room to further reduce exposure to triggers. The selection, training, and supervision of CHWs is described in sections 3.1.7.1 and 3.1.7.2.
2.3.3 Enhanced usual care
A usual care comparator provides the basis to evaluate the effectiveness of each of the two active interventions versus current practice. Comparisons with usual care also help to interpret results if there is no observed difference in outcomes between the active comparators (i.e., differentiating between similarly effective active comparators vs. similarly ineffective active comparators).
A potential challenge with a usual care group is that caregivers and children may not be willing to enroll in a usual care group. Also, stakeholders supported the need to enhance usual care so that all children, regardless of the randomized treatment assignment, benefited from participating in the CHICAGO Plan. Accordingly, children in the “enhanced usual care” group also received teaching about appropriate MDI technique and two MDI spacers free-of-charge.
Another challenge with the enhanced usual care group is that routine asthma care may be highly variable by clinical center or may change over time, both of which could limit inferences when comparing the effects of an intervention versus usual care. To address this potential challenge, electronic health records of participants in enhanced usual care and the two active intervention groups are accessed by a project manager masked to treatment assignment to abstract information included in the written discharge instructions provided to the child and caregiver prior to leaving the ED (see 2.4.2.3).(36) We acknowledge that documentation may be imperfect and could, in some instances, overstate or understate the care that was provided compared to direct observation; however, abstracting electronic health records is a non-obtrusive method to measure quality of care that has been used in other ED-based asthma studies (37) and is consistent with a design of a pragmatic trial.(38)
2.4 Outcomes
The selection of primary outcomes was based on several criteria: 1) patient-centeredness, defined as domains identified as important by children and their caregivers; 2) availability of validated measures in English and in Spanish that could be administered in person and by telephone; we reasoned that some patients may only be able to complete follow-up visits by telephone due to transportation or other barriers; 3) plausibility that such measures could be responsive to an effective intervention in the target population; and 4) limited burden (e.g., time) for study participants. Based on these criteria and feedback from stakeholders, the CHICAGO Plan Steering Committee selected two primary outcomes and several secondary outcomes. Outcomes are evaluated on enrollment and at each follow-up visit (1 month, 3 month, 6 month, and in some participants, 12 months; Table 2). Baseline (prior to randomization) and 6-month outcome data are collected in person by trained research staff who are not involved in the intervention. Outcomes at 1, 3, and 12 months are collected by trained research staff by telephone. All outcomes are collected by staff who are masked to treatment assignment.
Table 2.
Data collection schedule
|
|
Source | |||||
|---|---|---|---|---|---|---|
| Baseline | Follow-up visit | |||||
|
| ||||||
| Location | ED | Phone | Phone | Home | Phone | |
|
| ||||||
| Time since ED discharge | 0 | 1 month | 3 months | 6 months | 12 months | |
|
| ||||||
| Informed assent, consent | X | Child, caregiver | ||||
| Demographics | ||||||
| Gender, race/ethnicity, date of birth of child, caregiver's marital status, caregiver's highest level of education, caregiver's employment status, health insurance, household income | X | EHR, caregiver | ||||
| Contact information, including alternates | X | X | X | X | X | Caregiver |
| Name and contact information of primary asthma provider | X | X | X | X | X | Caregiver |
| Preferred language at home | X | Caregiver | ||||
| Medical history (child) | ||||||
| Height and weight | X | EHR | ||||
| Comorbid conditions | X | EHR | ||||
| Medications prescribed | X | EHR | ||||
| Attendance at follow-up appointments | X | EHR | ||||
| Self-management skills | ||||||
| Pharmacy dispensations for child | X | X | X | X | Pharmacy records | |
| Inhaler technique | X | X | ED coordinator review | |||
| Outcomes | ||||||
| Primary outcomes (PROMIS Asthma Impact Scale; PROMIS Satisfaction with participation in social roles); and secondary outcomes | X | X | X | X | X | Child, caregiver, EHR |
|
| ||||||
| Estimated duration of each visit | 60minutes | 20 minutes | 20 minutes | 45 minutes | 20 minutes | |
Data collection occurs on enrollment (in the ED), and then over a follow-up period of up to 12 months. Children enrolled in the first half of the 15-month recruitment periods complete follow-up assessments at 1 month (via phone), 3 months (via phone), and 6 months (home visit or via phone). Children enrolled early in the recruitment period are also invited to complete a follow-up visit at 12 months (via phone). Data may be collected from the Clinical center electronic health record (EHR), the caregiver, from the child, or from pharmacies. See 2.4 Outcomes, and 3.5 Data collection schedule for details. cACT=Childhood Asthma Control Test; ED= emergency department; EHR=Electronic health record; PACQLQ=Pediatric Asthma Caregiver Quality of Life; PROMIS= Patient-Reported Outcomes Measurement Information System.
2.4.1 Co-primary outcomes
A single primary outcome for the child and a separate single primary outcome for the caregiver were selected (i.e., we employed two co-primary outcomes), as there is extensive literature to indicate that a child’s asthma affects the caregiver, and that interventions that improve the child’s asthma control also improve a caregiver’s quality of life.(21) In addition, many of the interventions require active participation of the caregiver, so it was reasoned that assessing the impact of the intervention on the caregiver will build support for that intervention.
The NIH Patient-Reported Outcomes Measurement Information System (PROMIS) measures confer several distinct advantages for pragmatic trials: 1) comparability: measures have been standardized so there are common domains and metrics across conditions; 2) reliability and validity: metrics for each domain have been rigorously reviewed and tested; 3) flexibility: the information can be collected in a variety of ways (in person, telephone, or via computer adaptive testing); and 4) inclusiveness: all people, regardless of literacy, language, physical function or life course can be accommodated.(39)
Two PROMIS measures were selected for the two co-primary outcomes: 1) the Asthma Impact Scale (8 items) is used to assess the impact of asthma on the child in the past 7 days;(40) and 2) the Satisfaction with Participation in Social Roles scale (4 items) is used to assess the effects of the intervention on the caregiver’s level of satisfaction with his or her activities of daily living in the past 7 days.(41)
2.4.2 Secondary outcomes
Several measures were selected for secondary outcomes, in part to compare results of the CHICAGO Plan with previous studies, but also to meet recommendations of national asthma guidelines and expressed preferences of caregivers and other stakeholders.
2.4.2.1 Secondary outcomes in children
1) The Childhood Asthma Control Test (cACT),(42) and 2) acute care visits (number of all-cause and respiratory-related urgent care visits, emergency department visits, hospitalizations, based on self-report and review of electronic health records);(43)
2.4.2.2 Secondary outcomes in caregivers
1) The Pediatric Asthma Caregiver Quality of Life Questionnaire (PACQLQ);(44) and 2) the PROMIS measures for Anxiety, Depression, Fatigue, and Sleep disturbance;(40,45–47)
2.4.2.3
Guideline-recommendations for asthma care following ED discharge, documented in the electronic health records as information provided to the child and caregiver prior to leaving the ED: medications prescribed for use after ED discharge 1) systemic corticosteroids (yes/no), 2) inhaled corticosteroids or another controller medication (yes/no), 3) quick-relief medications (yes/no); 4) follow-up appointment scheduled by ED staff (yes/no); 5) instructions for appropriate inhaler technique (yes/no); 6) instructions for avoiding environmental triggers (yes/no); and 7) action plan about when to seek additional care. This information was abstracted by a project manager masked to treatment group.
2.4.2.4 Inhaler technique
Assessed on enrollment and at the 6-month in-person visit. The CHICAGO Plan ED Coordinator reviews the child’s MDI technique using a previously published inhaler checklist to identify inhaler misuse (misuse, defined as <75% steps correct, yes/no);(13)
2.4.2.5 Pharmacy dispensations for child
Filled prescriptions for systemic corticosteroids prescribed within 7 days of ED discharge (pharmacy data; yes/no), assessed in those who receive a new prescription on ED discharge. Filled prescription for inhaled corticosteroids or other asthma controller within 7 days of ED discharge (pharmacy data; yes/no), assessed in those who receive a new prescription on ED discharge. Data from pharmacies are not available in electronic health records at the clinical centers; we therefore request dispensation data from pharmacies identified by study participants as their source of medications. We acknowledge that pharmacy dispensation data are an imperfect measure of medication use and may be incomplete for some participants; we will follow the procedures used in previous studies.(48,49)
2.5 Settings
Children and their caregivers are recruited from EDs at six clinical centers that provide care to children living in the west and south sides of Chicago (Ann & Robert H. Lurie Children’s Hospital, Sinai Health System, John H. Stroger Jr. Hospital of Cook County Health & Hospitals System, Rush University Medical Center, University of Chicago Comer Children’s Hospital, and the University of Illinois Hospital & Health Sciences System; Figure 2). These institutions include a mix of public (John H. Stroger Jr. Hospital; University of Illinois Hospital & Health Sciences System) and private (all others) institutions. Some of the centers are pediatric EDs (Ann & Robert H. Lurie Children’s Hospital, John H. Stroger Jr. Hospital, University of Chicago Comer Children’s Hospital) and others are mixed pediatric-adult EDs.
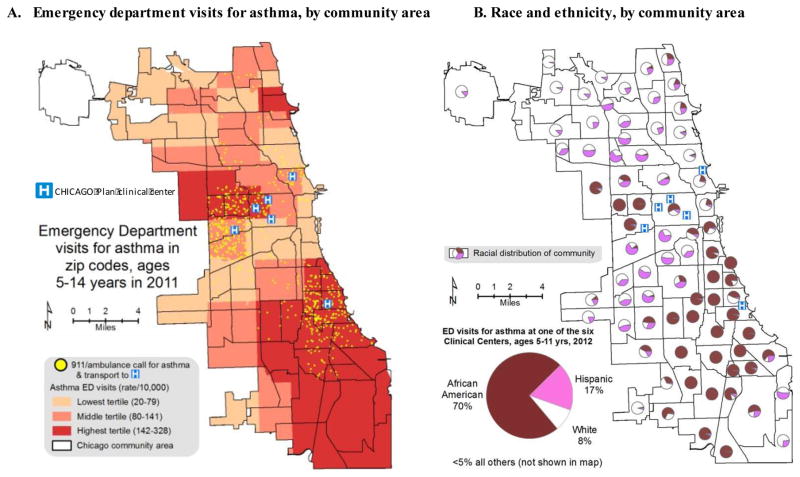
Figure 2. Setting for the CHICAGO Plan.
Data from the Chicago Department of Public Health indicate that emergency department visit rates are highest (dark red) in west and south sides of Chicago (Figure 2A), areas enriched with black (African-American) children (Figure 2B). The CHICAGO Plan will recruit from EDs in six Clinical centers, : Ann and Robert H. Lurie Children’s Hospital of Chicago, Sinai Health System’s Mount Sinai Hospital, John H. Stroger Jr. Hospital of Cook County Health & Hospitals System, Rush University Medical Center, University of Chicago Comer Children’s Hospital, and the University of Illinois Hospital & Health Sciences System. Locations of 911 ambulance calls for children 5 to 14 years with asthma who were transported to the six Clinical centers in 2011 are illustrated as yellow dots (Figure 2A).
3. PROCEDURE
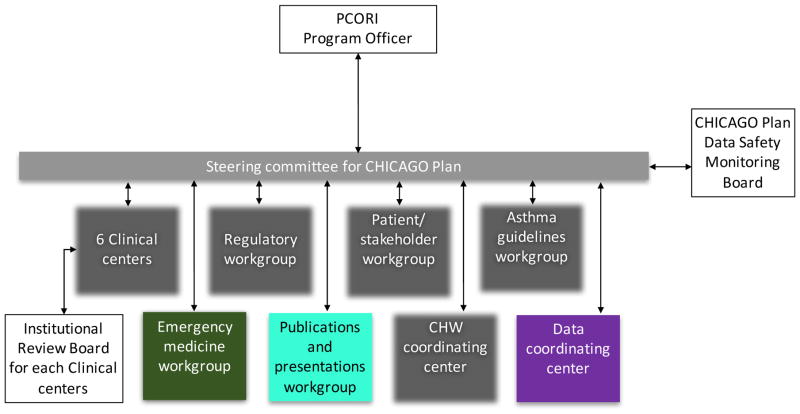
3.1 Study team organizational structure (Figure 3)
Figure 3. Organizational structure for the CHICAGO Plan.
The CHICAGO Plan consortium includes six Clinical center emergency departments that serve children living in the west and south sides of Chicago (see Figure 2 for list of Clinical centers), the Illinois Institute of Technology’s Institute of Design, two non-profit community-based organizations with established asthma programs (Chicago Asthma Consortium, Respiratory Health Association), a research organization with expertise in community health worker programs (CHW; Sinai Urban Health Institute), a representative of the Illinois Emergency Department Asthma Surveillance Project, and a representative of the Chicago Department of Public Health. Investigators and staff from these organizations collaborated in five workgroups (Regulatory, Patient/stakeholder, Asthma guidelines, Emergency medicine, and Publications and presentations), a CHW coordinating center, a Data coordinating center, and a Steering Committee; see 3.1 “Study team organizational structure,” for more information. Each Clinical center underwent initial and ongoing review of human subjects research activities by institution-specific institutional review boards. The CHICAGO Plan included an independent Data Safety Monitoring Board (see 3.6, Data safety monitoring”), and administrative oversight from a Program Officer at the Patient-Centered Outcomes Research Institute.
The CHICAGO Plan investigators are drawn from six clinical centers (2.5 Settings), Illinois Institute of Technology’s Institute of Design, two non-profit Chicago-based community-based organizations with a asthma programs (Chicago Asthma Consortium; Respiratory Health Association), a research organization with expertise in CHW programs (Sinai Urban Health Institute), a representative of the Illinois Emergency Department Asthma Surveillance Program, and a representative of the City of Chicago Department of Public Health. Investigators and staff in the CHICAGO Plan were organized into functional areas, including Clinical centers (3.1.1), multiple workgroups (3.1.2 to 3.1.6), a CHW coordinating center (3.1.7), and a Data coordinating center (3.1.8). A Steering Committee (3.1.9) facilitated communication and coordination across these functional areas.
3.1.1 Clinical centers
Each of six clinical centers includes a site principal investigator and a co-investigator (one of whom is a practicing ED clinician) who together provide oversight for research activities for that clinical center, a project manager (who provides administrative support, regulatory submissions to the institutional review board, quality control), an ED Coordinator (whose primary responsibility is recruitment, review of the CAPE with the child and caregiver in the ED, data entry), and a CHW (who conducts home visits in the ED plus home group).
3.1.2 Regulatory workgroup
This workgroup is led by a senior project manager and includes project managers from each clinical center, and two clinicians (one who practices in the ED and one who practices in the ambulatory setting). This workgroup is responsible for developing model documents for the institutional review boards and ensuring institutional compliance with U.S. laws and relevant agencies governing human subjects research.
3.1.3 Patient/stakeholder engagement workgroup
The CHICAGO Plan builds on nearly two decades of work performed by various asthma stakeholders in Chicago and surrounding areas of Illinois.(50–52) The Patient/stakeholder engagement workgroup is coled by the principal investigators of the Chicago Asthma Consortium(53) and the Respiratory Health Association.(54) The Chicago Asthma Consortium, formed in 1996, is a coalition of medical and public health professionals, business leaders, government agencies, community-based organizations, and individuals in Chicago dedicated to improving the quality of life for individuals with asthma through advocacy, education, and collaboration. The Respiratory Health Association, formed in 1906, leads numerous activities in Chicago, Illinois, and the U.S. to prevent lung disease and promote lung health through education, research, and policy change. Other members of the Patient/stakeholder engagement workgroup include design strategists from the Illinois Institute of Technology’s Institute of Design, and investigators from the Clinical centers.
The Patient/stakeholder engagement workgroup is responsible for facilitating early and continuous stakeholder engagement to inform the study design, assisting with the interpretation of results, and disseminating findings. The frequency of meeting of this workgroup varies according to the stage of the study. During the first 12 months, this workgroup met as frequently as every one to two weeks; during later stages of the study, the group meets every one to six months. Members of the Patient/stakeholder engagement workgroup are also part of the Steering committee, which facilitates communication with the rest of the study team.
In the first 6 months of the project period, the Patient/stakeholder engagement workgroup facilitated key informant interviews and focus groups with physicians and nurses in the ED and ambulatory care settings who provide care for children with asthma, African-American and Latino children with asthma and their caregivers, and CHWs with experience in conducting asthma interventions. In addition, this workgroup observed and interviewed children and caregivers in the EDs and in homes to understand the context and realities that affect how individuals provide, seek, receive, and use healthcare. This set of post-award stakeholder engagement activities provided the necessary opportunity to finalize the study design, including the development of the CAPE.
One to two times per year, the workgroup also meets with members of the Chicago Asthma Consortium’s Community Advisory Board,(55) which includes individuals with asthma, caregivers of children with asthma, community educators, and community leaders, to participate in the review and finalization of consent forms and patient education materials, and methods to enhance recruitment and retention into the study. Based on input from the Community Advisory Board, we revised the name of the study from the CHICAGO “Trial” to the CHICAGO “Plan”, because some community members suggested that the word “Trial” may incorrectly imply that the study was focused on civil or criminal court proceedings.
3.1.4 Asthma guidelines workgroup
The workgroup is led by a co-investigator with expertise in asthma clinical research and the national asthma guidelines, and includes other co-investigators with clinical experience in the management of asthma. This workgroup was developed to ensure that the study protocol in the ED and post-ED settings were scientifically supported and consistent with national asthma guidelines.
3.1.5 Emergency medicine workgroup
This workgroup is co-led by the investigator from the Illinois Emergency Department Asthma Surveillance Program and an ED clinician from the University of Illinois at Chicago and includes ED investigators from each Clinical center. This workgroup is responsible for collaborating with the Patient/stakeholder workgroup (see 3.1.3), Asthma guidelines workgroup (see 3.1.4), and the Operations unit (see 3.1.8) to inform the development of the study protocol around and manual of operations, as well as obtaining support from the ED leadership at the six Clinical centers for implementation of the study.
3.1.6 Publications and presentations workgroup
This workgroup includes investigators representing different stakeholder perspectives (community, ED clinical care, asthma clinical care, clinical research, communication design), and a representative from the Data coordinating center (DCC). The Steering Committee (see 3.1.9) selects the chair of this workgroup. This workgroup is responsible for recommending policies and procedures for the review and approval of research presentations, posters, abstracts, and publications, as well as information used for press releases and social media.
3.1.7 CHW coordinating center
The CHICAGO Plan CHW coordinating center is led by the site principal investigator from the Sinai Urban Health Institute, which is affiliated with the Sinai Health System.(56) The CHW coordinating center includes a CHW training specialist who is also a certified asthma educator, two CHW field supervisors, the principal investigator from the Chicago Department of Public Health, and investigators with clinical and research expertise using CHWs in asthma. Some but not all of the clinical centers had previously employed CHWs as part of their study teams. The CHW coordinating center serves as shared infrastructure for the CHICAGO Plan and is responsible for developing and implementing procedures for recruiting, training, and supervising CHWs deployed at the six clinical centers.
3.1.7.1 Recruiting and retaining CHWs
Job descriptions were developed by the CHW coordinating center and approved by each clinical center’s Human Resources Department and were posted in the communities where the majority of CHICAGO Plan participants were expected to reside (west and southside Chicago). Primary requirements for the CHW for the CHICAGO Plan include: General Educational Development (GED) or high school diploma, a passion for working in their communities, knowledge of their community, and a valid driver’s license. To promote interest in the community for the CHICAGO Plan and for CHWs in the study, the CHW coordinating center announced an informational and training session that included a discussion about the CHICAGO Plan, the CHW position, and a half-day training session about “asthma basics.” All interested candidates were strongly encouraged to attend the informational and training session. The session also provided the CHW coordinating center staff the opportunity to observe the CHW candidates in a group setting and to assess each candidate’s interpersonal skills, enthusiasm for the CHICAGO Plan and the CHW position, and ability to retain and teach basic asthma information; these personal attributes are vital for a CHW to be successful, but difficult to observe in a one-on-one interview session. All candidates completing the training were given instructions about how to apply for the CHW position at all of the six clinical centers. One-on-one interviews were then jointly conducted by CHW coordinating center staff and representatives of the clinical center. For individuals who were interested in the CHW position, but unable to attend the informational and training session, the interviews included a brief role-play exercise to assess interpersonal skills and ability to teach asthma self-management. With consultation from the CHW coordinating center, the clinical center made final decisions on the individual they hired for the CHW position.
The use of a CHW coordinating center was also used to facilitate retention of CHW staff in the study. Some Clinical centers did not generally employ CHWs, so it was possible that CHW at those sites would have insufficient supports, including training or supervision. The CHW coordinating center standardized the support of CHWs for all the sites, assisted sites in identifying appropriate salary ranges, and also assisted the sites with human resources functions to trouble shoot personnel issues.
3.1.7.2 Training, evaluating, and supervising CHWs
Newly hired CHWs enrolled in a 2.5 week, 86-hour, CHW asthma and self-management skills training workshop developed by SUHI and facilitated by the CHW training specialist, CHW field supervisors, and other experienced CHWs. Topics in the curriculum included asthma pathophysiology, symptom recognition, triggers and environmental control, and the principles of asthma management, including appropriate use of controller and quick-relief medications. The environmental control component focused mostly on cockroaches, rodents, and tobacco smoke, the primary triggers targeted by the CHICAGO Plan, but also incorporated information and techniques for reducing the presence of secondary triggers such as mold, dust mites, and irritants (e.g., certain cleaning products, strong odors). Self-management skills training included techniques for approaching and connecting with families, keys to successful home visits, motivational interviewing, patient self-management, CHW safety and proper documentation of interactions with program participants, and goal-setting.
Safety was an important aspect of the training and supervision of CHWs for the CHICAGO Plan. CHWs were advised to perform home visits during daylight hours, whenever possible. Supervisors accompanied CHWs during the training phase to model other appropriate safety precautions and CHWs were encouraged to contact caregivers before making home visits to ensure that home visits were scheduled at a time that was still appropriate. CHWs were advised to contact supervising CHWs if they felt unsafe and to schedule visits in a safe public location (e.g., nearby fast-food restaurant), if necessary.
The training sessions were interactive; participants learned through discussion, review of case studies, and role-play. The CHWs then participated in an additional 20-hour training session specific to home environmental assessment and remediation of triggers conducted by the Metropolitan Tenant’s Organization, a tenants’ rights organization contracted by the CHICAGO Plan.(57) Following the completion of their formal classroom-style training, new CHWs were provided the opportunity to shadow experienced asthma CHWs on two home visits. CHWs also underwent training on data collection procedures for the CHICAGO Plan and Clinical center-specific training in human subjects research and the Health Insurance and Portability and Accountability Act (HIPAA).
The CHW coordinating center also evaluated each CHW’s readiness to independently provide asthma self-management training using the CHICAGO Plan CHW manual of procedures. CHWs completed a series of three graded mock sessions. The first session took place one week after the CHW asthma and core skill training and required the CHW to provide a basic asthma overview to a mock participant. Subsequent sessions built in length and detail, with the final mock session incorporating all topics covered in an actual home visit for the CHICAGO Plan. In addition to the mock session evaluation, CHWs were required to perform a mock home environmental assessment. The focus of the mock assessment was on primary and secondary triggers identified for the CHICAGO Plan, and the CHW was expected to provide several ways in which to avoid or remediate each trigger. When necessary, the CHW coordinating center staff reviewed gaps in proficiency with the CHW and repeated the evaluation session a few days later. The CHW was permitted three attempts to achieve mastery on the CHICAGO Plan CHW-specific protocol in the mock sessions; as necessary, CHW-specific performance would be reviewed with the Clinical center project manager and investigators.
After successfully completing the mock asthma self-management training sessions, the CHWs were permitted to conduct home visits. The coordinating center field supervisors accompanied the CHWs on their first five home visits and reviewed the results of a performance evaluation completed after each home visit. Once a CHW demonstrated mastery on five home visits, the CHW was permitted to conduct home visits independently. Ongoing support and supervision of CHWs was provided by the CHW coordinating center by accompanying the CHW on their home visits once every two months (randomly selected) and by meeting with the CHW to review successes and problem-solve difficulties every two weeks.
We will invite a random 5 to 10% sample of caregivers to participate in a phone call to determine whether they were satisfied with the content, comprehension, and relevance of the home visit intervention to their needs. We will inform the participants that their responses will be anonymous and are being used to help determine how to improve the home visit intervention. We will also interview CHWs to identify barriers and facilitators to completing the intervention (e.g., space or time constraints when providing ED-based instruction; availability of participants at scheduled home visit times). These interviews will help identify suggestions to improve the feasibility of interventions.
3.1.8 Data coordinating center (DCC) and Operations unit
The CHICAGO Plan DCC is led by a senior biostatistician and includes the principal investigator for the CHICAGO Plan, master’s and PhD-level biostatisticians, data managers, a research nurse, and a project manager at the University of Illinois at Chicago. The DCC is responsible for developing the study protocol, the manual of operations, the data collection forms, the data management system, and conducting training for Project Managers and ED Coordinators on study enrollment and follow-up procedures, data collection and data entry into the electronic data capture system, developing and executing a randomization scheme; developing a quality control and quality assurance plan; developing and implementing the analysis plan for the specific aims; and assisting with developing reports for the data safety and monitoring board and institutional review board, as well as for scientific presentations. See more information about the DCC under “shared infrastructure.”
The CHICAGO Plan project manager and research nurse developed and co-lead an Operations unit that included the ED Coordinators, Project Managers, and CHWs from all six Clinical centers, as well as representatives from the Emergency medicine workgroup and the CHW coordinating center. The Operations unit is responsible for developing a detailed manual of procedures based on the study protocol, study staff training, and bi-directional communication between the Steering Committee and Clinical center staff, including identifying and trouble-shooting site-specific issues related to recruitment and retention of study participants.
3.1.9 Steering committee
The Steering Committee is composed of the contact principal investigator for the CHICAGO Plan (serves as chair of the Steering committee); investigators from the six Clinical centers, the Chicago Department of Public Health,(58) and the Illinois Institute of Technology’s Institute of Design; and representatives from the Regulatory workgroup, the Emergency medicine workgroup, the Publication and presentations workgroup, the Patient/Stakeholder workgroup, the CHW coordinating center, the Asthma guidelines workgroup, and the DCC. In some cases, an individual who led a Clinical center also led a workgroup (e.g., Asthma guidelines workgroup), which ensured that workgroups considered the perspectives of other functional teams in the CHICAGO Plan. Thus, the Steering committee includes individuals with expertise in the design and conduct of clinical trials, the clinical management of pediatric asthma, and those who could facilitate the subsequent dissemination and uptake of study results into policy and clinical routines. Inclusion of a representative of the Chicago Department of Public Health on the Steering committee provides the opportunity for the study to be aligned with public health efforts in the city of Chicago.(59,60) Co-investigators, project managers, and other staff were invited to participate in Steering Committee meetings as needed to facilitate communication.
Responsibilities of the Steering committee include the administrative, fiscal, and scientific oversight of the project as well as communication with the PCORI Program Officer, Data Safety Monitoring Board, External Advisory Committee, and other groups as needed. The Steering committee meets every two to four weeks, and makes decisions by consensus (i.e., general agreement). If necessary, each organization collaborating in the CHICAGO Plan is permitted one vote, with decisions made by majority vote. Minutes from the SC meetings are available by email and also uploaded to a secure, password-protected website.
3.2 Shared infrastructure
We employ an innovative shared research infrastructure to implement the CHW-led home visits component of the study. A detailed description of the CHW coordinating center is provided above (section 3.1.7). Use of a coordinating center for CHWs is helping to ensure the interventions delivered by the CHWs are standardized across the clinical centers. Also, all follow-up outcomes for the study are collected via telephone or in-person by study staff at the DCC (section 3.5, Data collection schedule). The DCC personnel are not involved in recruiting participants or conducting the interventions, providing the opportunity to mask staff collecting follow-up outcome data, to facilitate training and supervision, and to standardize data collection.
3.3 Recruitment and retention
The CHICAGO Plan was designed to take place as children with uncontrolled asthma receive care in the ED, a challenging setting in which to conduct research. We therefore sought stakeholder input and reviewed the published literature to implement several strategies to reduce barriers to recruitment and retention (Table 3).(61,62) Our on-site observations in the ED indicated that the time spent by children and caregivers in the ED occurs in several distinct phases, which altogether could vary between about 2 to 8 or more hours: 1) waiting room (0 to 2 hours), 2) triage desk (<5 minutes), 3) asthma treatment (2 to 6 hours, or more), and 4) receipt of discharge instructions (5 to 20 minutes). Based on these findings and interviews with caregivers, ED clinicians, and administrators, it was determined that children or caregivers may not be interested in the study if approached “too early,” when the child was too ill for the child or caregiver to participate in the informed consent process or for the treating clinicians to complete their initial assessments or treatment plan.
Table 3.
Strategies to increase recruitment and retention
| Number | Strategy |
|---|---|
| 1 | Design study so that the intervention and outcomes address the expressed needs of patients. |
| 2 | Demonstrate appreciation (verbal and non-verbal cues, offer reimbursements) when participants complete study activities |
| 3 | Minimize study burden (e.g., position recruitment so that it coincides with the “treatment room” phase of the ED stay, a period where there was sufficient time to present the study and obtain informed consent; reduce wait times and length of visits of follow-up visits; schedule visits around participant’s availability, such as weeknights or weekends; minimize in-person visits). |
| 4 | Provide education about inhaler technique for metered dose inhalers (MDI) and two free MDI spacers for all children 5 to 11 years who present for uncontrolled asthma, even if they subsequently are ineligible for the study or decline to participate in the study (“lead by teaching”). This approach builds support from clinicians for the study and provides the opportunity to explain the study to children and caregivers as an ED-supported program to understand how to improve the care and outcomes of children presenting with uncontrolled asthma. |
| 5 | Send reminders via text, phone, voice mail, or email (as per caregiver preference) for upcoming or missed follow-up visits. |
| 6 | For participants randomized to the ED plus home intervention, provide opportunities for participants to select the topics covered at home visits, or the number of home visits; provide option of completing “home visits” outside of the home if the participant is hesitant about completing a visit in the home (e.g., offer local fast food establishment, apartment complex courtyard). |
| 7 | Offers to complete follow-up outcomes assessments on a day and at a time convenient for the participant, including early morning or evening hours, and weekends. |
| 8 | Obtain multiple sources of contact information; if permitted by caregiver, add contact information for study staff to participant’s phone so that the participant is more likely to answer a call from CHICAGO Plan study staff. |
| 9 | Developed visually appealing materials, culturally tailored to the study population. |
| 10 | Hire and train culturally diverse study staff for study activities. |
Likewise, stakeholders indicated that approaching children and their caregivers at the end of their ED visit just prior to leaving the ED was not feasible. Children and their caregiver may be eager to return home and clinicians and administrators may be reluctant to use a patient care area within the ED for research activities after completing discharge processes, especially if there are other patients waiting to be seen. Based on this information, the CHICAGO Plan team elected to initiate recruitment activities at least 1 hour into the “treatment room” phase of the ED stay. After obtaining verbal assent from treating clinicians, the CHICAGO Plan ED Coordinator approaches children 5 to 11 years presenting with uncontrolled asthma and their caregivers. All such children and their caregivers first receive education about inhaler technique for metered dose inhalers (MDI) and two free MDI spacers, even if they are subsequently found to be ineligible for the study or decline to participate. Completing the assessments to evaluate eligibility and obtaining informed consent occurs after the teaching is completed and the caregiver is offered the MDI spacers. This “lead by teaching” approach is intended to build and sustain support from clinicians for the study and provides the opportunity to explain the study to children and caregivers as part of an ED-supported program to understand how to further improve the care and outcomes of children presenting with uncontrolled asthma. The positioning of recruitment to coincide with specific phases of the ED visit is an example of how the post-award stakeholder engagement activities helped to refine and finalize the study protocol.
To further promote recruitment and enhance retention rates, the CHICAGO Plan was designed to limit study burden for participants. For example, with the exception of the 6-month in-person follow-up visit in the participant’s home, all follow-up visits (at 1, 3, and 12-months after enrollment) occur by telephone (Table 2, Data collection schedule). Participants are also offered flexibility in scheduling the day and time of the follow-up visits, including evenings and weekends. Also, participants are offered $10 following enrollment and $35 per completed follow-up visit to compensate them for their time and effort.
3.4 Informed consent
IRB approval is obtained first at the prime site (University of Illinois at Chicago) and then submitted for approval at each clinical center. While consent forms are specific to the clinical center, the informed consent content about the study is standardized across the sites. We obtain informed assent from all children who are capable of providing assent (age varied based on local institutional review board [IRB] policies) and obtain written informed consent from each caregiver. Assent and consent procedures are performed in English or Spanish, whichever is the preferred language of the caregiver and child. All staff are trained in informed consent and assent procedures and are available to read the consent and assent forms to individuals with low literacy levels using IRB-approved procedures.
As participants in this study are aware of which treatment group they are assigned to following randomization, there is a risk for a Hawthorne effect (change in behavior as a result of monitoring alone), information bias as it relates to answering questions for the patient-reported outcomes (the co-primary outcomes and several secondary outcomes), or selection bias due to differential drop-outs across treatment groups. To minimize these threats to internal validity and because the research involves no more than minimal risk to participants, there is incomplete disclosure of the interventions in the CHICAGO study during informed consent.(63,64) In the IRB-approved consent documents, the study is described as testing different communication strategies combining written and verbal instructions. We also offered all study participants educational materials on a plasticized doorknob hanger (e.g., recommendations for influenza vaccinations; What is asthma?; How the lungs work); we adapted procedures successfully used in a previous study.(16) To further minimize the risk of bias, the DCC staff collecting the outcome data are masked to the treatment group. We are following the recommendations of the American Psychological Association and others regarding incomplete disclosure during consent and will conduct a full debriefing with participants at the conclusion of the study.(63,64)
3.5 Data collection schedule
Data are collected on enrollment (baseline) and at follow-up visits at 1 month, 3 months, and 6 months (i.e., end of the intervention period in children assigned to ED plus home intervention group). For participants enrolled in the first half of the 15-month recruitment period, we also collect outcome data at 12 months to evaluate evidence of sustainability in outcomes (Table 2).
3.6 Data safety and monitoring
We submitted the study protocol and consent form for review and approval by each IRB before initiating study activities at that clinical center; continuing annual reviews and adverse events are reported as specified by each IRB. Insofar as possible, the investigators remain masked to the treatment assignments of individual patients, unless it is judged that it is in the best interests of an individual patient.
This study includes an independent Data Safety Monitoring Board (DSMB) with five individuals who are not affiliated with any of the participating institutions. A senior researcher with experience in multi-center community-based asthma interventions serves as the Chair of the DSMB. Other members of the DSMB include two pediatricians with expertise in asthma clinical trials in the ED and in the ambulatory setting, one statistician with expertise in multi-center trials, and one caregiver of a child with asthma. The DSMB convenes to review and to approve the study protocol in year 1 and convenes two times per year in years 2 and 3 (3-year project period) to review study performance and safety data and make an affirmative recommendation to the study principal investigator whether to 1) continue without modifications, 2) continue with modifications, or 3) terminate the study early. Early termination is an option for the DSMB, particularly if there are serious concerns about patient safety or there is evidence of futility or sufficient evidence of efficacy. No pre-specified interim analyses of outcomes for efficacy or futility are planned.
4. ANALYSES
4.1 Experimental design and overview of analyses
The CHICAGO Plan is a pragmatic clinical trial with individuals randomized to one of three groups: ED-only intervention; ED-plus-home interventions; or enhanced usual care. Participants were recruited in the ED and followed for up to 12 months (observations collected at 0, 1, 3, 6, and [in about half the sample] 12 months.(65–67) The primary analysis will be conducted at 6 months (all study participants); durability of effects will be examined in a secondary analysis in which further data from 12-month observations are included (estimated to be about 50% of the overall trial population). For the whole sample we have a 3 group x 4 times design, but for half the sample there is an additional follow up, yielding a 3 group x 5 times design. Since the 3 x 4 design includes all patients, that was the basis for the power calculation described below.
The analyses will be according to the intent-to-treat principle (primary analysis), supplemented by more informative analyses of the sensitivity of results to different missing data processes. In addition to the linear mixed models relying on the normality assumption (appropriate for continuously scaled PROMIS measures), we anticipate using generalized linear mixed models and in particular generalized estimating equations for the between-groups (marginal) analysis of categorical responses, such as pharmacy dispensations. The analysis will additionally include examination of the heterogeneity of treatment effects as exploratory analyses, as specified in the PCORI Methodology Standards and relevant guidance.(68) The subgroups examined will include standard demographics such as race/ethnicity and gender, and all-cause acute care use prior to enrollment (less than vs. at least the median).
The sections below provide the alternate hypotheses for the two co-primary outcomes at 6 months after enrollment (the primary analyses); a similar approach will be used for the secondary outcomes (e.g., other PROMIS measures; all-cause ED visits). We also describe our methods to estimate sample size and our approach to missing data.
4.2 Hypotheses
4.2.1 Asthma Impact Scale (primary outcome for child)
4.2.1.1 Alternate hypothesis 1
At 6 months, children in the ED intervention group will report better asthma control (assessed using the PROMIS Asthma Impact Scale) than children in the enhanced usual care group.
4.2.1.2 Alternate hypothesis 2
At 6 months, children in the ED-plus-home interventions group will report better asthma control than children in the enhanced usual care group.
4.2.1.3 Alternate hypothesis 3
At 6 months, children in the ED-plus-home interventions group will report better asthma control than children in the ED-only intervention group.
4.2.2 Satisfaction with participation in social roles (primary outcome for caregiver)
4.2.2.1 Alternate hypothesis 4
At 6 months, caregivers in the ED-only intervention group will report higher satisfaction with participation in social roles (assessed using the PROMIS Satisfaction with Participation in Social Roles measure) than caregivers in the enhanced usual care group.
4.2.2.2 Alternate hypothesis 5
At 6 months, caregivers in the ED-plus-home intervention group will report higher satisfaction than caregivers in the enhanced usual care group.
4.2.2.3 Alternate hypothesis 6
At 6 months, caregivers in the ED-plus-home intervention group will report higher satisfaction than caregivers in the ED-only intervention group.
4.3 Power / sample size calculation
We proposed to enroll and randomize 640 participants over 15 months (~200–215 for each of the 3 treatment groups), representing ~25% of the expected pool of children 5–11 years presenting to the EDs, urgent care, or observation units locations across the CHICAGO Plan Clinical Centers with uncontrolled asthma. Assuming evaluable data in 80% of enrolled participants (n=512) at 6 months, sample size calculations suggest ample power for two co-primary outcomes. Our approach is based on the methods of Rochon,(69) with a Bonferroni adjustment for 3 pair-wise comparisons (2-sided α =.05/3 =.0167; enhanced usual care and two active intervention groups), power 80%, 4 measurements per individual (0, 30, 90, and 180 days), within individual correlation 0.80, correction for within ED clustering (design effect of 2), and a coefficient of determination (R2) for control of individual-level demographics = 0.15. Based on these considerations, a sample size of 426 (well within the expected sample size of 512) is sufficient for a minimum detectable difference of 0.35 SD units (midway between Cohen’s “small” (0.20) and “medium” (0.50) effect sizes) for two co-primary continuous outcomes compared pairwise across the three treatment groups.(70) The minimum detectable difference of 0.35 SD units corresponds (based on published SDs for the various outcome measures) to sufficient power to detect a minimum important difference in any two of the PROMIS measures (0.2 to 0.5 SD).(71–73) Analyses to examine heterogeneity of treatment effects have more limited power for the two co-primary outcomes, but results may inform further research and can be incorporated in later meta-analyses.
4.4 Missing data
We recognize that it is not possible to completely avoid missing data, particularly in a pragmatic trial designed to enroll and follow patients representative of those who seek care in emergency departments. We therefore deliberately designed the study to reduce study burden to participants and to study staff, and offer flexibility in completing study assessments (including via telephone). In cases where study participants elect to prematurely discontinue study interventions, we encourage participants to complete outcomes assessments to permit analysis according to the intention-to-treat principle. We will employ a multiple imputation strategy so that we can incorporate auxiliary information about missing data into the intention-to-treat primary analyses.(74) Such analyses, however, generally assume that data are missing at random. To test the robustness of this assumption, we will compare baseline and interval characteristics of patients with and without missing outcome data to help determine whether missing data were informative (e.g., patients with worse baseline health status). We will also employ a “pattern-mixture” approach to comparing interventions that assumes that participants with missing data have mean outcomes that deviates from that of participants who do not drop-out by an offset; we will explore the effect on the findings of various choices of offsets in the intervention groups. This strategy will be used to determine if the intervention effect(s) are qualitatively maintained for a range of offsets that are considered clinically plausible, as proposed by study investigators and with the oversight of the CHICAGO Plan Data Safety Monitoring Board.
5. DISCUSSION
The PCORI-funded CHICAGO Plan addresses evidence gaps about how best to improve the quality of care and outcomes in a predominantly minority population of children 5 to 11 years presenting to the ED with uncontrolled asthma. The CHICAGO Plan is the only project funded by the PCORI Asthma Disparities program targeted research initiative that enrolls children in the ED as they receive care for uncontrolled asthma.
We are employing a three-arm randomized pragmatic trial to compare an ED-focused intervention to improve the quality of care on ED discharge, and the same ED-focused intervention together with a CHW-led intervention at home to promote use of ED discharge instructions and other self-management skills, versus enhanced usual care, on a single primary outcome for the child (PROMIS Asthma Impact Scale) and for the caregiver (PROMIS Satisfaction with participation in social roles). All children, including those in the enhanced usual care group, receive education about the appropriate use of MDI devices and two MDI spacers free-of-charge in the ED to promote and sustain interest among children, caregivers, clinicians, and healthcare system administrators for the CHICAGO Plan.
Innovative features of the CHICAGO Plan include early and continuous stakeholder engagement to inform the design and implementation of the study, as well as a shared research infrastructure to support and coordinate CHW and data collection activities at multiple healthcare systems in Chicago. The study is designed to address the expressed needs of children with asthma and their caregivers, clinicians who care for them in the ED and in the post-ED settings, and other stakeholders. If successful, we believe the intervention could readily be integrated into existing care strategies in collaboration with community based organizations.
Acknowledgments
The CHICAGO Plan was funded through a PCORI award (contract #AS-1307-05420; Clinicialtrials.gov registration #NCT02319967). The statements in this report are solely the responsibility of the authors and do not necessarily represent the views of PCORI, the PCORI Board of Governors, or the PCORI Methodology Committee. The study was also partially supported by the University of Illinois at Chicago Center for Clinical and Translational Science, an award from the National Institutes of Health’s National Center for Advancing Translational Sciences (grant #UL1TR002003). We thank the children, caregivers, and clinicians who helped us to design the CHICAGO Plan.
We also acknowledge members of the CHICAGO Plan consortium: Ann and Robert H. Lurie Children’s Hospital of Chicago (Sana Ali, Janet Flores, Carmen Goralski, Rajesh Kumar, Michael Miller, Jacqueline Ortega, Zachary Pittsenbarger); Chicago Asthma Consortium (*Stacy Ignoffo, Joenell Henry-Tanner); Chicago Department of Public Health (Roderick Jones, Cortland Lohff); Illinois Emergency Department Asthma Surveillance Project (Michael McDermott); Illinois Institute of Technology’s Institute of Design (Kim Erwin, Tara Flippen, Thomas MacTavish, Sarah Norell, Jamie Rivera); John H. Stroger, Jr., Hospital of Cook County Hospitals and Health Sciences System (Maureen Damitz, David Massaquoi, Kenneth Soyemi, Thomas Senko, Trevonne Thompson); Respiratory Health Association (*Kate McMahon, Joel Africk, Amy O’Rourke); Rush University Medical Center (Jane Kramer, Rabia Malik, Pamela Manning, *Giselle Mosnaim); Sinai Health System (Jeanette Avila, Helen Margellos-Anast, Fatima Padron, Jessica Ramsay, Nazia Saiyed, Tala Schwindt, Gloria Seals, Leslie Zun); University of Chicago Medicine (Susannah Butters, Ashley Hull, S. Margaret Paik, Valerie Press, Julian Solway, Crystal Stevenson, John Kim, Nicole Twu, Nicole Woodrick); University of Illinois at Chicago (Michael Berbaum, Nina Bracken, Jennifer Buenrostro, Yi-Fan Chen, Julie DeLisa, David De La Torre-Dorado, Dameka Edwards, Alexander Frye, Kevin Gibbs, Maciej Grabarek, Sai Illendula, Hajwa Kim, Jerry Krishnan, Molly Martin, Sharmilee Nyenhuis, Trevonne Thompson). *Giselle Mosnaim is now at NorthShore University HealthSystem, Chicago, IL; Kate McMahon is now at the Chicago Department of Public Health; and Stacy Ignoffo is now at the Sinai Health System.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.U.S. Department of Health and Human Services (HHS) Expert panel report 3: Guidelines for the diagnosis and management of asthma. Bethesda, MD: HHS, National Heart, Lung and Blood Institute, National Institutes of Health; 2007. [accessed March 17, 2017]. Publication No. 07–4051. http://www.nhlbi.nih.gov/health-pro/guidelines/current/asthma-guidelines. [Google Scholar]
- 2.Centers for Disease Control and Prevention. [accessed March 17, 2017];AsthmaStats. http://www.cdc.gov/asthma/asthma_stats/
- 3.Moorman JE, Akinbami LJ, Bailey CM, et al. National Surveillance of Asthma: United States, 2001–2010. National Center for Health Statistics. Vital Health Stat. 2012;3(35) [PubMed] [Google Scholar]
- 4.Akinbami LJ, Moorman JE, Simon AE, Schoendorf KC. Trends in racial disparities for asthma outcomes among children 0 to 17 years, 2001–2010. J Allergy Clin Immunol. 2014 Sep;134(3):547–553. doi: 10.1016/j.jaci.2014.05.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Illinois Department of Public Health. [accessed March 17, 2017];Asthma burden update. 2013 http://www.iedasp.org/iedasp/AsthmaBurdenBrief_201305_final.pdf.
- 6.Cabana MD, Lara M, Shannon J. Racial and ethnic disparities in the quality of asthma care. Chest. 2007 Nov;132(5 Suppl):810S–817S. doi: 10.1378/chest.07-1910. [DOI] [PubMed] [Google Scholar]
- 7.Crocker D, Brown C, Moolenaar R, Moorman J, Bailey C, Mannino D, Holguin F. Racial and ethnic disparities in asthma medication usage and health-care utilization: data from the National Asthma Survey. Chest. 2009 Oct;136(4):1063–71. doi: 10.1378/chest.09-0013. [DOI] [PubMed] [Google Scholar]
- 8.Weiss KB, Shannon JJ, Sadowski LS, Sharp LK, Curtis L, Lyttle CS, Kumar R, Shalowitz MU, Weiselberg L, Catrambone CD, Evans A, Kee R, Miller J, Kimmel L, Grammer LC. The burden of asthma in the Chicago community fifteen years after the availability of national asthma guidelines: the design and initial results from the CHIRAH study. Contemp Clin Trials. 2009;30:246–55. doi: 10.1016/j.cct.2009.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Shalowitz MU, Sadowski LM, Kumar R, Weiss KB, Shannon JJ. Asthma burden in a citywide, diverse sample of elementary school children in Chicago. Ambul Pediatr. 2007;7:271–7. doi: 10.1016/j.ambp.2007.03.004. [DOI] [PubMed] [Google Scholar]
- 10.Patient-Centered Outcomes Research Institute. [accessed September 19, 2016]; http://www.pcori.org/news-release/pcori-approves-23-million-research-reduce-disparities-asthma-burden-and-outcomes.
- 11.Patient-Centered Outcomes Research Institute. [accessed March 17, 2017]; http://www.pcori.org/research-results/2013/coordinated-healthcare-interventions-childhood-asthma-gaps-outcomes-chicago.
- 12.Krishnan JA, Schatz M, Apter AJ. A call for action: Comparative effectiveness research in asthma. J Allergy Clin Immunol. 2011 Jan;127:123–7. doi: 10.1016/j.jaci.2010.08.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Press VG, Arora VM, Shah LM, Lewis SL, Ivy K, Charbeneau J, Badlani S, Nareckas E, Mazurek A, Krishnan JA. Misuse of respiratory inhalers in hospitalized patients with asthma or COPD. J Gen Intern Med. 2011;26:635–42. doi: 10.1007/s11606-010-1624-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Press VG, Arora VM, Trela KC, Adhikari R, Zadravecz FJ, Liao C, Naureckas E, White SR, Meltzer DO, Krishnan JA. Effectiveness of Interventions to Teach Metered-Dose and Diskus Inhaler Techniques. A Randomized Trial. Ann Am Thorac Soc. 2016;13:816–24. doi: 10.1513/AnnalsATS.201509-603OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Okelo SO, Butz AM, Sharma R, Diette GB, Pitts SI, King TM, Linn ST, Reuben M, Chelladurai Y, Robinson KA. Interventions to modify health care provider adherence to asthma guidelines: a systematic review. Pediatrics. 2013;132:517–34. doi: 10.1542/peds.2013-0779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ducharme FM, Zemek RL, Chalut D, McGillivray D, Noya FJ, Resendes S, Khomenko L, Rouleau R, Zhang X. Written action plan in pediatric emergency room improves asthma prescribing, adherence, and control. Am J Respir Crit Care Med. 2011;183(2):195–203. doi: 10.1164/rccm.201001-0115OC. [DOI] [PubMed] [Google Scholar]
- 17.Erwin K, Martin MA, Flippin T, Norell S, Shadlyn A, Yang J, Falco P, Rivera J, Ignoffo S, Kumar R, Margellos-Anast H, McDermott M, McMahon K, Mosnaim G, Nyenhuis SM, Press VG, Ramsay JE, Soyemi K, Thompson TM, Krishnan JA. Engaging stakeholders to design a comparative effectiveness trial in children with uncontrolled asthma. J Comp Eff Res. 2016;5:17–30. doi: 10.2217/cer.15.52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Martin MA, Press VG, Nyenhuis SM, Krishnan JA, Erwin K, Mosnaim G, Margellos-Anast H, Paik SM, Ignoffo S, McDermott M for the CHICAGO Plan Consortium. Care transition interventions for children with asthma in the emergency department. J Allergy Clin Immunol. 2016 Dec;138(6):1518–1525. doi: 10.1016/j.jaci.2016.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Witmer A. Community health workers: integral members of the health care work force. Am J Public Health. 1995;85:1055–58. doi: 10.2105/ajph.85.8_pt_1.1055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Krieger JW, Takaro TK, Song L, Weaver M. The Seattle-King County Healthy Homes Project: A randomized, controlled trial of a community health worker intervention to decrease exposure to indoor asthma triggers. Am J Public Health. 2005;95:652–9. doi: 10.2105/AJPH.2004.042994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Krieger J, Takaro TK, Song L, Beaudet N, Edwards K. A randomized controlled trial of asthma self-management support comparing clinic-based nurses and in-home community health workers: The Seattle-King County Healthy Homes II Project. Arch Pediatr Adolesc Med. 2009;163:141–9. doi: 10.1001/archpediatrics.2008.532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Campbell JD, Brooks M, Hosokawa P, Robinson J, Song L, Krieger J. Community Health Worker Home Visits for Medicaid-Enrolled Children With Asthma: Effects on Asthma Outcomes and Costs. Am J Public Health. 2015;105:2366–72. doi: 10.2105/AJPH.2015.302685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Postma J, Karr C, Kieckhefer G. Community health workers and environmental interventions for children with asthma: a systematic review. J Asthma. 2009;46:564–76. doi: 10.1080/02770900902912638. [DOI] [PubMed] [Google Scholar]
- 24.Parker EA, Israel BA, Robins TG, Mentz G, Lin Xihong, Brakefield-Caldwell W, Ramirez E, Edgren KK, Salinas M, Lewis TC. Evaluation of Community Action Against Asthma: a community health worker intervention to improve children's asthma-related health by reducing household environmental triggers for asthma. Health Educ Behav. 2008;35:376–95. doi: 10.1177/1090198106290622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mannino DM, Homa DM, Redd SC. Involuntary smoking and asthma severity in children: data from the Third National Health and Nutrition Examination Survey. Chest. 2002;122:409–15. doi: 10.1378/chest.122.2.409. [DOI] [PubMed] [Google Scholar]
- 26.Wilson SR, Yamada EG, Sudhakar R, Roberto L, Mannino D, Mejia C, Huss N. A controlled trial of an environmental tobacco smoke reduction intervention in low-income children with asthma. Chest. 2001;120:1709–22. doi: 10.1378/chest.120.5.1709. [DOI] [PubMed] [Google Scholar]
- 27.Klerman L. Protecting children: reducing their environmental tobacco smoke exposure. Nicotine Tob Res. 2004;6(Suppl 2):S239–53. doi: 10.1080/14622200410001669213. [DOI] [PubMed] [Google Scholar]
- 28.Rosenstreich DL, Eggleston P, Kattan M, Baker D, Slavin RG, Gergen P, Mitchell H, McNiff-Mortimer K, Lynn H, Ownby D, Malveaux F. The role of cockroach allergy and exposure to cockroach allergen in causing morbidity among inner-city children with asthma. N Engl J Med. 1997;336:1356–63. doi: 10.1056/NEJM199705083361904. [DOI] [PubMed] [Google Scholar]
- 29.Gruchalla RS, Pongracic J, Plaut M, Evans R, 3rd, Visness CM, Walter M, Crain EF, Kattan M, Morgan WJ, Steinbach S, Stout J, Malindzak G, Smartt E, Mitchell H. Inner City Asthma Study: relationships among sensitivity, allergen exposure, and asthma morbidity. J Allergy Clin Immunol. 2005;115:478–85. doi: 10.1016/j.jaci.2004.12.006. [DOI] [PubMed] [Google Scholar]
- 30.Pongracic JA, Visness CM, Gruchalla RS, Evans R, 3rd, Mitchell HE. Effect of mouse allergen and rodent environmental intervention on asthma in inner-city children. Ann Allergy Asthma Immunol. 2008;101:35–41. doi: 10.1016/S1081-1206(10)60832-0. [DOI] [PubMed] [Google Scholar]
- 31.Phipatanakul W, Eggleston PA, Wright EC, Wood RA. Mouse allergen. I. The prevalence of mouse allergen in inner-city homes. The National Cooperative Inner-City Asthma Study. J Allergy Clin Immunol. 2000;106:1070–4. doi: 10.1067/mai.2000.110796. [DOI] [PubMed] [Google Scholar]
- 32.Phipatanakul W, Matsui E, Portnoy J, Williams PB, Barnes C, Kennedy K, Bernstein D, Blessing-Moore J, Cox L, Khan D, Lang D, Nicklas R, Oppenheimer J, Randolph C, Schuller D, Spector S, Tilles SA, Wallace D, Sublett J, Bernstein J, Grimes C, Miller JD, Seltzer J Joint Task Force on Practice Parameters. Environmental assessment and exposure reduction of rodents: a practice parameter. Ann Allergy Asthma Immunol. 2012;109:375–87. doi: 10.1016/j.anai.2012.09.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.MacGregor K, Handley M, Wong S, Sharifi C, Gjeltema K, Schillinger D, Bodenheimer T. Behavior-change action plans in primary care: a feasibility study of clinicians. J Am Board Fam Med. 2006;19:215–23. doi: 10.3122/jabfm.19.3.215. [DOI] [PubMed] [Google Scholar]
- 34.Newcomb PA, McGrath KW, Covington JK, Lazarus SC, Janson SL. Barriers to patient-clinician collaboration in asthma management: the patient experience. J Asthma. 2010 Mar;47(2):192–7. doi: 10.3109/02770900903486397. [DOI] [PubMed] [Google Scholar]
- 35.McCullough AR, Ryan C, Macindoe C, Yii N, Bradley JM, O'Neill B, Elborn JS, Hughes CM. Behavior change theory, content and delivery of interventions to enhance adherence in chronic respiratory disease: A systematic review. Respir Med. 2016;116:78–84. doi: 10.1016/j.rmed.2016.05.021. [DOI] [PubMed] [Google Scholar]
- 36.Zwarenstein M, Treweek S, Gagnier JJ, Altman DG, Tunis S, Haynes B, Oxman AD, Moher D CONSORT group; Pragmatic Trials in Healthcare (Practihc) group. Improving the reporting of pragmatic trials: an extension of the CONSORT statement. BMJ. 2008;337:a2390. doi: 10.1136/bmj.a2390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Waseem M, Leber MJ, Wasserman EJ, Sullivan AF, Camargo CA, Jr, Hasegawa K. Factors associated with concordance with the non-level-A guideline recommendations for emergency department patients with acute asthma. J Allergy Clin Immunol Pract. 2015;3:618–20. doi: 10.1016/j.jaip.2015.03.007. [DOI] [PubMed] [Google Scholar]
- 38.Carson SS, Goss CH, Patel SR, Anzueto A, Au DH, Elborn S, Gerald JK, Gerald LB, Kahn JM, Malhotra A, Mularski RA, Riekert KA, Rubenfeld GD, Weaver TE, Krishnan JA American Thoracic Society Comparative Effectiveness Research Working Group. An official American Thoracic Society research statement: comparative effectiveness research in pulmonary, critical care, and sleep medicine. Am J Respir Crit Care Med. 2013;188:1253–61. doi: 10.1164/rccm.201310-1790ST. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.National Institutes of Health. [accessed October 1, 2016]; https://commonfund.nih.gov/promis/index.
- 40.Howell CR, Thompson LA, Gross HE, Reeve BB, DeWalt DA, Huang IC. Responsiveness to Change in PROMIS Measures among Children with Asthma: A Report from the PROMIS P ediatric Asthma Study. Value Health. 2016;19:192–201. doi: 10.1016/j.jval.2015.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hahn EA, Beaumont JL, Pilkonis PA, Garcia SF, Magasi S, DeWalt DA, Cella D. The PROMIS satisfaction with social participation measures demonstrated responsiveness in diverse clinical populations. J Clin Epidemiol. 2016;73:135–41. doi: 10.1016/j.jclinepi.2015.08.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Cloutier MM, Schatz M, Castro M, Clark N, Kelly HW, Mangione-Smith R, Sheller J, Sorkness C, Stoloff S, Gergen P. Asthma outcomes: composite scores of asthma control. J Allergy Clin Immunol. 2012;129(3 Suppl):S24–33. doi: 10.1016/j.jaci.2011.12.980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Akinbami LJ, Sullivan SD, Campbell JD, Grundmeier RW, Hartert TV, Lee TA, Smith RA. Asthma outcomes: healthcare utilization and costs. J Allergy Clin Immunol. 2012;129(3 Suppl):S49–64. doi: 10.1016/j.jaci.2011.12.984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Stelmach I, Podlecka D, Smejda K, Majak P, Jerzyńska J, Stelmach R, Janas A, Stelmach W. Pediatric asthma caregiver's quality of life questionnaire is a useful tool for monitoring asthma in children. Qual Life Res. 2012;21:1639–42. doi: 10.1007/s11136-011-0070-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.HealthMeasures. [accessed October 1, 2016];Patient-reported Outcomes Measurement Information System. http://www.healthmeasures.net/explore-measurement-systems/promis.
- 46.DeWalt DA, Gross HE, Gipson DS, Selewski DT, DeWitt EM, Dampier CD, Hinds PS, Huang IC, Thissen D, Varni JW. PROMIS pediatric self-report scales distinguish subgroups of children within and across six common pediatric chronic health conditions. Qual Life Res. 2015;24:2195–208. doi: 10.1007/s11136-015-0953-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Varni JW, Thissen D, Stucky BD, Liu Y, Magnus B, Quinn H, Irwin DE, DeWitt EM, Lai JS, Amtmann D, Gross HE, DeWalt DA. PROMIS® Parent Proxy Report Scales for children ages 5–7 years: an item response theory analysis of differential item functioning across age groups. Qual Life Res. 2014;23:349–61. doi: 10.1007/s11136-013-0439-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Otsuki M, Eakin MN, Rand CS, Butz AM, Hsu VD, Zuckerman IH, Ogborn J, Bilderback A, Riekert KA. Adherence feedback to improve asthma outcomes among inner-city children: a randomized trial. Pediatrics. 2009 Dec;124(6):1513–21. doi: 10.1542/peds.2008-2961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Butz AM, Tsoukleris M, Donithan M, Hsu VD, Mudd K, Zuckerman IH, Bollinger ME. Patterns of inhaled antiinflammatory medication use in young underserved children with asthma. Pediatrics. 2006 Dec;118(6):2504–13. doi: 10.1542/peds.2006-1630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.McDermott MF, Walter J, Catrambone C, Weis KB. Chest. 1999;116(suppl_2):196S–197S. doi: 10.1378/chest.116.suppl_2.196s. Asthma in Chicago. [DOI] [PubMed] [Google Scholar]
- 51.Lenhardt RO1, Catrambone CD, McDermott MF, Walter J, Williams SG, Weiss KB. Improving pediatric asthma care through surveillance: the Illinois Emergency Department Asthma Collaborative. Pediatrics. 2006;117(4 Pt 2):S96–105. doi: 10.1542/peds.2005-2000G. [DOI] [PubMed] [Google Scholar]
- 52. [accessed October 1, 2016];Illinois Emergency Department Asthma Surveillance Project (IEDASP) http://www.iedasp.org/
- 53.Chicago Asthma Consortium. [accessed March 17, 2017]; www.chicagoasthma.org/about.
- 54.Respiratory Health Association. [accessed March 17, 2017]; http://www.lungchicago.org/
- 55.Chicago Asthma Consortium Community Advisory Board. [accessed March 17, 2017]; http://chicagoasthma.org/about/community-advisory-board/
- 56.Sinai Urban Health Institute. [accessed March 17, 2017]; https://www.sinai.org/content/sinai-urban-health-institute-0.
- 57.Metropolitan Tenants Organization. [accessed March 17, 2017]; http://www.tenants-rights.org/
- 58.Chicago Department of Public Health. [accessed March 17, 2017]; https://www.cityofchicago.org/city/en/depts/cdph.html.
- 59.Chicago Department of Public Health. [accessed March 17, 2017];Healthy Chicago: Transforming the health of our city. https://www.cityofchicago.org/dam/city/depts/cdph/CDPH/PublicHlthAgenda2011.pdf.
- 60.Chicago Department of Public Health. [accessed March 17, 2017];Healthy Chicago 2.0: Partnering to Improve Health Inequity. https://www.cityofchicago.org/city/en/depts/cdph/provdrs/healthychicago/svcs/healthy-chicago-2-0--community-health-assessment-and-improvement.html.
- 61.Yancey AK, Ortega AN, Kumanyika SK. Effective recruitment and retention of minority research participants. Annu Rev Public Health. 2006;27:1–28. doi: 10.1146/annurev.publhealth.27.021405.102113. [DOI] [PubMed] [Google Scholar]
- 62.Nicholson LM, Schwirian PM, Klein EG, Skybo T, Murray-Johnson L, Eneli I, Boettner B, French GM, Groner JA. Recruitment and retention strategies in longitudinal clinical studies with low-income populations. Contemp Clin Trials. 2011;32:353–62. doi: 10.1016/j.cct.2011.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Bersoff DM, Bersoff DN. Ethics and self-report data. In: Stone A, Turkkan JS, Bachrach CA, Jobe JB, Kurtzman HS, Cain VS, editors. The science of self-report: implications for research and practice. Mahwah, NJ: Lawrence Erlbaum Associates; 1999. pp. 9–24. [Google Scholar]
- 64.American Psychological Association. Ethical principles of psychologists and code of conduct. Am Psychol. 1992;47:1597–1611. [Google Scholar]
- 65.Hedeker D, Gibbons RD. Longitudinal data analysis. Hoboken, NJ: John Wiley & Sons, Inc; 2006. [Google Scholar]
- 66.Diggle PJ, Heagerty P, Kung-Yee L, Zeger SL. Analysis of longitudinal data. 2. New York: Oxford University Press, Inc; 2002. [Google Scholar]
- 67.Little Roderick JA, Yau L. Intent-to-treat analysis in longitudinal studies with drop-outs. Biometrics. 1996;52:1324–1333. [PubMed] [Google Scholar]
- 68.Varadhan R, Segal J. Methodology Standards for the Analysis of Heterogeneity of Treatment Effect. Presentation at the AcademyHealth Conference; Baltimore, MD. June 25, 2013. [Google Scholar]
- 69.Rochon J. Sample size calculations for two-group repeated-measures experiments. Biometrics. 1991;47:1383–1398. [Google Scholar]
- 70.Cohen J. Statistical power analysis for the social sciences. 2. New York: Routledge; 1988. [Google Scholar]
- 71. [accessed March 17, 2017];Health measures: meaningful change. http://www.healthmeasures.net/score-and-interpret/interpret-scores/meaningful-change.
- 72.Thissen D, Liu Y, Magnus B, Quinn H, Gipson DS, Dampier C, Huang IC, Hinds PS, Selewski DT, Reeve BB, Gross HE, DeWalt DA. Estimating minimally important difference (MID) in PROMIS pediatric measures using the scale-judgment method. Qual Life Res. 2016;25:13–23. doi: 10.1007/s11136-015-1058-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Norman GR, Sloan JA, Wyrwich KW. Interpretation of changes in health-related quality of life: the remarkable universality of half a standard deviation. Med Care. 2003 May;41(5):582–92. doi: 10.1097/01.MLR.0000062554.74615.4C. [DOI] [PubMed] [Google Scholar]
- 74.Li P, Stuart EA, Allison DB. Multiple Imputation: A Flexible Tool for Handling Missing Data. JAMA. 2015;314(18):1966–1967. doi: 10.1001/jama.2015.15281. [DOI] [PMC free article] [PubMed] [Google Scholar]