Abstract
Dengue remains one the most important mosquito borne disease worldwide. Infection with one of the serologically related dengue viruses (DENV) can lead to a wide range of clinical manifestations and severity. Severe dengue is characterized by plasma leakage and abnormal bleeding that can lead to shock and death. There is currently no specific treatment for severe dengue due to gaps in understanding of the underlying mechanisms. The transient period of vascular leakage is usually followed by a rapid recovery and is suggestive of the effects of short lived biological mediators. Both the innate and the adaptive immune systems are activated in severe dengue and contribute to the cytokine production. We discuss the immunological events elicited during a DENV infection and identify candidate cytokines that may play a key role in the severe manifestations of dengue and possible interventions.
Keywords: Dengue, Dengue hemorrhagic fever, plasma leakage, innate immunity, adaptive immunity, cytokines
Introduction
Dengue is the most common vector borne disease worldwide. Approximately 350 million people are infected by dengue viruses (DENV) each year, and close to one million of these are symptomatic cases.[1] DENV are transmitted by the mosquito vectors Aedes aegypti and to a lesser extent Aedes albopictus, which inhabit tropical and subtropical areas. Although the highest burden of dengue is in Southeast Asia and Western Pacific Regions where 75% of dengue occurs, dengue is also endemic in Central and South America and parts of Africa. Major dengue outbreaks in South Asia and the Middle-East have been reported [2,3]. A few presumed locally transmitted dengue cases have been reported in Europe and the United States [4,5].
DENV, the etiologic agents of dengue, are four genetically and serologically related viruses belonging to the family Flaviviridae. Infection with DENV can lead to a wide spectrum of clinical illness from a nonspecific febrile syndrome to dengue hemorrhagic fever (DHF) characterized by increased vascular permeability, hemorrhage and shock [6]. Although the minority of dengue cases develops severe plasma leakage and bleeding, the need for close monitoring for timely detection and management of these severe manifestations puts a great strain on the public health system in endemic areas, many of which are resource-limited. There are no reliable markers to predict the development of severe manifestations or specific interventions currently available. The lack of predictive markers and specific treatments is a consequence of gaps in understanding of the underlying mechanisms of severe dengue disease.
The hallmark of severe dengue is a transient perturbation in blood vessel integrity and coagulation. Recovery is usually rapid and complete, suggesting that the key mechanisms are functional rather than structural changes in the vasculature; these changes are most likely due to effects of locally produced biological mediators, especially cytokines and other soluble factors released as a consequence of complex interactions between DENV and host innate and adaptive immune responses. In this article we review current understanding of events leading to the induction of the innate and adaptive immune responses in dengue and the consequences on these responses on the development of severe manifestations of dengue.
Dengue viruses and dengue clinical manifestations
DENV are small enveloped viruses containing a single-stranded RNA approximately 10 kilobases in length. The viral genome is a positive sense RNA that encodes a single polyprotein that is cleaved to produce 10 viral proteins, three structural proteins-C (capsid), prM (membrane), and E (envelope)- and seven nonstructural proteins: NS1, NS2A, NS2B, NS3, NS4A, NS4B, and NS5 [7,8]. The envelope protein plays an important role in viral binding and entry into host cells[9–11] and is the main target of neutralizing antibodies, which define the 4 DENV serotypes (DENV1, DENV2, DENV3, and DENV4) [7]. The nonstructural proteins of DENV function in viral polyprotein processing, RNA replication, and virion assembly [7]. Several nonstructural proteins also play a role in modifying host immune responses. NS2A, NS2B, NS4B and NS5 proteins have been shown to interfere with type I interferon (IFN) signaling [12,13]. NS5 and NS4B have been demonstrated to induce the production of chemokines and proinflammatory mediators [14,15]. NS proteins are important targets of the cell mediated immune system, especially CD8+ T cells.[16]
DENV infects a variety of cell types in vitro including epithelial cells, endothelial cells, hepatocytes, muscle cells, dendritic cells, monocytes and mast cells. However, cells of the immune system appear to be the major target for infection in vivo [17–21]. A number of studies have shown that C-type lectins including DC-SIGN (CD209) and CLEC5A expressed on dendritic cells and macrophages are cellular receptors of DENV [22,11]. DC-SIGN likely functions primarily as a target for viral attachment, since viral internalization occurs in cells expressing DC-SIGN mutated to lack its internalization sequence [23]. In contrast, DENV binding to CLEC5A has been shown to also induce the production of proinflammatory cytokines [22].
A primary DENV infection in young children is usually asymptomatic or manifests as a non-specific febrile illness. In older children and in adults, primary DENV infection results in Dengue Fever (DF), which is characterized by high fever, retroorbital pain, myalgia, leukopenia, thrombocytopenia, and hemorrhagic manifestations[24]. These symptoms are self-limited and the majority of DF cases recover within 4–7 days without requiring significant intervention. Following recovery, individuals develop long-lasting protective immunity to the same DENV serotype. There is cross reactivity at the humoral and cellular level for the other DENV serotypes, but it provides partial protection lasting only several months, following which individuals are fully susceptible to heterologous secondary DENV infection.
A minority of patients develop dengue hemorrhagic fever (DHF), which is characterized by fever, severe thrombocytopenia, hemorrhagic tendency, and plasma leakage [25]. Plasma leakage is the feature that distinguishes DHF from DF and is the principal cause of severity, with the potential to lead to circulatory insufficiency and death [25,26]. Plasma leakage in DHF characteristically occurs in the chest and abdominal cavities [27] around the time of defervescence. DHF is also strongly associated with secondary DENV infections; although only 2–3% of secondary DENV infections trigger DHF, multiple cohort studies have demonstrated a 15- to 80-fold increase in risk for DHF compared to primary DENV infections [28,29].
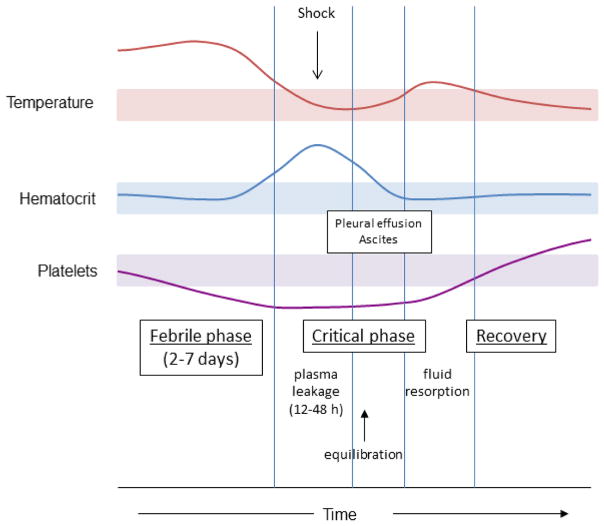
Figure 1 depicts the typical clinical progression of an individual with severe DHF. Plasma leakage occurs around the time of defervescence and at the nadir of platelet counts. Plasma leakage causes an increase in hematocrit (hemoconcentration). Severe plasma leakage can lead to shock and organ failure. Bleeding may occur at any time during the illness but is more common in patients with shock. Judicious use of intravenous fluid is paramount in the supportive care during this critical phase which usually lasts about 48–72 hours. Patients usually show a rapid improvement after this period. Pulmonary edema may develop in some cases after the critical phase when extravascular fluid is reabsorbed into the circulation.
Fig 1.
Clinical course of dengue hemorrhagic fever. Clinical events and laboratory findings during phases of illness from the febrile phase through the critical phase and into the recovery phase is shown. Hemoconcentration (an increase in hematocrit) occurs during the critical phase and is an indication of plasma leakage. Platelet counts decline during the illness reaching the lowest point around the time when plasma leakage occurs. Shaded areas represent the normal ranges for temperature, hematocrit, and platelet count.
Relevance and limitations of studies of dengue pathogenesis
In vitro studies of DENV infection using various cell types have elucidated the mechanisms of viral replication and immune evasion. These studies have also shown that the interaction between DENV and host cells results in the production and release of proinflammatory, antiviral, and immunoregulatory cytokines. DENV-infected macrophages and endothelial cells produced IL-8, an effect mediated by DENV NS5 and NS4B proteins [14,15,30]. DENV-infected endothelial cells also secreted IL-6, CXCL10, CXCL11, and RANTES [31]. These mediators can enhance permeability and have chemoattractant properties that could contribute to inflammation and plasma leakage in vivo [14]. DENV-infected dendritic cells produced matrix metalloproteinase (MMP)-2 and MMP-9, which enhance permeability of endothelial monolayers by downregulating VE-Cadherin expression [32]. However, the findings in these cell culture models do not necessarily reflect the relative contribution of these soluble factors to pathology in vivo. Studies with skin explants have also demonstrated that tissue macrophages and dendritic cells in the skin and keratinocytes may be targets after the initial inoculation of virus by mosquitoes [33,34].
Studies in animal models have provided further insights into disease process. Non-human primates infected with DENV develop viremia. However, viremia levels are low relative to humans, and these animals do not usually develop clinical disease [35,36]. A study reported bleeding manifestations in rhesus macaques after high dose intravenous inoculation, but this artificial approach to generating viremia may have limited relevance to natural DENV infection in humans [37]. A number of mouse models have also been developed. Severe combined immune deficient (SCID) mice transplanted with human hematopoietic stem cells generated human T cells, B cells, macrophages, dendritic cells and NK cells and produced a robust immune response to DENV infection [38–40]. Other mouse models including interferon receptor gene knock-out mice or mice infected with high dose wild-type or mouse-adapted DENV strains have demonstrated viremia and clinical signs after infection including thrombocytopenia, hemorrhage, and plasma leakage in the intestine [41–44]. Endothelial cell damage was associated with tissue infiltration of macrophages that secreted TNF-α [45,46]. Anti-TNF-α treatment inhibited hemorrhage in these models. However, findings in animal models must be interpreted with caution since none of these models closely mimics severe disease in humans.
Given the limitations of cell culture and animal models of dengue, human clinical studies remain critical to the understanding of the pathogenesis of dengue. Most studies have focused on identifying markers in the circulation that differ between DF and DHF or between severe and non-severe cases [47]. The finding of significantly different levels of biological markers between groups may provide clues regarding the mechanisms of severe manifestations of dengue, especially plasma leakage and bleeding, and may provide a rationale for testing specific interventions. In addition, these studies may provide predictors that can identify patients at risk for severe disease. However, there are caveats in interpreting findings from these studies: 1) different biomarkers exhibit distinct kinetics during the progression of the disease, therefore, the timing of sample collection is integral to interpreting and comparing findings from different studies, 2) the quality and completeness of clinical and laboratory data affect the accuracy of clinical classification, 3) differences in sample collection and processing may impact levels of certain biological molecules, particularly those that may be released or consumed during coagulation [48,49]. With these limitations taken into consideration, findings from these studies have provided important insights into the process leading to severe manifestations of dengue.
Limited studies of human tissue samples from fatal cases have provided unique and perhaps the most relevant information regarding dengue pathogenesis [50–52]. The most striking finding from these studies is the relative absence of tissue inflammation. Endothelial cell edema and perivascular edema have been the most common histological findings. DENV antigen and/or genome have been consistently found in monocyte, tissue macrophages and lymphocytes. Some studies have demonstrated viral antigen in other cell types including endothelial cells, hepatocytes, and cardiac muscle cells. However, most of these studies are small case series or cases with specific or unusual manifestations [53,54 ]. There has been limited evidence of endothelial injury measured as apoptosis or structural changes [54]. These findings together with the transient nature of plasma leakage and rapid recovery suggest that transient perturbation of vascular barrier integrity is the main mechanism underlying plasma leakage in DHF, and that the activation of endothelial cells and the coagulation system is likely mediated by cytokines produced by the innate and adaptive immune system.
Virus introduction and activation of the innate immune system
Although plasma leakage in DHF occurs at the end of the acute illness, there is substantial evidence that the pathophysiologic processes are set in motion at the earliest stages of infection (Figure 2). Introduction of DENV by mosquito bites activates the innate immune system. Cells that are initially infected include epidermal and dermal dendritic cells [34,55]. In vitro studies demonstrated the production of antiviral and proinflammatory cytokines by these cells when exposed to DENV. These cytokines include type I IFNs and chemotactic factors such as migration inhibition factor (MIF), monocyte chemotactic factor (MCP), and IL-8 [56–58]. This initial and focal cytokine response is not likely the cause of clinical symptoms but potentially plays an important role in regulating local viral replication and dissemination by recruiting virus-susceptible cells to the inoculation site. Infection of dendritic cells by DENV also induces the production of MMP-2 and MMP-9 which may facilitate migration of dendritic cells to the local lymph nodes where virus further replicates and subsequently enters the circulation.[32] Mast cells residing in the skin may also be involved in this early phase of infection [59]. DENV activate mast cell degranulation, a process that does not require replicating DENV. Elevated chymase levels in the blood of dengue patients indicate that mast cell degranulation occurs during the early phase of dengue [59]. Lower levels of serum chymase were found in DHF cases suggesting that mast cell activation is associated with protective effects, possibly through the recruitment of iNK T cells to the site of viral inoculation [60].
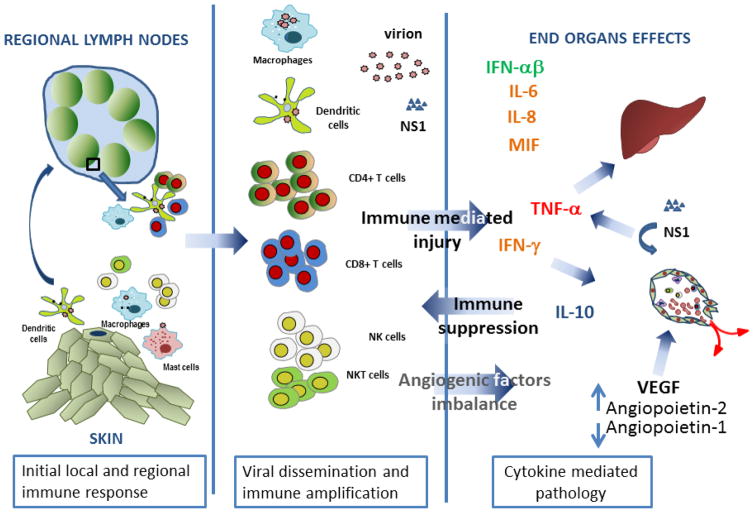
Fig 2.
Immunological events in DENV infection. DENV is introduced through mosquito bites and infects local immune cells including resident dendritic cells, mast cells, and blood-derived dendritic cells. Cytokines produced by these local immune cells regulate viral replication and further recruit immune cells to the site of infection. Infected cells migrate to local lymph nodes where DENV further replicates and activates B and T cells leading to differentiation of these cells into effector cells. DENV disseminates through the circulation and further amplified the innate and the adaptive immune responses. The cytokines produced by these immune cells activate endothelial cells resulting in the perturbation of vascular integrity and coagulopathy. NS1 protein may act directly on endothelial cells to enhance vascular permeability. These cytokines may affect other cell types including hepatocytes leading to liver injury.
Studies of biological markers have demonstrated that the early febrile phase is characterized by high levels of virus or soluble NS1 protein along with elevated levels of type I IFN [61,62]. This is consistent with results from sequential genome expression studies which showed that the early gene expression in peripheral blood cells of dengue patients is dominated by type I IFN-mediated response genes [63]. Higher levels of viremia and NS1 protein have been associated with more severe disease [64,61]. This may reflect a relatively defective innate immune response in patients with severe disease. Studies in rhesus monkeys have demonstrated that plasmacytoid dendritic cells, a major producer of type I IFN, are mobilized into the circulation after DENV infection. Furthermore, comparatively lower frequencies of plasmacytoid dendritic cells were found in the peripheral blood of DHF patients [62]. These findings indicate that the early response of type I IFN is critical in regulating viral replication and dissemination.
In addition to type I IFN, dendritic cells and monocyte/macrophages also produce proinflammatory cytokines that can increase vascular permeability. Interactions between DENV and CLEC5A, a selectin molecule expressed by macrophages, elicited TNF-α production and plasma leakage in a mouse model [22]. Macrophage derived TNF-α has been implicated in hemorrhage via production of reactive oxygen species in DENV-infected mice [46]. The NS1 protein has been shown to have a permeability enhancing effect on endothelial cells through activation of TLR4 and by inducing MIF production which increases permeability through mechanisms involving autophagy [65,66]. Treatment with antibody specific to NS1 prevented plasma leakage and death in mice [67]. Although findings from these studies are compelling, it is difficult to reconcile the observations that the permeability-enhancing effects of NS1 occur within hours of interaction with endothelial cells in vitro, whereas plasma leakage in dengue occurs at the time when virus and NS1 protein are cleared from the circulation, several days after peak levels in the circulation. Nevertheless, these findings provide a strong rationale for testing interventions targeted at NS1 in future studies.
Other populations of innate immune cells, namely NK and NK-like cells, are activated during DENV infection. Flow cytometric studies showed that the frequencies of activated NK cells are higher in patients with DHF compared to those with DF [68]. NK cells may be activated directly by DENV virions or through interaction with DENV-infected dendritic cells, a process which requires dendritic cell derived IFN-α and TNF-α [69]. An early increase in IL-15 levels accompanied the expansion of NK cells in dengue patients [70]. Cytolytic activity of NK cells mediated by the perforin-granzyme pathway or by the Fas-FasL pathway may provide antiviral effects but at the same time mediates tissue injury. In a mouse model, NK cell infiltration in the liver was observed during the very early phase of the infection and was associated with liver injury [71]. Consistent with this notion, elevated liver enzyme levels have been frequently observed in dengue patients especially those who subsequently developed DHF [72]. Human and animal studies have demonstrated that NK-like T cells, iNKT cells, are also activated during DENV infection and the frequencies of cells expressing an activation marker (CD69) correlated with disease severity [73]. iNKT cells are CD3+ T cells that express CD56 and recognize CD1-restricted lipids, and are potent secretors of cytokines especially IFN-γ and IL-4 [74]. Depletion of iNKT cells in DENV-infected immunocompetent mice prevented liver injury and plasma leakage, supporting a role in dengue pathogenesis [75]. Recruitment of iNKT cells appeared to require local mast cell activation by DENV and therefore may be important in limiting local viral replication [60].
In addition to their role as a key cellular target of proinflammatory cytokines in the pathogenesis of plasma leakage, endothelial cells also participate in antiviral defense and inflammation. Transcriptional analysis of DENV-infected endothelial cells showed activation of transcriptional programs related to cell death, type I IFN, angiogenesis, cytokines and chemokines, complement, and coagulation [76,77]. Cultures of human umbilical vein endothelial cells showed altered membrane integrity even though the frequencies of infected endothelial cells were low [78,79]. DENV infection of primary human endothelial cells also led to changes in cell surface receptors for vascular endothelial growth factor-A (VEGF-A) and an increase in VEGF-A responsiveness; this effect was observed in both DENV-infected cells and uninfected (bystander) cells in the same culture. These findings suggest that the biological response to DENV by endothelial cells may be widespread and occur in both infected and uninfected cells [80].
In summary, in the early phase of infection DENV activates a wide range of cells of the innate immune system through various mechanisms (Figure 2). The resulting cytokine environment affects local and systemic viral replication. If this early response fails to control viral replication, high levels of viremia and NS1 protein develop, and these viral products further activate the innate immune system leading to the amplification of cytokine production. As the infection progresses, bidirectional interactions between the innate and the adaptive immune system may lead to the exaggeration of immune responses and contribute to disease severity.
Activation of the adaptive immune system and its consequences
Both humoral and cellular adaptive immunity are activated during DENV infections and play critical roles in viral eradication as well as in disease pathogenesis. Due to the existence of multiple DENV serotypes and the lack of long term cross-serotype protective immunity, individuals in dengue-endemic areas are often infected more than once. Outbreak studies and prospective cohort studies have demonstrated that a previous DENV exposure is a strong predisposing factor for severity in a subsequent infection [29,81]. This observation implicates adaptive immune responses in severe dengue.
Primary infection with DENV stimulates naïve CD4+ and CD8+ T cells to become activated and differentiate into effector T cells that eradicate virus infection by direct lysis of virus-infected cells or by the production of cytokines.[82] Many different HLA-restricted T cell epitopes recognized on different proteins have been identified in DENV-immune individuals. Several studies indicate that non-structural proteins are more frequently recognized by CD8+ T cells, while structural proteins are better recognized by CD4+ T cells. [83,84] Depending on the epitope recognized, these T cells can respond to a second infection with a different DENV serotype. After a primary infection the strongest response is typically to the serotype of DENV that the subject has been exposed to, while responses after secondary infection are highly serotype cross-reactive [82]. Responses to the corresponding epitope variants of the heterologous DENV serotypes reveal different patterns of effector functions including cytolytic activity and cytokine production. A typical hierarchy of effector responses to heterologous epitopes has been production of MIP-1β> release of cytotoxic granules> production of TNF-α > production of IFN-γ [85]. In parallel with this in vitro observation, flow cytometric analysis of CD8+ T cells from patients with DHF demonstrated lower frequencies of cells with cytolytic function compared to those from DF cases, whereas the frequencies of cells producing proinflammatory cytokines were comparable or higher in DHF cases [86]. These finding suggest that defective or suboptimal cytolytic function during a secondary DENV infection in combination with enhanced cytokine production may be an important step in the development of severe disease. Most studies to date have described the production of Th1 cytokines and minimal production of Th2 cytokines by DENV-specific T cells. IL-17 and IL-21 secretion by DENV-specific T cells is just beginning to be described and therefore the roles of these cytokines in dengue pathogenesis are currently unknown [83].
Studies of acute dengue illness demonstrate high levels of T cell activation in vivo. Both the expression of activation markers on T cells and frequencies of DENV-specific T cells are markedly elevated during acute infection [68,87]. The kinetics of T cell activation remain the subject of some controversy. Expression of CD69, an early marker of activation, was highest soon after symptom onset [68], whereas other activation markers (e.g., CD38, HLA-DR, and CD71) and frequencies of tetramer-positive cells peaked at the time of defervescence or slightly thereafter [88]. The possibility that T cell activation is initially occurring in secondary lymphoid tissues prior to their appearance in the blood must be taken in consideration, however, confounding the interpretation of these results. Serum levels of soluble markers of T cell activation, such as sIL-2R, sTNFR, and sCD8, may reflect cellular activation throughout the body, and have been reported to be significantly higher in patients with more severe disease compared to individuals with milder disease [89,90]. These factors may also be produced by other immune cells, however.
B cells play a unique role in the immune response to dengue by secreting antibodies following activation through the B cell receptor. Antibodies to DENV can mediate multiple functions in vitro including neutralization, antibody dependent cell-mediated cytotoxicity (ADCC), antibody-dependent enhancement (ADE), and complement fixation [91]. The E, prM and NS1 proteins are major targets of antibodies generated during primary and secondary DENV infections. Antibodies to E and prM have been shown to enhance infection of Fc receptor-bearing cells in vitro via ADE, and this has been speculated to contribute to pathogenesis in vivo. DENV entry via ADE was also found to suppress the antiviral state of host cells by blocking IFN-α and to induce IL-10 production thus allowing increased replication [92]. These findings parallel the higher viremia with elevated IL-10 and low IFNs detected in patients with more severe dengue disease [64,93]. However other in vitro models have not found induction of IL-10 in the setting of ADE [94].
Studies of acute dengue illness also demonstrate high levels of B cell activation. Between 40–70% of all CD19+ B cells in the peripheral blood have markers associated with plasmablasts during severe acute secondary DENV infection, which is much higher than frequencies reported during a primary DENV infection or other acute viral illnesses [95–97]. In contrast, DENV-specific memory B cells exist at very low frequencies in the blood and do not actively secrete Ab. Memory B cells have been detected using fluorescently labeled virus [98], these memory B cells may be particularly relevant in subsequent DENV infections.
B cell activation during DENV infection may contribute to disease pathogenesis through mechanisms beyond antibody production. Activated B cells produce a number of cytokines including IL-6, IL-10, IL-35, CCL3, GM-CSF, and TNF-α. Some of these cytokines including IL-6, IFN-γ, and TNF-α regulate the differentiation of effector and memory CD4+ T cells, whereas IL-10 and IL-35 can negatively regulate immune responses [99]. B cell cytokine responses to DENV have largely been overlooked to date but are likely to be an important aspect of B cell biology to DENV.
Cytokine mediated pathology in severe dengue
Given the transient and reversible nature of vascular permeability and coagulopathy in dengue and the evidence of activation of innate and adaptive immunity discussed above, we and others have favored the model that severe dengue represents the culmination of a cascade of immune responses, with disease mediated by multiple soluble and short-lived immune effectors (Figure 2). Evidence to support this model has largely relied on the measurement of the levels of candidate biological mediators in dengue cases with varying severity. A number of cytokines and biological mediators have been implicated in dengue pathogenesis from these studies. However, conflicting results have been reported. Distinct study design, timing of sample collection, sample processing, and study populations likely contribute to these conflicting results, but the possibility that different pathways could produce similar clinical manifestations must also be considered.
TNF-α is one of the most studied cytokines in dengue both in human and in animal models. TNF-α may be released from either the innate immune system through virus interaction with monocytes/macrophages, and NK or iNKT cells, and by the adaptive immune system by activation of virus-specific CD4+ or CD8+ T cells. Exposure of endothelial cells to TNF-α in vitro resulted in increased permeability and cell death [100]. TNF-α also induces expression of tissue factor (TF) that can initiate the coagulation cascade. As such, TNF-α may play an important role in the two main pathological processes in severe dengue: plasma leakage and coagulopathy. In humans, elevated levels of TNF-α and soluble TNF-α receptors have been reported in DHF cases [70,89]. The relatively elevated levels of soluble forms of TNF receptors may reflect in vivo activation of cells through these receptors. Importantly, a prospective cohort study has shown that preillness PBMC from some patients that subsequently developed severe illness secreted TNF-α while those from individuals with subsequent milder dengue did not [101]. These findings provide a strong rationale for the use of TNF-α inhibitors for treatment in dengue. TNF-α is an attractive candidate molecule for intervention since currently there are a number of biological molecules including antibodies and receptor antagonists that are approved and have well-characterized safety profiles [102]. Side effects of TNF-α blockade, which include reactivation of mycobacterial infection, must be taken into consideration when designing an intervention study.
Vascular permeability is regulated by a functionally related set of cytokines that play key roles in angiogenesis. These include Vascular Endothelial Growth Factor-A (VEGF-A), angiopoietin-1, and angiopoietin-2 [103]. VEGF-A is the most potent permeability enhancing cytokine known, and elevated levels have been reported in DHF at the time of plasma leakage [80]. Changes in the levels of its soluble receptors including sVEGFR1 and sVEGFR2 have been reported in dengue [80]. In particular, decreased levels of sVEGFR2 were reported to correlate with the extent of plasma leakage and the levels of viral RNA in plasma of dengue patients. Angiopoietin-1 counteracts the permeability enhancing effect of VEGF-A [104]. Lower levels of angiopioetin-1 have been shown in dengue cases with plasma leakage. Interestingly, the levels of angiopoietin-1 antagonist, angiopoietin-2, had been shown to be elevated in DHF patents [105]. In addition to its permeability enhancing effect, VEGF-A is also a potent inducer of tissue factor (TF) expression by endothelial cells which may activate the coagulation system leading to coagulopathy.[106] This VEGF-A effect is also antagonized by angiopoietin-1 [106]. Taken together, these findings suggest that a concerted change in the levels of angiogenic cytokines occurs that may contribute to both increased vascular permeability and coagulopathy in severe dengue. The underlying mechanisms for these changes in angiogenic cytokine levels are not understood. The modulatory effects of DENV on VEGFR2 expression and signaling in endothelial cells mentioned above provide one potential mechanism, but it is not known whether the same effects also occur in vivo. Angiogenic cytokines are potential target for therapeutic intervention since there are a number of biologicals and small molecule inhibitors that are either in clinical use or in clinical trials for other indications.
Elevated levels of IL-10 have been a consistent finding in severe dengue [107,108]. IL-10 is a key immunoregulatory cytokine and is produced by a number of cells including monocyte/macrophages, dendritic cells, and regulatory T cells (Tregs). As noted earlier, monocytes have been shown to produce IL-10 when infected with DENV especially via ADE. Genetic polymorphisms of IL-10 gene have been reported to correlate with IL-10 produced by DENV-infected monocytes in vitro and associate with disease severity [109]. Lower frequencies of Tregs were reported in DHF cases compared to DF cases, [110] this would not explain the higher levels of IL-10 in DHF but will be consistent with the hypothesis that the immunopathology in DHF may be due to insufficient induction of Treg. Elevated IL-10 levels occur late in the course of illness and may reflect an enhanced induction of Tregs in DHF as a response to the intense cellular immune activation earlier in illness (Figure 2). On balance, a deleterious or beneficial effect of the immunosuppressive and anti-inflammatory functions of IL-10 may depend on the timing of its production. IL-10 produced by infected macrophages early in infection may facilitate viral replication and dissemination while IL-10 produced later in illness by regulatory T cells may be critical in attenuating the immune response and immune-mediated tissue injury.
A wide range of other cytokines have also been reported to be associated with dengue disease severity, but findings have been somewhat inconsistent and their relevance to disease pathogenesis is tenuous [47]. Those that seem more promising as potential contributors to disease include IL-6, and chemokines including IL-8, CCL2/MCP-1 and CXCL10/IP10 [108]. Chemokines play a key role in recruiting inflammatory cells to the site of viral infection and immune activation, and may thereby contribute to a cascade of cytokine production in dengue (Figure 2). The levels of a number of soluble cytokine receptors including sIL-1R, ST1, and sIL-2R have been shown to be elevated in severe dengue, but it is unclear whether these soluble receptors are a general marker of immune activation, a specific marker of cytokine signaling, or involved in disease pathogenesis through their biological functions. It is also possible that soluble receptors may interfere with the immunoassay for the cytokines resulting in falsely low levels. Elevated sIL-2R levels correlated with liver enzyme levels in dengue patients in one study [64] suggesting that this cytokine pathway plays some role in dengue severity.
Conclusion
Studies demonstrating exaggerated immune activation in severe dengue strongly suggest a critical role of the immune response in the pathogenesis of dengue. Both innate and adaptive immunity (and their interactions) are involved in this process. The current challenges in the clinical care of dengue are the lack of specific interventions to ameliorate the unwanted consequences of immune activation and the lack of reliable predictors for severe disease. Although a number of biological mediators have been implicated in disease severity, identifying the key molecule or molecules remains a challenge. There is a critical gap in the understanding of the relative contributions of various mediators in dengue pathogenesis which is required for the rational development for specific interventions. Due to the complexities and redundancy of the cytokine network, intervention strategies may include a combination of agents that target multiple cytokine pathways that play different roles at distinct stages of the disease. For example, treatment early in the infection might aim to control viral replication and attenuate the subsequent immune activation. The treatment in this phase may include antiviral agents including interferons. Treatment later in the course of the illness might target the amplification of the adaptive immune response using immunosuppressive agents. Finally, treatment might target mediators that contribute to specific manifestations of severe dengue including vascular leakage, coagulopathy, and end organ dysfunction. The prerequisite understanding of the disease process underscores the need for well-designed human studies with well-characterized patient populations and high quality clinical and laboratory data. New developments such as analytical platforms that allow simultaneous measurements of multiple analytes or transcription factors may help in providing a more global view of the biological process. However, interpretation of such data requires powerful and sophisticated analysis algorithms and must be done in the context of meaningful, objective, and quantifiable clinical indicators.
Acknowledgments
This work was supported in part by the National Institutes of Health (grant P01 AI034533). The opinions expressed are those of the authors and do not represent the official position of the National Institutes of Health.
Abbreviations
- CLEC5A
C-type lectin domain family 5 member A
- DCSIGN
dendritic cell-specific Intercellular adhesion molecule-3-grabbing non-integrin
- DENV
dengue virus
- DF
dengue fever
- DHF
dengue hemorrhagic fever
- IFN
interferon
- IL
interleukin
- LPS
lipopolysaccharide
- MCP
monocyte chemotactic factor
- MIF
migration inhibition factor
- MMP
matrix metalloproteases
- NS
nonstructural
- VE-Cadherin
vascular endothelial cadherin
- VEGF-A
vascular endothelial growth factor-A
- VEGFR1
vascular endothelial growth factor receptor 1
- VEGFR2
vascular endothelial growth factor receptor 2
References
- 1.Bhatt S, Gething PW, Brady OJ, Messina JP, Farlow AW, Moyes CL, Drake JM, Brownstein JS, Hoen AG, Sankoh O, Myers MF, George DB, Jaenisch T, Wint GR, Simmons CP, Scott TW, Farrar JJ, Hay SI. The global distribution and burden of dengue. Nature. 2013;496(7446):504–507. doi: 10.1038/nature12060. nature12060 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Amarasinghe A, Letson GW. Dengue in the Middle East: a neglected, emerging disease of importance. Trans R Soc Trop Med Hyg. 2012;106(1):1–2. doi: 10.1016/j.trstmh.2011.08.014. S0035-9203(11)00185-4 [pii] [DOI] [PubMed] [Google Scholar]
- 3.Baruah K, Singh PK, Mohalia MM, Dhariwal AC. A study on dengue outbreak during 2009 in Bhopal and Indore districts of Madhya Pradesh, India. J Commun Dis. 2010;42(4):273–279. [PubMed] [Google Scholar]
- 4.Franco C, Hynes NA, Bouri N, Henderson DA. The dengue threat to the United States. Biosecur Bioterror. 2010;8(3):273–276. doi: 10.1089/bsp.2010.0032. [DOI] [PubMed] [Google Scholar]
- 5.Morens DM, Fauci AS. Dengue and hemorrhagic fever: a potential threat to public health in the United States. Jama. 2008;299(2):214–216. doi: 10.1001/jama.2007.31-a. [DOI] [PubMed] [Google Scholar]
- 6.Nimmannitya S. Clinical manifestations of Dengue/Dengue Haemorrhagic Fever. In: Thongcharoen P, editor. Monograph on Dengue/Dengue Haemorrhagic Fever. World Health Organization; New Delhi: 1993. pp. 48–57. [Google Scholar]
- 7.Gubler D, Kuno G, Markoff L. Flavivirus, Field’s Virology. 5. Vol. 5. Lippincott Williams & Wilkins; 2007. [Google Scholar]
- 8.Lindenbach BD, Thiel H–J, Rice CM. Flaviviridae: The Viruses and Their Replication, Field’s Virology. 5. Lippincott Williams & Wilkins; 2007. [Google Scholar]
- 9.Leitmeyer KC, Vaughn DW, Watts DM, Salas R, Villalobos I, de C, Ramos C, Rico-Hesse R. Dengue virus structural differences that correlate with pathogenesis. J Virol. 1999;73(6):4738–4747. doi: 10.1128/jvi.73.6.4738-4747.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Modis Y, Ogata S, Clements D, Harrison SC. Structure of the dengue virus envelope protein after membrane fusion. Nature. 2004;427(6972):313–319. doi: 10.1038/nature02165. [DOI] [PubMed] [Google Scholar]
- 11.Tassaneetrithep B, Burgess TH, Granelli-Piperno A, Trumpfheller C, Finke J, Sun W, Eller MA, Pattanapanyasat K, Sarasombath S, Birx DL, Steinman RM, Schlesinger S, Marovich MA. DC-SIGN (CD209) mediates dengue virus infection of human dendritic cells. J Exp Med. 2003;197(7):823–829. doi: 10.1084/jem.20021840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jones M, Davidson A, Hibbert L, Gruenwald P, Schlaak J, Ball S, Foster GR, Jacobs M. Dengue virus inhibits alpha interferon signaling by reducing STAT2 expression. J Virol. 2005;79(9):5414–5420. doi: 10.1128/JVI.79.9.5414-5420.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Munoz-Jordan JL, Laurent-Rolle M, Ashour J, Martinez-Sobrido L, Ashok M, Lipkin WI, Garcia-Sastre A. Inhibition of alpha/beta interferon signaling by the NS4B protein of flaviviruses. J Virol. 2005;79(13):8004–8013. doi: 10.1128/JVI.79.13.8004-8013.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kelley JF, Kaufusi PH, Nerurkar VR. Dengue hemorrhagic fever-associated immunomediators induced via maturation of dengue virus nonstructural 4B protein in monocytes modulate endothelial cell adhesion molecules and human microvascular endothelial cells permeability. Virology. 2012;422(2):326–337. doi: 10.1016/j.virol.2011.10.030. S0042-6822(11)00517-4 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Medin CL, Fitzgerald KA, Rothman AL. Dengue virus nonstructural protein NS5 induces interleukin-8 transcription and secretion. J Virol. 2005;79(17):11053–11061. doi: 10.1128/JVI.79.17.11053-11061.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rothman AL. T lymphocyte responses to heterologous secondary dengue virus infections. Annals of the New York Academy of Sciences. 2009;1171(Suppl 1):E36–41. doi: 10.1111/j.1749-6632.2009.05055.x. NYAS5055 [pii] [DOI] [PubMed] [Google Scholar]
- 17.Arevalo MT, Simpson-Haidaris PJ, Kou Z, Schlesinger JJ, Jin X. Primary human endothelial cells support direct but not antibody-dependent enhancement of dengue viral infection. J Med Virol. 2009;81(3):519–528. doi: 10.1002/jmv.21408. [DOI] [PubMed] [Google Scholar]
- 18.Basu A, Jain P, Sarkar P, Gangodkar S, Deshpande D, Ganti K, Shetty S, Ghosh K. Dengue virus infection of SK Hep1 cells: inhibition of in vitro angiogenesis and altered cytomorphology by expressed viral envelope glycoprotein. FEMS Immunol Med Microbiol. 2011;62(2):140–147. doi: 10.1111/j.1574-695X.2011.00794.x. [DOI] [PubMed] [Google Scholar]
- 19.Brown MG, Hermann LL, Issekutz AC, Marshall JS, Rowter D, Al-Afif A, Anderson R. Dengue virus infection of mast cells triggers endothelial cell activation. J Virol. 2011;85(2):1145–1150. doi: 10.1128/JVI.01630-10. JVI.01630-10 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Paes MV, Lenzi HL, Nogueira AC, Nuovo GJ, Pinhao AT, Mota EM, Basilio-de-Oliveira CA, Schatzmayr H, Barth OM, Alves AM. Hepatic damage associated with dengue-2 virus replication in liver cells of BALB/c mice. Laboratory investigation; a journal of technical methods and pathology. 2009;89(10):1140–1151. doi: 10.1038/labinvest.2009.83. labinvest200983 [pii] [DOI] [PubMed] [Google Scholar]
- 21.Salgado DM, Eltit JM, Mansfield K, Panqueba C, Castro D, Vega MR, Xhaja K, Schmidt D, Martin KJ, Allen PD, Rodriguez JA, Dinsmore JH, Lopez JR, Bosch I. Heart and skeletal muscle are targets of dengue virus infection. Pediatr Infect Dis J. 2010;29(3):238–242. doi: 10.1097/INF.0b013e3181bc3c5b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chen ST, Lin YL, Huang MT, Wu MF, Cheng SC, Lei HY, Lee CK, Chiou TW, Wong CH, Hsieh SL. CLEC5A is critical for dengue-virus-induced lethal disease. Nature. 2008;453(7195):672–676. doi: 10.1038/nature07013. nature07013 [pii] [DOI] [PubMed] [Google Scholar]
- 23.Lozach PY, Burleigh L, Staropoli I, Navarro-Sanchez E, Harriague J, Virelizier JL, Rey FA, Despres P, Arenzana-Seisdedos F, Amara A. Dendritic cell-specific intercellular adhesion molecule 3-grabbing non-integrin (DC-SIGN)-mediated enhancement of dengue virus infection is independent of DC-SIGN internalization signals. J Biol Chem. 2005;280(25):23698–23708. doi: 10.1074/jbc.M504337200. [DOI] [PubMed] [Google Scholar]
- 24.Rigau-Perez JG, Laufer MK. Dengue-related deaths in Puerto Rico, 1992–1996: diagnosis and clinical alarm signals. Clin Infect Dis. 2006;42(9):1241–1246. doi: 10.1086/501355. [DOI] [PubMed] [Google Scholar]
- 25.WHO. Dengue hemorrhagic fever: diagnosis, treatment, prevention and control. 2. WHO; Geneva: 1997. [Google Scholar]
- 26.Dengue, guidelines for diagnosis, treatment, prevention and control. World Health Organization; 2009. [PubMed] [Google Scholar]
- 27.Srikiatkhachorn A, Krautrachue A, Ratanaprakarn W, Wongtapradit L, Nithipanya N, Kalayanarooj S, Nisalak A, Thomas SJ, Gibbons RV, Mammen MP, Jr, Libraty DH, Ennis FA, Rothman AL, Green S. Natural history of plasma leakage in dengue hemorrhagic fever: a serial ultrasonographic study. Pediatr Infect Dis J. 2007;26(4):283–290. doi: 10.1097/01.inf.0000258612.26743.10. discussion 291–282. [DOI] [PubMed] [Google Scholar]
- 28.Burke DS, Nisalak A, Johnson DE, Scott RM. A prospective study of dengue infections in Bangkok. Am J Trop Med Hyg. 1988;38(1):172–180. doi: 10.4269/ajtmh.1988.38.172. [DOI] [PubMed] [Google Scholar]
- 29.Sangkawibha N, Rojanasuphot S, Ahandrik S, Viriyapongse S, Jatanasen S, Salitul V, Phanthumachinda B, Halstead SB. Risk factors in dengue shock syndrome: a prospective epidemiologic study in Rayong, Thailand. I. The 1980 outbreak. Am J Epidemiol. 1984;120(5):653–669. doi: 10.1093/oxfordjournals.aje.a113932. [DOI] [PubMed] [Google Scholar]
- 30.Avirutnan P, Malasit P, Seliger B, Bhakdi S, Husmann M. Dengue virus infection of human endothelial cells leads to chemokine production, complement activation, and apoptosis. J Immunol. 1998;161(11):6338–6346. [PubMed] [Google Scholar]
- 31.Dalrymple NA, Mackow ER. Endothelial cells elicit immune-enhancing responses to dengue virus infection. J Virol. 2012;86(12):6408–6415. doi: 10.1128/JVI.00213-12. JVI.00213-12 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Luplertlop N, Misse D, Bray D, Deleuze V, Gonzalez JP, Leardkamolkarn V, Yssel H, Veas F. Dengue-virus-infected dendritic cells trigger vascular leakage through metalloproteinase overproduction. EMBO Rep. 2006;7(11):1176–1181. doi: 10.1038/sj.embor.7400814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Limon-Flores AY, Perez-Tapia M, Estrada-Garcia I, Vaughan G, Escobar-Gutierrez A, Calderon-Amador J, Herrera-Rodriguez SE, Brizuela-Garcia A, Heras-Chavarria M, Flores-Langarica A, Cedillo-Barron L, Flores-Romo L. Dengue virus inoculation to human skin explants: an effective approach to assess in situ the early infection and the effects on cutaneous dendritic cells. International journal of experimental pathology. 2005;86(5):323–334. doi: 10.1111/j.0959-9673.2005.00445.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wu SJ, Grouard-Vogel G, Sun W, Mascola JR, Brachtel E, Putvatana R, Louder MK, Filgueira L, Marovich MA, Wong HK, Blauvelt A, Murphy GS, Robb ML, Innes BL, Birx DL, Hayes CG, Frankel SS. Human skin Langerhans cells are targets of dengue virus infection. Nat Med. 2000;6(7):816–820. doi: 10.1038/77553. [DOI] [PubMed] [Google Scholar]
- 35.Freire M, Marchevsky R, Almeida L, Yamamura A, Caride E, Brindeiro P, Motta M, Nogueira R, Kubelka C, Bonaldo M, Galler R. Wild dengue virus types 1, 2 and 3 viremia in rhesus monkeys. Memorias do Instituto Oswaldo Cruz. 2007;102(2):203–208. doi: 10.1590/s0074-02762007005000011. [DOI] [PubMed] [Google Scholar]
- 36.Kraiselburd E, Gubler DJ, Kessler MJ. Quantity of dengue virus required to infect rhesus monkeys. Trans R Soc Trop Med Hyg. 1985;79(2):248–251. doi: 10.1016/0035-9203(85)90348-7. [DOI] [PubMed] [Google Scholar]
- 37.Onlamoon N, Noisakran S, Hsiao HM, Duncan A, Villinger F, Ansari AA, Perng GC. Dengue virus-induced hemorrhage in a nonhuman primate model. Blood. 2010;115(9):1823–1834. doi: 10.1182/blood-2009-09-242990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Jaiswal S, Pazoles P, Woda M, Shultz LD, Greiner DL, Brehm MA, Mathew A. Enhanced humoral and HLA-A2-restricted dengue virus-specific T-cell responses in humanized BLT NSG mice. Immunology. 2012;136(3):334–343. doi: 10.1111/j.1365-2567.2012.03585.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Akkina R. Human immune responses and potential for vaccine assessment in humanized mice. Current opinion in immunology. 2013;25(3):403–409. doi: 10.1016/j.coi.2013.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Mota J, Rico-Hesse R. Dengue virus tropism in humanized mice recapitulates human dengue fever. PLoS One. 2011;6(6):e20762. doi: 10.1371/journal.pone.0020762PONE-D-11-02788. [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Christofferson RC, McCracken MK, Johnson AM, Chisenhall DM, Mores CN. Development of a transmission model for dengue virus. Virol J. 2013;10:127. doi: 10.1186/1743-422X-10-127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Prestwood TR, Prigozhin DM, Sharar KL, Zellweger RM, Shresta S. A mouse-passaged dengue virus strain with reduced affinity for heparan sulfate causes severe disease in mice by establishing increased systemic viral loads. J Virol. 2008;82(17):8411–8421. doi: 10.1128/JVI.00611-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Shresta S, Sharar KL, Prigozhin DM, Beatty PR, Harris E. Murine model for dengue virus-induced lethal disease with increased vascular permeability. J Virol. 2006;80(20):10208–10217. doi: 10.1128/JVI.00062-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Tan GK, Ng JK, Trasti SL, Schul W, Yip G, Alonso S. A non mouse-adapted dengue virus strain as a new model of severe dengue infection in AG129 mice. PLoS Negl Trop Dis. 2010;4(4):e672. doi: 10.1371/journal.pntd.0000672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wu-Hsieh BA, Yen YT, Chen HC. Dengue hemorrhage in a mouse model. Annals of the New York Academy of Sciences. 2009;1171(Suppl 1):E42–47. doi: 10.1111/j.1749-6632.2009.05053.x. NYAS5053 [pii] [DOI] [PubMed] [Google Scholar]
- 46.Yen YT, Chen HC, Lin YD, Shieh CC, Wu-Hsieh BA. Enhancement by tumor necrosis factor alpha of dengue virus-induced endothelial cell production of reactive nitrogen and oxygen species is key to hemorrhage development. J Virol. 2008;82(24):12312–12324. doi: 10.1128/JVI.00968-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Srikiatkhachorn A, Green S. Markers of dengue disease severity. Curr Top Microbiol Immunol. 2010;338:67–82. doi: 10.1007/978-3-642-02215-9_6. [DOI] [PubMed] [Google Scholar]
- 48.Banks RE, Forbes MA, Kinsey SE, Stanley A, Ingham E, Walters C, Selby PJ. Release of the angiogenic cytokine vascular endothelial growth factor (VEGF) from platelets: significance for VEGF measurements and cancer biology. British journal of cancer. 1998;77(6):956–964. doi: 10.1038/bjc.1998.158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Harrison S, Vavken P, Kevy S, Jacobson M, Zurakowski D, Murray MM. Platelet activation by collagen provides sustained release of anabolic cytokines. The American journal of sports medicine. 2011;39(4):729–734. doi: 10.1177/0363546511401576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Jessie K, Fong MY, Devi S, Lam SK, Wong KT. Localization of dengue virus in naturally infected human tissues, by immunohistochemistry and in situ hybridization. J Infect Dis. 2004;189(8):1411–1418. doi: 10.1086/383043. [DOI] [PubMed] [Google Scholar]
- 51.Balsitis SJ, Coloma J, Castro G, Alava A, Flores D, McKerrow JH, Beatty PR, Harris E. Tropism of dengue virus in mice and humans defined by viral nonstructural protein 3-specific immunostaining. Am J Trop Med Hyg. 2009;80(3):416–424. 80/3/416 [pii] [PubMed] [Google Scholar]
- 52.Bhamarapravati N, Tuchinda P, Boonyapaknavik V. Pathology of Thailand haemorrhagic fever: a study of 100 autopsy cases. Ann Trop Med Parasitol. 1967;61(4):500–510. doi: 10.1080/00034983.1967.11686519. [DOI] [PubMed] [Google Scholar]
- 53.Couvelard A, Marianneau P, Bedel C, Drouet MT, Vachon F, Henin D, Deubel V. Report of a fatal case of dengue infection with hepatitis: demonstration of dengue antigens in hepatocytes and liver apoptosis. Hum Pathol. 1999;30(9):1106–1110. doi: 10.1016/s0046-8177(99)90230-7. [DOI] [PubMed] [Google Scholar]
- 54.Limonta D, Capo V, Torres G, Perez AB, Guzman MG. Apoptosis in tissues from fatal dengue shock syndrome. J Clin Virol. 2007;40(1):50–54. doi: 10.1016/j.jcv.2007.04.024. [DOI] [PubMed] [Google Scholar]
- 55.Taweechaisupapong S, Sriurairatana S, Angsubhakorn S, Yoksan S, Bhamarapravati N. In vivo and in vitro studies on the morphological change in the monkey epidermal Langerhans cells following exposure to dengue 2 (16681) virus. Southeast Asian J Trop Med Public Health. 1996;27(4):664–672. [PubMed] [Google Scholar]
- 56.Libraty DH, Pichyangkul S, Ajariyakhajorn C, Endy TP, Ennis FA. Human dendritic cells are activated by dengue virus infection: enhancement by gamma interferon and implications for disease pathogenesis. J Virol. 2001;75(8):3501–3508. doi: 10.1128/JVI.75.8.3501-3508.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Marovich M, Grouard-Vogel G, Louder M, Eller M, Sun W, Wu SJ, Putvatana R, Murphy G, Tassaneetrithep B, Burgess T, Birx D, Hayes C, Schlesinger-Frankel S, Mascola J. Human dendritic cells as targets of dengue virus infection. J Investig Dermatol Symp Proc. 2001;6(3):219–224. doi: 10.1046/j.0022-202x.2001.00037.x. [DOI] [PubMed] [Google Scholar]
- 58.Chuang YC, Lei HY, Liu HS, Lin YS, Fu TF, Yeh TM. Macrophage migration inhibitory factor induced by dengue virus infection increases vascular permeability. Cytokine. 2011;54(2):222–231. doi: 10.1016/j.cyto.2011.01.013. S1043-4666(11)00026-3 [pii] [DOI] [PubMed] [Google Scholar]
- 59.St John AL, Rathore AP, Raghavan B, Ng ML, Abraham SN. Contributions of mast cells and vasoactive products, leukotrienes and chymase, to dengue virus-induced vascular leakage. eLife. 2013;2:e00481. doi: 10.7554/eLife.00481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.St John AL, Rathore AP, Yap H, Ng ML, Metcalfe DD, Vasudevan SG, Abraham SN. Immune surveillance by mast cells during dengue infection promotes natural killer (NK) and NKT-cell recruitment and viral clearance. Proc Natl Acad Sci U S A. 2011;108(22):9190–9195. doi: 10.1073/pnas.1105079108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Libraty DH, Young PR, Pickering D, Endy TP, Kalayanarooj S, Green S, Vaughn DW, Nisalak A, Ennis FA, Rothman AL. High circulating levels of the dengue virus nonstructural protein NS1 early in dengue illness correlate with the development of dengue hemorrhagic fever. J Infect Dis. 2002;186(8):1165–1168. doi: 10.1086/343813. [DOI] [PubMed] [Google Scholar]
- 62.Pichyangkul S, Endy TP, Kalayanarooj S, Nisalak A, Yongvanitchit K, Green S, Rothman AL, Ennis FA, Libraty DH. A blunted blood plasmacytoid dendritic cell response to an acute systemic viral infection is associated with increased disease severity. J Immunol. 2003;171(10):5571–5578. doi: 10.4049/jimmunol.171.10.5571. [DOI] [PubMed] [Google Scholar]
- 63.Sun P, Garcia J, Comach G, Vahey MT, Wang Z, Forshey BM, Morrison AC, Sierra G, Bazan I, Rocha C, Vilcarromero S, Blair PJ, Scott TW, Camacho DE, Ockenhouse CF, Halsey ES, Kochel TJ. Sequential waves of gene expression in patients with clinically defined dengue illnesses reveal subtle disease phases and predict disease severity. PLoS Negl Trop Dis. 2013;7(7):e2298. doi: 10.1371/journal.pntd.0002298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Libraty DH, Endy TP, Houng HS, Green S, Kalayanarooj S, Suntayakorn S, Chansiriwongs W, Vaughn DW, Nisalak A, Ennis FA, Rothman AL. Differing influences of virus burden and immune activation on disease severity in secondary dengue-3 virus infections. J Infect Dis. 2002;185(9):1213–1221. doi: 10.1086/340365. [DOI] [PubMed] [Google Scholar]
- 65.Modhiran N, Watterson D, Muller DA, Panetta AK, Sester DP, Liu L, Hume DA, Stacey KJ, Young PR. Dengue virus NS1 protein activates cells via Toll-like receptor 4 and disrupts endothelial cell monolayer integrity. Sci Transl Med. 2015;7(304):304ra142. doi: 10.1126/scitranslmed.aaa3863. [DOI] [PubMed] [Google Scholar]
- 66.Chen HR, Chuang YC, Lin YS, Liu HS, Liu CC, Perng GC, Yeh TM. Dengue Virus Nonstructural Protein 1 Induces Vascular Leakage through Macrophage Migration Inhibitory Factor and Autophagy. PLoS Negl Trop Dis. 2016;10(7):e0004828. doi: 10.1371/journal.pntd.0004828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Beatty PR, Puerta-Guardo H, Killingbeck SS, Glasner DR, Hopkins K, Harris E. Dengue virus NS1 triggers endothelial permeability and vascular leak that is prevented by NS1 vaccination. Sci Transl Med. 2015;7(304):304ra141. doi: 10.1126/scitranslmed.aaa3787. [DOI] [PubMed] [Google Scholar]
- 68.Green S, Pichyangkul S, Vaughn DW, Kalayanarooj S, Nimmannitya S, Nisalak A, Kurane I, Rothman AL, Ennis FA. Early CD69 expression on peripheral blood lymphocytes from children with dengue hemorrhagic fever. J Infect Dis. 1999;180(5):1429–1435. doi: 10.1086/315072. [DOI] [PubMed] [Google Scholar]
- 69.Lim DS, Yawata N, Selva KJ, Li N, Tsai CY, Yeong LH, Liong KH, Ooi EE, Chong MK, Ng ML, Leo YS, Yawata M, Wong SB. The combination of type I IFN, TNF-alpha, and cell surface receptor engagement with dendritic cells enables NK cells to overcome immune evasion by dengue virus. J Immunol. 2014;193(10):5065–5075. doi: 10.4049/jimmunol.1302240. [DOI] [PubMed] [Google Scholar]
- 70.Azeredo EL, De Oliveira-Pinto LM, Zagne SM, Cerqueira DI, Nogueira RM, Kubelka CF. NK cells, displaying early activation, cytotoxicity and adhesion molecules, are associated with mild dengue disease. Clin Exp Immunol. 2006;143(2):345–356. doi: 10.1111/j.1365-2249.2006.02996.x. CEI2996 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Sung JM, Lee CK, Wu-Hsieh BA. Intrahepatic infiltrating NK and CD8 T cells cause liver cell death in different phases of dengue virus infection. PLoS One. 2012;7(9):e46292. doi: 10.1371/journal.pone.0046292PONE-D-12-16090. [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Kalayanarooj S, Vaughn DW, Nimmannitya S, Green S, Suntayakorn S, Kunentrasai N, Viramitrachai W, Ratanachu-eke S, Kiatpolpoj S, Innis BL, Rothman AL, Nisalak A, Ennis FA. Early clinical and laboratory indicators of acute dengue illness. J Infect Dis. 1997;176(2):313–321. doi: 10.1086/514047. [DOI] [PubMed] [Google Scholar]
- 73.Matangkasombut P, Chan-In W, Opasawaschai A, Pongchaikul P, Tangthawornchaikul N, Vasanawathana S, Limpitikul W, Malasit P, Duangchinda T, Screaton G, Mongkolsapaya J. Invariant NKT cell response to dengue virus infection in human. PLoS Negl Trop Dis. 2014;8(6):e2955. doi: 10.1371/journal.pntd.0002955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Juno JA, Keynan Y, Fowke KR. Invariant NKT cells: regulation and function during viral infection. PLoS Pathog. 2012;8(8):e1002838. doi: 10.1371/journal.ppat.1002838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Renneson J, Guabiraba R, Maillet I, Marques RE, Ivanov S, Fontaine J, Paget C, Quesniaux V, Faveeuw C, Ryffel B, Teixeira MM, Trottein F. A detrimental role for invariant natural killer T cells in the pathogenesis of experimental dengue virus infection. Am J Pathol. 2011;179(4):1872–1883. doi: 10.1016/j.ajpath.2011.06.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Calvert JK, Helbig KJ, Dimasi D, Cockshell M, Beard MR, Pitson SM, Bonder CS, Carr JM. Dengue Virus Infection of Primary Endothelial Cells Induces Innate Immune Responses, Changes in Endothelial Cells Function and Is Restricted by Interferon-Stimulated Responses. J Interferon Cytokine Res. 2015;35(8):654–665. doi: 10.1089/jir.2014.0195. [DOI] [PubMed] [Google Scholar]
- 77.Warke RV, Xhaja K, Martin KJ, Fournier MF, Shaw SK, Brizuela N, de Bosch N, Lapointe D, Ennis FA, Rothman AL, Bosch I. Dengue virus induces novel changes in gene expression of human umbilical vein endothelial cells. J Virol. 2003;77(21):11822–11832. doi: 10.1128/JVI.77.21.11822-11832.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Dewi BE, Takasaki T, Kurane I. In vitro assessment of human endothelial cell permeability: effects of inflammatory cytokines and dengue virus infection. J Virol Methods. 2004;121(2):171–180. doi: 10.1016/j.jviromet.2004.06.013. [DOI] [PubMed] [Google Scholar]
- 79.Liu P, Woda M, Ennis FA, Libraty DH. Dengue virus infection differentially regulates endothelial barrier function over time through type I interferon effects. J Infect Dis. 2009;200(2):191–201. doi: 10.1086/599795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Srikiatkhachorn A, Ajariyakhajorn C, Endy TP, Kalayanarooj S, Libraty DH, Green S, Ennis FA, Rothman AL. Virus-induced decline in soluble vascular endothelial growth receptor 2 is associated with plasma leakage in dengue hemorrhagic Fever. J Virol. 2007;81(4):1592–1600. doi: 10.1128/JVI.01642-06. JVI.01642-06 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Bravo JR, Guzman MG, Kouri GP. Why dengue haemorrhagic fever in Cuba? 1. Individual risk factors for dengue haemorrhagic fever/dengue shock syndrome (DHF/DSS) Trans R Soc Trop Med Hyg. 1987;81(5):816–820. doi: 10.1016/0035-9203(87)90041-1. [DOI] [PubMed] [Google Scholar]
- 82.Rothman AL. Immunity to dengue virus: a tale of original antigenic sin and tropical cytokine storms. Nature reviews Immunology. 2011;11(8):532–543. doi: 10.1038/nri3014. [DOI] [PubMed] [Google Scholar]
- 83.Rivino L, Kumaran EA, Jovanovic V, Nadua K, Teo EW, Pang SW, Teo GH, Gan VC, Lye DC, Leo YS, Hanson BJ, Smith KG, Bertoletti A, Kemeny DM, MacAry PA. Differential targeting of viral components by CD4+ versus CD8+ T lymphocytes in dengue virus infection. J Virol. 2013;87(5):2693–2706. doi: 10.1128/JVI.02675-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Weiskopf D, Cerpas C, Angelo MA, Bangs DJ, Sidney J, Paul S, Peters B, Sanches FP, Silvera CG, Costa PR, Kallas EG, Gresh L, de Silva AD, Balmaseda A, Harris E, Sette A. Human CD8+ T-Cell Responses Against the 4 Dengue Virus Serotypes Are Associated With Distinct Patterns of Protein Targets. J Infect Dis. 2015;212(11):1743–1751. doi: 10.1093/infdis/jiv289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Friberg H, Burns L, Woda M, Kalayanarooj S, Endy TP, Stephens HA, Green S, Rothman AL, Mathew A. Memory CD8+ T cells from naturally acquired primary dengue virus infection are highly cross-reactive. Immunology and cell biology. 2011;89(1):122–129. doi: 10.1038/icb.2010.61. icb201061 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Mongkolsapaya J, Duangchinda T, Dejnirattisai W, Vasanawathana S, Avirutnan P, Jairungsri A, Khemnu N, Tangthawornchaikul N, Chotiyarnwong P, Sae-Jang K, Koch M, Jones Y, McMichael A, Xu X, Malasit P, Screaton G. T cell responses in dengue hemorrhagic fever: are cross-reactive T cells suboptimal? J Immunol. 2006;176(6):3821–3829. doi: 10.4049/jimmunol.176.6.3821. [DOI] [PubMed] [Google Scholar]
- 87.Mongkolsapaya J, Dejnirattisai W, Xu XN, Vasanawathana S, Tangthawornchaikul N, Chairunsri A, Sawasdivorn S, Duangchinda T, Dong T, Rowland-Jones S, Yenchitsomanus PT, McMichael A, Malasit P, Screaton G. Original antigenic sin and apoptosis in the pathogenesis of dengue hemorrhagic fever. Nat Med. 2003;9(7):921–927. doi: 10.1038/nm887. [DOI] [PubMed] [Google Scholar]
- 88.Townsley E, Woda M, Thomas SJ, Kalayanarooj S, Gibbons RV, Nisalak A, Srikiatkhachorn A, Green S, Stephens HA, Rothman AL, Mathew A. Distinct Activation Phenotype of a Highly Conserved Novel HLA-B57-Restricted Epitope during Dengue Virus Infection. Immunology. 2013 doi: 10.1111/imm.12161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Braga EL, Moura P, Pinto LM, Ignacio SR, Oliveira MJ, Cordeiro MT, Kubelka CF. Detection of circulant tumor necrosis factor-alpha, soluble tumor necrosis factor p75 and interferon-gamma in Brazilian patients with dengue fever and dengue hemorrhagic fever. Memorias do Instituto Oswaldo Cruz. 2001;96(2):229–232. doi: 10.1590/s0074-02762001000200015. S0074-02762001000200015 [pii] [DOI] [PubMed] [Google Scholar]
- 90.Green S, Vaughn DW, Kalayanarooj S, Nimmannitya S, Suntayakorn S, Nisalak A, Lew R, Innis BL, Kurane I, Rothman AL, Ennis FA. Early immune activation in acute dengue illness is related to development of plasma leakage and disease severity. J Infect Dis. 1999;179(4):755–762. doi: 10.1086/314680. [DOI] [PubMed] [Google Scholar]
- 91.Lok SM. The Interplay of Dengue Virus Morphological Diversity and Human Antibodies. Trends Microbiol. 2016;24(4):284–293. doi: 10.1016/j.tim.2015.12.004. [DOI] [PubMed] [Google Scholar]
- 92.Ubol S, Phuklia W, Kalayanarooj S, Modhiran N. Mechanisms of immune evasion induced by a complex of dengue virus and preexisting enhancing antibodies. J Infect Dis. 2010;201(6):923–935. doi: 10.1086/651018. [DOI] [PubMed] [Google Scholar]
- 93.Green S, Vaughn DW, Kalayanarooj S, Nimmannitya S, Suntayakorn S, Nisalak A, Rothman AL, Ennis FA. Elevated plasma interleukin-10 levels in acute dengue correlate with disease severity. J Med Virol. 1999;59(3):329–334. [PubMed] [Google Scholar]
- 94.Huang X, Yue Y, Li D, Zhao Y, Qiu L, Chen J, Pan Y, Xi J, Wang X, Sun Q, Li Q. Antibody-dependent enhancement of dengue virus infection inhibits RLR-mediated Type-I IFN-independent signalling through upregulation of cellular autophagy. Sci Rep. 2016;6:22303. doi: 10.1038/srep22303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Xu M, Hadinoto V, Appanna R, Joensson K, Toh YX, Balakrishnan T, Ong SH, Warter L, Leo YS, Wang CI, Fink K. Plasmablasts generated during repeated dengue infection are virus glycoprotein-specific and bind to multiple virus serotypes. J Immunol. 2012;189(12):5877–5885. doi: 10.4049/jimmunol.1201688. [DOI] [PubMed] [Google Scholar]
- 96.Priyamvada L, Cho A, Onlamoon N, Zheng NY, Huang M, Kovalenkov Y, Chokephaibulkit K, Angkasekwinai N, Pattanapanyasat K, Ahmed R, Wilson PC, Wrammert J. B Cell Responses during Secondary Dengue Virus Infection Are Dominated by Highly Cross-Reactive, Memory-Derived Plasmablasts. J Virol. 2016;90(12):5574–5585. doi: 10.1128/JVI.03203-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Garcia-Bates TM, Cordeiro MT, Nascimento EJ, Smith AP, Soares de Melo KM, McBurney SP, Evans JD, Marques ET, Jr, Barratt-Boyes SM. Association between magnitude of the virus-specific plasmablast response and disease severity in dengue patients. J Immunol. 2013;190(1):80–87. doi: 10.4049/jimmunol.1103350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Woda M, Friberg H, Currier JR, Srikiatkhachorn A, Macareo LR, Green S, Jarman RG, Rothman AL, Mathew A. Dynamics of Dengue Virus (DENV)-Specific B Cells in the Response to DENV Serotype 1 Infections, Using Flow Cytometry With Labeled Virions. J Infect Dis. 2016;214(7):1001–1009. doi: 10.1093/infdis/jiw308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Shen P, Fillatreau S. Antibody-independent functions of B cells: a focus on cytokines. Nature reviews Immunology. 2015;15(7):441–451. doi: 10.1038/nri3857. [DOI] [PubMed] [Google Scholar]
- 100.Lopez-Ramirez MA, Fischer R, Torres-Badillo CC, Davies HA, Logan K, Pfizenmaier K, Male DK, Sharrack B, Romero IA. Role of caspases in cytokine-induced barrier breakdown in human brain endothelial cells. J Immunol. 2012;189(6):3130–3139. doi: 10.4049/jimmunol.1103460. [DOI] [PubMed] [Google Scholar]
- 101.Mangada MM, Endy TP, Nisalak A, Chunsuttiwat S, Vaughn DW, Libraty DH, Green S, Ennis FA, Rothman AL. Dengue-specific T cell responses in peripheral blood mononuclear cells obtained prior to secondary dengue virus infections in Thai schoolchildren. J Infect Dis. 2002;185(12):1697–1703. doi: 10.1086/340822. [DOI] [PubMed] [Google Scholar]
- 102.Ali T, Kaitha S, Mahmood S, Ftesi A, Stone J, Bronze MS. Clinical use of anti-TNF therapy and increased risk of infections. Drug, healthcare and patient safety. 2013;5:79–99. doi: 10.2147/DHPS.S28801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Augustin HG, Koh GY, Thurston G, Alitalo K. Control of vascular morphogenesis and homeostasis through the angiopoietin-Tie system. Nature reviews Molecular cell biology. 2009;10(3):165–177. doi: 10.1038/nrm2639. [DOI] [PubMed] [Google Scholar]
- 104.Gavard J, Patel V, Gutkind JS. Angiopoietin-1 prevents VEGF-induced endothelial permeability by sequestering Src through mDia. Dev Cell. 2008;14(1):25–36. doi: 10.1016/j.devcel.2007.10.019. [DOI] [PubMed] [Google Scholar]
- 105.Michels M, van der Ven AJ, Djamiatun K, Fijnheer R, de Groot PG, Griffioen AW, Sebastian S, Faradz SM, de Mast Q. Imbalance of angiopoietin-1 and angiopoetin-2 in severe dengue and relationship with thrombocytopenia, endothelial activation, and vascular stability. Am J Trop Med Hyg. 2012;87(5):943–946. doi: 10.4269/ajtmh.2012.12-0020. ajtmh.2012.12-0020 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Kim I, Oh JL, Ryu YS, So JN, Sessa WC, Walsh K, Koh GY. Angiopoietin-1 negatively regulates expression and activity of tissue factor in endothelial cells. FASEB J. 2002;16(1):126–128. doi: 10.1096/fj.01-0556fje. [DOI] [PubMed] [Google Scholar]
- 107.Chen LC, Lei HY, Liu CC, Shiesh SC, Chen SH, Liu HS, Lin YS, Wang ST, Shyu HW, Yeh TM. Correlation of serum levels of macrophage migration inhibitory factor with disease severity and clinical outcome in dengue patients. Am J Trop Med Hyg. 2006;74(1):142–147. 74/1/142 [pii] [PubMed] [Google Scholar]
- 108.Ferreira RA, de Oliveira SA, Gandini M, da Ferreira LC, Correa G, Abiraude FM, Reid MM, Cruz OG, Kubelka CF. Circulating cytokines and chemokines associated with plasma leakage and hepatic dysfunction in Brazilian children with dengue fever. Acta tropica. 2015;149:138–147. doi: 10.1016/j.actatropica.2015.04.023. [DOI] [PubMed] [Google Scholar]
- 109.Boonnak K, Dambach KM, Donofrio GC, Tassaneetrithep B, Marovich MA. Cell type specificity and host genetic polymorphisms influence antibody-dependent enhancement of dengue virus infection. J Virol. 2011;85(4):1671–1683. doi: 10.1128/JVI.00220-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Luhn K, Simmons CP, Moran E, Dung NT, Chau TN, Quyen NT, Thao le TT, Van Ngoc T, Dung NM, Wills B, Farrar J, McMichael AJ, Dong T, Rowland-Jones S. Increased frequencies of CD4+ CD25(high) regulatory T cells in acute dengue infection. J Exp Med. 2007;204(5):979–985. doi: 10.1084/jem.20061381. [DOI] [PMC free article] [PubMed] [Google Scholar]