Abstract
Rationale
Long living individuals show delay of aging, which is characterized by the progressive loss of cardiovascular homeostasis, along with reduced endothelial nitric oxide synthase activity, endothelial dysfunction, and impairment of tissue repair after ischemic injury.
Objective
Exploit genetic analysis of long living individuals to reveal master molecular regulators of physiological aging and new targets for treatment of cardiovascular disease.
Methods and Results
We show that the polymorphic variant rs2070325 (Ile229Val) in bactericidal/permeability-increasing fold-containing-family-B-member-4 (BPIFB4) associates with exceptional longevity, under a recessive genetic model, in 3 independent populations. Moreover, the expression of BPIFB4 is instrumental to maintenance of cellular and vascular homeostasis through regulation of protein synthesis. BPIFB4 phosphorylation/activation by protein-kinase-R–like endoplasmic reticulum kinase induces its complexing with 14-3-3 and heat shock protein 90, which is facilitated by the longevity-associated variant. In isolated vessels, BPIFB4 is upregulated by mechanical stress, and its knock-down inhibits endothelium-dependent vasorelaxation. In hypertensive rats and old mice, gene transfer of longevity-associated variant-BPIFB4 restores endothelial nitric oxide synthase signaling, rescues endothelial dysfunction, and reduces blood pressure levels. Furthermore, BPIFB4 is implicated in vascular repair. BPIFB4 is abundantly expressed in circulating CD34+ cells of long living individuals, and its knock-down in endothelial progenitor cells precludes their capacity to migrate toward the chemoattractant SDF-1. In a murine model of peripheral ischemia, systemic gene therapy with longevity-associated variant-BPIFB4 promotes the recruitment of hematopoietic stem cells, reparative vascularization, and reperfusion of the ischemic muscle.
Conclusions
Longevity-associated variant-BPIFB4 may represent a novel therapeutic tool to fight endothelial dysfunction and promote vascular reparative processes.
Keywords: aging, endothelial progenitor cell, endothelial function, endothelial nitric oxide synthase, longevity, vascular reactivity
Aging is an independent risk factor associated with endothelial dysfunction, impaired angiogenesis, and loss of protein homeostasis, or proteostasis, the decline of which is contrasted by adaptive cellular responses.1 The mechanisms that help cells adapt and survive under stressful conditions include the activation of heat shock factor protein 1–controlled heat shock proteins (HSPs), ribosomal biogenesis, and protein synthesis and are at least in part orchestrated by growth factors, as observed in Caenorhabditis elegans, in which heat shock factor protein 1 is under the control of insulin/IGF-1-like signaling.2 Importantly, IGF-1-like signaling modulation of heat shock factor protein 1 is necessary for controlling longevity3 and through modulation of FOXO (forkhead box O) transcription factors, also for stem cell maintenance.4,5 Therefore, a spectrum of shared mechanisms modulate development, response to stress, and aging.6 Furthermore, IGF-1-like signaling regulates activation of endothelial nitric oxide synthase (eNOS), a fundamental step for the beneficial effects of calorie restriction and exercise—environmental components that influence health and life-span—as underlined by the abolishment of the positive effects of caloric restriction in eNOS-knockout mice.7–9 eNOS has a key role in maintaining vascular function and integrity by generating nitric oxide (NO), a potent vasorelaxant and an inducer of angiogenesis and vasculogenesis.10–12 Dysfunction of eNOS signaling is observed in several pathological conditions, such as cardiovascular, immunologic, metabolic, and neural diseases.13
Exceptional longevity is an inheritable trait; long-lived individuals (LLIs), when compared with younger populations, have a reduced incidence of cardiovascular disease14,15 and a delayed ageing, a phenomenon that is in part genetically driven.16 Thus, nonhypothesis-based genetic approaches could uncover novel proteins that control aging and disease resistance.
Here, we report genetic findings that have allowed us to unequivocally identify BPI fold–containing family B member 4/palate lung and nasal epithelium clone 4 (BPIFB4/LPLUNC4)—a member of a family of proteins involved in innate immunity, but whose function remains elusive17—as a gene associated with exceptional longevity. Translating these findings into therapeutic outcomes, we found that BPIFB4 is downregulated in old mice and that forced expression of the longevity-associated variant (LAV) of BPIFB4 rescues age-related endothelial dysfunction. Additionally, LAV-BPIFB4 restores endothelial function and blood pressure levels in a hypertensive model and old mice and promotes reparative angiogenesis and recruitment of stem cells to the ischemic limb muscle of mice subjected to unilateral femoral artery ligation. Thus, LAV-BPIFB4 may constitute a novel treatment for vascular diseases, hypertension, and ischemia.
Methods
A supplemental section for more detailed methods is available online at http://circres.ahajournals.org.
LLI Populations
The LLI populations are described extensively elsewhere.18–21
Transcriptome Analysis
Transcriptome analysis was performed on total RNA of transfected HEK293T (human embryonic kidney 293T) cells using BeadChip Illumina. Complete data sets are available in Gene Expression Omnibus database (www.ncbi.nlm.nih.gov/geo) in GSE63912.
Endothelial Progenitor Cell Migration Assay
To perform migration assay, mononuclear cells (MNCs) were isolated and endothelial progenitor cells (EPC) enriched as described previously.22,23 Antigenic profile was assessed using a FACS Canto flow cytometer and FACS Diva software (Becton, Dickinson and Company). Migration was performed as described elsewhere.22,24,25
Evaluation of Vascular Reactivity
To evaluate the vascular reactivity in ex vivo transfected mouse vessels, second-order branches of the mesenteric arterial tree were removed from C57BL6 mice and transfected as described previously.26
Vasoconstriction was assessed with KCl or phenylephrine in control conditions and after NG-nitro-L-arginine methyl ester (L-NAME) exposure. Responses were tested before and after transfection. Endothelium-dependent and -independent relaxations were assessed by measuring the dilatory responses of mesenteric arteries to cumulative concentrations of acetylcholine or nitroglycerine, respectively, in vessels precontracted with U46619.27 Caution was taken to avoid endothelial damage; functional integrity was reflected by the response to acetylcholine (10−6 mol/L).28
Infection of Hypertensive Rats
Femoral arteries of AAV-infected spontaneously hypertensive rats (SHR) were analyzed in this study. The experimental protocol for this project was approved by the Istituto Neurologico Mediterraneo Neuromed, Italy, and complies with National Institute Health guidelines for care and use of laboratory animals. Femoral arteries were excised and placed on a wire system28 to perform vascular reactivity studies. Blood pressure was evaluated in another experimental series of SHRs (11-week-old) by tail-cuff plethysmography for 1 week as previously described.28
Infection of Hindlimb Ischemia Mice Models
Infection with AAV and measurement of superficial blood flow and neovascularization were performed in accordance with the Guide for the Care and Use of Laboratory Animals (The Institute of Laboratory Animal Resources, 1996) and with approval of the British Home Office and the University of Bristol.
Foot blood flow was measured immediately after ischemia and then at 7, 14, and 21 days (n=10 mice/group). Muscle cryosections with thickness of 5 μm were used for immunohistochemical analyses.
Evaluation of the modulation in recruitment of total and short-term and long-term reconstituting Lin− Sca-1+cKit+ cells in the ischemic muscles of AAV-injected mice was assessed using a multicolor flow cytometry.
Analysis of gene expression was performed on ischemic muscles and femoral arteries collected 1 week from induction of ischemia and gene therapy.
Statistical Analyses
The reader may refer to Malovini et al21 for details about the statistical methods and procedures applied to the analysis of data deriving from the genome-wide scan. For all statistical detailed information, refer the statistical method sections in the Online Data Supplement.
Results
rs2070325 in BPIFB4 Associates With Exceptional Longevity in Three Independent Populations
To follow up our previous hypothesis-generating genome-wide association study (Online Text I),21 we designed a 2-stage replication effort on 4 variations reported among the top findings (P<1×10−4) of that study. To this end, 2 nonsynonymous SNPs—that is, rs2070325 and rs571391—and 2 intronic markers—that is, rs7583529 and rs285097, which tagged the functional variants rs7917 and rs16955011 (r2>0.8 in the HapMap CEU panel), respectively—were tested for association in 2 independent cohorts, the first of which was recruited for the German Longevity Study20 and the second for a US-based effort18,19 (Online Text II).
Of the 4 variations tested with TaqMan assays, only rs2070325—which induces the amino acid change Ile229Val in BPIFB4 (identifier: P59827-2)—replicated the association observed in the screening cohort under the recessive genetic model (OR=2.42; 95% CI=1.56–3.77; P=5.8×10−5; power >0.85) in the German Longevity Study (OR=1.43; 95% CI=1.12–1.80; P=0.0036). This variant was found associated with the longevity phenotype also in the US replication set (OR=1.60; 95% CI=1.14–2.24; P=0.0063; Online Table I). Meta-analysis of the 2 populations confirmed this finding (3060 LLIs and 1609 controls: OR=1.49; 95% CI=1.22–1.81; P=7.59×10−5; power>0.90) and that of the screening and replication sets combined (3464 LLIs and 2160 controls; Bonferroni-adjusted significance threshold: P<3.22×10−7; OR=1.61; 95% CI=1.34–1.92; P=2.4×10−7; power >0.80; Online Figure I and Online Text III).
rs2070325 Is Associated With a Quadruple-SNP Haplotype
Haplotype analysis revealed patterns of strong linkage disequilibrium (LD: r2>0.8, D′>0.9) within the BPIFB4 genomic locus, delimiting a region highly enriched in nonsynonymous SNPs: the rs2070325 variation of BPIFB4 tagged rs2889732 (Asn281Thr), rs11699009 (Leu488Phe), and rs11696307 (Ile494Thr), codifying, respectively, for wild-type (WT; allele frequency, 66%) and LAV (allele frequency, 29.5%) isoforms (Online Text IV).
CD34+ Cells From LLIs Donors Abundantly Express BPIFB4
BPIFB4 has been found expressed in olfactory epithelium, MNCs, and Bowman’s gland.29 We found BPIFB4 also expressed in germline, stem, progenitor, and fetal cells (Online Figures II and IIIA and Online Text V). Moreover, circulating CD34+ cells, which are enriched with proangiogenic progenitors, had higher BPIFB4 RNA levels in LLIs as compared with ethnically matched young controls, suggesting a possible implication of BPIFB4 in cellular mechanisms of vascular repair (Online Figure IIIB). To further evaluate the involvement of BPIFB4 in proangiogenic cell function, we studied the impact of BPIFB4 knock-out on in vitro migration activity of culture-selected EPCs from healthy human donors. We observed a lack of migration toward the classical chemoattractant SDF-1a in EPCs lacking BPIFB4 (Online Figure IV).
BPIFB4 Overexpression Induces an Adaptive Stress Response and Proteostasis
To gain information on the role of BPIFB4 and its LAV in gene expression regulation, we performed genome-wide transcriptional profiling of HEK293T cells transfected with an empty-, WT- or LAV-BPIFB4-encoding vector. BPIFB4 isoforms activated adaptive stress responses and proteostasis, 2 key aspects for improved organism survival9 and stem cell maintenance,30 and potentiated small noncoding RNAs supportive of the spliceosome, genomic integrity machinery, and telomere maintenance (Online Figures V and VIA, Online Text VI and Online Tables III–VII). Altogether these data are indicative of a role of BPIFB4 in organism homeostasis.
PERK Modulates the Complexing of LAV-BPIFB4 With 14-3-3
To further dissect the molecular determinants underlying the mechanism of action of BPIFB4, we analyzed the structure of the protein motifs in its sequence. We identified a protein kinase R–like endoplasmic reticulum kinase (PERK) substrate motif (amino acids 73–80: EXSXRXXR/EGSIRDLR).31,32 PERK is a known transducer of the unfolded protein response, reducing endoplasmic reticulum protein loading through the inhibition of protein synthesis mediated by phosphorylation of eukaryotic translation initiation factor 2-alpha.33 Thus, a PERK substrate motif on BPIFB4 would indicate a role of BPIFB4 as a downstream effector. Specifically, we observed a reduction of eukaryotic translation initiation factor 2-alpha phosphorylation on transfection with BPIFB4 (Online Figure VIB). These findings suggest that BPIFB4 is part of a cascade of events orchestrated by PERK aimed at reducing endoplasmic reticulum stress. Further sequence analysis revealed the presence of an atypical 14-3-3 binding motif (amino acids 80–86: RXSXXXS/RNSGYRS).17 14-3-3 modulates cell signaling by binding and retaining proteins within the cytoplasm depending on their phosphorylation status.34
We next compared LAV-BPIFB4 and WT-BPIFB4 for potential interaction with 14-3-3 in vitro. Immunoprecipitation and confocal analyses revealed that LAV-BPIFB4 was mainly localized in the cytoplasm and efficiently formed a complex with 14-3-3, whereas WT-BPIFB4 was mostly nuclear (Online Figures VII–IX and Online Text VII). Cells transfected with LAV-BPIFB4 mutated either at serine 75 (LAV-BPIFB4mutPERK) or at serine 82 (LAV-BPIFB4mut14-3-3), which is part of the 14-3-3 binding motif, failed to immunoprecipitate 14-3-3, thus indicating a role of these sites in 14-3-3 recruitment (Online Figure VII).
Further characterization of LAV-BPIFB4 interactions revealed that it forms a complex with heat shock protein 90 (HSP90), a phenomenon that does not take place in cells transfected with LAV-BPIFB4mutPERK or LAV-BPIFB4mut14-3-3 (Online Figure VII). Pharmacological inhibition of PERK with GSK2606414 after transfection with LAV-BPIFB4 impeded the immunoprecipitation of 14-3-3 and HSP90 (Online Figure VII). These findings link PERK-mediated phosphorylation of LAV-BPIFB4 to its 14-3-3 binding activity, which is central for the recruitment of HSP90, a known eNOS activator.35 Of note, WT-BPIFB4 coimmunoprecipitated with HSP90, but not with 14-3-3, as detected by Western blotting. However, WT-BPIFB4mut14-3-3 failed to immunoprecipitate with HSP90 (Online Figure VII). This indicates that WT-BPIFB4 has a reduced, rather than no ability to recruit 14-3-3, which is a step needed for forming a complex with HSP90.
eNOS Is Phosphorylated in Homozygotic rs2070325 MNCs
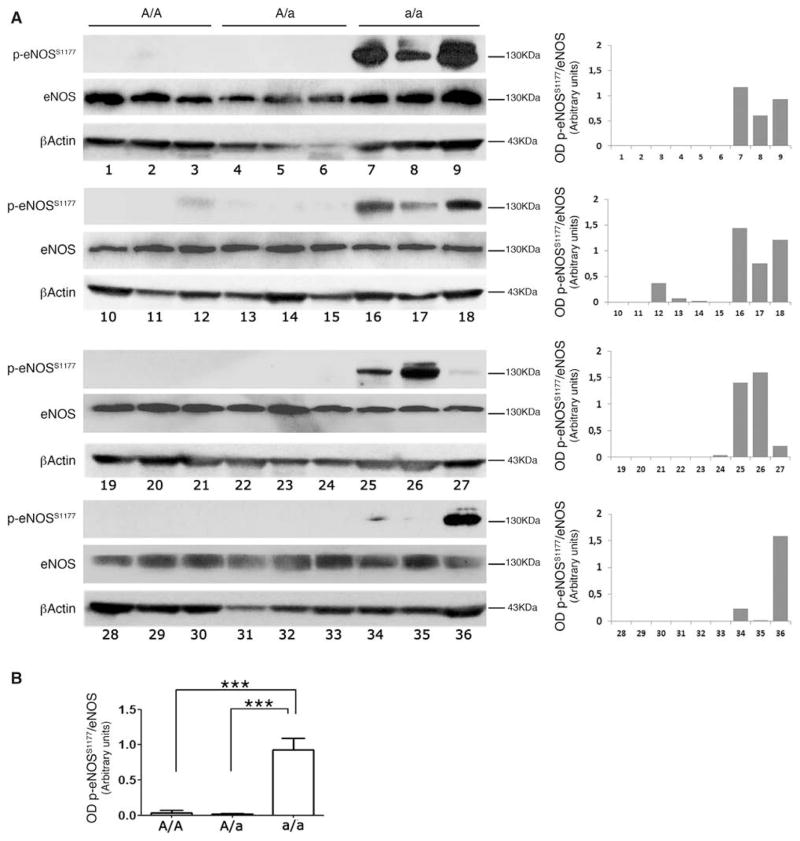
Aging is generally associated with a significant reduction in NO bioavailability, which leads to endothelial dysfunction.36 Based on the above observations of HSP90 recruitment by BPIFB4 and the latter being expressed in MNCs, we assessed the effect of the A/A (homozygotic major allele), A/a (heterozygotic allele), and a/a (homozygotic minor allele) genotypes of rs2070325 on the activity status of eNOS in MNCs from healthy blood donors. We found increased eNOS phosphorylation at serine 1177—an activation site of the enzyme—in a/a carriers (Figure 1).
Figure 1. Evaluation of endothelial nitric oxide synthase (eNOS) phosphorylation in subjects carrying variations on BPIFB4.
A, Phosphorylation status of eNOS on serine 1177 in ex vivo mononuclear cells (MNCs) of subjects A/A (N=12), A/a (N=12), or a/a (N=12) for rs2070325. Right plots give the optical density ratio between the phosphorylated and total forms of eNOS. B, Plot of average values. Values are means±SEM. Statistical analysis was performed with Student’s t test; ***P<0.001. BPIFB4 indicates bactericidal/permeability-increasing fold-containing-family-B-member-4.
BPIFB4 Is Present in the Vessel Wall and Modulates Vascular Tone
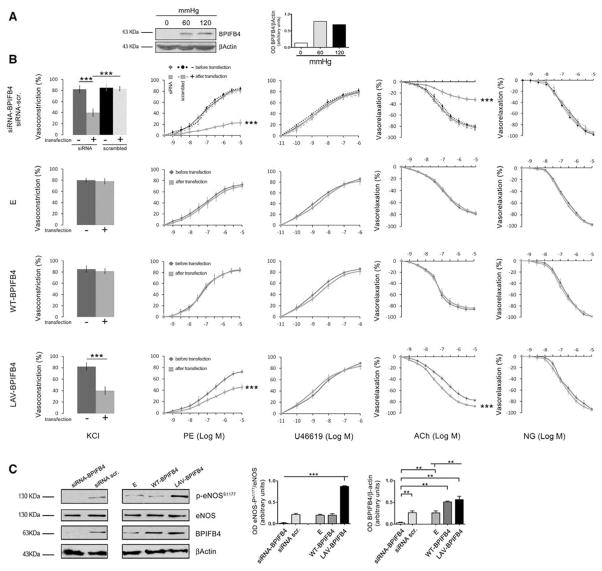
Based on the evidence of enhanced eNOS phosphorylation in MNCs with an rs2070325 genotype, we explored the role of BPIFB4 in the modulation of vascular tone, a process in which NO plays a prominent role.36 In mouse mesenteric arteries—a typical resistance vessel involved in blood pressure homeostasis—expression of the protein was upregulated on application of a biomechanical stress (ie, increased intraluminal pressure for 1 hour; Figure 2A). Inhibition of BPIFB4 expression had a detrimental effect on vascular function: in fact, siRNA-mediated knockdown of BPIFB4 induced marked reductions in phenylephrine- and potassium-evoked vasoconstrictions and acetylcholine-evoked endothelial vasorelaxation (Figure 2B). These effects were associated with the inhibition of eNOS phosphorylation at serine 1177 (Figure 2C). In contrast, silencing of BPIFB4 expression did not affect the endothelium-independent vasorelaxation response evoked by nitroglycerin (NG).
Figure 2. Expression of BPIFB4 in perfused vessels and effects of its variants on vascular reactivity and on phosphorylation of endothelial nitric oxide synthase (eNOS) in mesenteric arteries.
A, BPIFB4 protein expression in ex vivo mouse mesenteric arteries perfused with increasing pressure levels. The right graph gives quantification of BPIFB4 protein. B, Graphs show, from left to right, the ex vivo response of mouse mesenteric arteries to potassium (80 mmol/L KCl) and the dose responses to phenylephrine (PE), the thromboxane agonist U46619, endothelium-dependent vasorelaxant acetylcholine (ACh), and endothelium-independent vasorelaxant nitroglycerin (NG). In the first row, vascular responses are measured before (
 −) and after (
−) and after (
 +) transfection with short interfering (si) RNA for BPIFB4, and before (
+) transfection with short interfering (si) RNA for BPIFB4, and before (
 −) and after (
−) and after (
 +) transfection with scrambled siRNA. In the consecutive rows, before (
+) transfection with scrambled siRNA. In the consecutive rows, before (
 −) and after (
−) and after (
 +) transfection with an empty (E) plasmid or with wild-type (WT) or the longevity-associated variant (LAV) BPIFB4-encoding plasmids. Values are means±SEM. N=7. C, Western blot of 7 pooled experiments on ex vivo mouse mesenteric arteries. Graphs show quantification of eNOS phosphorylation and BPIFB4. Values are means±SEM, N=3 pools of experiments. Statistics was performed using ANOVA; **P<0.01; ***P<0.001. BPIFB4 indicates bactericidal/permeability-increasing fold-containing-family-B-member-4.
+) transfection with an empty (E) plasmid or with wild-type (WT) or the longevity-associated variant (LAV) BPIFB4-encoding plasmids. Values are means±SEM. N=7. C, Western blot of 7 pooled experiments on ex vivo mouse mesenteric arteries. Graphs show quantification of eNOS phosphorylation and BPIFB4. Values are means±SEM, N=3 pools of experiments. Statistics was performed using ANOVA; **P<0.01; ***P<0.001. BPIFB4 indicates bactericidal/permeability-increasing fold-containing-family-B-member-4.
Transfection of Arteries With LAV-BPIFB4 Enhances eNOS Phosphorylation and eNOS-Dependent Vasomotion Through PERK and 14-3-3 Binding
We transfected mesenteric arteries ex vivo with plasmids encoding a variant tagged with green fluorescent protein. Overexpression of WT-BPIFB4 did not interfere with vascular reactivity (Figure 2B), whereas the expression of LAV-BPIFB4 significantly enhanced acetylcholine-evoked vasorelaxation (Figure 2B) and promoted phosphorylation of eNOS at serine 1177 (Figure 2C). Of note, acetylcholine-evoked vasorelaxation was more efficiently inhibited with NG-nitro-L-arginine methyl ester (L-NAME) in LAV-BPIFB4–expressing resistance vessels than in vessels exposed to an empty vector, indicating a higher dependency on eNOS in the former. In contrast, forced BPIFB4 expression did not affect the endothelium-independent vasorelaxation response evoked by NO (Online Figure X and Online Text VIII).
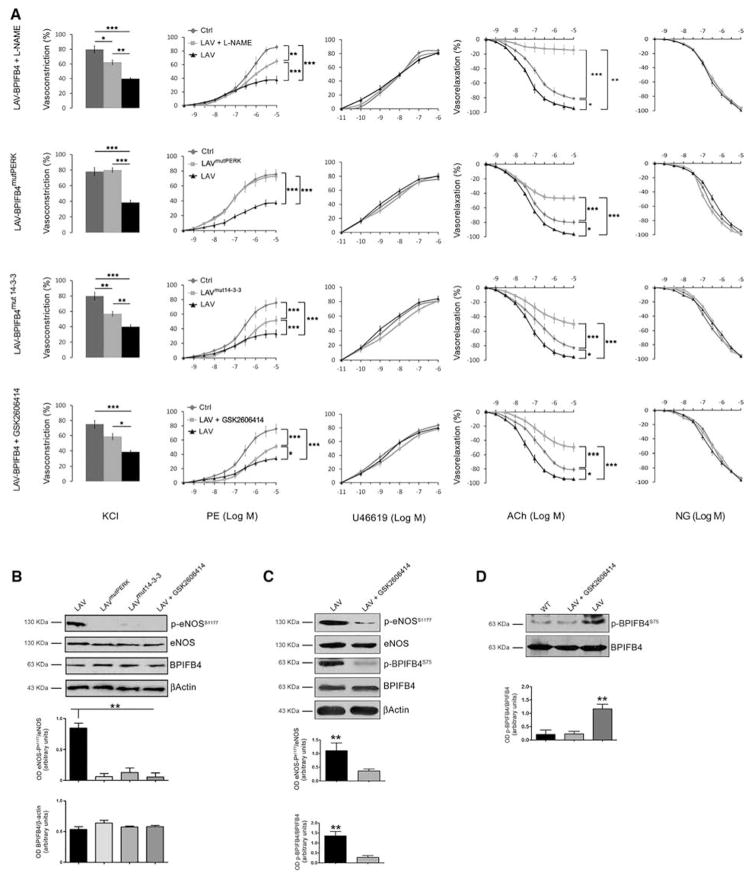
We then assessed the role of PERK and 14-3-3 binding in the activation of eNOS-dependent vascular responses by LAV-BPIFB4. To this aim, we transfected mouse mesenteric arteries ex vivo with LAV-BPIFB4mutPERK or LAV-BPIFB4mut14-3-3 and observed these mutants are devoid of the features manifested by LAV-BPIFB4 (Figure 3A and 3B). Likewise, pharmacological inhibition of PERK with GSK2606414 negated the effect of LAV-BPIFB4 on eNOS phosphorylation and vascular reactivity (Figure 3A–3C).
Figure 3. Effect of the nitric oxide (NO) inhibitor L-NAME, LAV-BPIFB4mutPERK, LAV-BPIFB4mut14-3-3, and the PERK inhibitor GSK2606414 on LAV-BPIFB4-mediated vascular reactivity and on phosphorylation of eNOS in mesenteric arteries.
A, Graphs show, from left to right, the vascular response of ex vivo mouse mesenteric arteries to potassium (80 mmol/L KCl) and the dose responses to phenylephrine (PE), the thromboxane agonist U46619, acetylcholine (ACh), to nitroglycerin (NG) before (
 ) and after transfection with LAV-BPIFB4 (
) and after transfection with LAV-BPIFB4 (
 ), or after transfection with LAV-BPIFB4 plus L-NAME (300 μM) in the first row, LAV-BPIFB4mutPERK in the second row, LAV-BPIFB4mut14-3-3 in the third row, and LAV-BPIFB4 plus GSK2606414 (0.5 μM;
), or after transfection with LAV-BPIFB4 plus L-NAME (300 μM) in the first row, LAV-BPIFB4mutPERK in the second row, LAV-BPIFB4mut14-3-3 in the third row, and LAV-BPIFB4 plus GSK2606414 (0.5 μM;
 ) in the last row. Values are means±SEM. N=7 experiments per group. Statistics was performed using ANOVA; *P<0.05; **P<0.01; ***P<0.001 before vs after transfection or treatment. B, Western blot of 7 pooled experiments on ex vivo mouse mesenteric arteries transfected with LAV-BPIFB4, LAV-BPIFB4 mutPERK, LAV-BPIFB4mut14-3-3, or LAV-BPIFB4 plus GSK2606414. Graphs show quantification of eNOS phosphorylation and BPIFB4. Values are means±SEM, N=2 pools of experiments. C, Western blot of 4 pooled experiments on ex vivo mouse mesenteric arteries with LAV-BPIFB4 or LAV-BPIFB4 plus GSK2606414. Graphs show quantification of eNOS and BPIFB4 phosphorylation. N=3 pool of experiments. D, Western blot of 4 pooled experiments on ex vivo mouse mesenteric arteries with WT-BPIFB4, LAV-BPIFB4 alone LAV-BPIFB4 plus GSK2606414. Graphs show quantification of eNOS and BPIFB4 phosphorylation. N=3 pool of experiments. For B, C, and D, statistics was performed using ANOVA; **P<0.01. BPIFB4 indicates bactericidal/permeability-increasing fold-containing-family-B-member-4; and LAV, longevity-associated variant.
) in the last row. Values are means±SEM. N=7 experiments per group. Statistics was performed using ANOVA; *P<0.05; **P<0.01; ***P<0.001 before vs after transfection or treatment. B, Western blot of 7 pooled experiments on ex vivo mouse mesenteric arteries transfected with LAV-BPIFB4, LAV-BPIFB4 mutPERK, LAV-BPIFB4mut14-3-3, or LAV-BPIFB4 plus GSK2606414. Graphs show quantification of eNOS phosphorylation and BPIFB4. Values are means±SEM, N=2 pools of experiments. C, Western blot of 4 pooled experiments on ex vivo mouse mesenteric arteries with LAV-BPIFB4 or LAV-BPIFB4 plus GSK2606414. Graphs show quantification of eNOS and BPIFB4 phosphorylation. N=3 pool of experiments. D, Western blot of 4 pooled experiments on ex vivo mouse mesenteric arteries with WT-BPIFB4, LAV-BPIFB4 alone LAV-BPIFB4 plus GSK2606414. Graphs show quantification of eNOS and BPIFB4 phosphorylation. N=3 pool of experiments. For B, C, and D, statistics was performed using ANOVA; **P<0.01. BPIFB4 indicates bactericidal/permeability-increasing fold-containing-family-B-member-4; and LAV, longevity-associated variant.
Phosphorylation of BPIFB4 at serine 75 was higher in mesenteric vessels transfected with LAV-BPIFB4 than in those transfected with WT-BPIFB4 (Figure 3D). Treatment with GSK2606414 reduced the level of phosphorylation at serine 75 on LAV-BPIFB4, as well as at serine 1177 on eNOS, supporting the role of PERK in potentiating the phosphorylation status of LAV-BPIFB4 and eNOS (Figure 3C). Because mesenteric vessels transfected with WT-BPIFB4mut14-3-3 also displayed endothelial dysfunction, it is plausible that WT-BPIFB4, similarly to LAV-BPIFB4, requires binding to 14-3-3 for activation of eNOS (Online Figure XI). Additionally, HSP90 seems to be implicated in LAV-BPIFB4–induced potentiation of eNOS, as documented by results showing LAV-BPIFB4’s failure to modulate eNOS/vascular responses on inhibition of HSP90 with SNX5422 (Online Figure XII). As a result, we hypothesize a model in which BPIFB4 needs to be phosphorylated by PERK to recruit 14-3-3, thus allowing the formation of a complex with HSP90 for final activation of eNOS. This cascade of events is potentiated in the presence of LAV-BPIFB4 (Online Figure XIII).
We also observed apparently paradoxical reductions in potassium- and phenylephrine-evoked vasoconstrictions by LAV-BPIFB4 compared with WT-BPIFB4 (Figure 2B). This effect was partially rescued by the eNOS inhibitor L-NAME, suggesting that LAV-BPIFB4–mediated enhancement of NO production modulated adrenergic and potassium vascular responses (Figure 3B). Additionally, as shown in Online Figure XIV, L-NAME enhanced phenylephrine and KCl vascular responses without affecting U46619 vasoconstriction. These results indicate that the NO signaling is not involved in the vascular response evoked by U46619.
Forced Expression of LAV-BPIFB4 In Vivo Enhances eNOS Function and Reduces Blood Pressure
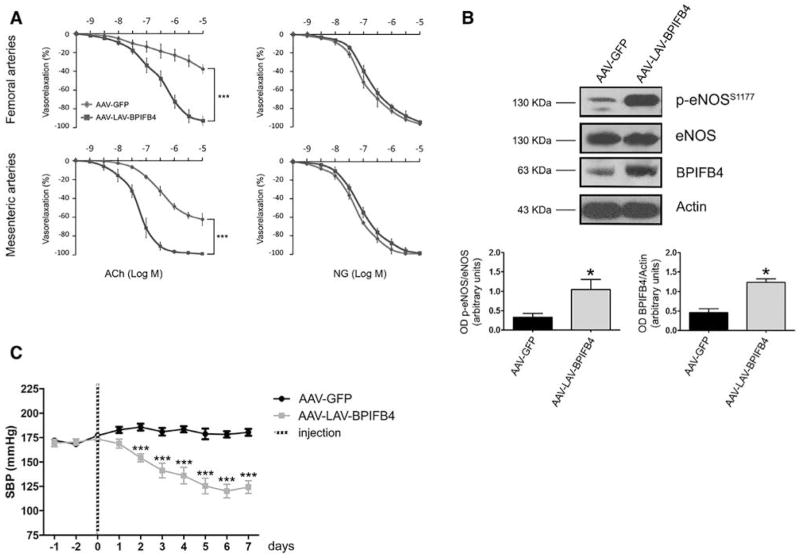
To evaluate the in vivo relevance of the findings obtained through plasmid transfection, we generated WT-BPIFB4-, LAV-BPIFB4-, and green fluorescent protein–encoding adeno-associated viral vectors (AAV serotype 9 with a TBG promoter) and used them to transduce normotensive mice through the femoral artery.37 We found that AAV-LAV-BPIFB4 enhances NO-mediated vasorelaxation in femoral arteries and also in mesenteric arteries, a vascular district different from the one used for gene delivery (Online Figure XVA). Both vessels represent the prototype of resistance vessels involved in blood pressure homeostasis.28,38–41 As expected, enhanced vasorelaxation by AAV-LAV-BPIFB4 was associated with potentiation of eNOS phosphorylation (Online Figure XVB) and interestingly with a reduction of both systolic and diastolic blood pressure (Online Figure XVI). In contrast, transduction with AAV-WT-BPIFB4 did not exert molecular and vasorelaxant effects in this model (Online Figure XV). It should again acknowledge that the observed effects were endothelium-dependent as no potentiation of NG-induced vasorelaxation was observed.
Investigation of the association between duration of transgene expression and functional/molecular outcomes indicates persistence of LAV-BPIFB4 expression ≤3 weeks after systemic delivery of the AVV vector, along with enhancement of endothelial vasorelaxation (Online Figure XVIIA) and eNOS phosphorylation (Online Figure XVIIB). On the other hand, the effect of LAV-BPIFB4 infection on blood pressure reduction was counteracted by compensatory mechanisms after 1 week (Online Figure XVIIC).
Analyses of different tissues from AAV-LAV-BPIFB4–infected mice showed overexpression of BPIFB4 in femoral bone marrow, brain, adipose tissue, and endothelium, but not in liver, blood serum, and MNCs (see details in Online Figures XVIII and XIX and Online Text IX). In addition, we quantified the abundance of CD31+ endothelial cells expressing BPIFB4 and found a significant increase of CD31+/BPIFB4+ endothelial cells AAV-LAV-BPIFB4–infected mice in comparison with AAV-green fluorescent protein–infected mice (Online Text IX).
Next, to strengthen the involvement of eNOS/NO signaling in the in vivo vascular effects of AAV-LAV-BPIFB4, we injected the vector in eNOS-knock-out and control mice. Results show that AAV-LAV-BPIFB4 fails to exert hemodynamic effects in conditions of eNOS deficiency (Online Figure XXA), despite an efficient LAV-BPIFB4 overexpression was confirmed in these animals by Western blot analysis (Online Figure XXB).
In Vivo Gene Therapy With LAV-BPIFB4 Rescues a Genetic Hypertensive Trait
We next studied the antihypertensive effect of LAV-BPIFB4 gene therapy in SHR, a model of essential hypertension characterized by endothelial dysfunction.42 Systemic delivery of LAV-BPIFB4 improved endothelial vasorelaxation and enhanced eNOS phosphorylation in vessels, along with normalized blood pressure (Figure 4).
Figure 4. Treatment of hypertensive rats with AAV-LAV-BPIFB4 results in pressure normalization.
A, Graph of the vascular response to acetylcholine (ACh) and nitroglycerin (NG) of ex vivo femoral and mesenteric arteries from spontaneously hypertensive rats (SHRs) injected with AAV-GFP (
 ; n=5) or AAV-LAV-BPIFB4 (
; n=5) or AAV-LAV-BPIFB4 (
 ; n=5). Values are means±SEM. B, Representative Western blot for endothelial nitric oxide synthase (eNOS) phosphorylation and BPIFB4 in femoral arteries from SHRs injected with AAV-GFP or AAV-LAV-BPIFB4. The right graph gives the quantification of eNOS phosphorylation and BPIFB4 of 3 pooled experiments. C, Systolic blood pressure (SBP) of SHR treated with AAV-GFP (
; n=5). Values are means±SEM. B, Representative Western blot for endothelial nitric oxide synthase (eNOS) phosphorylation and BPIFB4 in femoral arteries from SHRs injected with AAV-GFP or AAV-LAV-BPIFB4. The right graph gives the quantification of eNOS phosphorylation and BPIFB4 of 3 pooled experiments. C, Systolic blood pressure (SBP) of SHR treated with AAV-GFP (
 ; n=5) or AAV-LAV-BPIFB4 (
; n=5) or AAV-LAV-BPIFB4 (
 ; n=5). Values are means±SEM. Statistics was performed using ANOVA; *P<0.05; ***P<0.001. BPIFB4 indicates bactericidal/permeability-increasing fold-containing-family-B-member-4; GFP, green fluorescent protein; and LAV, longevity-associated variant.
; n=5). Values are means±SEM. Statistics was performed using ANOVA; *P<0.05; ***P<0.001. BPIFB4 indicates bactericidal/permeability-increasing fold-containing-family-B-member-4; GFP, green fluorescent protein; and LAV, longevity-associated variant.
We next explored if forced LAV-BPIFB4 expression has an impact on hypertensive vascular remodeling. As expected, and in agreement with previous reports, SHRs show an increase in media:lumen ratio and media thickness as compared with normotensive WKY rats,43,44 indicating hypertrophic remodeling. Intriguingly, although capable of normalizing blood pressure levels in SHRs, LAV-BPIFB4 failed to rescue vascular remodeling. These data indicate that the hypertrophically remodeled vasculature of chronically hypertensive rats remains functionally sensitive to the vasodilatory action of LAV-BPIFB4 (Online Figure XXI).
In Vivo Gene Therapy With LAV-BPIFB4 Reverts Vascular Ageing
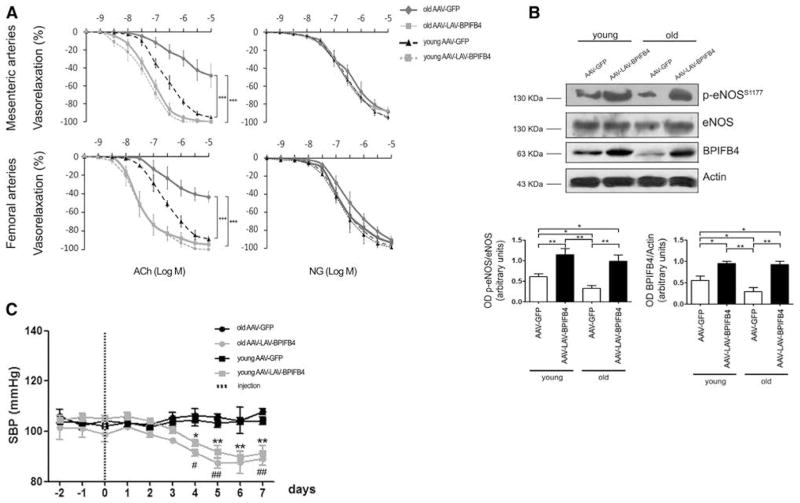
Aging is an independent cardiovascular risk factor associated with an impairment of endothelial function because of an imbalance between vasodilator and vasoconstriction substances and a progressive reduction in NO bioavailability.36,45,46 We confirm these typical features in old mice and provide new evidence of their association with downregulation of BPIFB4 (Figure 5B), thus strengthening the rationale for LAV-BPIFB4 gene therapy. Importantly, systemic delivery of AAV-LAV-BPIFB4 upregulates BPIFB4 and restores endothelial vasorelaxation and eNOS phosphorylation to the levels found in young animals (Figure 5A and 5B). The vasoactive effect was endothelium-dependent as denoted in experiments using NG (Figure 5A). Additionally, administration of AAV-LAV-BPIFB4 reduces blood pressure levels in both young and old mice (Figure 5C).
Figure 5. Effect of AAV-LAV-BPIFB4 on blood pressure, vascular reactivity, and endothelial nitric oxide synthase (eNOS) phosphorylation in old and young mice.
A, Graphs show vascular responses to acetylcholine (ACh) and nitroglycerin (NG) of mesenteric and femoral arteries from old and young mice injected with AAV-GFP (
 n=5;
n=5;
 n=5) or AAV-LAV-BPIFB4 (
n=5) or AAV-LAV-BPIFB4 (
 n=5;
n=5;
 ;n=5). Values are means±SEM. B, Western blot of 5 pooled experiments on mesenteric arteries injected from mice given AAV-GFP or AAV-LAV-BPIFB4. Graphs show quantification of eNOS phosphorylation and BPIFB4. Values are means±SEM, N=3 pools of experiments. C, Systolic blood pressure (SBP) in old and young mice treated with AAV-GFP (
;n=5). Values are means±SEM. B, Western blot of 5 pooled experiments on mesenteric arteries injected from mice given AAV-GFP or AAV-LAV-BPIFB4. Graphs show quantification of eNOS phosphorylation and BPIFB4. Values are means±SEM, N=3 pools of experiments. C, Systolic blood pressure (SBP) in old and young mice treated with AAV-GFP (
 n=5; -
n=5; -
 n=5) or AAV-LAV-BPIFB4 (
n=5) or AAV-LAV-BPIFB4 (
 n=5;
n=5;
 n=5). Values are means±SEM. Statistics was performed using ANOVA; *P<0.05; **P<0.01. BPIFB4 indicates bactericidal/permeability-increasing fold-containing-family-B-member-4; GFP, green fluorescent protein; and LAV, longevity-associated variant.
n=5). Values are means±SEM. Statistics was performed using ANOVA; *P<0.05; **P<0.01. BPIFB4 indicates bactericidal/permeability-increasing fold-containing-family-B-member-4; GFP, green fluorescent protein; and LAV, longevity-associated variant.
In Vivo Gene Therapy With LAV-BPIFB4 Enhances Postischemic Reparative Neovascularization and Reperfusion
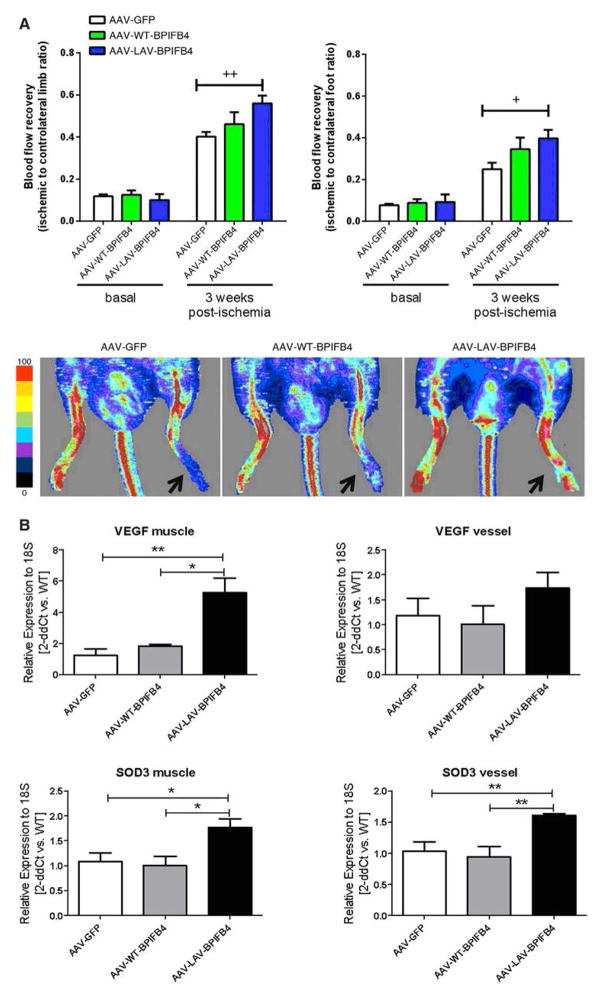
We then evaluated the therapeutic effect of AAV-LAV-BPIFB4 on revascularization, a process reduced during aging and 1 dependent on efficient homing of circulating proangiogenic cells.47 We investigated whether circulating progenitor cell homing to the ischemic muscles was enhanced by AAV-LAV-BPIFB4. To this end, lineage negative cKit+Sca-1+ cells and subpopulations of CD34− and CD34+ Lin− Sca-1+cKit+ cells—which in the mouse represent long-term and short-term reconstituting hematopoietic stem cells, respectively—were counted in enzymatically digested ischemic muscles 3 days after induction of ischemia (Online Figure XXIIA). The gating strategy for identification of these progenitor cell populations is shown in Online Figures XXIIA and XXIII. AAV-LAV-BPIFB4 increased the recruitment of total and both short- and long-term reconstituting Lin− Sca-1+cKit+ cells to ischemic muscle (Online Figure XXIIB). This finding suggests a therapeutic effect of LAV-BPIFB4 in ischemic vascular disease. Accordingly, in the mouse hindlimb ischemia model, AAV-LAV-BPIFB4 induced a significant recovery of superficial blood flow, as assessed by laser Doppler scanning at the level of the foot and whole limb 3 weeks after unilateral ligature of the femoral artery (Figure 6A; Online Figure XXIV). A tendency of blood flow to improve was also observed in mice given AAV-WT-BPIFB4, but the effect did not reach statistical significance. The hemodynamic benefit exerted by AAV-LAV-BPIFB4 corresponded with an increase in capillary and arteriole density (Online Figure XXV and Online Tables VIII and IX). In this model, AAV-LAV-BPIFB4 was injected systemically through the tail vein. To confirm transgene expression, we performed additional immunohistochemistry studies that confirm the transgene expression in limb muscles, at the level of capillaries and arterioles (Online Figure XXVI and Online Text IX). Additionally, we investigated the impact of AAV-LAV-BPIFB4 on the expression of proangiogenic and oxidative stress scavenging factors. Results show the upregulation of vascular endothelial growth factor (VEGF) and SOD-3 in ischemic muscles of AAV-LAV-BPIFB4-injected mice. Furthermore, similar inductive phenomena were observed in femoral arteries (Figure 6B).
Figure 6. Effect of longevity-associated variant (LAV)-BPFIB4 on postischemic recovery of superficial blood flow in mice.
A, Bar graph and representative images of blood flow recovery at the level of limb and foot, as assessed by laser Doppler flowmetry; arrows indicate the ischemic side 3 weeks postprocedure. Values are mean±SE of N=10 per group. B, Bar graphs show expression of vascular endothelial growth factor (VEGF) and SOD-3 in muscles and femoral arteries 1 week postischemia (N=4 per group). BPIFB4 indicates bactericidal/permeability-increasing fold-containing-family-B-member-4.
To be noted, we observed a transient reduction in blood pressure after the intravenous administration of AAV-LAV-BPIFB4 similarly to what observed with intra-arterial procedure, as shown in Online Figure XXVII. This is in keeping with improved native angiogenic response induced by vasodilator peptides, proteins, and drugs impacting on NO formation, like kinins, kallikrein, and converting enzyme inhibitors, which also favor blood pressure reduction.48–50
Discussion
Recent landmark studies have significantly improved our understanding of the genetic determinants of slow aging.51–53 Unfortunately, only apolipoprotein E (APOE) ε4 has reached the penalizing genome-wide threshold of significance to date.20 Therefore, to identify genes and genetic variants that influence human health through interaction with environmental factors, alternative approaches are needed, such as multistage study designs aimed at reducing the statistical penalty.18,20,54,55 In line with this, the present study adopted a combination of a multistage genetic and functional approach to investigate whether a candidate gene, that is, BPIFB4, is associated with exceptional longevity. Our findings strongly indicate the pivotal role of BPIFB4 in preserving the ability to activate an adaptive stress response and proteostasis, 2 physiological processes negatively affected by aging.6
BPIFB4 belongs to the superfamily of bactericidal BPI/PLUNC proteins, which are central to the host innate immune response against bacteria in regions of significant bacterial exposure, like the mouth, nose, and lungs. The expression of the activity-enhanced polymorphic variant LAV-BPIFB4 might initially produce privileged survival through better resistance to infectious diseases. The variant may offer additional advantages because of its ability to activate the NO-releasing enzyme eNOS. NO and peroxynitrite, which is generated by the interaction of NO with O2− during the respiratory burst, are mediators of the bactericidal effects of macrophages through inactivation of heme-containing enzymes, including cytochrome oxidases and cytochrome P450. Interestingly, development and refining of this prosurvival mechanism has facilitated exceptional longevity by ensuring better adaptation to stress conditions through improved function of ribosomal biogenesis, protein synthesis, and cardiovascular homeostasis.
Data from gene arrays of HEK293T cells indicate that BPIFB4 activates adaptive stress responses and proteostasis, 2 key aspects for improved organism survival,9 as well as stem cell maintenance.30 Analysis of BPIFB4’s sequence revealed a phosphorylation site for PERK, which is a further link between BPIFB4 and the stress response. A key element that makes LAV-BPIFB4 uniquely suited to confer physiological advantages is its peculiar ability to be superiorly phosphorylated by PERK and, thereby recruit the eNOS-activating factor HSP90. Although both LAV-BPIFB4 and WT-BPIFB4 can form an activated complex with HSP90, only LAV-BPIFB4 is capable of making this complex more productive for eNOS activation through post-transcriptional modulation by 14-3-3. In this regard, BPIFB4 sequence analysis revealed an atypical 14-3-3 binding site. Thus, 14-3-3 may modulate BPIFB4-mediated cell signaling by binding and retaining the latter within the cytoplasm on the basis of its phosphorylation status at serine 75. In fact, immunoprecipitation and confocal microscopy studies showed the LAV-BPIFB4 complexes efficiently with 14-3-3, a phenomenon that apparently did not occur with WT-BPIFB4. In support of the importance of PERK-mediated phosphoryla-tion at serine 75 and of serine 82 for LAV-BPIFB4’s binding to 14-3-3, LAV-BPIFB4mut14-3-3, LAV-BPIFB4mutPERK, or LAV-BPIFB4 with GSK2606414 (PERK inhibitor) failed to immu-noprecipitate 14-3-3.
A landmark finding of this study is the recognition that BPIFB4 plays a crucial role in maintenance of vascular ho-meostasis and that LAV-BPIFB4 exerts extraprotection. We show that biomechanical stress caused by increased arterial pressure upregulates BPIFB4 expression and that BPIFB4 silencing induces eNOS/endothelial dysfunction. Moreover, we found that LAV-BPIFB4 potentiated eNOS signaling in mouse vessels. In line with cellular data, studies in isolated vessels showed that the LAV-BPIFB4/HSP90 complex was dependent on PERK phosphorylation and 14-3-3 binding, with en-dothelial dysfunction ensuing on transfection of vessels with LAV-BPIFB4mut14-3-3, LAV-BPIFB4mutPERK, or LAV-BPIFB4 with GSK2606414. Similarly, WT-BPIFB4 binds to HSP90 and modulates endothelial function through a 14-3-3-mediated mechanism, as shown by mutagenesis of serine 82 (WT-BPIFB4mut14-3-3). However, LAV-BPIFB4 may have a more potent vasculoprotective effect because of its increased ability to bind 14-3-3.
Endothelial dysfunction is a systemic pathological state characterized by a reduction in the bioavailability of and responsiveness to vasodilators, and altered vascular wall metabolism. Because of its major causal role in cardiovascular diseases, such as hypertension and ischemia, therapeutic agents that restore endothelial function are of clinical interest. In this context, a body of evidence pinpoints eNOS as a major player in blood pressure homeostasis and eNOS downregulation as a hallmark of cardiovascular disease.56 Hence, we evaluated the effect of LAV-BPIFB4 in a rat model of essential hypertension characterized by impaired endothelial function. The in vivo administration of LAV-BPIFB4 normalized blood pressure levels and endothelial function in genetically hypertensive rats, without influencing vascular remodeling.
It is well known that cardiovascular risk is markedly increased in aged individuals. Our results show the association of dysfunctional endothelial-dependent vasorelaxation, reduced eNOS phosphorylation, and BPIFB4 downregulation in old mice. Importantly, we also newly demonstrate that forced BPIFB4 expression by AAV-LAV-BPIFB4 restores endothelium-dependent vasorelaxation, eNOS phosphorylation, and reduces blood pressure levels in old mice.
These results strongly candidate LAV-BPIFB4 as new therapeutic agent able to enhance endothelial NO release and reduce high blood pressure levels. We also recorded the higher expression of BPIFB4 in the CD34+ cells of LLIs and the absence of migration ability in EPCs after SDF-1 stimulation in the absence of BPIFB4. Thus, high levels of BPIFB4 could protect LLIs by improving revascularization, a process dependent on the migration ability of EPCs.47 In line, in a mouse model of hindlimb ischemia, LAV-BPIFB4 exerted a beneficial effect, significantly increasing homing of progenitor cells, enhancing blood flow, and spontaneous revascularization in the affected limb. Of note, only the LAV isoform confers substantial protection from acute ischemia through potentiation of vascular repair, which is associated with activation of VEGF and SOD3 in ischemic skeletal muscles, suggesting a role of proangiogenic and ROS scavenging mechanisms. More studies are needed to clarify the role of the effect of blood pressure lowering on this vascular phenotype. In conclusion, BPIFB4 is a multitasking protein involved in processes that are important for cellular and organism homeostasis and whose role in modulating eNOS is potentiated by the variations harbored by the LAV isoform. We have unraveled the molecular determinants linking functional vasculature responses, endothelial function, and a specific genetic trait and have identified BPIFB4 as a potential tool for innovative therapeutic strategies for aging, vascular repair, and endothelial dysfunction. Finally, we have found that genetic traits enriched in centenarians might be exploited to combat and attenuate cardiovascular disease at earlier stages of life. Thus, investigating exceptional longevity could bestow us with new knowledge instrumental to extending a clinical benefit to the population at large.
Supplementary Material
Novelty and Significance.
What Is Known?
The impaired production and release of nitric oxide (NO) from endothelial cells and the decline of vascular function are hallmarks of aging and predispose the development of cardiovascular diseases.
Exceptionally long-living individuals share common genetic features associated with protection against the onset of cardiovascular illness. Thus, a study of their genetic code could shed light on new mechanisms that control aging and disease resistance.
What New Information Does This Article Contribute?
Through the study of multiple populations of long-living individuals, we have identified the longevity-associated variant (LAV) of the gene BPIFB4 (bactericidal/permeability-increasing fold-containing family-B-member-4).
The forced expression of LAV-BPIFB4 both in old mice and in animal model of hypertension reduced blood pressure and rescued age-related endothelial dysfunction stimulating endothelial nitric oxide synthase activation.
In a mouse model of hindlimb ischemia, LAV-BPIFB4 facilitated reparative angiogenesis.
Identification and characterization of molecular regulators associated with longevity are important to develop new therapies aimed at reducing cardiovascular diseases. Based on a hypothesis-free genetic approach, we identified a novel exceptional longevity-associated variant of the gene BPIFB4, which was replicated in 3 independent populations. Moreover, we found that forced expression of LAV-BPIFB4 in cells activates stress response and proteostasis, 2 hallmarks of improved cellular homeostasis. Furthermore, in comparison with young controls, LAV-BPIFB4 expression was increased in cells from centenarian subjects, indicating its protective role. On phosphorylation by the stress kinase protein-kinase-R–like endoplasmic reticulum kinase, LAV-BPIFB4 modulates endothelial nitric oxide synthase activity through 14-3-3/heat shock protein 90 complex. Finally, in the hindlimb ischemia, LAV-BPIFB4 overexpression promotes the angiogenic reparative process and the recruitment of hematopoietic stem cells. These findings identify a new therapeutic target for preventing vascular aging and related cardiovascular diseases.
Acknowledgments
We thank the Doctorate School of Translational and Molecular Medicine (DIMET), Università degli Studi di Milano–Bicocca, Italy (F. Villa), and the Doctorate School of Molecular Medicine, Università degli Studi di Milano, Italy (A. Ferrario and C.C. Spinelli).
Sources of Funding
This work was supported by FIRB grants from the Italian Ministry of University and Research (AUTOMED–RBAP11Z3YA to A.A. Puca); Italian Ministry of Health (Giovani Ricercatori 2010 2302354); ITALBIONET (Italian Bioinformatics Network to R. Bellazzi); by Fondazione Umberto Veronesi fellowship (to A. Ferrario), and British Heart Foundation and Medical Research Council (to P. Madeddu).
Nonstandard Abbreviations and Acronyms
- BPIFB4
bactericidal/permeability-increasing fold-containing- family-B-member-4
- EPC
endothelial progenitor cells
- HSP90
heat shock protein 90
- LAV
longevity-associated variant
- LLIs
long living individuals
- MNC
mononuclear cells
- PERK
protein kinase R–like endoplasmic reticulum kinase
- SHR
spontaneously hypertensive rats
Footnotes
The online-only Data Supplement is available with this article at http://circres.ahajournals.org/lookup/suppl/doi:10.1161/CIRCRESAHA.117.305875/-/DC1.
Disclosures
A.A. Puca and C. Vecchione have filed a patent. The other authors report no conflicts.
References
- 1.Balch WE, Morimoto RI, Dillin A, Kelly JW. Adapting proteostasis for disease intervention. Science. 2008;319:916–919. doi: 10.1126/science.1141448. [DOI] [PubMed] [Google Scholar]
- 2.Preissler S, Deuerling E. Ribosome-associated chaperones as key players in proteostasis. Trends Biochem Sci. 2012;37:274–283. doi: 10.1016/j.tibs.2012.03.002. [DOI] [PubMed] [Google Scholar]
- 3.Hsu AL, Murphy CT, Kenyon C. Regulation of aging and age-related disease by DAF-16 and heat-shock factor. Science. 2003;300:1142–1145. doi: 10.1126/science.1083701. [DOI] [PubMed] [Google Scholar]
- 4.Tothova Z, Kollipara R, Huntly BJ, Lee BH, Castrillon DH, Cullen DE, McDowell EP, Lazo-Kallanian S, Williams IR, Sears C, Armstrong SA, Passegué E, DePinho RA, Gilliland DG. FoxOs are critical mediators of hematopoietic stem cell resistance to physiologic oxidative stress. Cell. 2007;128:325–339. doi: 10.1016/j.cell.2007.01.003. [DOI] [PubMed] [Google Scholar]
- 5.Paik JH, Ding Z, Narurkar R, et al. FoxOs cooperatively regulate diverse pathways governing neural stem cell homeostasis. Cell Stem Cell. 2009;5:540–553. doi: 10.1016/j.stem.2009.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rando TA. Stem cells, ageing and the quest for immortality. Nature. 2006;441:1080–1086. doi: 10.1038/nature04958. [DOI] [PubMed] [Google Scholar]
- 7.Mercken EM, Carboneau BA, Krzysik-Walker SM, de Cabo R. Of mice and men: the benefits of caloric restriction, exercise, and mimetics. Ageing Res Rev. 2012;11:390–398. doi: 10.1016/j.arr.2011.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zhang QJ, McMillin SL, Tanner JM, Palionyte M, Abel ED, Symons JD. Endothelial nitric oxide synthase phosphorylation in treadmill-running mice: role of vascular signalling kinases. J Physiol. 2009;587:3911–3920. doi: 10.1113/jphysiol.2009.172916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nisoli E, Tonello C, Cardile A, Cozzi V, Bracale R, Tedesco L, Falcone S, Valerio A, Cantoni O, Clementi E, Moncada S, Carruba MO. Calorie restriction promotes mitochondrial biogenesis by inducing the expression of eNOS. Science. 2005;310:314–317. doi: 10.1126/science.1117728. [DOI] [PubMed] [Google Scholar]
- 10.Kolluru GK, Bir SC, Kevil CG. Endothelial dysfunction and diabetes: effects on angiogenesis, vascular remodeling, and wound healing. Int J Vasc Med. 2012;2012:918267. doi: 10.1155/2012/918267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bir SC, Xiong Y, Kevil CG, Luo J. Emerging role of PKA/eNOS pathway in therapeutic angiogenesis for ischaemic tissue diseases. Cardiovasc Res. 2012;95:7–18. doi: 10.1093/cvr/cvs143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Walsh T, Donnelly T, Lyons D. Impaired endothelial nitric oxide bio-availability: a common link between aging, hypertension, and atherogenesis? J Am Geriatr Soc. 2009;57:140–145. doi: 10.1111/j.1532-5415.2008.02051.x. [DOI] [PubMed] [Google Scholar]
- 13.Moncada S, Higgs EA. Molecular mechanisms and therapeutic strategies related to nitric oxide. FASEB J. 1995;9:1319–1330. [PubMed] [Google Scholar]
- 14.Terry DF, Sebastiani P, Andersen SL, Perls TT. Disentangling the roles of disability and morbidity in survival to exceptional old age. Arch Intern Med. 2008;168:277–283. doi: 10.1001/archinternmed.2007.75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Perls TT, Wilmoth J, Levenson R, Drinkwater M, Cohen M, Bogan H, Joyce E, Brewster S, Kunkel L, Puca A. Life-long sustained mortality advantage of siblings of centenarians. Proc Natl Acad Sci U S A. 2002;99:8442–8447. doi: 10.1073/pnas.122587599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Heyn H, Li N, Ferreira HJ, et al. Distinct DNA methylomes of newborns and centenarians. Proc Natl Acad Sci U S A. 2012;109:10522–10527. doi: 10.1073/pnas.1120658109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bingle CD, Seal RL, Craven CJ. Systematic nomenclature for the PLUNC/PSP/BSP30/SMGB proteins as a subfamily of the BPI fold-containing superfamily. Biochem Soc Trans. 2011;39:977–983. doi: 10.1042/BST0390977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Novelli V, Viviani Anselmi C, Roncarati R, Guffanti G, Malovini A, Piluso G, Puca AA. Lack of replication of genetic associations with human longevity. Biogerontology. 2008;9:85–92. doi: 10.1007/s10522-007-9116-4. [DOI] [PubMed] [Google Scholar]
- 19.Geesaman BJ, Benson E, Brewster SJ, Kunkel LM, Blanché H, Thomas G, Perls TT, Daly MJ, Puca AA. Haplotype-based identification of a microsomal transfer protein marker associated with the human lifespan. Proc Natl Acad Sci U S A. 2003;100:14115–14120. doi: 10.1073/pnas.1936249100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nebel A, Kleindorp R, Caliebe A, et al. A genome-wide association study confirms APOE as the major gene influencing survival in long-lived individuals. Mech Ageing Dev. 2011;132:324–330. doi: 10.1016/j.mad.2011.06.008. [DOI] [PubMed] [Google Scholar]
- 21.Malovini A, Illario M, Iaccarino G, et al. Association study on long-living individuals from Southern Italy identifies rs10491334 in the CAMKIV gene that regulates survival proteins. Rejuvenation Res. 2011;14:283–291. doi: 10.1089/rej.2010.1114. [DOI] [PubMed] [Google Scholar]
- 22.Spinetti G, Fortunato O, Cordella D, Portararo P, Kränkel N, Katare R, Sala-Newby GB, Richer C, Vincent MP, Alhenc-Gelas F, Tonolo G, Cherchi S, Emanueli C, Madeddu P. Tissue kallikrein is essential for invasive capacity of circulating proangiogenic cells. Circ Res. 2011;108:284–293. doi: 10.1161/CIRCRESAHA.110.236786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Urbich C, Dimmeler S. Endothelial progenitor cells: characterization and role in vascular biology. Circ Res. 2004;95:343–353. doi: 10.1161/01.RES.0000137877.89448.78. [DOI] [PubMed] [Google Scholar]
- 24.Kränkel N, Katare RG, Siragusa M, et al. Role of kinin B2 receptor signaling in the recruitment of circulating progenitor cells with neovascularization potential. Circ Res. 2008;103:1335–1343. doi: 10.1161/CIRCRESAHA.108.179952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Urbich C, Dimmeler S. Endothelial progenitor cells functional characterization. Trends Cardiovasc Med. 2004;14:318–322. doi: 10.1016/j.tcm.2004.10.001. [DOI] [PubMed] [Google Scholar]
- 26.Vecchione C, Patrucco E, Marino G, et al. Protection from angiotensin II-mediated vasculotoxic and hypertensive response in mice lacking PI3Kgamma. J Exp Med. 2005;201:1217–1228. doi: 10.1084/jem.20040995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Vecchione C, Aretini A, Marino G, Bettarini U, Poulet R, Maffei A, Sbroggiò M, Pastore L, Gentile MT, Notte A, Iorio L, Hirsch E, Tarone G, Lembo G. Selective Rac-1 inhibition protects from diabetes-induced vascular injury. Circ Res. 2006;98:218–225. doi: 10.1161/01.RES.0000200440.18768.30. [DOI] [PubMed] [Google Scholar]
- 28.Zacchigna L, Vecchione C, Notte A, et al. Emilin1 links TGF-beta maturation to blood pressure homeostasis. Cell. 2006;124:929–942. doi: 10.1016/j.cell.2005.12.035. [DOI] [PubMed] [Google Scholar]
- 29.Andrault JB, Gaillard I, Giorgi D, Rouquier S. Expansion of the BPI family by duplication on human chromosome 20: characterization of the RY gene cluster in 20q11.21 encoding olfactory transporters/antimicrobial-like peptides. Genomics. 2003;82:172–184. doi: 10.1016/s0888-7543(03)00102-2. [DOI] [PubMed] [Google Scholar]
- 30.Vilchez D, Simic MS, Dillin A. Proteostasis and aging of stem cells. Trends Cell Biol. 2014;24:161–170. doi: 10.1016/j.tcb.2013.09.002. [DOI] [PubMed] [Google Scholar]
- 31.Amanchy R, Periaswamy B, Mathivanan S, Reddy R, Tattikota SG, Pandey A. A curated compendium of phosphorylation motifs. Nat Biotechnol. 2007;25:285–286. doi: 10.1038/nbt0307-285. [DOI] [PubMed] [Google Scholar]
- 32.Liu YC, Liu Y, Elly C, Yoshida H, Lipkowitz S, Altman A. Serine phosphorylation of Cbl induced by phorbol ester enhances its association with 14-3-3 proteins in T cells via a novel serine-rich 14-3-3-binding motif. J Biol Chem. 1997;272:9979–9985. doi: 10.1074/jbc.272.15.9979. [DOI] [PubMed] [Google Scholar]
- 33.Zhang K, Kaufman RJ. Signaling the unfolded protein response from the endoplasmic reticulum. J Biol Chem. 2004;279:25935–25938. doi: 10.1074/jbc.R400008200. [DOI] [PubMed] [Google Scholar]
- 34.Sato S, Fujita N, Tsuruo T. Regulation of kinase activity of 3-phos-phoinositide-dependent protein kinase-1 by binding to 14-3-3. J Biol Chem. 2002;277:39360–39367. doi: 10.1074/jbc.M205141200. [DOI] [PubMed] [Google Scholar]
- 35.Averna M, Stifanese R, De Tullio R, Passalacqua M, Salamino F, Pontremoli S, Melloni E. Functional role of HSP90 complexes with endothelial nitric-oxide synthase (eNOS) and calpain on nitric oxide generation in endothelial cells. J Biol Chem. 2008;283:29069–29076. doi: 10.1074/jbc.M803638200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Puca AA, Carrizzo A, Ferrario A, Villa F, Vecchione C. Endothelial nitric oxide synthase, vascular integrity and human exceptional longevity. Immun Ageing. 2012;9:26. doi: 10.1186/1742-4933-9-26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zincarelli C, Soltys S, Rengo G, Rabinowitz JE. Analysis of AAV serotypes 1–9 mediated gene expression and tropism in mice after systemic injection. Mol Ther. 2008;16:1073–1080. doi: 10.1038/mt.2008.76. [DOI] [PubMed] [Google Scholar]
- 38.Schiffrin EL. Reactivity of small blood vessels in hypertension: relation with structural changes. State of the art lecture. Hypertension. 1992;19(2 suppl):II1–9. doi: 10.1161/01.hyp.19.2_suppl.ii1-a. [DOI] [PubMed] [Google Scholar]
- 39.Welsh DG, Morielli AD, Nelson MT, Brayden JE. Transient receptor potential channels regulate myogenic tone of resistance arteries. Circ Res. 2002;90:248–250. doi: 10.1161/hh0302.105662. [DOI] [PubMed] [Google Scholar]
- 40.Carnevale D, Vecchione C, Mascio G, et al. PI3K inhibition reduces blood pressure by a vasorelaxant Akt/L-type calcium channel mechanism. Cardiovasc Res. 2012;93:200–209. doi: 10.1093/cvr/cvr288. [DOI] [PubMed] [Google Scholar]
- 41.Litteri G, Carnevale D, D’Urso A, et al. Vascular smooth muscle Emilin-1 is a regulator of arteriolar myogenic response and blood pressure. Arterioscler Thromb Vasc Biol. 2012;32:2178–2184. doi: 10.1161/ATVBAHA.112.254664. [DOI] [PubMed] [Google Scholar]
- 42.OKAMOTO K, AOKI K. Development of a strain of spontaneously hypertensive rats. Jpn Circ J. 1963;27:282–293. doi: 10.1253/jcj.27.282. [DOI] [PubMed] [Google Scholar]
- 43.Rezzani R, Porteri E, De Ciuceis C, Bonomini F, Rodella LF, Paiardi S, Boari GE, Platto C, Pilu A, Avanzi D, Rizzoni D, Agabiti Rosei E. Effects of melatonin and Pycnogenol on small artery structure and function in spontaneously hypertensive rats. Hypertension. 2010;55:1373–1380. doi: 10.1161/HYPERTENSIONAHA.109.148254. [DOI] [PubMed] [Google Scholar]
- 44.Intengan HD, Thibault G, Li JS, Schiffrin EL. Resistance artery mechanics, structure, and extracellular components in spontaneously hypertensive rats: effects of angiotensin receptor antagonism and converting enzyme inhibition. Circulation. 1999;100:2267–2275. doi: 10.1161/01.cir.100.22.2267. [DOI] [PubMed] [Google Scholar]
- 45.Najjar SS, Scuteri A, Lakatta EG. Arterial aging: is it an immutable cardiovascular risk factor? Hypertension. 2005;46:454–462. doi: 10.1161/01.HYP.0000177474.06749.98. [DOI] [PubMed] [Google Scholar]
- 46.Hoffmann J, Haendeler J, Aicher A, Rössig L, Vasa M, Zeiher AM, Dimmeler S. Aging enhances the sensitivity of endothelial cells toward apoptotic stimuli: important role of nitric oxide. Circ Res. 2001;89:709–715. doi: 10.1161/hh2001.097796. [DOI] [PubMed] [Google Scholar]
- 47.Asahara T, Murohara T, Sullivan A, Silver M, van der Zee R, Li T, Witzenbichler B, Schatteman G, Isner JM. Isolation of putative progenitor endothelial cells for angiogenesis. Science. 1997;275:964–967. doi: 10.1126/science.275.5302.964. [DOI] [PubMed] [Google Scholar]
- 48.Emanueli C, Minasi A, Zacheo A, Chao J, Chao L, Salis MB, Straino S, Tozzi MG, Smith R, Gaspa L, Bianchini G, Stillo F, Capogrossi MC, Madeddu P. Local delivery of human tissue kallikrein gene accelerates spontaneous angiogenesis in mouse model of hindlimb ischemia. Circulation. 2001;103:125–132. doi: 10.1161/01.cir.103.1.125. [DOI] [PubMed] [Google Scholar]
- 49.Emanueli C, Salis MB, Stacca T, Gaspa L, Chao J, Chao L, Piana A, Madeddu P. Rescue of impaired angiogenesis in spontaneously hypertensive rats by intramuscular human tissue kallikrein gene transfer. Hypertension. 2001;38:136–141. doi: 10.1161/01.hyp.38.1.136. [DOI] [PubMed] [Google Scholar]
- 50.Murohara T, Asahara T, Silver M, Bauters C, Masuda H, Kalka C, Kearney M, Chen D, Symes JF, Fishman MC, Huang PL, Isner JM. Nitric oxide synthase modulates angiogenesis in response to tissue ischemia. J Clin Invest. 1998;101:2567–2578. doi: 10.1172/JCI1560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Willcox BJ, Donlon TA, He Q, Chen R, Grove JS, Yano K, Masaki KH, Willcox DC, Rodriguez B, Curb JD. FOXO3A genotype is strongly associated with human longevity. Proc Natl Acad Sci U S A. 2008;105:13987–13992. doi: 10.1073/pnas.0801030105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Schächter F, Faure-Delanef L, Guénot F, Rouger H, Froguel P, Lesueur-Ginot L, Cohen D. Genetic associations with human longevity at the APOE and ACE loci. Nat Genet. 1994;6:29–32. doi: 10.1038/ng0194-29. [DOI] [PubMed] [Google Scholar]
- 53.Flachsbart F, Caliebe A, Kleindorp R, Blanché H, von Eller-Eberstein H, Nikolaus S, Schreiber S, Nebel A. Association of FOXO3A variation with human longevity confirmed in German centenarians. Proc Natl Acad Sci U S A. 2009;106:2700–2705. doi: 10.1073/pnas.0809594106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Sebastiani P, Timofeev N, Dworkis DA, Perls TT, Steinberg MH. Genome-wide association studies and the genetic dissection of complex traits. Am J Hematol. 2009;84:504–515. doi: 10.1002/ajh.21440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Ferrario A, Villa F, Malovini A, Araniti F, Puca AA. The application of genetics approaches to the study of exceptional longevity in humans: potential and limitations. Immun Ageing. 2012;9:7. doi: 10.1186/1742-4933-9-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Hu Z, Xiong Y, Han X, Geng C, Jiang B, Huo Y, Luo J. Acute mechanical stretch promotes eNOS activation in venous endothelial cells mainly via PKA and Akt pathways. PLoS One. 2013;8:e71359. doi: 10.1371/journal.pone.0071359. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.