Abstract
Chilling stress is an important constraint for maize seedling establishment in the field. To examine the role of salicylic acid (SA) and hydrogen peroxide (H2O2) in response to chilling stress, we investigated the effects of seed priming with SA, H2O2, and SA+H2O2 combination on maize resistance under chilling stress (13°C). Priming with SA, H2O2, and especially SA+H2O2 shortened seed germination time and enhanced seed vigor and seedling growth as compared with hydropriming and non-priming treatments under low temperature. Meanwhile, SA+H2O2 priming notably increased the endogenous H2O2 and SA content, antioxidant enzymes activities and their corresponding genes ZmPAL, ZmSOD4, ZmAPX2, ZmCAT2, and ZmGR expression levels. The α-amylase activity was enhanced to mobilize starch to supply metabolites such as soluble sugar and energy for seed germination under chilling stress. In addition, the SA+H2O2 combination positively up-regulated expressions of gibberellic acid (GA) biosynthesis genes ZmGA20ox1 and ZmGA3ox2, and down-regulated GA catabolism gene ZmGA2ox1 expression; while it promoted GA signaling transduction genes expressions of ZmGID1 and ZmGID2 and decreased the level of seed germination inhibitor gene ZmRGL2. The abscisic acid (ABA) catabolism gene ZmCYP707A2 and the expressions of ZmCPK11 and ZmSnRK2.1 encoding response receptors in ABA signaling pathway were all up-regulated. These results strongly suggested that priming with SA and H2O2 synergistically promoted hormones metabolism and signal transduction, and enhanced energy supply and antioxidant enzymes activities under chilling stress, which were closely relevant with chilling injury alleviation and chilling-tolerance improvement in maize seed.
Highlights:Seed germination and seedling growth were significantly improved under chilling stress by priming with SA+H2O2 combination, which was closely relevant with the change of reactive oxygen species, metabolites and energy supply, hormones metabolism and regulation.
Keywords: maize, seed priming, chilling tolerance, salicylic acid, H2O2, GA biosynthesis, ABA catabolism, antioxidant enzymes
Introduction
Chilling stress is one of the most severe abiotic stresses inhibiting obviously seed germination and plant growth (Allen and Ort, 2001). Maize, one of sensitive crops to chilling stress (Hola et al., 2007), often encounters low temperature when sowed in early spring, resulting in reduction of seed emergence and seedling growth (Farooq et al., 2008b; Guan et al., 2009; Imran et al., 2013). Therefore, it is necessary to develop novel methods to enhance the chilling tolerance of maize seed (Maslak et al., 2007).
Hydrogen peroxide (H2O2) at proper concentration was conducive to seed dormancy-broken and germination enhancement (Müller et al., 2009; Lariguet et al., 2013). However, the over-accumulation of H2O2 easily caused cell injury (Mittler et al., 2004; Bailly et al., 2008; Kumar et al., 2015). H2O2 signal directly or indirectly involved in many signaling pathways of SA, GA, ABA, and so on (Durner and Klessig, 1999; Diaz-Vivancos et al., 2013; Maarouf et al., 2015; Qi et al., 2015). Antioxidant enzymes produced in plants such as superoxide dismutase (SOD), catalase (CAT), ascorbate peroxidase (APX), and glutathione reductase (GR) contributed to the endogenous H2O2 balance (Noctor and Foyer, 1998; Slesak et al., 2007). However, there are few reports on chilling resistance improvement of maize seeds through H2O2 application.
Salicylic acid (SA) has been well known as a signaling molecule in inducing the plant defensive system to biotic or abiotic stresses (Horvath et al., 2007; Rivas-San Vicente and Plasencia, 2011; Dong et al., 2014). Under low temperature, the enhanced biosynthesis of endogenous SA was closely relevant with the increasing antioxidant enzymes activities and seedling growth in maize seeds (Wang et al., 2013). Guan et al. (2015) reported that as seedling suffering from chilling stress (5°C), dry weight of roots and shoots, and protective enzymes activities in SA-treated coating maize seeds were obviously higher than those SA-untreated ones. Although SA was found to enhance chilling tolerance of maize, and H2O2 could promote seed germination, few reports concentrated on the combination effect of SA and H2O2 on maize chilling tolerance enhancement, and the mechanism involved in their combination effect remained completely unclear.
During maize seed germination, the α-amylase played an important role in starch decomposing into soluble sugar (Sun et al., 2015). In addition, seed germination was generally inhibited by high ABA concentration and promoted by GA in many species (Olszewski et al., 2002; Yamauchi et al., 2004). The dynamic balance of synthesis and catabolism of ABA and GA was crucial for seed germination (Footitt et al., 2011), The NCED encoding 9-cis-epoxycarotenoiddioxygen-ase was known as the key gene in ABA biosynthesis, and CYP707A encoding abscisic acid 8-hydroxylases as the essential gene in ABA catabolism (Footitt et al., 2011). ABA endogenous messenger genes CPK11 and SnRK2.1, respectively, encoding calcium-dependent protein kinase and sucrose non-fermenting related protein kinase, were reported to be up-regulated under drought, salt and chilling stresses (Mao et al., 2010; Chen et al., 2012; Yang et al., 2014). The GA20ox (encoding GA 20-oxidase) and GA3ox (encoding GA 3-oxidase) were main genes during bioactive GA biosynthesis. In contrast, GA2ox encoding GA 2-oxidase antagonizes could active GAs to inactive GAs. Plant growth repressor DELLA proteins (DELLAs) consisting of GAI (GA-insensitive), RGA (Repressor of ga1-3) and RGL1-3 (RGA-like1-3) were inhibitors in GA signal pathway. In which, RGL2 was considered as the key repressor of seed germination (Stamm et al., 2012). The bond of GA with GID (gibberellin insensitive dwarf protein, a receptor of GA signaling) could trigger the degradation of RGL2, resulting in fast seed germination (Cao et al., 2006).
In this experiment, we found that maize seeds germinated obviously faster after SA+H2O2 combined priming as compared with priming treatment with SA or H2O2 alone. Therefore, antioxidant enzymes activities, α-amylase activities and corresponding genes expression involving in metabolism and signal transduction were determined after priming with SA, H2O2, and SA+H2O2 combination to acquire better understandings on the potential mechanism of SA+H2O2 priming in maize seed chilling tolerance enhancement.
Materials and Methods
Materials
Maize seeds of Meiyu No.3 with 10.1% moisture content were obtained from HaiNan Lv Chuan Seed Co., LTD. SA and H2O2 were purchased from Shanghai Dingguo Biotech Co., Ltd., Shanghai, China.
Seed Priming
Seeds were surface sterilized with 0.5% NaClO solution for 15 min and then washed three times with sterilized distilled water to remove the residue of disinfectant. The sterilized seeds were primed with priming solutions (1:5, w/v) at 20°C in darkness for 24 h, without changing the priming solution during the process. Then all primed seeds were air-dried at 25°C for 48 h to their original moisture contents. In this study, four priming solutions including distilled water (hydropriming), 0.5 mM SA, 50 mM H2O2, and 0.5 mM SA+50 mM H2O2 were carried out.
Seed Germination and Seedling Quality Measurement
All primed maize seeds were germinated in rolled towels moistened with water for 7 days in growth chambers at chilling stress (13°C), with a photosynthetic active photon flux density of 250 mmol m-2⋅s-1 and a photoperiod of 12 h light (L):12 h dark (D) (Wang et al., 2013). Those treatments were, respectively, indicated by HP+CS (hydropriming and chilling stress), SA+CS (SA priming and chilling stress), H2O2+CS (H2O2 priming and chilling stress), and SA+H2O2+CS (SA+H2O2 priming and chilling stress). Six replications of 50 seeds each for each treatment were used. The geminated seeds (5 mm radicle penetrated through seed coat) were counted daily for 7 days, and then germination energy (GE) and germination percentage (GP) was calculated on day 4 and day 7, respectively (ISTA, 2004). The germination index (GI) was measured as GI = Σ (Gt/Tt) (Hu et al., 2005). The mean germination time (MGT) was calculated as MGT = Σ (Gt × Tt)/ΣGt, where Gt is the number of new germinated seeds in time Tt. Shoot height and root length were measured manually with a ruler. The seedling dry weight (DW) was weighed directly after drying at 80°C for 24 h. Measurements were made on thirty randomly selected seedlings per replication. Vigor index (VI) was determined as VI = GI × DW (Hu et al., 2005).
Hydrogen Peroxide, Antioxidant Enzymes, Malondialdehyde, α-Amylase, and Soluble Sugar Measurements
During seed germination after sowing, 10 seed embryos per replication and 6 replications for each treatment at each sampling time (0, 6, 24, 48, and 72 h after sowing) were collected for physiological parameters determination. The H2O2 content was measured according to the method of Doulis et al. (1997). The activities of SOD, POD, APX, and CAT were determined through the methods described by Qiu et al. (2005). POD, APX, and CAT activity was, respectively, calculated as μmol ascorbate decomposition min-1⋅g-1⋅FW and μmol H2O2 decomposition min-1⋅g-1⋅FW; while one unit of SOD activity was defined as 50% inhibition of nitro-blue tetrazolium (NBT) photochemical reaction and the results were expressed in U g-1⋅FW. GR was quantitated by measuring the decrease in absorbance at 340 nm due to the oxidation of nicotinamide adenine dinucleotide phosphate (NADPH), and GR activity was calculated as μmol NADPH oxidation min-1⋅g-1⋅FW (Smith and Johnson, 1988). The malondialdehyde (MDA) content was measured using the thiobarbituric acid reaction method (Gao et al., 2009). The activity of α-amylase was conducted using 3,5-dinitrosalicylic acid colorimetric method (Li et al., 2009). The soluble sugar content in seeds was measured by anthrone colorimetric method (Li and Li, 2013) with slight modification.
Endogenous SA Measurements
Endogenous SA content was analyzed using HPLC according to the method of Wang et al. (2013). Each sample was ground into powder with liquid nitrogen and then 0.5 g of powder was homogenized in 1 ml aliquot of 90% (v/v) methanol. After centrifugation at 10,000 × g for 15 min, the supernatant was dried by nitrogen purging at 35°C. The residue was dissolved in 0.25 ml of 5% (w/v) TCA and the upper phase containing SA was concentrated by nitrogen purging at 35°C. In addition, the released SA was extracted from a lower aqueous phase. The SA collected from the upper phase and the lower phase were mixed and dissolved in a 600 μl of mobile phase consisting of 0.2 M sodium acetate buffer (pH 5.5) (90%) and methanol (10%), which was then filtered through a 0.22 μm membrane filter and analyzed by HPLC (Waters 600, Waters 717 automatic sampler, Waters, Milford, United States) under fluorescence detection (Waters 474). The excitation and emission wavelengths were 305 and 407 nm, respectively. Six replications of embryos for each treatment were collected at each sampling time.
Total RNA Extraction and Quantitative Real-time PCR (RT-qPCR) Analysis
Total RNA was isolated from seed embryo using Trizol reagent (Huayueyang, Beijing, China) and reverse transcribed using a Rever Tra Ace qPCR RT kit (Toyobo, Osaka, Japan) following the manufacturer’s instructions. Gene specific RT-PCR primers were designed based on their cDNA sequences (Supplementary Table S1). Six genes involved in stress responses, i.e., ZmSOD4, ZmAPX2, ZmCAT2, and ZmGR. ZmAMY (α-amylase gene) involved in seed germination. Six genes involved in GA signal, i.e., ZmGA20ox1, ZmGA3ox2, ZmGA2ox1, ZmGID1, ZmGID2, and ZmRGL2. Four genes involved in ABA signal, i.e., ZmNCED1, ZmCYP707A2, ZmCPK11, and ZmSnRK2.1. ZmPAL (encoding phenylalanine ammonia-lyase) involved in SA biosynthesis. The RT-qPCR was performed using Roche real-time PCR detection system (Roche life science, United States). Each reaction (20 μL) consisted of 10 μL of SYBR Green PCR Master Mix (Takara, Chiga, Japan), 1 μL of diluted cDNA and 0.1 μM forward and reserve primers. The PCR cycling conditions were as follows: 95°C for 3 min, followed by 40 cycles of 95°C for 10 s and 58°C for 45 s. The maize Actin gene was used as an internal control. Relative gene expression was calculated according to Livak and Schmittgen (2001).
Statistical Analysis
Data were analyzed by analysis of variance (ANOVA) using the Statistical Analysis System (SAS) (version 9.2) followed by calculation of the Least Significant Difference (LSD, p < 0.05). Percentage data were arc-sin-transformed prior to analysis.
Results
Seed Priming Enhanced Seed Germination Speed and Seedling Growth under Chilling Stress
As compared with NP+Cn, the seed germination was inhibited obviously under low temperature, and none of priming treatments reached the same germination speed and seedling growth compared to NP+Cn (Figure 1). The germination percentage of NP+Cn was 96% on the third day, when that of NP+CS was 0%. Seed priming treatments significantly promoted seed germination under chilling stress as compared with NP+CS. Seeds treated with SA+H2O2+CS started germinating at 1 day after sowing, whereas seeds treated with SA+CS or H2O2+CS began to germinate on the second day, while those seeds treated with HP+CS delayed germination until the third day after sowing (Figure 1 and Supplementary Figure S2). On the third day, the maximum germination percentage (80.0%) was recorded in SA+H2O2 under chilling stress, as well as GE, GI, DW, and VI, which were also significantly higher than other priming treatments (Table 1). All priming treatments owned longer RL than NP+CS did, in which SA+H2O2 had the relatively higher level (6.05 cm). Priming treatments showed less of an effect on SH, in which H2O2 and SA+H2O2 significantly heighten SH compared to NP+CS (Table 1).
FIGURE 1.
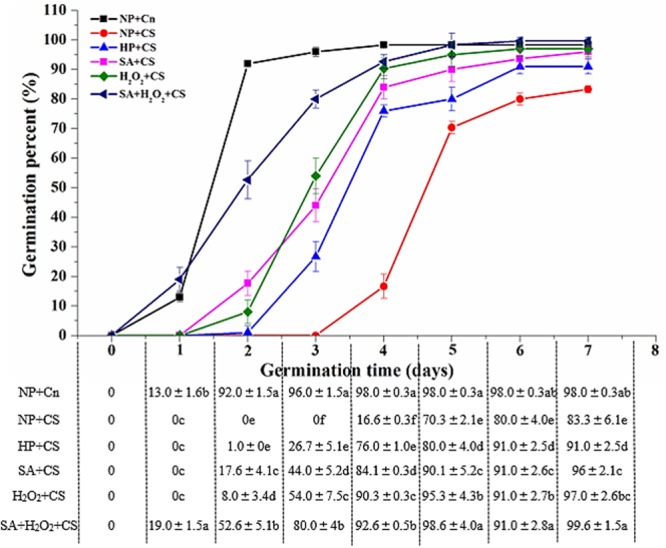
Seed priming enhanced seed germination speed under chilling stress (13°C). Vertical bars above mean indicated standard error of six replicates of 50 seeds each for each treatment. Values were mean ± SE (n = 6). Different small letter(s) following the values indicated significant difference (p < 0.05, LSD) among treatments. Other explanations were shown in Table 1.
Table 1.
Seed priming enhanced seed germination speed and seedling growth under chilling stress (13°C).
| Treatments | GE (%) | GI | MGT (d) | SH (cm) | RL (cm) | DW (g/10plants) | VI |
|---|---|---|---|---|---|---|---|
| NP+Cn∗ | 98.0 ± 0.3a | 54.4 ± 0.2a | 1.94 ± 0.02a | 4.64 ± 0.12a | 7.77 ± 0.09a | 1.0102 ± 0.0225a | 57.3 ± 1.01a |
| NP+CS | 16.6 ± 0.3f | 16.9 ± 0.2c | 4.98 ± 0.02f | 1.05 ± 0.09d | 2.11 ± 0.11f | 0.0320 ± 0.0009d | 0.55 ± 0.02d |
| HP+CS | 76.0 ± 1.0c | 24.4 ± 0.1bc | 3.99 ± 0.01e | 1.08 ± 0.10d | 3.24 ± 0.08e | 0.0569 ± 0.0018c | 1.36 ± 0.04cd |
| SA+CS | 84.1 ± 0.3d | 20.8 ± 0.5bc | 3.57 ± 0.03d | 1.10 ± 0.09d | 4.24 ± 0.09d | 0.0670 ± 0.0018c | 1.40 ± 0.04cd |
| H2O2+CS | 90.3 ± 0.3c | 29.6 ± 0.2b | 3.45 ± 0.04c | 1.30 ± 0.10c | 5.16 ± 0.10c | 0.0597 ± 0.0011c | 1.77 ± 0.03c |
| SA+H2O2+CS | 92.6 ± 0.5b | 49.5 ± 0.3a | 2.55 ± 0.05a | 1.51 ± 0.11b | 6.05 ± 0.06b | 0.0889 ± 0.0004b | 4.40 ± 0.02b |
∗Values were mean ± SE (n = 6). Different small letter (s) following the values indicated significant difference (p < 0.05, LSD) among treatments. The germination standard is 5 mm radicle penetrated through seed coat. GE, germination energy; GI, germination index; MGT, mean germination time; SH, shoot height; RL, root length; DW, the seedling dry weight; VI, vigor index. NP+Cn, no priming and no stress (25°C); NP+CS, no priming and chilling stress (13°C); HP+CS, hydropriming (water priming) and chilling stress; SA+CS, 0.5 mM salicylic acid priming and chilling stress; H2O2+CS, 50 mM hydrogen peroxide priming and chilling stress; SA+H2O2+CS, 0.5 mM salicylic acid and 50 mM hydrogen peroxide combination priming and chilling stress.
Seed Priming Increased Endogenous H2O2 and Decreased MDA Content under Chilling Stress
Hydrogen peroxide concentration gradually increased under normal condition (NP+Cn) and were significantly higher than NP+CS from 24 to 72 h after sowing (Figure 2A). The H2O2 content in NP+CS kept a stably low level and showed no visibly difference during seed germination (Supplementary Figure S1A). As comparing with NP+CS, the H2O2 concentration of H2O2+CS was significantly higher at 0 and 48 h; while SA+CS obviously improved H2O2 concentration from 48 to 72 h. SA+H2O2+CS enhanced H2O2 level in general, but did not show distinct difference with NP+CS and HP+CS from 6 to 72 h.
FIGURE 2.
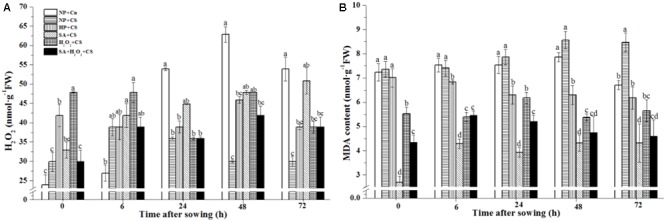
Seed priming increased endogenous H2O2 (A) and decreased MDA content (B) under chilling stress. Seed embryos were collected, respectively, at 0, 6, 24, 48, and 72 h after sowing, and six replications for each treatment at each sampling time were used. Different small letter(s) on the top of the bars indicated significant differences (p < 0.05, LSD) among treatments at the same time. Error bars indicated ± SE of mean (n = 6). For additional explanations, please see Table 1.
In addition, NP+Cn and NP+CS showed relatively higher MDA levels during seed germination than all priming treatments did (Figure 2B and Supplementary Figure S1B). The MDA content of NP+Cn decreased significantly at 72 h compared to NP+CS. Except for 24 and 72 h, HP had obviously higher MDA content than other three priming treatments. SA primed seeds showed the lowest MDA levels from 0 to 72 h, followed by SA+H2O2 and H2O2 primed seeds under chilling stress.
Seed Priming Increased Endogenous SA Contents and Up-regulated ZmPAL Expression under Chilling Stress
Non-primed seeds had prominently higher SA concentrations under 25°C than 13°C during seed germination (Figure 3A), and the notable increase in SA contents happened after 24 h after sowing. Under chilling stress, SA concentrations treated by SA+CS, H2O2 +CS, and SA+H2O2+CS rapidly increased as compared with NP+CS which were all significantly higher than HP+CS. All priming treatments increased SA contents markedly from 0 to 72 h under chilling stress.
FIGURE 3.
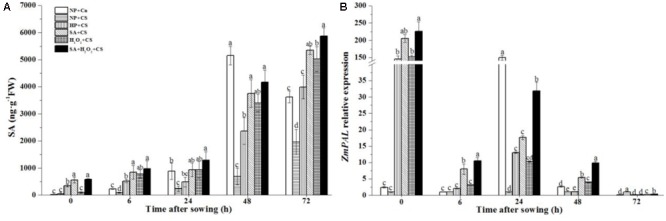
Seed priming increased endogenous SA contents (A) and up-regulated ZmPAL expression (B) under chilling stress. Seed embryos were collected, respectively, at 0, 6, 24, 48, and 72 h after sown, and six replications for each treatment at each sampling time were used. Different small letter(s) on the top of the bars indicated significant differences (p < 0.05, LSD) among treatments at the same time. Error bars indicated ± SE of mean (n = 6). For additional explanations, please see Table 1.
The ZmPAL expression of NP+Cn reached its maximum level at 24 h after sowing (160-fold higher than NP+CS) (Figure 3B); while ZmPAL expression of NP+CS stayed at relatively low levels during 72 h after sowing. Under chilling stress, priming seeds significantly enhanced ZmPAL expression compared to NP+CS and NP+Cn at 0 h, and also up-regulated ZmPAL expression compared to NP+CS at 24 h. ZmPAL expression was obviously higher in case of SA+H2O2 combination than other three priming treatments from 6 to 48 h after sowing.
Seed Priming Stimulated Antioxidant Enzymes Activities in Response to Chilling Stress
All of the antioxidant enzymes activities including SOD, APX, CAT, and GR of six treatments showed a stepped upward tendency during seed germination and reached highest levels at 72 h after sowing (Figure 4). These four enzymes activities of NP+CS were generally lower and had significant differences at 72 h as compared with those of NP+Cn. On the whole, all priming treatments improved four tested antioxidant enzymes activities compared to NP+CS, with a few exceptions such as HP+CS at 24 h in SOD and H2O2+CS at 72 h in GR. The relative higher levels of four antioxidant enzymes were all recorded in SA+H2O2 among four priming treatments under chilling stress.
FIGURE 4.
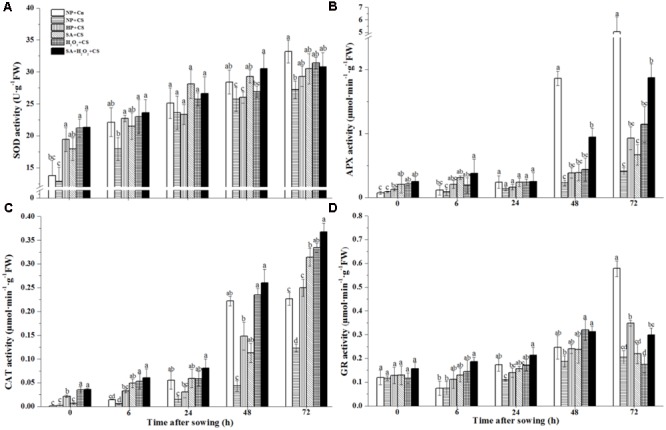
Seed priming stimulated antioxidant enzymes activities including superoxide dismutase (A, SOD), ascorbate peroxidase (B, APX), catalase (C, CAT) and glutathione reductase (D, GR) in response to chilling stress. Seed embryos were collected, respectively, at 0, 6, 24, 48 and 72 h after sowing, and six replications for each treatment at each sampling time were used. Different small letter(s) on the top of the bars indicated significant differences (p < 0.05, LSD) among treatments at the same time. Error bars indicated ± SE of mean (n = 6). For additional explanations, please see Table 1.
Seed Priming Enhanced ZmSOD4, ZmAPX2, ZmCAT2, and ZmGR Expression in Response to Chilling Stress
The ZmSOD4 expression of NP+Cn increased and got the highest level at 48 h, which was about six-fold higher than NP+CS (Figure 5A). At the end of seed priming, SA+H2O2+CS, H2O2+CS, and HP+CS significantly enhanced ZmSOD4 expression compared to NP+Cn and NP+CS, and the higher ZmSOD4 level was recorded in SA+H2O2. At 48 h, ZmSOD4 expression in primed seeds increased fleetly, and SA+H2O2 had significantly higher ZmSOD4 level than other treatments during seed germination except 6 and 24 h.
FIGURE 5.
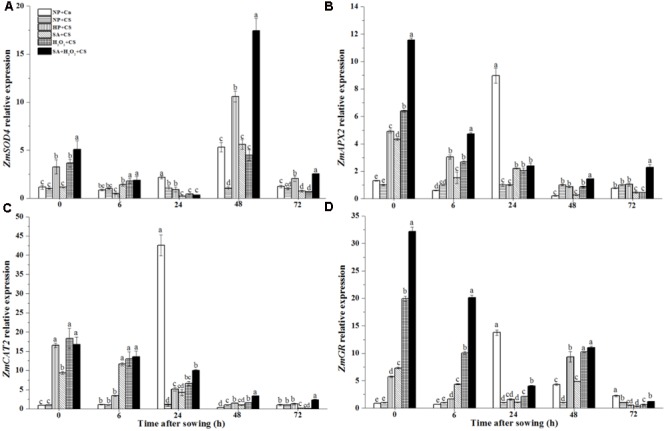
Seed priming enhanced ZmSOD4 (A), ZmAPX2 (B), ZmCAT2 (C), and ZmGR (D) expression in response to chilling stress. Seed embryos were collected, respectively, at 0, 6, 24, 48, and 72 h after sowing, and six replications for each treatment at each sampling time were used. Different small letter(s) on the top of the bars indicated significant differences (p < 0.05, LSD) among treatments at the same time. Error bars indicated ± SE of mean (n = 6). For additional explanations, please see Table 1.
The expressions of ZmCAT2 and ZmAPX2 in NP+Cn and NP+CS remained at low levels during seed germination after sowing; however, sudden increases happened in NP+Cn at 24 h, which were notably higher than those in NP+CS and priming treatments (Figures 5B,C). All four priming treatments improved ZmCAT2 and ZmAPX2 expression compared with NP+Cn and NP+CS at 0 h, of which SA+H2O2+CS kept the higher levels during seed imbibition than other priming treatments.
The ZmGR expression of NP+Cn increased from 0 to 24 h after sowing, and the highest expression were recorded at 24 h in NP+Cn which was about 14-fold higher than NP+CS (Figure 5D). At the end of seed priming, the expression levels of ZmGR were significantly up-regulated, of which SA+H2O2 had the highest ZmGR level (32-fold higher than NP+CS) among the four priming treatments (Figure 5D, 0 h). In addition, ZmGR expression in SA+H2O2+CS was visibly higher than other three priming treatments from 0 to 24 h after sowing.
Seed Priming Enhanced α-Amylase Activity and Soluble Sugar Content and Up-regulated ZmAMY Expression under Chilling Stress
The activity of α-amylase in NP+Cn increased gradually during seed germination, and reached higher level at 48 and 72 h than those in NP+CS and other priming treatments (Figure 6A). At the end of priming (0 h), α-amylase activities were improved obviously by primed treatments compared to those non-primed seeds, and seeds treated by SA, H2O2, and SA+H2O2 owned higher α-amylase levels than those treated by HP.
FIGURE 6.
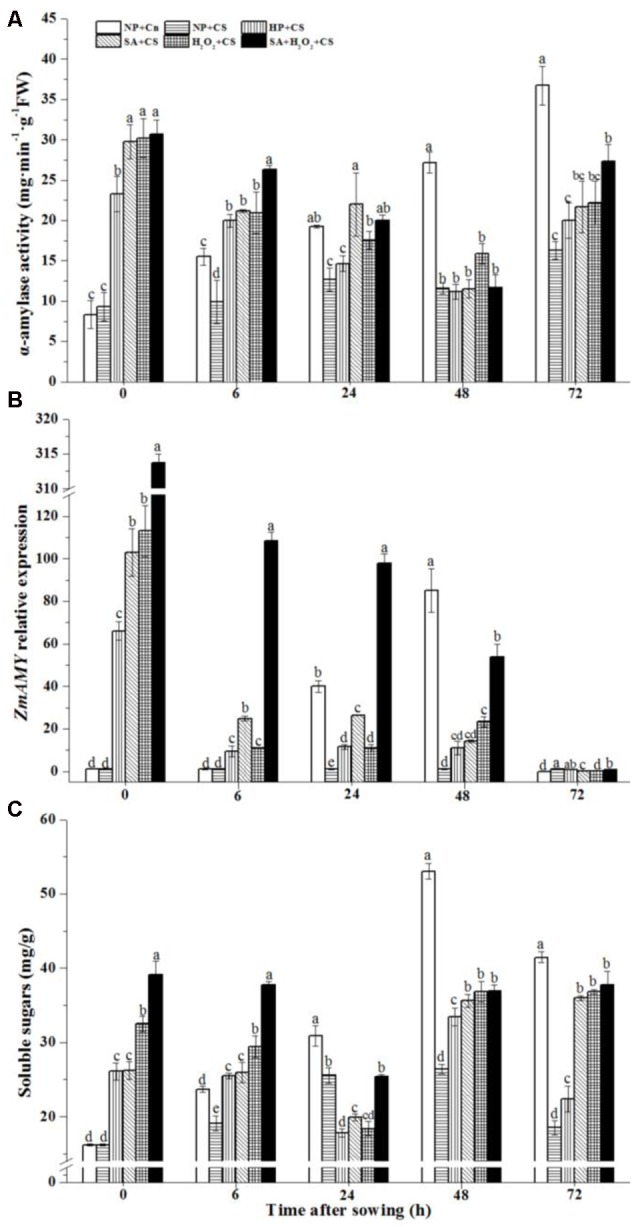
Seed priming enhanced α-amylase activity (A) and soluble sugar content (C) and up-regulated ZmAMY expression under (B) chilling stress. Seed embryos were collected, respectively, at 0, 6, 24, 48, and 72 h after sowing, and six replications for each treatment at each sampling time were used. Different small letter(s) on top of the bars indicated significant differences (p < 0.05, LSD) among treatments at same sown time. Error bars indicated ± SE of mean (n = 6). For additional explanations, please see Table 1.
The expression of ZmAMY in NP+Cn was increased gradually from 0 to 48 h, and the highest value was recorded at 48 h which was about 90-fold higher than that in NP+CS (Figure 6B). ZmAMY expression were up-regulated significantly at the end of priming treatments (Figure 6B, 0 h), then sharply decreased from 0 to 6 h. SA+H2O2 remained ZmAMY expression above 100-fold more than NP+CS from 0 to 48 h. After sowing for 72 h, ZmAMY expression of all treatments fell back to a low level.
In addition, the soluble sugar content of NP+Cn increased rapidly to a maximum value at 48 h after sowing, and then accompanied by a slightly decline (Figure 6C). Except 24 h after sowing, priming treatments obviously improved soluble sugar content in maize seeds compared with NP+CS. During seed germination, SA+H2O2+CS kept a relatively higher level than other priming treatments except 48 h (Figure 6C).
Seed Priming Regulated ZmNCED1, ZmCYP707A2, ZmSnRK2.1, and ZmCPK11 Expression under Chilling Stress
The ZmNCED1 expression of NP+Cn had its relatively higher value at 48 h as compared with other times after sowing (Figure 7A). At the end of priming (0 h), four priming treatments increased obviously the ZmNCED1 expressions than non-primed seeds, among which the highest level was recorded in HP. During seed germination from 0 to 6 h under chilling stress, ZmNCED1 expression in priming treatments rapidly decreased; however, from 24 to 72 h, SA, H2O2, and SA+H2O2 presented a gradual upward trend.
FIGURE 7.
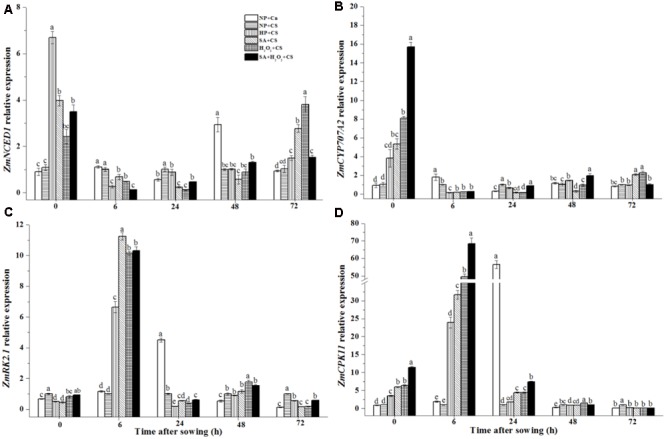
Seed priming regulated ZmNCED1 (A), ZmCYP707A2 (B), ZmSnRK2.1 (C), and ZmCPK11 (D) expression under chilling stress. Seed embryos were collected respectively at 0, 6, 24, 48 and 72 h after sowing, and six replications for each treatment at each sampling time were used. Different small letter(s) on the top of the bars indicated significant differences (p < 0.05, LSD) among treatments at same sown time. Error bars indicated ± SE of mean (n = 6). For additional explanations, please see Table 1.
ZmCYP707A2 expressions of priming treatments were up-regulated significantly at 0 h as compared with NP+Cn and NP+CS (Figure 7B), in which SA+H2O2 up-regulated the expression of ZmCYP707A2 by almost 16-fold than NP+CS. However, ZmCYP707A2 expression decreased rapidly and stayed at low levels during time after sowing in different treatments.
The changing trends of ZmSnRK2.1 and ZmCPK11 expressions in four priming treatments were similar during seed germination (Figures 7C,D). At 6 h after sowing, they reached their highest levels, andZmSnRK2.1 expression in SA+CS, H2O2+CS, SA+H2O2+CS, and HP+CS were, respectively, 11-fold, 10-fold, 10-fold, and 6-fold higher than NP+CS; while ZmCPK11 expression was, respectively, 33-fold, 50-fold, 65-fold, and 22.5-fold higher than NP+CS. In addition, ZmSnRK2.1 and ZmCPK11 expressions in NP+Cn reached maximum at 24 h, which was, respectively, 35-fold and 58-fold higher than NP+CS.
Seed Priming Up-regulated ZmGA20ox1, ZmGA3ox2, ZmRGL2, ZmGID1, and ZmGID2 Expression and Down-regulated ZmGA2ox1 Expression under Chilling Stress
During seed germination, ZmGA20ox1 and ZmGA3ox2 genes were involved in GA biosynthesis. ZmGA20ox1 expression of NP+Cn reached the highest level at 24 h (about 11-fold higher than NP+CS), and was significantly higher than NP+CS from 24 to 48 h (Figure 8A). At the end of priming treatments (0 h), the expression levels of ZmGA20ox1 were up-regulated visibly. SA+H2O2+CS reached the highest ZmGA20ox1 expression level (about 25-fold higher than NP+CS) which were distinctly higher than H2O2+CS, SA+CS, and HP+CS treatments. At 6 h after sowing, ZmGA20ox1 expression remained increasing and reached obviously highest levels which were, respectively, 110-fold in SA+H2O2+CS, 100-fold in H2O2+CS, 90-fold in SA+CS, and 70-fold in HP+CS higher than NP+CS, and then they decreased rapidly.
FIGURE 8.
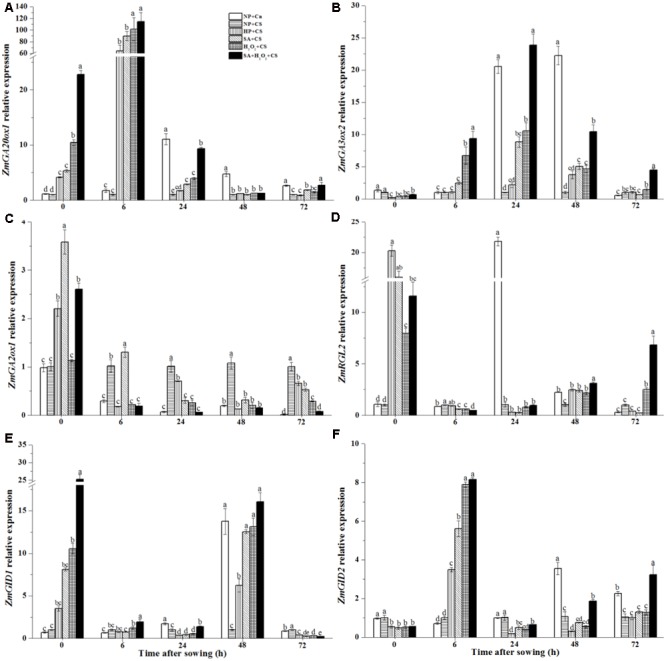
Seed priming up-regulated ZmGA20ox1 (A), ZmGA3ox2 (B), ZmRGL2 (D), ZmGID1 (E) and ZmGID2 (F) expression and down-regulated ZmGA2ox1 (C) expression under chilling stress. Seed embryos were collected respectively at 0, 6, 24, 48, and 72 h after sowing, and six replications for each treatment at each sampling time were used. Different small letter(s) on the top of the bars indicated significant differences (p < 0.05, LSD) among treatments at the same time. Error bars indicated ± SE of mean (n = 6). For additional explanations, please see Table 1.
The highest ZmGA3ox2 expression of NP+Cn was recorded at 48 h, which was significantly higher than NP+CS and other treatments (Figure 8B). Four priming treatments up-regulated ZmGA3ox2 expression compared with NP+CS. Seeds treated with SA, H2O2, and SA+H2O2 owned higher levels than those treated by HP from 6 to 48 h under chilling stress. And the significantly highest value was recorded in SA+H2O2+CS.
The ZmGA2ox1 expression in NP+Cn was always significantly lower than that in NP+CS from 6 to 72 h (Figure 8C). Four priming treatments increased ZmGA2ox1 expression after priming, which, however, decreased rapidly at the beginning of seed germination and significantly down-regulated as compared with NP+CS from 24 to 72 h. SA+H2O2+CS owned the lowest value among the four priming treatments.
The ZmRGL2 expressions in NP+Cn and NP+CS stayed at low levels after priming (Figure 8D), the markedly highest level was showed at 24 h in NP+Cn which was about 22-fold higher than NP+CS and other treatments. The expression levels of ZmRGL2 were significantly up-regulated at the end of priming treatments as compared with NP+CS, but then decreased rapidly.
The ZmGID1 and ZmGID2 genes are two positive regulators to mediate GA signaling pathways. The maximum levels of ZmGID1 and ZmGID2 expressions in NP+Cn were observed at 48 h, which were, respectively, about 13-fold and 3-fold higher than NP+CS (Figures 8E,F). After four priming treatments, ZmGID1 and ZmGID2 expressions were significantly up-regulated as compared with NP+Cn and NP+CS at 0 and 6 h, respectively. Seeds treated with SA+H2O2, H2O2, and SA had higher ZmGID1 and ZmGID2 expression values than those treated by HP (at 48 and 6 h, respectively) and the highest value was recorded in SA+H2O2 under chilling stress.
Discussion
Chilling, one of the major abiotic stresses, exerts severe influences on growth and development of plant. In the present study, 13°C was applied as chilling temperature according to the report by Luo et al. (2015), and under chilling stress (NP+CS), germination of maize was delayed by ≥3 days after sowing compared with that in normal temperature (NP+Cn) (Figure 1). It was consistent with the results concluded by Farooq et al. (2008a) that the time to 50% emergence of hybrid maize was about 1.5 days shorter under normal condition than that under chilling stress. In comparison with the germination time of non-primed seed under 13°C (NP+CS), seeds primed with SA+H2O2 germinated 3 days in advance, seeds primed with SA or H2O2 germinated 2 days in advance and while seeds treated with HP germinated 1 day in advance (Figure 1 and Supplementary Figure S2). Although the seedling quality of four priming treatments under chilling stress could not reach the NP+Cn level, they increased significantly the RL and DW compared to the NP+CS and among which SA+H2O2 had the highest values of SH, RL, DW, and VI. Obviously, priming treatments are useful (Elkoca et al., 2007; Pereira et al., 2009; Xu et al., 2011), which could be used for maize seeds germination acceleration and seedling quality improvement under chilling stress. Especially, SA and H2O2 had synergistical effect on seed germination enhancement.
The germinated ability of seeds seemed to be linked to the H2O2 accumulation to a critical level (Hossain et al., 2015; Huang et al., 2017). And H2O2 content was related with endosperm cap weakening and embryo elongation during lettuce seed germination (Zhang Y. et al., 2014). Our data showed that NP+Cn accumulated more H2O2 content from 24 to 72 h than NP+CS and other priming treatments; while four kind of priming seeds had relatively higher H2O2 levels than NP+CS from 48 to 72 h (Figure 2). However, over accumulation of H2O2 easily led to oxidative damage of membrane lipids, proteins, and nucleic acids (Das and Roychoudhury, 2014). The MDA content as an index of oxidative damage degree was consequently analyzed and the results showed that priming treatments obviously down-regulated MDA accumulation during seed germination under chilling stress. In addition, MDA concentrations in NP+CS and NP+Cn kept similarly high level from 0 to 24 h after sowing. However, with the extended germination time, MDA content in NP+Cn gradually decreased, while that in NP+CS continuously increased. It was suggested that the H2O2 accumulation in non-primed seeds under normal temperature or induced by priming under chilling stress did not cause an oxidative damage to seed embryo. However, NP+CS with the cumulative MDA under low temperature easily caused a harmful influence on the cell membrane integrity (Allen and Ort, 2001). Interestingly, priming seeds under chilling stress had even lower MDA content than non-primed seed under normal temperature, which might be due to seed membrane and organelle after priming had been repaired in advance, however, unprimed seed needed time to repair during seed germination.
Priming treatments significantly improved endogenous SA concentration during seed germination under chilling stress, which might be closely relevant with the up-regulated ZmPAL expression (Figure 3). Especially for SA+H2O2 combination, its SA content and ZmPAL expression level were obviously higher than those in SA or H2O2. Increased H2O2 and SA as signaling molecules could easily induce antioxidant defense system in plant (Mittler, 2002; Kang et al., 2003; Horvath et al., 2007). Comparing with NP+CS, the activities of antioxidant enzymes and their biosynthesis related genes expressions significantly enhanced after priming treatments (Figures 4, 5). Luo et al. (2011) also reported that the beneficial effect of SA on alleviating postharvest chilling injury of ‘Qingnai’ plum fruit closely related to the increased antioxidant enzymes activities and decreased MDA concentrations. Therefore, SA and H2O2 might synergistically promote activities of antioxidant enzymes to protect maize embryo against injury derived from low temperature.
During seed germination, starch in seed could be decomposed by α-amylase into maltodextrin, sucrose, glucose, etc. to provide metabolites and energy for seed germination and seedling growth, and so germination speed was highly associated with the starch metabolism (Hussain et al., 2015). The slower germination speed of maize seeds soaked at 10°C was closely related to the lower α-amylase activity as compared with seeds treated under 25°C (Silva-Neta et al., 2015). In this study, NP+Cn started to germinate at 24 h of after sowing, accompanied by obvious increase of α-amylase activity and its synthetic gene ZmAMY expression. However, chilling stress reduced a-amylase activity and total soluble sugars and seed reserves were not metabolized during the first 72 h after sowing, so non-primed seeds (NP+CS) did not germinate during this time. Therefore the rapidly increased α-amylase activity and enhanced soluble sugar content might be part of the reasons for the fast seed germination after priming treatments. It’s worth noting that at 24 h after sowing, the soluble sugar content had a sudden decrease in primed seeds, which might be the result of soluble sugars consumption by activated endogenous metabolism for germination preparation after priming. The ZmAMY expression involving in α-amylase synthesis were significantly up-regulated in priming treatments, even the SA+H2O2 improved the ZmAMY expression 113-fold higher than NP+CS. It indicated that priming treatments might enhance α-amylase activity though up-regulating ZmAMY expression under chilling stress. Seed germination was usually accompanied by the increases in ABA catabolism and GA biosynthesis (Liu et al., 2014; Zhang H. et al., 2014). The cyp707a2 mutant, encoding the abscisic acid 80-hydroxylases, accumulated much more ABA and showed stronger dormancy than the wild type (Zhang H. et al., 2014). The over-expression of bean PvNCED1 resulted in delayed seed germination and increased ABA content (Qin and Zeevaart, 2002). In our study, the expression levels of ZmNCED1 and ZmCYP707A2 increased significantly at the end of seed priming and then decreased rapidly during seed germination, which might contribute to the lower ABA concentration and faster germination in primed seeds than non-primed seeds under low temperature (Figure 7). The expressions of ZmCPK11 and ZmSnRK2.1 involving in ABA signal pathway notably up-regulated by seed priming compared with NP+CS under chilling stress. It was consistent with the results reported by Mao et al. (2010) that enhanced resistances to drought, salt and chilling were observed in ZmCPK11 and ZmSnRK2.1 over-expressing plants.
GA is another key regulator of seed germination in many species. A total of 136 different GAs have been identified in plants; however, only a few of GAs are biologically active (such as GA1 and GA4) and the predominantly active GA is usually species dependent (Olszewski et al., 2002; Yamauchi et al., 2004). Seeds from GA3ox deletion mutant could not germinate even under normal condition (Mitchum et al., 2006) and the transcript level of GA3ox2 increased 40-fold in dormancy-broken seeds as compared with dormant ones (Finch-Savage et al., 2007). Whereas GA2ox1 expression kept at obviously higher level in highly dormant seeds than in non-dormant seeds (Finch-Savage et al., 2007). After SA, H2O2, and SA+H2O2 priming, the expression levels of ZmGA20ox1 and ZmGA3ox2 involving in GA synthesis were up-regulated and those of ZmGA2ox1 involving in GA catabolism were significantly down-regulated during maize seed imbibition at chilling condition (Figure 8). In addition, the expression of ZmRGL2 involving in GA signal transduction was up-regulated at the end of priming, and then down-regulated sharply during germination. It was consistent with the model that GA improved germination by stimulating the SCF (SLY1) E3 ubiquitin ligase complex to trigger ubiquitination and destruction of RGL2 (Ariizumi and Steber, 2007). RGL2 protein was inferred as the inhibitor of seed germination (Lee et al., 2002). Ariizumi and Steber (2007) reported that seeds failing to germination in the GA biosynthesis mutant ga1-3 could be rescued by exogenous GA application or by knocking out the DELLA gene RGL2. ZmGID1 and ZmGID2 positively regulated GA signaling transduction. The expression of ZmGID1 was significantly up-regulated at 0 and 48 h, while those of ZmGID2 significantly up-regulated at 6 h under chilling stress in this study. Therefore, enhanced GA signal transduction might be another reason for faster seed germination and better seedling establishment in primed seeds than non-primed seeds under chilling stress.
In addition, it is generally known that radicle breakout through seed coat and embryo eruption are two key points during seed germination (Dekkers et al., 2013). Radicle of maize seeds usually broke through seed coat after 24 h under normal temperature and embryo erupted after 48 h (Figure 1). Correspondingly, violently physiological and biochemical changes occurred usually during 24 to 48 h, such as GAs and ABA metabolism and signal transduction, antioxidant enzymes activities and α-amylase activities. As non-primed seed germinating at 13°C, radicle breakout delayed until 96 h of imbibition and embryo erupted happened until 7 days of germination, endogenous substances therefore had no dramatic changes during 72 h. However, the time for radicle breakout under low temperature predated to 24 h after seeds treated with SA+H2O2, the times predated to 48 h after seeds treated with SA or H2O2; while the time predated to 72 h after seeds treated with HP. Therefore, violent changes of endogenously physiological metabolism were correspondingly in advance at the end of priming.
Seed vigor and seedling establishment under chilling stress were notably improved in SA+H2O2 combination in tested maize seeds. There might underline several possible mechanisms (Figure 9). (1) Changes of reactive oxygen species: endogenous H2O2 and SA content increased and antioxidant enzymes activities enhanced after seed priming; (2) metabolism and energy supply: the enhancement of α-amylase activity indicated rapid mobilization of starch in seeds, which supplied metabolites and energy for seed germination and seedling growth under chilling stress; (3) hormones metabolism and regulation: GA biosynthesis genes ZmGA20ox1 and ZmGA3ox2 up-regulated, GA catabolism gene ZmGA2ox1 and ABA biosynthesis gene ZmNCED1 down-regulated. Meanwhile, GA signaling transduction genes ZmGID1 and ZmGID2 expression increased and seed germination inhibitor gene ZmRGL2 level decreased after seed priming. In addition, the expression of ZmCPK11 and ZmSnRK2.1 encoding response receptors in ABA signaling pathway were up-regulated.
FIGURE 9.
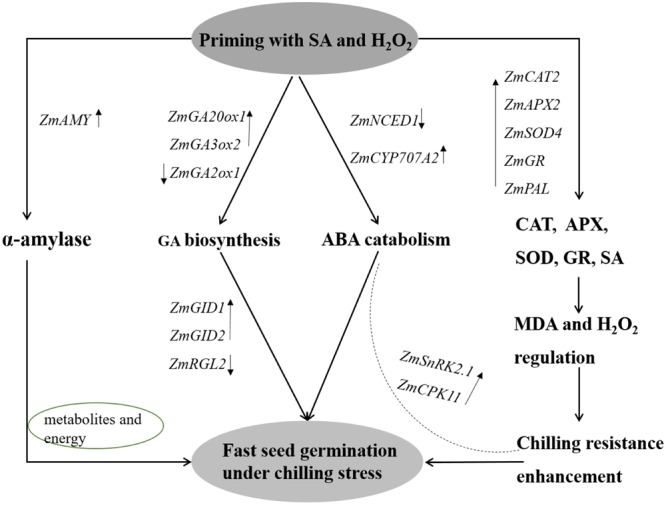
Proposed model of priming by SA and H2O2 on chilling tolerance improvement during maize (Zea mays L.) seed germination. The α-amylase was enhanced to decompose starch to provide metabolisms and energy for seed germination under chilling stress. Priming seeds with SA and H2O2 positively up-regulated GA biosynthesis and ABA catabolism, and promoted GA signaling transduction. SA content and antioxidant enzymes activities increased to alleviate chilling stress. ZmCPK11 and ZmSnRK2.1 expressions were up-regulated to improve the tolerance to chilling stress. SA, salicylic acid, H2O2, hydrogen peroxide.
Conclusion
Chilling tolerance of maize seeds could be significantly improved by SA+H2O2, which was obviously superior to SA or H2O2. However, the effect of SA+H2O2 on chilling-resistance improvement was not the simple addition of single effects of SA and H2O2. It definitely indicated that, based on the physiological and molecular data, SA and H2O2 could mutually induce and maintain homeostasis to exert synergistic effect on maize seeds chilling resistance. Furthermore, the underlined synergies of SA and H2O2 suggested that there were different mechanisms in chilling resistance induction by SA or H2O2; however, there existed interaction effects among different physiological and molecular mechanisms under chilling stress, which still needed further study.
Author Contributions
Conceived and designed the experiments: ZL and YaG. Performed the experiments: ZL, JX, WH, and CW. Analyzed the data: ZL, YuG, MS. Contributed reagents/materials/analysis tools: JH, YL, YH, GG, and WH. Wrote the paper: ZL and YaG.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
This research was supported by the National Natural Science Foundation of China (No. 31371708, 31201279, 31671774), Zhejiang Provincial Natural Science Foundation (No.LY15C130002, LZ14C130002), the Fundamental Research Funds for the Central Universities (No. 2015QNA6019) and Jiangsu Collaborative Innovation Center for Modern Crop Production, China.
Supplementary Material
The Supplementary Material for this article can be found online at: http://journal.frontiersin.org/article/10.3389/fpls.2017.01153/full#supplementary-material
References
- Allen D. J., Ort D. R. (2001). Impacts of chilling temperatures on photosynthesis in warm-climate plants. Trends Plant Sci. 6 36–42. 10.1016/S1360-1385(00)01808-2 [DOI] [PubMed] [Google Scholar]
- Ariizumi T., Steber M. (2007). Seed germination of GA-insensitive sleepy1 mutants does not require RGL2 protein disappearance in Arabidopsis. Plant Cell 19 791–804. 10.1105/tpc.106.048009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bailly C., El-Maarouf-Bouteau H., Corbineau F. (2008). From intracellular signaling networks to cell death: the dual role of reactive oxygen species in seed physiology. C. R. Biol. 331 806–814. 10.1016/j.crvi.2008.07.022 [DOI] [PubMed] [Google Scholar]
- Cao D., Cheng H., Wu W., Soo H. M., Peng J. (2006). Gibberellin mobilizes distinct DELLA-dependent transcriptomes to regulate seed germination and floral development in Arabidopsis. Plant Physiol. 142 509–525. 10.1104/pp.106.082289 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen J. H., Jiang H. W., Hsieh E. J., Chen H. Y., Chien C. T., Hsieh H. L., et al. (2012). Drought and salt stress tolerance of an Arabidopsis glutathione S-transferase U17 knockout mutant are attributed to the combined effect of glutathione and abscisic acid. Plant Physiol. 158 340–351. 10.1104/pp.111.181875 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Das K., Roychoudhury A. (2014). Reactive oxygen species (ROS) and response of antioxidants as ROS-scavengers during environmental stress in plants. Front. Environ. Sci. 2:53 10.3389/fenvs.2014.00053 [DOI] [Google Scholar]
- Dekkers B., Pearce S., Veldkamp R., Marshall A., Widera P., Gilbert J. (2013). Transcriptional dynamics of two seed compartments with opposing roles in arabidopsis seed germination. Plant Physiol. 163 205–215. 10.1104/pp.113.223511 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diaz-Vivancos P., Barba-Espin G., Hernandez J. A. (2013). Elucidating hormonal/ROS networks during seed germination: insights and perspectives. Plant Cell Rep. 32 1491–1502. 10.1007/s00299-013-1473-7 [DOI] [PubMed] [Google Scholar]
- Dong C. J., Li L., Shang Q. M., Liu X. Y., Zhang Z. G. (2014). Endogenous salicylic acid accumulation is required for chilling tolerance in cucumber (Cucumis sativus L.) seedlings. Planta 240 687–700. 10.1007/s00425-014-2115-1 [DOI] [PubMed] [Google Scholar]
- Doulis A. G., Debian N., Kingston-Smith A. H., Foyer C. H. (1997). Differential localization of antioxidants in maize leaves. Plant Physiol. 114 1031–1037. 10.1007/s00425-014-2115-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Durner J., Klessig D. F. (1999). Nitric oxide as a signal in plants. Curr. Opin. Plant Biol. 2 369–374. 10.1104/pp.114.3.1031 [DOI] [PubMed] [Google Scholar]
- Elkoca E., Haliloglu K., Esitken A., Ercisli S. (2007). Hydro-and osmo-priming improve chickpea germination. Acta Agric. Scand. B Soil Plant Sci. 57 193–200. 10.1016/S1369-5266(99)00007-2 [DOI] [Google Scholar]
- Farooq M., Aziz T., Basra S. M. A., Cheema M. A., Rehman H. (2008a). Chilling tolerance in hybrid maize induced by seed priming with salicylic acid. J. Agron. Crop Sci. 194 161–168. 10.1111/j.1439-037X.2008.00300.x [DOI] [Google Scholar]
- Farooq M., Aziz T., Basra S. M. A., Wahid A., Khaliq A., Cheema M. A. (2008b). Exploring the role of calcium to improve chilling tolerance in hybrid maize. J. Agron. Crop Sci. 194 350–359. 10.1111/j.1439-037X.2008.00322.x [DOI] [Google Scholar]
- Finch-Savage W. E., Cadman C. S., Toorop P. E., Lynn J. R., Hilhorst H. W. (2007). Seed dormancy release in Arabidopsis Cvi by dry after-ripening, low temperature, nitrate and light shows common quantitative patterns of gene expression directed by environmentally specific sensing. Plant J. 51 60–78. 10.1111/j.1365-313X.2007.03118.x [DOI] [PubMed] [Google Scholar]
- Footitt S., Douterelo-Soler I., Clay H., Finch-Savage W. E. (2011). Dormancy cycling in Arabidopsis seeds is controlled by seasonally distinct hormone-signaling pathways. Proc. Natl. Acad. Sci. U.S.A. 108 20236–20241. 10.1073/pnas.1116325108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao C. H., Hu J., Zhang S., Zheng Y. Y., Knapp A. (2009). Association of polyamines in governing the chilling sensitivity of maize genotypes. Plant Growth Regul. 57 31–38. 10.1007/s10725-008-9315-2 [DOI] [Google Scholar]
- Guan Y. J., Hu J., Wang X. J., Shao C. X. (2009). Seed priming with chitosan improves maize germination and seedling growth in relation to physiological changes under low temperature stress. J. Zhejiang Univ. Sci. B 10 427–433. 10.1631/jzus.B0820373 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guan Y. J., Li Z., He F., Huang Y. T., Song W. J., Hu J. (2015). “on-off” thermoresponsive coating agent containing salicylic acid applied to maize seeds for chilling tolerance. PLoS ONE 10:e0120695 10.1371/journal.pone.0120695 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hola D., Kocova M., Rothova O., Wilhelmova N., Benesova M. (2007). Recovery of maize (Zea mays L.) inbreds hybrids from chilling stress of various duration: photosynthesis and antioxidant enzymes. J. Plant Physiol. 164 868–877. 10.1016/j.jplph.2006.04.016 [DOI] [PubMed] [Google Scholar]
- Horvath E., Szalai G., Janda T., Paldi E. (2007). Induction of abiotic stress tolerance by salicylic acid signaling. J. Plant Growth Regul. 26 290–300. 10.1007/s00344-007-9017-4 [DOI] [Google Scholar]
- Hossain M. A., Bhattacharjee S., Armin S. M., Qian P., Xin W., Li H. Y. (2015). Hydrogen peroxide priming modulates abiotic oxidative stress tolerance: insights from ROS detoxification and scavenging. Front. Plant Sci. 6:420 10.3389/fpls.2015.00420 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu J., Zhu Z. Y., Song W. J., Wang J. C., Hu W. M. (2005). Effects of sand priming on germination and field performance in direct-sown rice (Oryza sativa L.). Seed Sci. Technol. 33 243–248. 10.15258/sst.2005.33.1.25 [DOI] [Google Scholar]
- Huang Y. T., Lin C., He F., Li Z., Guan Y. J., Hu J. (2017). Exogenous spermidine improves seed germination of sweet corn via involvement in phytohormone interactions, H2O2 and relevant gene expression. BMC Plant Biol. 17:1 10.1186/s12870-016-0951-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hussain S., Zheng M., Khan F., Khaliq A., Fahad S., Peng S., et al. (2015). Benefits of rice seed priming are offset permanently by prolonged storage and the storage conditions. Sci. Rep. 5:8101 10.1038/srep08101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imran S., Afzal I., Basra S. M. A., Saqib M. (2013). Integrated seed priming with growth promoting substances enhances germination and seedling vigour of spring maize at low temperature. Int. J. Agric. Biol. 15 1251–1257. [Google Scholar]
- ISTA (2004). Annexe to Chapter 5. Germination Test in International Rules for Seed Testing (Bassersdorf: International Seed Testing Association; ). [Google Scholar]
- Kang G., Wang C., Sun G., Wang Z. (2003). Salicylic acid changes activities of H2O2-metabolizing enzymes and increases the chilling tolerance of banana seedlings. Environ. Exp. Bot. 50 9–15. 10.1016/S0098-8472(02)00109-0 [DOI] [Google Scholar]
- Kumar S. P. J., Prasad S. R., Banerjee R., Thammineni C. (2015). Seed birth to death: dual functions of reactive oxygen species in seed physiology. Ann. Bot. 116 663–668. 10.1093/aob/mcv098 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lariguet P., Ranocha P., De Meyer M., Barbier O., Penel C., Dunand C. (2013). Identification of a hydrogen peroxide signalling pathway in the control of light-dependent germination in Arabidopsis. Planta 238 381–395. 10.1007/s00425-013-1901-5 [DOI] [PubMed] [Google Scholar]
- Lee S., Cheng H., King K., Wang W., He Y., Hussain A., et al. (2002). Gibberellin regulates Arabidopsis seed germination via RGL2, a GAI/RGA-like gene whose expression is up-regulated following imbibition. Genes Dev. 16 646–658. 10.1101/gad.969002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li X. X., Li J. Z. (2013). Determination of the content of soluble sugar in sweet corn with optimized anthrone colorimetric method. Storage Process 13 24–27. [Google Scholar]
- Li Y., Gao F., Shan F., Bian J., Zhao C. (2009). Study on the interaction between 3 flavonoid compounds and α-amylase by fluorescence spectroscopy and enzymatic kinetics. J. Food Sci. 74 199–203. 10.1111/j.1750-3841.2009.01080.x [DOI] [PubMed] [Google Scholar]
- Liu Y., Fang J., Xu F., Chu J., Yan C., Schlappi M., et al. (2014). Expression patterns of ABA and GA metabolism genes and hormone levels during rice seed development and imbibition: a comparison of dormant and non-dormant rice cultivars. J. Genet. Genomics 41 327–338. 10.1016/j.jgg.2014.04.004 [DOI] [PubMed] [Google Scholar]
- Livak K. J., Schmittgen T. D. (2001). Analysis of relative gene expression data using real-time quantitative PCR and the 2- ΔΔCT method. Methods 25 402–408. 10.1006/meth.2001.1262 [DOI] [PubMed] [Google Scholar]
- Luo Y., Guan Y. J., Huang Y. T., Li J., Li Z., Hu J. (2015). Single counts of radicle emergence provides an alternative method to test seed vigour in sweet corn. Seed Sci. Technol. 43 519–525. 10.15258/sst.2015.43.3.02 [DOI] [Google Scholar]
- Luo Z., Chen C., Xie J. (2011). Effect of salicylic acid treatment on alleviating postharvest chilling injury of ‘Qingnai’plum fruit. Postharvest Biol. Technol. 62 115–120. 10.1016/j.postharvbio.2011.05.012 [DOI] [Google Scholar]
- Maarouf B., Sajjad Y., Bazin J., Langlade N., Cristescu S. M., Balzergue S., et al. (2015). Reactive oxygen species, abscisic acid and ethylene interact to regulate sunflower seed germination. Plant Cell Environ. 38 364–374. 10.1111/pce.12371 [DOI] [PubMed] [Google Scholar]
- Mao X., Zhang H., Tian S., Chang X., Jing R. (2010). TaSnRK 2.4, an SNF1-type serine/threonine protein kinase of wheat (Triticum aestivum L.), confers enhanced multistress tolerance in Arabidopsis. J. Exp. Bot. 61 683–696. 10.1093/jxb/erp331 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maslak J., Baczek-Kwinta R., Oleksiewicz A., Grzesiak M. T., Grzesiak S. (2007). Seed germination, and growth and development of maize (Zea mays L.) seedlings in chilling conditions. Acta Physiol. Plant. 1 S82 10.1016/j.jplph.2016.01.012 [DOI] [Google Scholar]
- Mitchum M. G., Yamaguchi S., Hanada A., Kuwahara A., Yoshioka Y., Kato T., et al. (2006). Distinct and overlapping roles of two gibberellin 3-oxidases in Arabidopsis development. Plant J. 45 804–818. 10.1111/j.1365-313X.2005.02642.x [DOI] [PubMed] [Google Scholar]
- Mittler R. (2002). Oxidative stress, antioxidants and stress tolerance. Trends Plant Sci. 7 405–410. 10.1016/S1360-1385(02)02312-9 [DOI] [PubMed] [Google Scholar]
- Mittler R., Vanderauwera S., Gollery M., Van Breusegem F. (2004). Reactive oxygen gene network of plants. Trends Plant Sci. 9 490–498. 10.1016/j.tplants.2004.08.009 [DOI] [PubMed] [Google Scholar]
- Müller K., Linkies A., Vreeburg R. A., Fry S. C., Krieger-Liszkay A., Leubner-Metzger G. (2009). In vivo cell wall loosening by hydroxyl radicals during cress seed germination and elongation growth. Plant Physiol. 150 1855–1865. 10.1104/pp.109.139204 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noctor G., Foyer C. H. (1998). Ascorbate and glutathione: keeping active oxygen under control. Annu. Rev. Plant Biol. 49 249–279. 10.1146/annurev.arplant.49.1.249 [DOI] [PubMed] [Google Scholar]
- Olszewski N., Sun T., Gubler F. (2002). Gibberellin signaling biosynthesis, catabolism, and response pathways. Plant Cell 14 61–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pereira M. D., dos Santos Dias D. C. F., dos Santos Dias L. A., Araújo E. F. (2009). Primed carrot seeds water performance under stress temperature. Sci. Agric. 66 174–179. 10.1590/S0103-90162009000200005 [DOI] [Google Scholar]
- Qi W., Zhang L., Feng W., Xu H., Wang L., Jiao Z. (2015). ROS and ABA signaling are involved in the growth stimulation induced by low-dose gamma irradiation in Arabidopsis seedling. Appl. Biochem. Biotechnol. 175 1490–1506. 10.1007/s12010-014-1372-6 [DOI] [PubMed] [Google Scholar]
- Qin X., Zeevaart J. A. D. (2002). Overexpression of a 9-cis-epoxycarotenoid dioxygenase gene in Nicotiana plumbaginifolia increases abscisic acid and phaseic acid levels and enhances drought tolerance. Plant Physiol. 128 544–551. 10.1104/pp.010663 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qiu J., Wang R., Yan J., Hu J. (2005). Seed film coating with uniconazole improves rape seedling growth in relation to physiological changes under waterlogging stress. Plant Growth Regul. 47 75–81. 10.1007/s10725-005-2451-z [DOI] [Google Scholar]
- Rivas-San Vicente M., Plasencia J. (2011). Salicylic acid beyond defence: its role in plant growth and development. J. Exp. Bot. 62 3321–3338. 10.1093/jxb/err031 [DOI] [PubMed] [Google Scholar]
- Silva-Neta I. C., Pinho E., Veiga A., Pinho R., Guimaraes M., Caixeta F., et al. (2015). Expression of genes related to tolerance to low temperature for maize seed germination. Genet. Mol. Res. 14 2674–2690. 10.4238/2015.March.30.28 [DOI] [PubMed] [Google Scholar]
- Slesak I., Libik M., Karpinska B., Karpinski S., Miszalski Z. (2007). The role of hydrogen peroxide in regulation of plant metabolism and cellular signalling in response to environmental stresses. Acta Biochim. Pol. 54 39–50. [PubMed] [Google Scholar]
- Smith D. B., Johnson K. S. (1988). Single-step purification of polypeptides expressed in Escherichia coli as fusions with glutathione S-transferase. Gene 67 31–40. 10.1016/0378-1119(88)90005-4 [DOI] [PubMed] [Google Scholar]
- Stamm P., Ravindran P., Mohanty B., Tan E., Yu H., Kumar P. (2012). Insights into the molecular mechanism of RGL2-mediated inhibition of seed germination in Arabidopsis thaliana. BMC Plant Biol. 12:179 10.1186/1471-2229-12-179 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun J., Wu D., Xu J., Rasmussen S. K., Shu X. (2015). Characterisation of starch during germination and seedling development of a rice mutant with a high content of resistant starch. J. Cereal Sci. 62 94–101. 10.1016/j.jcs.2015.01.002 [DOI] [Google Scholar]
- Wang Y., Wen T., Hu J., Han R., Zhu Y., Guan Y., et al. (2013). Relationship between endogenous salicylic acid and antioxidant enzyme activities in maize seedlings under chilling stress. Exp. Agric. 49 295–308. 10.1017/S0014479712001329 [DOI] [Google Scholar]
- Xu S., Hu J., Li Y., Ma W., Zheng Y., Zhu S. (2011). Chilling tolerance in Nicotiana tabacum induced by seed priming with putrescine. Plant Growth Regul. 63 279–290. 10.1007/s10725-010-9528-z [DOI] [Google Scholar]
- Yamauchi Y., Ogawa M., Kuwahara A., Hanada A., Kamiya Y., Yamaguchi S. (2004). Activation of gibberellin biosynthesis and response pathways by low temperature during imbibition of Arabidopsis thaliana seeds. Plant Cell 16 367–378. 10.1105/tpc.018143 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang R., Yang T., Zhang H., Qi Y., Xing Y., Zhang N., et al. (2014). Hormone profiling and transcription analysis reveal a major role of ABA in tomato salt tolerance. Plant Physiol. Biochem. 77 23–34. 10.1016/j.plaphy.2014.01.015 [DOI] [PubMed] [Google Scholar]
- Zhang H., Zhang N., Yang R., Wang L., Sun Q., Li D., et al. (2014). Melatonin promotes seed germination under high salinity by regulating antioxidant systems, ABA and GA4 interaction in cucumber (Cucumis sativus L.). J. Pineal Res. 57 269–279. 10.1111/jpi.12167 [DOI] [PubMed] [Google Scholar]
- Zhang Y., Chen B., Xu Z., Shi Z., Chen S., Huang X., et al. (2014). Involvement of reactive oxygen species in endosperm cap weakening and embryo elongation growth during lettuce seed germination. J. Exp. Bot. 65 3189–3200. 10.1093/jxb/eru167 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


