Abstract
Background
Rapid population growth and catastrophic harvesting methods of wild medicinal plants especially trees, result in the exploitation of natural sources and its management is the need of the hour. Dashamoolarishta is an amalgam of roots of ten plants of a popular Ayurvedic FDC formulation consisting of the root of Premna latifolia Roxb. as one of its ingredients. Presently, their populations like many other trees are under threat due to extensive use of the roots by the herbal drug industry.
Objective
With an aim to conserve the biodiversity, a systematic study based on a rational approach by substituting root/root bark with alternative and renewable parts was conducted.
Materials and methods
The fingerprint profile together with anti-inflammatory and analgesic effect of different parts of the plant was established for comparison.
Results
The results based on chemical and biological study indicated close similarity between the roots and the leaves and suggest the possible use of latter over root/root bark.
Conclusion
The study proposes that the substitution of the root with alternate renewable parts of the same plant shall form the best strategy towards conservation of the trees like P. latifolia.
Keywords: Ayurveda, Dashamoolarishta, Premna latifolia Roxb., Conservation, Plant parts, Chemical and biological profile
Highlights
-
•
We examined the use of alternate plant parts as suitable means to conserve medicinal plants.
-
•
Possible use of renewable plant parts like leaves in place of root/bark/stem.
-
•
Chemical profiling was correlated with the pharmacological findings.
-
•
Observed results suggest leaf can be effectively used in place of root in herbal formulations.
-
•
The study signifies the importance of plant parts substitution based approach for effective conservation of medicinal plants.
1. Introduction
Drugs of natural origin are well known for their beneficial role in the health care system and continue to play a significant role in the treatment of many diseases worldwide. Majority of the population believes in traditional herbal medicine because of easy accessibility and lesser side effects. But regardless of their importance, medicinal plants are still misused with no concern of their conservation. Over-exploitation of medicinal plants is a result of speedy population growth and increasing urbanization which affects ecosystem and biodiversity especially for the slow growing plants like trees. Further, the illegal and indiscriminate harvesting methods of the medicinal plants result in the depletion of natural resources [1]. The different possible strategies available to conserve the natural resources are:
-
(i)
Establishment of more conservation areas and enforcement of laws against bark and root collection.
-
(ii)
Promoting awareness about plant diversity and its role in sustainable livelihood and healthcare.
-
(iii)
Large scale cultivation including those of slow growing plants.
-
(iv)
Whereever possible, use of alternative vegetative renewable plant parts such as leaves, young stems and fruits in place of bark and underground parts like root, rhizome etc.
There are a few reports focussing on the last suggestion of plant or plant parts substitution. To implement this policy, there is a dire need to do more case studies and create awareness about its practical usefulness towards conservation and sustainability of medicinal plants. The most reasonable perspective for carrying out a validated case study is by phyto-pharmacological evaluation of different plant parts and their relation with each other as a pre-requisite of this policy [2]. Very few publications relevant to major thrust of this manuscript have been published during the last fifteen years. The most comprehensive report came in the year 2000 when Zschocke et al. addressed this issue while working on four plants viz. Eucomis autumnalis (Mill.) Chitt, Siphonochilus aethiopicus (Schweif.) B.L. Burt, Ocotea bullata (Burch.) Baill., and Warburgia salutaris (G.Bertol.) Chiov. of South Africa. The authors demonstrated how the strategy of plant part substitution can be carried out in the laboratory and also showed that the potential for plant part substitution is highly plant specific [2]. Most of the subsequent reports were also from South Africa on Pelargonium sidoides DC. (aerial parts in place of underground roots and tubers) [3], [4], Hypoxis hemerocallidea Fisch., C.A.Mey. & Ave-Lall. (leaf in place of corms) [5], Curtisia dentata (Burm.f.) C.A.Sm. (leaf in place of stem bark) [6], and one of the reports on W. salutaris (G.Bertol.) Chiov. is published simply on phytochemical basis [7]. The concept of only phytochemical approach has been recently studied in Aegle marmelos (L.) Correa (stem in place of root) [8]. Of the two relevant studies from India, Venkatasubramanian et al. reported that Cyperus rotundus L. (Musta) can be substituted in place of Aconitum heterophyllum Wall. ex Royle (Ativisha) on the concept of both phytochemical and pharmacological approach [9]. The second report provides useful information wherein Ayurvedic practitioners use Cryptocoryne spiralis (Retz.) Fisch ex Wydler (Naattu Atividayam) and Cyperus scariosus R. Br. (Nagaramusta) as substitutes for A. heterophyllum Wall. ex Royle (Ativisha) and C. rotundus L. (Musta), respectively [10]. These studies demand an equal awareness coming from other countries as well and strongly emphasize the need of evaluating plant part substitution both chemically and biologically.
Hence, in the present study, an indigenous tree Premna latifolia Roxb. (Verbenaceae) was selected which is one of the ten ingredients of an Ayurvedic formulation “Dashamoolarishta”. Dashamoolarishta is popularly regarded as immune modulator and general restorative tonic in geriatrics and for women having problem with conception and pregnancy [11]. The methanolic extract of leaves is reported to have anti-inflammatory [12], anticalculogenic [13], and antifeedant activity [14]. An all-encompassing literature survey publicized that a lot of phytochemical work has been done on root bark and leaves from which iridoids [15], sesquiterpenoids [16], diterpenoids [17], furanoid [18], and flavonoids [19], have been reported.
The plant is under threat due to extensive use of its roots in herbal formulations and requires an obvious necessity to re-examine the fixed-dose-combination used in Ayurveda following suitable scientific approach. The roots of the plant are collected by destructive method which reduces the opportunity for rejuvenation and affects plant demography. A number of possible strategies are proposed from time to time for plants under-threat to curtail overharvesting, among which the most promising is the use of renewable plant parts as alternative to bark, roots and rhizomes for medicinal purposes. With an aim to accomplishing this objective and to conserve the biodiversity, a replacement of root/root barks of P. latifolia Roxb. with its alternative and renewable vegetative parts (young stems, leaves) was studied chemically and pharmacologically. In this study, we determined how different or close various parts of P. latifolia Roxb. are based on the TLC fingerprinting together with anti-inflammatory and analgesic activities. The results are in a definitive direction to promote the use of aerial parts as a suitable initiative towards plant conservation and ensuring sustainable harvesting.
2. Materials and methods
2.1. Plant material
The plant material comprising of different parts of P. latifolia was collected during the month of July 2009 from Hamirpur district, Himachal Pradesh, India. The identity of the plant sample was confirmed on the basis of detailed study of taxonomic characters and by comparison with authentic sample available at Medicinal Plants Garden of University Institute of Pharmaceutical Sciences, Panjab University, Chandigarh. The identity of the sample was further confirmed by NISCAIR vide Ref-no. NISCAIR/RHMD/Consult/-2010-11/1456/54. A specimen of the plant has been deposited in the Museum-cum-Herbarium of University Institute of Pharmaceutical Sciences, Centre for Advanced Studies, Panjab University, Chandigarh, India, under voucher no 1465.
2.2. Preparation of extracts
All extractions were carried out at room temperature by macerating 100 g of coarsely powdered plant material with 500 ml of methanol till exhaustion. The methanolic extract of whole root (11 g), root bark (8.5 g), root wood (7 g) young stem (9.5 g) and leaf (10 g) was obtained by evaporating the solvent under reduced pressure in rotary vacuum evaporator and used for chemical and pharmacological studies.
2.3. Statistical analysis
Statistical analysis was performed using Jandel Sigma Stat statistical software. Significance of difference between two groups was evaluated by two-way analysis of variance (ANOVA) followed by Bonferroni-test. Results were considered significant if p values were ≤ 0.05.
2.4. Chemical studies
2.4.1. Comparative chemical profiling
The comparative TLC chromatograms and fingerprint profiles were developed using pre-coated silica gel F254 plates [E. Merck (India) Ltd., alumina base, and 0.2 mm thickness]. A large number of various combinations of solvent systems were used during the present studies and the best resolution was obtained in a solvent system of toluene:ethyl acetate:acetic acid (7.4:2.4:0.2). Extracts were applied as bands using Camag Linomat 5 available in the laboratory. The running distance was kept at 8 cm and anisaldehyde sulphuric acid reagent was used as derivatizing agent followed by heating at 110 °C for 5 min or till the bands developed colour. TLC fingerprint profiles were recorded as images under UV at 254 and 366 nm before spray and under white light after derivatization on Camag Reprostar fitted with D × A 252 16 mm camera.
2.5. Pharmacological studies
2.5.1. Animals and experimental design
Animals were procured from Central Animal House of Panjab University, Chandigarh, India and kept under controlled environmental conditions with room temperature (22 ± 2 °C) and humidity (50 ± 10%). The studies were approved by Institutional Animal Ethics Committee (IAEC) vide ref no 978 dated 08.02.2010 and animals were used according to the CPCSEA (Committee For the Purpose of Control and Supervision of Experimentation on Animals) guidelines. Experiments were performed on male Wistar rats (200–220 g) and LACA mice (20–30 g). Animals were randomized into nine groups each consisting of six animals with free access to food and water ad libitum. The 12 h light and dark cycle was maintained throughout the study. Animals were acclimatized for one week prior to the commencement of the experiments. The doses were administered orally with the help of an oral cannula fitted on a tuberculine syringe. Anti-inflammatory activity and analgesic activity were evaluated by carrageenan induced paw oedema model and tail flick method, respectively [20]. The experimental animals were divided into groups of control, standard and test of six animals each. The control group animals received only vehicle, the standard group animals received ibuprofen [21] or pentazocine [22] for comparison and the test group animals received the test materials (extracts).
2.6. Evaluation of anti-inflammatory activity
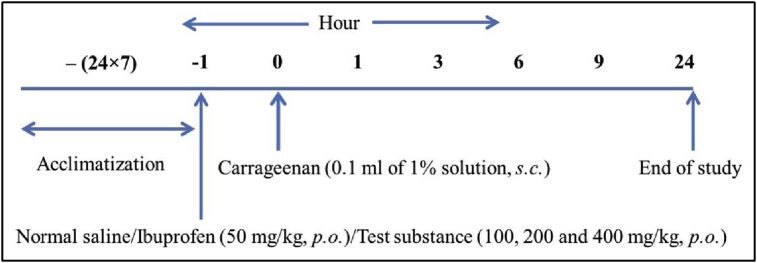
Anti-inflammatory activity was determined using carrageenan-induced paw oedema in rats [23]. The animals were divided into different groups of control, standard (ibuprofen) and test of six animals each. All animals were starved overnight. Acute oedema was induced in left hind paw of rats by injecting 0.1 ml of freshly prepared working solution of carrageenan (1%) under plantar region of left hind paw. The control group received only the vehicle while standard and test groups received ibuprofen and test substances, respectively. The paw was marked with ink at the level of the lateral malleolus and immersed in solution up to this mark for noting the paw volume. The complete experimental design is outlined in Scheme I.
Scheme I.
Experimental design for anti-inflammatory activity.
The increase in paw volume was measured using Plethysmometer (water displacement, UGO-Basile, Varese, Italy) at 0, 1, 3, 6, 9 and 24 h after carrageenan challenge. Percent protection of paw oedema was calculated from the following formula:
| % Protection of paw oedema = [(Vt − V0)control − (Vt − V0)treated/(Vt − V0)control] × 100 |
(V0) = volume of the paw before treatment with carrageenan.
(Vt) = volume of paw after carrageenan administration at 1, 3, 6, 9 and 24 h.
2.7. Evaluation of analgesic activity
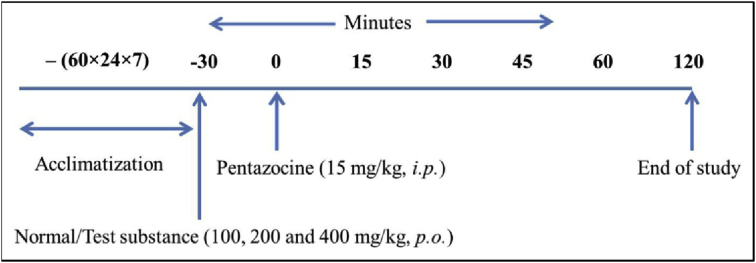
Analgesic activity was determined by tail flick method [24] using analgesiometer (Imcorp, Ambala, India). Mice were divided into different groups of six animals each. All the animals in the respective groups were individually exposed to analgesiometer maintained at 55 °C. The base line tail flick latency averaged 2–4 s in all animals. The tail withdrawal from the heat (flicking response) was taken as the end point. Pentazocine (15 mg/kg) was used as a standard drug for comparison. Cut off time was set as 10 s for animals. The response of the drug was observed at time intervals of 0, 15, 30, 45, 60 and 120 min after drug administration and expressed in terms of maximum protection effect (MPE), which was calculated from the following formula:
| % Maximum protection effect = [Reaction time−Basal reading/Cut off time−Basal reading] × 100 |
| Cut off time = 10 s |
The complete experimental design is outlined in Scheme II.
Scheme II.
Experimental design for analgesic activity.
3. Results
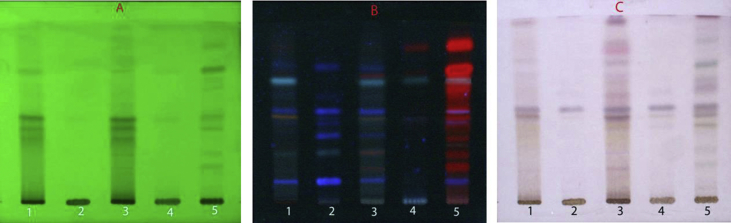
The TLC fingerprinting of methanolic extract of the whole root, root bark, root wood, young stem and leaf of P. latifolia Roxb. was developed for comparative chemical profiling. The best resolution of all the plant parts was obtained in a solvent system of toluene:ethyl acetate:acetic acid (7.4:2.4:0.2) and the recorded fingerprint profiles are shown in Fig. 1(A–C). The different plant parts showed distinct patterns under UV-light at 254 and 366 nm as well as after derivatizing with anisaldehyde-sulphuric acid reagent. The chromatogram of the whole root was characterized by fifteen bands. The nine major bands at Rf 0.18, 0.35, 0.46, 0.50, 0.56, 0.66, 0.70, 0.84 and 0.86 occurred as dark spots under UV254, blue and red spots under UV366 and turned pink/light pink after derivatization. The chromatogram of the root bark showed close similarity to that of the whole root under white light with seven corresponding bands at Rf 0.18, 0.35, 0.46, 0.50, 0.70, 0.84 and 0.86. In case of the root wood, only one major band appeared at Rf 0.50 along with two minor bands as observed under white light. The young stem showed three major and two minor spots under white light in which the bands at Rf 0.46, 0.50 and 0.56 matched to that of the whole root. The TLC chromatogram of the leaf extract was largely dominated by chlorophyll pigment and its degradation products (red fluorescence under UV 366 nm). However, its chromatogram was analogous in profile to that of the whole root both under UV254 and after derivatization, with six major bands appearing at matching Rf 0.18, 0.40, 0.46, 0.50, 0.66 and 0.70.
Fig. 1.
Comparative TLC fingerprint profile of methanolic extract of different parts of P. latifolia Roxb. 1: Root bark, 2: Root wood, 3: Whole root, 4: Young stem, 5: Leaf, (A) and (B): under UV254 and 366 nm, (C): under white light after derivatization with anisaldehyde-sulphuric reagent.
The comparative anti-inflammatory and analgesic activity of the methanolic extract of different plant parts was carried out at a dose of 100, 200 and 400 mg/kg. In the paw oedema and tail flick models, all the parts showed statistically significant activity (p ≤ 0.05) and were found to be dose dependent. In the carrageenan induced paw oedema model, various plant parts at different doses showed significant decrease in paw volume at variable time intervals as shown in Table 1. The whole root and leaf showed same level of inhibition of oedema (48%) at 6 h at a dose of 400 mg/kg in comparison to 59% inhibition shown by ibuprofen while young stem was less active.
Table 1.
Anti-inflammatory activity of different parts of P. latifolia Roxb. in carrageenan induced paw oedema model.
| Treatment | Dose mg/kg | Paw volume (mL) at different time intervals (h) ± S.E.M (percentage of maximum effect) |
|||||
|---|---|---|---|---|---|---|---|
| 0 h | 1 h | 3 h | 6 h | 9 h | 24 h | ||
| Control | Vehicle | 0.129 ± 0.03 | 0.402 ± 0.02 | 0.632 ± 0.01 | 0.674 ± 0.01 | 0.586 ± 0.01 | 0.450 ± 0.01 |
| Ibuprofen | 50 | 0.049 ± 0.02a | 0.212 ± 0.01a (40.29) | 0.302 ± 0.01a (49.7) | 0.274 ± 0.01a (58.62) | 0.270 ± 0.02a (51.46) | 0.245 ± 0.01a (38.86) |
| Whole root | 100 | 0.032 ± 0.01a | 0.261 ± 0.02a (16.11) | 0.419 ± 0.01ab (23.06) | 0.442 ± 0.02ab (24.77) | 0.402 ± 0.01ab (19.03) | 0.315 ± 0.02ab (11.83) |
| 200 | 0.043 ± 0.01a | 0.262 ± 0.02a (19.78) | 0.397 ± 0.02ab (29.62) | 0.384 ± 0.03ab (37.43) | 0.355 ± 0.03ab (31.72) | 0.297 ± 0.02a (20.87) | |
| 400 | 0.078 ± 0.01 | 0.287 ± 0.02ab (23.44) | 0.394 ± 0.01ab (37.17) | 0.359 ± 0.02ab (48.44) | 0.377 ± 0.01ab (34.57) | 0.329 ± 0.02ab (21.8) | |
| Stem | 100 | 0.028 ± 0.01a | 0.269 ± 0.01a (11.72) | 0.455 ± 0.02a (15.10) | 0.464 ± 0.02ab (20.0) | 0.405 ± 0.03ab (17.5) | 0.306 ± 0.02a (13.4) |
| 200 | 0.065 ± 0.02a | 0.290 ± 0.01ab (17.5) | 0.438 ± 0.02ab (25.84) | 0.426 ± 0.01ab (33.76) | 0.358 ± 0.02ab (35.88) | 0.318 ± 0.01ab (21.18) | |
| 400 | 0097 ± 0.01 | 0.315 ± 0.02ab (20.14) | 0.460 ± 0.02abc (27.83) | 0.446 ± 0.02abc (35.96) | 0.368 ± 0.02ab (40.70) | 0.344 ± 0.02ab (23.05) | |
| Leaf | 100 | 0.038 ± 0.01a | 0.274 ± 0.01a (13.55) | 0.442 ± 0.01ab (19.68) | 0.469 ± 0.02ab (20.91) | 0.394 ± 0.02ab (22.10) | 0.324 ± 0.03ab (10.90) |
| 200 | 0.077 ± 0.01 | 0.298 ± 0.02ab (19.04) | 0.446 ± 0.02ab (26.64) | 0.443 ± 0.01ab (32.84) | 0.40 ± 0.01ab (29.32) | 0.348 ± 0.02ab (15.57) | |
| 400 | 0.108 ± 0.02 | 0.317 ± 0.02ab (23.44) | 0.431 ± 0.02ab (35.78) | 0.392 ± 0.02ab (47.88) | 0.389 ± 0.02ab (38.51) | 0.373 ± 0.01ab (17.45) | |
Values are expressed as mean ± SEM. Statistical analysis was done by two-way ANOVA followed by Bonferroni-test (p < 0.05). a when compared with control group, b when compared with ibuprofen and c when compared with whole root.
In the tail flick method, all the test samples at an oral dose of 100, 200 and 400 mg/kg showed significant (p ≤ 0.05) increase in latency time at different time intervals (15, 30, 45, 60 and 120 min), which differed as compared to baseline values. The whole root and leaf significantly increased percentage reaction time by 43 and 42% at 400 mg/kg dose level, as compared to 62% shown by the pentazocine group Table 2.
Table 2.
Average response time (in seconds) of different parts of P. latifolia Roxb. and standard in the tail flick model.
| Group | Dose (mg/kg) | Mean basal reading ± S.E.M | Mean reaction time (sec) ± S.E.M (percentage reaction time) |
||||
|---|---|---|---|---|---|---|---|
| 15 min | 30 min | 45 min | 60 min | 120 min | |||
| Control | Vehicle | 2.23 ± 0.15 | 2.30 ± 0.17 | 2.40 ± 0.12 | 2.27 ± 0.18 | 2.47 ± 0.15 | 2.31 ± 0.14 |
| Pentazocine | 15 | 3.11 ± 0.38 | 4.85 ± 0.21 (25.25) | 7.35 ± 0.29 (61.54) | 6.5 ± 0.46 (49.20) | 6.05 ± 0.36 (42.71) | 4.84 ± 0.19 (25.21) |
| Whole root | 100 | 2.95 ± 0.45 | 3.46 ± 0.38a (7.23) | 4.50 ± 0.46a (22.0) | 4.70 ± 0.45 (24.82) | 4.30 ± 0.22 (19.14) | 4.07 ± 0.44 (15.88) |
| 200 | 2.6 ± 0.39 | 3.58 ± 0.45a (13.24) | 4.26 ± 0.30a (22.42) | 5.44 ± 0.46 (38.37) | 4.4 ± 0.42a (24.3) | 3.96 ± 0.35 (18.3) | |
| 400 | 2.38 ± 0.15 | 3.46 ± 0.38a (14.17) | 4.55 ± 0.46a (28.47) | 5.67 ± 0.33 (43.17) | 4.93 ± 0.22 (33.46) | 4.0 ± 0.43 (21.25) | |
| Stem | 100 | 2.14 ± 0.40 | 2.40 ± 0.21a (3.35) | 2.30 ± 0.31ab (13.86) | 4.03 ± 0.35ab (24.04) | 3.49 ± 0.35ab (17.17) | 2.81 ± 0.32a (14.75) |
| 200 | 2.60 ± 0.42 | 3.25 ± 0.40 (8.78) | 4.10 ± 0.35a (20.27) | 4.35 ± 0.42a (23.64) | 4.65 ± 0.39 (27.70) | 3.72 ± 0.46 (17.43) | |
| 400 | 2.0 ± 0.25 | 3.04 ± 0.16a (13.0) | 3.83 ± 0.31a (22.8) | 4.11 ± 0.40ab (26.3) | 4.57 ± 0.35a (32.12) | 3.44 ± 0.36a (18.0) | |
| Leaf | 100 | 2.51 ± 0.63 | 3.13 ± 0.34a (8.22) | 3.71 ± 0.44ac (16.02) | 4.51 ± 0.44a (26.7) | 3.96 ± 0.34a (19.35) | 3.81 ± 0.43 (17.35) |
| 200 | 2.7 ± 0.17 | 3.53 ± 0.21 (11.36) | 4.35 ± 0.36a (22.6) | 5.29 ± 0.41 (35.47) | 4.43 ± 0.45a (23.69) | 3.93 ± 0.44 (16.84) | |
| 400 | 2.5 ± 0.41 | 3.55 ± 0.38 (14.0) | 4.77 ± 0.38a (30.26) | 5.62 ± 0.37c (41.6) | 5.01 ± 0.44 (33.46) | 4.03 ± 0.36 (20.4) | |
Values are expressed as mean ± SEM. Statistical analysis was done by two-way ANOVA followed by Bonferroni-test (p < 0.05). a when compared with pentazocine, b when compared with whole root and c when compared with whole stem.
4. Discussion
TLC is an established tool of choice for handling complex task involving herbal drug standardization for its simplicity, flexibility, reliability and cost efficient separation. The comparative TLC analysis revealed there is significant similarity among the different plant parts under UV and in day light as observed by the intensity of the major bands. The analogous fingerprint profile of the roots and leaves indicated the presence of similar type of compounds. In the carrageenan induced paw oedema and tail flick model, both whole root and leaf exhibited nearly same level of protection. The stem too showed significant anti-inflammatory and analgesic activities as compared to the standard drug but lesser than whole root and leaf.
An assessment of the similarities and differences by chemical and pharmacological methods of various parts of a plant acts as a significant tool towards the suggested strategies of the plant part substitution for conservation of biodiversity. A careful look at the results obtained in the present investigations clearly indicated that the leaf and root are comparable chemically and biologically and the root can be safely substituted or replaced with the leaf on the basis of similarities in chemical profile together with the biological activity.
Regardless of all the progress in modern healthcare systems, plants are still an essential source of medicinal preparations, drug discovery and development. Most of the plants are collected from the wild sources and majority of the developing countries believe in herbal remedies. The international agencies such as World Health Organization, World Wildlife Fund, International Union for Conservation of Nature, International Plant Genetic Resources Institute and many national agencies play an increasing role in the medicinal plant conservation and cultivation. The present time therefore offers a unique opportunity for implementing the scientific research methods and policies to regulate conservation, cultivation, processing and marketing of medicinal plants which will further bridge the gap between affordable healthcare and conservation of diversity.
5. Conclusion
The study suggests that the substitution of root with leaf, a renewable plant part, shall form the best strategy towards conservation of bioresources and sustainable bioprospecting especially of medicinal plants.
Conflict of interest
None declared.
Footnotes
Peer review under responsibility of Transdisciplinary University, Bangalore.
References
- 1.Cunningham A.B. Development of a conservation policy on commercially exploited medicinal plants: a case study from southern Africa. In: Heywood V., Synge H., Akerele O., editors. Conservation of medicinal plants. Cambridge University Press; Cambridge; UK: 1991. pp. 337–358. [Google Scholar]
- 2.Zschocke S., Rabe T., Taylor J.L.S., Jager A.K., Staden J.V. Plant part substitution-a way to conserve endangered medicinal plants? J Ethnopharmacol. 2000;71:281–292. doi: 10.1016/s0378-8741(00)00186-0. [DOI] [PubMed] [Google Scholar]
- 3.Lewu F.B., Grierson D.S., Afolayan A.J. The leaves of Pelargonium sidoides may substitute for its roots in the treatment of bacterial infections. Biol Conserv. 2006;128:582–584. [Google Scholar]
- 4.Moyo M., Aremu A.O., Gruz J., Subrtova M., Szucova L., Dolezal K. Conservation strategy for Pelargonium sidoides DC: phenolic profile and pharmacological activity of acclimatized plants derived from tissue culture. J Ethnopharmacol. 2013;149:557–561. doi: 10.1016/j.jep.2013.07.021. [DOI] [PubMed] [Google Scholar]
- 5.Katerere D.R., Eloff J.N. Anti-bacterial and anti-oxidant activity of Hypoxis hemerocallidea (Hypoxidaceae): can leaves be substituted for corms as a conservation strategy? S Afr J Bot. 2008;74:613–616. [Google Scholar]
- 6.Shai L.J., McGaw L.J., Eloff J.N. Extracts of the leaves and twigs of the threatened tree Curtisia dentate (Cornaceae) are more active against Candida albicans and other microorganisms than the stem bark extract. S Afr J Bot. 2009;75:363–366. [Google Scholar]
- 7.Drewes S.E., Crouch N.R., Mashimbye M.J., de Leeuw B.M. A phytochemical basis for the potential use of Warburgia salutaris (pepper-bark tree) leaves in the place of bark. S Afr J Sci. 2001;97:383–386. [Google Scholar]
- 8.Sulaiman C.T., Balachandran I. Plant part substitution for medicinal use in Aegle marmelos-a phytochemical approach. J Trop Med Plants. 2013;14:19–22. [Google Scholar]
- 9.Venkatasubramanian P., Kumar S.K., Nair V.S. Cyperus rotundus, a substitute for Aconitum heterophyllum: studies on the Ayurvedic concept of Abhava Pratinidhi Dravya (drug substitution) J Ayurveda Integr Med. 2010;1:33–39. doi: 10.4103/0975-9476.59825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nagarajan M., Kuruvilla G.R., Kumar K.S., Venkatasubramanian P. Pharmacology of Ativisha, Musta and their substitutes. J Ayurveda Integr Med. 2015;6:121–133. doi: 10.4103/0975-9476.146551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sarin Y.K. Council of Scientific and Industrial Research and Indian Council of Medical Research; New Delhi: 1996. Illustrated manual of herbal drugs used in Ayurveda; p. 2. [Google Scholar]
- 12.Mahire N.B., Tote M.V., Jain A.P., Undale V.R., Bhosle A.V. Anti-inflammatory effect of Premna latifolia leaves. Pharmacologyonline. 2009;3:929–937. [Google Scholar]
- 13.Aravindakshan C., Jayanti B.N. Effect of Premnalatifolia Roxb. and Imperata arundinacea Cyril. on in vitro oxalate crystal growth. Ind J Clin Biochem. 1996;11:42–45. [Google Scholar]
- 14.Kumar A., Tamta M.L., Negi N., Chandrasekhar K., Negi D.S. Phytochemical investigation and antifeedant activity of Premna latifolia leaves. Nat Prod Res. 2011;25:1680–1686. doi: 10.1080/14786419.2010.511620. [DOI] [PubMed] [Google Scholar]
- 15.Rao C.H.B., Vijaykumar E.K.S., Vijayalakshmi K.V. Iridoids from Premna latifolia Roxb. Planta Med. 1981;41:80–83. doi: 10.1055/s-2007-971680. [DOI] [PubMed] [Google Scholar]
- 16.Rao C.H.B., Subbaraju G.V., Gopalakrishna P. Chemical examination of Premna species: part VIII-spirosesquiterpenes from Premna latifolia Roxb. var. cuneata. Indian J Chem. 1982;21B:267–268. [Google Scholar]
- 17.Rao C.H.B., Suseela K., Subbaraju G.V. Chemical examination of Premna species: part IX-pimaradienols from Premna latifolia Roxb. var. mollissima. Indian J Chem. 1984;23B:177–179. [Google Scholar]
- 18.Rao C.H.B., Vijaykumar E.K.S. Premnalatin, a new furanoid from leaves of Premna latifolia Roxb. Indian J Chem. 1980;19B:240–241. [Google Scholar]
- 19.Rao C.H.B., Subbaraju G.V. New flavone glycosides from the leaves of Premna latifolia Roxb. Curr Sci. 1981;50:180–181. [Google Scholar]
- 20.Vogel G.H. Analgesic, anti-inflammatory and anti-pyretic activity. In: Vogel W.H., Scholkens B.A., Sandow J., Muller G., Vogel W.F., editors. Drug discovery and evaluation of pharmacological assays. Springer Publications; Germany: 2002. pp. 725–756. [Google Scholar]
- 21.Gautam R., Srivastava A., Jachak S.M., Saklani A. Anti-inflammatory, cyclooxygenase (COX)-2, COX-1 inhibitory and antioxidant effects of Dysophylla stellata Benth. Fitoterapia. 2010;81:45–49. doi: 10.1016/j.fitote.2009.07.004. [DOI] [PubMed] [Google Scholar]
- 22.Clarke G., Wright D.M. A comparison of analgesia and suppression of oxytocin release by opiates. Br J Pharmacol. 1984;83:799–806. doi: 10.1111/j.1476-5381.1984.tb16235.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Karan M., Sarup P., Suneja V., Vasisht K. Effect of traditional purification processes of guggulu on carrageenan induced paw oedema in rats. J Pharm Biomed Sci. 2012;21:1–5. [Google Scholar]
- 24.Karan M., Sarup P., Vasisht K. Evaluation of antioxidant and antinociceptive potential of raw and purified guggulu. J Pharm Biomed Sci. 2013;31:1150–1158. [Google Scholar]