Abstract
Background
The use of herbal plant extracts in wound healing is known through decades, but it is necessary to provide scientific data through reverse pharmacology.
Objective
The aim of the present study is to find the mechanism behind the healing of wounds using in vitro and in vivo assays.
Material and methods
The study was designed to determine proliferation and mobilization of fibroblast and keratinocytes at the site of injury, angiogenesis at the site of healing and reduction in oxidative stress while healing. In our earlier studies it was observed that herbal extract of Vitex negundo L. (VN), Emblica officinalis Gaertn (EO), and Tridax procumbens L. (TP) showed rapid regeneration of skin, wound contraction and collagen synthesis at the site of injury in excision wound model. In the present study the cell mobilization was monitored in the scratch assay on L929 fibroblastic cell line and HaCaT keratinocytes cell line under the influence of aqueous plant extracts and its formulation. This formulation was also assessed for its angiogenic potential using CAM assay. Study was carried out to probe synergistic effect of polyherbal formulation using excision model in rat.
Results
The formulation was found to contain high amount of flavonoids, tannins and phenols which facilitate wound healing. At 20 μg/ml concentration of formulation, significant increase in tertiary and quaternary vessels were observed due to angiogenic potential of formulation. Formulation at the concentration of 3 μg/ml and 5 μg/ml showed significant mobilization of keratinocytes and fibroblasts respectively at the site of injury. Polyherbal formulation showed rapid regeneration of skin and wound contraction. Biochemical parameters like hydroxyproline, hexosamine and collagen turnover was increased in test drug treated animals as compared to untreated, whereas antioxidants such as catalase and GSH were increased significantly and decreased amount of tissue MDA was observed.
Conclusion
Polyherbal formulation prepared from the plant extracts accelerates wound healing process by proliferation and mobilization of fibroblast and keratinocytes, and angiogenesis at the site of injury. It also shows fast contraction of wound with its beneficial improvement in tissue biochemical and antioxidant parameters.
Keywords: Wound healing, Polyherbal formulation, Scratch assay, Fibroblast, Keratinocytes, Angiogenesis, Excision wound
1. Introduction
Wound healing is a process of reconstruction of injured skin, coordinated by interaction of various epithelial and mesenchymal cells with cytokines, chemokines and growth factors [1]. Keratinocyte growth factor (KGF) is a paracrine growth factor synthesized by fibroblasts, endothelial cells, smooth muscle cells and dendritic epidermal T-cells [2]. KGF also known to induce mitogen activated protein activation and directly acts as angiogenic factor in vitro [3]. Natural plant products play major role in proliferation of fibroblasts and keratinocytes [4]. Plant products were reported to contain growth factors, cell signaling molecules and cell adhesion molecules [5], [6]. In our previous studies aqueous extract of Vitex negundo L. (VN), Emblica officinalis Gaertn (EO) and Tridax procumbens L. (TP) showed rapid regeneration of skin along with collagen turnover [7], [8], [9].
Leaves of VN are useful in toothache, inflammation, leucoderma, skin-ulcers, rheumatoid arthritis, and the methanolic extract of leaves was studied for wound healing activity [10]. Leaves of Tridax have effect on blood pressure, heart rate, immunomodulation, anti-microbial properties, and arrests bleeding from cuts and bruises [11], [12].
EO is reported to have antimicrobial, antioxidant, anti-inflammatory, analgesic and antipyretic, adaptogenic, hepatoprotective, antitumor and antiulcerogenic activities [13]. The properties found in VN, EO and TP may be supporting characteristics as wound healing properties which were useful as selection criteria of plants for wound healing activity.
In the present studies we have used in vitro assays to evaluate efficacy of polyherbal formulation in terms of mobilization of fibroblasts and keratinocytes to the site of injury and their angiogenic potential as well as its in vivo efficacy.
2. Material and methods
2.1. Cell lines, drugs and reagents
L929 mouse skin fibroblasts and HaCaT (Human keratinocytes) cell lines were kindly supplied by Cell Repository of National Centre for Cell Science (NCCS) Pune, India. Cipladine, a standard used for effective wound healing was obtained from Cipla Ltd, Mumbai, India. Day zero fertilized chicken eggs were obtained from Venkateshwara Hatcheries, Pune, India. Media and reagents used in this study were of analytical grade and procured from Sigma-Aldrich, St. Louis, USA.
2.2. Animals
Eighteen Wistar rats of either sex, weight range of 150–200 gms were procured from National Toxicology Centre, Pune. All the animals were provided with water and food ad libitum. Rats were housed in standard laboratory condition as per CPCSEA norms. Animals were divided into three groups (control, standard, and polyherbal formulation treated group) of 6 animals each.
2.3. Collection and authentication of plant materials
Leaves of V. negundo, bark of E. officinalis and whole plant of T. procumbens were collected from Kem village, Maharashtra, India, Pune. The herbarium was made and authentication was carried out at Botanical Survey of India (BSI), Pune. No TAY3.BSI/WRC/Tech/2011. One copy of the herbarium specimens were submitted to APT Research Foundation, Pune, India.
2.4. Extraction of the plants
The leaves, stem and bark of respective plants were cleaned and shade dried prior to extraction. The dried plant material was then ground to powder using electric blender. The plant powders obtained were extracted with water at 80° C to obtain aqueous extracts using Soxhlet apparatus. These extracts were then concentrated in a Rota evaporator under reduced pressure and constant temperature at 60° C. and dried to powder and their extractive yields were measured [14].
2.5. Quantification of total flavonoids, phenols and tannins
Total flavonoids [15], phenols [16] and tannins [17] from aqueous extracts were determined using standard protocols.
2.6. Determination of antioxidant potential
The antioxidant effect of the extracts were studied using ABTS (2, 2-azino-bis-3 ethyl benzathiazoline-6-sulphonic acid) radical cations (ABTS+) decolourisation assay according to protocol of Shirwaikar et al., [18]. The concentration equivalent to ascorbic acid was determined from the standard curve of ascorbic acid. The percent inhibition was calculated.
2.7. Preparation of polyherbal formulation
The aqueous extracts of three plants were mixed in equal proportion, to obtain the best formulation in order to increase the acceptability and adoptability of herbal medicine for wound healing [19], [20]. Liquid paraffin 20% was added in 30% emulsifying wax and 50% white soft paraffin (oily phase) was kept warm. Warm aqueous phase i.e 30% (emulsifying ointment) 1% chlorocrysol and 69.9% double distilled water were added in warm oily phase and stirred gently until cooled [21], [22]. The cream was homogenized using mortar and pestle. It was stored in wide mouth glass bottle and placed in cool place.
2.8. Quality control parameters of formulation
Quality control of the formulation at different concentrations was carried out to evaluate the pH, spreadability, and extrudability. Acute dermal toxicity for individual aqueous extracts of three plants was carried out and was found safe at limit dose (2000 mg/kg). The pH of various formulations was determined by using digital pH meter. One gram of cream was dissolved in 100 ml of distilled water and stored for 2 h. The measurement of pH of each formulation was done in triplicate. The cream was placed in between the slides under the direction of certain load. The spreadability was expressed in terms of time in seconds taken by two slides to slip off from cream. The extrudability of cream formulations was determined in terms of weight in grams required to extrude a 0.5 cm ribbon of cream in 10 s. External characters of developed cream formulation were also noted, such as color, odor, smoothness and grittiness.
2.9. Angiogenesis activity
Angiogenetic potential of polyherbal formulation was determined using chick embryo chorioallantoic membrane assay (CAM) described by Surekha et al. [23]. It is a unique assay to study the blood vessels sprouting in response to angiogenic agent. The surfaces of freshly laid fertilized chicken eggs were wiped with 70% ethanol. On 4th day a small window of one centimeter square was made at the blunt end to puncture the air sac. Windows were sealed with durapore sealing tape and kept horizontally in the incubator till day nine. On 10th day Whatman filter paper rings treated with various concentrations of drug formulations (5 ng, 10 ng, and 20 ng were placed in each embryo). Eggs treated with equal volume of phosphate buffered saline (PBS) served as normal control. After further incubation for 72 h, CAMs were excised from the eggs and fixed in 4% ice cold paraformaldehyde/PBS for 30 min. The membranes were placed on glass slides and image of control and treated CAMs were captured for comparative analysis. Increase in number of primary, secondary and tertiary vessels shall be the main criteria for analysis of angiogenesis effect [24].
2.10. Scratch assay with fibroblast and keratinocytes cell line
The fibroblast L929 (1 × 104 cells) and keratinocytes HaCaT (2 × 104 cells) were seeded in 24-well cell culture plate. Linear scratch was made in confluent cell monolayer using 200 μl pipette tip. Cell debris were washed out with plain DME medium [25]. Formulation made of three plant extracts was diluted to 5 μg/ml. The standard drug Cipladine at 5 μg/ml used as positive control. After addition of formulations and standard drug, images of cellular gap were captured periodically on Nikon Eclipse TS100 inverted microscope. The cellular gap in the cell monolayer was measured at different times.
2.11. Excision wound model
The animals were anaesthetized by injecting intramuscularly ketamine hydrochloride and xylazine in 1:1 concentration. The dorsal fur of the animals was shaved with trimmer and impression was made on dorsal region with circular stainless stencil using picric acid.
Using toothed forceps and pointed scissors circular excision wound of 300–400 mm2 and 2 mm depth were made by cutting out layer of skin from the shaven area. In the control group, the wound was left open [26], whereas, the standard drug Cipladine [27] and test drug (polyherbal formulation) applied topically on excised wound.
Wound areas were measured by tracing the wound on transparency sheet with permanent marker and by using millimeter based graph paper on days 0, 3, 6, 9, 12 and 15 for all groups. Percentage reduction in wound area with respect to initial wound area [28].
At the end of the study the skin was divided into three parts. One part was used for histopathology, the second part was used for biochemical parameters and third part was used for evaluation of antioxidant enzymes.
2.11.1. Biochemical parameters
2.11.1.1. Estimation of Hydroxyproline
Healed tissue was excised and dried in glass vials in a 110 °C oven for 48 h. 5 mg of lyophilized sample was hydrolyzed with 5 ml of 6 N HCl at 110 °C for 18–20 h in a sealed tube for estimation of hydroxyproline as per method described by Agarwal [29].
2.11.1.2. Estimation of collagen
The hydroxyproline content may be converted to its equivalent collagen through multiplication by the factor 7.46 [30].
2.11.1.3. Estimation of hexosamine
It was carried out as per Nithya et al. [31]. Briefly, 5 mg of lyophilized tissue sample was hydrolyzed with 5 ml of 2 N HCl at 110 °C for 6–7 h., evaporated to dryness and the residue was dissolved in known amount of water. The solution was treated with 1 ml of freshly prepared 2% acetyl acetone in 0.5 M sodium carbonate and boiled for 15 min. After cooling 5 ml of 95% ethanol, 1 ml of Ehrlich's reagent was added and mixed thoroughly. The purple red color developed was read after 30 min at 530 nm spectrophotometrically.
2.11.2. Antioxidant enzymes
2.11.2.1. Catalase
To the 10% tissue homogenate in pH 7, a detergent e.g. (1% Triton X-100) was added and it was further diluted with phosphate buffer pH-7 (1:100). Estimation of catalase was carried out as described by Aebi et al. [32]; Ramazan et al. [33].
2.11.2.2. Reduced glutathione assay
A tissue homogenate was prepared with 0.5 g of the skin tissue with 2.5 ml of 5% TCA. The precipitated protein was centrifuged at 1000 rpm for 10 min. The supernatant (0.1 ml) was used for the estimation of GSH [34].
2.11.2.3. Lipid peroxidation assay
After addition of 1 ml tissue homogenate to TCA–TBA HCl, all tubes were vortexed for few seconds in boiling water bath, cooled to room temperature and centrifuged for 15 min. Supernatants were pipetted out in cuvette and OD was measured at 535 nm against blank [35].
2.11.3. Histopathology
The skin tissues were then processed by undergoing a series of steps that include fixation, dehydration, clearing, wax impregnation, embedding & subsequent sectioning on the microtome [36], [37], with slight modifications. Five micrometer thick sections were stained with haematoxylin and eosin. All slides were observed under microscope.
2.12. Statistical methods
Results obtained in vivo excision model expressed as Mean ± SD and were compared with the corresponding control group by one way ANOVA test for assessing statistical significance.
3. Results
3.1. Quantification of flavonoids, phenols and tannins
The total flavonoid content in the aqueous formulation was found to be 76.80 ± 0.4 mg/gm when compared with standard flavonoid i.e. quercetin. The phenol content in the formulation was 154.3 ± 3.2 mg/gm equivalent to standard gallic acid whereas; tannins were 366.9 ± 1.4 mg/gm equivalent to standard catechin.
3.2. ABTS radical scavenging assay
The aqueous extract was found to scavenge the superoxides generated by photoreduction of riboflavin in dose-dependent manner. The extracts were investigated in comparison with the known antioxidant such as ascorbic acid. The formulation made by combining three plant extracts showed 69.9% inhibition at 250 μg/ml. Increasing concentrations of formulation showed increasing order of ABTS radical scavenging activity.
3.3. Quality control of formulation
The polyherbal formulation passed all the quality control parameters such as pH, spreadability-time in seconds, extrudability, color, odour, smoothness, and grittiness. Cream formed was consistent and nicely applicable to skin.
3.3.1. HPTLC analysis of catechin
Catechin was used as standard tannin and observed at 455 nm. Concentration used for HPTLC was 50 μg/ml. Linearity was showed by catechin using volume 2 μl, 4 μl, 6 μl, 8 μl, 10 μl. Tannins present in polyherbal formulation were analyzed by comparing to catechin, and observations were carried out in duplicate. Area observed for formulation showed 1441.6.
3.3.2. HPTLC analysis of flavonoids
Quercetin was used as standard flavonoid and observed at 254 nm. Concentration used for HPTLC was 50 μg/ml. Linearity was showed by quercetin using volume 2 μl, 4 μl, 6 μl, 8 μl, 10 μl. Flavonoids present in polyherbal formulation were analyzed by comparing to quercetin. Observations were carried out in duplicate. Area observed for formulation showed 21817.2.
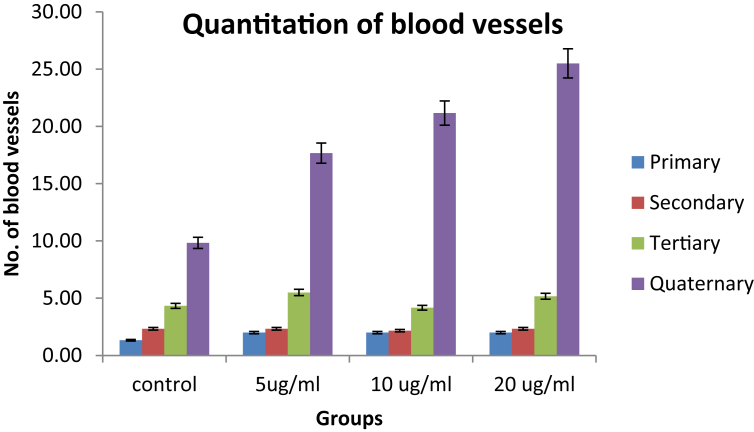
3.4. Angiogenesis activity
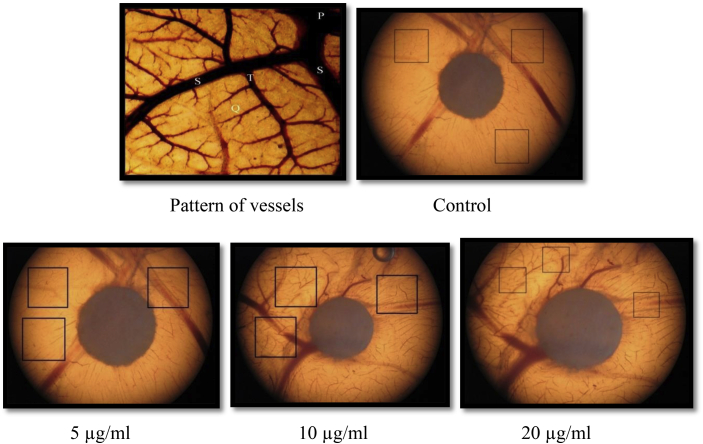
The angiogenesis activity of polyherbal formulation was evaluated by CAM assay. The quantification was carried out in chick embryo by counting the primary vessels and vessels sprouted from primary branches e.g. secondary, tertiary and quaternary branches manually in selected areas (N = 5). Comparison for quantification of blood vessels in control-treated, test-treated embryos was carried out (Fig. 1). At 20 μg/ml concentration of formulation, significant increase in tertiary and quaternary vessels angiogenesis was observed as compared to control group p < 0.01. Fig. 2 indicates pattern for normal angiogenesis as well as pattern for treated embryos with control, 5 μg/ml, 10 μg/ml, 20 μg/ml.
Fig. 1.
Quantification of blood vessels.
Fig. 2.
Blood vessels in egg embryo in control group and formulation treated embryos at 5 μg, 10 μg and 20 μg.
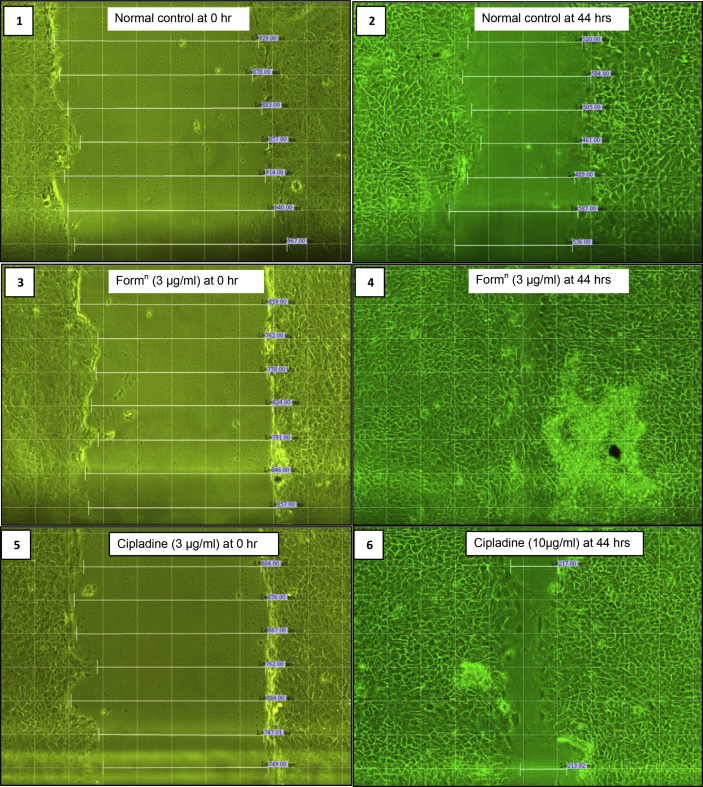
3.5. Scratch assay to determine mobilization of fibroblast and keratinocytes
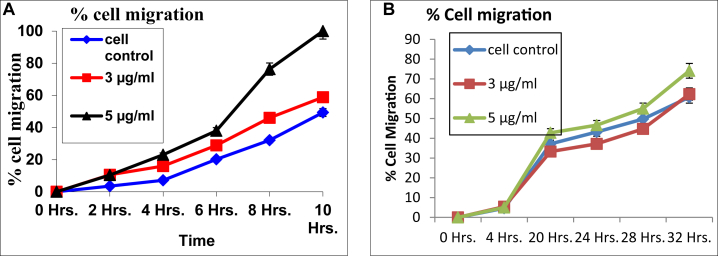
In the present study, L929 (fibroblast) and HaCaT (keratinocytes) cells were used in scratch assay. Using image analyzer, the time required to close the gap in the confluent cell monolayer in presence of different concentrations of formulation were studied. The time taken to close the gap was plotted and compared with untreated cell culture. The experimental results showed that formulation at 5 μg/ml of concentration closed the gap in the scratch of fibroblasts more efficiently as compared to control group p < 0.05. Fig. 3A showed % fibroblast cell migration at specific time interval and comparison of control with 3 μg/ml and 5 μg/ml of formulation. However keratinocytes are fastidious cells and normally take 48 h to close the gap in cell monolayer. In the present study, formulation at the concentration of 3 μg/ml showed significant mobilization of keratinocytes and closed the gap as compared to the control without addition of formulation. Fig. 3B indicates % keratinocyte cell migration at specific time interval and comparison between control, 3 μg/ml and 5 μg/ml of formulation.
Fig. 3.
A: Cell migration using scratch assay in L929 cell line. B: Cell migration using scratch assay in HaCaT cell line
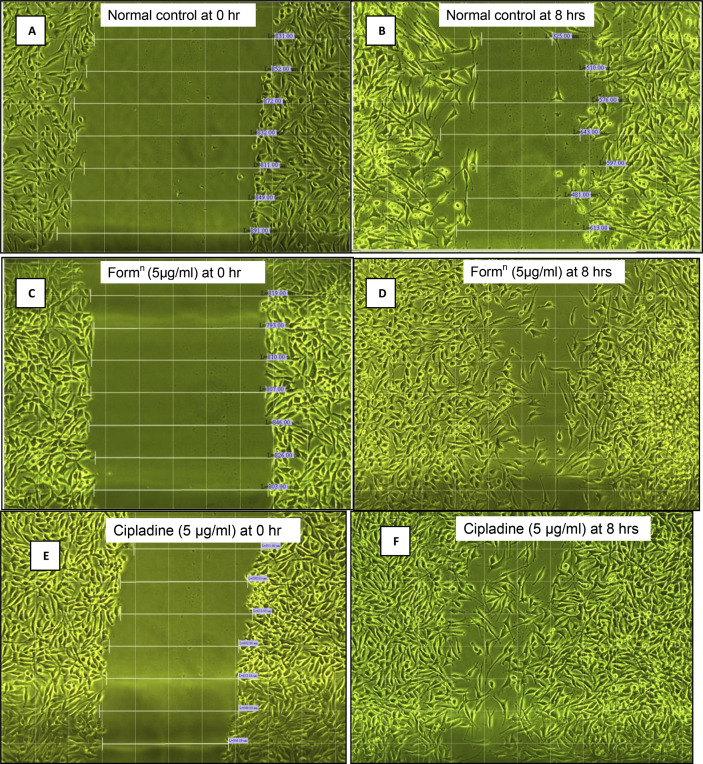
Photographs indicating comparative cell migration in non-treated, standard drug-treated and formulation-treated is showed in Fig. 4 for L929 (fibroblast) cells at 0 h and 8 h time interval and Fig. 5 for HaCaT (keratinocyte) cells at 0 h and 44 h time interval to heal gap completely.
Fig. 4.
Scratch assay on L929 cells (Fibroblast cell line).
Fig. 5.
Scratch assay HaCaT (keratinocytes cell line).
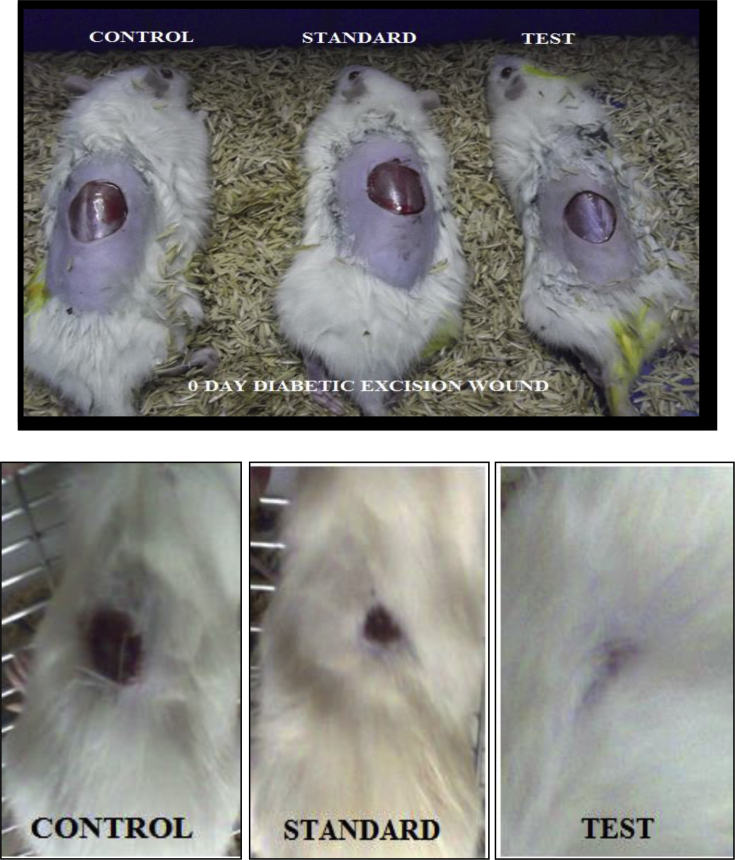
3.6. Excision wound model
Control rats showed delayed contraction rate of the circular excised wound when compared with the standard drug Cipladine-treated animal as well as test formulation-treated animals (p < 0.001) (Table 1).
Table 1.
Effect of the polyherbal formulation on excision wound contraction in mm2
| Groups | 0 day | 3rd day | 6th day | 9th day | 12th day | 15th day |
|---|---|---|---|---|---|---|
| Control | 388.50 ± 7.06 | 325.00 ± 5.83 | 292.67 ± 5.54 | 141.33 ± 8.09 | 60.50 ± 6.16 | 45.33 ± 7.92 |
| Std. | 405.33 ± 7.92 | 226.67 ± 6.25∗∗∗ | 149.83 ± 9.83∗∗∗ | 61.83 ± 6.43∗∗∗ | 23.33 ± 3.67∗∗∗ | 6.50 ± 2.43∗∗∗ |
| Polyherbal formulation | 389.17 ± 7.39 | 298.50 ± 4.97∗∗∗ | 204.67 ± 4.46∗∗∗ | 115.00 ± 6.32∗∗∗ | 43.17 ± 5.53∗∗∗ | 3.00 ± 1.79∗∗∗ |
Values are expressed as Mean ± SD (N = 6). Statistical analysis was done by one way ANOVA.
p < 0.05 when compared with control.
The aqueous extract formulation showed 99.2% wound contraction which was greater than standard drug which showed 98.2% contraction of wound at the end of 15 days.
Both the test and standard drug treated animals showed significant reduction in wound size as compared with control group animals which showed 88.3% reduction (Fig. 6).
Fig. 6.
Healing pattern in excision wound at day 0 and day 15
3.6.1. Biochemistry of wound healing
The aqueous extract formulation showed an increase in hydroxyproline, collagen and hexosamine levels per gram of dried regenerated tissue compared to control group indicating efficient wound healing. Hydroxyproline values of control animals were 32.3 ± 3.5 μg/g, standard drug treated animals showed 48.9 ± 3.9 μg/g and test drug treated animals showed 63.6 ± 5.6 μg/g of tissue.
Tissue hexosamine was observed 6.5 ± 2.6 mg/gm in control group, 21.40 ± 1.30 mg/gm in standard drug-treated group and 28.4 ± 2.2 mg/gm in test drug-treated animals, Collagen synthesis is always directly proportional to hexosamine; control group showed 240.7 ± 26.1 μg/gm of tissue, standard drug-treated animals showed 365.0 ± 29.3 μg/gm whereas 474.5 ± 42.2 μg/g of tissue collagen in test drug-treated group was observed (Table 2). Our observations suggest that the polyherbal formulation showed better and efficient wound healing properties than individual plant extracts as evident.
Table 2.
Effect of polyherbal formulation biochemistry of wound healing
| Groups | Hydroxyproline (μg/gm) | Collagen (μg/gm) | Hexosamine (mg/gm) |
|---|---|---|---|
| Control | 32.3 ± 3.5 | 240.7 ± 26.1 | 6.5 ± 2.6 |
| Std. drug | 48.9 ± 3.9*** | 365.0 ± 29.3*** | 21.4 ± 1.3*** |
| Polyherbal formulation | 63.6 ± 5.6*** | 474.5 ± 42.2*** | 28.4 ± 2.2*** |
Values are expressed as Mean ± SD (N = 6). Statistical analysis was done by one way ANOVA.
∗∗∗p < 0.001 when compared with Control.
3.6.2. Antioxidant activity in wound healing
The polyherbal formulation showed significant increase in catalase, and GSH levels as compared to the control group. Control group showed 1.82 ± 0.6 U/gm, standard drug-treated animals showed 3.88 ± 0.6 U/gm and aqueous formulation-treated animals showed 5.44 ± 0.8 U/gm of catalase in dry tissue. GSH values observed in control group, standard drug group and test drug groups were found to be 173.3 ± 37.3 mol/gm, 331.7 ± 42.5 mol/gm and 564.6 ± 46.4 mol/gm respectively. Whereas the tissue MDA values were higher in the control group 256.6 ± 24.5 U/gm, but standard drug-treated group showed 109.9 ± 9.6 U/gm as well as the formulation- treated group showed 83.8 ± 5.6 U/gm i. e. the significant reduction in levels of MDA noted using polyherbal formulation (p < 0.001) (Table 3).
Table 3.
Effect of polyherbal formulation on tissue antioxidant parameters
| Groups | Catalase (U/gm) | MDA (U/gm) | GSH (moles/gm) |
|---|---|---|---|
| Control | 1.82 ± 0.6 | 256.6 ± 24.5 | 173.3 ± 37.3 |
| Std. drug | 3.88 ± 0.6∗∗∗ | 109.9 ± 9.6∗∗∗ | 331.7 ± 42.5∗∗∗ |
| Polyherbal formulation | 5.44 ± 0.8∗∗∗ | 83.8 ± 5.6∗∗∗ | 564.6 ± 46.4∗∗∗ |
Values are expressed as Mean ± SD (N = 6). Statistical analysis was done by one way ANOVA.
∗∗∗p < 0.001 when compared with Control.
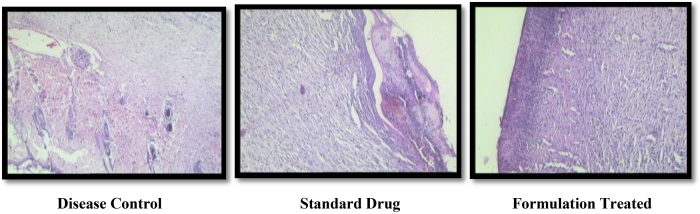
3.6.3. Histopathology using regenerated skin tissue
Neovascularisation, re-epithelialisation of epidermis and sub-epidermal cells were seen in regenerated tissue of polyherbal formulation treated animal. Also fibroblastic proliferation was observed at the site of wound healing. Adjoining skin in standard and test drug treated animals showed normal epidermis with presence of increased amount of collagen. When wound was completely healed, fibroblastic proliferation and vascular proliferation covered with granulation tissue was also observed in test and standard drug treated animals.
Fibrous proliferation seen under normal epidermis dipper down showed collagen fibers. Large area of scab formation with entrapped polymorphs beneath the scab. Fibroblastic and vascular proliferation with scanty mononuclear cell infiltrate was observed in disease control (non-treated) animals (Fig. 7).
Fig. 7.
Histopathology using regenerated skin tissue.
4. Discussion
A process of wound healing comprises of three phases i.e. inflammation, angiogenesis and collagen deposition. An angiogenesis refers to the formation of new capillaries formed as band like structures from pre-existing vessels adjacent to the wound. Dose-dependant increase in blood vessels was evident by CAM assay indicating active and effective angiogenesis activity in chick embryos treated with the polyherbal formulation. Epidermal keratinocytes undergo differentiation in response to several stimuli to form the confined envelope that contributes the barrier function of the skin. The predominant cell populations in mammalian skin are fibroblasts and keratinocytes. Several in vitro studies utilize either or both of these cell types as effective tools to directly visualize cellular interaction [38], [39]. In this study, it was observed that L929 fibroblasts and HaCaT cells mobilize towards the artificially created cell injury under the influence of plant extract formulation at different speed. Keratinocytes were found to be fastidious as compared to L929 fibroblasts according to their inherent characteristics.
Phytochemical analysis showed presence of flavonoids, phenols and tannins in the formulation. Tannins are phenolic compounds that typically act as astringent and are found in a variety of plant products used in wound healing. The astringent property is responsible for wound contraction and accelerates rate of epithelialization at the granulation formation and scar remodeling phases [40]. Therapeutic potential of a tannic acid cross-linked collagen scaffolds is reported earlier and demonstrated significant effect in wound closure and wound healing rate [41].
The present formulation showed significant wound contraction as compared to control untreated group at the end of 15 days. For healing of wounds, collagen is an important constituent of extra cellular matrix. Collagen synthesis is always directly proportional to hydroxyproline.
In the present study, hydroxyproline levels in newly formed tissue were found to be significantly increased in polyherbal formulation treated animals as compared to control group. Synthesis of collagen formation accelerated in newly formed tissue indicating increased collagen turnover after treatment with formulation i.e. 200% over control and 130% over standard drug.
The conventional assays to determine efficacy of plant products for wound healing comprises of painful invasive procedures in animal models. In vitro assays described in this study enable us to screen large number of plant products having antioxidant, cell mobilization and angiogenic properties essential for wound healing. It was reported earlier that wound healing and antioxidant properties co-exist in plant products [42]. Most often wound healing processes can be aided by the presence of antioxidants.
Thus minimum essential parameters of wound healing properties of any herbal preparation could be screened using in vitro assays described in the present study, and unnecessary usage of experimental animals could be avoided.
5. Conclusion
Polyherbal formulation prepared from the plant extracts (V. negundo, E. officinalis and T. procumbens) accelerates wound healing process by proliferation and mobilization of fibroblast and keratinocytes, and promotes angiogenesis at the site of injury.
Source of support
Project was self funded.
Conflict of interest
None declared.
Acknowledgement
Contributors thank Dr. S. Ghaskadbi and Dr. Surekha K. for their help in carrying out CAM assay. Contributors also thank to National Toxicology Centre for providing their lab facility.
Footnotes
Peer review under responsibility of Transdisciplinary University, Bangalore.
References
- 1.Martin P., Leibovich S.J. Inflammatory cells during wound repair: the good, the bad and the ugly. Trends Cell Biol. 2005;15:599–607. doi: 10.1016/j.tcb.2005.09.002. [DOI] [PubMed] [Google Scholar]
- 2.Pierce G.F., Yanagihara D., Klopchin K., Danilenko D.M., Hsu E., Kenney W.C. Stimulation of all epithelial elements during skin regeneration by keratinocyte growth factor. J Exp Med. 1994;179:831–840. doi: 10.1084/jem.179.3.831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Paul G., Ushma S., Olga V.V., Benilde J., Christopher M.W., Ralph J.P. Keratinocyte growth factor induces angiogenesis and protects endothelial barrier function. J Cell Sci. 1999;112:2049–2057. doi: 10.1242/jcs.112.12.2049. [DOI] [PubMed] [Google Scholar]
- 4.Tao S., Phil M.M., Simon C., Mike H., Rod S., Sheila M.N. An integrated systems biology approach to understanding the rules of keratinocyte colony formation. J R Soc Interface. 2007;4(17):1077–1092. doi: 10.1098/rsif.2007.0227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Obara K., Sumi K., Fukuda H. The use of multiple transcription starts causes the dual targeting of Arabidopsis putative monodehydro ascorbate reductase to both mitochondria and chloroplasts. Plant Cell Physiol. 2001;43:697–705. doi: 10.1093/pcp/pcf103. [DOI] [PubMed] [Google Scholar]
- 6.Thomas E., Stefan K., Kerstin P., Michael A., Rolf R., Herbert B. Phytochemistry and pharmacogenomics of natural products derived from traditional chinese medicine and chinese materia medica with activity against tumor cells. Mol Cancer Ther. 2008;7:152–161. doi: 10.1158/1535-7163.MCT-07-0073. [DOI] [PubMed] [Google Scholar]
- 7.Talekar Y.P., Das B., Paul T., Talekar D.Y., Apte K.G., Parab P.B. Wound healing potential of Vitex negundo Linn in experimental animals. Int J Pharm Pharm Sci. 2012;4(4):543–546. [Google Scholar]
- 8.Talekar Y.P., Das B., Paul T., Talekar D.Y., Apte K.G., Parab P.B. Wound healing activity of aqueous and ethanolic extract of the bark of Emblica officinalis in Wistar rats. Inven Rapid Planta Act. 2012;4:1–5. [Google Scholar]
- 9.Talekar Y.P., Das B., Paul T., Talekar D.Y., Apte K.G., Parab P.B. Evaluation of wound healing potential of aqueous and ethanolic extracts of Tridax procumbens Linn. in Wistar rats. Asian J Pharm Clin Res. 2012;5(4):141–145. [Google Scholar]
- 10.Roosewelt C., Vincent S., Sujith K., Darwin C.R. Wound healing activity of methanolic extract of Vitex negundo leaves in albino Wistar rats. J Pharm Res. 2011;4(8):2553–2555. [Google Scholar]
- 11.Salahdeen H.M., Yemitan O.K., Alada A.R. An Effect of aqueous leaf extract of Tridax procumbens on blood pressure and heart rate in rats. Afr J Biomed Res. 2004;7:27–29. [Google Scholar]
- 12.Udupa A.L., Kulkarni D.R., Udupa S.L. Effect of Tridax procumbens extracts on wound healing. Int J Pharmacogn. 1995;33(1):37–40. [Google Scholar]
- 13.Gaire B.P., Subedi L. Phytochemistry, pharmacology and medicinal properties of Phyllanthus emblica Linn. Chin J Integr Med. 2015;2:1–8. doi: 10.1007/s11655-014-1984-2. [DOI] [PubMed] [Google Scholar]
- 14.Sharma M.C., Sharma S. Phytochemical and pharmacological screening of combined Mimosa pudica Linn and Tridax procumbens for in vitro antimicrobial activity. Int J Microbiol Res. 2010;1:171–174. [Google Scholar]
- 15.Lobo R., Sodde V., Dashora N., Gupta N., Prabhu K. Quantification of flavonoid and phenol content from Macrosolen parasiticus (L.) danser. J Nat Prod Plant Res. 2011;1:96–99. [Google Scholar]
- 16.Habila J.D., Bello I.A., Dzikwi A.A., Musa H., Abubakar N. Total phenolics and antioxidant activity of Tridax procumbens Linn. Afr J Pharma Pharmacol. 2010;4:123–126. [Google Scholar]
- 17.Butler L.G., Price M.L., Brothertod J.E. Vanillin assay for proanthocyanidins (condensed tannins): modification of the solvent for estimation of the degree of polymerization. J Agri Food Chem. 1982;30:1087–1089. [Google Scholar]
- 18.Shirwaikar A., Prabhu K.S., Punitha I.S.R. In vitro antioxidant studies of Sphaeranthu indicus (Linn) Ind J Expt Biol. 2006;44:993–996. [PubMed] [Google Scholar]
- 19.Ramasamy T.N., Jesuthankaraj G.N., Arunagiri C., Suneera L., Melda S., Divya D. Evaluation of antibacterial, antioxidant and wound healing properties of seven traditional medicinal plants from India in experimental animals. Asian Pac J Trop Biomed. 2012:1245–1253. [Google Scholar]
- 20.Ishaque S., Rizwani G.H., Shareef H., Khursheed R. Formulation and pharmaceutical evaluation of polyherbal capsule (Femitex-SP 4) for treating menorrhagia. Int J Pharma Pharm Sci. 2011;3:149–154. [Google Scholar]
- 21.Pawar A., Gaud R.S. Modern dispensing pharmacy. Career Publ. 2009;3:256–261. [Google Scholar]
- 22.Jain N.K., Gupta G.D. vol. 1. Pharma Book Syndicate; 2008. p. 235. (Modern Dispensing Pharmacy). [Google Scholar]
- 23.Surekha K.L., Waghchoude M., Ghaskadbi S. Enhancement of angiogenesis by a 27 KDa Lectin from perivitelline fluid of horseshoe crab embryos through upregulation of VEGF and its receptor. J Nat Prod. 2013;76:117–120. doi: 10.1021/np3005198. [DOI] [PubMed] [Google Scholar]
- 24.Zilberberg L., Shinkaruk S., Lequin O., Rousseau B., Hagedorn M., Costa F. Structure and inhibitory effects on angiogenesis and tumor development of a new vascular endothelial growth inhibitor. J Biol Chem. 2003;278:35564–35573. doi: 10.1074/jbc.M304435200. [DOI] [PubMed] [Google Scholar]
- 25.Chun-Chi L., Ann Y.P., Jun-Lin G. In vitro scratch assay: a convenient and inexpensive method for analysis of cell migration in vitro. J Nat Prot. 2007;2:329–333. doi: 10.1038/nprot.2007.30. [DOI] [PubMed] [Google Scholar]
- 26.Shenoy C., Patil M.B., Kumar R., Patil S. Preliminary phytochemical investigation and wound healing activity of Allium cepa Linn (liliaceae) Int J Pharm Pharm Sci. 2009;2:167–175. [Google Scholar]
- 27.Tayade P.M., Borde S.N., Chandrasekar N., Jagtap S.A., Joshi A.S. Evaluation of wound healing properties of Psoreliya corolifolia Linn. in diabetic rats. Pharmacologyonline. 2011;1:282–288. [Google Scholar]
- 28.Jain S., Jain N., Tiwari A., Balekar N., Jain D.K. Simple evaluation of wound healing activity of polyherbal formulation of roots of Ageratum conyzoides Linn. Asian J Res Chem. 2009;2:135–138. [Google Scholar]
- 29.Agarwal P.K., Singh A., Gaurav K., Goel S., Khanna H.D., Goel R.K. Evaluation of wound healing activity of extracts of plantain banana (Musa sapientum var. paradisiaca) in rats. Indian J Exp Biol. 2009;47:32–40. [PubMed] [Google Scholar]
- 30.Neuman R.E., Logan M.A. The determination of collagen and elastin in tissues. J Biochem. 1972;186:549–556. [PubMed] [Google Scholar]
- 31.Nithya V., Brinda P., Anand K.V. Wound healing activity of Leonotis nepetaefolia R. Br., in Wistar albino rats. Asian J Pharm Clin Res. 2011;4:23–36. [Google Scholar]
- 32.Aebi H. Catalase in vitro. In: Parker L., editor. vol. 105. Academic Press; New York: 1984. pp. 121–126. (Methods in enzymology). [Google Scholar]
- 33.Ramazan M., Kenan G., Fienol D., Fatih A. The effect of pre-injury supplementation with selenium or vitamin E on lipid peroxidation and antioxidant enzymes in burn injury. Turk J Med Sci. 2006;36(3):141–146. [Google Scholar]
- 34.Moron M.A., Mannervick B. Levels of glutathione, glutathione s-transferase activities in rat liver. Biochim Biophys Acta. 1979;582:67–78. doi: 10.1016/0304-4165(79)90289-7. [DOI] [PubMed] [Google Scholar]
- 35.Chandramohan G., Al-Numair K.S., Pugalendi K.V. Restoration of altered plasma, erythrocyte and liver anti oxidants levels by 3-hydroxymethyl xyliton in streptozotocin-diabetic rats. Int J Integr Biol. 2009;5:176–181. [Google Scholar]
- 36.Sadaf F., Saleem R., Ahmed M., Ahmad S.I., Navaid-Ul Z. Healing potential of cream containing extract of Sphaeranthus indicius on dermal wounds in Guinea pigs. J Ethnopharmacol. 2006;107:161–163. doi: 10.1016/j.jep.2006.02.022. [DOI] [PubMed] [Google Scholar]
- 37.Gadekar R., Saurabh K.S., Thakur G.S., Saurabh A. Study of formulation, characterisation and wound healing potential of transdermal patches of curcumin. Asian J Pharm Clin Res. 2012;5(4):225–230. [Google Scholar]
- 38.Oberringer M., Meins C., Bubel M., Pohlemann T. A new in vitro wound model based on the co-culture of human dermal microvascular endothelial cells and human dermal fibroblasts. Biol Cell. 2007;99(4):197–207. doi: 10.1042/BC20060116. [DOI] [PubMed] [Google Scholar]
- 39.Khorshid F., Ali S.S., Alsofyani T., Albar H. Plectranthus tenuiflorus (shara) promotes wound healing: in vitro and in vivo studies. Int J Bot. 2010;6(2):69–80. [Google Scholar]
- 40.Bin Li, Wang James H.C. Fibroblasts and myofibroblasts in wound healing: force generation and measurement. J Tissue Viab. 2011;20(4):108–120. doi: 10.1016/j.jtv.2009.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Natarajan V., Krithica N., Madhan B., Sehgal P.K. Preparation and properties of tannic acid cross-linked collagen scaffold and its application in wound healing. J Biomed Mater Res B Appl Biomater. 2013;101(4):560–567. doi: 10.1002/jbm.b.32856. [DOI] [PubMed] [Google Scholar]
- 42.Suntar I., Kupeli A.E., Nahar L., Satyajit D., Sarker S.D. Wound healing and antioxidant properties: do they coexist in plants. Fr Rad Antioxid. 2012;2(2):1–7. [Google Scholar]