Abstract
Aims/Introduction
Low aerobic capacity is a strong and independent predictor of all‐cause mortality in patients with metabolic syndrome (MetS). Here, we investigated the effects of pioglitazone treatment on whole‐body aerobic capacity and skeletal muscle energy metabolism in MetS patients.
Materials and Methods
A total of 14 male patients with MetS received oral pioglitazone 15 mg/day for 4 months. To assess whole‐body aerobic capacity, exercise testing with a bicycle ergometer was carried out before and after pioglitazone treatment. To assess skeletal muscle energy metabolism, intramyocellular lipid in the resting leg and high‐energy phosphates in the calf muscle during plantar‐flexion exercise were measured using 1proton‐ and 31phosphorus magnetic resonance spectroscopy, respectively.
Results
Pioglitazone significantly increased peak oxygen uptake (25.1 ± 4.9 mL/kg/min pretreatment vs 27.2 ± 3.9 mL/kg/min post‐ treatment, P < 0.05) and anaerobic threshold (12.7 ± 1.9 mL/kg/min pretreatment vs 13.6 ± 1.6 mL/kg/min post‐treatment, P < 0.05), although daily physical activity was comparable before and after the treatment. Intramyocellular lipid content was significantly reduced after pioglitazone treatment by 26%, indicating improved skeletal muscle fatty acid metabolism. Pioglitazone also significantly decreased the muscle phosphocreatine loss during exercise by 13%, indicating improved skeletal muscle high‐energy phosphate metabolism. Notably, the increase in anaerobic threshold; that is, submaximal aerobic capacity, closely correlated with the decrease in intramyocellular lipid content after pioglitazone treatment.
Conclusions
Pioglitazone significantly improved the MetS patients’ whole‐body aerobic capacity and skeletal muscle energy metabolism. The beneficial effect of pioglitazone on whole‐body aerobic capacity might be at least in part through improved fatty acid metabolism in the skeletal muscle.
Keywords: Clinical, Metabolic syndrome, Treatment drug
Introduction
Metabolic syndrome (MetS) is a multifactorial condition characterized mainly by obesity and insulin resistance, which increases the risk of the development of type 2 diabetes and cardiovascular disease. The worldwide prevalence of the MetS is increasing dramatically, leading to both medical and public health crises worldwide. Furthermore, the rapid growth of the numbers of individuals with type 2 diabetes worldwide over the past decade highlights the necessity of early pharmacological intervention to prevent type 2 diabetes and its complications, such as macrovascular and microvascular diseases1, 2.
Pioglitazone, an insulin‐sensitizing thiazolidinedione, is widely used for the treatment of type 2 diabetes. Thiazolidinediones are known to activate peroxisome proliferator‐activated receptor‐γ, which is distributed not only in adipose tissue but also in skeletal muscle, and plays an important role in fatty acid and glucose metabolism3. Among the various antidiabetic drugs, pioglitazone has a strong effect on insulin resistance, and is considered a potential candidate treatment for patients with MetS4.
Large clinical trials have shown that pioglitazone reduces the risk of the development of type 2 diabetes in patients with impaired glucose tolerance5, and reduces the risk of stroke and acute myocardial infarction in insulin‐resistant patients with a history of ischemic stroke or transient ischemic attack6.
Low aerobic capacity is a more powerful predictor of all‐cause mortality than other established risk factors for cardiovascular diseases in healthy individuals7. We previously showed that aerobic capacity is lowered in association with impaired skeletal muscle energy metabolism in MetS patients8, 9. Low aerobic capacity could independently increase the risks of cardiovascular diseases and death in obese and insulin‐resistant MetS patients10, 11. These findings support the importance of treatment to improve the aerobic capacity in patients with MetS.
The first‐line treatments for MetS are lifestyle interventions, including exercise and calorie restriction. However, most MetS patients cannot maintain high physical activity and/or their optimal bodyweight over a sustained period of time, and consequently they remain at high risk for developing type 2 diabetes and cardiovascular disease. Therefore, additional treatment to improve aerobic capacity is clinically beneficial for MetS patients.
We recently showed that pioglitazone increased aerobic capacity with improved skeletal muscle mitochondrial function in diet‐induced obese and insulin‐resistant mice12, which raises the possibility that pioglitazone can improve aerobic capacity in patients with MetS. However, to our knowledge, no study showing the effect of pioglitazone on aerobic capacity in humans has been published.
In the present study, therefore, we examined whether pioglitazone could improve the aerobic capacity and skeletal muscle energy metabolism in MetS patients. We also determined whether the effects of pioglitazone on aerobic capacity are associated with improved skeletal muscle energy metabolism.
Materials and Methods
Patients
A total of 14 male MetS patients who did not engage in habitual exercise and who were diagnosed with MetS according to the International Diabetes Federation criteria participated in the study. All 14 patients were in good health with no evidence of cardiovascular, hepatic or renal disease, as determined by medical history and physical examination including screening blood tests, electrocardiograms and cardiac ultrasounds before the study. A total of 10 of the 14 patients were treated with antihypertensive drugs: a calcium antagonist, β‐blocker, angiotensin‐converting enzyme inhibitor, angiotensin II receptor blocker or diuretic. One patient was also receiving statin therapy. These medications were not altered for at least 3 months before enrolment or during the study period. The present report is a part of a large study investigating the impairment of skeletal muscle energy metabolism in MetS, and thus, part of the data are from the same patients whose data were published previously, but in a different context8, 9. All patients gave written informed consent before the study, which was approved by the Medical Ethics Committee of Hokkaido University Hospital, and all investigations were carried out according to the guidelines in the Declaration of Helsinki.
Study design
The patients underwent blood tests to evaluate their insulin sensitivity and lipid profiles after a 10‐h overnight fast, followed by clinical and anthropometric measurements including body composition determined by an air displacement plethysmograph (BOD POD® Body Composition System; Life Measurement Instruments, Concord, California, USA), and 1proton (1H) ‐magnetic resonance spectroscopy (MRS) studies to measure the intramyocellular lipid (IMCL) content in the resting leg muscle.
The patients also underwent an exercise test with a bicycle ergometer to assess their aerobic capacity. 31Phosphorus (31P)‐MRS studies were carried out to assess the high‐energy phosphate metabolism in the leg muscle during exercise on another day. Each patient's daily physical activity was monitored by a pedometer with an accelerometry sensor (Lifecorder Plus; Suzuken, Nagoya, Japan) for at least 1 week before and after the pioglitazone treatment. The patients were instructed not to change any aspect of their lifestyle including diet and physical activity during the study period. Each patient received oral pioglitazone 15 mg/day for 4 months. After 4 months of pioglitazone treatment, the patients underwent the same tests to evaluate the effects of pioglitazone.
Systemic oxidative stress
Each patient's level of serum thiobarbituric acid reactive substances, which are lipid peroxides known as a marker of oxidative damage, were measured, as described13. We also measured the patients’ systemic anti‐oxidant defense capacity including serum thiols and enzymatic activities of superoxide dismutase, glutathione peroxidase and glutathione reductase, as described13.
Whole‐body aerobic capacity
Whole‐body aerobic capacity was assessed by respiratory gas analysis (Aeromonitor AE‐300S; Minato Medical Science, Osaka, Japan) with a bicycle ergometer. A ramp protocol of 25 watts/min (after a 3‐min warm‐up) was used for the exercise testing. The respiratory exchange ratio was calculated as the ratio of carbon dioxide production/oxygen uptake (VO2). For the measurement of their peak VO2, the patients were asked in advance to attain their symptom‐limited maximal point. As an index of perceived effort, the rating of perceived exertion was evaluated with the 10‐point Borg scale. The anaerobic threshold (AT) was determined by the V‐slope method14, except in one patient.
IMCL content in skeletal muscle
We measured IMCL content in the patients’ resting tibialis anterior muscle at the level of the muscle belly of the calf using 1H‐MRS, as described8, 9. The IMCL content from one of the 14 patients could not be measured because of technical difficulties.
High‐energy phosphate metabolism in skeletal muscle
Before the measurement of high‐energy phosphate metabolism in skeletal muscle, one‐repetition maximum (1‐RM) was determined, as described8, 9. The calf flexor muscle cross‐sectional area at the level of the muscle belly was also measured using magnetic resonance imaging. After the patient rested for ≥30 min, the high‐energy phosphate metabolism in the calf muscle was measured at rest and during a plantar flexion exercise with the patient in the supine position on the original apparatus equipped with a 1.5‐Tesla (T) whole‐body scanner system (Magnetom Vision VB33G; Siemens, Erlangen, Germany), using 31P‐MRS, as described8, 9. The exercise protocol was a constant load of 20% 1‐RM at the pace of 40 times/min for 4 min. Phosphocreatine (PCr) was standardized as (PCr)/([PCr] + [Pi]) on the basis of the notion that (PCr) + (Pi) is constant at rest and during exercise, where (PCr) indicates the concentration of PCr and (Pi) indicates the concentration of inorganic phosphate (Pi). In addition, the degree of PCr change (i.e., PCr loss) during exercise was calculated as PCr loss = standardized PCrrest − standardized PCrlowest, where PCrrest indicates the PCr level at rest and PCrlowest indicates the lowest PCr level during exercise.
Statistical analysis
Data are expressed as mean ± standard deviation. The values obtained before and after treatment were compared using paired t‐tests. We examined correlations by carrying out a linear regression analysis using Pearson's correlation coefficient. Statistical analyses were carried out using GRAPHPAD PRISM v5.01 (GraphPad Software, San Diego, California, USA), and significance was defined as P < 0.05.
Results
Patient characteristics
The patients’ bodyweight, body mass index, percent fat, lean body mass, waist circumference and blood pressure did not change after the pioglitazone treatment (Table 1). There was no significant change in the cross‐sectional area of the calf muscle after pioglitazone treatment (57.9 ± 9.5 cm2 before treatment vs 58.2 ± 7.4 cm2 after treatment).
Table 1.
Patient characteristics before and after pioglitazone treatment
| Before (n = 14) | After (n = 14) | |
|---|---|---|
| Age (years) | 52 ± 11 | – |
| Body weight (kg) | 77.5 ± 11.1 | 77.0 ± 10.3 |
| Body mass index (kg/m2) | 26.6 ± 3.3 | 26.4 ± 3.0 |
| Percent fat (%) | 28.0 ± 3.9 | 28.8 ± 4.2 |
| Lean body mass (kg) | 55.2 ± 7.9 | 54.6 ± 6.3 |
| Waist circumference (cm) | 94.1 ± 9.0 | 93.5 ± 8.0 |
| Systolic blood pressure (mmHg) | 143 ± 13 | 138 ± 15 |
| Diastolic blood pressure (mmHg) | 83 ± 10 | 83 ± 7 |
| Daily steps (steps/day) | 7617 ± 3871 | 6739 ± 2374 |
| MCC (kcal/day) | 259 ± 176 | 221 ± 92 |
Data are means ± standard deviation. MCC, movement‐related calorie consumption.
The patients’ daily physical activity was characterized by the number of steps taken and the movement‐related calorie consumption, which were both measured by a pedometer. These two parameters’ values were comparable before and after pioglitazone treatment (Table 1), suggesting that similar daily physical activity occurred during the study period.
The 4‐month pioglitazone treatment significantly reduced the patients’ fasting blood glucose, insulin, homeostasis model assessment of insulin resistance and triglyceride levels, whereas it did not change the levels of glycohemoglobin, high‐density lipoprotein cholesterol, low‐density lipoprotein cholesterol or free fatty acids (Table 2).
Table 2.
Blood biochemistry before and after pioglitazone treatment
| Before (n = 14) | After (n = 14) | |
|---|---|---|
| Fasting blood glucose (mmol/L) | 6.4 ± 1.0 | 5.9 ± 0.7* |
| Insulin (pmol/L) | 70 ± 60 | 38 ± 17* |
| HOMA‐IR | 3.8 ± 3.4 | 1.7 ± 0.8* |
| HbA1c (%) | 5.7 ± 0.6 | 5.6 ± 0.4 |
| HDL cholesterol (mmol/L) | 1.34 ± 0.25 | 1.43 ± 0.28 |
| LDL cholesterol (mmol/L) | 3.18 ± 0.71 | 3.52 ± 0.67 |
| Triglyceride (mmol/L) | 1.74 ± 0.94 | 1.14 ± 0.59* |
| Free fatty acids (g/L) | 0.17 ± 0.09 | 0.16 ± 0.10 |
Data are mean ± standard deviation. *P < 0.05 vs Before. HbA1c, glycohemoglobin; HDL, high‐density lipoprotein; HOMA‐IR, homeostasis model assessment of insulin resistance; LDL, low‐density lipoprotein.
Systemic oxidative stress
The pioglitazone treatment did not change the patients’ serum thiobarbituric acid reactive substances (16.5 ± 4.4 μmol/L before vs 15.4 ± 3.1 μmol/L after treatment). There were no significant changes in the systemic anti‐oxidant defense capacity including total thiols (8.4 ± 1.5 units/g protein vs 7.6 ± 2.5 units/g protein), superoxide dismutase activity (0.86 ± 0.22 units/g protein vs 0.86 ± 0.27 units/g protein), glutathione peroxidase activity (13.1 ± 1.7 units/g protein vs 13.5 ± 1.4 units/g protein) and glutathione reductase activity (0.99 ± 0.18 units/g protein vs 0.99 ± 0.16 units/g protein) from before to after pioglitazone treatment.
Whole‐body aerobic capacity
Pioglitazone significantly increased the patients’ peak VO2 (Figure 1a) and AT (Figure 1b), indicating that pioglitazone improved the MetS patients’ aerobic capacity. In addition, there were no significant changes in the patients’ peak respiratory exchange ratio, peak heart rate or rating of perceived exertion after the pioglitazone treatment (peak respiratory exchange ratio: 1.24 ± 0.10 before treatment vs 1.22 ± 0.11 after treatment; peak heart rate: 144 ± 26 b.p.m. before treatment vs 143 ± 23 b.p.m. after treatment; rating of perceived exertion: 7.3 ± 1.8 before treatment vs 7.8 ± 1.6 after treatment), suggesting that the effort at peak exercise was similar.
Figure 1.
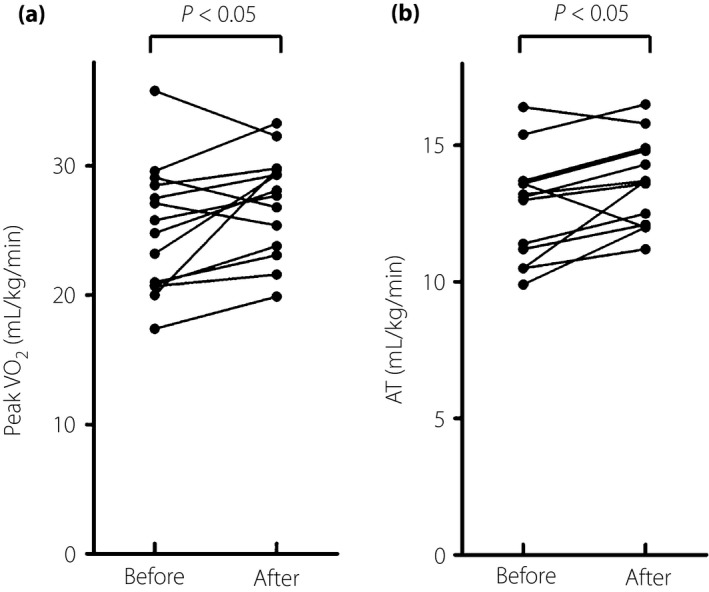
The metabolic syndrome patients’ aerobic capacity before and after pioglitazone treatment. (a) Peak oxygen uptake (VO2; n = 14). (b) Anaerobic threshold (AT; n = 13).
IMCL content in skeletal muscle
The IMCL content in the resting leg muscles of the MetS patients was significantly reduced after the pioglitazone treatment (Figure 2a).
Figure 2.
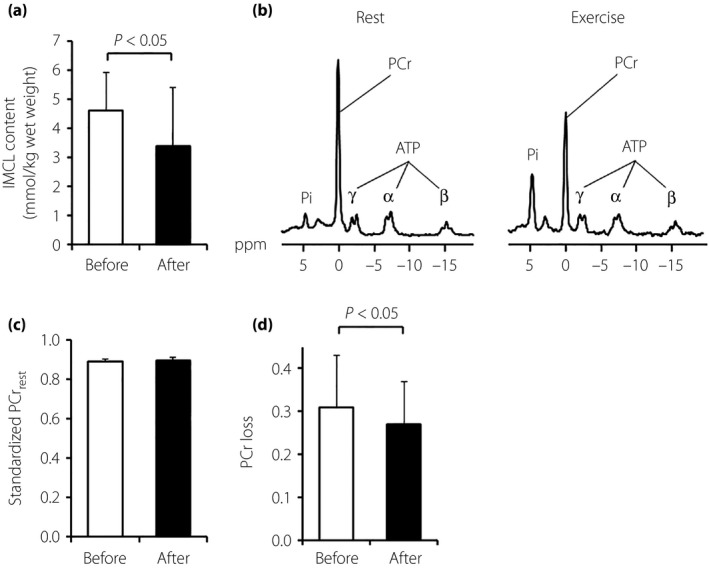
The metabolic syndrome patients’ skeletal muscle energy metabolism before and after pioglitazone treatment. The skeletal muscle energy metabolism was evaluated using (a) 1proton‐magnetic resonance spectroscopy and (b–e) 31phosphorus‐magnetic resonance spectroscopy. (a) intramyocellular lipid (IMCL) content. (b) Representative spectra of 31phosphorus‐magnetic resonance spectroscopy at rest (left panel) and during plantar flexion exercise (right panel) in the calf muscle. (c) Standardized phosphocreatine (PCr) level at rest. (d) Muscle PCr loss during exercise. Data are mean ± standard deviation (n = 14, except for IMCL content, which is n = 13). ATP, adenosine triphosphate; Pi, inorganic phosphate.
High‐energy phosphate metabolism in skeletal muscle
We measured 1‐RM in MetS patients (42.6 ± 6.1 kg) only before the pioglitazone treatment, assuming that 1‐RM would not be changed throughout the study period, which is supported by the unchanged cross‐sectional area of the calf muscle after the treatment. After the initiation of a constant load of 20% 1‐RM exercise, the PCr level in the patients’ calf muscle started to decrease, and was finally stabilized within a few minutes in all experiments. The representative spectra of 31P‐MRS at rest and during the plantar flexion exercise are shown in Figure 2b. The standardized muscle PCr level at rest was similar before and after the treatment (Figure 2c). The pioglitazone significantly decreased the muscle PCr loss during exercise (Figure 2d), suggesting that the MetS patients’ intramuscular high‐energy phosphate metabolism was improved after the pioglitazone treatment.
Relationships between the changes in aerobic capacity and skeletal muscle energy metabolism after pioglitazone treatment
There was an inverse correlation between the changes in AT and the changes in IMCL content after the treatment (Figure 3b), whereas there was no significant correlation between the changes in peak VO2 and the IMCL content (Figure 3a). The decrease in muscle PCr loss did not correlate with the increase in peak VO2 and AT (data not shown).
Figure 3.

Relationships between the changes in aerobic capacity and intramyocellular lipid (IMCL) content after pioglitazone treatment. AT, anaerobic threshold; VO2, oxygen uptake.
Discussion
The major finding of the present study was that the 4‐month pioglitazone treatment (15 mg/day) improved the aerobic capacity characterized by increased peak VO2 and AT in patients with MetS. The pioglitazone treatment decreased the resting IMCL content and PCr loss during exercise in the patients’ leg muscle, indicating that pioglitazone improved skeletal muscle energy metabolism in these MetS patients. There was also a significant relationship between the increase in AT and the decrease in IMCL content after pioglitazone treatment. The improved skeletal muscle fatty acid metabolism might thus have contributed to the MetS patients’ increased aerobic capacity after the pioglitazone treatment.
Low aerobic capacity has been well documented to be a strong and independent predictor of all‐cause mortality in patients with obesity and insulin resistance10, 11. It has also been shown that the increase in maximal aerobic capacity by each 1‐metabolic equivalent, equivalent to a 3.5‐mL/kg/min increase of peak VO2, confers a 12% improvement in survival7. The modest, but significant, increase in aerobic capacity in the present study's MetS patients after the pioglitazone treatment is thus clinically relevant. In addition, there was no significant difference in the number of daily steps or movement‐related calorie consumption between before and after pioglitazone treatment, indicating that the increased aerobic capacity after the pioglitazone treatment was independent of the patients’ daily physical activity.
We observed a decrease in the IMCL content in the MetS patients after the pioglitazone treatment, which is consistent with previous studies of patients with impaired glucose tolerance15 and patients with type 2 diabetes16. The content of IMCL, which is triglycerides within the muscle cells, is regulated by both uptake of fatty acids and fatty acid oxidation in skeletal muscle, and an accumulation of IMCL might be attributable to a reduced capacity for fatty acid oxidation rather than increased fatty acid uptake in skeletal muscle in obese patients with insulin resistance17. In addition, it was shown that pioglitazone increases the gene expression involved in fatty acid oxidation in the skeletal muscle in patients with type 2 diabetes18. These findings support the hypothesis that pioglitazone might decrease the IMCL content in individuals with MetS through an increased capacity for fatty acid oxidation in skeletal muscle.
The results of one of our previous studies showed that MetS patients had impaired high‐energy phosphate metabolism characterized by greater muscle PCr loss during aerobic exercise with the constant load of 20% 1‐RM8. Muscle PCr works as an energy reserve in the cytosol and is converted to adenosine triphosphate (ATP) through creatine kinase reaction to keep the ATP level constant in the muscle cell19.
PCr + adenosine diphosphate → ATP + creatine
When mitochondrial ATP production cannot meet the energy demand under aerobic conditions, the increased amount of PCr is converted to ATP, which might result in greater PCr loss. In the present study, the pioglitazone treatment significantly decreased muscle PCr loss during low‐intensity exercise in the MetS patients. It was shown that pioglitazone activates AMP‐activated protein kinase, which is known as a key regulator of mitochondrial biogenesis, and that pioglitazone increases the gene expression involved in mitochondrial function in human skeletal muscle18. It was also reported that pioglitazone improves mitochondrial respiratory capacity in skeletal muscle in patients with type 2 diabetes20. Taken together, all of these findings show that improved mitochondrial function as well as increased substrate utilization in skeletal muscle might contribute to the improvement in skeletal muscle energy metabolism after pioglitazone treatment.
We observed that fasting blood glucose, insulin, homeostasis model assessment of insulin resistance, and serum triglycerides were decreased after pioglitazone treatment without changing waist circumference and body composition in MetS patients, although pioglitazone has been reported to reduce visceral fat volume in patients with impaired glucose tolerance or type 2 diabetes21. It has been reported that pioglitazone treatment improves muscle insulin sensitivity as well as adipose tissue insulin sensitivity22. Several studies have shown that IMCL content is inversely correlated with muscle insulin sensitivity23. Therefore, in the present study, pioglitazone treatment increased systemic insulin sensitivity and decreased serum triglycerides at least in part through improved skeletal muscle energy metabolism including fatty acid metabolism in MetS patients.
In the present study's MetS patients, the increase in AT, but not the increase in peak VO2, correlated with the decrease in IMCL content after the pioglitazone treatment. As fatty acids are the primary substrate for ATP production in skeletal muscle during low‐ to moderate‐intensity exercise24, it seems reasonable that there was a relationship between the increase in submaximal aerobic capacity and the improvement in skeletal muscle fatty acid metabolism after pioglitazone treatment in our MetS patients. However, as the intensity of exercise is further increased, particularly near or at peak intensity, the main energy sources can be switched to glucose and lactate, which might be the reason why the increase in peak VO2 did not significantly correlate with the improvement in skeletal muscle fatty acid metabolism after pioglitazone treatment.
In addition to improved fatty acid metabolism in the skeletal muscle, other mechanisms could also contribute to the increased aerobic capacity of MetS patients after pioglitazone treatment. It has been shown that insulin sensitivity per se is associated with aerobic capacity25. However, in the present study, the improvement in insulin sensitivity markers including fasting blood glucose, insulin and homeostasis model assessment of insulin resistance did not correlate with the increase in aerobic capacity produced by pioglitazone treatment. Pioglitazone has been reported to improve endothelial function26, which can improve aerobic capacity through an increased O2 supply into skeletal muscle. We cannot exclude the contribution of changes in peripheral blood flow by pioglitazone, because we did not assess endothelial function.
There are some limitations that should be acknowledged. First, the small sample size (n = 14) might limit our interpretation and discussion. Second, the study was not a double‐blind study using a matching placebo. Further randomized clinical trials with larger sample sizes are required to confirm the data of the present study.
In conclusion, this is the first study to show that pioglitazone treatment improved MetS patients’ whole‐body aerobic capacity and skeletal muscle energy metabolism. The increased submaximal aerobic capacity might be at least in part attributable to the improved fatty acid metabolism in skeletal muscle after the pioglitazone treatment. Although there is no doubt that lifestyle interventions, such as exercise and diet therapy, are the most desirable treatment for MetS, the present findings raise the possibility that pioglitazone can be a potential drug treatment for obese and insulin‐resistant patients whose exercise capacity is lowered.
Disclosure
The authors declare no conflict of interest.
Acknowledgments
We thank Miwako Fujii, Akiko Aita and Kaoruko Kawai for their technical assistance. This study was supported by grants from the Ministry of Education, Culture, Sports, Science and Technology of Japan (grant no. 18790487, 17390223, 20117004 and 21390236); the Meiji Yasuda Life Foundation of Health and Welfare; the Mitsui Life Social Welfare Foundation; the Uehara Memorial Foundation; the Mochida Memorial Foundation for Medical and Pharmaceutical Research; and the Center of Innovation Program from Japan Science and Technology Agency (JST).
J Diabetes Investig 2017; 8: 535–541
References
- 1. DeFronzo RA, Abdul‐Ghani M. Type 2 diabetes can be prevented with early pharmacological intervention. Diabetes Care 2011; 34(Suppl 2): S202–S209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Grundy SM. Pre‐diabetes, metabolic syndrome, and cardiovascular risk. J Am Coll Cardiol 2012; 59: 635–643. [DOI] [PubMed] [Google Scholar]
- 3. Yki‐Jarvinen H. Thiazolidinediones. N Engl J Med 2004; 351: 1106–1118. [DOI] [PubMed] [Google Scholar]
- 4. Schernthaner G, Currie CJ, Schernthaner GH. Do we still need pioglitazone for the treatment of type 2 diabetes? A risk‐benefit critique in 2013. Diabetes Care 2013; 36(Suppl 2): S155–S161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. DeFronzo RA, Tripathy D, Schwenke DC, et al Pioglitazone for diabetes prevention in impaired glucose tolerance. N Engl J Med 2011; 364: 1104–1115. [DOI] [PubMed] [Google Scholar]
- 6. Kernan WN, Viscoli CM, Furie KL, et al Pioglitazone after ischemic stroke or transient ischemic attack. N Engl J Med 2016; 374: 1321–1331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Myers J, Prakash M, Froelicher V, et al Exercise capacity and mortality among men referred for exercise testing. N Engl J Med 2002; 346: 793–801. [DOI] [PubMed] [Google Scholar]
- 8. Yokota T, Kinugawa S, Okita K, et al Lower aerobic capacity was associated with abnormal intramuscular energetics in patients with metabolic syndrome. Hypertens Res 2009; 34: 1029–1034. [DOI] [PubMed] [Google Scholar]
- 9. Yokota T, Kinugawa S, Yamato M, et al Systemic oxidative stress is associated with lower aerobic capacity and impaired skeletal muscle energy metabolism in patients with metabolic syndrome. Diabetes Care 2013; 36: 1341–1346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Wei M, Gibbons LW, Kampert JB, et al Low cardiorespiratory fitness and physical inactivity as predictors of mortality in men with type 2 diabetes. Ann Intern Med 2000; 132: 605–611. [DOI] [PubMed] [Google Scholar]
- 11. Wei M, Kampert JB, Barlow CE, et al Relationship between low cardiorespiratory fitness and mortality in normal‐weight, overweight, and obese men. JAMA 1999; 282: 1547–1553. [DOI] [PubMed] [Google Scholar]
- 12. Takada S, Hirabayashi K, Kinugawa S, et al Pioglitazone ameliorates the lowered exercise capacity and impaired mitochondrial function of the skeletal muscle in type 2 diabetic mice. Eur J Pharmacol 2014; 740: 690–696. [DOI] [PubMed] [Google Scholar]
- 13. Yamato M, Shiba T, Yoshida M, et al Fatty acids increase the circulating levels of oxidative stress factors in mice with diet‐induced obesity via redox changes of albumin. FEBS J 2007; 274: 3855–3863. [DOI] [PubMed] [Google Scholar]
- 14. Beaver WL, Wasserman K, Whipp BJ. A new method for detecting anaerobic threshold by gas exchange. J Appl Physiol 1986; 60: 2020–2027. [DOI] [PubMed] [Google Scholar]
- 15. Rasouli N, Raue U, Miles LM, et al Pioglitazone improves insulin sensitivity through reduction in muscle lipid and redistribution of lipid into adipose tissue. Am J Physiol Endocrinol Metab 2005; 288: E930–E934. [DOI] [PubMed] [Google Scholar]
- 16. Bajaj M, Baig R, Suraamornkul S, et al Effects of pioglitazone on intramyocellular fat metabolism in patients with type 2 diabetes mellitus. J Clin Endocrinol Metab 2010; 95: 1916–1923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Kelley DE, Goodpaster B, Wing RR, et al Skeletal muscle fatty acid metabolism in association with insulin resistance, obesity, and weight loss. Am J Physiol 1999; 277: E1130–E1141. [DOI] [PubMed] [Google Scholar]
- 18. Coletta DK, Sriwijitkamol A, Wajcberg E, et al Pioglitazone stimulates amp‐activated protein kinase signalling and increases the expression of genes involved in adiponectin signalling, mitochondrial function and fat oxidation in human skeletal muscle in vivo: a randomised trial. Diabetologia 2009; 52: 723–732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Blei ML, Conley KE, Kushmerick MJ. Separate measures of atp utilization and recovery in human skeletal muscle. J Physiol 1993; 465: 203–222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Rabol R, Boushel R, Almdal T, et al Opposite effects of pioglitazone and rosiglitazone on mitochondrial respiration in skeletal muscle of patients with type 2 diabetes. Diabetes Obes Metab 2010; 12: 806–814. [DOI] [PubMed] [Google Scholar]
- 21. Kodama N, Tahara N, Tahara A, et al Effects of pioglitazone on visceral fat metabolic activity in impaired glucose tolerance or type 2 diabetes mellitus. J Clin Endocrinol Metab 2013; 98: 4438–4445. [DOI] [PubMed] [Google Scholar]
- 22. Cusi K, Orsak B, Bril F, et al Long‐term pioglitazone treatment for patients with nonalcoholic steatohepatitis and prediabetes or type 2 diabetes mellitus: a randomized trial. Ann Intern Med 2016; 165: 305–315. [DOI] [PubMed] [Google Scholar]
- 23. Krssak M, Falk Petersen K, Dresner A, et al Intramyocellular lipid concentrations are correlated with insulin sensitivity in humans: a 1 h nmr spectroscopy study. Diabetologia 1999; 42: 113–116. [DOI] [PubMed] [Google Scholar]
- 24. Ruby BC, Robergs RA. Gender differences in substrate utilisation during exercise. Sports Med 1994; 17: 393–410. [DOI] [PubMed] [Google Scholar]
- 25. Clausen JO, Borch‐Johnsen K, Ibsen H, et al Insulin sensitivity index, acute insulin response, and glucose effectiveness in a population‐based sample of 380 young healthy caucasians. Analysis of the impact of gender, body fat, physical fitness, and life‐style factors. J Clin Invest. 1996; 98: 1195–1209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Rizza S, Cardellini M, Porzio O, et al Pioglitazone improves endothelial and adipose tissue dysfunction in pre‐diabetic cad subjects. Atherosclerosis 2011; 215: 180–183. [DOI] [PubMed] [Google Scholar]


