Abstract
Aims/Introduction
The distributer of the anti‐glutamic acid decarboxylase antibody assay kit using radioimmunoassay (RIA) recently announced its discontinuation, and proposed an alternative kit using enzyme‐linked immunosorbent assay (ELISA). The aim of the present study was to investigate the diagnostic values of the anti‐glutamic acid decarboxylase antibody by RIA and ELISA among type 1 diabetes mellitus patients and control participants.
Materials and Methods
A total of 79 type 1 diabetes mellitus patients and 79 age‐matched controls were enrolled and assessed using RIA and ELISA. Sensitivity, specificity, positive predictive values and negative predictive values were calculated for cut‐off values (RIA = 1.5 U/mL and ELISA = 5.0 U/mL, respectively). Kappa coefficients were used to test for agreements between the RIA and ELISA methods regarding the diagnosis of type 1 diabetes mellitus.
Results
The sensitivity, specificity, positive predictive values, and negative predictive values for diagnosing type 1 diabetes mellitus were 57.0, 97.5, 95.7, and 69.4% by RIA, and 60.8, 100.0, 100.0 and 71.8% by ELISA, respectively. The diagnosis of type 1 diabetes mellitus using the RIA and ELISA methods showed substantial agreement with the kappa values of 0.74 for all participants, and of 0.64 for the acute type; however, there was moderate agreement with the kappa value of 0.56 for the slowly progressive type.
Conclusions
The present study suggests that both anti‐glutamic acid decarboxylase antibody by RIA and ELISA was useful for diagnosing type 1 diabetes mellitus. However, in the slowly progressive type, the degree of agreement of these two kits was poorer compared with those in all participants or in the acute type.
Keywords: Anti‐glutamic acid decarboxylase antibody, Enzyme‐linked immunosorbent assay, Type 1 diabetes mellitus
Introduction
Type 1 diabetes mellitus is caused by an autoimmune mechanism that destroys β‐cells in pancreatic islets, resulting in absolute deficiency in insulin1, 2. The detection of auto‐antibodies to islet antigens in serum is one of the key elements in the diagnosis of type 1 diabetes mellitus3. Among the auto‐antibodies identified in patients with type 1 diabetes mellitus, the anti‐glutamic acid decarboxylase (GAD) antibody is the most frequently used in clinical settings. An anti‐GAD antibody assay kit using radioimmunoassay (RIA) has been widely used as a standard method4. However, the distributer of this kit (Cosmic Corporation, Tokyo, Japan) recently announced its discontinuation, and proposed an alternative kit using enzyme‐linked immunosorbent assay (ELISA)5. Therefore, we compared the characteristics of these two anti‐GAD antibody assay kits among type 1 diabetes mellitus patients and control participants.
Materials and Methods
Study participants
In the present cross‐sectional study, 79 patients with type 1 diabetes mellitus and 79 age‐matched controls from National Hospital Organization Kyoto Medical Center, Kyoto, Japan, were selected. The control participants were originally recruited for another study regarding obesity. Among them, 27 had type 2 diabetes mellitus and 52 were non‐diabetic. Inclusion criteria were: aged no younger than 20 years, and patients with or without type 1 diabetes mellitus. A diagnosis was reached according to the criteria of the Japan Diabetes Society6. Based on the manner of onset and progression, patients were classified to fulminant, acute‐onset or slowly progressive type 1 diabetes7, 8, 9.
Ethics
The study was approved (15‐119) by the ethical committee of NHO Kyoto Medical Center.
Data collection and measurements
Serum samples were obtained and frozen at −80°C. Relevant clinical and demographic data were collected for each patient. Hemoglobin A1c (HbA1c) levels were measured using an ADAMS A1c HA‐8180 automatic glycohemoglobin analyzer (Arkray Inc., Kyoto, Japan)10. Anti‐GAD antibody levels were measured using RIA and ELISA at the LSI Medience Corporation (Tokyo, Japan) using commercial kits (manufacturer: RSR, Cardiff, UK; distributer: Cosmic Corporation)11, 12. The cut‐off value for the kit using RIA is 1.5 U/mL, and that of ELISA is 5.0 U/mL. The coefficient of variation for the kit using RIA is <19%, and that of ELISA is <15%.
Statistical analysis
The background characteristics of patients with and without type 1 diabetes mellitus were compared using Fisher's exact test and Student's t‐test. Sensitivity, specificity, positive predictive values and negative predictive values were calculated for the assigned cut‐off values. Kappa coefficients were used to test for agreements between the RIA and ELISA methods regarding the diagnosis of type 1 diabetes mellitus. The kappa coefficient was used as a measure of concordance for categorical data, and was interpreted as follows: <0 was considered less than chance agreement, 0.01–0.20 was considered slight agreement, 0.21–0.40 fair agreement, 0.41–0.60 moderate agreement, 0.61–0.80 substantial agreement and 0.81–0.99 almost perfect agreement13. The distributions of anti‐GAD antibody titers by RIA and ELISA were skewed; therefore, Pearson's correlation coefficients were calculated using log‐transformed values instead of raw data. Patients with type 1 diabetes mellitus were divided into four groups (group 1, RIA‐positive and ELISA‐positive; group 2, RIA‐positive and ELISA‐negative; group 3, RIA‐negative and ELISA‐positive; and group 4, RIA‐negative and ELISA‐negative).
The normal distribution of variables was checked by the Kolmogorov–Smirnov test. Categorical variables were compared among the four groups using χ2‐tests. In order to identify significant differences between groups after χ2‐tests, we consecutively carried out a residual error analysis. We compared continuous variables among the four groups using an analysis of variance (anova test) and post‐hoc comparison test (Tukey test).
P‐values <0.05 were considered to be significant. Statistical analyses were carried out with spss (SPSS 20.0; IBM, Armonk, NY, USA) and EZR15 (Saitama Medical Center, Jichi Medical University, Saitama, Japan)14.
Results
Clinical characteristics of the participants are shown in Table 1. All the participants with type 1 diabetes mellitus classified as the slowly progressive type were treated with insulin. In two cases of non‐diabetic participants, RIA showed very low titers (1.7 and 1.8 U/mL) and ELISA were negative. The type 2 diabetic participants were neither RIA‐positive nor ELISA‐positive. In one case of fulminant type 1 diabetes, RIA‐negative, but ELISA‐positive, results were observed. The anti‐GAD antibody by ELISA correlated with the anti‐GAD antibody by RIA, excluding the participants with RIA‐negative or ELISA‐negative participants (r = 0.832, P < 0.001; Figure 1).
Table 1.
Participant characteristics
| Descriptive variables | Type 1 diabetes | Control | P‐value |
|---|---|---|---|
| n | 79 | 79 | – |
| Classification | – | ||
| Acute | 49 | – | |
| Slowly progressive | 29 | – | |
| Fulminant | 1 | – | |
| Age (years) | 54.9 (16.6) | 57.6 (14.9) | 0.283 |
| Male (%) | 34.2 | 32.9 | 0.866 |
| Diabetes duration (years) | 15.6 (11.3) | – | – |
| HbA1c (%) | 7.7 (1.0) | 6.5 (1.6) | <0.001* |
Data are presented as a number, percent and mean (SD). *P < 0.05. HbA1c, Hemoglobin A1c.
Figure 1.
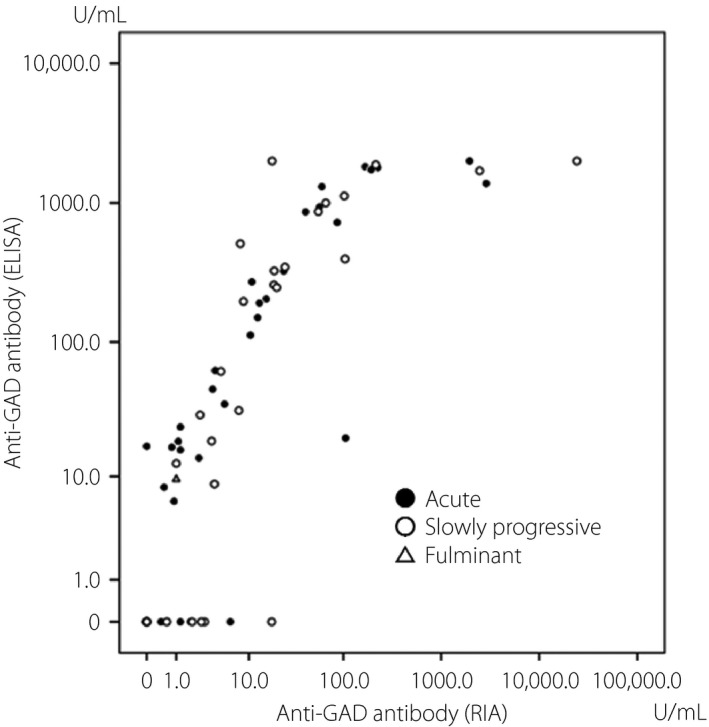
Correlation between the anti‐glutamic acid decarboxylase (GAD) antibody by radioimmunoassay (RIA) and enzyme‐linked immunosorbent assay (ELISA).
The sensitivity, specificity, positive predictive values, and negative predictive values for the diagnosis of type 1 diabetes mellitus were 57.0, 97.5, 95.7 and 69.4% by RIA, and 60.8, 100.0, 100.0 and 71.8% by ELISA, respectively (Table 2). Diagnosis for type 1 diabetes mellitus using the RIA or ELISA method showed substantial agreement with kappa values of 0.74 (95% confidence interval 0.63–0.86) for all participants. There was a substantial agreement with a kappa value of 0.64 (95% confidence interval 0.42–0.85) for the acute type although there was a moderate agreement with a kappa value of 0.56 (95% confidence interval 0.20–0.91) for the slowly progressive type.
Table 2.
Accuracy parameters of radioimmunoassay and enzyme‐linked immunosorbent assay tests
| Category | Tests | Sensitivity (%) | Specificity (%) | PPV (%) | NPV (%) | Diagnostic accuracy (%) | Likelihood ratio of a positive test | Likelihood ratio of a negative test |
|---|---|---|---|---|---|---|---|---|
| All | RIA | 57.0 (45.3–68.1) | 97.5 (91.4–99.7) | 95.7 (85.5–99.5) | 69.4 (59.9–77.8) | 77.2 (69.9–83.5) | 22.5 (5.7–89.6) | 0.4 (0.3–0.6) |
| ELISA | 60.8 (49.1–71.6) | 100.0 (93.2–100.0) | 100.0 (89.1–100.0) | 71.8 (62.4–80.0) | 80.4 (73.3–86.3) | – | 0.4 (0.3–0.5) | |
| Acute type | RIA | 44.9 (30.7–59.8) | 97.5 (91.2–99.7) | 91.7 (73.0–99.0) | 74.0 (64.5–82.1) | 77.3 (69.1–84.3) | 17.7 (4.4–72.1) | 0.6 (0.4–0.7) |
| ELISA | 55.1 (40.2–69.3) | 100.0 (93.2–100.0) | 100.0 (81.7–100.0) | 78.2 (68.9–85.8) | 82.8 (75.1–88.9) | – | 0.4 (0.3–0.6) | |
| Slowly progressive type | RIA | 79.3 (60.3–92.0) | 97.5 (91.2–99.7) | 92.0 (74.0–99.0) | 92.8 (84.9–97.3) | 92.6 (85.9–96.7) | 31.3 (7.9–124.6) | 0.2 (0.1–0.4) |
| ELISA | 69.0 (49.2–84.7) | 100.0 (93.2–100.0) | 100.0 (76.2–100.0) | 89.8 (81.5–95.2) | 91.7 (84.8–96.1) | – | 0.3 (0.2–0.5) |
Data are presented as percent, ratio and number (95% confidence interval). ELISA, enzyme‐linked immunosorbent assay; NPV, negative predictive value; PPV, positive predictive value; RIA, radioimmunoassay.
The prevalence of the slowly progressive type was higher in group 1, and lower in group 4 (Table 3). The duration of diabetes was shorter in group 1 than in group 4 (Table 4). The clinical characteristics of patients in groups 1–4 in the acute type and in the slowly progressive type were separately analyzed (Tables 5 and 6).
Table 3.
Classification and sex according to groups
| Variables | Group 1 (RIA‐positive and ELISA‐positive) | Group 2 (RIA‐positive and ELISA‐negative) | Group 3 (RIA‐negative and ELISA‐positive) | Group 4 (RIA‐negative and ELISA‐negative) | P‐value |
|---|---|---|---|---|---|
| n | 39 | 6 | 8 | 25 | – |
| Classification | 0.021 | ||||
| Acute type (%) | 40.8 | 4.1 | 14.3 | 40.8 | |
| Slowly progressive type (%) | 65.5* | 13.8 | 3.4 | 17.2* | |
| Male (%) | 35.9 | 16.7 | 37.5 | 36.0 | 0.817 |
| Acute type (%) | 35.0 | 50.0 | 42.9 | 45.0 | 0.920 |
| Slowly progressive type (%) | 36.8 | 0 | 0 | 0 | 0.183 |
Data are presented as a number, percent and mean (SD). *P < 0.05 (vs the acute type). ELISA, enzyme‐linked immunosorbent assay; RIA, radioimmunoassay.
Table 4.
Patient characteristics according to groups
| Variables | Group 1 (RIA‐positive and ELISA‐positive) | Group 2 (RIA‐positive and ELISA‐negative) | Group 3 (RIA‐negative and ELISA‐positive) | Group 4 (RIA‐negative and ELISA‐negative) | F‐value |
|---|---|---|---|---|---|
| n | 39 | 6 | 8 | 25 | – |
| Age (years) | 52.7 (16.6) | 64.8 (15.3) | 60.8 (19.4) | 55.1 (15.3) | 0.281 |
| BMI (kg/m2) | 22.9 (3.2) | 24.1 (2.2) | 22.0 (3.7) | 21.9 (3.0) | 0.349 |
| Diabetes duration (years) | 11.3 (8.5)** | 21.8 (8.4) | 20.6 (14.1) | 19.6 (12.6) | 0.005 |
| HbA1c (%) | 7.5 (1.0) | 8.3 (0.6) | 7.6 (1.0) | 7.8 (1.0) | 0.296 |
Data are presented as a number, percent and mean (SD). **P < 0.05 (vs group 4). BMI, body mass index; ELISA, enzyme‐linked immunosorbent assay; HbA1c, hemoglobin A1c; RIA, radioimmunoassay.
Table 5.
Patient characteristics according to groups in the acute type
| Variables | Group 1 (RIA‐positive and ELISA‐positive) | Group 2 (RIA‐positive and ELISA‐negative) | Group 3 (RIA‐negative and ELISA‐positive) | Group 4 (RIA‐negative and ELISA‐negative) | F‐value |
|---|---|---|---|---|---|
| n | 20 | 2 | 7 | 20 | – |
| Age (years) | 43.9 (11.6) | 36, 69 | 57.9 (19.0) | 52.3 (15.4) | 0.132 |
| BMI (kg/m2) | 22.7 (2.5) | 21.1, 23.1 | 22.6 (3.6) | 21.3 (3.0) | 0.473 |
| Diabetes duration (years) | 9.5 (8.7) | 26, 30 | 21.7 (14.8) | 18.8 (11.9) | 0.011 |
| HbA1c (%) | 7.5 (1.2) | 7.5, 8.5 | 7.5 (1.0) | 7.7 (1.1) | 0.833 |
Data are presented as a number, percent and mean (SD). BMI, body mass index; ELISA, enzyme‐linked immunosorbent assay; HbA1c, hemoglobin A1c; RIA, radioimmunoassay.
Table 6.
Patient characteristics according to groups in the slowly progressive type
| Variables | Group 1 (RIA‐positive and ELISA‐positive) | Group 2 (RIA‐positive and ELISA‐negative) | Group 3 (RIA‐negative and ELISA‐positive) | Group 4 (RIA‐negative and ELISA‐negative) | F‐value |
|---|---|---|---|---|---|
| n | 19 | 4 | 1 | 5 | – |
| Age (years) | 62.0 (16.3) | 71.0 (7.4) | 81.0 | 66.4 (8.1) | 0.441 |
| BMI (kg/m2) | 23.0 (3.8) | 25.1 (1.9) | 18.0 | 24.0 (2.3) | 0.302 |
| Diabetes duration (years) | 13.1 (8.2) | 18.8 (8.8) | 13.0 | 23.0 (16.2) | 0.247 |
| HbA1c (%) | 7.5 (0.9) | 8.4 (0.5) | 8.3 | 7.9 (0.3) | 0.168 |
Data are presented as a number, percent, and mean (SD). BMI, body mass index; ELISA, enzyme‐linked immunosorbent assay; HbA1c, hemoglobin A1c; RIA, radioimmunoassay.
Discussion
In the present study, we showed that the two different commercial assay kits for measuring the anti‐GAD antibody by RIA or ELISA were almost equivalent for sensitivity, specificity, positive predictive values, negative predictive values, and diagnostic accuracy. These results are in accordance with previous reports15. Therefore, we suggest that the ELISA method can be used as an alternative method to the discontinued RIA method. However, it is important to note that the kappa value was lower for the slowly progressive type than those for all participants and the acute type, suggesting that the degree of the agreement was poorer for the slowly progressive type. These discrepancies might be related to the different reactivities of the antibodies against GAD used in these two kits; a truncated recombinant protein lacking amino acids 2–45 in the N‐terminal region in the RIA kit and a full‐length recombinant protein in the ELISA kit16. The anti‐GAD antibodies in patients with the slowly progressive type might differ in epitope recognition from those in patients with the acute type17. The present study showed that the patients who tested positive for GAD antibody on RIA, but negative for GAD antibody on ELISA, were mainly slowly progressive diabetic patients (4.1% of acute type cases vs 13.8% of slowly progressive type cases). Further studies on epitope mapping of anti‐GAD antibodies would resolve these issues. The reason why the duration of diabetes was shorter in group 1 than in group 4 might be due to the anti‐GAD antibody more likely becoming negative in some patients as the duration of diabetes increases18.
The current cut‐off value for the ELISA method was provided by the manufacturer according to data collected during the development of the kit. In a previous study comparing these two kits, participants with low anti‐GAD antibody titers detected by the RIA method were found to be negative by the ELISA method5. Further investigations are required in order to establish whether the current cut‐off value for the ELISA kit is appropriate to diagnose different types of type 1 diabetes mellitus; that is, the acute onset type, slowly progressive type and fulminant type, in relation to the clinical prognosis of the disease.
As most control participants were non‐diabetic, mean HbA1c levels were significantly lower in control participants than in patients with type 1 diabetes mellitus. The limitations of the present study were that it was a cross‐sectional survey with a relatively small sample size, and did not include data of serum or urine C‐peptide as a marker for remaining intrinsic insulin secretory capability from the pancreas, the insulin dosage and the human leukocyte antigen typing.
In conclusion, the present study showed that the anti‐GAD antibody kit using ELISA as an alternative to the anti‐GAD antibody kit using RIA was useful for the diagnosis of type 1 diabetes mellitus. However, these two kits did not agree completely, especially for the slowly progressive type, in the present study. Further investigations including prospective studies are required in order to address these issues.
Disclosure
The authors declare no conflict of interest.
Acknowledgments
This study was supported by a Grant‐in‐Aid from NHO. The anti‐GAD assay was carried out either in ordinary clinical practice or at the expense of LSI Medience Corporation. The authors thank Akiko Suganuma for her assistance in data processing, and all of the colleagues at the Diabetes Center, NHO Kyoto Medical Center for their help with this study.
J Diabetes Investig 2017; 8: 475–479
References
- 1. McLaughlin RJ, Spindler MP, van Lummel M, et al Where, how, and when: Positioning posttranslational modification within type 1 diabetes pathogenesis. Curr Diab Rep 2016; 16: 63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Pipi E, Marketou M, Tsirogianni A. Distinct clinical and laboratory characteristics of latent autoimmune diabetes in adults in relation to type 1 and type 2 diabetes mellitus. World J Diabetes 2014; 5: 505–510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Kawasaki E. Type 1 diabetes and autoimmunity. Clin Pediatr Endocrinol 2014; 23: 99–105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Wyatt R, Williams AJ. Islet autoantibody analysis: Radioimmunoassays. Methods Mol Biol 2016; 1433: 57–83. [DOI] [PubMed] [Google Scholar]
- 5. Oikawa Y, Tanaka M, Horie I, et al A study on the correlation between anti‐GAD antibody titers measured by ELISA kit and RIA kit. Igaku Yakugaku 2015; 72: 1551–1560 (Japanese). [Google Scholar]
- 6. Seino Y, Nanjo K, Tajima N, et al Report of the committee on the classification and diagnostic criteria of diabetes mellitus. J Diabetes Investig 2010; 1: 212–228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Kawasaki E, Maruyama T, Imagawa A, et al Diagnostic criteria for acute‐onset type 1 diabetes mellitus (2012): Report of the Committee of Japan Diabetes Society on the Research of Fulminant and Acute‐onset Type 1 Diabetes Mellitus. J Diabetes Investig 2014; 5: 115–118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Imagawa A, Hanafusa T, Awata T, et al Report of the Committee of the Japan Diabetes Society on the Research of Fulminant and Acute‐onset Type 1 Diabetes Mellitus: New diagnostic criteria of fulminant type 1 diabetes mellitus (2012). J Diabetes Investig 2012; 3: 536–539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Tanaka S, Ohmori M, Awata T, et al Diagnostic criteria for slowly progressive insulin‐dependent (type 1) diabetes mellitus (SPIDDM) (2012): Report by the Committee on Slowly Progressive Insulin‐Dependent (Type 1) Diabetes Mellitus of the Japan Diabetes Society. Diabetol Int 2015; 6: 1–7. [Google Scholar]
- 10. Kashiwagi A, Kasuga M, Araki E, et al Committee on the Standardization of Diabetes Mellitus‐Related Laboratory Testing of Japan Diabetes Society. International clinical harmonization of glycated hemoglobin in Japan: From Japan Diabetes Society to National Glycohemoglobin Standardization Program values. J Diabetes Investig 2012; 3: 39–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Cosmic Corporation . GADAb RIA “Cosmic” – Instructions for use, 5th edn Tokyo: Cosmic, 2014. (Japanese). [Google Scholar]
- 12. Cosmic Corporation . GADAb ELISA “Cosmic” – Instructions for use, 5th edn Tokyo: Cosmic, 2015. (Japanese). [Google Scholar]
- 13. Viera AJ, Garrett JM. Understanding interobserver agreement: The kappa statistic. Fam Med 2005; 37: 360–363. [PubMed] [Google Scholar]
- 14. Kanda Y. Investigation of the freely available easy‐to‐use software ‘EZR’ for medical statistics. Bone Marrow Transplant 2013; 48: 452–458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Rahmati K, Lernmark A, Becker C, et al A comparison of serum and EDTA plasma in the measurement of glutamic acid decarboxylase autoantibodies (GADA) and autoantibodies to islet antigen‐2 (IA‐2A) using the RSR radioimmunoassay (RIA) and enzyme linked immunosorbent assay (ELISA) kits. Clin Lab 2008; 54: 227–235. [PubMed] [Google Scholar]
- 16. Powell M, Prentice L, Asawa T, et al Glutamic acid decarboxylase autoantibody assay using 125I‐labelled recombinant GAD65 produced in yeast. Clin Chim Acta 1996; 256: 175–188. [DOI] [PubMed] [Google Scholar]
- 17. Hampe CS, Kockum I, Landin‐Olsson M, et al GAD65 antibody epitope patterns of type 1.5 diabetic patients are consistent with slow‐onset autoimmune diabetes. Diabetes Care 2002; 25: 1481–1482. [DOI] [PubMed] [Google Scholar]
- 18. Kong YH, Kim MS, Lee DY. Comparison of the prevalence of islet autoantibodies according to age and disease duration in patients with type 1 diabetes mellitus. Ann Pediatr Endocrinol Metab 2013; 18: 65–70. [DOI] [PMC free article] [PubMed] [Google Scholar]


