Abstract
The specific sodium–glucose cotransporter 2 inhibitors (SGLT2 inhibitors) inhibit glucose reabsorption in proximal renal tubular cells, and both fasting and postprandial glucose significantly decrease because of urinary glucose loss. As a result, pancreatic β‐cell function and peripheral insulin action significantly improve with relief from glucose toxicity. Furthermore, whole‐body energy metabolism changes to relative glucose deficiency and triggers increased lipolysis in fat cells, and fatty acid oxidation and then ketone body production in the liver during treatment with SGLT2 inhibitors. In addition, SGLT2 inhibitors have profound hemodynamic effects including diuresis, dehydration, weight loss and lowering blood pressure. The most recent findings on SGLT2 inhibitors come from results of the Empagliflozin, Cardiovascular Outcomes and Mortality in Type 2 Diabetes trial. SGLT2 inhibitors exert extremely unique and cardio‐renal protection through metabolic and hemodynamic effects, with long‐term durability on the reduction of blood glucose, bodyweight and blood pressure. Although a site of action of SGLT2 inhibitors is highly specific to inhibit renal glucose reabsorption, whole‐body energy metabolism, and hemodynamic and renal functions are profoundly modulated during the treatment of SGLT2 inhibitors. Previous studies suggest multifactorial clinical benefits and safety concerns of SGLT2 inhibitors. Although ambivalent clinical results of this drug are still under active discussion, the present review summarizes promising recent evidence on the cardio‐renal and metabolic benefits of SGLT2 inhibitors in the treatment of type 2 diabetes.
Keywords: Oral hypoglycemic drugs, Sodium‐glucose cotransporter 2 inhibitors, Type 2 diabetes mellitus
Introduction
Sodium‐glucose cotransporter 2 (SGLT2) inhibitors are a new class of glucose‐lowering drug. There are six SGLT2 inhibitors now available in Japan. These SGLT2 inhibitors share a similar chemical structure with low‐affinity, high‐capacity, highly specific inhibitors against SGLT2, which is located at the apical surface of the S1 portion of the proximal renal tubule1, 2. This transporter plays a specific role in the renal tubular reabsorption of more than 90% of glucose filtrated through glomeruli. Therefore, complete inhibition of SGLT2 results in overloading of glucose to SGLT1 at the downstream S3 portion of the proximal renal tubule2, 3, and approximately 60 and 100 g of unabsorbed glucose are excreted in the urine of healthy people and diabetes patients, respectively4, 5. Among six SGLT2 inhibitors, there are some differences in chemical and relative selectivity against SGLT2/SGLT1 proteins, as well as bioavailability (Table 1)6, 7, 8, 9, 10. Although clinical usefulness and safety concerns are reported to be roughly similar, previous review articles in this journal indicate that further studies with larger sample sizes and long‐term clinical evidences are required to fully evaluate SGLT2 inhibitors as a standard treatment for patients with type 2 diabetes11, 12. Recent clinical studies have shown multifactorial clinical benefits and unexpected marked protective effects on cardio‐renal events with treatment of SGLT2 inhibitors13, 14. Many metabolic and hemodynamic characteristics of SGLT2 inhibitors are summarized based on recent knowledge, as detailed in many review articles15, 16.
Table 1.
Chemical and pharmacological characteristics of SGLT2 inhibitors available in Japan
| Generic name | Ipragliflozin | Dapagliflozin | Luseogliflozin | Tofogliflozin | Canagliflozin | Empagliflozin |
|---|---|---|---|---|---|---|
| Structural formula |
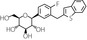
|
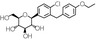
|
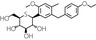
|
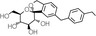
|
|
|
| Initial marketing | 2014 April | 2014 May | 2014 May | 2014 May | 2014 September | 2015 Ferbruary |
| Dosage and administration | 50 mg once daily (to 100 mg once daily) | 5 mg once daily (to 10 mg once daily) | 2.5 mg once daily (to 5 mg once daily) | 20 mg once daily | 100 mg once daily | 10 mg once daily (to 25 mg once daily) |
| SGLT2/SGLT1 | 860 | 610 | 1,770 | 2,900 | 290 | 2,680–5,000 |
| Half‐life (h) | 11.71 | 12.1 | 11.2 | 5.4 | 10.6 | 9.88–11.7 |
| Bioavailability | 90.20% | 78% | 90% | 97.50% | 65% | NA |
| Protein binding | 94.6–96.5% | 91% | 96.0–96.3% | 82.3–82.6% | 99% | 86.20% |
| Metabolism | UGT2B7 | UGT1A9 | CYP3A4/5, 4A11, 4F2, 4F3B, UGT1A1 | CYP3A4/5, 4A11, 4F3B | UGT1A9, 2B4, 3A4, CYP2D6 | UGT2B7, 1A3, 1A9 |
| Excretion |
Urinary ex. 67.9% Fecal ex 32.7% |
Urinary ex. 75.0% Fecal ex. 21.0% |
Urinary ex. 44.2% Fecal ex. 50.0% |
Urinary ex. 76.2% Fecal ex. 21.4% |
Urinary ex. 32.5% Fecal ex. 60.4% |
Urinary ex. 54.4% Fecal ex. 41.2% |
CYP, cytochrome P450 superfamily; N/A, not available; UGT, UDP‐glucosyltransferase.
In the present review article, we summarize recent advances in our understanding of metabolic and hemodynamic benefits, and safety issues regarding the ambivalent clinical characteristics of SGLT2 inhibitors.
Characteristics in Glycemic Control
Glucose excretion in the urine of patients with diabetes treated with SGLT2 inhibitors is generally reported to be 80–100 g/day5. In a phase 2 randomized clinical trial (RCT)17, patients with type 2 diabetes mellitus were treated with 12.5–100 mg ipragliflozin once daily for 12 weeks. Glycated hemoglobin (HbA1c) levels in the ipragliflozin group dose‐dependently decreased by a maximum of −1.31% compared with the placebo group. Similar placebo‐adjusted mean changes from baseline HbA1c (−1.24%) were also found in a phase 3 RCT using ipragliflozin18. Similarly, patients with type 2 diabetes treated with dapagliflozin 2.5–50 mg once daily, metformin or a placebo for 12 weeks showed a placebo‐adjusted mean change in HbA1c to a maximum −0.9% in the dapagliflozin group and −0.73% in the metformin group19. In a pooled analysis of phase 2 and 3 trials that included monotherapy or add‐on studies with other oral hypoglycemic drugs20, placebo‐adjusted mean changes in HbA1c in patients with type 2 diabetes significantly decreased by −1.17% in the ipragliflozin group. Reductions in HbA1c by ipragliflozin were only weakly associated with reductions in bodyweight. However, reductions in HbA1c were greater in patients with poor glycemic control when compared with good glycemic control groups17, 20. Consistently, patients with near normal baseline HbA1c did not show further reduction with ipragliflozin treatment.
Clinical usefulness of SGLT2 Inhibitors in Combination with other Oral Hypoglycemic Drugs
A similar effectiveness of placebo‐adjusted reduction in HbA1c was observed in add‐on studies using 50 mg ipragliflozin once daily in combination with other oral hypoglycemic drugs, such as sulfonylureas21, metformin22 or pioglitazone23. Consistently, a similar add‐on RCT study (phase 3) was reported that used 100 and 300 mg canagliflozin as compared with a placebo in patients with both metformin plus sulfonylurea for 26 weeks24. HbA1c significantly reduced in the canagliflozin vs the placebo groups (100 mg, −0.85%; 300 mg, −1.06 vs −0.13%). Efficacy and safety of canagliflozin vs glimepiride in patients with type 2 diabetes inadequately controlled with metformin25 in a 52‐week, phase 3, non‐inferiority RCT, and only canagliflozin 300 mg was found to be superior to glimepiride. Similarly, canagliflozin 300 mg compared with a placebo and sitagliptin 100 mg were studied for 26 weeks in patients with type 2 diabetes who were inadequately treated with metformin26. Only canagliflozin 300 mg showed statistical superiority to sitagliptin in lowering HbA1c (−0.88 vs −0.73%). Furthermore, in the Continuous Glucose Monitoring study27, luseogliflozin 2.5 mg once daily for 7 days shifted the area under curve (AUC) for glucose to lower levels, and both AUC and peak glucose levels significantly reduced in patients with mildly impaired glycemic control. Similarly, Yamada et al.28 reported that the ipragliflozin treatment improved the entire 24‐h glucose AUC without causing hypoglycemia. Finally, Japanese patients with type 2 diabetes inadequately controlled with sulfonylureas were randomly assigned to receive luseogliflozin 2.5 mg or a placebo for 24 weeks, and the placebo‐adjusted mean reduction in HbA1c was −0.88% in the treatment group29.
Effects of Glomerular Function on Glycemic Control
The hypoglycemic effect is mediated by inhibiting SGLT2‐dependent reabsorption of glucose in proximal renal tubular cells. Therefore, the usefulness of SGLT2 inhibitors to control hyperglycemia is affected by the glomerular filtration rate of the patients30, 31, 32. A RCT study was carried out using 252 type 2 diabetes patients with inadequately controlled HbA1c and moderately impaired renal function (estimated glomerular filtration rate 30–60 mL/min/1.73 m2), who were treated with dapagliflozin and placebo for 24 weeks, respectively. Dapagliflozin at any dose did not show a significant reduction compared with the placebo30. However, interestingly, both bodyweight and blood pressure significantly decreased in the dapagliflozin group vs the placebo group. However, another RCT examining canagliflozin using 269 patients with inadequately controlled type 2 diabetes with moderately impaired renal function (estimated glomerular filtration rate 30–50 mL/min/1.73 m2) showed that canagliflozin significantly decreased HbA1c compared with the placebo group. Furthermore, consistently, both bodyweight and blood pressure also decreased with canagliflozin treatment31. A similar renal function‐dependent reduction in HbA1c in patients with type 2 diabetes was also reported using luseogliflozin 2.5 mg daily32.
Long‐term Durability of Glycemic Control
Glycemic control with SGLT2 inhibitors is characterized by long‐term durability of excellent glycemic control in not only monotherapy, but also add‐on studies with other oral hypoglycemic drugs33, 34, 35. Glucose‐lowering effects of canagliflozin 300 mg were compared with sitagliptin 100 mg, both once daily in a study using type 2 diabetes patients inadequately controlled with metformin and sulfonylureas. In this randomized, double‐blind, active‐controlled, phase 3 trial, canagliflozin showed non‐inferiority in the control of HbA1c. Then in a subsequent assessment, canagliflozin was found to be superior to sitagliptin for the long‐term durability of glycemic control33. Furthermore, the stable and continuous reductions in both bodyweight and blood pressure were also found with canagliflozin treatment. Similar long‐term durability of glycemic improvement, as well as bodyweight reduction, was also reported when compared with glimepiride in a randomized, double‐blind study34. A total of 1,450 patients with type 2 diabetes, who were inadequately controlled, were assigned to receive canagliflozin 300 mg or glimepiride for a 52‐week core period followed by a 52‐week extension. At week 104, reductions in HbA1c from baseline values were −0.74 and −0.55% with canagliflozin 300 mg and glimepiride, respectively. Furthermore, reductions in both bodyweight and blood pressure were also well maintained over 104 weeks compared with glimepiride. Long‐term durability of glycemic control over 2 years was also found in the dapagliflozin group compared with the glipizide group. In a further extension study, dapagliflozin compared with glipizide showed further sustained reductions of HbA1c, bodyweight and systolic blood pressure over 208 weeks35. The long‐term durability of better glycemic control, as well as reductions of bodyweight and systolic blood pressure, are major characteristics of SGLT2 inhibitors, and could have a specific usefulness in suppressing chronic diabetic vascular complications.
Improvements in Insulin Secretion, Insulin Sensitivity and Glucose Toxicity with Enhanced Glucagon Secretion
Ferrannini et al.36 measured whole‐body glucose utilization using the double‐tracer glucose administration method after single‐dose and chronic empagliflozin 25 mg once daily for 4 weeks compared with baseline levels in 66 patients with type 2 diabetes. Empagliflozin treatment resulted in glucose loss in urine (single 7.8 g/3 h and chronic 9.2 g/3 h during fasting, and 29 g/5 h and 28.2 g/5 h after a meal, respectively). After a 3‐h fast, empagliflozin increased endogenous glucose production by 25%, whereas plasma glucose was significantly lower than baseline. After a meal, endogenous glucose production remained higher in the empagliflozin group compared with the placebo group. In contrast, the total glucose disposal rate significantly reduced because of reductions in both glucose oxidation and non‐oxidative glucose disposal, with concomitant increases in lipid oxidation during treatment with empagliflozin. At these metabolic conditions after a meal, both glucose and insulin AUCs decreased, whereas the glucagon response increased during empagliflozin treatment. Under such conditions, it is apparent that empagliflozin improves both β‐cell function and insulin sensitivity, despite the fall in insulin secretion. Merovci et al.37 also reported a similar improvement of insulin sensitivity in muscle, despite increased fasting glucagon concentration and endogenous glucose production in 18 men with type 2 diabetes, who were randomized to receive either dapagliflozin (n = 12) or a placebo (n = 6) for 2 weeks. Dapagliflozin treatment significantly reduced fasting plasma glucose, and increased insulin‐mediated tissue glucose disposal by approximately 18% using the hyperinsulinemic glucose clamp technique as shown in previous rodent models38, 39. These results provide the first definitive evidence of the applicability of the glucose toxicity hypothesis to humans with type 2 diabetes37, 38.
This interesting evidence on paradoxical increases in endogenous glucose production and glucagon secretion despite an overall reduction of fasting plasma glucose after treatment with SGLT2 inhibition has been comprehensively reviewed40. Recent evidence suggests that SGLT2 is expressed in glucagon‐secreting α‐cells of the pancreatic islets, and suppresses glucagon secretion40. Then, expression of SLC5A2, which encodes SGLT2, is downregulated under chronic hyperglycemic conditions with consequently upregulated glucagon gene expression. Therefore, treatment with SGLT2 inhibitors, which suppresses SGLT2 function in α‐cells, might directly further promote glucagon secretion from pancreatic α‐cells in type 2 diabetes patients with poor glycemic control41. Similar improvements of β‐cell function are reported in patients with type 2 diabetes treated with canagliflozin 300 mg daily for 52 weeks42 and ipragliflozin 50 mg daily for 4 weeks43. SGLT2 inhibitors decreased both plasma glucose and insulin levels, and showed significant improvements in insulin resistance as well as insulin secretion. Consistently, Leiter et al.44 reported that canagliflozin provides significant improvements in liver function, such as improved serum levels of liver enzymes compared with the placebo and sitagliptin groups. These reductions might be closely related to improvements in fatty liver in patients with type 2 diabetes.
Clinical Significance of Increased Ketone Body Production
Increased ketone body production is one of characteristics of treatment with SGLT2 inhibitors. Ketone body production in the liver is dependent on a relative ratio of action potentials between insulin and its counterregulatory hormones. Namely, ketone body production increases in the case of relatively insufficient insulin action against insulin counterregulatory hormones, or relatively increased action of counterregulatory hormones against insulin. The increased production is also related to insufficient glucose intake with a low carbohydrate diet and severe energy restriction, as well as increased glucose energy loss in urine45, 46. Enhanced glycosuria in patients treated with SGLT2 inhibitors could result in relative glucose energy deficiency in vivo with a concomitant effective reduction in plasma glucose levels, a concurrent lack of insulin and an excess of glucagon in plasma. Accelerated lipolysis in adipose tissue and the release in free fatty acid results in increased ketone body production in the liver with SGLT2 inhibitor treatment47. Pooled analysis of phase 2 and 3 RCTs with tofogliflozin treatment in Japanese patients with type 2 diabetes showed dose‐dependent increases in the levels of acetoacetate and β‐hydroxybutylic acid48. Recently, the US Food and Drug Administration warned that SGLT2 inhibitors for the treatment of diabetes might result in an increased risk for diabetic ketoacidosis (DKA), with mild‐to‐moderate glucose elevations (euglycemic DKA)49. Euglycemic DKA is defined as DKA without marked hyperglycemia. Most cases of DKA are reported in insulin‐treated type 2 diabetes patients. The European Medicines Agency has announced that the Pharmacovigilance Risk Assessment Committee has started a review of all three approved SGLT2 inhibitors (canagliflozin, dapagliflozin and empagliflozin) to evaluate the risk of DKA in type 2 diabetes, and have noted 101 cases of DKA have been reported. Although no clinical details are provided, all cases were serious, with some requiring hospitalization and some thought to be type 1 diabetes50. Erondu et al.51 reported a relatively low frequency of DKA in the Canagliflozin Cardiovascular Assessment Study, with an estimated incidence rate is 0.8 and 0.2 per 1,000 patient‐years with canagliflozin 300 mg and a comparator, respectively. Rosenstock et al.52 reported euglycemic DKA cases as a predictable, detectable and preventable concern with SGLT2 inhibitors. A recent review article53 reported the incidence of DKA to be <0.1% in the clinical trials using dapagliflozin effect on cardiovascular events (DECLARE) and empagliflozin, cardiovascular outcomes and mortality in type 2 diabetes trial (EMPAREGOUTCOME), and in the other reports in both patients with type 1 and type 2 diabetes54. Therefore, patients with insulin‐treated diabetes who develop nausea, vomiting and malaise during treatment with SGLT2 inhibitors should be promptly evaluated for the possible coexistence of ketoacidosis.
Hemodynamic and Renal Effects with Blood Pressure Lowering
Hypertension and type 2 diabetes mellitus are major risk factors for cardiovascular events, and frequently coexist. For example, 56% of Japanese type 2 diabetes patients have hypertension55. It is well established that lowering blood pressure reduces not only cardiovascular events56, but also has renoprotective effects in patients with type 2 diabetes57. Concerted reductions in both bodyweight and blood pressure are unique characteristics of SGLT2 inhibitors, as shown in many clinical trials, pooled analyses, systemic reviews and meta‐analyses58, 59, 60. Both systolic blood pressure (SBP) and diastolic blood pressure (DBP) significantly reduced by 2.8 and 1.6 mmHg, respectively in pooled analysis of five RCT studies61. Blood pressure reduction by ipragliflozin was also consistently found in patients not undergoing cotreatment with other antihypertensive medication61. Reductions of both SBP and DBP were significantly greater in patients with baseline SBP ≥140 mmHg than patients with SBP <140 mmHg in a pooled analysis of six RCTs61. The percentage reduction of blood pressure was not affected by baseline body mass index. In a systematic review and meta‐analysis, SGLT2 inhibitors consistently reduced both SBP and DBP relative to the placebo, with a weighted mean difference of −4 and −1.6 mmHg, respectively62.
The potential underling mechanisms behind the blood pressure‐lowering effects of SGLT2 inhibitors are thought to link multiple factors, such as bodyweight reduction63, osmotic diuresis and natriuresis58, 59, 64, 65, 66, and increased degradation of adipose tissue and muscle mass25, 67. An interesting RCT on the effects of dapagliflozin on 24‐h blood pressure, plasma volume and hematocrit compared with hydrochlorothiazide was reported in patients with type 2 diabetes. Dapagliflozin when compared with hydrochlorothiazide showed a significant reduction in plasma volume with resultant hemoconcentration, and increases in hemoglobin, reticulocyte and hematocrit counts59. This increase in red cell counts was explained in part by an increase in plasma erythropoietin concentration in the dapagliflozin treatment group. Hydrochlorothiazide did not show any such increases59. However, further studies are required to understand the exact mechanisms behind blood pressure reductions and increases in hematocrit with treatment with SGLT2 inhibitors.
Major Adverse Events: Hypoglycemia, Skin Disorders, and Genital Mycotic and Urinary Tract Infection
A summary of overall safety and selected adverse events in different clinical trials is shown in Table 2.
Table 2.
Summary of overall safety and selected adverse events selected adverse events
| Adverse events | Pooled analysis of ipragliflozin20 | EMPA‐REG OUTCOME trial13 | STELLA‐ELDER70Japanese type 2 diabetes mellitus102 | Lavalle‐Gonzalez FJ. Type 2 diabetes mellitus study26 | ||||
|---|---|---|---|---|---|---|---|---|
| Placebo | Ipragliflozin | Placebo | Empagliflozin | Ipragliflozin | Dapagliflozin | Placebo/sitagliptin | Canagliflozin | |
| No. patients |
322 n (%) |
509 n (%) |
2,333 n (%) |
4,687 n (%) |
8,505 n (%) |
728 n (%) |
183 n (%) |
735 n (%) |
| TEAEs | 216 (67.1) | 361 (70.9) | 2,139 (91.7) | 4,230 (90.2) | 1,438 (16.9) | 544 (74.7) | 122 (66.7) | 496 (67.5) |
| Hypoglycemia | 3 (0.9) | 5 (1) | 650 (27.9) | 1,303 (27.8) | 58 (0.68) | 25 (3.4) | 5 (2.7) | 50 (6.8) |
| Genital infection | ||||||||
| Male | 1 (0.5) | 3 (0.9) | 25 (1.5) | 166 (5.0) | Male+female | Male+female | 1 (1.1) | 13 (3.8) |
| Female | 2 (1.9) | 8 (5.3) | 17 (2.6) | 135 (10.0) | 166 (1.95) | 19 (2.6) | 1 (1.1) | 42 (10.7) |
| Urinary tract infection | ||||||||
| Male | 1 (0.5) | 2 (0.6) | 158 (9.4) | 350 (10.5) | Male+female | Male+female | Male+female | Male+female |
| Female | 7 (6.5) | 8 (4.7) | 265 (40.6) | 492 (36.4) | 118 (1.38) | 20 (2.7) | 12 (6.6) | 47 (6.4) |
| Volume depletion | 12 (3.8) | 72 (14.2) | 115 (4.9) | 239 (5.1) | 436 (5.13) | 49 (6.7) | 2 (1.0) | 41 (5.6) |
| Skin complications | NA | NA | NA | NA | 269 (3.16) | 23 (3.17) | NA | NA |
NA, not available; TEAEs, treatment‐emergent adverse events.
Hypoglycemia
Monotherapy of any SGLT2 inhibitor is not a cause of clinically significant hypoglycemia. If SGLT2 inhibitors are used in combination with either sulfonylureas or insulin, hypoglycemia becomes a clinical problem in general practice68, 69. However, patients treated with multiple oral hypoglycemic drugs have fewer hypoglycemic episodes using SGLT2 inhibitors compared with sulfonylureas34, 35. Real‐world evidence on the safety of ipragliflozin in 8,505 elderly Japanese patients with type 2 diabetes Specified drug use resulTs survEy of ipragLifLozintreAtment in ELDERly type 2 diabetes patients (STELLA‐ELDER) has been reported (Table 2)70. Adverse drug reactions of special interest related to hypoglycemia were found in 0.68% of patients. The incidence of adverse hypoglycemic events was 1 in 10 in the dapagliflozin group compared with the sulfonylurea group71. However, in another study, patients treated with a combination of dapagliflozin and insulin showed a higher frequency of hypoglycemia compared with the placebo group, although the dose of insulin was generally 50% of the original dose68.
Skin disorders with pruritus
A variety of skin disorders with pruritus have been reported in Japanese clinical trials, with an incidence rate of approximately 3–3.5% in both the STELLA‐ELDER and preapproval clinical trials70. In a phase 3 RCT for glycemic control study with ipragliflozin using Korean patients with type 2 diabetes, skin and subcutaneous tissue disorders were found in 2.3% of patients72. In contrast, in clinical trials in Western countries, the adverse events related to skin disorders have not been described, and appear higher in Asian people25, 33, 69, 70, 71, 72, 73. Skin disorders in patients treated with SGLT2 inhibitors are often reported between 2 to 4 weeks after initiation of the drug. Although very few cases are suspected to be drug eruption, most skin disorders are possibly diagnosed as dehydration‐related dyshidrotic eczema by skin specialists. There are some racial differences in the expression of various adverse events74.
Genital mycotic infection and urinary tract infection
It is well recognized that the prevalence of genital infections in people with diabetes is higher than that of non‐diabetic people75. Genital infections were confirmed to have a higher incidence in an empagliflozin‐treated group (men 5.0%, women 10.0%) compared with a placebo group (men 1.5%, women 2.6%), respectively13, and a canagliflozin‐treated group (men 3.8%, women 10.7%) compared with a placebo group26. Pooled analysis of the incidence of genital infection in Japanese female patients showed a higher incidence in an ipragliflozin‐treated group (5.3%) compared with a placebo group (1.9%)20. It is generally accepted that genital infections are seen at higher frequencies in female than male diabetes patients (Table 2). The high incidence rate of urinary tract infections is not further increased by treatment with SGLT2 inhibitors, as shown in both pooled analysis of ipragliflozin RCTs20 and the EMPA‐REG OUTCOME trial13. However, post‐marketing reports for SGLT2 inhibitors show possible risks for complications of acute pyelonephritis and sepsis from a urological origin. Therefore, screening for signs and symptoms of urinary tract infections might be necessary during treatment with SGLT2 inhibitors.
Cardiovascular Benefits Lessons from the EMPA‐REG OUTCOME Trial
Beneficial effects for prevention of cardiovascular events by intensive glucose control with medical treatment can only be observed after long‐term good control of hyperglycemia in both type 1 and type 2 diabetes mellitus patients76, 77. In the Action to Control Cardiovascular Risk in Diabetes (ACCORD) study, patients with near normal glycemic control with intensive medical treatments had a 22% higher risk for death compared with the standard group78. Therefore, there are unsolved cardiovascular safety concerns with glucose‐lowering treatment.
In the EMPA‐REG OUTCOME trial13, 7,020 patients with type 2 diabetes at high risk of cardiovascular diseases with 75–80% coronary artery disease and 23–25% stroke were randomly assigned into three groups (placebo 10 mg/day and empagliflozin 20 mg/day), and were followed for a median of 3.1 years. Mean baseline HbA1c was 8.1%, and more than 57% of patients had greater than a 10‐year diabetes duration. Background diabetes treatment was first unchanged for 12 weeks, and was then permitted to change depending on each patient's glycemic conditions. Placebo‐adjusted mean reductions in HbA1c in the empagliflozin treatment groups were −0.54% at 12 weeks and −0.24% at 206 weeks. The primary end‐points were defined as three‐point major adverse cardiovascular events, including death from cardiovascular causes, non‐fatal myocardial infarction and non‐fatal stroke, decreased by 14% (P = 0.04). For secondary end‐points, there were significantly lower rates of death from cardiovascular causes (by 38%), hospitalization for heart failure (by 35%) and death from any causes (by 35%). However, there were no significant differences in the rate of myocardial infarction or stroke between the placebo and the treatment groups13. Prevention of cardiovascular death in the empagliflozin group was found even in patients who were additionally treated with renin–angiotensin system (RAS) inhibitors (more than 80% of patients), β‐blocker (63–68% of patients), diuretics (37 or 59% of patients), statins (75–80% of patients) and/or aspirin (80–83% of patients). In subanalysis of heart failure‐related outcomes in the EMPA‐REG OUTCOME trial, empagliflozin effectively decreased both re‐hospitalization and new hospitalization for heart failure irrespective of the baseline state of heart failure. The number of patients needed to treat to prevent one heart failure hospitalization or cardiovascular death was calculated to be 35 over 3 years79.
Two possible mechanisms are suggested as the likely mechanisms behind the cardiovascular benefits of SGLT2 inhibitors: (i) hemodynamic effects including blood pressure lowering, diuretic effects and increased hematocrit58, 64, 65, 66; and (ii) metabolic effects relating to negative glucose and energy balance including ketone body production47, 48, and reductions in atherogenic risk factors including reductions of HbA1c, bodyweight, uric acid80 and triglyceride, as well as increases in HDL‐C17, 18, 19, 20, 25 (Figure 1). However, the very rapid emergence of cardiac benefits, which were detected 3 months after initiation of the clinical trial, suggests that hemodynamic improvements are a more likely mechanism for the beneficial outcome.
Figure 1.
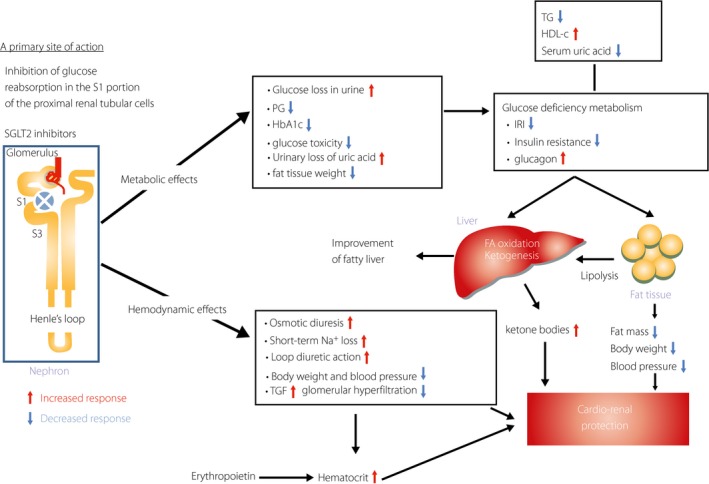
Multifactorial metabolic and hemodynamic effects of sodium–glucose cotransporter 2 (SGLT2) inhibitors to protect cardio‐renal outcomes. A primary site of action of SGLT2 inhibitors is specifically located in the S1 portion of the proximal renal tubular cells, and they inhibit Na/glucose cotransport and then the increased urinary glucose loss. This specific urinary glucose loss triggers profound metabolic and hemodynamic effects in vivo. The main parts of these multifactorial effects are shown: (i) reductions of plasma glucose, insulin and body fat mass, as well as bodyweight; (ii) osmotic diuresis and loop diuretic action with reductions of bodyweight and blood pressure, and activation of tubulo‐glomerular feedback mechanisms with consequently decreased glomerular hyperfiltration. In addition to those effects, increased plasma glucagon secretion is directly activated with the SGLT2 inhibitor treatment41, increased hematocrit is possibly related to erythropoietin secretion with an unknown mechanism59 and serum uric acid decreases80. Increased response is shown by a red arrow, and decreased response is shown by a blue arrow. FA, fatty acid; IRI, immunoreactive insulin; TGF, tubulo‐glomerular feedback.
SGLT2 inhibitors as diuretics might be expected to prevent cardiovascular events and heart failure. The mechanisms behind SGLT2 inhibitors as diuretics have been extensively discussed, and SGLT2 inhibitors have a similar effect as loop diuretics without induction of hypokalemia66, 81. SGLT2 inhibitors increase glucose concentration, and gradually decrease Cl– concentration in tubular fluid delivered to the loop of Henle because of continuous proximal tubular reabsorption of Cl– in the presence of a high concentration of unreabsorbed glucose. The reduction of Cl– concentration in the downstream tubular fluid inhibits reabsorption in the loop of Henle through inhibition of Na+‐K+‐2Cl– transporter, as a rate‐limiting step for reabsorption of water and solute in the loop of Henle is the intraluminal Cl– concentration66. Furthermore, this reduction of Cl‐concentration in the tubular fluid as well as osmotic diuresis with volume depletion and natriuresis may additively stimulate renin secretion and result in RAS activation59. Interestingly, heat failure hospitalization or cardiovascular death were effectively protected by co‐administration with a RAS inhibitor, but not with mineral corticoid receptor antagonists in the EMPA‐REG OUTCOME trial13.
The second hypothetical mechanisms are raised by Ferrannini et al.82 and Mudaliar et al.83. In the failing heart, the conservation of oxygen consumption is essential to maintain cardiac function. In terms of oxygen cost, the energy yield of beta‐hydroxybutyrate (β‐HB) is comparable with that of glucose and pyruvate, and lower than palmitate84. In perfused working hearts, β‐HB addition is readily taken up through a monocarboxylate transporter into the brain, heart and kidney85, and preferentially oxidized in mitochondria. β‐HB is exclusively produced in the liver, and the serum levels are reported to be increased in people with diabetes treated with SGLT2 inhibitors47, 48; a finding that might offer significant cardio‐protection in the failing heart in type 2 diabetes patients with high coronary risk factors86. A similar significant protection of a failing heart with β‐HB is reported in patients with chronic heart failure87, 88. These changes in energy metabolism are beneficial for the protection of chronic heart failure in type 2 diabetes. An increased percentage of hematocrit with SGLT2 inhibitors59 is another possible underling mechanism for energy supply to the failing heart. These proposed mechanisms could be related to the marked beneficial cardiac outcome in the EMPA‐REG OUTCOME trial13.
Renal Benefits Lessons from EMPA‐REG OUTCOME Trial
It is generally accepted that both strict glycemic and blood pressure control are essential for protection against the initiation and for the slow‐down of diabetic nephropathy. Furthermore, RAS inhibitors might also provide additional benefits for the prevention of diabetic nephropathy in type 2 diabetes89. However, there are still substantial residual risks for progression to the advanced stage of renal disease with present medical treatment90.
In a recent analysis of the EMPA‐REG OUTCOME trial, the prespecified secondary microvascular outcomes regarding incident or worsening nephropathy were studied14. The end‐points occurred in 12.7% in the empagliflozin group and 18.8% in the placebo group (hazard ratio in the empagliflozin group 0.61, P < 0.001). Hazard ratios for progression to macroalbuminuria, doubling of the serum creatinine level, and initiation of renal‐replacement therapy in the empagliflozin group were 0.62 (P < 0.001), 0.56 (P < 0.001) and 0.45 (P < 0.04), respectively. In contrast, there was no significant difference in the incidence rate for microalbuminuria in patients with normal albuminuria at baseline between the placebo and treatment groups. The authors concluded that empagliflozin had potent effects preventing the progression from pre‐ and early diabetic nephropathy into overt diabetic kidney disease, as well as further progression of the advanced stage of diabetic kidney disease in patients with type 2 diabetes at high cardiovascular risk. Interestingly, these results were observed in patients whose blood pressure was well managed with extensive use of RAS inhibitors.
Regarding these beneficial effects of empagliflozin in this trial, increased supply of Na+ deliveries to the macula densa might exert strong benefits through activating tubulo‐glomerular feedback, leading to afferent arteriolar vasocontraction and then a decrease in glomerular hyperfiltration91. It has been consistently reported that empagliflozin reduces the intraglomerular hyperfiltration in patients with type 1 diabetes as a result of tubule‐glomerular feedback regulation92, 93. It is possible that beneficial renal effects in the EMPA‐REG OUTCOME trial might be partly explained by multifactorial risk reductions including modest diuresis with lowered blood pressure, and reductions of HbA1c, bodyweight and uric acid in the empagliflozin‐treatment group compared with the placebo group.
High glucose concentrations in proximal renal tubular cells stimulate Na+/glucose reabsorption through SGLT2, which activates Na+/K+ ATPase and results in increased tubular oxygen consumption, and might be related to tubular hypoxia94. In terms of renoprotective action, renal tubular hypoxia should be improved as part of the treatment target in diabetes. A recent study suggests that tubulo‐interstitial hypoxia is a significant common pathway in the progression to end‐stage renal disease95. Renal cortical hypoxia improved with administration of a non‐specific SGLT inhibitor, phlorizin96. Thus, SGLT2 inhibitors in the treatment of diabetes could lead to less hypoxic stress on the diabetic kidney.
To prevent hypoxia in renal tubular cells, ketone bodies in renal cells are a more efficient fuel than glucose and free fatty acids on a molar basis97. Serum ketone concentrations are known to increase twice the level of placebo groups47, 48, 86. Ketone bodies are therefore the preferred renal fuel under the increased supply with treatment with SGLT2 inhibitors. Ketone bodies inhibit the pyruvate oxidation, as well as the uptake and oxidation of oleate, and modulate gene expression to promote resistance to oxidative stress98, 99. SGLT2 inhibitors can improve fuel efficiency, thereby lowering oxygen consumption, which relieves hypoxic stress, improves renal function and prevents progression of kidney disease in part through an increase of ketone body production.
In terms of renal tubular hypoxia protection in diabetes with treatment with SGLT2 inhibitors, another possible mechanism is suggested to relate to increased hematocrit after treatment. An increased hematocrit concentration might be associated with oxygen delivery to hypoxic tissues. One explanation for the elevated hematocrit in patients treated with SGLT2 inhibitors is generally accepted to be as a result of hemoconcentration due to osmotic diuresis. However, it seems unlikely that hemoconcentration during diuretic therapy is a major cause of increased hematocrit100, 101. Another new interesting possible mechanism for increased hematocrit is proposed to be an increased erythropoietin concentration with treatment with dapagliflozin in diabetes59. In terms of renoprotective effects of SGLT2 inhibitors found in the EMPA‐REG OUTCOME trial, multifactorial mechanisms proposed include: (i) activation of a renal factor, such as a tubule‐glomerular feedback mechanism; (ii) protection from renal tubular hypoxia through increased ketone body production and an increase in hematocrit concentration; and (iii) improvements of renal risk factors including HbA1c, a specific diuretic action with lowering blood pressure and bodyweight reduction, as well as the serum uric acid concentration in the long‐term renal benefits.
Conclusion
SGLT2 inhibitors are classified as unique oral glucose‐lowering drugs. They have potent glucose‐lowering effects, which are insulin‐independent and show a negative glucose energy balance because of urinary glucose loss, and then fatty acid oxidation and ketone body production are activated in the liver. They also improve insulin secretion and insulin sensitivity based on relief of glucose toxicity. SGLT2 inhibitors also have a loop‐like diuretic action, which is associated with reductions of blood pressure and bodyweight, and then increase in hematocrit with undefined mechanisms. These metabolic and hemodynamic effects of SGLT2 inhibitors showed profound beneficial effects in the prevention of cardio‐renal events, as shown in the EMPA‐REG OUTCOME trial. However, it should be noted that SGLT2 inhibitors can induce ketoacidosis, increased risks for genital and urinary tract infections, and skin disorders in some patients. The beneficial effects should be further confirmed in future studies using people of different ethnicities and in patients with different stages of type 2 diabetes, as well as in patients treated with other SGLT2 inhibitors.
Disclosure
A Kashiwagi is a consultant for and has received consulting fees/honoraria from Astellas Pharma Inc. and Sunstar Group. H Maegawa has received lecture fees from MSD, Nippon Boehringer Ingelheim, Astellas Pharma, Ono Pharmaceutical, Mitsubishi‐Tanabe Pharma, Sanofi, Taisho‐Toyama Pharmaceutical, Takeda Pharmaceutical, Kowa Pharmaceutical, Daiichi Sankyo, AstraZeneca, Sanwa Kagaku Kenkyusho, Novartis Pharma and Eli Lilly; research support from Astellas Pharma and AstraZeneca; and grants from Takeda Pharmaceutical, Astellas Pharma, MSD, Nippon Boehringer Ingelheim, Kyowa Hakko Kirin, Taisho‐Toyama Pharmaceutical, Kowa Pharmaceutical, Ono Pharmaceutical, Daiichi Sankyo, Sanofi, Mitsubishi‐Tanabe Pharma, Shionogi, Chugai, Sunstar, Otsuka Pharmaceutical, Sanwa Kagaku Kenkyusho, Dainippon‐Sumitomo Pharma, Eizai, Pfizer, Novo Nordisk, Mochida Pharmaceutical, Novartis Pharma and Bristol‐Myers Squibb.
J Diabetes Investig 2017; 8: 416–427
References
- 1. Kanai Y, Lee WS, You G, et al The human kidney low affinity Na+/glucose cotransporter SGLT2, Delineation of the major renal reabsorptive mechanism for D‐glucose. J Clin Invest 1994; 93: 397–404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Wright EM, Loo DD, Hirayama BA. Biology of human sodium glucose transporters. Physiol Rev 2011; 91: 733–794. [DOI] [PubMed] [Google Scholar]
- 3. Lee WS, Kanai Y, Wells RG, et al The high affinity Na+/glucose cotransporter. J Biol Chem 1994; 269: 12032–12039 [PubMed] [Google Scholar]
- 4. Bailey CJ. Renal glucose reabsorption inhibitors to treat diabetes. Trends Pharmacol Sci 2011; 32: 63–71. [DOI] [PubMed] [Google Scholar]
- 5. Abdul‐Ghani M, DeFronzo R, Norton L. Novel hypothesis to explain why SGLT2 inhibitors inhibit only 30‐50% of filtered glucose load in humans. Diabetes 2013; 62: 324–3328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Isaji M. SGLT2 inhibitors: molecular design and potential differences in effect. Kidney Int 2011; 79(Suppl 120): S14–S19. [DOI] [PubMed] [Google Scholar]
- 7. Kadokura T, Akiyama N, Kashiwagi A, et al Pharmacokinetic and pharmaco dynamic study of ipragliflozin in Japanese patients with type 2 diabetes mellitus: a randomized, double‐blind, placebo‐controlled study. Diab Res Clin Pract 2014; 106: 50–56. [DOI] [PubMed] [Google Scholar]
- 8. Sha S, Polidori D, Farrell K, et al Pharmacodynamic differences between canagliflozin and dapagliflozin: results of a randomized, double‐blind, crossover study. Diabetes Obesity Metab 2015; 17: 188–197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Sarashina A, Koiwai K, Seman LJ, et al Safety, tolerability, pharmacokinetics and pharmacodynamics of single doses of empagliflozin, a sodium glucose cotransporter 2 (SGLT2) inhibitor, in healthy Japanese subjects. Drug Metab Pharamacokinet 2013; 28: 213–219. [DOI] [PubMed] [Google Scholar]
- 10. Rosenwasser RF, Rosenwasser JN, Sutton D, et al Tofogliflozin: a highly serective SGLT2 inhibitor for the treatment of type 2 diabetes. Drug of Today 2014; 50: 739–745. [DOI] [PubMed] [Google Scholar]
- 11. Fujita Y, Inagaki N. Renal sodium glucose cotransporter 2 inhibitors as a novel therapeutic approach to treatment of type 2 diabetes: clinical data and mechanism of action. J Diabetes Investig 2014; 5: 265–275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Yabe D, Nishikino R, Kaneko M, et al Short‐term impacts of sodium/glucose co‐transporter 2 inhibitors in Japanese clinical practice: considerations for their appropriate use to avoid serious adverse events. Expert Opin Drug Saf 2015; 14: 1–6. [DOI] [PubMed] [Google Scholar]
- 13. Zinman B, Christoph W, Lachin JM, et al Empagliflozin, cardiovascular outcomes and mortality in type 2 diabetes. N Engl J Med 2015; 373: 2117–2128. [DOI] [PubMed] [Google Scholar]
- 14. Wanner C, Inzucchi SE, Lachin JM, et al Empagliflozin and progression of kidney disease in type 2 diabetes. N Engl J Med 2016; 375: 323–334. [DOI] [PubMed] [Google Scholar]
- 15. Inzucchi SE, Zinman B, Wanner C, et al SGLT‐2 inhibitors and cardiovascular risk: proposed pathway and review of on‐going outcome trials. Diab Vasc Dis Res 2015; 12: 90–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Ferrannini G, Hach T, Crowe S, et al Energy balance after sodium–glucose cotransporter 2 inhibition. Diabetes Care 2015; 38: 730–1735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Kashiwagi A, Kazuta K, Yoshida S, et al Randomized, placebo‐controlled, double‐blind glycemic control trial of novel sodium‐dependent glucose cotransporter 2 inhibitor ipragliflozin in Japanese patients with type 2 diabetes mellitus. J Diabetes Investig 2014; 5: 382–391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Kashiwagi A, Kazuta K, Takinami Y, et al Ipragliflozin improves glycemic control in Japanese patients with type 2 diabetes mellitus: the BRIGHTEN study. Diabetol Int 2015; 6: 8–18. [Google Scholar]
- 19. List JF, Woo V, Morales E, et al Sodium‐glucose cotransport inhibition with dapagliflozin in type 2 diabetes. Diabetes Care 2009; 32: 650–657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Kashiwagi A, Yoshida S, Nakamura I, et al Efficacy and safety of ipragli‐flozin in Japanese patients with type 2 diabetes stratified by body mass index: a subgroup analysis of five randomized clinical trials. J Diabetes Investig 2016; 7: 544–554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Kashiwagi A, Akiyama N, Shiga T, et al Efficacy and safety of ipragliflozin as an add‐on to a sulfonylurea in Japanese patients with inadequately controlled type 2 diabetes: results of the randomized, placebo‐controlled, double blind, phase III EMIT study. Diabetol Int 2015; 6: 125–138. [Google Scholar]
- 22. Kashiwagi A, Kazuta K, Goto K, et al Ipragliflozin in combination with metformin for the treatment of Japanese patients with type 2 diabetes: ILLUMINATE, a randomized, double‐blind, placebo‐controlled study. Diabetes Obes Metab 2015; 17: 304–308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Kashiwagi A, Shiga T, Akiyama N, et al Efficacy and safety of ipragliflozin as an add‐on to pioglitazone in Japanese patients with inadequately controlled type 2 diabetes: a randomized, double‐blind, placebo‐controlled study (the SPOTLIGHT study). Diabetol Int 2015; 6: 104–116. [Google Scholar]
- 24. Wilding JPH, Charpentier G, Hollander P, et al Efficacy and safety of canagliflozin in patients with type 2 diabetes mellitus inadequately controlled with metformin and sulphonylurea: a randomized trial. Int J Clinical Pract 2013; 67: 1267–1282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Cefalu WT, Leiter LA, Yoon KH, et al Efficacy and safety of canagliflozin versus glimepiride in patients with type 2 diabetes inadequately controlled with metformin (CANTATA‐SU): 52 week results from a randomised, double‐blind, phase 3 non‐inferiority trial. Lancet 2013; 382: 941–950. [DOI] [PubMed] [Google Scholar]
- 26. Lavalle‐González FJ, Januszewicz A, Davidson J, et al Efficacy and safety of canagliflozin compared with placebo and sitagliptin in patients with type 2 diabetes on background metformin monotherapy: a randomised trial. Diabetologia 2013; 56: 2582–2592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Nishimura B, Tanaka Y, Koiwai K, et al Effect of empagliflozin monotherapy on postprandial glucose and 24‐hour glucose variability in Japanese patients with type 2 diabetes mellitus: a randomized, double blind, placebo‐controlled, crossover study. Diabetes Obes Metab 2015; 17: 800–804.25930989 [Google Scholar]
- 28. Yamada A, Nakayama H, Yoshinobu S, et al Effects of a sodium glucose co‐transporter 2 selective inhibitor, ipragliflozin, on the diurnal profile of plasma glucose in patients with type 2 diabetes: a study using continuous glucose monitoring. J Diabetes Investig 2015; 6: 699–707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Seino Y, Inagaki N, Haneda M, et al Efficacy and safety of luseogliflozin added to various oral anti‐diabetic drugs in Japanese patients with type 2 diabetes. J Diabetes Investig 2015; 6: 443–453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Kohna DE, Fioretto P, Tang W, et al Long‐term study of patients with type 2 diabetes and moderate renal impairment shows that dapagliflozin reduces weight and blood pressure but does not improve glycemic control. Kidney Int 2014; 85: 962–971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Yale J‐F, Bakris G, Cariou B, et al Efficacy and safety of canagliflozin over 52 weeks in patients with type 2 diabetes mellitus and chronic kidney disease. Diabetes Obes Metab 2014; 16: 1016–1027. [DOI] [PubMed] [Google Scholar]
- 32. Haneda M, Seino Y, Inagaki N, et al Influence of renal function on the 52 week efficacy and safety of the sodium glucose cotransporter 2 inhibitor luseogliflozin in Japanese patients with type 2 diabetes mellitus. Clin Ther 2016; 38: 66–88. [DOI] [PubMed] [Google Scholar]
- 33. Schernthaner G, Gross JL, Rosenstock J, et al Canagliflozin compared with sitagliptin for patients with type 2 diabetes who do not have adequate glyce mic control with metformin plus sulfonylurea. A 52‐week randomized trial. Diabetes Care 2013; 36: 2508–2515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Leiter LA, Yoon K‐H, Arios P, et al Canagliflozin provides durable glycemic improvements and body weight reduction over 104 weeks versus glimepiride in patients with type 2 diabetes on metformin: a randomized, double‐blind, phase 3 study. Diabetes Care 2015; 38: 355–364. [DOI] [PubMed] [Google Scholar]
- 35. Prato SD, Nauck M, Duran‐Garcia S, et al Long‐term glycaemic response and tolerability of dapagliflozin versus a sulfonylurea as add‐on therapy to metformin in patients with type 2 diabetes: 4‐year data. Diabetes Obes Metab 2015; 17: 581–590. [DOI] [PubMed] [Google Scholar]
- 36. Ferrannini E, Muscelli E, Frascerra S, et al Metabolic response to sodium‐glucose cotransporter 2 inhibition in type 2 diabetic patients. J Clin Invest 2013; 124: 499–508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Merovci A, Solis‐Herrera C, Daniele G, et al Dapagliflozin improves muscle insulin sensitivity but enhances endogenous glucose production. J Clin Invest 2014; 124: 509–514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Rossetti L, Smith D, Shulman GI, et al Correction of hyperglycemia with phlorizin normalizes tissue sensitivity to insulin. J Clin Invest 1987; 79: 1510–1515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Rossetti L, Giaccari A, DeFronzo RA. Glucose toxicity. Diabetes Care 1990; 13: 610–630. [DOI] [PubMed] [Google Scholar]
- 40. Cefalu WT. Paradoxical insights into whole body metabolic adaptations following SGLT2 inhibition. J Clin Invest 2015; 124: 485–487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Bonner C, Kerr‐Conte J, Gmyr V, et al Inhibition of the glucose transporter SGLT2 with dapagliflozin in pancreatic alpha cells triggers glucagon secretion. Nat Med 2015; 21: 512–517. [DOI] [PubMed] [Google Scholar]
- 42. Polidori D, Mari A, Ferrannini E. Canagliflozin, a sodium glucose co‐transporter 2 inhibitor, improves model‐based indices of beta cell function in patients with type 2 diabetes. Diabetologia 2014; 57: 891–901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Takahara M, Shiraiwa T, Matsuoka T, et al Ameliorated pancreatic β cell dysfunction in type 2 diabetic patients treated with a sodium‐glucose cotransporter 2 inhibitor ipragliflozin. Endocrine J 2015; 62: 77–86. [DOI] [PubMed] [Google Scholar]
- 44. Leiter LA, Forst T, Polidori D, et al Effect of canagliflozin on liver function tests in patients with type 2 diabetes. Diabetes Metab 2016; 42: 25–32. [DOI] [PubMed] [Google Scholar]
- 45. McPherson PA, McEneny J. The biochemistry of ketogenesis and its role in weight management, neurological disease and oxidative stress. J Physiol Biochem 2011; 68: 141–151. [DOI] [PubMed] [Google Scholar]
- 46. Laffel L. Ketone bodies: a review of physiology, pathophysiology and application of monitoring to diabetes. Diabetes Metab Res Rev 1999; 15: 412–426. [DOI] [PubMed] [Google Scholar]
- 47. Taylor SI, Blau JE, Rother KI. SGLT2 Inhibitors. Predispose to Ketoacidosis. J Clin Endocrinol Metab 2015; 100: 2849–2852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Kaku K, Watada H, Iwamoto Y, et al Efficacy and safety of monotherapy with the novel sodium/glucose cotransporter‐2 inhibitor tofogliflozin in Japanese patients with type 2 diabetes mellitus: a combined Phase 2 and 3 randomized, placebo‐controlled, double‐blind, parallel‐group comparative study. Cardiovasc Diabetol 2014; 13: 65–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. US Food and Drug Administration . Drug Safety Communication: FDA warns that SGLT2 inhibitors for diabetes may result in a serious condition of too much acid in the blood [Internet], 15 May 2015. Available from: http://www.fda.gov/downloads/Drugs/DrugSafety/UCM446954.pdf Accessed June 22, 2015.
- 50. European Medicines Agency . Review of diabetes medicines called SGLT2 inhibitors started: risk of diabetic ketoacidosis to be examined [Internet], 12 June 2015. Available from: http://www.ema.europa.eu/docs/en_GB/document_library/Referrals_document/SGLT2_inhibitors_20/Procedure_started/WC500187926 Accessed June 12, 2015.
- 51. Erondu N, Desai M, Ways K, et al Diabetic ketoacidosis and related events in the canagliflozin type 2 diabetes clinical program. Diabetes Care 2015; 38: 1680–1686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Rosenstock J, Ferrannini E. Euglycemic Diabetic Ketoacidosis: a predictable, detectable, and preventable safety concern with SGLT2 inhibitors. Diabetes Care 2015; 38: 1638–1642. [DOI] [PubMed] [Google Scholar]
- 53. Peters AL, Buschur EO, Buse JB, et al Euglycemic diabetic ketoacidosis: a potential complication of treatment with sodium–glucose cotransporter 2 inhibition. Diabetes Care 2015; 38: 1687–1693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Hayami T, Kato Y, Kamiya H, et al Case of ketoacidosis by a sodium‐glucose cotransporter 2 inhibitor in a diabetic patient with a low carbohydrate diet. J Diabetes Investig 2015; 6: 587–590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Ministry of Health, Labor, and Welfare, Japan . Patient Survey. 2011. (Disease and Injury) (Japanese). Available at: http://www.mhlw.go.jp/toukei/saikin/hw/kanja/10syoubyo Accessed April 2015.
- 56. Emdin CA, Rahimi K, Neal B, et al Blood pressure lowering in type 2 diabetes: a systematic review and meta‐analysis. JAMA 2015; 313: 603–615. [DOI] [PubMed] [Google Scholar]
- 57. UK Prospective Diabetes Study Group . Tight blood pressure control and risk of macrovascular and microvascular complications in type 2 diabetes: UKPDS 38. BMJ 1998; 317: 703–713. [PMC free article] [PubMed] [Google Scholar]
- 58. Oliva RV, Bakris GL. Blood pressure effects of sodium‐glucose co‐transport 2 (SGLT2) inhibitors. J Am Soc Hypertens 2014; 8: 330–339. [DOI] [PubMed] [Google Scholar]
- 59. Lambers Heerspink HJ, de Zeeuw D, Wie L, et al Dapagliflozin a glucose‐regulating drug with diuretic properties in subjects with type 2 diabetes. Diabetes Obes Metab 2013; 15: 853–862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Tikkanen I, Narko K, Zeller C, et al Empagliflozin reduces blood pressure in patients with type 2 diabetes and hypertension. Diabetes Care 2015; 38: 420–428. [DOI] [PubMed] [Google Scholar]
- 61. Kashiwagi A, Yoshida S, Kawamura K, et al Effects of ipragliflozin, a selective sodium–glucose co‐transporter 2 inhibitor on blood pressure in Japanese patients with type 2 diabetes mellitus: a pooled analysis of six randomized, placebo‐controlled clinical trials. Diabetol Int 2017; 8: 76–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Baker WL, Smyth LR, Riche DM, et al Effects of sodium‐glucose co‐transporter 2 inhibitors on blood pressure: a systematic review and meta‐analysis. J Am Soc Hypertens 2014; 8: 262–275. [DOI] [PubMed] [Google Scholar]
- 63. Sha S, Polidori D, Heise T, et al Effect of the sodium glucose co‐transporter 2 inhibitor canagliflozin on plasma volume in patients with type 2 diabetes mellitus. Diabetes Obes Metab 2016; 16: 1087–1095. [DOI] [PubMed] [Google Scholar]
- 64. Maliha G, Townsend RR. SGLT2 inhibitors: their potential reduction in blood pressure. J Am Soc Hypertens 2015; 9: 48–53. [DOI] [PubMed] [Google Scholar]
- 65. Majewski C, Bakris GL. Blood pressure reduction: an added benefit of sodium‐glucose cotransporter 2 inhibitors in patients with type 2 diabetes. Diabetes Care 2015; 38: 429–430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Kimura G. Importance of inhibiting sodium‐glucose cotransporter and its compelling indicator in type 2 diabetes: pathophysiological hypothesis. J Am Soc Hypertension 2016; 10: 271–278. [DOI] [PubMed] [Google Scholar]
- 67. Bolinder J, Ljunggren O, Kullberg J, et al Effects of dapagliflozin on body weight, total fat mass, and regional adipose tissue distribution in patients with type 2 diabetes mellitus with inadequate glycemic control on metformin. J Clin Endocrinol Metab 2012; 97: 1020–1031. [DOI] [PubMed] [Google Scholar]
- 68. Wilding JP, Norwood PT, Joen C, et al A study of dapagliflozin in patients with type 2 diabetes receiving high doses of insulin plus insulin sensitizers: applicability of a novel insulin‐independent treatment. Diabetes Care 2009; 32: 1656–1662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Moses RG, Colagiuri S, Pollock C. SGLT2 inhibitors: new medicines for addressing unmet needs in type 2 diabetes. Australas Med J 2014; 7: 405–415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Yokote K, Terauchi Y, Nakamura I, et al Real‐world evidence for the safety of ipragliflozin in elderly Japanese patients with type 2 diabetes mellitus (STELLA‐ELDER): final results of a post‐marketing surveillance study. Expert Opin Pharmacother 2016; 17: 1995–2003. [DOI] [PubMed] [Google Scholar]
- 71. Nauck MA, Del Prato S, Meier U, et al Dapagliflozin versus glipizide as add‐on therapy in patients with type 2 diabetes who have inadequate glycemic control with metformin: a randomized, 52‐week, double‐blind, active‐controlled noninferiority trial. Diabetes Care 2011; 34: 2015–2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Lu C‐H, Min KW, Chuang L‐M, et al Efficacy, Safety, and tolerability of ipragliflozin in Asian patients with type 2 diabetes mellitus and inadequate glycemic control with metformin: results of phase 3 randomized placebo‐controlled, double‐blind, multicenter trial. J Diabetes Investig 2016; 7: 366–373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Dailey G. Empagliflozin for the treatment of type 2 diabetes mellitus: an overview of safety and efficacy based on phase 3 trials. J Diabetes 2015; 7: 448–461. [DOI] [PubMed] [Google Scholar]
- 74. Gavin JR III, Davies MJ, Davies M, et al The efficacy and safety of canagliflozin across racial groups in patients with type 2 diabetes mellitus. Curr Med Res Opin 2015; 31: 1693–1702. [DOI] [PubMed] [Google Scholar]
- 75. Hirji I, Andersson SW, Guo Z, et al Incidence of genital infection among patients with 2 diabetes in the UK General Practice Research Database. J Diabetes Complications 2012; 26: 501–505. [DOI] [PubMed] [Google Scholar]
- 76. Holman RR, Paul SK, Bethel MA, et al 10‐year follow‐up of intensive glucose control in type 2 diabetes. N Engl J Med 2008; 359: 1577–1589. [DOI] [PubMed] [Google Scholar]
- 77. The Diabetes Control and Complications Trial/Epidemiology of Diabetes . In terventions and Complications Research Group. Effect of intensive therapy on the microvascular complications of type 1 diabetes mellitus. JAMA 2002; 287: 2563–2569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Gerstein HC, Miller ME, Byington RP, et al Action to control cardiovascular risk in diabetes study group. Effects of intensive glucose lowering in type 2 diabetes. N Engl J Med 2008; 358: 2545–2559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Fitchett D, Zinman B, Christone W, et al Heart failure outcomes with empagliflozin in patients with type 2 diabetes at high cardiovascular risk: results of the EMPA‐REG OUTCOME trial. Eur Heart J 2016; 37: 1526–1534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Chino Y, Samukawa Y, Sakai S, et al SGLT2 inhibitor lowers serum uric acid through alteration of uric acid transport activity in renal tubule by increased glycosuria. Biopharm Drug Dispos 2014; 35: 391–404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Kimura G. Diuretic action of sodium‐glucose cotransporter 2 inhibitors and its importance in the management of heart failure. Circ J 2016; 80: 2277–2281. [DOI] [PubMed] [Google Scholar]
- 82. Ferrannini E, Mark M, Mayoux E. CV protection in EMPA‐REG OUTCOME trial: A “thrifty substrate”. Diabetes Care 2016; 39: 29–35. [DOI] [PubMed] [Google Scholar]
- 83. Mudaliar S, Alloju S, Henry RR. Can a shift in fuel energetics explain the beneficial cardiorenal outcomes in the EMPA‐REG OUTCOME study? A unifying hypothesis Diabetes Care 2016; 39: 36–43. [DOI] [PubMed] [Google Scholar]
- 84. Sato K, Kashiwaya Y, Keon CA, et al Insulin, ketone bodies, and mitochondrial energy transduction. FASEB J 1995; 9: 651–658. [DOI] [PubMed] [Google Scholar]
- 85. Balasse EO, Fery F. Ketone body production and disposal: effects of fasting, diabetes, and exercise. Diabetes Metab Rev 1989; 5: 247–270. [DOI] [PubMed] [Google Scholar]
- 86. Ferrannini E, Baldi S, Frascerra S, et al Shift to fatty substrate utilization in response to sodium‐glucose cotransporter 2 inhibition in subjects without diabetes and patients with type 2 diabetes. Diabetes 2016; 65: 1190–1195. [DOI] [PubMed] [Google Scholar]
- 87. Lommi J, Kupari M, Koskinen P, et al Blood ketone bodies in congestive heart failure. J Am Coll Cardiol 1996; 28: 665–6729. [DOI] [PubMed] [Google Scholar]
- 88. Janardhan A, Chen J, Crawford PA. Altered systemic ketone body metabolism in advanced heart failure. Tex Heart Inst J 2011; 38: 533–538. [PMC free article] [PubMed] [Google Scholar]
- 89. Brenner BM, Cooper ME, deZeew D, et al Effects of losartan on renal and cardiovascular outcomes in patients with type 2 diabetes and nephropathy. N Engl J Med 2001; 345: 861–869. [DOI] [PubMed] [Google Scholar]
- 90. Fioretto P, Dodson PM, Ziegler D, et al Residual microvascular risk in diabetes: unmet needs and future directions. Nat Rev Endocrinol 2010; 6: 19–25. [DOI] [PubMed] [Google Scholar]
- 91. Vallon V, Gerasimova M, Rose MA, et al SGLT2 inhibitor empagliflozin reduces renal growth and albuminuria in proportion to hyperglycemia and prevents glomerular hyperfiltration in diabetic Akita mice. Am J Physiol Renal Physiol 2014; 306: F194–F204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Skric M, Yang GK, Perkins BA, et al Characterization of glomerular haemodynamic responses to SGLT2 inhibition in patients with type 1 diabetes and renal hyperfiltration. Diabetologia 2014; 57: 2599–2602. [DOI] [PubMed] [Google Scholar]
- 93. Cherney DZ, Perkins BA, Soleymalon N, et al Renal hemodynamic effect of sodium‐glucose cotransporter 2 inhibition in patients with type 1 diabetes mellitus. Circulation 2014; 129: 587–597. [DOI] [PubMed] [Google Scholar]
- 94. Korner A, Ekiof AC, Celsi G, et al Increased renal metabolism in diabetes. Mechanism and functional implications. Diabetes 1994; 43: 629–633. [DOI] [PubMed] [Google Scholar]
- 95. Nangaku M. Chronic hypoxia and tubulointerstitial injury: a final common pathway to end‐stage renal failure. J Am Soc Nephrol 2006; 17: 17–25. [DOI] [PubMed] [Google Scholar]
- 96. O'Neill J, Fasching A, Pihl L, et al Acute SGLT inhibition normalizes O2 tension in the renal cortex but causes hypoxia in the renal medulla in anaesthetized control and diabetic rats. Am J Physiol Renal Physiol 2015; 309: F227–F234. [DOI] [PubMed] [Google Scholar]
- 97. Cahill GF Jr. Fuel metabolism in starvation in starvation. Ann Rev Nutr 2006; 26: 1–22. [DOI] [PubMed] [Google Scholar]
- 98. Guder WG, Wagner S, Wirthensohn G, et al Metabolic fuels along the nephron: pathways and intracellular mechanisms of interaction. Kidney Int 1986; 29: 41–45. [DOI] [PubMed] [Google Scholar]
- 99. Shimazu T, Hirschey MD, Newman J, et al Suppression of oxidative stress by beta‐hydroxybutyrate, an endogenous histone deacetylase inhibitor. Science 2013; 339: 211–214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Greene SJ, Gheorghiade M, Vaduganathan M, et al Hemoconcentration, renal function, and post‐discharge outcomes among patients hospitalized for heart failure with reduced ejection fraction: insights from the EVEREST trial. Eur J Heart Fail 2013; 15: 1401–1411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Sano M, Takei M, Shiraishi Y, et al Increased hematocrit during sodium‐glucose cotransporter 2 inhibitor therapy indicates recovery of tubulointerstitial function in diabetic kidneys. J Clin Med Res 2016; 8: 844–847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Kaku K, Maegawa H, Tanizawa Y, et al Dapagliflozin as monotherapy or combination therapy in Japanese patients with type 2 diabetes: an open‐label study. Diabetes Ther 2014; 5: 415–433. [DOI] [PMC free article] [PubMed] [Google Scholar]


