Abstract
Aims/Introduction
Epidemiological evidence for the effect of omega‐3 fatty acids on the risk of type 2 diabetes is controversial. A meta‐analysis based on prospective cohorts was carried out to evaluate this issue.
Materials and Methods
Pooled diabetic risk was calculated using a fixed or random effects model. The dose–response relationship was assessed by meta‐regression analysis.
Results
The study showed that consumption of single omega‐3 was associated with an increased risk of type 2 diabetes (relative risk [RR] = 1.45, P < 0.001); whereas the RR for mixed omega‐3 was statistically insignificant. The dose–response curve presented an inverted U‐shape of diabetes risk corresponding to the dose of omega‐3 consumption. Subanalysis showed that omega‐3 was inversely associated with type 2 diabetes risk in Asians (RR = 0.82, P < 0.001); whereas the risk was increased in Westerners (RR = 1.30, P < 0.001). Studies with follow‐up duration ≥16 years and baseline age ≥54 years showed a positive association between type 2 diabetes risk and omega‐3 intake.
Conclusions
The present findings suggest that dosage and composition of omega‐3, ethnicity, trial duration, and age could influence the effect of omega‐3 on type 2 diabetes progression.
Keywords: Meta‐analysis, Omega‐3 fatty acids, Type 2 diabetes
Introduction
Type 2 diabetes is a complex metabolic disorder characterized by chronic hyperglycemia, the prevalence of which is estimated to rise from 171 million in 2000 to 366 million in 2030 worldwide1, 2, 3. There are various factors contributing to the growth of diabetic incidence, and daily diet stands out as an important factor4, 5.
Since the year 1966, it has been reported that the incidence of type 2 diabetes has been significantly reduced with high fish and seafood consumption6, 7, 8 in northwestern Greenland, which might be attributed to the effect of omega‐3 fatty acids, the predominant fatty acid composition of seafood. In the past decades, notwithstanding plenty of reports that have been published about the effects of omega‐3 fatty acids on diabetes prevention, discrepancies still remain. Several cohort studies showed that high intake of omega‐3 fatty acids related to a lower prevalence of type 2 diabetes5, 9, 10, 11; whereas some other studies showed positive12, 13 or null associations14, 15. Inconsistent results were also reported in clinical trials that investigated the effect of fish oil supplementation on glucose homeostasis16, 17, 18, 19, 20.
Recent published systematic reviews reported that omega‐3 fatty acids supplementation was either positively or insignificantly associated with type 2 diabetes development21, 22, 23, 24, 25. These conclusions suggest an unfavorable effect of omega‐3 supplementation on people who are prone to develop to diabetes; for example, people with obesity, insulin resistance and hyperlipidemia. However, some other systematic reviews reported that omega‐3 has beneficial effects on metabolic‐related diseases; it exerts a cardioprotective effect, reduces ischemic stroke risk, corrects high triglycerides level and increases insulin sensitivity21, 26. These contradicting notions might confound physicians and nutritionists on dietary guidance. Additionally, these meta‐analysis papers failed to dissect the source that results in this contradiction. We therefore carried out a meta‐analysis with a dose–response model and subgroup analysis in prospective cohort studies to evaluate the potential factors that influence the effect of omega‐3 fatty acids consumption on type 2 diabetes incidence.
Methods
Data sources and searches
A comprehensive literature search was carried out of the Pubmed, Cochrane Library, Medline, SIGLE and EMBASE databases, and National Research Register with the last date of inclusion to be the end of May 2016. The Medical Subject Heading terms and keywords for database searching included (i) omega‐3 or n‐3 or ω‐3 fatty acids; (ii) docosapentaenoic acid or DPA; (iii) eicosapentaenoic acid or EPA; (iv) docosahexaenoic acid or DHA; and (v) fish oil(s). We combined these terms with diabetes mellitus, type 2 diabetes or T2D, which was described in detail in our previous work26. Cross‐references of studies or reviews were also examined manually.
Two investigators (Shao and Chen) worked independently to determine the eligible studies by reviewing the titles, abstracts and keywords. The manuscripts were obtained in full‐text version for further assessment if the study: (i) was a cohort design investigating the association between omega‐3 supplementation and the incidence of type 2 diabetes; (ii) analyzed relative risk (RR), hazard ratios (HR) or odds ratios (OR) with 95% confidence interval (CI); (iii) reported at least three quantitative exposure levels of omega‐3 for dose–response analysis; (iv) showed the method of dietary assessment; and (v) included the participants at baseline who were not diagnosed as type 2 diabetes.
Data extraction
Data extraction was independently carried out by Shao and Chen. The extracted information included composition and intake amounts of omega‐3, number of cases and person‐years of follow up in each exposure category, follow‐up years, study setting, diabetes diagnosis, baseline characteristics of included participants (number of participants, age at recruitment, sex and ethnicity), and the adjusted RR with 95% CI. For studies with OR or HR data, we converted OR and HR into RR using a previously published formula27. The corresponding CI values were also converted. When studies reported results with different models for variable adjustment, data were extracted from models including the most potential confounders.
Quality assessment
Quality assessment of cohort studies was carried out based on the Newcastle–Ottawa Scale Criteria28, which were performed by Shao and Chen independently with discrepancies resolved by Yang. The maximum score that can be assigned by the Newcastle–Ottawa Scale is 9 points in three broad items: (i) selection of study groups (up to 4 points); (ii) comparability of groups (up to 2 points); and (iii) assessment of exposure and outcomes (up to 3 points). The overall evaluation of included trials is presented in Table S1.
Statistical analysis
The meta‐analysis was carried out using stata 11.0 (StataCorp, College Station, TX, USA). Extracted data from cohort studies were analyzed with a fixed effects model to calculate the pooled RR comparing the highest versus the lowest intake of eicosapentaenoic acid (EPA), docosahexaenoic acid (DHA) or mixed omega‐3 with 95% CI. P < 0.05 was considered to be statistically significant. Heterogeneity was assessed using the χ2 method29. A random effects model was used when heterogeneity was statistically significant (P < 0.1)30.
The methods proposed by Greenland and Orsini31, 32 were used in our dose–response analysis. To assess the possible non‐linear trends between ω‐3 fatty acid intake and type 2 diabetes risk, we used a restricted cubic spline model, in which four fixed knots at the 5th, 35th, 65th and 95th percentile of the exposure distribution were set up33, 34. The regression coefficients of the second and third splines were assumed equal to zero to test the non‐linearity using the Wald test31. Linear analysis was chosen in the subsequent calculating steps if P ≥ 0.05; otherwise, a non‐linear model was used.
The generalized least‐square model was used to estimate the RR of type 2 diabetes for daily dose increment of ω‐3 fatty acid consumption; and a random‐effects model was applied to synthesize the study‐specific regression coefficients35, 36. The mean or median value of each exposure level was allocated to the corresponding RR36. The lowest exposure category was defined as a referent; whereas other categories were centralized to the referent dose. Incomplete data was calculated using an improved method of Bekkering et al 37.
Subgroup analysis was carried out according to: (i) duration of studies (<16 years vs ≥16 years); (ii) ethnicity (Asian vs USA/European); (iii) age at the initial stage of studies (<54 years vs ≥54 years).
The potential source of heterogeneity between included studies was investigated through meta‐regression analysis with a P‐value <0.1 as statistical significance. The items including type of omega‐3 fatty acids, study duration, ethnicity and age at recruitment were analyzed in the regression model. A funnel plot with Egger's linear regression analysis was used to determine the risk of publication bias by assessing the asymmetry of the funnel plot. A P‐value <0.1 was considered to be of significant bias38, 39.
Results
Description of studies
The initial search obtained 1,450 publications in 2016, of which 30 studies were investigated in full‐text articles (Figure S1). Finally, five articles with 10 cohort trials were potentially eligible5, 9, 10, 12, 13.
The scores of included studies, according to the Newcastle–Ottawa Scale Criteria, ranged from 5 to 8 (Table S1). A total of 426,852 participants were included. The individual sample size varied from 35,988 to 91,669 participants, and the follow‐up duration was 4.1–18 years. These participants were non‐diabetic at the beginning of each study, and were aged from 26 to 78 years. The Validated Food Frequency Questionnaire was used to assess the dietary method. EPA, DHA and mixed omega‐3 fatty acids were examined as dietary factors, the amounts of which were organized in five quintiles. The pooled RR for type 2 diabetes was calculated by comparing the RR corresponding to the highest exposure category of omega‐3 with that of the lowest one.
Omega‐3 fatty acids intake and risk of Type 2 diabetes
A total of 10 cohort studies with three dietary factors (EPA, DHA and mixed omega‐3) were included in the present meta‐analysis. As shown in Figure 1, the overall effect of total omega‐3 fatty acids on the risk of type 2 diabetes was insignificant (RR = 1.14, 95% CI 0.99–1.31, P = 0.062). Furthermore, we analyzed the association of mixed omega‐3 fatty acids supplementation with type 2 diabetes, which was found to be insignificant (RR = 1.07, 95% CI 0.92–1.25, P = 0.35) as well. Interestingly, despite the limited included studies, the consumption of single omega‐3 subtype (either EPA or DHA) was related to an increased risk of type 2 diabetes (Figure 1) with the pooled effect size being 1.45 (95% CI 1.31–1.60, P < 0.001).
Figure 1.
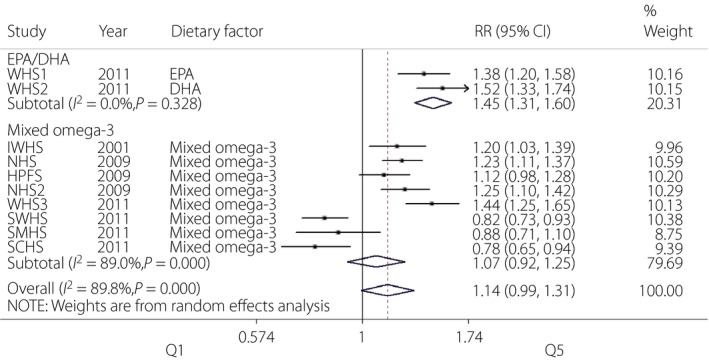
Forest plot of meta‐analysis for type 2 diabetes risk and omega‐3 fatty acids supplementation. CI, confidence interval; DHA, docosahexaenoic acid; EPA, eicosapentaenoic acid; HPFS, Health Care Professionals Study; IWHS, Iowa Women's Health Study; NHS, Nurses' Health Study; NHS2, Nurses' Health Study II; RR, relative risk; SCHS, Singapore Chinese Health Study; SMHS, Shanghai Men's Health Study; SWHS, Shanghai Women's Health Study; WHS3, Women's Health Study.
Dose–response analysis
Among the 10 included studies, a significant non‐linear association was identified (P < 0.001 for non‐linear test). The dose–response curve showed an inverted U‐shape with 0.43 g/day as the peak point. Specifically, within people consuming 0.10–0.43 g n‐3 fatty acids per day, an increment dose of fatty acids intake was generally associated with a significant higher type 2 diabetes risk as compared with the referent dosage (0 g/day). However, supplementation of n‐3 fatty acids with 0.43–0.75 g/day showed a decreased tendency in the type 2 diabetes risk as compared with the 0.10–0.43 g/day dose range. RR in the >0.75 g/day category (0.75–1.08 g/day) was further decreased with no statistical significance (Figure 2a).
Figure 2.
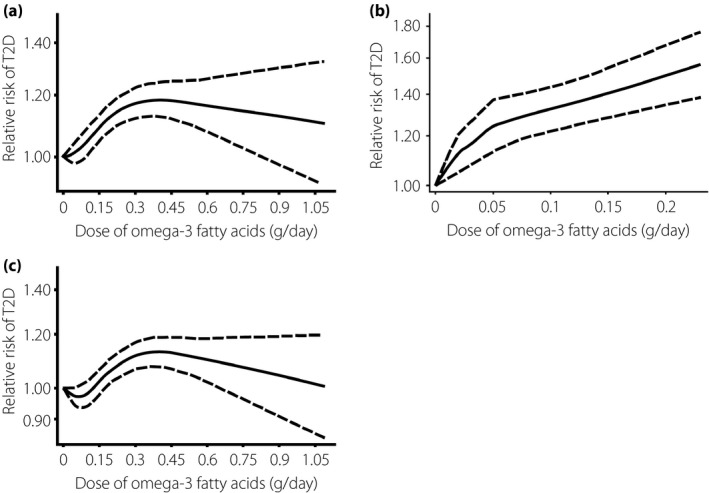
Non‐linear dose–response relationship between omega‐3 fatty acids consumption and type 2 diabetes (T2D) risk based on (a) total omega‐3, (b) single omega‐3 and (c) mixed omega‐3.
Two studies investigated the association of type 2 diabetes risk and single omega‐3 fatty acids intake. In accordance with results we obtained in Figure 1, a significant positive association between EPA/DHA with type 2 diabetes risk was observed in the non‐linear model (Figure 2b). To exclude the possible interference of single n‐3 fatty acids, we carried out a non‐linear analysis in eight studies with mixed omega‐3 as the dietary factor (Figure 2c) and a similar curve was obtained with that in Figure 2a (P < 0.001 for non‐linear test, P < 0.001 for overall association).
Subgroup analysis and heterogeneity evaluation
A high degree of heterogeneity was observed in the overall analysis (I 2 = 89.8%, P < 0.001). Thus, we carried out further subanalysis according to ethnicity, study duration and age of participants at recruitment to explore the source of heterogeneity. As shown in Figure 3a, studies based on Asian populations showed a protective effect of omega‐3 fatty acids intake against the development of type 2 diabetes (pooled RR = 0.82, P < 0.001). Conversely, studies on Western populations showed increased risk of type 2 diabetes (pooled RR = 1.30, P < 0.001).
Figure 3.
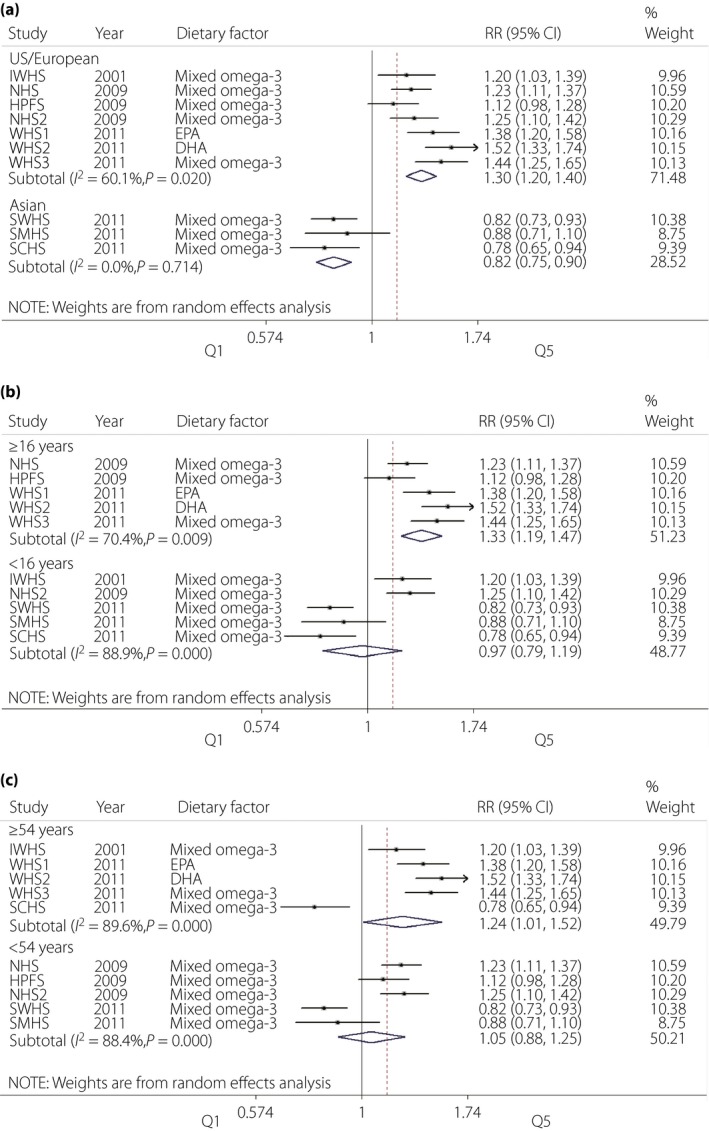
Forest plot of meta‐analysis for type 2 diabetes risk and omega‐3 fatty acids supplementation according to (a) Asian/Western populations, (b) duration of follow up and (c) age of participants at baseline. CI, confidence interval; DHA, docosahexaenoic acid; EPA, eicosapentaenoic acid; HPFS, Health Care Professionals Study; IWHS, Iowa Women's Health Study; NHS, Nurses' Health Study; NHS2, Nurses' Health Study II; RR, relative risk; SCHS, Singapore Chinese Health Study; SMHS, Shanghai Men's Health Study; SWHS, Shanghai Women's Health Study; WHS3, Women's Health Study.
We chose the median values as the cut‐off points of study duration (16 years) and participants' age (54 years). There was no significant association between omega‐3 fatty acids intake and type 2 diabetes incidence in studies with <16 years of follow up (pooled RR = 0.97, P = 0.782); whereas studies with more than 16 years of follow up (including 16 years) showed an increased risk of type 2 diabetes (pooled RR = 1.33, P < 0.001; Figure 3b). In subgroup analysis according to age at recruitment (Figure 3c), individuals who were recruited at the age of >54 years presented increased risk of type 2 diabetes (pooled RR = 1.24, P = 0.04). No significant association was observed in a subgroup that included individuals with initial age <54 years (pooled RR = 1.05, P = 0.574).
According to the aforementioned data, a high degree of heterogeneity was still observed in all subgroup analyses, except in studies with Asian participants. Subsequently, we carried out a meta‐regression analysis according to the following covariates: ethnicity, age of participants at baseline, omega‐3 composition and follow‐up duration (Table S2). Univariate analysis showed that no significant association was observed in the covariate of age (P = 0.783); whereas ethnicity (P < 0.01), omega‐3 composition (P = 0.10) and study duration (P = 0.01) were possible factors that caused the heterogeneity. Thus, we selected these factors for multivariate analysis, and identified ethnicity (P = 0.007) and omega‐3 composition (P = 0.064) as the major factors that contribute to the heterogeneity between studies involved (Table 1).
Table 1.
Results of source meta‐regression analysis to explore heterogeneity (multivariate analysis)
| Covariates | Exp (b) | SE | P |
|---|---|---|---|
| Ethnicity | 0.63 | 0.07 | 0.01 |
| Omega‐3 composition | 0.86 | 0.06 | 0.06 |
| Follow‐up duration | 0.99 | 0.01 | 0.65 |
The dependent variable is the ln(relative risk) (lnRR) for type 2 diabetes incidence from each study. Weights were assigned according to the estimated variance of lnRR. exp (b), relative risk of estimates; RR, relative risk; SE, standard error of relative risk of estimates.
Furthermore, we carried out subgroup analysis in USA/European populations according to omega‐3 composition. As shown in Figure 4, no significant heterogeneity was observed in the single (I 2 = 0.0%, P = 0.328) or mixed (I 2 = 42.1%, P = 0.141) omega‐3 subgroups, which further confirmed the source of heterogeneity identified in the present study.
Figure 4.
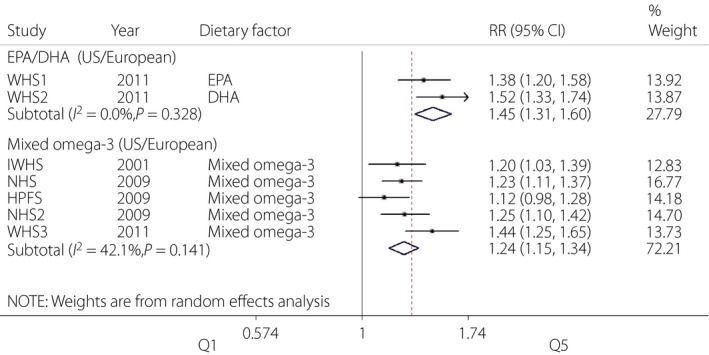
Forest plot of meta‐analysis for type 2 diabetes risk and omega‐3 fatty acids supplementation based on omega‐3 composition in USA/European population. CI, confidence interval; DHA, docosahexaenoic acid; EPA, eicosapentaenoic acid; HPFS, Health Care Professionals Study; IWHS, Iowa Women's Health Study; NHS, Nurses' Health Study; NHS2, Nurses' Health Study II; RR, relative risk; WHS3, Women's Health Study.
Publication bias evaluation
As shown in Figure 5, the risk of publication bias was evaluated using the method of funnel plot with Egger's linear regression line. Although the graph of the funnel plot showed an asymmetrical shape, no publication bias was observed in Egger's test (P = 0.411).
Figure 5.
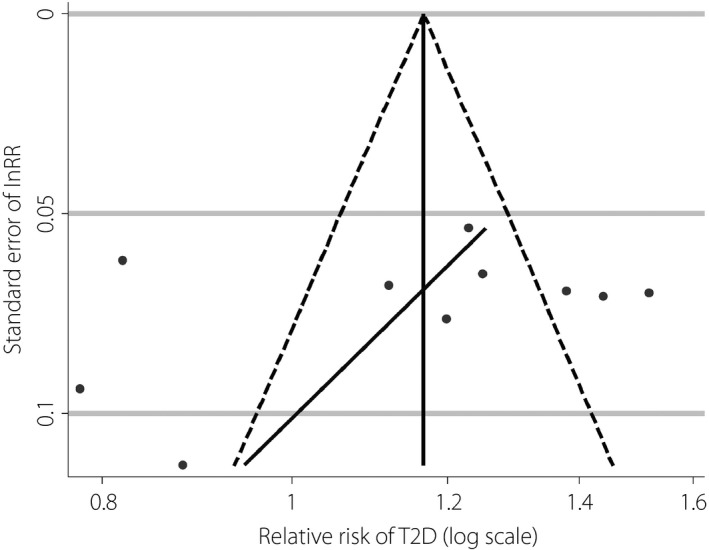
Funnel plot with Egger's regression line for risk ratio of type 2 diabetes (T2D). lnRR, ln(relative risk).
Discussion
This meta‐analysis pooled 10 cohort studies with 426,852 participants to explore the association between omega‐3 consumption and type 2 diabetes risk. It is known that omega‐3 fatty acids include EPA, docosapentaenoic acid (DPA), DHA, α‐linolenic acid and so on40. Dietary factors included in these cohort studies were EPA, DHA and mixed omega‐3. Although the overall effect of total omega‐3 fatty acids was insignificant on type 2 diabetes development, the supplementation of a single omega‐3 subtype was correlated to an increased risk of type 2 diabetes. The dose–response analysis presented an inverted U‐shaped curve of type 2 diabetes risk, with the peak point at 0.43 g/days of omega‐3 supplementation. Subgroup analysis identified that omega‐3 consumption only showed beneficial effects in Asian subjects.
It was identified that individuals with single omega‐3 supplementation presented a more obvious trend on type 2 diabetes progression when compared with mixed omega‐3 intake. Although the included trials were limited, this finding still impelled us to assume whether synergic action in vivo of different omega‐3 individuals could alleviate the detrimental effects of single omega‐3 subtype intake on type 2 diabetes development. Mixed omega‐3 fatty acids used in the Women's Health Study included EPA, DHA and DPA12. In the Shanghai Women's Health Study, participants were supplemented with omega‐3 mixtures of EPA and DHA5. The composition of omega‐3 fatty acids in the Singapore Chinese Health Study was EPA, DHA and α‐linolenic acid9. However, in the Nurses' Health Study, Nurses' Health Study II, Health Care Professionals Study, Shanghai Men's Health Study and Iowa Women's Health Study, detailed information of mixed omega‐3 was unavailable10, 13. Additionally, the percentage of the individual fatty acid in mixed omega‐3 supplementation is not clear. Our previous study found that a high ratio of EPA/DHA could improve insulin resistance26. It is known that insulin resistance is the major pathophysiological feature of type 2 diabetes27. Therefore, we assumed that the varied compositions and proportions of omega‐3 subtypes might explain, at least in part, the divergences of their effects on type 2 diabetes prevention and development. Our heterogeneity analysis also identified that the composition of omega‐3 contributed a great deal to the high degree of heterogeneity between involved trails. More investigations focusing on this assumption remain to be carried out, which might bring a new concept to omega‐3 supplementation.
According to the inverted U‐shaped dose–response curve with ~1 g/day dose range (Figure 2), we presume that daily intake of approximately 0.43 g omega‐3 fatty acids might impose the most significant detrimental effect on type 2 diabetes development. Either a lower or higher supplementation dose showed a decreasing tendency. In contrast, several studies with a higher dose of omega‐3 fatty acids intake have shown beneficial effects on some diseases that share similar pathological processes and/or risk factors with type 2 diabetes, such as hyperlipidemia41, insulin sensitivity42, 43, 44, obesity45, 46, non‐alcoholic fatty liver disease47, inflammatory reaction48 and cardiovascular diseases49. Pirillo et al.41 suggested that the optimal dosage should be 3–4 g/day to achieve a significant lipid‐lowering effect. Daily consumption of 0.85–1.8 g/day omega‐3 was advised to provide a protective efficacy in people with documented cardiovascular disease50, 51. Additionally, 0.7–5.1 g/day of n‐3 fatty acids supplementation was associated with a greater reduction on urine protein excretion52. These findings together with the dose–response curve in the present study led us to the concept that higher omega‐3 intake amount (more than 0.43 g/day at least) might provide a protective effect, or at least a risk‐lowering tendency in type 2 diabetes. An appropriate dose range to achieve a beneficial effect on type 2 diabetes is awaiting identification.
Furthermore, our subgroup analysis showed that omega‐3 fatty acids exerted beneficial effects in Asian populations, but were detrimental in Western populations. However, there were just two studies that included Asian populations (Chinese people in particular). More investigations across different Asian groups were required. Nevertheless, the distinct lifestyle between these two populations, especially the cooking style, might result in the contrary observations. It is speculated that cooking style (e.g., fried in Asian style or raw in Western style) can influence the generation of omega‐3 derived lipid mediators and resultantly change the physiological effects of omega‐3 fatty acids in vivo. Additionally, Eastern and Western populations share totally different dietary patterns. It would be significant to identify the biochemical interactions between omega‐3 fatty acids and other multifarious dietary factors. Furthermore, fish oil‐related gene polymorphisms in different ethnicities could also contribute to the opposite effects observed in USA/European and Asian populations. A recent study reported that carriers of ELOVL2 single nucleotide polymorphism minor alleles with a daily intake of 1.8 g omega‐3 showed a more obvious increase of plasma EPA and DHA when compared with non‐carriers. Thus, these carriers might benefit from high levels of plasma omega‐353. It is known that adiponectin has a beneficial effect on the improvement of insulin sensitization54. Alsaleh et al.55 reported that the ADIPOQ gene polymorphism interacted with fish oil to affect plasma adiponectin levels. More studies that assess the relationship between omega‐3 fatty acids and single nucleotide polymorphisms in different ethnicities might shed light on the complicated effects of omega‐3 on different ethnicities.
Subanalysis according to follow‐up duration recognized that studies with >16 years of follow up showed a positive effect of omega‐3 intake on type 2 diabetes development; whereas insignificant findings were identified in studies with <16 years of follow up. Due to aging as one of the primary risk factors for type 2 diabetes56, 57, there should be no question on this conclusion, as longer follow‐up duration implies more aged participants. The results from subanalyses by age with a cut‐off point of 54 years obtained consistent results. These data show that early supplementation of omega‐3 fatty acids might achieve a beneficial outcome for type 2 diabetes prevention.
Furthermore, we evaluated the risk of publication bias with the method of funnel plot. Although there was some suggestion of asymmetry from visual inspection, no statistical significance was identified. Thus, it is assumed that such asymmetrical shape should not be a marked factor that affects our conclusions. An asymmetrical funnel plot usually indicates a possible publication bias; nevertheless, there are other factors that can cause the asymmetry of a funnel plot, such as the involvement of small‐size studies and marked heterogeneity between included studies58. As shown in Figure 5, dots in the funnel plot, which represent included studies, are spread widely, suggesting large heterogeneity between these studies. Thus, it is estimated that the asymmetry of the funnel plot might result from the study heterogeneity, but not publication bias.
In the present study, a high degree of heterogeneity can be observed either in overall or in subgroup analysis, which might overestimate or underestimate the effect of omega‐3 fatty acids on type 2 diabetes risk. Such heterogeneity could be attributed to several limitations of the included studies. First, the included studies contained people from different ethnicities. Just three trials showed the protective effect of omega‐3 fatty acids on type 2 diabetes, all of which chose Asian populations as recruited participants. Second, various dietary factors (single or mixed omega‐3) were used in these studies; however, the detailed information about the composition and percentage of supplemented omega‐3 was not available. Third, the included studies ranged from 1990 to 2011; hence, the progression of techniques and the update of testing devices might affect the obtained data. Thus, we carried out meta‐regression analysis to trace the source of heterogeneity. Ethnicity and omega‐3 composition were identified as the major factors.
In conclusion, the present data are relevant to clinicians and nutritionists adopting optimized dietary guidance for diabetes‐prone populations. We assumed that dietary supplementation with different subtypes of omega‐3 results in varied effects on type 2 diabetes prevention. However, the present study did not provide evidence to discourage the use of omega‐3 fatty acids, because the analyzed studies have shown the decreased incidence of type 2 diabetes with mixed omega‐3 supplementation in Asian populations. The appropriate dosage and compositions of omega‐3, the optimized cooking method, and early omega‐3 supplementation might be beneficial for type 2 diabetes prevention.
Disclosure
The authors declare no conflict of interest.
Supporting information
Figure S1 ¦ Flow chart of article selection process.
Table S1 ¦ Characteristics and quality assessment of included cohort studies.
Table S2 ¦ Results of source meta‐regression analysis to explore heterogeneity (univariate analysis).
Acknowledgments
This work was supported by a grant from National Natural Science Foundation of China (81100581 to S Shao), CIMF‐Novo Nordisk China diabetes Yingcai Funding (grant number 2014SShao) and the Fundamental Research Funds for the Central Universities (grant number 0118540208).
J Diabetes Investig 2017; 8: 480–488
Cai Chen and Yan Yang contributed equally to this work.
References
- 1. American Diabetes A . Diagnosis and classification of diabetes mellitus. Diabetes Care 2009; 32 Suppl 1: S62–S67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Shaw JE, Sicree RA, Zimmet PZ. Global estimates of the prevalence of diabetes for 2010 and 2030. Diabetes Res Clin Pract 2010; 87: 4–14. [DOI] [PubMed] [Google Scholar]
- 3. American Diabetes A . (13) Diabetes care in the hospital, nursing home, and skilled nursing facility. Diabetes Care 2015; 38 Suppl: S80–S85. [DOI] [PubMed] [Google Scholar]
- 4. Ley SH, Hamdy O, Mohan V, et al Prevention and management of type 2 diabetes: Dietary components and nutritional strategies. Lancet 2014; 383: 1999–2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Villegas R, Xiang YB, Elasy T, et al Fish, shellfish, and long‐chain n‐3 fatty acid consumption and risk of incident type 2 diabetes in middle‐aged Chinese men and women. Am J Clin Nutr 2011; 94: 543–551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Sagild U, Littauer J, Jespersen CS, et al Epidemiological studies in Greenland 1962–1964. I. Diabetes mellitus in Eskimos. Acta Med Scand 1966; 179: 29–39. [DOI] [PubMed] [Google Scholar]
- 7. Bang HO, Dyerberg J, Sinclair HM. The composition of the Eskimo food in north western Greenland. Am J Clin Nutr 1980; 33: 2657–2661. [DOI] [PubMed] [Google Scholar]
- 8. Kromann N, Green A. Epidemiological studies in the Upernavik district, Greenland. Incidence of some chronic diseases 1950–1974. Acta Med Scand 1980; 208: 401–406. [PubMed] [Google Scholar]
- 9. Brostow DP, Odegaard AO, Koh WP, et al Omega‐3 fatty acids and incident type 2 diabetes: The Singapore Chinese Health Study. Am J Clin Nutr 2011; 94: 520–526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Meyer KA, Kushi LH, Jacobs DR, et al Dietary fat and incidence of type 2 diabetes in older Iowa women. Diabetes Care 2001; 24: 1528–1535. [DOI] [PubMed] [Google Scholar]
- 11. Patel PS, Sharp SJ, Luben RN, et al Association between type of dietary fish and seafood intake and the risk of incident type 2 diabetes: The European prospective investigation of cancer (EPIC)‐Norfolk cohort study. Diabetes Care 2009; 32: 1857–1863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Djousse L, Gaziano JM, Buring JE, et al Dietary omega‐3 fatty acids and fish consumption and risk of type 2 diabetes. Am J Clin Nutr 2011; 93: 143–150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Kaushik M, Mozaffarian D, Spiegelman D, et al Long‐chain omega‐3 fatty acids, fish intake, and the risk of type 2 diabetes mellitus. Am J Clin Nutr 2009; 90: 613–620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Djousse L, Biggs ML, Lemaitre RN, et al Plasma omega‐3 fatty acids and incident diabetes in older adults. Am J Clin Nutr 2011; 94: 527–533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. van Woudenbergh GJ, van Ballegooijen AJ, Kuijsten A, et al Eating fish and risk of type 2 diabetes: A population‐based, prospective follow‐up study. Diabetes Care 2009; 32: 2021–2026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Fasching P, Ratheiser K, Waldhausl W, et al Metabolic effects of fish‐oil supplementation in patients with impaired glucose tolerance. Diabetes 1991; 40: 583–589. [DOI] [PubMed] [Google Scholar]
- 17. Morgan WA, Raskin P, Rosenstock J. A comparison of fish oil or corn oil supplements in hyperlipidemic subjects with NIDDM. Diabetes Care 1995; 18: 83–86. [DOI] [PubMed] [Google Scholar]
- 18. Puhakainen I, Ahola I, Yki‐Jarvinen H. Dietary supplementation with n‐3 fatty acids increases gluconeogenesis from glycerol but not hepatic glucose production in patients with non‐insulin‐dependent diabetes mellitus. Am J Clin Nutr 1995; 61: 121–126. [DOI] [PubMed] [Google Scholar]
- 19. Sirtori CR, Paoletti R, Mancini M, et al N‐3 fatty acids do not lead to an increased diabetic risk in patients with hyperlipidemia and abnormal glucose tolerance. Italian Fish Oil Multicenter Study. Am J Clin Nutr 1997; 65: 1874–1881. [DOI] [PubMed] [Google Scholar]
- 20. Vessby B, Karlstrom B, Boberg M, et al Polyunsaturated fatty acids may impair blood glucose control in type 2 diabetic patients. Diabet Med 1992; 9: 126–133. [DOI] [PubMed] [Google Scholar]
- 21. Li D. Omega‐3 polyunsaturated fatty acids and non‐communicable diseases: Meta‐analysis based systematic review. Asia Pac J Clin Nutr 2015; 24: 10–15. [DOI] [PubMed] [Google Scholar]
- 22. Wu JH, Micha R, Imamura F, et al Omega‐3 fatty acids and incident type 2 diabetes: A systematic review and meta‐analysis. Br J Nutr 2012; 107(Suppl 2): S214–S227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Zhang M, Picard‐Deland E, Marette A. Fish and marine omega‐3 polyunsatured fatty acid consumption and incidence of type 2 diabetes: A systematic review and meta‐analysis. Int J Endocrinol 2013; 2013: 501015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Zheng JS, Huang T, Yang J, et al Marine N‐3 polyunsaturated fatty acids are inversely associated with risk of type 2 diabetes in Asians: A systematic review and meta‐analysis. PLoS One 2012; 7: e44525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Zhou Y, Tian C, Jia C. Association of fish and n‐3 fatty acid intake with the risk of type 2 diabetes: A meta‐analysis of prospective studies. Br J Nutr 2012; 108: 408–417. [DOI] [PubMed] [Google Scholar]
- 26. Chen C, Yu X, Shao S. Effects of omega‐3 fatty acid supplementation on glucose control and lipid levels in Type 2 diabetes: A meta‐analysis. PLoS One 2015; 10: e0139565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Tangvarasittichai S. Oxidative stress, insulin resistance, dyslipidemia and type 2 diabetes mellitus. World J Diabetes 2015; 6: 456–480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Terrin N, Schmid CH, Lau J. In an empirical evaluation of the funnel plot, researchers could not visually identify publication bias. J Clin Epidemiol 2005; 58: 894–901. [DOI] [PubMed] [Google Scholar]
- 29. Higgins JP, Thompson SG, Deeks JJ, et al Measuring inconsistency in meta‐analyses. BMJ 2003; 327: 557–560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. DerSimonian R, Laird N. Meta‐analysis in clinical trials. Control Clin Trials 1986; 7: 177–188. [DOI] [PubMed] [Google Scholar]
- 31. Greenland S, Longnecker MP. Methods for trend estimation from summarized dose‐response data, with applications to meta‐analysis. Am J Epidemiol 1992; 135: 1301–1309. [DOI] [PubMed] [Google Scholar]
- 32. Orsini N, Li R, Wolk A, et al Meta‐analysis for linear and nonlinear dose‐response relations: Examples, an evaluation of approximations, and software. Am J Epidemiol 2012; 175: 66–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Bagnardi V, Zambon A, Quatto P, et al Flexible meta‐regression functions for modeling aggregate dose‐response data, with an application to alcohol and mortality. Am J Epidemiol 2004; 159: 1077–1086. [DOI] [PubMed] [Google Scholar]
- 34. Durrleman S, Simon R. Flexible regression models with cubic splines. Stat Med 1989; 8: 551–561. [DOI] [PubMed] [Google Scholar]
- 35. Orsini N, Bellocco R, Greenland S. Generalized least squares for trend estimation of summarized dose‐response data. Stata J 2005; 6: 40–57. [Google Scholar]
- 36. Berlin JA, Longnecker MP, Greenland S. Meta‐analysis of epidemiologic dose‐response data. Epidemiology 1993; 4: 218–228. [DOI] [PubMed] [Google Scholar]
- 37. Bekkering GE, Harris RJ, Thomas S, et al How much of the data published in observational studies of the association between diet and prostate or bladder cancer is usable for meta‐analysis? Am J Epidemiol 2008; 167: 1017–1026. [DOI] [PubMed] [Google Scholar]
- 38. Begg CB, Mazumdar M. Operating characteristics of a rank correlation test for publication bias. Biometrics 1994; 50: 1088–1101. [PubMed] [Google Scholar]
- 39. Egger M, Davey Smith G, Schneider M, et al Bias in meta‐analysis detected by a simple, graphical test. BMJ 1997; 315: 629–634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Lands B. Historical perspectives on the impact of n‐3 and n‐6 nutrients on health. Prog Lipid Res 2014; 55: 17–29. [DOI] [PubMed] [Google Scholar]
- 41. Pirillo A, Catapano AL. Omega‐3 polyunsaturated fatty acids in the treatment of hypertriglyceridaemia. Int J Cardiol 2013; 170(Suppl 1): S16–S20. [DOI] [PubMed] [Google Scholar]
- 42. Fedor D, Kelley DS. Prevention of insulin resistance by n‐3 polyunsaturated fatty acids. Curr Opin Clin Nutr Metab Care 2009; 12: 138–146. [DOI] [PubMed] [Google Scholar]
- 43. Storlien LH, Jenkins AB, Chisholm DJ, et al Influence of dietary fat composition on development of insulin resistance in rats. Relationship to muscle triglyceride and omega‐3 fatty acids in muscle phospholipid. Diabetes 1991; 40: 280–289. [DOI] [PubMed] [Google Scholar]
- 44. Storlien LH, Kraegen EW, Chisholm DJ, et al Fish oil prevents insulin resistance induced by high‐fat feeding in rats. Science 1987; 237: 885–888. [DOI] [PubMed] [Google Scholar]
- 45. Kelly OJ, Gilman JC, Kim Y, et al Long‐chain polyunsaturated fatty acids may mutually benefit both obesity and osteoporosis. Nutr Res 2013; 33: 521–533. [DOI] [PubMed] [Google Scholar]
- 46. Liu M, Montgomery MK, Fiveash CE, et al PPARalpha‐independent actions of omega‐3 PUFAs contribute to their beneficial effects on adiposity and glucose homeostasis. Sci Rep 2014; 4: 5538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Boyraz M, Pirgon O, Dundar B, et al Long‐term treatment with n‐3 polyunsaturated fatty acids as a monotherapy in children with nonalcoholic fatty liver disease. J Clin Res Pediatr Endocrinol 2015; 7: 121–127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Ellulu MS, Khaza'ai H, Patimah I, et al Effect of long chain omega‐3 polyunsaturated fatty acids on inflammation and metabolic markers in hypertensive and/or diabetic obese adults: A randomized controlled trial. Food Nutr Res 2016; 60:29268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Sposito AC, Caramelli B, Fonseca FA, et al IV Brazilian guideline for Dyslipidemia and atherosclerosis prevention: Department of Atherosclerosis of Brazilian Society of Cardiology. Arq Bras Cardiol 2007; 88(Suppl 1): 2–19. [DOI] [PubMed] [Google Scholar]
- 50. Marchioli R, Silletta MG, Levantesi G, et al Omega‐3 fatty acids and heart failure. Curr Atheroscler Rep 2009; 11: 440–447. [DOI] [PubMed] [Google Scholar]
- 51. Yokoyama M, Origasa H, Matsuzaki M, et al Effects of eicosapentaenoic acid on major coronary events in hypercholesterolaemic patients (JELIS): A randomised open‐label, blinded endpoint analysis. Lancet 2007; 369: 1090–1098. [DOI] [PubMed] [Google Scholar]
- 52. Miller ER 3rd, Juraschek SP, Appel LJ, et al The effect of n‐3 long‐chain polyunsaturated fatty acid supplementation on urine protein excretion and kidney function: Meta‐analysis of clinical trials. Am J Clin Nutr 2009; 89: 1937–1945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Alsaleh A, Maniou Z, Lewis FJ, et al ELOVL2 gene polymorphisms are associated with increases in plasma eicosapentaenoic and docosahexaenoic acid proportions after fish oil supplement. Genes Nutr 2014; 9: 362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Takashima S, Nishii N, Kato A, et al Molecular cloning of feline resistin and the expression of resistin, leptin and adiponectin in the adipose tissue of normal and obese cats. J Vet Med Sci 2016; 78: 23–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Alsaleh A, Crepostnaia D, Maniou Z, et al Adiponectin gene variant interacts with fish oil supplementation to influence serum adiponectin in older individuals. J Nutr 2013; 143: 1021–1027. [DOI] [PubMed] [Google Scholar]
- 56. Kirkman MS, Briscoe VJ, Clark N, et al Diabetes in older adults. Diabetes care 2012; 35: 2650–2664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Yehuda AB, Zinger A, Durso S. The older patient with diabetes: A practical approach. Diabetes Metab Res Rev 2014; 30: 88–95. [DOI] [PubMed] [Google Scholar]
- 58. Thornton A, Lee P. Publication bias in meta‐analysis: Its causes and consequences. J Clin Epidemiol 2000; 53: 207–216. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1 ¦ Flow chart of article selection process.
Table S1 ¦ Characteristics and quality assessment of included cohort studies.
Table S2 ¦ Results of source meta‐regression analysis to explore heterogeneity (univariate analysis).


