Abstract
Aims/Introduction
Studies have been carried out to evaluate the correlation between TCF7L2 genetic polymorphisms and gestational diabetes mellitus (GDM) risk. However, the conclusions from these studies are incomplete, because partial single nucleotide polymorphisms (SNPs) were analyzed. We carried out a meta‐analysis aimed to systematically evaluate TCF7L2 gene polymorphisms and GDM susceptibility in all population and racial/ethnic subgroups to afford a foundation for future research.
Materials and Methods
Published studies censoring TCF7L2 variants and GDM risk were captured from the EMBASE, PubMed, CNKI and Wanfang databases. The meta‐analysis was processed using software of RevMan 5.2 and Stata13. The relationship between TCF7L2 polymorphism and GDM occurrence was evaluated by pooled odds ratios. Stratified analysis based on race/ethnicity was also carried out. The allele‐specific odds ratios and 95% confidence intervals were counted, and based on homogeneity evaluated using the I 2‐test, fixed‐ or random‐effects pooled measures were selected.
Results
A total of 22 studies were covered, capturing eight TCF7L2 SNPs and involving 5,573 cases and 13,266 controls. Six of eight SNPs showed significant relationships with GDM occurrence, of which the SNPs rs7903146, rs12255372 and rs7901695 were the most powerful. Stratified analysis by race/ethnicity showed discrepant results in these three SNPs. In Caucasians and other races, all these SNPs were found to have a significant association with GDM risk, but in Asians, only SNP rs7903146 showed a significant association.
Conclusions
Six of eight SNPs were found to have significant associations between TCF7L2 variants and GDM risk in the overall population, with the most powerful in SNPs being rs7903146, rs12255372 and rs7901695, but the contribution of these SNPs to GDM risk were variable among different racial/ethnic groups.
Keywords: Gestational diabetes mellitus, Polymorphism, TCF7L2
Introduction
Gestational diabetes mellitus (GDM), defined as impaired glucose tolerance with onset or first identification during pregnancy, is one of the most frequent complications of pregnancy. The prevalence is estimated to be 5–10%, and the incidence rate has shown a gradual upward trend1, 2, making it a growing health issue3. GDM increases the risk of adverse pregnancy outcomes, and has adverse effects for both mothers and their children, including susceptibility to obesity, metabolic syndrome and type 2 diabetes mellitus in later life3, 4, 5. Furthermore, GDM occurrence is confused in different populations or ethnic groups. Therefore, it is necessary to systematically evaluate TCF7L2 gene polymorphisms and GDM susceptibility in all population and racial/ethnic subgroups.
GDM is a heterogeneous metabolic disorder, with mixed genetic etiology and phenotypes6. Insulin resistance is regarded as an important factor to contribute to GDM. Furthermore, there are heritable elements, and GDM could share some risk factors of genetics with type 2 diabetes mellitus7. Transcription factor 7‐like 2 (TCF7L2) expresses in pancreatic β‐cells8, and belongs to the high mobility group‐box family as a transcription factor9 that plays a crucial role in the maintenance of glucose homeostasis. Recent findings suggest that the TCF7L2 contributes to GDM risk10.
So far, TCF7L2 has multiple single nucleotide polymorphisms (SNPs), eight of which have been reported, including rs790314611, 12, 13, 14, 15, 16, 17, 18, 19, 20, 21, 22, 23, 24, 25, 26, 27, 28; rs1225537216, 17, 18, 19, 20, 21, 26, 28, 29, 30; rs790169518, 20, 25, 29, 31; rs29048728, 32, 33; rs1119620528, 32; rs1119621832, 34; rs1224332625, 29; and rs4506565.25, 29 The SNP rs7903146 has been most widely researched, and has been associated with an increased risk of GDM in Scandinavian women7. A study of Mexican Americans showed that SNP rs12255372 of TCF7L2 has a relationship with GDM risk and affected the insulin response to oral glucose in propends with GDM30, and rs7901695 has been shown to benefit individuals with type 2 diabetes mellitus and GDM risk18. The SNPs rs7903146 C>T, rs12255372 G>T and rs7901695 T>C are the strongest to be beneficial to GDM risk35, and lie within a well‐defined linkage disequilibrium block36, 37, 38. A study has also shown that SNPs were susceptible to type 2 diabetes mellitus occurrence through damaging insulin secretion, possibly attributable to a potential mechanism in β‐cells39. The other five SNPs have been less studied, and conflicting findings exist. Furthermore, individual studies might be limited by relatively inadequate sample sizes, and might be unable to receive convincing results. Therefore, we carried out a meta‐analysis to examine the association between TCF7L2 polymorphisms and the risk of GDM.
Materials and Methods
Literature search
A retrieve was carried out for related available articles published in four databases including PubMed and EMBASE for English articles, and China National Knowledge Infrastructure and Wanfang for Chinese articles. ‘TCF7L2’, ‘transcription factor 7‐like 2’, ‘polymorphism’, ‘SNP’, ‘GDM’, ‘gestational diabetes mellitus’, ‘Gestational diabetes’ and ‘Screening for gestational diabetes’ as keywords were used to search. We also evaluated the references in the retrieved studies, and recognized extra‐published articles not captured in review articles by search strategy from these databases.
Inclusion criteria
The following was the inclusion criteria, including: (i) case–control or cohort study published as an original study to assess the relationship between TCF7L2 polymorphisms with GDM risk; (ii) precise numbers for each genotype reported in case and control groups or exposed and unexposed groups, or countable data from these numbers in the published papers; and (iii) participants from the same ethnicity and the same time in each research; and (iv) the nearest and integrated article was selected if a paper had been published more than once.
Data extraction
According to the inclusion criteria listed above, information was drawn from all eligible papers by two reviewers (Chang Shaoyan and Wang Zhen) independently, and the validation was tested by a third reviewer if there was disagreement. The literature were eliminated as follows: overviews or editorials, studies with cell culture or animal research, studies based on family, studies of the primary outcome exclusive of GDM, and studies with no evaluation of the relationships of GDM and TCF7L2 polymorphisms, and studies that excluded a quality control group, or insufficient data for influence evaluations of the genetic relationships. Data were drawn from each published article as follows: first author, publication year, ethnicity, country, mean age, genotyping method, size of sample, number of cases and controls, study design, genetic variants, minor allele and allele distribution by GDM situation. If odds ratios (ORs) were accessible, but the genotype and allele distributions on the basis of GDM situation were not covered in the primitive study, the corresponding authors were contacted by email.
Quality assessment
Risk of bias was devoted to evaluate the methodological reliability of the incorporated studies. Quality was accessed based on five questions, using different colors for low risk (green), unclear risk (yellow), and high risk (read), with more green manifesting better research quality.
Statistical analysis
A meta‐analysis was carried out by RevMan version 5.2 (Cochrane Collaboration, Oxford, UK) and Stata13 software (StataCorp, College Station, Texas, USA). During the data analysis, we carried out independent meta‐analyses for GDM and TCF7L2 variants. The ORs of individual research were recounted from the accessible genotype distributions based on an allelic model, pooled using random‐effects models (REMs) or fixed‐effect models (FEMs) and visualized using forest plots. Heterogeneity in all qualified comparisons was evaluated by the I 2‐value and the χ2‐test to evaluate the P‐value. I 2 was the percentage of total degree of variation observed among the studies due to actual differences between the trials rather than to sampling error (chance), where I 2 > 50% is regarded as a significant heterogeneity.
Results
Description of the included studies
The search generated 147 articles of which 21 were eligible for meta‐analysis (Figure 1). The 22 eligible studies captured eight TCF7L2 SNPs, and included 5,573 cases and 13,266 controls. There were 18 studies about rs7903146, 10 about rs12255372, 5 about rs7901695, 3 about rs290487, 2 about rs11196205, 2 about rs11196218, 2 about rs12243326 and 2 about rs4506565. Characteristics of the included studies, and the genotype and allele distributions of SNPs among GDM cases and controls are shown in Tables 1 and 2.
Figure 1.
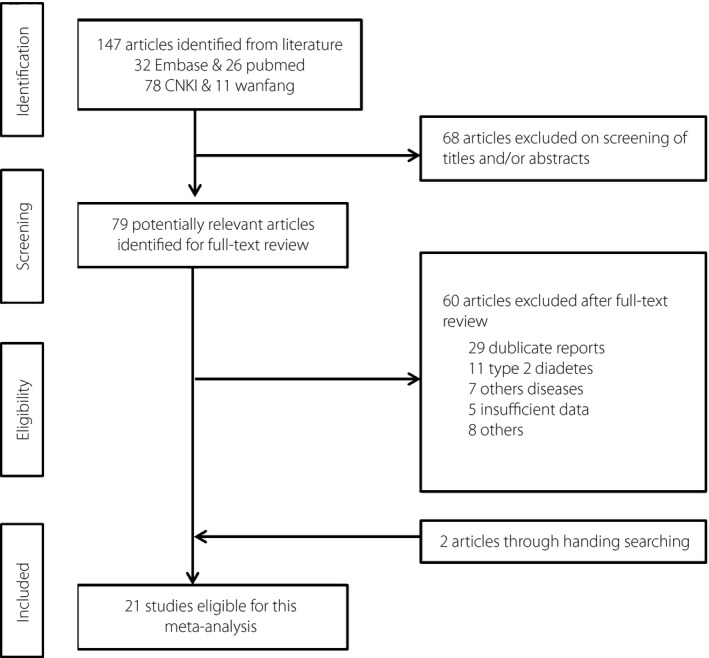
Selection of studies for inclusion in the meta‐analysis.
Table 1.
Characteristics of the included studies on the association between TCF7L2 and gestational diabetes mellitus risk
| Author, (year) reference | Ethnicity | Country | Mean age (cases/controls) | Genotyping method | GDM criteria |
|---|---|---|---|---|---|
| Aris, (2012)27 | Asian | Malaysia | 29.7/28.5 | NA | ADA |
| Cho, (2009)17 | Asian | Korea | 32/64.7 | Allelic discrimination assay | IWCGDM |
| de Melo, (2015)26 | Hispanic/Latino | Brazil | 32/24 | SNP Genotyping Assay | ADA |
| Freathy, (2010)12 | Mixed | Australia and UK | NA | Illumina Golden Gate platform | OGTT |
| Kan, (2014)15 | Asian | China | 30.7/30.9 | Allelic discrimination assay | OGTT |
| Huerta‐Chagoya, (2015)25 | Hispanic/Latino | Mexico | 35/28 | SNP genotype (LGC) | OGTT |
| Hui, (2011)32 | Asian | China | 32/30 | PCR‐LDR | OGTT |
| Klein, (2012)19 | Caucasian | Australia | 28.2/30.1 | NA | OGTT |
| Liu, (2014)33 | Asian | China | 31.88/28.78 | Mass spectrometry | OGTT |
| Pagan, (2014)29 | Caucasian | Spain | 34.31/31.2 | Sequencing | OGTT |
| Papadopoulou, (2011)20 | Caucasian | Sweden | NA | TaqMan allelic discrimination assay | OGTT |
| Reyes‐López, (2014)21 | Mexican | Mexico | 29/31 | PCR | ADA |
| RIZK, (2011)16 | Caucasian | Qatar | NA | TaqMan allelic discrimination assay | NA |
| Shaat, (2007)14 | Caucasian | Scandinavia | 32.3/30.5 | TaqMan allelic discrimination assay | OGTT |
| Shi, (2014)28 | Asian | China | 30/29 | AS‐PCR | OGTT |
| Stuebe, (2014)31 | African‐American | USA | 24.1 (total) | Sequenom iPLEX platform | OGTT |
| Thomas, (2014)24 | Asian | India | NA | NA | NA |
| Vcelak, (2012)18 | Caucasian | Czech Republic | 32.8/29.9 | TaqMan allelic discrimination assay | WHO |
| Wang, (2013)39 | Asian | China | NA | PCR‐LDR | OGTT |
| Watanabe, (2007)30 | Mexican‐American | USA | 35.0/33.4 | TaqMan allelic discrimination assay | OGTT |
| Zhang, (2015)23 | Asian | China | 30.58/28.75 | PCR‐RFLP | OGTT |
ADA, American Diabetes Association; AS‐PCR, allele‐specific polymerase chain reaction; GDM, gestational diabetes mellitus; IWCGDM, International Workshop‐Conference on Gestational Diabetes Mellitus; LGC, LGC Bioresearch Technologies; OGTT, oral glucose tolerance test; NA, not available; PCR, polymerase chain reaction; PCR‐LDR, ligase detection reaction–polymerase chain reaction; SNP, single nucleotide polymorphism; WHO, World Health Organization.
Table 2.
TCF7L2 allele distribution among GDM cases and controls in the included studies
| Author (year) | Variant (minor allele) | No. cases | Genotypes in GDM case | Genotypes in GDM control | Minor allele frequency (%) | P for HWE | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Case | Control | AA | AB | BB | AA | AB | BB | Case | Control | |||
| Aris (2012) | rs7903146(T) | 173 | 114 | 129 | 43 | 1 | 99 | 15 | 0 | 87 | 93.4 | 0.686 |
| Cho (2009) | 868 | 627 | 2 | 63 | 803 | 0 | 31 | 596 | 3.9 | 2.5 | 1 | |
| de Melo (2015) | 200 | 200 | 20 | 104 | 76 | 16 | 86 | 98 | 36 | 29.5 | 0.633 | |
| Freathy (2010) | 614 | 3,811 | 75 | 246 | 293 | 370 | 1,557 | 1,884 | 32.2 | 30.1 | 0.29 | |
| Freathy (2010) | 384 | 1,332 | 0 | 46 | 338 | 3 | 108 | 1,211 | 6 | 4.3 | 0.73 | |
| Huerta‐Chagoya (2015) | 408 | 342 | 19 | 124 | 265 | 10 | 67 | 265 | 19.9 | 12.7 | 0.03 | |
| Kan (2014) | 100 | 100 | 1 | 15 | 84 | 0 | 5 | 95 | 8.5 | 5 | >0.05 | |
| Klein (2012) | 125 | 125 | 5 | 112 | 8 | 8 | 107 | 10 | 48.8 | 49.2 | NA | |
| Lauenborg (2009) | 276 | 2,353 | 33 | 125 | 118 | 198 | 863 | 1,292 | 34.6 | 26.8 | >0.05 | |
| Pagan (2014) | 45 | 24 | 8 | 18 | 19 | 2 | 12 | 10 | 38 | 33 | 1 | |
| Papadopoulou (2011) | 803 | 1,110 | 88 | 352 | 363 | 82 | 384 | 644 | 32.9 | 24.7 | 0.02 | |
| PAPPA (2011) | 148 | 107 | 18 | 81 | 49 | 7 | 38 | 62 | 39.53 | 24.29 | 0.792 | |
| Reyes‐López (2014) | 90 | 108 | 6 | 29 | 55 | 4 | 23 | 81 | 23 | 14 | NA | |
| RIZK (2011) | 40 | 74 | 6 | 18 | 16 | 8 | 37 | 29 | 37.5 | 37.2 | 0.706 | |
| Shaat (2007) | 585 | 1,111 | 59 | 255 | 271 | 69 | 392 | 650 | 31.9 | 23.8 | 0.363 | |
| Shi (2014) | 100 | 100 | 24 | 36 | 40 | 7 | 38 | 55 | 42 | 26 | >0.05 | |
| Thomas (2014) | 117 | 49 | 16 | 46 | 55 | 4 | 18 | 27 | 33.3 | 26.5 | 0.452 | |
| Vcelak (2012) | 261 | 376 | 17 | 102 | 142 | 35 | 185 | 156 | 33.8 | 26.1 | 0.067 | |
| Zhang (2015) | 113 | 115 | 0 | 17 | 96 | 0 | 5 | 110 | 7.52 | 2.17 | >0.05 | |
| Cho (2009) | rs12255372(T) | 867 | 630 | 0 | 7 | 860 | 0 | 2 | 628 | 0.4 | 0.2 | NA |
| de Melo (2015) | 200 | 200 | 20 | 88 | 92 | 23 | 75 | 102 | 32 | 30.3 | 0.633 | |
| Klein (2012) | 125 | 125 | 39.6 | 28 | NA | |||||||
| Pagan (2014) | 45 | 25 | 6 | 20 | 19 | 2 | 14 | 9 | 36 | 36 | 0.4095 | |
| Papadopoulou (2011) | 801 | 1,102 | 81 | 333 | 387 | 84 | 385 | 633 | 30.9 | 25.1 | 0.02 | |
| Reyes‐López (2014) | 90 | 108 | 7 | 23 | 60 | 2 | 5 | 101 | 20 | 5 | NA | |
| RIZK (2011) | 40 | 74 | 6 | 28 | 6 | 11 | 38 | 25 | 50 | 40.5 | 0.108 | |
| Shi (2014) | 100 | 100 | 0 | 0 | 100 | 0 | 0 | 100 | 0 | 0 | >0.05 | |
| Vcelak (2012) | 260 | 376 | 22 | 115 | 124 | 23 | 147 | 206 | 30 | 25.7 | 0.067 | |
| Watanabe (2007) | 94 | 58 | 39.4 | 20.7 | NA | |||||||
| Huerta‐Chagoya (2015) | rs7901695(C) | 408 | 342 | NA | NA | NA | NA | NA | NA | 20.3 | 13.6 | 0.03 |
| Pagan (2014) | 45 | 25 | 8 | 20 | 17 | 2 | 13 | 10 | 40 | 34 | 0.6626 | |
| Papadopoulou (2011) | 794 | 1,102 | 95 | 356 | 343 | 90 | 405 | 607 | 34.4 | 26.5 | 0.02 | |
| Stuebe (2014) | 56 | 842 | 9 | 30 | 17 | 70 | 357 | 415 | 42.9 | 29.5 | >0.05 | |
| Stuebe (2014) | 24 | 366 | 4 | 15 | 5 | 79 | 162 | 121 | 47.9 | 43.7 | >0.05 | |
| Vcelak (2012) | 261 | 376 | 25 | 130 | 106 | 24 | 147 | 205 | 34.4 | 26.9 | 0.067 | |
| Hui (2011) | rs290487(C) | 480 | 631 | 90 | 220 | 170 | 88 | 282 | 261 | 41.7 | 36.3 | 0.2076 |
| Shi (2014) | 100 | 90 | 12 | 36 | 52 | 6 | 34 | 50 | 30 | 33 | >0.05 | |
| Wang (2013) | 70 | 70 | 11 | 37 | 22 | 9 | 33 | 28 | 42.2 | 36.5 | NA | |
| Hui (2011) | rs11196205(G) | 479 | 623 | 461 | 18 | 0 | 591 | 32 | 0 | 98.1 | 97.4 | 1 |
| Shi (2014) | 100 | 100 | 99 | 0 | 1 | 100 | 0 | 0 | 99 | 100 | >0.05 | |
| Hui (2011) | rs11196218(A) | 471 | 625 | 30 | 201 | 240 | 43 | 256 | 326 | 27.7 | 27.4 | 0.1449 |
| Liu (2014) | 144 | 144 | 9 | 58 | 77 | 5 | 53 | 86 | 26 | 22 | >0.05 | |
| Huerta‐Chagoya (2015) | rs12243326(C) | 408 | 342 | NA | NA | NA | NA | NA | NA | 16.8 | 9.6 | 0.03 |
| Pagan (2014) | 45 | 25 | 7 | 20 | 18 | 2 | 13 | 10 | 38 | 34 | 0.6626 | |
| Huerta‐Chagoya (2015) | rs4506565(T) | 408 | 342 | NA | NA | NA | NA | NA | NA | 21.3 | 14 | 0.03 |
| Pagan (2014) | 45 | 25 | 9 | 20 | 16 | 0 | 12 | 13 | 42 | 24 | 0.2762 | |
GDM, gestational diabetes mellitus; HWE, Hardy–Weinberg equilibrium; NA, not available.
Meta‐analysis results
The SNP rs7903146 of TCF7L2 was the most extensively studied variant, and showed a conflicting correlation across different populations. The results of the allele distribution are described in detail in Table 2. We found a significant correlation between the T allele of SNP rs7903146 and the risk of GDM (REM, OR 1.36, 95% CI: 1.17–1.57) by meta‐analysis. Further subgroup analyses were carried out based on race/ethnicity, and the results showed significant relationships in all populations including in Caucasian (REM, OR 1.27, 95% CI: 1.04–1.55), Asian (REM, OR 1.55, 95% CI: 1.05–2.31) and other populations (REM, OR1.39, 95% CI: 1.08–1.80; Figure 2).
Figure 2.
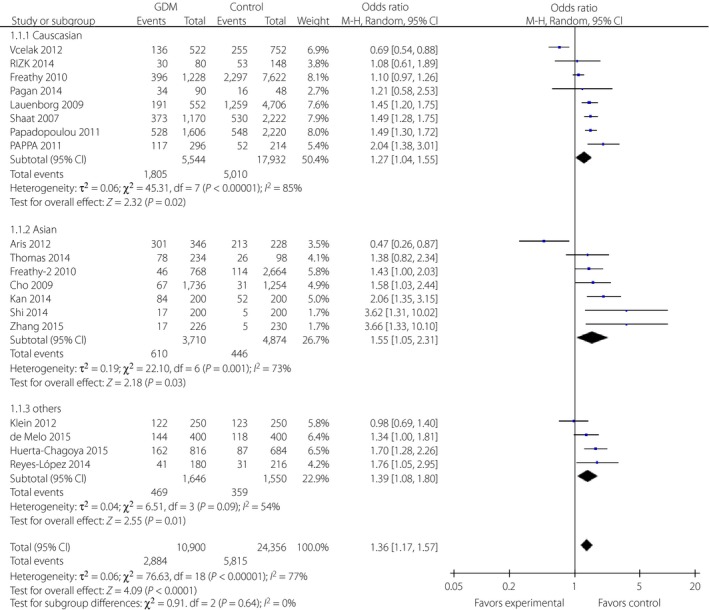
Forest plots of the relationship between TCF7L2 rs7903146 polymorphism and the risk of gestational diabetes mellitus across different populations. Black diamonds denote the pooled odds ratio. Blue squares indicate the odds ratio in each study, with square sizes inversely proportional to the standard error of the odds ratio. Horizontal lines represent 95% confidence intervals (CI).
The rs12255372 polymorphism was also widely studied with the results of different relationships with GDM risk in different populations. There was a significant relationship between the T allele of SNP rs12255372 and the risk of GDM (REM, OR 1.55, 95% CI: 1.23–1.96) by meta‐analysis. Further subgroup analyses showed significant correlations in Caucasian (REM, OR 1.32, 95% CI: 1.17–1.48) and other populations (REM, OR 2.11, 95% CI: 1.17–3.82), but no correlation in Asian populations (REM, OR 2.55, 95% CI: 0.53–12.29; Figure 3).
Figure 3.
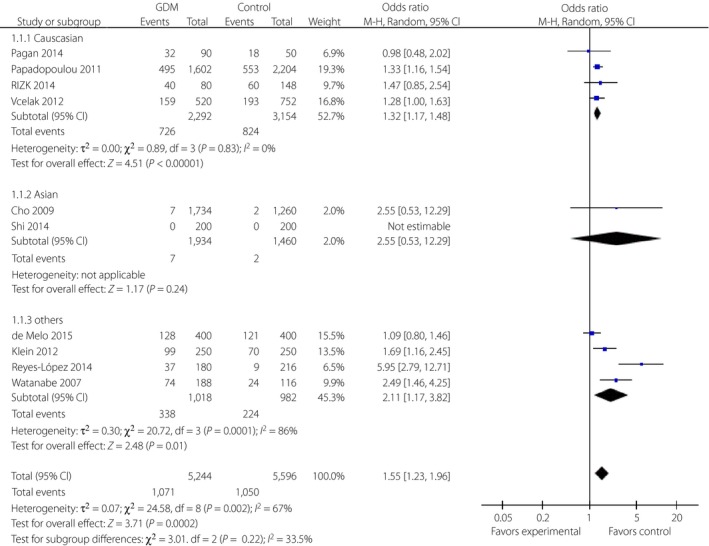
Forest plots of the relationship between TCF7L2 rs12255372 polymorphism and the risk of gestational diabetes mellitus across different populations. Black diamonds denote the pooled odds ratio. Blue squares indicate the odds ratio in each study, with square sizes inversely proportional to the standard error of the odds ratio. Horizontal lines represent 95% confidence intervals (CI).
Additionally, a significant relationship was discovered between the C allele of SNP rs7901695 and GDM risk by meta‐analysis with 1.49 (95% CI: 1.34–1.66) of overall OR (FEM). Further subgroup analyses showed significant correlations in Caucasian (FEM, OR 1.48, 95% CI: 1.32–1.66) and other populations (FEM, OR 1.53, 95% CI: 1.19–1.97; Figure 4).
Figure 4.
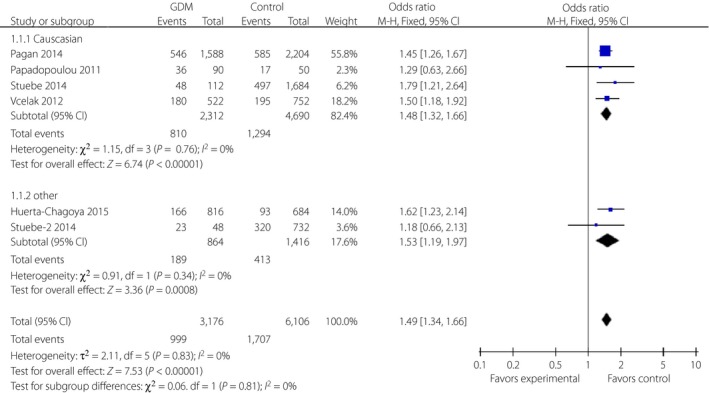
Forest plots of the relationship between TCF7L2 rs7901695 polymorphism and the risk of gestational diabetes mellitus across different populations. Black diamonds denote the pooled odds ratio. Blue squares indicate the odds ratio in each study, with square sizes inversely proportional to the standard error of the odds ratio. Horizontal lines represent 95% confidence intervals (CI).
Three variants in the TCF7L2 gene, including rs290487, rs11196205 and rs11196218, were researched only in Chinese populations (Figures 5, 6, 7). There was a significant correlation between rs290487 (FEM, OR 1.25, 95% CI: 1.08–1.46) and GDM risk, but no correlation with rs11196205 (FEM, OR 1.24, 95% CI: 0.71–2.18) and rs11196218 (FEM, OR 1.06, 95% CI: 0.90–1.26). Two other SNPs in the TCF7L2 gene were found only in two literature research articles with different ethnicity, and they had significant correlations between rs4506565 (FEM, OR 1.72, 95% CI: 1.33–2.23) with GDM, and rs12243326 (FEM, OR 1.76, 95% CI: 1.32–2.34) with GDM (Figures 8 and 9).
Figure 5.
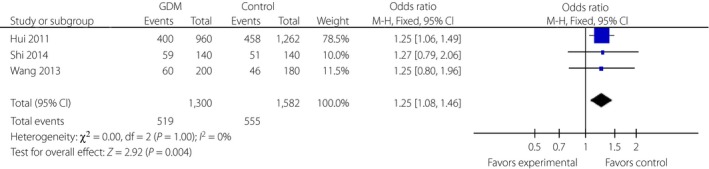
Forest plots of the relationship between TCF7L2 rs290487 polymorphism and the risk of gestational diabetes mellitus across different populations. Black diamonds denote the pooled odds ratio. Blue squares indicate the odds ratio in each study, with square sizes inversely proportional to the standard error of the odds ratio. Horizontal lines represent 95% confidence intervals (CI).
Figure 6.
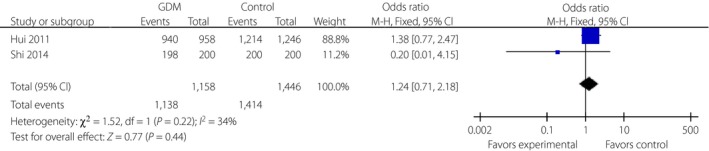
Forest plots of the relationship between TCF7L2 rs11196205 polymorphism and the risk of gestational diabetes mellitus across different populations. Black diamonds denote the pooled odds ratio. Blue squares indicate the odds ratio in each study, with square sizes inversely proportional to the standard error of the odds ratio. Horizontal lines represent 95% confidence intervals (CI).
Figure 7.
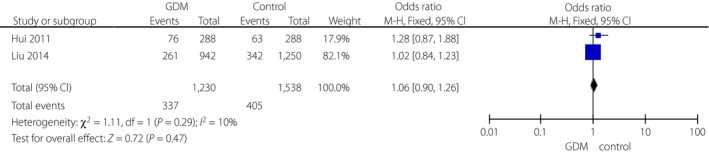
Forest plots of the relationship between TCF7L2 rs11196218 polymorphism and risk of gestational diabetes mellitus (GDM) across different populations. Black diamonds denote the pooled odds ratio. Blue squares indicate the odds ratio in each study, with square sizes inversely proportional to the standard error of the odds ratio. Horizontal lines represent 95% confidence intervals (CI).
Figure 8.
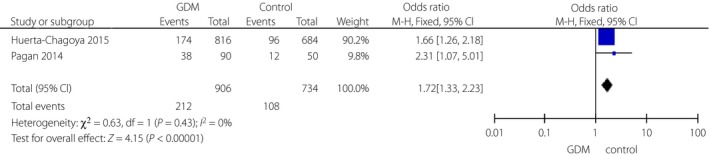
Forest plots of the relationship between TCF7L2 rs4506565 polymorphism and the risk of gestational diabetes mellitus (GDM) across different populations. Black diamonds denote the pooled odds ratio. Blue squares indicate the odds ratio in each study, with square sizes inversely proportional to the standard error of the odds ratio. Horizontal lines represent represent 95% confidence intervals (CI).
Figure 9.
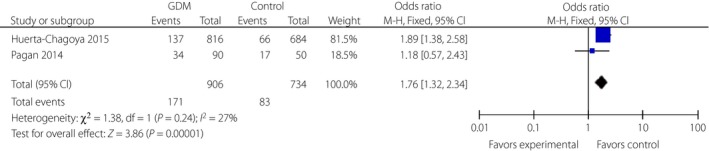
Forest plots of the relationship between TCF7L2 rs12243326 polymorphism and risk of gestational diabetes mellitus across different populations. Black diamonds denote the pooled odds ratio. Blue squares indicate the odds ratio in each study, with square sizes inversely proportional to the standard error of the odds ratio. Horizontal lines represent 95% confidence intervals (CI).
Heterogeneity
Heterogeneity between studies was measured in all comparisons. There was no heterogeneity in rs7901695, rs4506565 and rs290487 with I 2 = 0%, slight heterogeneity in rs11196205 (I 2 = 34%), rs11196218 (I 2 = 10%) and rs12243326 (I 2 = 27%), but severe heterogeneity in rs12255372 (I 2 = 67%) and rs7903146 (I 2 = 77%). Except for rs12255372 and rs7903146, all SNPs in the TCF7L2 gene were analyzed using the FEM. Although subgroup analysis according to ethnicity of rs12255372 and rs7903146 was carried out, heterogeneity was still severe. Therefore, the use of REMs was justified in these analyses.
Publication bias analysis
Publication bias of all TCF7L2 SNPs was determined using a funnel plot of Revman 5.2, but vague results of SNPs rs7903146 and SNPs rs7903146 were found. Therefore, Egger of Stata13 was used to further confirm the results. The results showed that no evidence of statistically significant publication bias was detected for the studies (Figure [Link], [Link], [Link], [Link], [Link], [Link], [Link], [Link]; Tables [Link], [Link], [Link], [Link]).
Discussion
An increasing number of studies have shown that TCF7L2 variants are related to GDM risk. However, the results of the studies are inconsistent and incomplete, and might have limited statistical power with individual studies having relatively small sample sizes and the analysis of partial SNPs. Therefore, we carried out the meta‐analysis with the aim to provide a more comprehensive summary of the currently available evidence with respect to the relationship between TCF7L2 variants and GDM risk. Overall, 22 eligible studies captured eight TCF7L2 SNPs, including 5,573 cases and 13,266 controls. The SNPs rs7903146 C>T, rs12255372 G>T and rs7901695 T>C were the most powerful to assess the relationship between TCF7L2 polymorphism and the risk of GDM. The SNPs rs7903146 and rs7901695 showed significant relationships with GDM risk in the overall and subgroup analyses; however, the relationship of rs12255372 was significant and conflicting among different ethnicities.
GDM can progress when a genetic susceptibility to pancreatic islet β‐cell injury is exposed by incremental insulin resistance during pregnancy40. Among the most widely studied genes involved in GDM risk, TCF7L2 is identified as regulating β‐cell action41. It is well known that TCF7L2 expresses in pancreatic β‐cells, and belongs to the supernal mobility group‐box transcription factors family and plays a vital role in maintaining glucose homeostasis. Eight SNPs in the TCF7L2 gene have been reported, including rs7903146, rs12255372, rs7901695, rs290487, rs4506565, rs11196205, rs11196218 and rs12243326.
The most widely studied SNP was rs7903146, and a previous meta‐analysis regarding this SNP found a 1.65‐fold increased risk of GDM, based on six studies42, and 1.63‐fold in 16 studies43. We identified 18 studies assessing the association, and the results were consistent with the previous analysis. Furthermore, we carried out subgroup analysis based on ethnicity, and found a significant correlation in Caucasian and Asian ethnicities and others, as seen in the previous study. Combined with all results, T allele of the SNP rs7903146 of TCF7L2 was related to GDM risk.
The rs12255372 variant was also widely studied with the results of different relationships with GDM risk in different populations. A systematic review of four studies found a significant relationship between this SNP and GDM44. We extended the sample to nine studies, and increased the number of participants to carry out a relatively comprehensive assessment. The significant relationship remained, with rs12255372 associated with GDM, consistent with previous research. However, findings of the subgroup analysis differed, with a significant correlation in Caucasian and other groups, but no association in Asian groups. Overall, this suggests that the T allele of rs12255372 might be related to GDM risk, but to varying degrees among different ethnicities.
A significant relationship was discovered in the meta‐analysis regarding the C allele of rs7901695 and the risk of GDM (OR 1.49, 95% CI: 1.34–1.66), with subgroup analysis showing a significant correlation in Caucasian, Asian and other groups.
In conclusion, different relationships between TCF7L2 variants and GDM risk among different ethnic groups might be attributable to genetic characteristics and sample size, or selection standards of participants, but could also be because of different growth environments, body structure and variations in genetic background. Therefore, ongoing research and systematic analysis is important. Because we were unable to completely eliminate potential bias and heterogeneous factors in the present meta‐analysis, further studies with rich sample capacity and using normalized unbiased genotyping methods are required to verify the present findings.
Disclosure
The authors declare no conflict of interest.
Supporting information
Figure S1 | Funnel plots of publication bias for the relationship of TCF7L2 rs7903146 polymorphism and risk of gestational diabetes mellitus across different populations.
Figure S2 | Funnel plots of publication bias for the relationship of TCF7L2 rs12255372 polymorphism and risk of gestational diabetes mellitus across different populations.
Figure S3 | Funnel plots of publication bias for the relationship of TCF7L2 rs7901695 polymorphism and risk of gestational diabetes mellitus across different populations.
Figure S4 | Funnel plots of publication bias for the relationship of TCF7L2 rs290487 polymorphism and risk of gestational diabetes mellitus across different populations.
Figure S5 | Funnel plots of publication bias for the relationship of TCF7L2 rs4506565 polymorphism and risk of gestational diabetes mellitus across different populations.
Figure S6 | Funnel plots of publication bias for the relationship of TCF7L2 rs11196205 polymorphism and risk of gestational diabetes mellitus across different populations.
Figure S7 | Funnel plots of publication bias for the relationship of TCF7L2 rs11196218 polymorphism and risk of gestational diabetes mellitus across different populations.
Figure S8 | Funnel plots of publication bias for the relationship of TCF7L2 rs12243326 polymorphism and risk of gestational diabetes mellitus across different populations.
Table S1 | Publication bias list for the relationship of TCF7L2 rs7903146 polymorphism and risk of GDM across different populations.
Table S2 | Egger's test of publication bias for the relationship of TCF7L2 rs7903146 polymorphism and risk of GDM across different populations.
Table S3 | Publication bias list for the relationship of TCF7L2 rs12255372 polymorphism and risk of GDM across different populations.
Table S4 | Egger's test of publication bias for the relationship of TCF7L2 rs12255372 polymorphism and risk of GDM across different populations.
Acknowledgments
The authors thank Zhen Wang for remarkable technical support, and Li Wang for carefully reviewing the manuscript. This work was supported by the National Natural Science Foundation of China (grant number 81370967) and Beijing Natural Science Foundation (7132036).
J Diabetes Investig 2017; 8: 560–570
References
- 1. Expert Committee on the Diagnosis and Classification of Diabetes Mellitus . Report of the expert committee on the diagnosis and classification of diabetes mellitus. Diabetes Care 2003; 26(Suppl 1): S5–S20. [DOI] [PubMed] [Google Scholar]
- 2. Dabelea D, Snell‐Bergeon JK, Hartsfield CL, et al Increasing prevalence of gestational diabetes mellitus (GDM) over time and by birth cohort: Kaiser Permanente of Colorado GDM Screening Program. Diabetes Care 2005; 28: 579–584. [DOI] [PubMed] [Google Scholar]
- 3. Reece EA, Leguizamon G, Wiznitzer A. Gestational diabetes: the need for a common ground. Lancet 2009; 373: 1789–1797. [DOI] [PubMed] [Google Scholar]
- 4. Association AD. Gestational diabetes mellitus. Diabetes Care 2004; 27(Suppl 1): S88–S90. [DOI] [PubMed] [Google Scholar]
- 5. Bellamy L, Casas JP, Hingorani AD, et al Type 2 diabetes mellitus after gestational diabetes: a systematic review and meta‐analysis. Lancet 2009; 373: 1773–1779. [DOI] [PubMed] [Google Scholar]
- 6. Lapolla A, Dalfra MG, Fedele D. Diabetes related autoimmunity in gestational diabetes mellitus: is it important? Nutr Metab Cardiovascu Dis 2009; 19: 674–682. [DOI] [PubMed] [Google Scholar]
- 7. Shaat N, Groop L. Genetics of gestational diabetes mellitus. Curr Med Chem 2007; 14: 569–583. [DOI] [PubMed] [Google Scholar]
- 8. Cauchi S, Meyre D, Dina C, et al Transcription factor TCF7L2 genetic study in the French population: expression in human beta‐cells and adipose tissue and strong association with type 2 diabetes. Diabetes 2006; 55: 2903–2908. [DOI] [PubMed] [Google Scholar]
- 9. Duval A, Busson‐Leconiat M, Berger R, et al Assignment of the TCF‐4 gene (TCF7L2) to human chromosome band 10q25.3. Cytogenet Cell Genet 2000; 88: 264–265. [DOI] [PubMed] [Google Scholar]
- 10. Ekelund M, Shaat N, Almgren P, et al Genetic prediction of postpartum diabetes in women with gestational diabetes mellitus. Diabetes Res Clin Pract 2012; 97: 394–398. [DOI] [PubMed] [Google Scholar]
- 11. Pappa KI, Gazouli M, Economou K, et al Gestational diabetes mellitus shares polymorphisms of genes associated with insulin resistance and type 2 diabetes in the Greek population. Gynecol Endocrinol 2011; 27: 267–272. [DOI] [PubMed] [Google Scholar]
- 12. Freathy RM, Hayes MG, Urbanek M, et al Hyperglycemia and Adverse Pregnancy Outcome (HAPO) Study: common genetic variants in GCK and TCF7L2 are associated with fasting and postchallenge glucose levels in pregnancy and with the new consensus definition of gestational diabetes mellitus from the International Association of Diabetes and Pregnancy Study Groups. Diabetes 2010; 59: 2682–2689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Lauenborg J, Grarup N, Damm P, et al Common type 2 diabetes risk gene variants associate with gestational diabetes. J Clin Endocrinol Metab 2009; 94: 145–150. [DOI] [PubMed] [Google Scholar]
- 14. Shaat N, Lernmark A, Karlsson E, et al A variant in the transcription factor 7‐like 2 (TCF7L2) gene is associated with an increased risk of gestational diabetes mellitus. Diabetologia 2007; 50: 972–979. [DOI] [PubMed] [Google Scholar]
- 15. Kan Lin LA‐F, Feng‐ying F, Yu‐jie T, et al Study of the association in transcription factor 7‐like2 (TCF7L2) gene polymorphism with gestational diabetes mellitus. Chin J Birth Health Hered 2014; 22: 41–43 (Chinese). [Google Scholar]
- 16. Nasser M, RIZK AAR, Rooshenas FA, et al The Rs12255372 Variant of Transcription Like Factor 7–Like 2 [TCF7L2] Is Associated with an Increased Risk of Gestational Diabetes Mellitus in Arab Women. Diabetes 2011; 60 (SUPPL. 1): A643. [Google Scholar]
- 17. Cho YM, Kim TH, Lim S, et al Type 2 diabetes‐associated genetic variants discovered in the recent genome‐wide association studies are related to gestational diabetes mellitus in the Korean population. Diabetologia 2009; 52: 253–261. [DOI] [PubMed] [Google Scholar]
- 18. Včelák J, Vejražková D, Vaňková M, et al T2D Risk Haplotypes of the TCF7L2 Gene in the Czech Population Sample: the Association With Free Fatty Acids Composition. Physiol Res 2012; 61: 229–240. [DOI] [PubMed] [Google Scholar]
- 19. Klein K, Haslinger P, Bancher‐Todesca D, et al Transcription factor 7‐like 2 gene polymorphisms and gestational diabetes mellitus. J Maternal‐fetal Neonatal Med 2012; 25: 1783–1786. [DOI] [PubMed] [Google Scholar]
- 20. Papadopoulou A, Lynch KF, Shaat N, et al Gestational diabetes mellitus is associated with TCF7L2 gene polymorphisms independent of HLA‐DQB1*0602 genotypes and islet cell autoantibodies. Diabetic Med 2011; 28: 1018–1027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Reyes‐Lopez R, Perez‐Luque E, Malacara JM. Metabolic, hormonal characteristics and genetic variants of TCF7L2 associated with development of gestational diabetes mellitus in Mexican women. Diabet Metab Res Rev 2014; 30: 701–706. [DOI] [PubMed] [Google Scholar]
- 22. Kwak SH, Kim S‐H, Cho YM, et al A Genome‐Wide Association Study of Gestational Diabetes Mellitus in Korean Women. Diabetes Care 2012; 61: 531–541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Hongmei ZXJ. Relationship between rs7903146‐T/C polymorphism of TCFTL2 gene and gestational diabetes mellitus. J Chin Phys 2015; 17: 65–67 (Chinese). [Google Scholar]
- 24. Thomas N, Mahesh D, Chapla A, et al Does TCF7L2 polymorphisms increase the risk of gestational diabetes mellitus in South Indian population?. Endocrine Abstracts 2014; 34: P270. [Google Scholar]
- 25. Huerta‐Chagoya A, Vazquez‐Cardenas P, Moreno‐Macias H, et al Genetic determinants for gestational diabetes mellitus and related metabolic traits in Mexican women. PLoS One 2015; 10: e0126408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. de Melo SF, Frigeri HR, dos Santos‐Weiss IC, et al Polymorphisms in FTO and TCF7L2 genes of Euro‐Brazilian women with gestational diabetes. Clin Biochem 2015; 48: 1064–1067. [DOI] [PubMed] [Google Scholar]
- 27. Aris NKM, Ismai NAM, Mahdy ZA, et al An Analysis of Targeted Single Nucleotide Polymorphisms for the Risk Prediction of Gestational Diabetes Mellitus in a Cohort of Malaysian Patients. Asia‐Pacific J Mol Med 2011; 1: 1–8. [Google Scholar]
- 28. Shi Xiling CZ. Association of single nucleotide polymorphism of transcription factor 7 like 2 gene with gestafional diabetes mellitus. Chin J Birth Health Hered 2014; 22: 9–11 (Chinese). [Google Scholar]
- 29. Pagan A, Sabater‐Molina M, Olza J, et al A gene variant in the transcription factor 7‐like 2 (TCF7L2) is associated with an increased risk of gestational diabetes mellitus. Eur J Obstet Gynecol Reprod Biol 2014; 180: 77–82. [DOI] [PubMed] [Google Scholar]
- 30. Watanabe RM, Allayee H, Xiang AH, et al Transcription factor 7‐like 2 (TCF7L2) is associated with gestational diabetes mellitus and interacts with adiposity to alter insulin secretion in Mexican Americans. Diabetes 2007; 56: 1481–1485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Stuebe AM, Wise A, Nguyen T, et al Maternal genotype and gestational diabetes. Am J Perinatol 2014; 31: 69–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Yuan‐chi H, Fang P, Wei L, et al Association of single nucleotide polymorphism of transcription factor 7·like 2 gene wim gestafional diabetes mellitus. Chin J Endocrinol Metab 2011; 27: 32–35 (Chinese).. [Google Scholar]
- 33. Qingling W, Wang Y, Jian G, et al Association of the rs290487 polymorphism in transcription factor 7‐like 2 (TCF7L2) gene with gestational diabetes mellitus. Matern Child Health Care China 2013; 28: 4374–4375 (Chinese). [Google Scholar]
- 34. Xiaohui L, LF, Huanling Z. Study on the correlation between gestational diabetes mellitus andChinese gene TCF7L2 polymorphism. China Health Indust 2012; 12: 7–9 (Chinese). [Google Scholar]
- 35. Tong Y, Lin Y, Zhang Y, et al Association between TCF7L2 gene polymorphisms and susceptibility to type 2 diabetes mellitus: a large Human Genome Epidemiology (HuGE) review and meta‐analysis. BMC Med Genet 2009; 10: 15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Grant SF, Thorleifsson G, Reynisdottir I, et al Variant of transcription factor 7‐like 2 (TCF7L2) gene confers risk of type 2 diabetes. Nat Genet 2006; 38: 320–323. [DOI] [PubMed] [Google Scholar]
- 37. Helgason A, Palsson S, Thorleifsson G, et al Refining the impact of TCF7L2 gene variants on type 2 diabetes and adaptive evolution. Nat Genet 2007; 39: 218–225. [DOI] [PubMed] [Google Scholar]
- 38. Scott LJ, Bonnycastle LL, Willer CJ, et al Association of transcription factor 7‐like 2 (TCF7L2) variants with type 2 diabetes in a Finnish sample. Diabetes 2006; 55: 2649–2653. [DOI] [PubMed] [Google Scholar]
- 39. Lyssenko V, Lupi R, Marchetti P, et al Mechanisms by which common variants in the TCF7L2 gene increase risk of type 2 diabetes. J Clin Investig 2007; 117: 2155–2163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Lambrinoudaki I, Vlachou SA, Creatsas G. Genetics in gestational diabetes mellitus: association with incidence, severity, pregnancy outcome and response to treatment. Curr Diabet Rev 2010; 6: 393–399. [DOI] [PubMed] [Google Scholar]
- 41. Schafer SA, Machicao F, Fritsche A, et al New type 2 diabetes risk genes provide new insights in insulin secretion mechanisms. Diabetes Res Clin Pract 2011; 93(Suppl 1): S9–S24. [DOI] [PubMed] [Google Scholar]
- 42. Kang S, Xie Z, Zhang D. Association of the rs7903146 polymorphism in transcription factor 7‐like 2 (TCF7L2) gene with gestational diabetes mellitus: a meta‐analysis. Gynecol Endocrinol 2013; 29: 873–877. [DOI] [PubMed] [Google Scholar]
- 43. Lin PC, Lin WT, Yeh YH, et al Transcription Factor 7‐Like 2 (TCF7L2) rs7903146 polymorphism as a risk factor for gestational diabetes mellitus: a meta‐analysis. PLoS One 2016; 11: e0153044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Zhang C, Bao W, Rong Y, et al Genetic variants and the risk of gestational diabetes mellitus: a systematic review. Human Reproduct Update 2013; 19: 376–390. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1 | Funnel plots of publication bias for the relationship of TCF7L2 rs7903146 polymorphism and risk of gestational diabetes mellitus across different populations.
Figure S2 | Funnel plots of publication bias for the relationship of TCF7L2 rs12255372 polymorphism and risk of gestational diabetes mellitus across different populations.
Figure S3 | Funnel plots of publication bias for the relationship of TCF7L2 rs7901695 polymorphism and risk of gestational diabetes mellitus across different populations.
Figure S4 | Funnel plots of publication bias for the relationship of TCF7L2 rs290487 polymorphism and risk of gestational diabetes mellitus across different populations.
Figure S5 | Funnel plots of publication bias for the relationship of TCF7L2 rs4506565 polymorphism and risk of gestational diabetes mellitus across different populations.
Figure S6 | Funnel plots of publication bias for the relationship of TCF7L2 rs11196205 polymorphism and risk of gestational diabetes mellitus across different populations.
Figure S7 | Funnel plots of publication bias for the relationship of TCF7L2 rs11196218 polymorphism and risk of gestational diabetes mellitus across different populations.
Figure S8 | Funnel plots of publication bias for the relationship of TCF7L2 rs12243326 polymorphism and risk of gestational diabetes mellitus across different populations.
Table S1 | Publication bias list for the relationship of TCF7L2 rs7903146 polymorphism and risk of GDM across different populations.
Table S2 | Egger's test of publication bias for the relationship of TCF7L2 rs7903146 polymorphism and risk of GDM across different populations.
Table S3 | Publication bias list for the relationship of TCF7L2 rs12255372 polymorphism and risk of GDM across different populations.
Table S4 | Egger's test of publication bias for the relationship of TCF7L2 rs12255372 polymorphism and risk of GDM across different populations.


