Abstract
Aims/Introduction
It has not been reported whether chronic hepatitis B virus infection (CHB) is associated with a specific type of diabetes. We sought to investigate the prevalence of CHB status in different diabetes subtypes among a Chinese population.
Materials and Methods
This was a cross‐sectional study. A total of 381 patients with adult‐onset autoimmune diabetes, 1,365 patients with type 2 diabetes and 1,365 non‐diabetic controls were recruited from June 2005 to February 2014. The exclusion criteria included: (i) hepatitis C virus antibody positive; (ii) hepatic cirrhosis; and (iii) malignant neoplasm and severe renal dysfunction (serum creatinine >450 μmol/L). Patients were grouped as hepatitis B virus‐negative and CHB status.
Results
Patients with type 2 diabetes had a higher prevalence of CHB than the controls in the overall population (13.5 vs 10.0%, P = 0.004) and among patients with normal hepatic function (13.3 vs 8.8%, P = 0.002). There was no difference in the prevalence of CHB status between patients with adult‐onset autoimmune diabetes and the controls. Multiple logistic regression analysis showed that the odds ratio of CHB increased by ~1.5‐fold in patients with type 2 diabetes than in the control group after adjustment for age, sex and body mass index, regardless of hepatic function status.
Conclusions
CHB status was more prevalent in patients with type 2 diabetes than in individuals with adult‐onset autoimmune diabetes and the controls among the Chinese population. Further research is required to ascertain whether CHB status increases the risk of developing type 2 diabetes, or whether type 2 diabetes, but not adult‐onset autoimmune diabetes, increases the risk of CHB.
Keywords: Adult‐onset autoimmune diabetes, Hepatitis B virus, Type 2 diabetes
Introduction
Traditionally, the development of diabetes has been viewed to be closely associated with aging, obesity and lack of physical activity1. However, research has also shown that hepatitis virus infections could be a potential risk factor for diabetes. Previous studies have already shown that hepatitis C virus (HCV) infection is a risk factor for developing type 2 diabetes2, 3. Some studies have shown that chronic hepatitis B virus (HBV) infection (CHB) is also related to an increased risk of diabetes in certain ethnic populations, such as Asian Americans4, and associated with increased impaired fasting glucose among Nigerians5. A study in Hong Kong showed that hepatitis B surface antigen (HBsAg) carrier status was an independent risk factor for gestational diabetes6. Furthermore, HBV infection has been associated with impaired hepatic insulin signaling and insulin resistance7, 8. Meanwhile, other studies have reported that CHB was not associated with diabetes9, 10.
However, few studies have examined the relationship between CHB and diabetes while fully controlling for the effects of liver cirrhosis or hepatic dysfunction, conditions that are well recognized to be associated with diabetes11, 12, 13, 14, 15; more than 30% of patients with liver cirrhosis have diabetes11, 12. Additionally, it has not been reported whether CHB status is associated with a specific type of diabetes. Adult‐onset autoimmune diabetes, which accounts for 5–10% of those with diabetes16, was defined as: age at diagnosis of diabetes ≥18 years and the presence of the islet auto‐antibodies glutamic acid decarboxylase and/or protein tyrosine phosphatase IA‐2 in the present study. In the present study, we explored the association of CHB status with adult‐onset autoimmune diabetes and type 2 diabetes among Chinese patients, taking hepatic function into consideration.
Materials and Methods
Patients
The present study was carried out at Shanghai Jiao Tong University Affiliated Sixth People's Hospital, Shanghai, China. A 10‐year hospital‐based dataset (from June 2005 to February 2014) comprising clinical, medication and laboratory data was analyzed. All cases with adult‐onset diabetes (age at diagnosis of diabetes ≥18 years) were recruited from the Department of Endocrinology and Metabolism. A total of 1,797 adult inpatients with diabetes were assessed in the study according to the following inclusion criteria: (i) aged 20–75 years; (ii) with complete islet autoantibody data; and (iii) screening markers of HBV and HCV. Patients with any of the following abnormalities were excluded: HCV antibody‐positive (n = 10); a history of cirrhosis or cirrhosis detected by ultrasonography (n = 10); malignant neoplasm (n = 19); and severe renal dysfunction with serum creatinine ≥450 μmol/L (n = 12). Finally, 1,746 diabetes patients (381 with adult‐onset autoimmune diabetes and 1,365 with type 2 diabetes) were enrolled in the present study (Figure 1). The controls were collected from the Department of Traumatology and Department of General Surgery. These patients were hospitalized for trauma or diseases requiring general surgery, who were admitted in our hospital during the same period as those with diabetes, and were matched with patients with type 2 diabetes according to the criteria of similar age, sex and approximate hepatic function (normal or abnormal hepatic function). Those patients with HCV antibody positivity, severe renal dysfunction, cancer or hepatic cirrhosis were excluded according to the study protocol. A total of 1,365 individuals without diabetes were enrolled as the controls.
Figure 1.
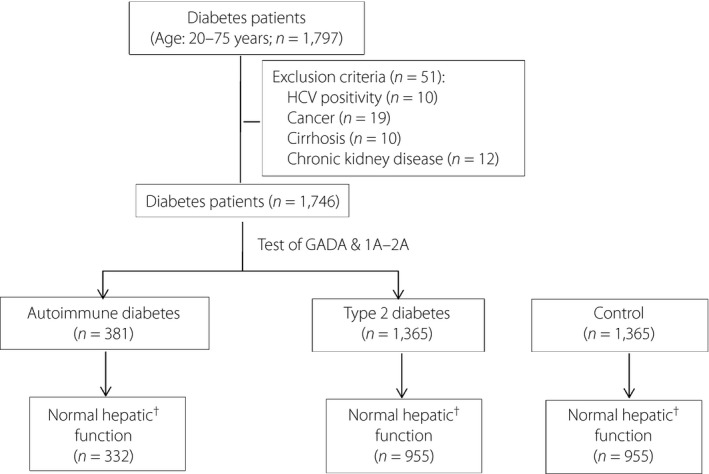
Flowchart of participants enrolled. †Normal hepatic function: alanine amino transferase <65 U/L and aspartate amino transferase <37 U/L. GADA, auto‐antibody to glutamic acid decarboxylase; HCV, hepatitis C virus; IA‐2A, auto‐antibody to protein tyrosine phosphatase IA‐2.
The protocol of the research project was approved by the institutional review board of Shanghai Jiao Tong University Affiliated Sixth People's Hospital in accordance with the principles of the Helsinki Declaration in 1995. Written consent was obtained from all the patients included in this study.
Anthropometric and biochemical measurements
Height and weight were measured at hospital admission while the patients were barefoot and wearing light clothing. Body mass index (BMI) was calculated as weight divided by height squared (kg/m2).
Alanine aminotransferase (ALT) was measured using the ultraviolet lactate dehydrogenase method, aspartate amino transferase (AST) using the ultraviolet‐malic dehydrogenase method, gamma‐glutamyl transpeptidase using the L‐γ‐glutamyl‐ 3‐carboxy‐4 nitranilide method and alkaline phosphatase using an Alkaline Phosphatase Kit (Shanghai Kehua Bio‐Engineering, Shanghai, China). Total bilirubin was determined using the vanadate oxidation method (Wako Pure Chemical Industries Ltd, Osaka, Japan). Serum albumin was determined by nephelometry, and activated partial thromboplastin time was determined using Dade Actin Activated Cephaloplastin Reagent (Siemens Healthcare Diagnostics Products, Marburg, Germany).
Autoantibodies to glutamic acid decarboxylase and/or protein tyrosine phosphatase IA‐2 were assessed using a radioligand assay (RSR Limited, Cardiff, UK) between June 2005 and March 2007, and enzyme‐linked immunosorbent assay (Euroimmun, Luebeck, Germany) from April 2007 onwards. Autoantibodies were defined according to the manufacturer's instructions.
Analysis of HBV serum markers
HBV serum markers, including HBsAg, hepatitis B surface antibody, hepatitis B e antigen, hepatitis B e antibody and hepatitis B core antibody were measured by enzyme‐linked immunosorbent assay (Shanghai Kehua Bio‐Engineering, Shanghai, China) before November 2008, and chemiluminescence particle immunoassays (Architect, Sligo, Ireland) after November 2008. HCV antibody was measured using an enzyme‐linked immunosorbent assay (Shanghai Kehua Bio‐Engineering). All assays and classification of the aforementioned parameters were carried out according to the manufacturers' instructions.
Definitions of chronic HBV infection status and normal hepatic function
CHB status was defined as HBsAg seropositivity, and all other patients were defined as HBV negative. Normal hepatic function was defined as a normal level of ALT (<65 U/L) and AST (<37 U/L).
Definition of glycemic status
Diabetes was defined as fasting plasma glucose ≥7.0 mmol/L and/or a 2‐h post‐load plasma glucose ≥11.1 mmol/L, or receiving medical treatment for diabetes. Adult‐onset autoimmune diabetes was defined as age at diagnosis of diabetes ≥18 years, and the presence of the islet auto‐antibodies of glutamic acid decarboxylase and/or protein tyrosine phosphatase IA‐2. Non‐diabetes was identified according to the criteria of negative diabetic history, normal fasting plasma glucose and possibly glycated hemoglobin <6.5%1.
Statistical analysis
The distribution of continuous variables was assessed using the Kolmogorov–Smirnov test. Data are expressed as median values (interquartile range) for continuous variables and as frequency (%) for categorical variables. The differences between continuous variables were examined using the Mann–Whitney U‐test; categorical variables were examined using the χ2‐test. The association of HBV infection status with diabetes subtypes was assessed using multinomial logistic regression analyses using the entry method; odds ratios and P‐values are shown (Tables 3 and 4). All statistical analyses were carried out using spss 12.0 (SPSS, Chicago, Illinois, USA); two‐sided P‐values <0.05 were considered statistically significant.
Table 3.
Association of CHB with diabetes subtypes in the total population
| Variable | Prevalence of CHB (%) | Model 1 | Model 2 | ||
|---|---|---|---|---|---|
| Crude OR | P‐value | Adjusted OR | P‐value | ||
| Total | |||||
| Glycemic status | |||||
| Non‐diabetes | 10.0 | 1 | – | 1 | – |
| Adult‐onset autoimmune diabetes | 7.6 | 0.75 (0.49–1.13) | 0.171 | 0.92 (0.59–1.45) | 0.733 |
| Type 2 diabetes | 13.5 | 1.41 (1.11–1.78) | 0.004 | 1.51 (1.11–2.05) | 0.009 |
| Men | |||||
| Glycemic status | |||||
| Non‐diabetes | 11.6 | 1 | – | 1 | – |
| Adult‐onset autoimmune diabetes | 6.9 | 0.57 (0.32–1.02) | 0.058 | 0.69 (0.37–1.29) | 0.242 |
| Type 2 diabetes | 14.9 | 1.34 (1.01–1.77) | 0.045 | 1.41 (0.97–2.04) | 0.072 |
| Women | |||||
| Glycemic status | |||||
| Non‐diabetes | 7.3 | 1 | – | 1 | – |
| Adult‐onset autoimmune diabetes | 8.4 | 1.16 (0.62–2.17) | 0.636 | 1.4 (0.71–2.78) | 0.335 |
| Type 2 diabetes | 11.2 | 1.59 (1.04–2.44) | 0.033 | 1.77 (1.02–3.09) | 0.042 |
Odds ratios (OR) were derived from multinomial logistic regression. ORs in model 2 were adjusted for sex (men), age (years) and body mass index (kg/m2). CHB, chronic hepatitis B virus infection.
Table 4.
Association of hepatitis B virus infection status with diabetes subtypes among patients with normal hepatic function
| Variable | Prevalence of CHB (%) | Model 1 | Model 2 | ||
|---|---|---|---|---|---|
| Crude OR | P‐value | Adjusted OR | P‐value | ||
| Total | |||||
| Glycemic status | |||||
| Non‐diabetes | 8.8 | 1 | – | 1 | – |
| Adult‐onset autoimmune diabetes | 7.3 | 0.89 (0.55–1.45) | 0.642 | 0.81 (0.51–1.30) | 0.383 |
| Type 2 diabetes | 13.3 | 1.47 (1.05–2.07) | 0.026 | 1.59 (1.19–2.13) | 0.002 |
| Men | |||||
| Glycemic status | |||||
| Non‐diabetes | 10.3 | 1 | – | 1 | – |
| Adult‐onset autoimmune diabetes | 6.2 | 0.58 (0.30–1.12) | 0.106 | 0.66 (0.34–1.29) | 0.223 |
| Type 2 diabetes | 14.1 | 1.43 (1.00–2.03) | 0.050 | 1.30 (0.86–1.97) | 0.214 |
| Women | |||||
| Glycemic status | |||||
| Non‐diabetes | 6.5 | 1 | – | 1 | – |
| Adult‐onset autoimmune diabetes | 8.4 | 1.34 (0.66–2.70) | 0.418 | 1.37 (0.66–2.82) | 0.397 |
| Type 2 diabetes | 12.1 | 2.00 (1.19–3.35) | 0.009 | 1.89 (1.04–3.42) | 0.036 |
Odds ratios (OR) were derived from multinomial logistic regression. ORs in model 2 were adjusted for sex (men), age (years) and body mass index (kg/m2). CHB, chronic hepatitis B virus infection.
Results
Clinical characteristics of the two groups
As shown in Table 1, patients with adult‐onset autoimmune diabetes and type 2 diabetes had higher levels of fasting plasma glucose and BMI than the controls (P < 0.05). However, patients with type 2 diabetes had higher levels of BMI, fasting C‐peptide, ALT, AST, gamma‐glutamyl transpeptidase and triglyceride, but shorter duration of diabetes than patients with adult‐onset autoimmune diabetes (all P < 0.01).
Table 1.
Clinical characteristics of patients with adult‐onset autoimmune diabetes and type 2 diabetes
| Variables | Control (group A: n = 1,365) | Adult‐onset autoimmune diabetes (group B: n = 381) | Type 2 diabetes (group C: n = 1,365) | P‐value | ||
|---|---|---|---|---|---|---|
| A vs B | A vs C | B vs C | ||||
| Sex (men) | 847 (62.1) | 202 (53.0) | 847 (62.1) | 0.001 | 1.000 | 0.001 |
| Age (years) | 54 (45–63) | 55 (43‐63) | 54 (45–63) | 0.422 | 0.987 | 0.417 |
| BMI (kg/m2) | 22.0 (18.7–22.7) | 22.2 (19.0–24.2) | 25.3 (22.8–27.7) | 0.002 | <0.001 | <0.001 |
| DM duration (years) | – | 5 (1–10) | 3 (0.1–10) | – | – | <0.001 |
| FPG (mmol/L) | 5.01 (4.71–5.3) | 8.35 (6.10–11.56) | 8.28 (6.73–10.76) | <0.001 | <0.001 | 0.543 |
| HbA1c (%) | – | 8.4 (7.1–10.3) | 8.4 (6.8–10.1) | – | – | 0.636 |
| FCP (ng/mL) | – | 0.57 (0.12–1.69) | 2.07 (1.32–2.92) | – | – | <0.001 |
| ALT (U/L) | 18 (12–36) | 17 (12–28) | 27 (16–59) | 0.235 | <0.001 | <0.001 |
| AST (U/L) | 22 (17–38) | 19 (16–26) | 23 (17–39) | <0.001 | 0.270 | <0.001 |
| GGT (U/L) | 23 (16–36) | 19 (13–32) | 33 (21–61) | <0.001 | <0.001 | <0.001 |
| Serum ALB (g/L) | 43 (41–46) | 44 (41–49) | 44 (41–47) | <0.001 | 0.001 | 0.007 |
| ALP (U/L) | 68 (57–83) | 69 (57–90) | 72 (58–92) | 0.326 | <0.001 | 0.146 |
| Total BIL (μmol/L) | 12 (9–15) | 12 (9–16) | 13 (10–16) | 0.402 | <0.001 | 0.084 |
| APTT (s) | 25.7 (23.7–28.3) | 25.4 (24.2‐28.4) | 25.9 (23.2–28.7) | 0.653 | 0.927 | 0.638 |
| Blood platelet (109/L) | 192 (159–230) | 192 (161–234) | 183 (151–223) | 0.500 | <0.001 | 0.001 |
| TC (mmol/L) | – | 4.78 (4.00–5.41) | 4.65 (3.99–5.41) | – | – | 0.524 |
| TG (mmol/L) | – | 0.96 (0.66–1.58) | 1.55 (1.07–2.24) | – | – | <0.001 |
| LDL (mmol/L) | – | 2.80 (2.25–3.55) | 2.92 (2.38–3.58) | – | – | 0.120 |
| HDL (mmol/L) | – | 1.26 (1.00–1.63) | 1.03 (0.88–1.22) | – | – | <0.001 |
| SCr (μmol/L) | 67 (59–78) | 62 (52–75) | 64 (54–77) | <0.001 | <0.001 | 0.072 |
| HBV markers | ||||||
| HBsAg positive | 136 (10.0) | 29 (7.6) | 184 (13.5) | 0.169 | 0.004 | 0.002 |
| HBsAb positive | 601 (44.0) | 175 (46.1) | 504 (37.0) | 0.483 | <0.001 | 0.001 |
| HBeAg positive | 17 (1.2) | 2 (0.6) | 28 (2.1) | 0.277 | 0.097 | 0.057 |
| HBeAb positive | 377 (27.6) | 106 (27.9) | 456 (33.4) | 0.915 | 0.001 | 0.041 |
| HBcAb positive | 817 (59.9) | 209 (55.0) | 851 (62.3) | 0.089 | 0.182 | 0.010 |
Data are expressed as median (interquartile) or number (%). The difference between proportions was tested using the χ2‐test, and the difference between medians was examined using the Mann–Whitney U‐test. ALP, alkaline phosphatase; ALT, alanine amino transferase; APTT, activated partial thromboplastin time; AST, aspartate amino transferase; BMI, body mass index; FCP, fasting C peptide; FPG, fasting plasma glucose; GGT, gamma‐glutamyl transpeptidase; HbA1c, glycated hemoglobin; HBcAb, hepatitis B core antibody; HBeAb, hepatitis B e antibody; HBeAg, hepatitis B e antigen; HBsAb, hepatitis B surface antibody; HBsAg, hepatitis B surface antigen; HBV, hepatitis B virus; HDL‐C, high‐density lipoprotein cholesterol; LDL‐C, low‐density lipoprotein cholesterol; SCr, serum creatinine; TC, total cholesterol; TG, triglyceride; Total BIL, total bilirubin.
Chronic HBV infection status in various groups
For the HBV markers, patients with type 2 diabetes had higher prevalence of HBsAg and hepatitis B e antibody, but lower rates of hepatitis B surface antibody compared with the controls (all P < 0.05). With regard to HBV infection status (shown in Tables 2 and 4), patients with type 2 diabetes had a higher prevalence of CHB status than the controls in the overall population (13.5 vs 10.0%, P = 0.004) and among patients with normal hepatic function (13.3 vs 8.8%, P = 0.002). The proportion of CHB in patients with autoimmune diabetes was similar to that in the control group in both men and women, but lower than that in type 2 diabetes in men in the total population (P = 0.003). The aforementioned phenomena existed even after adjustment for the population composition of Shanghai, China, in 2013 (Shown in Table S1).
Table 2.
Prevalence of chronic hepatitis B virus carrier in various groups in the total population
| Sex | Age group (years) | Control (group A: n = 1,365) | Adult‐onset autoimmune diabetes (group B: n = 381) | Type 2 diabetes (group C: n = 1,365) | P‐value | ||
|---|---|---|---|---|---|---|---|
| A vs B | A vs C | B vs C | |||||
| Total | 20–39 | 11.8 | 6.8 | 14.9 | 0.239 | 0.371 | 0.079 |
| 40–59 | 12.0 | 7.3 | 14.5 | 0.076 | 0.181 | 0.012 | |
| 60–75 | 6.3 | 8.5 | 11.5 | 0.372 | 0.004 | 0.326 | |
| Total (crude) | 10.0 | 7.6 | 13.5 | 0.169 | 0.004 | 0.002 | |
| Total (adjusted)† | 10.8 | 7.4 | 14.0 | – | – | – | |
| Men | 20–39 | 12.8 | 6.3 | 15.6 | 0.215 | 0.495 | 0.099 |
| 40–59 | 13.6 | 8.4 | 16.2 | 0.165 | 0.268 | 0.051 | |
| 60–75 | 7.0 | 5.1 | 11.9 | 0.601 | 0.063 | 0.127 | |
| Total (crude) | 11.6 | 6.9 | 14.9 | 0.055 | 0.045 | 0.003 | |
| Total (adjusted)† | 12.0 | 7.0 | 15.1 | – | – | – | |
| Women | 20–39 | 9.3 | 8.0 | 13.0 | 0.855 | 0.54 | 0.518 |
| 40–59 | 8.8 | 6.1 | 10.9 | 0.446 | 0.442 | 0.205 | |
| 60–75 | 5.5 | 11.3 | 11.1 | 0.092 | 0.029 | 0.962 | |
| Total (crude) | 7.3 | 8.4 | 11.2 | 0.636 | 0.032 | 0.298 | |
| Total (adjusted)† | 8.3 | 7.8 | 11.7 | – | – | – | |
P‐values were derived from the χ2‐test. †Data were adjusted for the local population composition (Shanghai, China 2013).
Association of HBV infection status with diabetes subtypes
Univariate regression analysis showed that CHB status was significantly associated with type 2 diabetes in the overall population and among patients with normal hepatic function in comparison with the controls (Tables 3 and 4, all P < 0.05). When considering sex, the crude odds ratios of CHB increased in both men and women with type 2 diabetes in comparison with those without diabetes (all P < 0.05). Multiple regression analysis also showed that the odds ratio of CHB for type 2 diabetes was approximately 1.5‐fold higher after adjustment for traditional risk factors, such as sex, age and BMI, in the overall population, and patients with normal hepatic function in comparison with the controls (all P < 0.01). However, there was no significant association between HBV infection status and adult‐onset autoimmune diabetes, and the odds ratio of CHB for adult‐onset autoimmune diabetes was similar to the control group in both men and women.
Discussion
There have been few reports to date on the relationship between CHB status and diabetes subtypes, and we drew a few interesting conclusions from the present study of Chinese patients aged 20–75 years. First, CHB status was significantly more prevalent in patients with type 2 diabetes than in the control group and patients with adult‐onset autoimmune diabetes; for patients with adult‐onset autoimmune diabetes and type 2 diabetes, the respective CHB status rates were 7.6 and 13.5% for the overall population, and 7.3 and 13.3% for patients with normal hepatic function. Most importantly, however, multiple logistic regression analysis showed that patients with type 2 diabetes had a 1.5‐fold higher risk of CHB compared with non‐diabetic controls, even after adjustment for confounding factors, such as age, sex, BMI and hepatic function.
Previous studies supported the suggestion that CHB increases the risk of diabetes. CHB was associated with a 9.7‐fold (95% confidence interval: 3.30–28.69) increased risk of diabetes among Asian Americans compared with patients without HBV infection4. It was also reported that Chinese women with HBsAg carrier status had a 3.3‐fold increased risk of gestational diabetes compared with HBsAg‐negative Chinese women6. Additionally, impaired fasting glucose was also reported to be more common in hepatitis B e antigen‐seropositive patients5. However, these studies did not fully exclude the effect of active hepatitis or advanced liver disease (cirrhosis or hepatic decompensation). In the present study, the odds ratio of CHB status was 1.59‐fold higher in type 2 diabetes patients than the controls after adjustment for age, sex and BMI in patients with normal hepatic function.
The mechanism of the action of HBV infection on the presence of diabetes was investigated in previous studies. It is well recognized that liver cirrhosis is associated with hepatogenous diabetes, and more than 30% of patients with cirrhosis have diabetes11, 12. Additionally, among patients with CHB, the prevalence of diabetes is higher in cases with active hepatitis infection and advanced liver fibrosis13, 14. As a complication of liver cirrhosis, hepatogenous diabetes develops gradually as a result of insulin resistance and increased endogenous glucose production, which might lead to pancreatic β‐cell dysfunction among patients with advanced liver disease8, 17, 18. However, in patients without advanced liver disease, insulin resistance could also explain the increased risk of diabetes in individuals with CHB. It was also reported that, in HBV x‐transgenic mice, the HBV X protein decreased insulin receptor substrate‐1 protein expression, augmented the expression of suppressor of cytokine signaling‐3 and induced ubiquitination of insulin receptor substrate‐1, and thus interfered with insulin signaling activation7. However, insulin resistance was also reported to be no more common in patients with chronic HBV infection than in the general population19, and HBV infection was reported to be associated with decreased triglyceride levels and a lower presence of metabolic syndrome20, 21. Furthermore, it has been shown that HBV can directly invade pancreatic islet cells. The HBV S, C and X genes were found to be expressed in pancreatic tissues, and expression of HBV C antigen (HBcAg) was confirmed in pancreatic islets22. Therefore, it was speculated that the pathophysiological mechanism by which HBV infection causes diabetes might be different from the pathogenesis of traditional type 2 diabetes, which is characterized as a metabolic disorder.
In addition, it has been reported that HBV‐related risk behaviors, such as assisted blood glucose monitoring and shared insulin pen use, increase the risk of HBV infection in patients with diabetes23. Usually, patients with adult‐onset autoimmune diabetes are presumed to be more likely to receive such medical care than their counterparts with type 2 diabetes. However, the present study hints that such HBV‐related risk behaviors did not seem to explain the significantly higher prevalence of CHB status in patients with type 2 diabetes than in patients with adult‐onset autoimmune diabetes.
The present study had a number of strengths. First, we examined the association between CHB and diabetes while fully controlling for the effect of hepatic dysfunction. Second, we reported for the first time that HBV carrier status was significantly associated with type 2 diabetes rather than adult‐onset autoimmune diabetes. However, the present study also had its limitations. First, this was a retrospective study, and further prospective studies are required to ascertain whether CHB status increases the risk of developing type 2 diabetes or whether type 2 diabetes, but not adult‐onset autoimmune diabetes, increases the risk of CHB. Second, selection bias might exist in this study; because it was hospital‐based, most patients included in this study were almost all local residents of Shanghai, China. Caution should be taken when extrapolating from this study's conclusions, and multicenter studies are required.
In conclusion, CHB status was more prevalent in patients with type 2 diabetes than in the controls, but the proportion of CHB was similar in patients with both adult‐onset autoimmune diabetes and non‐diabetes. CHB status might be a risk factor for type 2 diabetes rather than adult‐onset immune diabetes.
Disclosure
The authors declare no conflict of interest.
Supporting information
Table S1| Population composition in Shanghai (2013).
Acknowledgments
This work was funded by grants from The Key Program of the Shanghai Municipality for Basic Research (11JC1409600); The Major State Basic Research Development Program of China (973 Program; 2011CB504001); The National Major Scientific and Technological Special Project for “Significant New Drugs Development” (2011ZX09307‐001‐02).
J Diabetes Investig 2017; 8: 619–625
References
- 1. American Diabetes A . Diagnosis and classification of diabetes mellitus. Diabetes Care 2010; 33(Suppl 1): S62–S69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Wang CS, Wang ST, Yao WJ, et al Hepatitis C virus infection and the development of type 2 diabetes in a community‐based longitudinal study. Am J Epidemiol 2007; 166: 196–203. [DOI] [PubMed] [Google Scholar]
- 3. Naing C, Mak JW, Ahmed SI, et al Relationship between hepatitis C virus infection and type 2 diabetes mellitus: meta‐analysis. World J Gastroenterol 2012; 18: 1642–1651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Li‐Ng M, Tropp S, Danoff A, et al Association between chronic hepatitis B virus infection and diabetes among Asian Americans and Pacific Islanders. Dig Liver Dis 2007; 39: 549–556. [DOI] [PubMed] [Google Scholar]
- 5. Iroezindu MO, Isiguzo GC, Young EE. Prevalence and predictors of impaired fasting glucose among Nigerian patients with hepatitis B virus infection. Diabetes Res Clin Pract 2012; 98: 338–345. [DOI] [PubMed] [Google Scholar]
- 6. Lao TT, Tse KY, Chan LY, et al HBsAg carrier status and the association between gestational diabetes with increased serum ferritin concentration in Chinese women. Diabetes Care 2003; 26: 3011–3016. [DOI] [PubMed] [Google Scholar]
- 7. Kim K, Kim KH, Cheong J. Hepatitis B virus X protein impairs hepatic insulin signaling through degradation of IRS1 and induction of SOCS3. PLoS One 2010; 5: e8649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Lee JG, Lee S, Kim YJ, et al Association of chronic viral hepatitis B with insulin resistance. World J Gastroenterol 2012; 18: 6120–6126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Huang ZS, Huang TS, Wu TH, et al Asymptomatic chronic hepatitis B virus infection does not increase the risk of diabetes mellitus: a ten‐year observation. J Gastroenterol Hepatol 2010; 25: 1420–1425. [DOI] [PubMed] [Google Scholar]
- 10. Spradling PR, Simons B, Narayanan M, et al Incidence of diabetes mellitus in a population‐based cohort of persons with chronic hepatitis B virus infection. J Viral Hepat 2013; 20: 510–513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Petit JM, Hamza S, Rollot F, et al Impact of liver disease severity and etiology on the occurrence of diabetes mellitus in patients with liver cirrhosis. Acta Diabetol 2014; 51: 455–460. [DOI] [PubMed] [Google Scholar]
- 12. Hickman IJ, Macdonald GA. Impact of diabetes on the severity of liver disease. Am J Med 2007; 120: 829–834. [DOI] [PubMed] [Google Scholar]
- 13. Papatheodoridis GV, Chrysanthos N, Savvas S, et al Diabetes mellitus in chronic hepatitis B and C: prevalence and potential association with the extent of liver fibrosis. J Viral Hepat 2006; 13: 303–310. [DOI] [PubMed] [Google Scholar]
- 14. Matsumoto N, Arase Y, Seko Y, et al Prevalence and predictive factors of diabetes in hepatitis virus positive liver cirrhosis with fasting plasma glucose level of <126 mg/dL. Hepatol Res 2012; 42: 558–563. [DOI] [PubMed] [Google Scholar]
- 15. Gao F, Pan JM, Hou XH, et al Liver enzymes concentrations are closely related to prediabetes: findings of the Shanghai Diabetes Study II (SHDS II). Biomed Environ. Sci 2012; 25: 30–37. [DOI] [PubMed] [Google Scholar]
- 16. American Diabetes A . Diagnosis and classification of diabetes mellitus. Diabetes Care 2012; 35(Suppl 1): S64–S71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Garcia‐Compean D, Jaquez‐Quintana JO, Gonzalez‐Gonzalez JA, et al Liver cirrhosis and diabetes: risk factors, pathophysiology, clinical implications and management. World J Gastroenterol 2009; 15: 280–288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Kim MG. Choi WC [Differential diagnosis of diabetes mellitus caused by liver cirrhosis and other type 2 diabetes mellitus]. Korean J Hepatol 2006; 12: 524–529. [PubMed] [Google Scholar]
- 19. Pais R, Rusu E, Ratziu V. The impact of obesity and metabolic syndrome on chronic hepatitis B and drug‐induced liver disease. Clin Liver Dis 2014; 18: 165–178. [DOI] [PubMed] [Google Scholar]
- 20. Luo B, Wang Y, Wang K. Association of metabolic syndrome and hepatitis B infection in a Chinese population. Clin Chim Acta 2007; 380: 238–240. [DOI] [PubMed] [Google Scholar]
- 21. Jan CF, Chen CJ, Chiu YH, et al A population‐based study investigating the association between metabolic syndrome and hepatitis B/C infection (Keelung Community‐based Integrated Screening study No. 10). Int J Obes (Lond) 2006; 30: 794–799. [DOI] [PubMed] [Google Scholar]
- 22. Jin Y, Gao H, Chen H, et al Identification and impact of hepatitis B virus DNA and antigens in pancreatic cancer tissues and adjacent non‐cancerous tissues. Cancer Lett 2013; 335: 447–454. [DOI] [PubMed] [Google Scholar]
- 23. Kirkman MS, Schaffner W. Another shot to protect people with diabetes: add hepatitis B vaccination to the checklist. Diabetes Care 2012; 35: 941–942. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1| Population composition in Shanghai (2013).


