Abstract
The present study examined the long‐term efficacy of insulin pump therapy for type 1 diabetes patients when carried out using carbohydrate counting with bolus calculators for 1 year. A total of 22 type 1 diabetes patients who had just started continuous subcutaneous insulin infusion were examined and divided into two groups: one that was educated about carbohydrate counting using bolus calculators (n = 14); and another that did not use bolus calculators (n = 8). After 1 year, the hemoglobin A1c levels of the patient group that used bolus calculators decreased persistently and significantly (P = 0.0297), whereas those of the other group did not. The bodyweight, total daily dose of insulin and bolus percentage of both groups did not change. Carbohydrate counting using bolus calculators is necessary to achieve optimal and persistent glycemic control in patients undergoing continuous subcutaneous insulin infusion.
Keywords: Bolus calculator, Continuous subcutaneous insulin infusion, Type 1 diabetes
Introduction
The Diabetes Control and Complication Trial showed that both intensive insulin therapy, which is carried out by multiple daily injections or continuous subcutaneous insulin infusion (CSII), and carbohydrate counting (CC) were necessary to achieve optimal glycemic control to prevent diabetic complications in type 1 diabetes patients1, 2. There are two levels of CC: basic and advanced2. In basic CC, patients eat a fixed amount of carbohydrates and inject the same amount of insulin, whereas the goal of advanced CC is to match the amount of carbohydrate intake and bolus injection2.
Bolus insulin in CC is determined using the carbohydrate–insulin ratio, insulin sensitivity factor and target blood glucose levels2, 3. Patients using CSII could calculate bolus insulin using software (bolus calculator [BC]). The calculator considers active insulin; therefore, patients can avoid hypoglycemia4. CC's efficacy, especially its long‐term efficacy, using a BC during CSII therapy remains unclear.
We aimed to investigate CC's efficacy using a BC compared with not using one on glycemic control and change in bodyweight (BW) in type 1 diabetes patients.
Materials and Methods
Ethical statement
The present study was approved by the Gunma University Institutional Review Board. Patients provided written informed consent before any study‐related procedures.
Materials and participants
Data for 26 patients new to CSII therapy or using a BC in the Department of Internal Medicine, Division of Endocrinology and Diabetes, Gunma University Hospital, Maebashi, Japan, from 2011 to 2015 were reviewed. Patients who were pregnant (n = 2) or undergoing hemodialysis (n = 2) at the introduction of CSII were excluded. Three of 14 patients from the group who carried out CC using a BC (Patients+) had already used CSII without a BC (Patients−). Dietary counseling was provided for ≥30 min using a CC list or the Japanese food exchange list. All patients were provided with the Minimed Paradigm 722 or Minimed 620G (Medtronic, Northridge, California, USA) insulin pump. A short‐term assessment with continuous glucose monitoring (CGM) using Medtronic iPro2 (Medtronic) was typically carried out for one period of 3–5 days. Medtronic iPro2 was introduced at our hospital in July 2013; beforehand, we could not carry out CGM. Therefore, the group who did not use BC did not undergo CGM. Conversely, Patients+ participated in 1.4 ± 1.1 (mean ± standard deviation) assessments of CGM (Table 1). For these reasons, there was no intentional patient classification, and the backgrounds of patients included in this study were similar, except for the differences noted above (Table 1).
Table 1.
Baseline characteristics of participants analyzed
| All | Bolus calculator (+) | Bolus calculator (−) | (+) vs (−) | |
|---|---|---|---|---|
| n | 22 | 14 | 8 | NS |
| Age (years) | 40 ± 10.9 | 40 ± 11.2 | 40 ± 11.1 | NS |
| Sex (% female) | 20 (91) | 13 (93) | 7 (88) | NS |
| Age of onset (years) | 19 ± 12.3 | 19 ± 11.2 | 20 ± 15.0 | NS |
| Duration of diabetes (years) | 21 ± 11.0 | 21 ±10.9 | 21 ± 12.0 | NS |
| Duration of introduction after onset of diabetes (years) | 19 ± 11.3 | 20 ± 11.0 | 17 ± 12.3 | NS |
| BW | 60 ± 7.2 | 60 ± 7.2 | 56 ± 10.0 | NS |
| BMI | 24 ± 3.2 | 24 ± 3.2 | 23 ± 4.2 | NS |
| Dietary counseling | ||||
| Before introduction (times) | 1.9 ± 2.4 | 1.7 ± 2.7 | 2.1 ± 1.8 | NS |
| After introduction (times) | 1.3 ± 1.1 | 1.1 ± 1.3 | 1.5 ± 0.2 | NS |
| Continuous glucose monitoring (times) | 0.9 ± 1.1 | 1.4 ± 1.1 | 0 | P = 0.001 |
| A1C (%) | 8.6 ± 1.5 | 8.4 ± 1.1 | 8.9 ± 2.1 | NS |
| TDD | 41.4 ± 18.0 | 37.1 ± 5.9 | 48.9 ± 28.3 | P = 0.07 |
| % Bolus | 60.5 ± 10.2 | 59.5 ± 7.2 | 62.1 ± 14.5 | NS |
| % Hypoglycemia (<70 mg/dL) | ||||
| Before introduction | 7.9 ± 7.1 | 7.1 ± 6.8 | 9.0 ± 7.9 | NS |
| After introduction | 6.4 ± 5.4 | 5.1 ± 4.0 | 8.3 ± 6.7 | NS |
A1C, hemoglobin A1c; BMI, body mass index; BW, bodyweight; TDD, total daily insulin dose.
While specialists individually set the BC monthly, the initial settings were normally carried out according to the protocols outlined previously3. The amount of bolus and basal insulin was checked monthly using internal CSII software. We used a team approach for intensive patient management, and used hemoglobin A1c (HbA1c) levels as the measure for overall glycemic control; BW and body mass index were monitored to avoid weight gain.
Statistical analysis
All results are expressed as the mean ± standard deviation for continuous variables, and as absolute numbers and relative percentages for categorical variables. Associations between continuous variables were examined using Spearman's coefficients. All tests for significance and the resulting P‐values were two‐sided with a 5% significance level. Statistical analyses were carried out using jmp 9.0.2 (SAS Institute, Cary, North Carolina, USA).
Results
We considered HbA1c levels as the measure for overall glycemic control. The HbA1c levels of Patients+ reduced gradually, but persistently and significantly (Figure 1a). Importantly, the rate of hypoglycemia did not increase after introduction (Table 1). Notably, this effect was retained over a year. However, Patients– had no significant decrease in HbA1c levels. After 1 year, their HbA1c levels rebounded, even though dietary counseling was provided regularly (Table 1 and Figure 1b).
Figure 1.
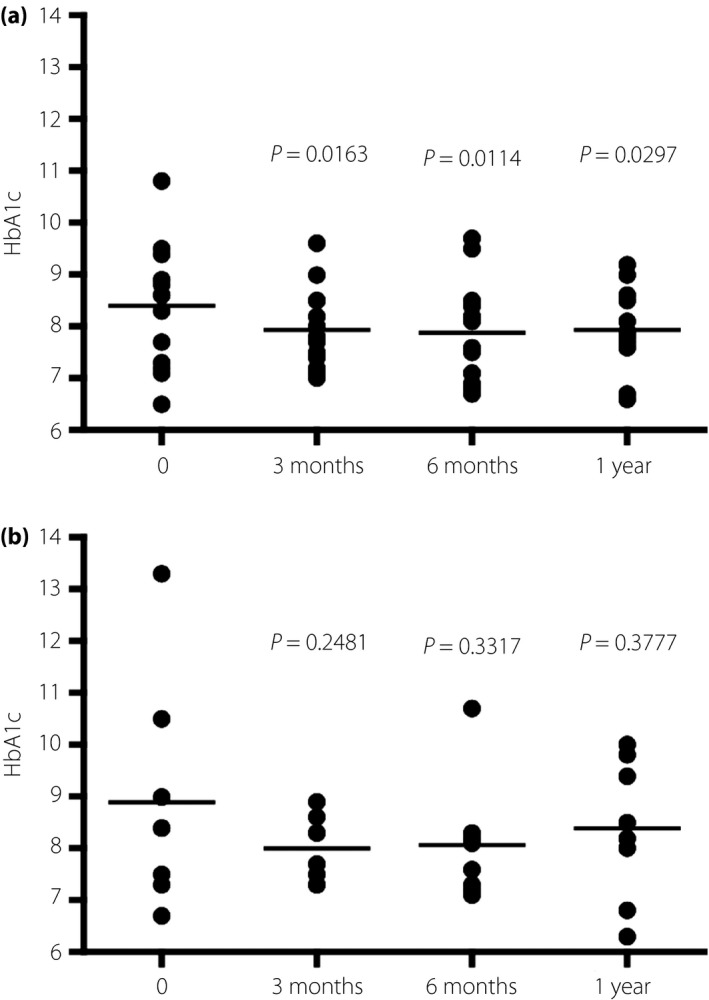
The changes in hemoglobin A1c (HbA1c) levels in patients who (a) carried out carbohydrate counting using bolus calculators and (b) those who carried it out without bolus calculators.
Next, we examined the effect of CC using a BC on BW. There was no significant change over a year in both groups, indicating that BW was not affected by CSII or the method of bolus injection (Figure 2). The hypoglycemia rate did not differ between the groups (Table 1), consistent with the idea that hypoglycemia usually results in unnecessary food intake, thereby causing undesirable BW gain. Notably, the total daily insulin dose (TDD) and the bolus dose as a percentage of TDD (% bolus), which were critical factors for glycemic control in type 1 diabetes patients, did not change for a year in both groups (Figure 3), but glycemic control in these groups was different (Figure 1).
Figure 2.
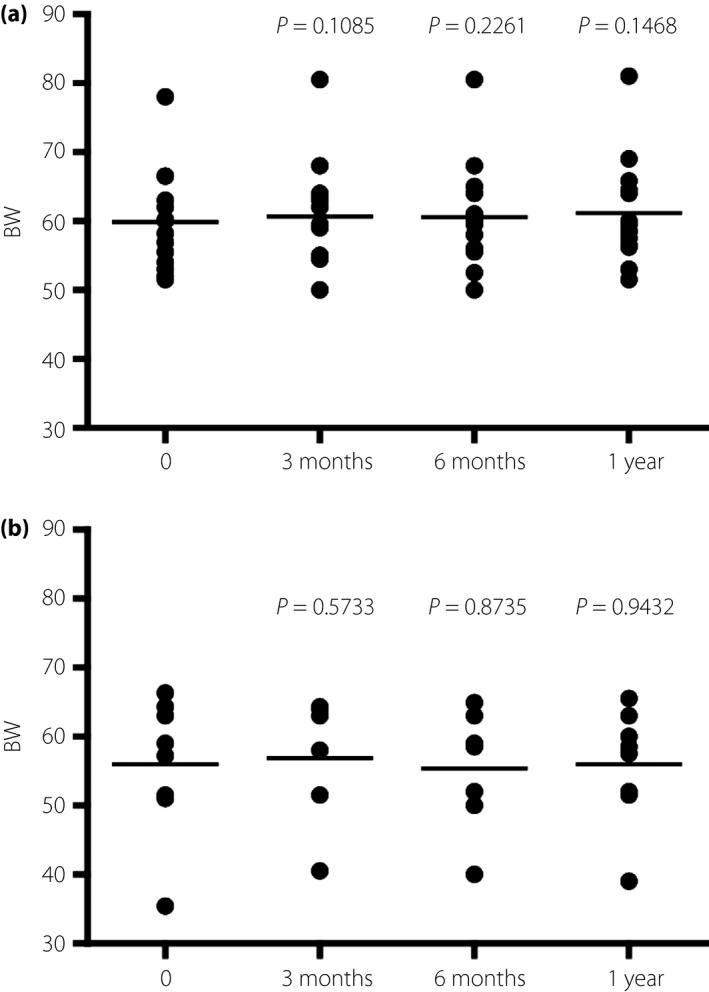
The changes in the bodyweight of patients who (a) carried out carbohydrate counting using bolus calculators and (b) those who carried it out without bolus calculators.
Figure 3.
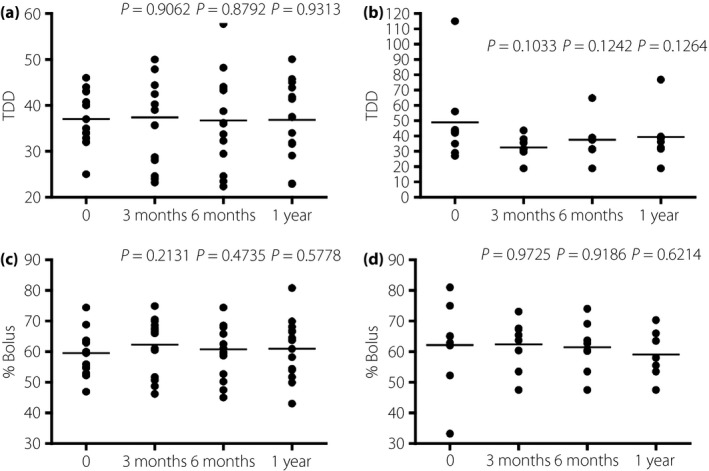
The change in (a,b) total daily dose (TDD) and (c,d) % bolus in patients who (a,c) carried out carbohydrate counting using bolus calculators and (b,d) those who carried it without bolus calculators.
Discussion
We examined the effect of CC using a BC on CSII. Our data showed that glycemic control in Patients+ was better than in Patients−. Significantly, the HbA1c levels of Patients+ decreased even after 1 year, whereas that of Patients– rebounded. As it was reported that neither CC nor a BC alone could consistently improve glycemic control in type 1 diabetes patients, both strategies were implemented simultaneously in the present study2, 5, 6.
Strict glycemic control could increase the risk of hypoglycemia, resulting in an increase in BW2, 7. Furthermore, CSII use increased BW7. Therefore, we examined patients' BW and body mass index changes; there was no change over a year in both patient groups, suggesting that CSII itself could not be a critical factor of weight gain, and appropriate education and examination could reduce the risk of weight gain.
To achieve good glycemic control, type 1 diabetes patients require optimal insulin dosing8, 9. TDD, which is usually estimated by BW, and % bolus are important factors in achieving good glycemic control9. As patients with BCs consumed as much food they desired, bolus injections could be increased. Therefore, we examined the TDD and % bolus, and showed that there were no significant changes in both patient groups; therefore, appropriate education could also reduce the risk of increasing TDD and % bolus. Significantly, using CSII software, we could check the amount of carbohydrate the patients consumed between examinations; therefore, we could actually control the intake of carbohydrates and other nutrients in Patients+ more easily than in Patients−.
Several studies showed that bolus calculators could improve glucose variability, but not glycemic control (HbA1c) levels, especially in patients with type 1 diabetes10. The present study might have differed from previous studies, because Patients– did not undergo GGM. Although Patients+ participated in just 1.4 ± 1.1 short‐term assessments of CGM during the year, and although short‐term CGM appears ineffective for persistently good glycemic control11, 12, 13, synergic effects could have occurred between the CC/BC and CGM. We carried out a team‐approach education program, which combined CC, BC and CGM. This program contributed to optimal and persistent glycemic control in our patients. Although the frequency of self‐monitoring of blood glucose might be crucial for good glycemic control14, 15, 16, it was very similar for both groups, showing that self‐monitoring of blood glucose frequency was not responsible for the between‐group differences. Furthermore, a recent report showed that the frequency of BC use correlates positively with glycemic control17. In the present study, Patients+ used approximately <10% manual bolus, suggesting that almost all boluses were determined by calculator, which might have resulted in good glycemic control.
The present study had several limitations. This study had a cross‐sectional retrospective design; it had a small sample size and we only evaluated cases from our hospital. The patient parameters might be different from those at other hospitals in Japan, especially regarding the sex ratio and treatment. Additionally, although a cause‐and‐effect relationship could not be discerned, the present study included a longitudinal follow‐up study for a year.
In summary, we investigated the efficacy of CC using a BC during CSII therapy. Patients+ achieved optimal glycemic control without any change in BW or TDD, whereas Patients– did not have this outcome. Importantly, this effect was maintained over 1 year. Future studies including more patients are necessary to reconfirm these results.
Disclosure
The authors declare no conflict of interest.
J Diabetes Investig 2017; 8: 496–500
References
- 1. The DCCT Research Group . Diabetes Control and Complications Trial (DCCT): results of feasibility study. Diabetes Care 1987; 10: 1–19. [DOI] [PubMed] [Google Scholar]
- 2. Gillespie SJ, Kulkarni KD, Daly AE. Using carbohydrate counting in diabetes clinical practice. J Am Dietetic Assoc 1998; 98: 897–905. [DOI] [PubMed] [Google Scholar]
- 3. Walsh J, Roberts R, Bailey T. Guidelines for insulin dosing in continuous subcutaneous insulin infusion using new formulas from a retrospective study of individuals with optimal glucose levels. J Diabetes Sci Technol 2010; 4: 1174–1181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Schmidt S, Norgaard K. Bolus calculators. J Diabetes Sci Technol 2014; 8: 1035–1041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Yeh HC, Brown TT, Maruthur N, et al Comparative effectiveness and safety of methods of insulin delivery and glucose monitoring for diabetes mellitus: a systematic review and meta‐analysis. Ann Int Med 2012; 157: 336–347. [DOI] [PubMed] [Google Scholar]
- 6. Joshi M, Choudhary P. Multiple daily injections or insulin pump therapy: choosing the best option for your patient—an evidence‐based approach. Curr Diabetes Rep 2015; 15: 81. [DOI] [PubMed] [Google Scholar]
- 7. The DCCT Research Group . Weight gain associated with intensive therapy in the diabetes control and complications trial. Diabetes Care 1988; 11: 567–573. [DOI] [PubMed] [Google Scholar]
- 8. Hahr AJ, Molitch ME. Optimizing insulin therapy in patients with type 1 and type 2 diabetes mellitus: optimal dosing and timing in the outpatient setting. Dis Mon 2010; 56: 148–162. [DOI] [PubMed] [Google Scholar]
- 9. King AB, Kuroda A, Matsuhisa M, et al A review of insulin‐dosing formulas for continuous subcutaneous insulin infusion (CSII) for adults with type 1 diabetes. Curr Diabetes Rep 2016; 16: 83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Ramotowska A, Golicki D, Dzygalo K, et al The effect of using the insulin pump bolus calculator compared to standard insulin dosage calculations in patients with type 1 diabetes mellitus ‐ systematic review. Exp Clin Endocrinol Diabetes 2013; 121: 248–254. [DOI] [PubMed] [Google Scholar]
- 11. Leinung M, Nardacci E, Patel N, et al Benefits of short‐term professional continuous glucose monitoring in clinical practice. Diabetes Technol Ther 2013; 15: 744–747. [DOI] [PubMed] [Google Scholar]
- 12. Al Hayek AA, Robert AA, Al Dawish M, et al The evolving role of short‐term professional continuous glucose monitoring on glycemic control and hypoglycemia among Saudi patients with type 1 diabetes: a prospective study. Diabetes Ther 2015; 6: 329–337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Pepper GM, Steinsapir J, Reynolds K. Effect of short‐term iPRO continuous glucose monitoring on hemoglobin A1c levels in clinical practice. Diabetes Technol Ther 2012; 14: 654–657. [DOI] [PubMed] [Google Scholar]
- 14. Murata T, Tsuzaki K, Yoshioka F, et al The relationship between the frequency of self‐monitoring of blood glucose and glycemic control in patients with type 1 diabetes mellitus on continuous subcutaneous insulin infusion or on multiple daily injections. J Diabetes Investig 2015; 6: 687–691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Ziegler R, Heidtmann B, Hilgard D, et al Frequency of SMBG correlates with HbA1c and acute complications in children and adolescents with type 1 diabetes. Pediatr Diabetes 2011; 12: 11–17. [DOI] [PubMed] [Google Scholar]
- 16. Miller KM, Beck RW, Bergenstal RM, et al Evidence of a strong association between frequency of self‐monitoring of blood glucose and hemoglobin A1c levels in type 1 diabetes patients exchange clinic registry participants. Diabetes Care 2013; 36: 2009–2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Ziegler R, Rees C, Jacobs N, et al Frequent use of an automated bolus advisor improves glycemic control in pediatric patients treated with insulin pump therapy: results of the Bolus Advisor Benefit Evaluation (BABE) study. Pediatr Diabetes 2016; 17: 311–318. [DOI] [PubMed] [Google Scholar]


