Abstract
Aims/Introduction
Milk fat globule‐epidermal growth factor 8 (MFG‐E8) is the key mediator in anti‐inflammatory responses that facilitate phagocytosis of apoptotic cells, and play an essential role in type 2 diabetes and pregnancy, both of which are under a low‐grade inflammatory state. However, the action of MFG‐E8 in gestational diabetes mellitus (GDM) is unclear. We measured plasma MFG‐E8 levels in pregnancy and GDM for the first time, and elucidated possible relationships between its plasma levels and various metabolic parameters.
Materials and Methods
Plasma MFG‐E8 levels were quantified by enzyme‐linked immunosorbent assay in 66 women with GDM, 70 with normal pregnancy (p‐NGT) and 44 healthy non‐pregnant controls (CON), who were matched for age and body mass index. Inflammatory factors tumor necrosis factor‐α (TNF‐α) and C‐reactive protein levels were measured, oral glucose tolerance test was carried out and β‐cell function was evaluated.
Results
Plasma MFG‐E8 levels were remarkably higher in p‐NGT than in CON (P = 0.024), and were further elevated in GDM vs p‐NGT (P = 0.016). MFG‐E8 concentrations correlated positively with hemoglobin A1c, glucose levels and insulin resistance (homeostasis model assessment for insulin resistance), and correlated inversely with TNF‐α and insulin secretion evaluated by disposition indices in pregnancies. Fasting glucose levels, disposition index of first phase insulin secretion and TNF‐α were independent predictors of MFG‐E8 levels in pregnancies. Logistic regression analyses showed that women in the third tertile of MFG‐E8 levels had a markedly elevated risk of GDM.
Conclusions
Circulating MFG‐E8 levels are dramatically elevated in pregnancy, and are significantly higher in GDM vs p‐NGT. MFG‐E8 concentrations are significantly associated with TNF‐α, fasting glucose levels, homeostasis model assessment for insulin resistance and disposition indices. However, further studies are required to elucidate the regulation mechanism of MFG‐E8 during pregnancy and GDM.
Keywords: Gestational diabetes mellitus, Inflammation, Milk‐fat globule‐epidermal growth factor 8
Introduction
In recent years, there have been increased debates regarding the pivotal role of inflammation in metabolic disorders. It is a widely shared belief that human pregnancy is featured by a chronic, low‐grade inflammatory state in comparison with the non‐pregnant condition, which is mainly caused by the lack of physical exercise and a high calorie intake, leading to systemic insulin resistance. Gestational diabetes mellitus (GDM) occurs when the pancreatic β‐cell reserve is inadequate to compensate for the enhanced insulin resistance with the onset or first recognition of pregnancy, and is reported in approximately 7% of all pregnant women according to the 2014 American Diabetes Association. Although GDM disappears after parturition, it is generally related to an enhanced risk of progression to obesity and type 2 diabetes mellitus in women. Correlations between insulin resistance and inflammatory factors, such as tumor necrosis factor‐α (TNF‐α), C‐reactive protein (CRP) or interleukin‐6 have been identified in GDM, even in normal pregnant women1, 2. Kirwan et al.3 reported that in both normal pregnancies and GDM, TNF‐α was the most powerful predictor of insulin resistance during gestation, whereas estradiol, progesterone, human placental lactogen and other placenta‐derived hormones were not considered specific markers of insulin resistance.
Milk fat globule‐epidermal growth factor 8 (MFG‐E8; also known as lactadherin in humans) was originally characterized as a secreted glycoprotein of the milk‐fat globule, and was found to facilitate phagocytosis of apoptotic cells4, 5. MFG‐E8 binds to apoptotic cells through the phosphatidylserine in the C‐terminal, while it attaches to avβ3/β5 integrin expressed on activated macrophages through the arginine–glycine–aspartate motif of the N‐terminal epidermal growth factor repeats. Two isoforms of MFG‐E8 have been identified in murine mice, humans only have the short variant. The long form contains the proline/threonine‐rich domain between the second epidermal growth factor‐like repeat and the discoidin domain‐1, which distinguishes it from the short variant. In mice, the long variant shows a limited tissue distribution, and is predominant during the late stages of gestation and during lactation; whereas the short form is expressed in various tissues, and substantially decreases in a lactation‐dependent manner6. To date, MFG‐E8 has been implicated in various human inflammatory diseases, and shows an anti‐inflammation property in coronary atherosclerotic heart disease, chronic obstructive pulmonary disease, ulcerative colitis and rheumatoid arthritis7, 8, 9, 10, whereas inversely in brain ischemia11 and atherosclerosis12. Although known to be a mediator of inflammation, MFG‐E8 is a multifunctional molecule that is involved in several cell surface‐mediated regulatory events, such as tissue remodeling, tumor promotion, angiogenesis and facilitation of fertilization13, 14, 15, 16.
MFG‐E8 and its receptor, avβ3/β5 integrin, are predominantly localized to the human endometrial epithelium. Based on a microarray analysis, Rehman et al.17 reported that MFG‐E8 expression was 4.9‐fold higher in the myometrium of pregnant women than in non‐pregnant women, suggesting that MFG‐E8 might play an essential role during human pregnancy. Bocca et al.18 confirmed that MFG‐E8 functioned as a modulator of endometrial physiology and trophoblast adhesion and invasion by regulating the essential processes of remodeling, apoptosis, and angiogenesis in humans. Furthermore, MFG‐E8 is an epithelial cell secretory product, and has been detected in microvesicles in milk and seminal plasma, as well as in the peripheral circulation19. Previous studies have shown a significant relationship between MFG‐E8 and pregnancy in the uterus. However, there are no reports with data directly referring to MFG‐E8 and pregnancy with or without normal glucose tolerance in the human blood circulation. As MFG‐E8 is a secreted protein, we examined plasma MFG‐E8 levels using enzyme‐linked immunosorbent assay to determine its clinical significance in pregnant women with or without diabetes. Pro‐inflammatory cytokines originating from adipose tissue in obesity are a primary driver of inflammation; therefore, a state of adiposity before pregnancy is associated with the risk of pregnancy‐related hypertension and diabetes20. Women who were overweight had a body mass index (BMI) between 25 and 30 kg/m2, and those with a BMI ≥30 kg/m2 (regarded as obese, according to the 2004 World Health Organization criteria) before pregnancy were excluded from the current study to eliminate the interference of inflammation and insulin resistance.
Materials and Methods
Study population
From June 2015 to April 2016, non‐pregnant women were recruited from healthy volunteers, and gestational women were recruited from outpatients at the Department of Endocrinology and Metabolism and Department of Obstetrics and Gynecology at Shanghai Jiaotong University Affiliated Sixth People's Hospital, Shanghai, China. Informed consent was obtained from all participants, and the protocol was approved by the local ethics committee according to the Declaration of Helsinki as revised in 2008 before enrollment for the study. Women who met any of the following criteria were excluded from the study: family history of diabetes mellitus, multiple gestations, previous history of diabetes mellitus or other complications, hepatic disease, renal disease, thyroid dysfunction, hypertension, proteinuria, hematopathy or a pre‐pregnancy BMI (pre‐BMI; calculated as weight in kilograms divided by the square of height in meters) <18.5 or >25. At 24–28 weeks of gestation, pregnant women were recruited at the Department of Gynecology and Obstetrics of Sixth People's Hospital, Shanghai, China, when they visited for a routine prenatal examination, and were subjected to a standardized 50‐g glucose challenge test. Individuals with 1‐h plasma glucose level ≥7.2 mmol/L were considered glucose challenge test positive, and returned within 1 week for blood tests and screening tests after overnight fasting. A 75‐g oral glucose tolerance test (OGTT) was administered, and blood glucose and insulin levels at 0, 30, 60, 120, and 180 min were measured after glucose administration. GDM was diagnosed using the following threshold based on the American Diabetes Association 2013 guidelines: fasting plasma glucose levels ≥5.1 mmol/L and/or 60‐min plasma glucose ≥10.0 mmol/L and/or 120 min plasma glucose levels ≥8.5 mmol/L.
Based on all these thresholds, 66 pregnant women were defined as having GDM, whereas 70 age‐ and pre‐BMI‐matched pregnancies were classified under normal glucose tolerance. Meanwhile, healthy, lean non‐pregnant women with no previous history of pregnancy or diabetes were enrolled in the control group (CON). All controls underwent a 75‐g OGTT to exclude impaired glucose tolerance.
Anthropometric and biochemical measurements
The screening tests included a physical examination and a review of the clinical history of the participants. On the day of the test, the height, weight, systolic blood pressure (SP), diastolic blood pressure (DP), and gestational days of each participant were assessed and recorded. BMI was determined as the weight (in kilograms) at blood collection divided by the square of the height (in meters). Plasma glucose was determined using the oxidase method with a Glamour 2000 Automatic Biochemical Analyzer (Molecular Devices, Sunnyvale, CA, USA). Hemoglobin A1c was determined using high‐performance liquid chromatography with a VARIANT II Turbo Hemoglobin Testing System (Bio‐Rad Laboratories, Hercules, CA, USA). Glycosylated albumin was determined using the Glamour 2000 autoanalyzer and an enzyme‐based assay (Lucica GA‐L; Asahi Kasei Pharma, Tokyo, Japan). Plasma insulin and C‐peptide were measured by the chemiluminescent enzyme immunoassay (Insulin Elecsys; Roche Diagnostics, Alameda, CA, USA).The total serum cholesterol (TC), triglyceride (TG), high‐density lipoprotein and low‐density lipoprotein, alanine transaminase, aspartate aminotransferase, creatinine and uric acid concentrations were determined enzymatically (7600–020; Hitachi, Tokyo, Japan). C‐reactive protein (CRP) serum levels were determined using a commercially available latex particle‐enhanced immunonephelometry assay (Dade Behring Inc., Newark, NJ, USA). Inter‐ and intra‐assay coefficients of variation were <5% for the measurements. MFG‐E8 and TNF‐α levels were determined using commercially available enzyme‐linked immunosorbent assays, according to the manufacturer's instructions (Cloud‐Clone Corp. Houston, TX, USA). Inter‐ and intra‐assay coefficients of variation were <10% and <12%, respectively. All samples were run in duplicate and repeated if there was a > 15% difference between duplicates. The lower and upper limits of detection of the enzyme‐linked immunosorbent assay for MFG‐E8 were 31.25 and 2,000 pg/mL, respectively, and for TNF‐α were 6.25 and 1.00 pg/mL, respectively.
Insulin resistance and insulin secretion evaluation
The homeostasis model assessment (HOMA) was used to estimate steady‐state β‐cell secretion (HOMA‐β) and insulin resistance (HOMA‐IR). HOMA‐β = (20 × Ins0)/(Glu0 − 3.5)21 and HOMA‐IR = (Ins0 × Glu0)/22.522. Ins0 is fasting insulin levels and Glu0 is fasting glucose levels. Insulin sensitivity index (ISI) was calculated from OGTT according to Matsuda and DeFronzo (ISIM) as follows: Matsuda (ISIM) = 10,000/([Glu0 ×Ins0] × [Glumean × Insmean])1/2 23, which showed better correlation with insulin sensitivity derived using the euglycemic–hyperinsulinemic clamp technique than the HOMA‐IR in pregnant women24. The Stumvoll insulin secretion was derived using multiple linear regression models to predict directly measured first‐ and second‐phase insulin release during hyperglycemic clamp studies25, 26. The Stumvoll first‐phase insulin secretion: (1st‐phase secretion) = (1,194 + [4.724 × Ins0]−[117.0 × Glu60] + [1.414 × Ins60]) and second‐phase insulin response: (2nd‐phase secretion) = (295 + [0.349 ×I ns60] −[25.72 × Glu60] + [1.107 × Ins0]).
Finally, as the limitations of insulin secretion indices (such as HOMA‐β and Stumvoll) without controlling for the prevailing insulin resistance, we also calculated the insulin secretion sensitivity index using the standard OGTT, introduced by Retnakaranand validated in pregnancy27, which can be considered as a proxy for the disposition index (DI) and as a comprehensive index of β‐cell function in pregnancy. DI is defined as DI1st = Stumvoll1st × ISIM; DI2nd = Stumvoll2nd × ISIM.
Statistical analysis
All statistical analyses were carried out using spss version 19.0 (SPSS Inc., Chicago, IL, USA). Data are shown as mean ± standard deviation or median (25th and 75th percentiles) for continuous variables, or as percentage for categorical variables. When necessary, data that were not normally distributed (as determined using the Kolmogorov–Smirnov test) were log10 or ln transformed to achieve a satisfactory fit to the normal distribution or variance homogeneity. Unpaired independent Student's t‐test was used to compare differences between two groups, one‐way anova or ancova (adjusting for age, pre‐BMI, SP, DP, TC and TG) with post‐hoc analysis using Bonferroni correction was used to compare significant differences between groups. Categorical variables were examined by the χ2‐test. Relationships among MFG‐E8 and clinical parameters were examined by the calculation of Pearson's correlation and partial correlation coefficients. Bonferroni's correction was used to adjust P‐values for multiple comparisons. Multivariate regression models were fit for MFG‐E8 as a dependent variable, and only variables significantly related (P < 0.05) to MFG‐E8 by Pearson's correlation analyses were entered into the multiple linear stepwise regression analysis. Participants were divided into tertiles based on MFG‐E8 levels, and logistic regression analysis was carried out to create univariate and multivariate models for the odds of developing GDM. A two‐sided value of P < 0.05 was considered statistically significant.
Results
Baseline characteristics description
A total of 136 pregnant women (66 with GDM and 70 with p‐NGT) in gestational weeks 24–28, as well as 44 healthy lean age‐matched female controls (CON) were included in the present study. Anthropometric and biochemical characteristics of the subgroups studied are summarized in Table 1.
Table 1.
Anthropometric parameters and biochemical characteristics of healthy non‐pregnant controls, women with normal pregnancy and women with gestational diabetes mellitus
| Variables | CON | p‐NGT | GDM | P‐value | ||
|---|---|---|---|---|---|---|
| CON vs p‐NGT | p‐NGT vs GDM | CON vs GDM | ||||
| n | 44 | 70 | 66 | |||
| Age (years) | 29.39 ± 4.57 | 29.10 ± 3.92 | 29.83 ± 4.66 | 1.0 | 1.0 | 0.986 |
| BMI before pregnancy (kg/m2) | 21.06 ± 2.18 | 20.45 ± 2.38 | 21.24 ± 2.70 | 0.648 | 1.0 | 1.0 |
| BMI at blood collection (kg/m2) | 23.59 ± 2.49 | 24.34 ± 2.77 | 0.101 | |||
| Gestation days at OGTT (days) | 194.43 ± 11.05 | 194.41 ± 11.53 | 0.992 | |||
| Systolic blood pressure (mmHg) | 112.27 ± 12.29 | 111.94 ± 11.08 | 117.42 ± 12.50 | 1.0 | 0.085 | 0.025 |
| Diastolic blood pressure (mmHg) | 72.59 ± 8.55 | 66.59 ± 8.17 | 69.08 ± 9.87 | 0.002 | 0.135 | 0.320 |
| TC (mmol/L) | 4.10 (3.67–4.32) | 4.48 (3.96–5.23) | 4.68 (4.23–5.29) | 0.013 | 1.0 | 0.007 |
| TG (mmol/L) | 0.93 (0.78–1.21) | 1.32 (1.04–1.73) | 1.66 (1.30–2.46) | 0.001 | 0.078 | <0.001 |
| HDL (mmol/L) | 1.64 ± 0.37 | 1.61 ± 0.30 | 1.51 ± 0.35 | 0.343 | 0.508 | 0.032 |
| LDL (mmol/L) | 2.56 ± 0.60 | 2.32 ± 0.88 | 2.53 ± 0.81 | <0.001 | 0.552 | <0.001 |
| ALT (U/L) | 12.50 (8.25–15.00) | 11.00 (8.00–17.00) | 12.00 (8.75–21.00) | 1.0 | 1.0 | 1.0 |
| AST (U/L) | 19.00 (15.00–20.00) | 17.00 (14.00–20.00) | 17.00 (14.00–20.25) | 1.0 | 1.0 | 1.0 |
| Cr (umol/L) | 50.20 ± 10.48 | 45.14 ± 7.14 | 43.20 ± 6.23 | 0.048 | 0.813 | 0.009 |
| HbA1c (%)/(mmol/mol) | 5.28 ± 0.38 (34.24 ± 4.20) | 4.8 ± 0.27 (29.00 ± 2.80) | 5.02 ± 0.36 (31.36 ± 4.00) | <0.001 | 0.007 | 0.005 |
| GA (%) | 13.65 ± 1.94 | 11.26 ± 1.23 | 11.46 ± 1.45 | <0.001 | 0.181 | <0.001 |
| PG0 (mmol/L) | 4.70 ± 0.40 | 4.50 ± 0.33 | 5.13 ± 0.65 | 0.170 | <0.001 | 0.001 |
| PG60 (mmol/L) | 7.04 ± 1.69 | 7.65 ± 1.25 | 9.92 ± 1.89 | 0.568 | <0.001 | <0.001 |
| PG120 (mmol/L) | 5.23 ± 1.14 | 6.14 ± 1.14 | 8.06 ± 1.94 | 0.122 | <0.001 | <0.001 |
| INS0 (μU/mL) | 6.26 (5.22–7.91) | 8.63 (6.81–11.38) | 11.16 (7.28–16.25) | 0.001 | 0.110 | <0.001 |
| ISIM | 6.97 ± 2.49 | 5.83 ± 2.73 | 4.07 ± 1.97 | 0.844 | 0.001 | <0.001 |
| HOMA‐β | 106.65 (81.83–154.35) | 178.41 (140.36–233.05) | 142.32 (101.09–210.44) | <0.001 | 0.002 | 0.105 |
| HOMA‐IR | 1.28 (1.04–1.73) | 1.72 (1.32–2.37) | 2.51 (1.68–3.55) | 0.017 | 0.002 | <0.001 |
| Stumvoll 1st‐phase secretion (pmol/L) | 1,239.36 (1,028.85–1,457.89) | 1253.84 (1,059.77–1,539.46) | 1,202.09 (955.82–1,520.61) | 0.573 | 1.0 | 0.616 |
| Stumvoll 2nd‐phase secretion (pmol/L) | 329.26 (273.20–380.48) | 331.00 (285.60–398.84) | 329.54 (264.75–397.80) | 1.0 | 0.059 | 0.153 |
| DI1st | 8255.00 (6,460.58–10,973.78) | 6640.77 (5,610.11–8,099.58) | 4,738.90 (3,597.20–5,822.82) | 0.017 | <0.001 | <0.001 |
| DI2nd | 2147.47 (1,712.20–2,821.97) | 1,759.84 (1,479.22–2,135.92) | 1,246.02 (958.48–1,523.05) | 0.018 | <0.001 | <0.001 |
| CRP (mg/L) | 0.24 (0.18–0.30) | 1.99 (1.23–3.10) | 2.67 (1.77–3.95) | <0.001 | <0.001 | <0.001 |
| TNF‐α (pg/mL) | 14.82 ± 3.65 | 27.31 ± 9.99 | 35.13 ± 12.63 | <0.001 | <0.001 | <0.001 |
| MFG‐E8 (pg/mL) | 104.32 (103.94–116.49) | 150.36 (139.10–164.03) | 183.02 (169.32–235.05) | 0.024 | 0.016 | <0.001 |
Data are mean ± standard deviation, % or median (25th and 75th percentiles). The skewed distributions were log10 or ln transformed for comparison. ancova with post‐hoc analysis with Bonferroni's correction was used to compare significant differences between groups. Differences between two groups were tested by unpaired independent Student's t‐test. ALT, alanine transaminase; AST, aspartate aminotransferase; BMI, body mass index; CON, healthy non‐pregnant controls; Cr, creatinine; CRP, C‐reactive protein; DI, disposition index; GA, glycosylated albumin; GDM, gestational diabetes mellitus; HbA1c, glycosylated hemoglobin; HDL, high‐density lipoprotein cholesterol; HOMA‐β, homeostasis model assessment of insulin secretion; HOMA‐IR, homeostasis model assessment of insulin resistance; INS, insulin levels; ISIM, Matsuda index; LDL, low‐density lipoprotein cholesterol; MFG‐E8, milk fat globule‐epidermal growth factor 8; OGTT, 75‐g oral glucose tolerance test; PG, plasma glucose levels; p‐NGT, pregnancy with normal glucose tolerance; TC, total cholesterol; TG, triglyceride; TNF‐α, tumor necrosis factor‐α.
Women with p‐NGT and GDM were well matched with respect to age, gestational days and BMI. Pregnant women showed poor glucose homeostasis and strong insulin resistance, evidenced by significantly increased insulin levels and HOMA‐IR (P < 0.01 or P < 0.05 vs CON), which were further altered in GDM patients (P < 0.01 or P < 0.05 vs p‐NGT). Basic β‐cell secretory capacity was reserved, as suggested by increased HOMA‐β, Stumvoll1st and Stumvoll2nd in normal pregnancy, whereas β‐cell secretory capacity was shown to decrease as a result of progression to diabetes. Remarkably, after adjusting for HOMA‐IR, DI1st and DI2nd showed a significant reduction in p‐NGT compared with CON, which was further decreased in the GDM group (P < 0.01).
The median (25th and 75th percentiles) plasma MFG‐E8 level in all participants was 153.79 pg/mL (123.56–189.25 pg/mL). Circulating plasma concentrations of MFG‐E8 were significantly higher in pregnant participants, and were further increased in GDM patients, after correcting for age, pre‐BMI, SP, DP, TC and TG, as shown in Table 1 (all P < 0.05).
Correlation of MFG‐E8 with clinical parameters analysis
Next, the relationship of circulating MFG‐E8 levels with various anthropometric parameters was investigated using Pearson's and partial correlations. MFG‐E8 levels were neither related to pre‐BMI, alanine transaminase, aspartate aminotransferase and glycosylated albumin nor to insulin secretion indices evaluated by HOMA‐β, Stumvoll1st, and Stumvoll2nd, as summarized in Table 2, and Tables S1 and S2.
Table 2.
Pearson's and partial correlations of milk fat globule‐epidermal growth factor 8 with anthropometric parameters and biochemical characteristics as well as glucose metabolism in pregnant participants
| Variables | Pregnancies | |||||
|---|---|---|---|---|---|---|
| r | P‐value | Age, pre‐BMI, SP, DP, TC and TG adjusted | ||||
| Bonferroni P‐value† | r | P‐value | Bonferroni P‐value† | |||
| Age | 0.208 | 0.015 | 1.0 | |||
| BMI before pregnancy | 0.141 | 0.102 | 1.0 | |||
| BMI BMI at blood collection | 0.201 | 0.019 | 0.532 | 0.145 | 0.105 | 1.0 |
| Gestation days at OGTT | −0.153 | 0.076 | 1.0 | −0.133 | 0.138 | 1.0 |
| Systolic blood pressure | 0.298 | <0.001 | <0.001 | |||
| Diastolic blood pressure | 0.238 | 0.006 | 0.168 | |||
| TC | 0.234 | 0.007 | 0.196 | |||
| TG | 0.216 | 0.012 | 0.336 | |||
| HDL | 0.186 | 0.032 | 0.896 | 0.181 | 0.043 | 1.0 |
| LDL | 0.231 | 0.007 | 0.175 | 0.003 | 0.971 | 1.0 |
| ALT | 0.143 | 0.100 | 1.0 | 0.054 | 0.545 | 1.0 |
| AST | 0.046 | 0.600 | 1.0 | −0.036 | 0.691 | 1.0 |
| Cr | 0.102 | 0.237 | 1.0 | 0.083 | 0.354 | 1.0 |
| HbA1c | 0.394 | <0.001 | <0.001 | 0.324 | <0.001 | <0.001 |
| GA | −0.002 | 0.978 | 1.0 | 0.033 | 0.714 | 1.0 |
| PG0 | 0.453 | <0.001 | <0.001 | 0.358 | <0.001 | <0.001 |
| PG60 | 0.383 | <0.001 | <0.001 | 0.294 | 0.001 | 0.028 |
| PG120 | 0.291 | 0.001 | 0.028 | 0.204 | 0.022 | 0.616 |
| INS0 | 0.348 | <0.001 | <0.001 | 0.253 | 0.004 | 0.112 |
| ISIM | −0.376 | <0.001 | <0.001 | −0.322 | <0.001 | <0.001 |
| HOMA‐β | −0.065 | 0.450 | 1.0 | −0.071 | 0.429 | 1.0 |
| HOMA‐IR | 0.405 | <0.001 | <0.001 | 0.309 | <0.001 | <0.001 |
| Stumvoll 1st‐phase secretion | 0.092 | 0.286 | 1.0 | 0.063 | 0.485 | 1.0 |
| Stumvoll 2nd‐phase secretion | −0.084 | 0.329 | 1.0 | −0.111 | 0.217 | 1.0 |
| DI1st | −0.451 | <0.001 | <0.001 | −0.320 | <0.001 | <0.001 |
| DI2nd | −0.447 | <0.001 | <0.001 | −0.331 | 0.000 | <0.001 |
| CRP | −0.137 | 0.112 | 1.0 | −0.169 | 0.058 | 1.0 |
| TNF‐α | −0.280 | 0.001 | 0.028 | −0.346 | <0.001 | <0.001 |
Skewed distributions were log10 or ln transformed. Correlation between variables was analyzed by Pearson's correlation test and partial correlation test (adjusted by age, sex, body mass index [BMI], systolic blood pressure [SP], diastolic blood pressure [DP], total cholesterol [TC] and triglyceride [TG]). Bonferroni's correction was applied for multiple testing (28 times) to adjust P‐value†. ALT, alanine transaminase; AST, aspartate aminotransferase; CON, healthy non‐pregnant controls; Cr, creatinine; CRP, C‐reactive protein; DI, disposition index; GA, glycosylated albumin; GDM, gestational diabetes mellitus; HbA1c, glycosylated hemoglobin; HDL, high‐density lipoprotein cholesterol; HOMA‐β, homeostasis model assessment of insulin secretion; HOMA‐IR, homeostasis model assessment of insulin resistance; INS, insulin levels; ISIM, Matsuda index; LDL, low‐density lipoprotein cholesterol; MFG‐E8, milk fat globule‐epidermal growth factor 8; OGTT, 75‐g oral glucose tolerance test; PG, plasma glucose levels; pre‐BMI, body mass index before pregnancy; p‐NGT, pregnancy with normal glucose tolerance; TNF‐α, tumor necrosis factor‐α.
No correlation was found between MFG‐E8 plasma levels and any variable in the control group (Table S1). In all pregnant participants, MFG‐E8 had a significantly positive correlation with age, BMI at blood collection, blood pressures and lipid profiles (Figure 1). Nevertheless, the correlation was no longer significant after Bonferroni's correction was carried out, except for SP. In addition, MFG‐E8 correlated positively with hemoglobin A1c, fasting and postprandial glucose levels, fasting insulin levels, and HOMA‐IR, whereas it was inversely correlated with ISIM and insulin secretion evaluated by disposition indices (DI1st and DI2nd). It is noteworthy that all of these correlations remained statistically significant, despite being adjusted for age, pre‐BMI, SP, DP, TC and TG. After Bonferroni's correction was applied, the statistically significant association persisted, except for glucose levels at 120 min and fasting insulin levels.
Figure 1.
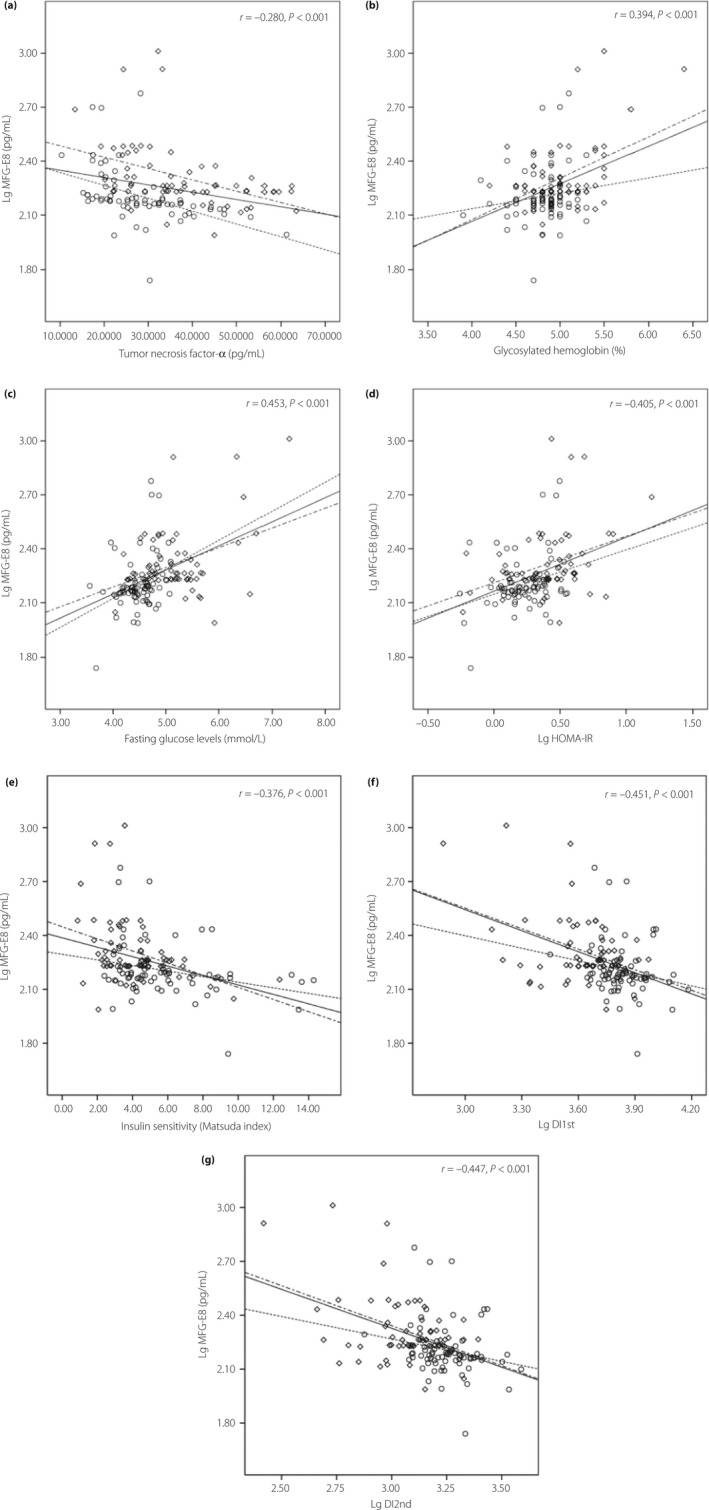
Correlation of plasma milk fat globule‐epidermal growth factor 8 (MFG‐E8) levels (log10 transformed) with (a) tumor necrosis factor‐α levels, (b) glycosylated hemoglobin, (c) fasting glucose levels (d) homeostasis model assessment of insulin resistance, log10 transformed (Lg HOMA‐IR), (e) insulin sensitivity index, (f) disposition index of first phase insulin secretion, log10 transformed (Lg DI1st) and (g) disposition index of second phase insulin secretion, log10 transformed (Lg DI2nd). Circles represent normal pregnancy, diamonds represent gestational diabetes mellitus.
However, the situation was different after subgroup analysis. MFG‐E8 levels were positively associated with age, BMI at blood collection, lipid profiles, fasting glucose and insulin levels, and HOMA‐IR in the normal pregnant group, whereas MFG‐E8 levels were inversely associated with ISIM and disposition indices in the same group. However, all parameters except BMI at blood collection lost the statistically significant correlation with MFG‐E8 after adjusted for age, pre‐BMI, SP, DP, TC and TG. In the diabetic group, a positive correlation between MFG‐E8 and blood pressure, hemoglobin A1c, fasting and postprandial glucose levels, fasting insulin levels, HOMA‐IR, and creatinine, as well as a negative association with gestation days, ISIM and disposition indices were observed, and the association remained statistically significant after interference factors were adjusted, except for glucose levels at 120 min, fasting insulin levels and HOMA‐IR. After Bonferroni's correction was applied for multiple testing (28 times) to adjust P‐values, all significance was eliminated.
There was a significantly negative correlation between circulating MFG‐E8 and TNF‐α in all pregnancies or subgroups. After Bonferroni correction was carried out and interference factors were adjusted, the statistical significance still remained. In addition, MFG‐E8 was negatively correlated with CRP only in the GDM group, and lost statistical significance after multiple comparison.
When participants were divided by tertiles of HOMA‐IR, ISIM and disposition indices, MFG‐E8 concentration increased progressively from the first to the third tertile of HOMA‐IR, whereas MFG‐E8 concentration decreased progressively from the first to the third tertile of ISIM, DI1st and DI2nd (all P < 0.05; Figure 2).
Figure 2.
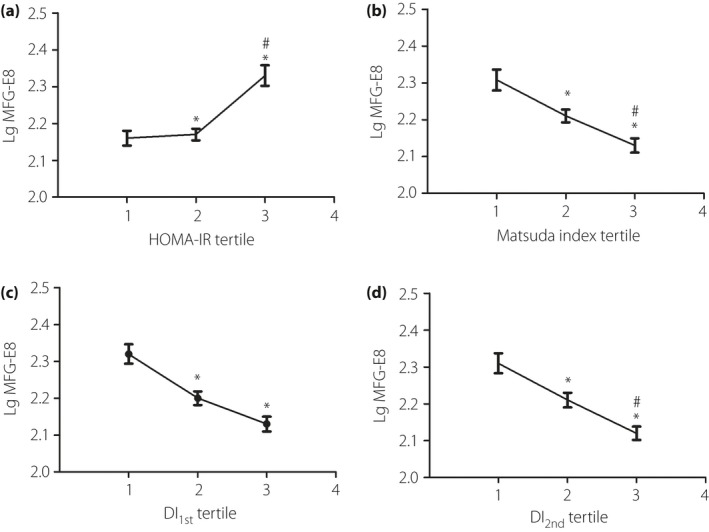
Log10 transformed milk fat globule‐epidermal growth factor 8 (LgMFG‐E8) in tertiles of (a) homeostasis model assessment of insulin resistance (HOMA‐IR), (b) Matsuda index, (d) disposition index of first phase insulin secretion (DI 1st) and (d) disposition index of second phase insulin secretion (DI 2nd). Data were shown as mean ± standard error of the men. MFG‐E8 was logarithmically transformed for comparison. Differences between groups were assessed by one‐way ancova adjusted for age, pre‐pregnancy body mass index, systolic blood pressure, diastolic blood pressure, total serum cholesterol and triglyceride. *P < 0.05 vs the first tertile, # P < 0.05 vs the second tertile.
Multiple regression analysis
The multivariate stepwise linear regression analysis showed that independent factors influencing MFG‐E8 levels were fasting glucose levels (β = 0.092; P < 0.001), DI1st (β = −0.300; P < 0.001) and TNF‐α (β = −0.005; P < 0.001). The adjusted R 2 value was 0.371.
Logistic regression analysis
Finally, pregnant women were stratified into tertiles based on MFG‐E8 levels; women in the third tertile had an 8.62‐fold risk of developing GDM compared with women in the first tertile. This odds ratio merely decreased a 0.37‐fold risk after adjustment for age, pre‐BMI, SP, DP, TC and TG, and remained statistically significant (P < 0.01; Figure 3).
Figure 3.
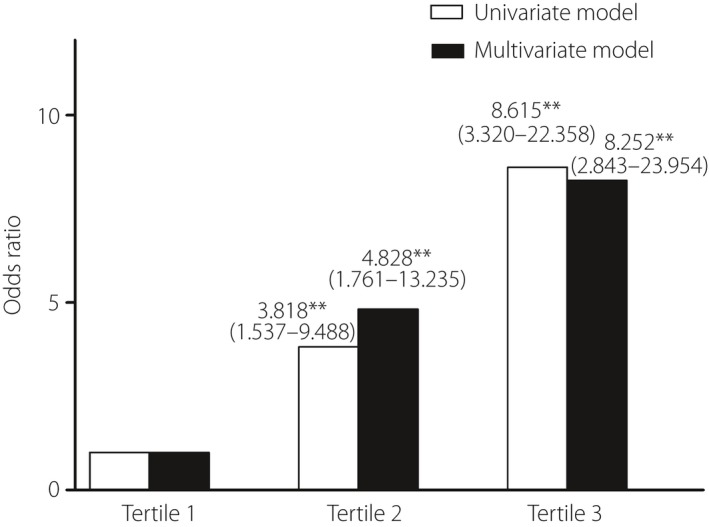
Odds of developing gestational diabetes mellitus by milk fat globule‐epidermal growth factor 8 (MFG‐E8) tertiles. Participants were divided into tertiles based on the MFG‐E8 levels. Univariate (white) and multivariate (dark) logistic regression analyses were used to determine odds ratios for the development of gestational diabetes mellitus in each tertile. The multivariate logistic regression model includes adjustment for age, pre‐pregnancy body mass index, systolic blood pressure, diastolic blood pressure, total serum cholesterol and triglyceride. **Significantly different from the reference tertile at the P < 0.01 level.
Discussion
To the best of our knowledge, this is the first study to show that plasma MFG‐E8 levels were significantly increased in pregnant women vs non‐pregnant women (P < 0.05). This suggests a similarity in alteration in MFG‐E8 activity that was found in the myometrium, as well as in the endometrium during human pregnancy17, 28, which was probably due to MFG‐E8 functioning as an extracellular growth factor during gestation that promoted expansion of the connective tissue framework supporting the hypertrophied bundles of smooth muscle. Furthermore, we established that the plasma concentrations of MFG‐E8 were greater in GDM than that observed in normal pregnant women, which was in consist with the elevated MFG‐E8 levels that have been confirmed in db/db mice and type 2 diabetes patients29, 30. However, the exact mechanism of elevated MFG‐E8 concentrations during human gestation and GDM has not been completely elucidated yet, and further research is required for it to be clearly understood. In the current study, when pregnancies were divided into tertiles based on MFG‐E8 levels, women with the highest levels of MFG‐E8 showed a markedly elevated risk of GDM compared with women with the lowest MFG‐E8 levels. This estimate of risk was consistent with, but did not prove the involvement of, MFG‐E8 in GDM pathophysiology.
In the hypothesized direction, pregnant women were under a state of subclinical chronic inflammation as evidenced by significantly higher TNF‐α and CRP levels compared with controls, which were further altered in GDM. MFG‐E8 is now accepted as a major inflammatory mediator stimulating anti‐inflammatory reprogramming of human and murine endothelial cells and macrophages31. MFG‐E8 alleviates the inflammation by suppressing lipopolysaccharide‐toll like receptor 4 signaling and quenching TNF‐α production32, 33. MFG‐E8 has been reported to alleviate the inflammation, and negatively regulate the production of TNF‐α in patients with rheumatoid arthritis, sepsis and ischemia reperfusion injury10, 34, 35. Consistent with these results, the present study showed that TNF‐α was negative, and independently correlated with plasma MFG‐E8 concentrations during gestation. However, both MFG‐E8 and TNF‐α circulating levels were increased during normal gestation and GDM in the present study. The reason for the discrepancy is unclear and is probably because of the complexity in patients during pregnancy, as it has been shown that endometrial MFG‐E8 gene expression is significantly upregulated by TNF‐α during gestation, which is a critical step required for the endometrial changes during embryonic attachment and invasion36. Future studies are needed to elucidate this point. Furthermore, contrary to the results of Dai et al.7 that found circulating MFG‐E8 concentrations were negatively associated with CRP in patients with coronary artery disease, we did not find a significant association between MFG‐E8 and CRP levels, and were unable to support that conclusion in this current study, which focused on patients with pregnancy and diabetes.
In the current study, normal pregnancy was associated with impaired β‐cell function evidenced by enhanced insulin resistance and inadequate insulin secretion. This situation was even worse in women with GDM. Interestingly, as there are few studies available on the topic in general, the present results not only replicated the finding that MFG‐E8 was negatively associated with TNF‐α, but also showed that MFG‐E8 correlated positively with indices of insulin resistance, including HOMA‐IR and ISIM. Importantly, we excluded potentially confusing effects in the association of MFG‐E8 with pregnancy and GDM, such as age, BMI, blood pressure and lipid profiles. In addition, we also ruled out the interaction effect for multiple comparisons by carrying out Bonferroni's correction. Nevertheless, the negative correlation of MFG‐E8 and insulin secretion was only observed by using disposition indices that reflected accurate insulin secretion controlling for insulin resistance, while the first phase insulin secretion (DI1st) was predictive of MFG‐E8 levels during gestation. Furthermore, MFG‐E8 concentration increased progressively from the first to the third tertile of HOMA‐IR, whereas MFG‐E8 concentration increased inversely in ISIM, DI1st and DI2nd. However, riding on these mechanistic tools, it is not clear whether elevated MFG‐E8 level is a compensatory response or a marker of insulin resistance and insulin secretion. Accumulating data suggested that low‐grade inflammation was a central feature of the insulin resistance syndrome, and that TNF‐α was the most powerful predictor of insulin resistance and was inversely correlated to insulin secretion during pregnancy37. Consistent with plasma MFG‐E8 concentrations being negatively correlated with TNF‐α in the present study, therefore, we speculated that MFG‐E8 might be associated with insulin resistance and insulin secretion through an inflammatory pathway.
Overall, considering the complexity of the mechanisms of MFG‐E8 functioning, we cannot exclude the possibility that the MFG‐E8 levels might be associated with other unknown intermediate factors, which could affect insulin resistance and insulin secretion. Further studies are required to delineate the mechanisms of MFG‐E8 in GDM and insulin resistance. Correlations between MFG‐E8 and parameters of β‐cell function were not statistically significant in each group individually, which might be due to the relatively small sample size.
There were still some limitations in the study that need to be emphasized. First, our sample size was relatively small. Second, the cross‐sectional design of the study prevented us from extracting safe conclusions on the possible etiological relationship between MFG‐E8 and pregnancy and diabetes. Third, the study was carried out during the 24–28 weeks of pregnancy without considering the whole duration of gestation. Fourth, the study excluded the effects of hormone levels, such as prolactin, estrogen, cortisol and progesterone. Larger prospective studies are necessary for the validation of these conclusions and to elucidate the clinical roles of MFG‐E8.
Disclosure
The authors declare no conflict of interest.
Supporting information
Table S1 | Pearson's and partial correlations of milk fat globule‐epidermal growth factor 8 with anthropometric parameters and biochemical characteristics as well as glucose metabolism in healthy controls
Table S2 | Pearson's and partial correlations of Milk fat globule‐epidermal growth factor 8 with anthropometric parameters and biochemical characteristics as well as glucose metabolism in subgroups of pregnancies
Acknowledgments
We are grateful to the research individuals for their participation in this study. We are also grateful for all the nurses who contributed to data collection. This study was funded by grants from the application foundation planning project of Wuhan Science and Technology Bureau (2015061701011619).
J Diabetes Investig 2017; 8: 571–581
References
- 1. Ramsay JE, Ferrell WR, Crawford L, et al Maternal obesity is associated with dysregulation of metabolic, vascular, and inflammatory pathways. J Clin Endocrinol Metab 2002; 87: 4231–4237. [DOI] [PubMed] [Google Scholar]
- 2. Walsh JM, McGowan CA, Byrne JA, et al The association between TNF‐α and insulin resistance in euglycemic women. Cytokine 2013; 64: 208–212. [DOI] [PubMed] [Google Scholar]
- 3. Kirwan JP, Hauguel‐De Mouzon S, Lepercq J, et al TNF‐α is a predictor of insulin resistance in human pregnancy. Diabetes 2002; 51: 2207–2213. [DOI] [PubMed] [Google Scholar]
- 4. Oshima K, Aoki N, Kato T, et al Secretion of a peripheral membrane protein, MFG‐E8, as a complex with membrane vesicles. Eur J Biochem 2002; 269: 1209–1218. [DOI] [PubMed] [Google Scholar]
- 5. Hanayama R, Tanaka M, Miwa K, et al Identification of a factor that links apoptotic cells to phagocytes. Nature 2002; 417: 182–187. [DOI] [PubMed] [Google Scholar]
- 6. Oshima K, Aoki N, Negi M, et al Lactation‐dependent expression of an mRNA splice variant with an exon for a multiply O‐glycosylated domain of mouse milk fat globule glycoprotein MFG‐E8. Biochem Biophys Res Commun 1999; 254: 522–528. [DOI] [PubMed] [Google Scholar]
- 7. Dai W, Li Y, Lv Y, et al The roles of a novel anti‐inflammatory factor, milk fat globule‐epidermal growth factor 8, in patients with coronary atherosclerotic heart disease. Atherosclerosis 2014; 233: 661–666. [DOI] [PubMed] [Google Scholar]
- 8. Zhang S, Xie JG, Su BT, et al MFG‐E8, a clearance glycoprotein of apoptotic cells, as a new marker of disease severity in chronic obstructive pulmonary disease. Braz J Med Biol Res 2015; 48: 1032–1038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Zhao QJ, Yu YB, Zuo XL, et al Milk fat globule‐epidermal growth factor 8 is decreased in intestinal epithelium of ulcerative colitis patients and thereby causes increased apoptosis and impaired wound healing. Mol Med 2012; 18: 497–506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Albus E, Sinningen K, Winzer M, et al Milk fat globule‐epidermal growth factor 8 (MFG‐E8) is a novel anti‐inflammatory factor in rheumatoid arthritis in mice and humans. J Bone Miner Res 2016; 31: 596–605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Neher JJ, Emmrich JV, Fricker M, et al Phagocytosis executes delayed neuronal death after focal brain ischemia. Proc Natl Acad Sci USA 2013; 110: E4098–E4107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Leonardi‐Essmann F, Emig M, Kitamura Y, et al Fractalkine‐upregulated milk‐fat globule EGF factor‐8 protein in cultured rat microglia. J Neuroimmunol 2005; 160: 92–101. [DOI] [PubMed] [Google Scholar]
- 13. Bu HF, Zuo XL, Wang X, et al Milk fat globule‐EGF factor 8/lactadherin plays a crucial role in maintenance and repair of murine intestinal epithelium. J Clin Invest 2007; 117: 3673–3683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Ellert‐Miklaszewska A, Wisniewski P, Kijewska M, et al Tumour‐processed osteopontin and lactadherin drive the protumorigenic reprogramming of microglia and glioma progression. Oncogene 2016; 35: 6366–6377. [DOI] [PubMed] [Google Scholar]
- 15. Silvestre JS, Théry C, Hamard G, et al Lacthadherin promotes VEGF‐dependent neovascularization. Nat Med 2005; 11: 499–506. [DOI] [PubMed] [Google Scholar]
- 16. Ensslin MA, Shur BD. Identification of mouse sperm SED1, a bimotif EGF repeat and discoidin‐domain protein involved in sperm‐egg binding. Cell 2003; 114: 405–417. [DOI] [PubMed] [Google Scholar]
- 17. Rehman KS, Yin S, Mayhew BA, et al Human myometrial adaptation to pregnancy: cDNA microarray gene expression profiling of myometrium from non‐pregnant and pregnant women. Mol Hum Reprod 2003; 9: 681–700. [DOI] [PubMed] [Google Scholar]
- 18. Bocca SM, Anderson S, Amaker B, et al Milk fat globule epidermal growth factor 8 (MFG‐E8): a novel protein in the mammalian endometrium with putative roles in implantation and placentation. Placenta 2012; 33: 795–802. [DOI] [PubMed] [Google Scholar]
- 19. Yamaguchi H, Takagi J, Miyamae T, et al Milk fat globule EGF factor 8 in the serum of human patients of systemic lupus erythematosus. J Leukoc Biol 2008; 83: 1300–1307. [DOI] [PubMed] [Google Scholar]
- 20. Retnakaran R, Hanley AJ, Raif N, et al C‐reactive protein and gestational diabetes: the central role of maternal obesity. J Clin Endocrinol Metab 2003; 88: 3507–3512. [DOI] [PubMed] [Google Scholar]
- 21. Matthews DR, Hosker JP, Rudenski AS, et al Homeostasis model assessment: insulin resistance and beta‐cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia 1985; 28: 412–419. [DOI] [PubMed] [Google Scholar]
- 22. Gupta D, Krueger CB, Lastra G. Over‐nutrition, obesity and insulin resistance in the development of beta‐cell dysfunction. Curr Diabetes Rev 2012; 8: 76–83. [DOI] [PubMed] [Google Scholar]
- 23. Matsuda M, DeFronzo RA. Insulin sensitivity indices obtained from oral glucose tolerance testing: comparison with the euglycemic insulin clamp. Diabetes Care 1999; 22: 1462–1470. [DOI] [PubMed] [Google Scholar]
- 24. Kirwan JP, Huston‐Presley L, Kalhan SC, et al Clinically useful estimates of insulin sensitivity during pregnancy: validation studies in women with normal glucose tolerance and gestational diabetes mellitus. Diabetes Care 2001; 24: 1602–1607. [DOI] [PubMed] [Google Scholar]
- 25. Stumvoll M, Van Haeften T, Fritsche A, et al Oral glucose tolerance test indexes for insulin sensitivity and secretion based on various avail abilities of sampling times. Diabetes Care 2001; 24: 796–797. [DOI] [PubMed] [Google Scholar]
- 26. Stumvoll M, Mitrakou A, Pimenta W, et al Use of the oral glucose tolerance test to assess insulin release and insulin sensitivity. Diabetes Care 2000; 23: 295–301. [DOI] [PubMed] [Google Scholar]
- 27. Retnakaran R, Hanley AJ, Raif N, et al Adiponectin and beta cell dysfunction in gestational diabetes: pathophysiological implications. Diabetologia 2005; 48: 993–1001. [DOI] [PubMed] [Google Scholar]
- 28. Mirkin S, Arslan M, Churikov D, et al In search of candidate genes critically expressed in the human endometrium during the window of implantation. Hum Reprod 2005; 20: 2104–2117. [DOI] [PubMed] [Google Scholar]
- 29. Cheng M, Li BY, Li XL, et al Correlation between serum lactadherin and pulse wave velocity and cardiovascular risk factors in elderly patients with type 2 diabetes mellitus. Diabetes Res Clin Pract 2012; 95: 125–131. [DOI] [PubMed] [Google Scholar]
- 30. Yin W, Li B, Li X, et al Anti‐inflammatory effects of grape seed procyanidin B2 on a diabetic pancreas. Food Funct 2015; 6: 3065–3071. [DOI] [PubMed] [Google Scholar]
- 31. Brissette MJ, Lepage S, Lamonde AS, et al MFG‐E8 released by apoptotic endothelial cells triggers anti‐inflammatory macrophage reprogramming. PLoS ONE 2012; 7: e36368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Mukundan L, Odegaard JI, Morel CR, et al PPAR‐delta senses and orchestrates clearance of apoptotic cells to promote tolerance. Nat Med 2009; 15: 1266–1272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Aziz MM, Jacob A, Wu R, et al MFG‐E8 attenuates LPS‐induced TNF‐α production in macrophages via STAT3‐mediated SOCS3 activation. PLoS ONE 2011; 6: e27685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Matsuda A, Jacob A, Wu R, et al Milk fat globule‐EGF factor VIII in sepsis and ischemia‐reperfusion injury. Mol Med 2011; 17: 126–133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Yang WL, Sharma A, Zhang F, et al Milk fat globule epidermal growth factor‐factor 8‐derived peptide attenuates organ injury and improves survival in sepsis. Crit Care 2015; 19: 375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Yu L, Anderson S, Oehninger S, et al Tumor necrosis factor α up‐regulates endometrial milk fat globule‐epidermal growth factor 8 protein production via nuclear factor κB activation, resulting in cell migration of epithelial cells. Fertil Steril 2014; 101: 52–59. [DOI] [PubMed] [Google Scholar]
- 37. Festa A, D'Agostino R Jr, Howard G, et al Chronic subclinical inflammation as part of the insulin resistance syndrome: the Insulin Resistance Atherosclerosis Study (IRAS). Circulation 2000; 102: 42–47. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1 | Pearson's and partial correlations of milk fat globule‐epidermal growth factor 8 with anthropometric parameters and biochemical characteristics as well as glucose metabolism in healthy controls
Table S2 | Pearson's and partial correlations of Milk fat globule‐epidermal growth factor 8 with anthropometric parameters and biochemical characteristics as well as glucose metabolism in subgroups of pregnancies


