Abstract
Aims/Introduction
We evaluated the impact of postprandial hyperglycemia at clinic visits on the incidence of cardiovascular diseases (CVD) and all‐cause mortality independently of mean glycosylated hemoglobin in type 2 diabetes patients in a real‐world setting.
Materials and Methods
The present retrospective observational cohort study included 646 type 2 diabetes patients. All of the participants had their initial consultations at the Institute for Diabetes Care and Research, Asahi Life Foundation affiliated Marunouchi Hospital, Tokyo, Japan, during the period from 1995 to 1996, visited the clinic ≥4 times, had their 2‐h post‐breakfast blood glucose (2h‐PBBG) levels measured and were followed up for ≥1 year. The 646 patients were followed up for survival. Of the 646 patients, 618 had no history of CVD at the first visit and had measured 2h‐PBBG until the first CVD onset or censorings. These two cohorts were followed up through June 2012, and subsequently questionnaires were mailed. Multivariate Cox proportional hazard models were used to evaluate the risk of CVD incidence and death.
Results
CVD occurred in 78 patients, and 56 patients died. The median follow‐up periods of the CVD cohort and the mortality cohort were 15.6 and 15.9 years, respectively. The mean 2h‐PBBG is a significant predictor of the CVD incidence and all‐cause mortality after adjusting for the mean glycosylated hemoglobin, the number of 2h‐PBBG measurements, age, sex and classical risk factors.
Conclusions
Postprandial hyperglycemia represented by the mean level of 2h‐PBBG at clinic visits is associated with CVD incidence and all‐cause mortality independently of the mean glycosylated hemoglobin level in type 2 diabetes patients. Prospective interventional trials are warranted to confirm the present findings.
Keywords: Cardiovascular disease, Mortality, Postprandial blood glucose
Introduction
Evidence exists that postload or postchallenge hyperglycemia plays a key role in cardiovascular diseases (CVD) and mortality. This evidence is mainly based on the results of epidemiological studies carried out in non‐diabetes patients1, 2, 3, 4, 5, 6, and has been confirmed by meta‐analyses7, 8, 9 and reviewed10, 11, 12, 13. However, the results obtained from non‐diabetes patients cannot be transferred to diabetes patients. Furthermore, postload or postchallenge glycemia obtained using an oral glucose tolerance test cannot be equated to postprandial glycemia. Epidemiological studies evaluating the association between the risk of adverse events and postprandial hyperglycemia in diabetes patients are scarce. In such a situation, postprandial hyperglycemia is considered to be a relevant therapeutic target in diabetes patients, as shown by many current guidelines. However, the interventional trial in type 2 diabetes patients after acute myocardial infarction showed no definite proof that targeting postprandial hyperglycemia results in a more beneficial outcome of CVD14, except for the post‐hoc subgroup analysis in older patients15.
In Japanese patients with diabetes, the most frequent cause of death is cancer16, 17, followed by infections and CVD. Acute glucose excursions rather than sustained hyperglycemia triggers oxidative stress18, 19. Oxidative stress generates DNA damage20, increased inflammation21 and vascular endothelial damage, which might contribute to cancer progression and atherosclerosis.
Thus, we aimed to evaluate the impact of postprandial hyperglycemia at clinic visits on the CVD incidence and all‐cause mortality independently of mean glycosylated haemoglobin (HbA1c) in type 2 diabetes patients in a real‐world setting.
Materials and Methods
Definitions of postprandial glycemia
Blood glucose and HbA1c levels were measured irrespective of fasting or postprandial status. At each visit, patients were asked when they had started eating their previous meal, and capillary blood was drawn to determine the blood glucose and HbA1c levels. A medical technologist calculated the postprandial time interval and classified it according to 15‐min units. Blood glucose levels at 2 h ± 15 min after breakfast were defined as 2‐h post‐breakfast blood glucose (2h‐PBBG).
Postprandial blood glucose levels are generally standardized at 2 h after starting meals. Peter et al.22 reported higher glycemic excursion in response to the first meal of the day compared with the subsequent two meals on the same day, irrespective of glycemic control. Monnier et al.23 carried out continuous glucose monitoring and similarly reported that in most groups of patients the post‐meal glucose excursion was more marked after breakfast than after lunch or dinner. Therefore, we concluded that determination of the 2h‐PBBG was appropriate for detecting postprandial hyperglycemia.
Study participants
This was a retrospective observational cohort study. Between January 1995 and December 1996, a total of 1,912 patients underwent initial consultations at the outpatient clinic of the Institute for Diabetes Care and Research, Asahi Life Foundation affiliated Marunouchi Hospital, Tokyo, Japan. Of these, 832 were diagnosed with type 2 diabetes and visited our clinic ≥4 times. A total of 816 of these patients were followed up for ≥1 year, of whom 646 had their 2h‐PBBG levels measured and so were enrolled in the present study. The survival of these participants was assessed during the follow‐up period. Of the participants, 618 had never suffered from CVD at the time of their initial consultation and underwent 2h‐PBBG measurements before the onset of CVD or censoring (Figure 1). Both the mortality and CVD cohorts were followed up until the end of June 2012, and the participants who had been lost to follow up were subsequently sent questionnaires.
Figure 1.
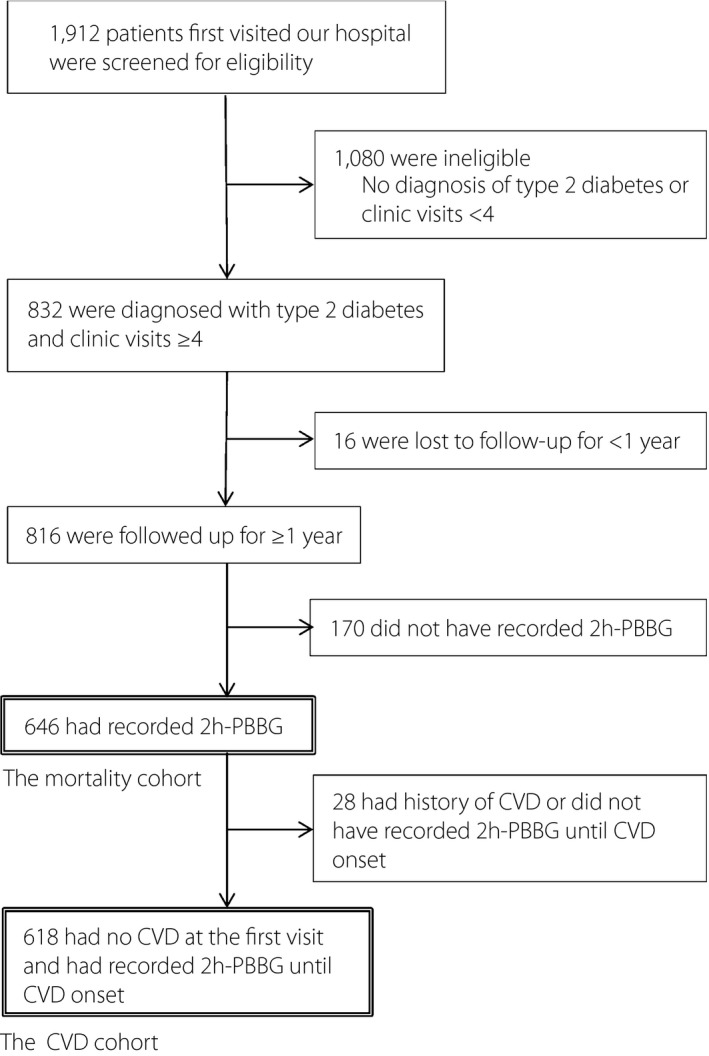
Diagram of the mortality and cardiovascular disease (CVD) cohorts. 2 h‐PBBG, 2‐h post‐breakfast blood glucose.
Of the 646 patients, 314 continued to regularly undergo treatment at our clinic and 30 died. The remaining 302 patients were sent a questionnaire in June 2012. A total of 130 patients responded. As a result, it was found that 26 patients had died. The remaining 172 patients were censored at the time of their last visit. Therefore, the overall follow‐up rate was 73.4% (474/646), and a total of 56 patients died. The significant difference in baseline characteristics between patients who completed (474 patients) and did not complete (172 patients) follow up was only found in the proportion of patients who were current smokers (44.9% completed vs 36.1% did not complete, P = 0.043).
A total of 21 patients died of cancer, whereas 18 patients died of CVD, six of infections, three of digestive conditions, one of kidney failure, three of other conditions (senility, heat stroke and a subdural hematoma) and four of unknown causes. Of the 21 patients who died of cancer, six died of lung cancer, five of pancreatic cancer, two of gastric cancer and eight of other (one case each of liver, breast, kidney, bladder, prostate, oral, endocrine and blood) cancer.
The effects of sex, age, duration of diabetes, body mass index (BMI), blood pressure (BP), HbA1c levels, smoking status, alcohol consumption, serum levels of lipid and creatinine (SCr), estimated glomerular filtration rate (eGFR), use of lipid‐lowering and/or antihypertensive drugs, and treatment for diabetes were analyzed. Any treatment that was first administered before, during or within 6 months of the patient's initial consultation was classified as an initial therapy. Patients that were administered combination treatment involving insulin plus oral antidiabetic medication were classified as insulin‐treated patients.
The study protocol, which was approved by the institutional review board, met the requirements of the Declaration of Helsinki and the Japanese government's Ethical Guidelines Regarding Epidemiological Studies.
End‐point definition
Death and CVD onset were used as end‐points. The definition of CVD was as follows: fatal or non‐fatal acute myocardial infarction, coronary artery procedure (bypass surgery or angioplasty) or a stroke (hemorrhagic or ischemic) that resulted in hospitalization. We chose these definitions based on a thorough review of medical records and the patients' questionnaire responses.
In the CVD cohort, patients that had not suffered any CVD events, including those that had died from any non‐CVD cause, were censored at the time of their death or the time of their last visit to our clinic. In the mortality cohort, the surviving patients and those that were lost to follow up were censored at the time of their last visit to our clinic.
Clinical examination and laboratory methods
The glucose‐oxidase method (Fuji DRI‐CHEM; Fuji Film, Tokyo, Japan) was used to determine the patients' capillary blood glucose levels. The patients' blood glucose data are shown as plasma equivalents. Diabetes analyzers (Tosoh Bioscience, Tokyo, Japan) were used to obtain the HbA1c data. A high‐performance liquid chromatography technique standardized by the Japan Diabetes Society was used to assess the patients' HbA1c levels. Linear regression equations were used to convert the HbA1c data obtained before January 2007 to the Japan Diabetes Society standard HbA1c values. The National Glycohemoglobin Standardization Program‐certified method was used from June 2012. All previously acquired HbA1c (%) data were converted to National Glycohemoglobin Standardization Program values (%) in June 201224.
In general, BP was assessed once per visit, as was bodyweight. BP was measured by a trained medical technologist using an electronic sphygmomanometer (Omron, Kyoto, Japan) while the patient was seated. The patients' lipid levels were assessed at least once every few visits, regardless of their postprandial or fasting status. The total cholesterol (TC) to high‐density lipoprotein cholesterol (HDL‐C) ratio (TC/HDL‐C) was used for the analysis, because it is the best lipid‐based predictor of CVD among men with type 2 diabetes25, 26. Until 11 June 1995, the Jaffe rate method was used to assess the patients' SCr levels, whereas they were subsequently evaluated with an enzymatic technique. A linear regression equation based on data from duplicated assays was used to convert the Jaffé rate method‐derived SCr data to enzymatic‐method equivalents. The following equation, which was developed by the Japanese Society of Nephrology, was used to calculate the eGFR: eGFR (mL/min/1.73 m2) = 194 × SCr−1.094 × age−0.287 (×0.739 if a woman)27.
Statistical analysis
Baseline clinical characteristics of the CVD cohort and the mortality cohort were compared with those for all the 832 type 2 diabetes patients, using the Student's t‐test and Wilcoxon rank‐sum test for continuous variables, and the χ2‐test for categorical variables. Differences between the patients who developed and did not develop a CVD event, and those between survivors and decedents were also analyzed using the Student's t‐test, Wilcoxon rank‐sum test and the χ2‐test. Age was adjusted using logistic regression analysis. Hazard ratios (HRs) for the CVD incidence and all‐cause mortality associated with mean 2h‐PBBG were calculated using multivariate Cox proportional hazard models. Covariates included mean HbA1c, number of 2h‐PBBG measurements, age and sex in model 1. In model 2, duration of diabetes, mean BMI, mean systolic BP (SBP), mean TC/HDL‐C and smoking status were added to model 1. The values for the number of intrapersonal 2h‐PBBG measurements were ln‐transformed for inclusion in the model.
Sensitivity analysis was carried out in several ways for the assessment of postprandial glycemia. Blood glucose levels at 1 h ± 15 min and those from 1 h − 15 min to 2 h + 15 min after breakfast were defined as 1‐h post‐breakfast blood glucose (1 h‐PBBG) and 1–2‐h post‐breakfast blood glucose (1–2h‐PBBG), respectively. In addition, blood glucose levels at 1 h ± 15 min and those at 2 h ± 15 min after lunch were defined as 1‐h post‐lunch blood glucose (1 h‐PLBG) and 2‐h post‐lunch blood glucose (2 h‐PLBG), respectively. Furthermore, for comparison with postprandial glycemia, fasting blood glucose (FBG) levels were also analyzed.
All analyses were carried out using the statistical software Sas (version 9.4; SAS Institute, Cary, North Carolina, USA), and P‐values of <0.05 derived from two‐tailed tests were considered to be significant.
Results
Table 1 compares baseline clinical characteristics of the CVD cohort and the mortality cohort with those for all the 832 type 2 diabetes patients who first visited our clinic between 1995 and 1996, and visited at least four times. There was no significant difference between the CVD cohort and all the 832 participants, and between the mortality cohort and all the 832 participants.
Table 1.
Baseline characteristics of the patients
| All (n = 832) | CVD cohort (n = 618) | P‐value CVD cohort vs all | Mortality cohort (n = 646) | P‐value Mortality cohort vs all | |
|---|---|---|---|---|---|
| Men (%) | 684 (82.2) | 501 (81.2) | 0.63 | 524 (81.1) | 0.59 |
| Age (years) | 54.5 ± 9.9 | 54.4 ± 9.9 | 0.81 | 54.7 ± 10.0 | 0.68 |
| Duration of diabetes (years) | 5.5 ± 6.6 | 5.5 ± 6.6 | 0.85 | 5.7 ± 6.6 | 0.54 |
| BMI (kg/m2) | 23.6 ± 3.4 | 23.4 ± 3.3 | 0.35 | 23.4 ± 3.3 | 0.35 |
| HbA1c (%) | 8.0 ± 1.7 | 8.1 ± 1.7 | 0.20 | 8.1 ± 1.7 | 0.15 |
| HbA1c (mmol/mol) | 64 ± 18 | 65 ± 19 | 0.20 | 65 ± 19 | 0.15 |
| SBP (mmHg) | 132.8 ± 20.7 | 131.4 ± 19.2 | 0.19 | 131.8 ± 19.4 | 0.30 |
| DBP (mmHg) | 77.5 ± 12.5 | 76.8 ± 11.8 | 0.29 | 76.8 ± 11.8 | 0.30 |
| TC (mmol/L) | 5.46 ± 1.00 | 5.47 ± 0.98 | 0.80 | 5.48 ± 0.99 | 0.62 |
| HDL‐C (mmol/L) | 1.28 ± 0.34 | 1.28 ± 0.34 | 0.79 | 1.28 ± 0.34 | 0.93 |
| eGFR (mL/min/1.73 m2) | 80.6 ± 19.0 | 81.1 ± 19.4 | 0.62 | 80.9 ± 19.5 | 0.73 |
| Current smoker | 350 (42.1) | 265 (42.9) | 0.76 | 275 (42.6) | 0.85 |
| Alcohol intake | 627 (75.5) | 469 (75.9) | 0.85 | 485 (75.1) | 0.87 |
| Oral antidiabetic agents† | 333 (40.0) | 251 (40.6) | 0.82 | 267 (41.3) | 0.61 |
| Insulin‡ | 102 (12.3) | 84 (13.6) | 0.45 | 91 (14.1) | 0.30 |
| Antihypertensive agents | 174 (20.9) | 123 (19.9) | 0.64 | 140 (21.7) | 0.72 |
| Lipid‐lowering agents | 91 (10.9) | 61 (9.9) | 0.51 | 69 (10.7) | 0.88 |
Values are numbers (percentages) or means ± standard deviation. †Excludes patients treated with oral antidiabetic agents and insulin. ‡Includes patients treated with oral antidiabetic agents and insulin. BMI, body mass index; DBP, diastolic blood pressure; eGFR, estimated glomerular filtration rate; HbA1c, glycosylated hemoglobin; HDL‐C, high‐density lipoprotein cholesterol; SBP, systolic blood pressure; TC, total cholesterol.
In total, 56 (8.7%) patients died (15 women and 41 men), and 78 (12.6%) patients (11 women and 67 men) experienced CVD events during the follow‐up period. The median (interquartile range [IQR]) follow‐up period, mean 2h‐PBBG level and number of 2h‐PBBG measurements was 15.6 years (IQR 8.3–16.4 years), 9.93 mmol/L (IQR 8.38–11.88 mmol/L) and 6 (IQR 2–15) for the CVD cohort; and 15.9 years (IQR 10.9–16.6 years), 9.93 mmol/L (IQR 8.41–11.85 mmol/L) and 6 (2–16) for the mortality cohort; respectively. A correlation between mean 2h‐PBBG and mean HbA1c was observed in the CVD cohort (r = 0.489, P < 0.0001) and the mortality cohort (r = 0.490, P < 0.0001).
Table 2 summarizes the baseline clinical characteristics of the patients classified according to the CVD incidence and mortality. The patients who suffered a CVD event were older, had longer duration of diabetes, higher TC levels, lower HDL‐C levels, and were more likely to be using an oral antidiabetic agent, an antihypertensive agent and a lipid‐lowering agent compared with the patients without CVD events. After adjusting for age, the patients with a CVD event had longer duration of diabetes, higher TC levels, lower HDL‐C levels and showed a higher frequency of oral antidiabetic agent use than the patients without CVD events. The difference of the proportion of baseline oral antidiabetic agent users seems to depend on the duration of diabetes, because after adjusting for the duration of diabetes in addition to age, there was no longer a significant relationship. The decedents were older, had longer duration of diabetes, higher SBP, lower eGFR and were less likely to drink alcohol, and more likely to be using insulin and an antihypertensive agent compared with the survivors. After adjusting for age, the decedents had higher HbA1c level, and showed a significantly higher frequency of insulin use compared with the survivors. The difference of the proportion of baseline insulin users seems to depend on baseline HbA1c, because after adjusting for baseline HbA1c in addition to age, there was no significant difference anymore.
Table 2.
Baseline characteristics of the patients classified according to the cardiovascular disease incidence and mortality
| CVD cohort | Mortality cohort | |||||||
|---|---|---|---|---|---|---|---|---|
| No event | Event | P‐value | Adjusted§ P‐value | Survivors | Decedents | P‐value | Adjusted§ P‐value | |
| n | 540 | 78 | – | – | 590 | 56 | – | – |
| Men (%) | 435 (80.6) | 67 (85.9) | 0.26 | 0.086 | 483 (81.9) | 41 (73.2) | 0.11 | 0.74 |
| Age (years) | 54.0 ± 10.0 | 57.3 ± 8.8 | 0.005 | – | 53.8 ± 9.5 | 64.7 ± 9.2 | <0.0001 | – |
| Duration of diabetes (years) | 5.1 ± 6.3 | 8.6 ± 7.7 | <0.0001 | 0.0004 | 5.5 ± 6.3 | 7.5 ± 9.2 | 0.51 | 0.92 |
| BMI (kg/m2) | 23.4 ± 3.4 | 23.1 ± 2.8 | 0.46 | 0.73 | 23.4 ± 3.3 | 22.9 ± 3.2 | 0.24 | 0.68 |
| HbA1c (%) | 8.1 ± 1.7 | 8.3 ± 1.6 | 0.41 | 0.24 | 8.1 ± 1.7 | 8.5 ± 1.8 | 0.089 | 0.033 |
| HbA1c (mmol/mol) | 65 ± 19 | 67 ± 17 | 0.41 | 0.24 | 65 ± 19 | 69 ± 20 | 0.089 | 0.033 |
| SBP (mmHg) | 131.1 ± 19.4 | 133.6 ± 17.5 | 0.29 | 0.61 | 131.2 ± 19.1 | 137.9 ± 21.6 | 0.013 | 0.40 |
| DBP (mmHg) | 76.7 ± 11.9 | 77.1 ± 10.7 | 0.79 | 0.91 | 76.8 ± 11.8 | 76.8 ± 11.8 | 0.995 | 0.997 |
| TC (mmol/L) | 5.43 ± 0.96 | 5.75 ± 1.03 | 0.006 | 0.008 | 5.49 ± 0.99 | 5.38 ± 0.99 | 0.41 | 0.10 |
| HDL‐C (mmol/L) | 1.29 ± 0.35 | 1.19 ± 0.30 | 0.016 | 0.004 | 1.27 ± 0.33 | 1.32 ± 0.42 | 0.47 | 0.97 |
| eGFR (mL/min/1.73 m2) | 81.5 ± 19.6 | 78.2 ± 17.7 | 0.16 | 0.75 | 81.6 ± 19.5 | 74.3 ± 18.3 | 0.008 | 0.54 |
| Current smoker | 233 (43.2) | 32 (41.0) | 0.72 | 0.72 | 256 (43.4) | 19 (33.9) | 0.17 | 0.27 |
| Alcohol intake | 413 (76.5) | 56 (71.8) | 0.37 | 0.77 | 450 (76.3) | 35 (62.5) | 0.023 | 0.73 |
| Oral antidiabetic agents† | 210 (38.9) | 41 (52.6) | 0.022 | 0.038 | 242 (41.0) | 25 (44.6) | 0.60 | 0.93 |
| Insulin‡ | 73 (13.5) | 11 (14.1) | 0.89 | 0.82 | 76 (12.9) | 15 (26.8) | 0.004 | 0.009 |
| Antihypertensive agents | 99 (18.3) | 24 (30.8) | 0.010 | 0.066 | 118 (20.0) | 22 (39.3) | 0.0008 | 0.26 |
| Lipid‐lowering agents | 48 (8.9) | 13 (16.7) | 0.031 | 0.066 | 61 (10.3) | 8 (14.3) | 0.36 | 0.76 |
Values are numbers (percentages) or means ± standard deviation. †Excludes patients treated with oral antidiabetic agents and insulin. ‡Includes patients treated with oral antidiabetic agents and insulin. §Age adjusted P‐value. BMI, body mass index; CVD, cardiovascular disease; DBP, diastolic blood pressure; eGFR, estimated glomerular filtration rate; HbA1c, glycosylated hemoglobin; HDL‐C, high‐density lipoprotein cholesterol; SBP, systolic blood pressure; TC, total cholesterol.
Table 3 shows the HRs for the CVD incidence and all‐cause mortality associated with mean 2h‐PBBG levels calculated using multivariate Cox proportional hazard models. The covariates of model 1 and 2 were described in the Methods section. With respect to the CVD incidence, mean 2h‐PBBG and age were significant predictors in model 1, and mean 2h‐PBBG, age, duration of diabetes and mean TC/HDL‐C were significant predictors in model 2. With respect to all‐cause mortality, mean 2h‐PBBG, and age were significant predictors in model 1 and 2.
Table 3.
Multivariate Cox proportional hazard models for the incidence of cardiovascular disease and all‐cause mortality
| CVD incidence (event/patients 78/618) | All‐cause mortality (event/patients 56/646) | |||||||
|---|---|---|---|---|---|---|---|---|
| Model 1 | Model 2 | Model 1 | Model 2 | |||||
| HR (95% CI) | P‐value | HR (95% CI) | P‐value | HR (95% CI) | P‐value | HR (95% CI) | P‐value | |
| Mean 2h‐PBBG (1 mmol/L) | 1.13 (1.04–1.23) | 0.004 | 1.11 (1.02–1.21) | 0.013 | 1.14 (1.04–1.25) | 0.007 | 1.15 (1.05–1.26) | 0.003 |
| Mean HbA1c (%) | 0.99 (0.72–1.37) | 0.95 | 0.85 (0.61–1.18) | 0.33 | 0.97 (0.65–1.43) | 0.81 | 0.99 (0.65–1.51) | 0.98 |
| No. 2h‐PBBG measurements† | 0.84 (0.69–1.02) | 0.083 | 0.85 (0.70–1.03) | 0.088 | 0.80 (0.63–1.01) | 0.062 | 0.79 (0.63–1.01) | 0.055 |
| Age (10 years) | 1.74 (1.32–2.28) | <0.0001 | 1.42 (1.06–1.89) | 0.019 | 3.58 (2.67–4.80) | <0.0001 | 3.93 (2.79–5.54) | <0.0001 |
| Women/men | 0.61 (0.32–1.19) | 0.15 | 0.66 (0.33–1.33) | 0.25 | 0.94 (0.50–1.76) | 0.85 | 0.91 (0.46–1.79) | 0.78 |
| Duration of diabetes (5 years) | – | – | 1.32 (1.15–1.53) | <0.0001 | – | – | 0.94 (0.80–1.11) | 0.45 |
| Mean BMI (kg/m2) | – | – | 0.94 (0.85–1.03) | 0.18 | – | – | 0.97 (0.87–1.08) | 0.60 |
| Mean SBP (10 mmHg) | – | – | 1.15 (0.95–1.40) | 0.16 | – | – | 1.22 (0.97–1.55) | 0.091 |
| Mean TC/HDL‐C | – | – | 1.77 (1.41–2.22) | <0.0001 | – | – | 0.85 (0.64–1.15) | 0.29 |
| Smoking status | – | – | 0.73 (0.44–1.22) | 0.23 | – | – | 1.28 (0.67–2.46) | 0.46 |
†ln‐transformed. 2h‐PBBG, 2‐h post‐breakfast blood glucose; BMI, body mass index; CI, confidence interval; CVD, cardiovascular disease; HbA1c, glycosylated hemoglobin; HR, hazard ratio; SBP, systolic blood pressure; TC/HDL‐C, total cholesterol/high‐density lipoprotein cholesterol.
As age greatly affected the outcome, stratified analysis by age was carried out. In the patients aged 60 years or older, HR for the CVD incidence (events/patients: 25/157) associated with mean 2h‐PBBG (1‐mmol/L increment) was 1.26 (95% confidence interval [CI]: 1.08–1.47) after adjusting for mean HbA1c, number of 2h‐PBBG, and sex. The HR for all‐cause mortality (events/patients: 36/173) associated with mean 2h‐PBBG (1‐mmol/L increment) was 1.16 (95% CI: 1.02–1.32) after adjusting for the above covariates. Likewise, in the patients aged younger than 60 years, the HR associated with mean 2h‐PBBG (1‐mmol/L increment) was 1.09 (95% CI: 0.99–1.21) for the CVD incidence (events/patients: 53/461) and 1.17 (95% CI: 1.00–1.36) for all‐cause mortality (events/patients: 20/473) after adjusting for the aforementioned covariates.
The sensitivity analysis was also carried out. Because of the small sample size and the smaller number of events, 1 h‐PBBG was not able to be analyzed. By the use of 1–2h‐PBBG instead of 2h‐PBBG, similar results were obtained. In detail, HRs for the CVD incidence (events/patients: 82/653) associated with mean 1–2h‐PBBG (1‐mmol/L increment) were 1.17 (95% CI: 1.08–1.28) after adjusting for mean HbA1c, number of 1–2h‐PBBG measurements (ln‐transformed), age and sex, and 1.14 (95% CI: 1.04–1.25) after adjusting for mean HbA1c, number of 1–2 h‐PBBG measurements, age, sex, diabetes duration, mean BMI, mean SBP, mean TC/HDL and smoking status. Likewise, HRs for all‐cause mortality (events/patients: 57/679) associated with mean 1–2 h‐PBBG (1‐mmol/L increment) were 1.14 (95% CI: 1.04–1.26) and 1.15 (95% CI: 1.05–1.27) after adjusting for the aforementioned covariates, respectively. In contrast, neither 1 h‐PLBG nor 2 h‐PLBG predicted these adverse events (data not shown).
In addition, the effects of FBG at clinic visits on these adverse events were also analyzed for comparison with those of postprandial glycemia. HRs for the CVD incidence (events/patients: 84/676) associated with mean FBG (1‐mmol/L increment) were 1.26 (95% CI: 1.11–1.43) after adjusting for mean HbA1c, number of FBG measurements (ln‐transformed), age and sex, and 1.21 (95% CI: 1.05–1.39) after adjusting for mean HbA1c, number of FBG measurements, age, sex, diabetes duration, mean BMI, mean SBP, mean TC/HDL and smoking status. In contrast, HRs for all‐cause mortality (events/patients: 56/696) associated with mean FBG (1‐mmol/L increment) were 1.07 (95% CI: 0.87–1.31) and 1.03 (95% CI: 0.84–1.28) after adjusting for the aforementioned covariates, respectively. A correlation between mean FBG and mean HbA1c was observed in the CVD cohort (r = 0.61, P < 0.0001) and the mortality cohort (r = 0.60, P < 0.0001). From a perspective of the collinearity, when the mean HbA1c was deleted from these models, similar results were obtained. In detail, HRs for the CVD incidence were 1.26 (95% CI: 1.13–1.40) after adjusting for the number of FBG measurements, age and sex, and 1.17 (95% CI: 1.04–1.32) after adjusting for the number of FBG measurements, age, sex, diabetes duration, mean BMI, mean SBP, mean TC/HDL and smoking status. HRs for all‐cause mortality were 1.08 (95% CI: 0.91–1.27) and 1.03 (95% CI: 0.85–1.25) after adjusting for the aforementioned covariates, respectively.
Discussion
The present study showed that the mean 2h‐PBBG at clinic visits was a predictor of the CVD incidence and all‐cause mortality in type 2 diabetes patients independently of the mean HbA1c level: a 1‐mmol/L increase in the mean 2h‐PBBG resulted in a 11% increase in the risk of the CVD incidence, and also led to a 15% increase in the risk of all‐cause mortality. In addition, the association between 2h‐PBBG and the CVD incidence seemed to be stronger in the patients aged 60 years or older than that in the patients younger than 60 years.
Support for these findings is provided by data of the post‐hoc subgroup analysis of the Hyperglycemia and Its Effect After Acute Myocardial Infarction on Cardiovascular Outcomes in Patients With Type 2 Diabetes Mellitus (HEART2D) trial15, the Diabetes Intervention Study (DIS)28 and the San Luigi Gonzaga Diabetes Study29, 30. Those studies examined postprandial blood glucose, but not postload or postchallenge blood glucose in type 2 diabetes patients. However, the HEART2D post‐hoc subgroup analysis included only older (aged >65.7 years) acute myocardial infarction survivors, and used self‐monitored four‐ and seven‐point blood glucose profiles. The DIS did not evaluate HbA1c. The San Luigi Gonzaga Diabetes Study used 2‐h postprandial blood glucose measured by both clinic monitoring and home self‐monitoring, and showed that blood glucose 2 h after lunch predicts cardiovascular events and all‐cause mortality. In their study population in Italy, the blood glucose 2 h after lunch was more representative of the postprandial state than blood glucose 2 h after breakfast. Thus, the postprandial state seems to be affected by ethnic differences in dietary habits. To our knowledge, this is the only study to show the association between post‐breakfast hyperglycemia at clinic visits and the CVD incidence and all‐cause mortality in type 2 diabetes patients in a real‐life condition.
We previously reported that HbA1c variability was a predictor of CVD incidence and all‐cause mortality in type 2 diabetes patients independent of mean HbA1c level31, 32. Thus, these adverse events might be influenced by both short‐term glucose excursions and long‐term glycemic variability in type 2 diabetes patients.
The systematic review of the few observational studies in diabetes patients showed that postprandial hyperglycemia was associated with increased all‐cause and cardiovascular mortality, incidence of CVD, and progression of diabetic retinopathy33. However, it also noted that further studies with adequate sample size, long‐term follow up and reliable methods for assessment of postprandial glycemia are strongly required. We also carried out the sensitivity analysis in several ways for the assessment of postprandial glycemia. By the use of 1–2h‐PBBG, the similar results were obtained. Thus, the sensitivity analysis certified the current results. However, neither 1 h‐PLBG nor 2 h‐PLBG predicted these adverse events. These discordant results between PBBG and PLBG might be due to the differences in the length of fasting time, and partly in variation of dietary intake.
The effects of FBG at clinic visits on these adverse events were also analyzed. Consequently, the mean FBG was a significant predictor of the incidence of CVD, but not all‐cause mortality. It seems that both fasting and postprandial hyperglycemia contribute to the incidence of CVD, whereas, only postprandial hyperglycemia might affect all‐cause mortality in patients with type 2 diabetes.
The strengths of the present study include the use of postprandial glycemia in a real‐world setting and a long‐term follow‐up period. In contrast, the present study had a number of limitations. First, it had a retrospective observational cohort design; therefore, it was not possible to draw any direct causal links from our findings. Differences in the examination techniques, self‐reported postprandial time intervals and the number of 2h‐PBBG measurements carried out were all potential sources of bias. However, linear regression equations, which were based on data obtained from duplicated assays, were used to standardize the data acquired with the various measurement techniques. The number of 2h‐PBBG measurements was subjected to ln‐transformation, and was then included as a covariate in the model. Furthermore, the times when patients began to eat their meals were confirmed carefully by a medical technologist, and the postprandial time interval was calculated. The sensitivity analysis reinforced the current results. Second, the relatively low follow‐up rate for survival might lead to a bias. However, the significant difference in baseline characteristics between patients who completed and did not complete follow up was only found in the proportion of patients who were current smokers. The use of Cox regression analysis was considered to be statistically appropriate. Third, there were a small number of events including the events based on the responses of the questionnaire. With respect to CVD incidence, 65 (83.3%) events were determined according to a thorough review of medical records, and just 13 (16.7%) events were confirmed from the responses of the questionnaire. Further studies with larger sample size and a higher follow‐up rate are required to prove the present findings. Fourth, 2h‐PBBG was measured after an ordinary breakfast, without standardization of dietary intake. However, the aim of the present study was to evaluate the impact of postprandial hyperglycemia at clinic visits on adverse outcomes in real‐life conditions, and hence standardization of dietary intake was not considered. Fifth, we could not carry out an analysis of the patients' triglyceride levels, because their lipid levels were measured regardless of their fasting or postprandial status. However, TC/HDL‐C was used as a covariate in the analysis, as it is the best lipid‐based predictor of CVD in men with type 2 diabetes25, 26. Finally, all of the participants were treated at the same Japanese hospital. Thus, it will be necessary to carry out international multicenter trials in order to determine whether the present findings are generalizable to other patient groups.
In conclusion, postprandial hyperglycemia represented by the mean level of 2‐h post‐breakfast blood glucose at clinic visits is associated with the CVD incidence and all‐cause mortality independently of the mean HbA1c level in type 2 diabetes patients in a real‐world setting. Prospective interventional trials are warranted to confirm our findings.
Disclosure
The authors declare no conflict of interest.
Acknowledgments
The authors sincerely thank Kumiko Kimura, the Institute for Adult Diseases, Asahi Life Foundation, Tokyo, Japan, for her assistance with the collection of research data, and Nobumi Suzuki, the Institute for Adult Diseases, Asahi Life Foundation, Tokyo, Japan, for his technical assistance with revision of this paper.
J Diabetes Investig 2017; 8: 600–608
References
- 1. de Vegt F, Dekker JM, Ruhé HG, et al Hyperglycaemia is associated with all‐cause and cardiovascular mortality in the Hoorn population: the Hoorn Study. Diabetologia 1999; 42: 926–931. [DOI] [PubMed] [Google Scholar]
- 2. Donahue RP, Abbott RD, Reed DM, et al Postchallenge glucose concentration and coronary heart disease in men of Japanese ancestry: Honolulu Heart Program. Diabetes 1987; 36: 689–692. [DOI] [PubMed] [Google Scholar]
- 3. Lowe LP, Liu K, Greenland P, et al Diabetes, asymptomatic hyperglycemia, and 22‐year mortality in black and white men: the Chicago Heart Association Detection Project in Industry study. Diabetes Care 1997; 20: 163–169. [DOI] [PubMed] [Google Scholar]
- 4. The DECODE Study Group, the European Diabetes Epidemiology Group . Glucose tolerance and cardiovascular mortality: comparison of fasting and 2‐hour diagnostic criteria. Arch Intern Med 2001; 161: 397–405. [DOI] [PubMed] [Google Scholar]
- 5. Nakagami T, the DECODA Study Group . Hyperglycaemia and mortality from all causes and from cardiovascular disease in five populations of Asian origin. Diabetologia 2004; 47: 385–394. [DOI] [PubMed] [Google Scholar]
- 6. Meigs JB, Nathan DM, D'Agostino RB Sr, et al Fasting and postchallenge glycemia and cardiovascular disease risk: the Framingham Offspring Study. Diabetes Care 2002; 25: 1845–1850. [DOI] [PubMed] [Google Scholar]
- 7. Coutinho M, Gerstein HC, Wang Y, et al The relationship between glucose and incident cardiovascular events: a metaregression analysis of published data from 20 studies of 95,783 individuals followed for 12.4 years. Diabetes Care 1999; 22: 233–240. [DOI] [PubMed] [Google Scholar]
- 8. Balkau B, Shipley M, Jarrett RJ, et al High blood glucose concentration is a risk factor for mortality in middle‐aged nondiabetic men: 20‐year follow‐up in the Whitehall Study, the Paris Prospective Study, and the Helsinki Policemen Study. Diabetes Care 1998; 21: 360–367. [DOI] [PubMed] [Google Scholar]
- 9. Levitan EB, Song Y, Ford ES, et al Is nondiabetic hyperglycemia a risk factor for cardiovascular disease? A meta‐analysis of prospective studies. Arch Intern Med 2004; 164: 2147–2155. [DOI] [PubMed] [Google Scholar]
- 10. Bonora E, Muggeo M. Postprandial blood glucose as a risk factor for cardiovascular disease in type 2 diabetes: the epidemiological evidence. Diabetologia 2001; 44: 2107–2114. [DOI] [PubMed] [Google Scholar]
- 11. Heine RJ, Dekker JM. Beyond postprandial hyperglycaemia: metabolic factors associated with cardiovascular disease. Diabetologia 2002; 45: 461–475. [DOI] [PubMed] [Google Scholar]
- 12. Ceriello A. Postprandial hyperglycemia and diabetes complications: is it time to treat? Diabetes 2005; 54: 1–7. [DOI] [PubMed] [Google Scholar]
- 13. Ceriello A, Hanefeld M, Leiter L, et al Postprandial glucose regulation and diabetic complications. Arch Intern Med 2004; 164: 2090–2095. [DOI] [PubMed] [Google Scholar]
- 14. Raz I, Wilson PW, Strojek K, et al Effects of prandial versus fasting glycemia on cardiovascular outcomes in type 2 diabetes: the HEART2D trial. Diabetes Care 2009; 32: 381–386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Raz I, Ceriello A, Wilson PW, et al Post hoc subgroup analysis of the HEART2D trial demonstrates lower cardiovascular risk in older patients targeting postprandial versus fasting/premeal glycemia. Diabetes Care 2011; 34: 1511–1513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Hotta N, Nakamura J, Iwamoto Y, et al Causes of death in Japanese diabetics based on the results of a survey of 18,385 diabetics during 1991–2000: report of committee on cause of death in diabetes mellitus. J Japan Diab Soc 2007; 50: 47–61 (Japanese). [Google Scholar]
- 17. Nakamura J, Kamiya H, Haneda M, et al Causes of Death in Japanese Patients with Diabetes Based on the Results of a Survey of 45,708 Cases during 2001–2010 ‐Report from the Committee on the Cause of Death in Diabetes Mellitus‐. J Japan Diab Soc 2016; 59: 667–684 (Japanese). [Google Scholar]
- 18. Monnier L, Mas E, Ginet C, et al Activation of oxidative stress by acute glucose fluctuations compared with sustained chronic hyperglycemia in patients with type 2 diabetes. JAMA 2006; 295: 1681–1687. [DOI] [PubMed] [Google Scholar]
- 19. Sala LL, Cattaneo M, De Nigris V, et al Oscillating glucose induces microRNA‐185 and impairs an efficient antioxidant response in human endothelial cells. Cardiovasc Diabetol 2016; 15: 71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Akatsuka S, Toyokuni S. Genome‐wide assessment of oxidatively generated DNA damage. Free Radic Res 2012; 46: 523–530. [DOI] [PubMed] [Google Scholar]
- 21. Bondia‐Pons I, Ryan L, Martinez JA. Oxidative stress and inflammation interactions in human obesity. J Physiol Biochem 2012; 68: 701–711. [DOI] [PubMed] [Google Scholar]
- 22. Peter R, Dunseath G, Luzio SD, et al Daytime variability of postprandial glucose tolerance and pancreatic B‐cell function using 12‐h profiles in persons with type 2 diabetes. Diabet Med 2010; 27: 266–273. [DOI] [PubMed] [Google Scholar]
- 23. Monnier L, Colette C, Owens DR. Integrating glycemic variability in the glycaemic disorders of type 2 diabetes: a move towards a unified glucose tetrad concept. Diabetes Metab Res Rev 2009; 25: 393–402. [DOI] [PubMed] [Google Scholar]
- 24. Kashiwagi A, Kasuga M, Araki E, et al International clinical harmonization of glycated hemoglobin in Japan: from Japan Diabetes Society to National Glycohemoglobin Standardization Program values. J Diabetes Investig 2012; 3: 39–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Sone H, Tanaka S, Tanaka S, et al Comparison of various lipid variables as predictors of coronary heart disease in Japanese men and women with type 2 diabetes: subanalysis of the Japan Diabetes Complications Study. Diabetes Care 2012; 35: 1150–1157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Jiang R, Schulze MB, Li T, et al Non‐HDL cholesterol and apolipoprotein B predict cardiovascular disease events among men with type 2 diabetes. Diabetes Care 2004; 27: 1991–1997. [DOI] [PubMed] [Google Scholar]
- 27. Matsuo S, Imai E, Horio M, et al Revised equations for estimated GFR from serum creatinine in Japan. Am J Kidney Dis 2009; 53: 982–992. [DOI] [PubMed] [Google Scholar]
- 28. Hanefeld M, Fischer S, Julius U, et al Risk factors for myocardial infarction and death in newly detected NIDDM: the Diabetes Intervention Study, 11‐year follow‐up. Diabetologia 1996; 39: 1577–1583. [DOI] [PubMed] [Google Scholar]
- 29. Cavalot F, Petrelli A, Traversa M, et al Postprandial blood glucose is a stronger predictor of cardiovascular events than fasting blood glucose in type 2 diabetes mellitus, particularly in women: lessons from the San Luigi Gonzaga Diabetes Study. J Clin Endocrinol Metab 2006; 91: 813–819. [DOI] [PubMed] [Google Scholar]
- 30. Cavalot F, Pagliarino A, Valle M, et al Postprandial blood glucose predicts cardiovascular events and all‐cause mortality in type 2 diabetes in a 14‐year follow‐up: lessons from the San Luigi Gonzaga Diabetes Study. Diabetes Care 2011; 34: 2237–2243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Takao T, Matsuyama Y, Yanagisawa H, et al Association between HbA1c variability and mortality in patients with type 2 diabetes. J Diabetes Complications 2014; 28: 494–499. [DOI] [PubMed] [Google Scholar]
- 32. Takao T, Matsuyama Y, Suka M, et al The combined effect of visit‐to‐visit variability in HbA1c and systolic blood pressure on the incidence of cardiovascular events in patients with type 2 diabetes. BMJ Open Diabetes Res Care 2015; 3: e000129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Mannucci E, Monami M, Lamanna C, et al Post‐prandial glucose and diabetic complications: systematic review of observational studies. Acta Diabetol 2012; 49: 307–314. [DOI] [PubMed] [Google Scholar]


