Abstract
Aims/Introduction
To evaluate the glycometabolism and outcomes of gestational diabetes mellitus (GDM) patients according to the International Association of Diabetes and Pregnancy Study Group (IADPSG) criteria in China.
Materials and Methods
According to the results of a 75‐g oral glucose tolerance test, 1,683 pregnant women were divided into three groups: (i) an increment GDM group (patients meet the IADPSG criteria, but not the previous Chinese criteria); (ii) a stock GDM group (patients meet both criteria); and (iii) a normal glucose tolerance group. Their glycometabolism outcomes, prepregnancy and postpartum body mass index were compared, as were maternal–fetal outcomes.
Results
The IADPSG and previous Chinese criteria diagnosed 12.4% and 5.5% of women with GDM. Pairwise comparison showed significant differences in 1‐h plasma glucose, 2‐h plasma glucose, HbA1c values and area under curve of glucose among all groups (P < 0.01). The fasting plasma glucose and postpartum body mass index of the stock group were significantly higher than those of the other two groups (P < 0.01). The incidences of hypertensive disorder complicating pregnancy and cesarean section of the normal glucose tolerance group were significantly lower than those of the other two groups (P < 0.001). No significant differences in patient age, prepregnancy body mass index, duration of pregnancy, prevalence of premature labor, premature rupture of membranes, neonatal jaundice, neonatal asphyxia or Ponderal Index were observed, but significant differences in macrosomia and neonatal hypoglycemia were observed (P < 0.05).
Conclusions
The IADPSG criteria doubled the number of GDM patients. The cases of the increment patients were mild. The IADPSG criteria should be discussed fully before implementation in China.
Keywords: Diabetes, Heterogeneity, Pregnancy
Introduction
Gestational diabetes mellitus (GDM) is defined as ‘carbohydrate intolerance resulting in hyperglycemia of variable severity with onset or first recognition during pregnancy’1. GDM is caused by insufficient pancreatic β‐cell function that cannot meet the body's insulin needs, and results in increasing insulin resistance during pregnancy. Many different diagnostic tests2 are being used worldwide, which has caused much clinical confusion3. The use of different criteria makes it impossible to compare the prevalence and treatment results of GDM across various locations. A set of local Chinese criteria was adopted in China in 20074. The International Association of Diabetes and Pregnancy Study Group (IADPSG) recommended new diagnostic criteria in 20105, stating that a pregnant woman is diagnosed with GDM if her values on a 75‐g oral glucose tolerance test (OGTT) exceed a fasting plasma glucose (FPG) of 5.1 mmol/L (91.8 mg/dL), a 1‐h plasma glucose (PG) of 10.0 mmol/L (180 mg/dL) or a 2‐h PG of 8.5 mmol/L (153 mg/dL). The American Diabetes Association (ADA) has endorsed the IADPSG recommendations6 for GDM and continually backed it from 2012 to 20137, 8, whereas the American College of Obstetricians and Gynecologists has not9. The Obstetrics and Gynecology Subcommittee of the Chinese Medical Association recommended a modified version of the criteria in 201410. However, the new PG threshold values are lower than before, which means that the prevalence of GDM will increase significantly11. Meanwhile, a Canadian study found that women who were classified as having diabetes by the IADPSG criteria, but were not considered to have gestational diabetes according to the Canadian Diabetes Association Criteria, showed no differences in pregnancy outcomes from women without gestational diabetes12. China was not included among the observed locations in the global prospective multicenter study carried out by the IADPSG5. We have summarized the data of pregnant women in Shanghai Seventh People's Hospital, Shanghai, China, in recent years based on the new criteria, and discuss their glucose metabolism and pregnancy outcomes by comparison with the previous Chinese criteria in this study.
Methods
Patients
The present retrospective observational study was carried out from 1 January 2009 to 31 December 2011 in Shanghai Seventh People's Hospital (Shanghai, China), and received approval from the research ethics committee of Shanghai Seventh People's Hospital. Participants provided written informed consent. All pregnant women, with a mean age of 28.6 years (range 19–36 years), were included in the present study. All of the women were pregnant with a singleton pregnancy at the first pregnancy and were in good physical health, without any serious acute or chronic diseases, particularly obesity, hypertension or other metabolic disorders. Babies who weigh ≥4,000 g at birth are considered fetal macrosomia in China.
Test method
Each pregnant woman was subjected to a 75‐g OGTT between 24 and 28 weeks of gestation. An acceptable daily carbohydrate intake (≥150 g) was ensured the day before the test, and each patient fasted for 10–12 h before testing. All participants avoided strenuous movements, and finished the sugar water in 3–5 min. The timing started when they finished the sugar water. We tested their FPG, 1‐ and 2‐h PG levels. Each patient's HbA1c was tested using an AEROSET 2000 clinical analyzer (Sysmex GT, Kobe, Japan) on the day before the OGTT test.
Method of grouping
According to the IADPSG criteria, the 1,683 pregnant women were classified as normal glucose tolerance (NGT) and GDM. Patients with GDM were subdivided into the increment and stock groups (to express conveniently and vividly the difference in these groups, the term ‘increment’ is used to represent the patients who are classified as GDM by the IADPSG criteria, but who are considered non‐diabetic according to the previous Chinese criteria, and the term ‘stock’ represents the patients who are diagnosed as GDM by both criteria). The OGTT thresholds of the former Chinese criteria were as follows: FPG ≥5.6 mmol/L (101 mg/dL), 1‐h PG ≥10.3 mmol/L (185 mg/dL), 2‐h PG ≥8.6 mmol/L (155 mg/dL) and 3‐h PG ≥6.7 mmol/L (121 mg/dL). GDM was defined by two abnormal values on the OGTT. These 75‐g OGTT tests were carried out between 24 and 28 weeks of pregnancy.
Statistical analysis
The data of all pregnant women who met the inclusion criteria were collected retrospectively without requiring their doctors’ permission. We recorded their FPG, their 1‐h PG and 2‐h PG data from the OGTT, as well as their HbA1c levels, and we tracked their pregnancy outcomes.
The area under the curve of glucose (AUCG) was calculated using the trapezoidal approximation method9, 10 of the PG levels measured every hour as follows: AUCG = ½ (FPG + 2‐h PG) + 1‐h PG. In order to describe the physical status and nutrients intakes of children (aged 0–18 years) accurately, the Ponderal Index recommended by the World Health Organization is shown here, as is body mass index (BMI) to adults. The Ponderal Index was calculated as follows: PI = weight (kg)/length3 (m3). BMI was calculated as follows: BMI = weight (kg)/length2 (m2). Prepregnancy BMI was calculated based on the patients’ information. Postpartum BMI was measured when the patients were discharged from hospital.
All statistical analyses were carried out using Spss 17.0 (SPSS, Chicago, Illinois, USA). Continuous variables are expressed as mean ± standard deviation, whereas categorical variables are expressed as numbers and percentages. The χ2‐test, Fisher's exact probability test and the Student–Newman–Keuls test were used when appropriate. The results were considered statistically significant at P‐values <0.05. All figures were drawn by Excel 2010 (Microsoft, Redmond, Washington, USA).
Results
For the present study, a total of 1,683 pregnant women were included between 1 January 2009 and 31 December 2011. Among them, 208 women (12.4%) were classified as GDM according to the IADPSG criteria; however, just 92 were diagnosed as GDM according to the former Chinese criteria (P < 0.001; Figure 1). The study population was divided into three groups, with 116 in the increment GDM group (mean 28.03 ± 8.93 years), 92 in the stock GDM group (mean 29.12 ± 7.15 years) and 1,475 in the NGT group (mean 28.71 ± 7.21 years). There was no significant difference in age among all groups (P = 0.5314; Table 1). No significant difference was found in prepregnancy BMI among all groups. Two patients were treated with insulin in the increment group, and 21 were treated with insulin in the stock group.
Figure 1.
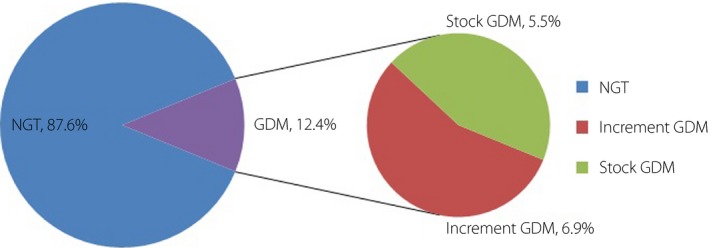
The gestational diabetes mellitus (GDM) incidence was 12.4%, and 6.9% were grouped into the increment GDM group and 5.5% in the stock GDM group. Because of the bias of pregnant women collected in the present study, the incidence of GDM was not accurate. However, the proportion of the women had a certain significance. The number of GDM has more than doubled. NGT, normal glucose tolerance.
Table 1.
Glucose metabolism results of the three study groups
| Group | NGT | Increment | Stock | P‐value |
|---|---|---|---|---|
| Cases | 1,475 | 116 | 92 | – |
| Age (years) | 28.71 ± 7.21 | 28.03 ± 8.93 | 29.12 ± 7.15 | 0.531 |
| FPG (mmol/L) | 4.67 ± 0.82a | 4.81 ± 0.74b | 5.23 ± 0.86ab | <0.001 |
| 1‐h PG (mmol/L) | 8.43 ± 1.43 | 9.82 ± 1.17 | 11.06 ± 1.36 | <0.001 |
| 2‐h PG (mmol/L) | 7.12 ± 1.58 | 8.37 ± 1.45 | 9.87 ± 1.52 | <0.001 |
| HbA1c (mmol/mol) | 34.6 ± 11.5 | 41.8 ± 15.2 | 49.3 ± 14.9 | <0.001 |
| AUCG (mmol/L × h) | 12.52 ± 6.81 | 17.31 ± 6.58 | 22.72 ± 7.37 | <0.001 |
| Prepregnancy BMI (kg/m2) | 20.6 ± 5.1 | 20.7 ± 4.4 | 21.1 ± 6.3 | 0.656 |
| Postpartum BMI (kg/m2) | 21.6 ± 3.2c | 22.3 ± 3.4d | 24.6 ± 4.3cd | <0.001 |
| Treatment methods | ||||
| Insulin | – | 2 | 21 | – |
| Non‐insulin | – | 114 | 71 | – |
P < 0.01 in fasting plasma glucose (FPG) and postpartum body mass index (BMI), P < 0.05 among all groups in 1‐h plasma glucose (PG), 2‐h PG, hemoglobin A1c (HbA1c) and area under the curve of glucose (AUCG). a,b,c,dThe differences between the values labeled with the same letter were observed through pairwise comparison. NGT, normal glucose tolerance.
Glucose metabolism
The glucose metabolism of all pregnant women was evaluated according to the outcome of an OGTT and HbA1c levels. The FPG, 1‐h PG, 2‐h PG, HbA1c and AUCG values decreased in a stepwise fashion among the groups (stock > increment > NGT). Pairwise comparison showed that the FPG and BMI of the stock group were significantly higher than those of the other groups (P < 0.01), but there were no significant differences between the increment and NGT groups (P > 0.05). Pairwise comparison also showed significant differences in 1‐h PG, 2‐h PG, and HbA1c values among all groups (P < 0.001; Table 1).
Pregnancy outcomes
The rate of hypertensive disorder complicating pregnancy (HDCP) and cesarean section increased progressively from one group to the next (NGT > increment > stock). Pairwise comparison showed no significant difference between the stock and increment groups (P > 0.05); however, a significant difference between the NGT and the other two groups was observed (P < 0.05). There was no significant difference in the rate of premature rupture of membranes (P = 0.0970), premature labor (P = 0.2213), neonatal jaundice (P = 0.1463) or neonatal asphyxia (P = 0.1515), and there was no significant difference in pregnancy duration (P = 0.1033) or neonatal Ponderal Index (P = 0.1118) among the three groups; however, a significant difference in the rate of macrosomia (NGT, 3.5% vs increment, 16.4% vs stock, 29.3%) and neonatal hypoglycemia (NGT, 0.7% vs increment, 12.1% vs stock, 26.1%) was observed (P < 0.05; Table 2).
Table 2.
Maternal–fetal outcomes in the three study groups
| Group | NGT | Increment | Stock | P‐value |
|---|---|---|---|---|
| Cases | 1475 | 116 | 92 | – |
| HDCP (%) | 1.4ab (n = 21) | 12.9a (n = 15) | 17.4b (n = 16) | <0.001 |
| PROM (%) | 5.9 (n = 87) | 8.6 (n = 10) | 10.9 (n = 10) | 0.097 |
| Cesarean section (%) | 66.9 cd (n = 987) | 87.1c (n = 101) | 93.5d (n = 86) | <0.001 |
| Premature labor (%) | 10.5 (n = 155) | 11.2 (n = 13) | 16.3 (n = 15) | 0.221 |
| Duration of pregnancy (weeks) | 38.18 ± 0.17 | 38.21 ± 0.14 | 38.20 ± 0.11 | 0.103 |
| Macrosomia (%) | 3.5 (n = 52) | 16.4 (n = 19) | 29.3 (n = 27) | <0.001 |
| Neonatal hypoglycemia (%) | 0.7 (n = 10) | 12.1 (n = 14) | 26.1 (n = 24) | <0.001 |
| Neonatal jaundice (%) | 2.8 (n = 41) | 5.2 (n = 6) | 5.4 (n = 5) | 0.146 |
| Neonatal asphyxia (%) | 4.5 (n = 66) | 6.0 (n = 7) | 8.7 (n = 8) | 0.152 |
| Ponderal Index (kg/m3) | 26.1 ± 3.4 | 25.7 ± 5.8 | 26.8 ± 6.1 | 0.112 |
P < 0.01 in hypertensive disorder complicating pregnancy (HDCP) and cesarean section, P < 0.05 among all groups in macrosomia, neonatal hypoglycemia. a,b,c,dThe differences between the values labeled with the same letter were observed through pairwise comparison. NGT, normal glucose tolerance; PROM, premature rupture of membranes.
Discussion
GDM is defined as diabetes diagnosed during pregnancy that is not clearly overt diabetes8. GDM is associated with adverse pregnancy outcomes, including fetal macrosomia, premature labor, dystocia and related neonatal disturbances, such as hyperinsulinemia, hypoglycemia and hyperbilirubinemia13. The ADA had adopted the IADPSG criteria in 2011, and continually backed it in 2012 and 2013, but this has since changed. Recently, two test methods and diagnostic criteria were recommended by the ADA and the US National Institutes of Health (NIH)14, 15. The NIH harbored the idea that the incidence of GDM would increase rapidly according to the IADPSG criteria, and that the corresponding diagnoses might be considered overdiagnoses, leading to a waste of medical resources and heavy burdens on patients. Furthermore, the NIH advocated that the criteria for GDM should be established on the basis of a full assessment15.
With the easing of GDM diagnostic criteria by the IADPSG, more patients will be diagnosed with GDM. In the present study, the number of GDM patients more than doubled. Similar findings have been reported, with an increase of 200% in GDM patients in China16, 17, although differences in GDM prevalence do exist in different regions in China17, 18. The incidence of GDM in the study by Liao's et al.17 is the highest (36.2% by the IADPSG criteria), and we speculate that one possible reason for this high incidence could be that the population in Sichuan province prefers a diet higher in fat, sugar and salt than populations in other regions of China. However, this speculation requires more empirical evidence to be confirmed. The incidence of GDM in the present study (12.4% by the IADPSG criteria) is closer to that of Shang and Lin16 (19.9% by the IADPSG criteria), but lower; the lower incidence in our study could be attributable to our stricter entry criterion that our participants were pregnant with a singleton pregnancy during their first pregnancy.
The present study found that the FPG and postpartum BMI in the stock group were significantly higher than in the other two groups, but there were no significant differences between the NGT and increment groups. The FPG of the increment group fell in between the other two groups, suggesting that in the case of fasting, the patients in the stock group have the most severe glucose metabolism disorder, but the increment and NGT groups have similar severities of glucose metabolism disorder. Measuring FPG is very important and useful3, 19, and can greatly simplify the diagnostic algorithm20, 21, but some studies argue against relying on a one‐time FPG result to diagnose GDM17, 22, and that postprandial PG is a better predictor of glycemic control23 and cardiovascular events24, 25 than FPG. Normal PG levels could be maintained in the increment group when they were fasting, but the balance was broken after a meal. Significant differences existed in the 1‐, 2‐h PG and AUCG among all groups. Our materials also showed that the values of HbA1c were significantly different (NGT, 34.6 ± 11.5 vs increment, 41.8 ± 15.2 vs stock, 49.3 ± 14.9; P < 0.001). HbA1c levels increase substantially when glucose metabolism disorders are exacerbated. The ADA showed that HbA1c is the gold standard to monitor diabetes26. Because FPG and postprandial PG reflect only glucose levels at a specific time, they are susceptible to variability caused by eating‐ and glucose metabolism‐related factors. Furthermore, HbA1c values stably and reliably reflect average PG values over 120 days. There is little interference by test timing, fasting, insulin use and other related factors. Therefore, the ADA recommended that HbA1c ≥6.5% (48 mmol/mol) was one diagnostic criterion for diabetes in non‐pregnant individuals in 201027. The World Health Organization concluded that an HbA1c of 48 mmol/mol can be used as a diagnostic cut‐off for diabetes28. Glycated albumin might be a useful marker for monitoring during treatment of patients with diabetes29. Therefore, even if it is not a diagnostic reference index, HbA1c is still recommended as one of the important indicators for monitoring GDM10.
The aforementioned conclusions showed that the glycometabolism of GDM patients was not identical. The degree of glucose metabolic disorder in the stock women was the most serious, and they were not able to naturally maintain blood glucose homeostasis, even in the case of fasting. The degree of glucose metabolic disorder in the increment women was milder than in the stock women, but their ability to control postprandial blood glucose was notably impaired.
The maternal–fetal outcomes of GDM patients were also analyzed. The present study's findings showed no significant difference in cesarean delivery rates between the increment and stock groups, but both were significantly higher than that in the NGT group. Although cesarean rates are related to social, political and personal factors17, the risk of maternal–fetal poor prognosis induced by glucose metabolic disturbance also affects women's behavior regarding their choice to deliver by cesarean section. In fact, China has one of the highest cesarean rates in the world, with most cesareans carried out without indication30. The present study's findings support this view, with the cesarean rates in the NGT group reaching 65%. Patients and families in China usually perceive cesarean delivery as safer30, so women with GDM and their families prefer to deliver by cesarean section, although their conditions are not serious and are considered impaired glucose tolerance rather than full‐blown GDM. The NIH has also considered the unintended consequences of the more stringent diagnostic criteria for GDM recommended by the IADPSG criteria, such as increases in cesarean deliveries and more intensive newborn assessments15. Meanwhile, because of the use of selective cesarean section for delivery in all groups, there was no significant difference in the prevalence of premature rupture of membranes among all groups. There was also no significant difference in the prevalence of HDCP between the increment and stock groups, but both were clearly higher than that in the NGT group. This trend suggests that there are common risk factors for HDCP and GDM; HDCP is closely related to GDM, and the rate of HDCP in women with GDM is significantly higher than that in NGT pregnant women.
The offspring of mothers with GDM are at higher risk for adverse outcomes than those without GDM, including macrosomia, premature labor, stillbirth and other GDM‐related complications, including hyperinsulinemia, hypoglycemia and hyperbilirubinemia13. The present study showed no significant difference in pregnancy duration, neonatal Ponderal Index, or the prevalence of premature labor, neonatal jaundice or neonatal asphyxia. Although glucose metabolism varied among all groups, pregnancy outcomes were partly similar, suggesting that patients with abnormal glucose tolerance had received effective clinical interventions. However, the treatments for GDM women were different. This difference in treatment indicates that the severity of the disease in the two groups was substantially different. Nevertheless, there were significant differences in macrosomia and neonatal hypoglycemia between the increment and stock groups, likely as a result of fetal hyperinsulinemia and significant differences in maternal serum insulin levels. To a certain extent, these outcomes reflect the substantial differences in maternal glucose metabolism among all groups. Meanwhile, it should be stressed that the disorder of maternal glucose metabolism and adverse effects on the fetus in the increment group do exist, because of the higher incidence of macrosomia and neonatal hypoglycemia in the increment group compared with the NGT group. The cases are mild, but proper treatment and monitoring is necessary.
Using the IADPSG criteria, the number of patients diagnosed as GDM increased markedly. The present study shows that the number of GDM diagnoses doubled by the IADPSG criteria, but the degree of glucose metabolic disorder in GDM patients is different. All women in the increment group were considered to have gestational impaired glucose tolerance by the former criteria, which also possesses heterogeneity31. So, it is more indispensable for us to practice hierarchical management for women with GDM diagnosed by the IADPSG criteria. In fact, the treatment for the increment women that we used involved diet and exercise therapy, and insulin only as appropriate; this is clearly a ‘lighter’ treatment than that used for those in the stock group. Under different treatment regimens, their maternal–fetal outcomes were partly similar, which proved that the treatment used was effective. In reality, many pregnant women and their families are very nervous when they are diagnosed with GDM, although they are only classified as gestational impaired glucose tolerance by the former criteria, which is relatively mild. The argument as to whether the new IADPSG criteria are reasonable and huge fruits can be reaped will still exist17, 32, 33, but it is generally universally agreed that obstetricians must pour attention into each patient's individual condition, and provide differentiated treatment based on the actual condition.
The present study had some limitations, including its retrospective observational design, potential group bias and lack of a thorough description of the treatment for GDM patients. We take the attitude that it is still open to question as to whether the so‐called GDM patients should be diagnosed as GDM according to the IADPSG criteria. More prospective and randomized multicenter studies need to be carried out in the future.
Disclosure
The authors declare no conflict of interest.
Acknowledgment
The authors extend their gratitude to the team at the Obstetrics and Gynecology Department, Shanghai Seventh People's Hospital. No funding was received.
J Diabetes Investig 2017; 8: 554–559
References
- 1. World Health Organization . Definition, Diagnosis and Classification of Diabetes Mellitus and its Complications. Part 1: Diagnosis and Classification of Diabetes Mellitus. Geneva: World Health Org; 1999. [Google Scholar]
- 2. Cutchie WA, Cheung NW, Simmons D. Comparison of international and New Zealand guidelines for the care of pregnant women with diabetes. Diabet Med 2006; 23: 460–468. [DOI] [PubMed] [Google Scholar]
- 3. Agarwal MM, Dhatt GS, Punnose J. Gestational diabetes: utility of fasting plasma glucose as a screening test depends upon the diagnostic criteria. Diabet Med 2006; 23: 1319–1326. [DOI] [PubMed] [Google Scholar]
- 4. The obstetric group of obstetrics and gynecology subcommittee of Chinese Medical Association . The gestational diabetes mellitus cooperative group of perinatology subcommittee of Chinese Medical Association. Guide for diagnosis and treatment of gestational diabetes mellitus (draft). Zhonghua Fu Chan Ke Za Zhi 2007; 42: 426–428. (Chinese). [Google Scholar]
- 5. International Association of Diabetes and Pregnancy Study Groups Consensus Panel , Metzger BE, Gabbe SG, et al International association of diabetes and pregnancy study groups recommendations on the diagnosis and classification of hyperglycemia in pregnancy. Diabetes Care 2010; 33: 676–681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. American Diabetes Association . Standards of medical care in diabetes‐2011. Diabetes Care 2011; 34(Suppl 1): s11–s61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. American Diabetes Association . Standards of medical care in diabetes‐2012. Diabetes Care 2012; 35(Suppl 1): s11–s63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. American Diabetes Association . Standards of medical care in diabetes‐2013. Diabetes Care 2013; 36(Suppl 1): s11–s66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. American College of Obstetricians and Gynecologists . Committee opinion no. 504: screening and diagnosis of gestational diabetes. Obstet Gynecol 2011; 118: 751–753. [DOI] [PubMed] [Google Scholar]
- 10. The obstetric group of obstetrics and gynecology subcommittee of Chinese Medical Association . The gestational diabetes mellitus cooperative group of Chinese perinatology subcommittee of Chinese Medical Association. Guide for diagnosis and treatment of gestational diabetes mellitus. Zhonghua Fu Chan Ke Za Zhi 2014; 49: 461–569 (Chinese). [Google Scholar]
- 11. Oriot P, Selvais J, Radikov J, et al Assessing the incidence of gestational diabetes and neonatal outcomes with the Carpenter and Coustan criteria in a Belgian general hospital. Acta Clin Belg 2014; 69: 8–11. [DOI] [PubMed] [Google Scholar]
- 12. Bodmer‐Roy S, Morin L, Cousineau J, et al Pregnancy outcomes in women with and without gestational diabetes mellitus according to the International Association of the Diabetes and Pregnancy Study Groups criteria. Obstet Gynecol 2012; 120: 746–752. [DOI] [PubMed] [Google Scholar]
- 13. O'Sullivan JB, Gellis SS, Dandrow RV, et al The potential diabetic and her treatment in pregnancy. Obstet Gynecol 1966; 27: 683–689. [PubMed] [Google Scholar]
- 14. American Diabetes Association . Standards of medical care in diabetes‐2014. Diabetes Care 2014; 37(Suppl 1): s14–s80. [DOI] [PubMed] [Google Scholar]
- 15. Vandorsten JP1, Dodson WC, Espeland MA, et al NIH consensus development conference: diagnosing gestational diabetes mellitus. NIH Consens State Sci Statements 2013; 29: 1–31. [PubMed] [Google Scholar]
- 16. Shang M, Lin L. IADPSG criteria for diagnosing gestational diabetes mellitus and predicting adverse pregnancy outcomes. J Perinatol 2014; 34: 100–104. [DOI] [PubMed] [Google Scholar]
- 17. Liao S, Mei J, Song W, et al The impact of the International Association of Diabetes and Pregnancy Study Groups (IADPSG) fasting glucose diagnostic criterion on the prevalence and outcomes of gestational diabetes mellitus in Han Chinese women. Diabet Med 2014; 31: 341–351. [DOI] [PubMed] [Google Scholar]
- 18. Yang H, Wei Y, Gao X, et al China National GDM Survey Working Group. Risk factors for gestational diabetes mellitus in Chinese women: a prospective study of 16286 pregnant women in China. Diabet Med 2009; 26: 1099–1104. [DOI] [PubMed] [Google Scholar]
- 19. Agarwal MM, Hughes PF, Punnose J, et al Fasting plasma glucose as a screening test for gestational diabetes in a multi‐ethnic, high‐risk population. Diabet Med 2000; 26: 760–765. [DOI] [PubMed] [Google Scholar]
- 20. Agarwal MM, Dhatt GS, Punnose J, et al Gestation diabetes in a high‐risk population: using the fasting plasma to simplify the diagnostic algorithm. Eur J Obstet Gynecol Reprod Biol 2005; 120: 39–44. [DOI] [PubMed] [Google Scholar]
- 21. Agarwal MM, Dhatt GS, Shah SM. Simplifying the international association of diabetes and pregnancy diagnostic algorithm using fasting plasma glucose. Diabetes Care 2010; 33: 2018–2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Waugh N, Royle P, Clar C, et al Screening for hyperglycemia in pregnancy: a rapid update for the National Screening Committee. Health Technol Assess 2010; 14: 1–183. [DOI] [PubMed] [Google Scholar]
- 23. Avignon A, Radauceanu A, Monnier L. Nonfasting plasma glucose is a better marker of diabetic control than fasting plasma glucose in type 2 diabetes. Diabet Care 1997; 20: 1822–1826. [DOI] [PubMed] [Google Scholar]
- 24. The Emerging Risk Factors Collaboration . Diabetes mellitus, fasting blood glucose concentration, and risk of vascular disease: a collaborative meta‐analysis of 102 prospective studies. Lancet 2010; 375: 2215–2222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Cavalot F, Petrelli A, Traversa M, et al Postprandial blood glucose is a stronger predictor of cardiovascular events than fasting blood glucose in type 2 diabetes mellitus, particularly in women: lessons from the San Luigi Gonzaga diabetes study. J Clin Endocr Metab 2006; 91: 813–819. [DOI] [PubMed] [Google Scholar]
- 26. American Diabetes Association . Standards of medical care in diabetes–2007. Diabet Care 2007; 30(Suppl 1): S4–S41. [DOI] [PubMed] [Google Scholar]
- 27. American Diabetes Association . Diagnosis and classification of diabetes mellitus. Diabetes Care 2010; 33: S62–S69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. World Health Organization . Use of glycated haemoglobin (HbA1c) in diagnosis of diabetes mellitus: abbreviated report of a WHO consultation. 2011. [PubMed]
- 29. Takahashi S, Uchino H, Shimizu T, et al Comparison of glycated albumin (GA) and glycated hemoglobin (HbA1c) in type 2 diabetic patients: usefulness of GA for evaluation of short‐term changes in glycemic control. Endocr J 2007; 54: 139–144. [DOI] [PubMed] [Google Scholar]
- 30. Hellerstein S, Feldman S, Duan T. Survey of Obstetric Care and Cesarean Delivery Rates in Shanghai, China. Birth. 2016; 43: 193–199. [DOI] [PubMed] [Google Scholar]
- 31. Retnakaran R, Zinman B, Connelly PW, et al Impaired glucose tolerance of pregnancy is a heterogeneous metabolic disorder as defined by glycemic response to the oral glucose tolerance test. Diabetes Care 2006; 29: 57–62. [DOI] [PubMed] [Google Scholar]
- 32. Coustan DR. Point: the American Diabetes Association and the International Association of Diabetes and Pregnancy study groups recommendations for diagnosing gestational diabetes should be used worldwide. Clin Chem 2012; 58: 1094–1097. [DOI] [PubMed] [Google Scholar]
- 33. Black SC. Counterpoint enough evidence to treat? The American College of Obstetricians and Gynecologists guidelines. Clin Chem 2012; 58: 1098–1100. [DOI] [PubMed] [Google Scholar]


