Abstract
Aims/Introduction
To evaluate the efficacy of weight changes from baseline of the sodium‐glucose cotransporter 2 (SGLT2) inhibitors treatment and glucagon‐like peptide‐1 (GLP‐1) analogs treatment after comparisons with a placebo in type 2 diabetes patients, and the associated factors.
Materials and Methods
Studies were searched from when recording began, June 2004, until June 2015, and re‐searched in July 2016, and placebo‐controlled randomized trials in type 2 diabetes patients with a study length of ≥12 weeks were included.
Results
A total of 97 randomized controlled trials were included. Compared with a placebo, treatment with SGLT2 inhibitors was associated with a significantly greater decrease in weight change from baseline (weighted mean differences −2.01 kg, 95% confidence interval −2.18 to −1.83 kg, P < 0.001). Compared with a placebo, changes with GLP ‐1 treatment were also associated with a comparable decrease in weight change from baseline (weighted mean differences −1.59 kg, 95% confidence interval −1.86 to −1.32 kg, P < 0.001). Meta‐regression analysis showed that the baseline age, sex, baseline glycated hemoglobin, diabetes duration or baseline body mass index were not associated with the weight change from baseline in SGLT2 inhibitors or in GLP‐1 treatment corrected by placebo. Comparisons of weight changes from baseline corrected by placebo between SGLT2 inhibitors and GLP‐1 treatment showed that the difference was not significant (P > 0.05).
Conclusions
According to the present meta‐analysis, treatment with SGLT2 inhibitors and treatment with GLP‐1 analogs led to comparable weight changes from baseline, which are both with significance when compared with placebo treatment.
Keywords: Bodyweight, Glucagon‐like peptide‐1 analogs, Sodium‐glucose cotransporter 2 inhibitors
Introduction
It is well known that type 2 diabetes is characterized by β‐cell dysfunction and insulin resistance. In type 2 diabetes management, addressing obesity is considered to be an important factor, which might help to improve both the insulin resistance and the glycemic control1, 2; therefore, weight loss is recommended for patients with type 2 diabetes3. It was suggested that approximately 5–10% of weight loss might improve glycemic control4, and other cardiovascular risk factors and comorbidities5, 6. However, although there are a number of antidiabetes agents currently available, we should confess that it is still very difficult for type 2 diabetes patients to achieve optimal weight reduction as well as glycemic control. It is suggested that metformin provides modest weight reduction, whereas sulfonylureas and thiazolidinediones lead to weight gain, and dipeptidyl peptidase‐4 inhibitors are associated with weight neutral1, 7.
Recently, it was reported in some clinical trials that glucagon‐like peptide‐1 (GLP‐1) analogs had a unique efficacy in weight reduction for both obesity and type 2 diabetes8, 9, 10. Sodium‐glucose cotransporter 2 (SGLT2) inhibitors, have a mechanism of causing urinary glucose excretion through inhibiting renal glucose reabsorption by SGLT2, also providing both glycemic control and bodyweight reduction11, 12, 13, 14. Both of the aforementioned kinds of antidiabetes agents might lead to bodyweight reductions different from other antidiabetes agents. However, which of the two kinds of agents is superior? So far, a head‐to‐head comparative study of these two kinds of antidiabetes agents has not been carried, out and no results could be found about weight changes. Therefore, to evaluate the efficacy of weight changes of GLP‐1 analogs and SGLT2 inhibitors, we carried out the present meta‐analysis.
Methods
Search strategy
We mainly searched data from MEDLINE® (PubMed), from 2004 until June 2015, and re‐searched in July 2016. The following terms were used: dapagliflozin; canagliflozin; empagliflozin; ipragliflozin; tofogliflozin; sodium glucose co‐transporter 2 inhibitors; exenatide; liraglutide; albiglutide; taspoglutide; lixisenatide; glucagon‐like peptide‐1 analogs; type 2 diabetes; randomized controlled trials. Furthermore, documents for medications (dapagliflozin, canagliflozin, empagliflozin, ipragliflozin, liraglutide, exenatide, albiglutide, taspoglutide, lixisenatide) were searched for trials at the clinical trials website.
Data selection and data extraction
Studies meeting the inclusion criteria were included in this meta‐analysis: (i) randomized trial of SGLT2 inhibitors treatment compared with placebo in type 2 diabetes participants as monotherapy or add‐on therapy; (ii) randomized trial of GLP‐1 analogs treatment compared with placebo in type 2 diabetes participants as monotherapy or add‐on therapy; (iii) study length should be more than 12 weeks; (iv) change in the weight from baseline was provided in both the antidiabetes agent group and the placebo group; and (v) baseline characteristics, such as as age, body mass index (BMI) or glycated hemoglobin (HbA1c), were reported in the trial. Monotherapy was defined as patients not receiving any hypoglycemic agent before being randomized into the clinical trials, and after randomization, they received active hypoglycemic agent or a placebo. Add‐on therapy was defined as patients receiving hypoglycemic agents before randomization, but not well controlled, then after randomization, they received another active hypoglycemic agent or placebo add‐on to their previous treatment as the protocol defined.
Based on the inclusion criteria, WY and YC evaluated the eligibility of the studies independently. When disagreements between the two authors arose, they consulted with another investigator (LZ). By using the Cochrane instrument, we evaluated the quality of each study. Details are shown in supplement figures.
By using a standard form, WY and YC independently carried out the data extraction. Study titles and authors, study design, the number of individuals, patients' age, diabetes duration, baseline HbA1c, dosage of the study drugs, duration of follow up, and the changes of bodyweight were all documented. If there was any disagreement, the two review authors (WY and YC) would discuss together with another investigator (LZ).
Statistical analysis
We used weighted mean difference (WMD) and 95% confidence intervals (CIs) to evaluate the placebo‐corrected weight changes in the treatment of SGLT2 inhibitors and GLP‐1 analogs separately. The statistical analysis has been reported previously15. Meta‐regression was carried out to find the association between the bodyweight changes and the baseline age, sex, duration of diabetes, baseline BMI or baseline HbA1c (P < 0.05 shows significance). The meta‐analyses were carried out by the Review Manager statistical software package (version 5.2; The Nordic Cochrane Center, The Cochrane Collaboration, Copenhagen, Denmark), and the meta‐regression analyses were carried out by the Stata statistical software package (version 11.0; StataCorp, College Station, Texas, USA).
Results
Characteristics of included studies
The flowchart of the study selection process is shown in Figure 1. In total, 97 studies were relevant, including 51 studies with SGLT2 inhibitors (SGLT2i) treatment (17 studies as monotherapy and 34 studies as add‐on therapy) and 46 studies with GLP‐1 analogs (GLP‐1) treatment (15 studies as monotherapy and 31 studies as add‐on therapy). A reference list and clinical characteristics of studies are presented as Table S1. Characteristics of the individuals receiving SGLT2i and GLP‐1 analogs treatment in this meta‐analysis are shown in Table 1. This meta‐analysis was based on data from 8,710 individuals in the SGLT2i treatment, and 7,409 individuals in the GLP‐1 analogs treatment.
Figure 1.
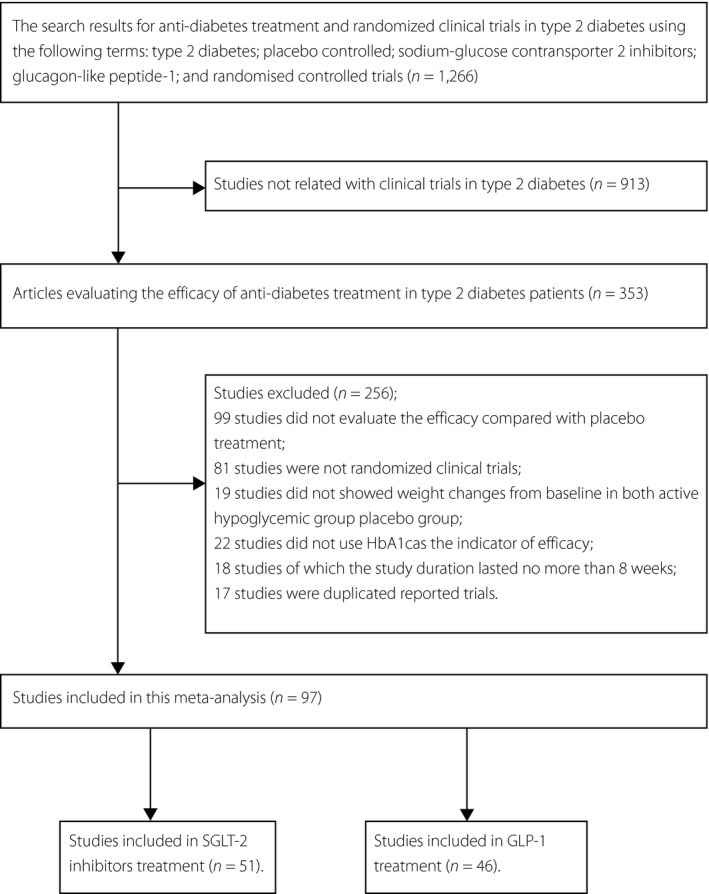
The flowchart of included studies. GLP‐1, glucagon‐like peptide‐1; HBA1c, glycated hemoglobin; SGLT2, sodium‐glucose cotransporter 2.
Table 1.
Baseline characteristics of studies included in this meta‐analysis in sodium‐glucose cotransporter 2 inhibitors treatment and glucagon‐like peptide‐1 analogs treatment
| SGLT2 inhibitors | GLP‐1 analogs | |
|---|---|---|
| No. studies | 51 | 46 |
| Age (years) | 57.1 ± 4.3 | 55.5 ± 2.2 |
| Male (%) | 44 | 47 |
| Baseline BMI (kg/m2) | 30.4 ± 2.7 | 31.1 ± 4.6 |
| Baseline weight (kg) | 84.7 ± 8.3 | 88.4 ± 12.3 |
| DM duration (year) | 7.5 ± 4.4 | 6.4 ± 2.8 |
| Baseline HbA1c (%) | 8.1 ± 0.4 | 8.0 ± 0.4 |
| Study duration (weeks) | 30.2 ± 22.0 | 26.4 ± 22.4 |
BMI, body mass index; DM, diabetes mellitus; GLP‐1, glucagon‐like peptide‐1; HBA1c, glycated hemoglobin; SGLT2, sodium‐glucose cotransporter 2.
Quality of methodology
The present meta‐analysis included studies that were randomized, placebo‐controlled and with double‐blind treatment. Most studies reported baseline age, sex, BMI, HbA1c and diabetes duration between the comparison groups. The visual inspection of the funnel plots showed an even distribution of the variables that were studied (Figures S5, S6). For the low level of heterogeneity, the fixed‐effects model was used, and for the high level of heterogeneity, the random‐effects model was used.
Weight changes in SGLT2 inhibitors treatment
When SGLT2 inhibitors treatment was compared with placebo treatment, analysis of the combined data suggested that SGLT2 inhibitors led a significantly greater change in the bodyweight (WMD −2.01 kg, 95% CI: −2.18 to −1.83 kg, P < 0.001, in random‐effects). Compared with a placebo, SGLT2 inhibitors as monotherapy also led a significantly greater decrease in bodyweight (WMD −1.95 kg, 95% CI: −2.13 to −1.77 kg, P < 0.001, in random‐effects). As add‐on therapy, compared with a placebo, SGLT2 inhibitors led a significantly greater decrease in bodyweight (WMD −2.04 kg, 95% CI: −2.26 to −1.82 kg, P < 0.001, in random‐effects). Details are shown in Table 2. Results from the meta‐regression analysis (Figure S3) suggested that the bodyweight changes in SGLT2 inhibitors treatment was not associated with baseline BMI (β 0.179, 95% CI: −0.804 to 1.162, P > 0.05), or baseline HbA1c (β −1.639, 95% CI: −8.24 to 4.96, P > 0.05), or HbA1c changes from baseline (β 0.001, 95% CI: −5.20 to 5.20, P > 0.05) or baseline bodyweight (β 0.026, 95% CI: −0.253 to 0.305, P > 0.05).
Table 2.
Comparisons of the weight changes from baseline between sodium‐glucose cotransporter 2 inhibitors treatment and glucagon‐like peptide‐1 analogs treatment
| Variables | SGLT2 inhibitors treatment | GLP‐1 analogs treatment | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| No. studies | No. participants (SGLT2i vs placebo) | WMD from baseline | 95% CI | P‐value | I 2‐value | No. studies | No. participants (GLP‐1 vs placebo) | WMD from baseline | 95% CI | P‐value | I 2‐value | |
| Weight change from baseline (kg) | ||||||||||||
| Monotherapy | 17 | 1,750/1,649 | −1.95a | −2.13, −1.77 | <0.001 | 96% | 9 | 671/700 | −1.22a | −1.61, −0.83 | <0.001 | 98% |
| Add‐on therapy | 34 | 6,972/6,520 | −2.04a | −2.26, −1.82 | <0.001 | 99% | 28 | 4,838/3,808 | −1.70a | −2.02, −1.39 | <0.001 | 100% |
| Total | 51 | 8,710/8,151 | −2.01a | −2.18, −1.83 | <0.001 | 99% | 37 | 5,509/4,508 | −1.59a | −1.86, −1.32 | <0.001 | 100% |
| HbA1c change from baseline (%) | ||||||||||||
| Monotherapy | 17 | 1,750/1,649 | −0.78a | −0.87, −0.70 | <0.001 | 98% | 15 | 1,674/1,030 | −1.05a | −1.25, −0.84 | <0.001 | 98% |
| Add‐on therapy | 34 | 6,972/6,520 | −0.58a | −0.62, −0.53 | <0.001 | 99% | 31 | 5,735/3,974 | −0.75a | −0.85, −0.66 | <0.001 | 100% |
| Total | 51 | 8,710/8,151 | −0.64a | −0.68, −0.60 | <0.001 | 99% | 46 | 7,409/5,004 | −0.84a | −0.94, −0.74 | <0.001 | 100% |
P < 0.001. CI, confidence interval; GLP‐1, glucagon‐like peptide‐1; HBA1c, glycated hemoglobin; SGLT2, sodium‐glucose cotransporter 2; WMD, weighted mean difference.
Subgroup analysis was based on the efficacy of bodyweight in different kinds of SGLT2 inhibitors treatment. The results showed that dapagliflozin treatment led to a significantly greater decrease in the bodyweight when compared with a placebo (WMD −1.92 kg, 95% CI: −2.11 to −1.72 kg, P < 0.001, in random‐effects); canagliflozin treatment was associated with a significantly greater bodyweight reduction when compared with a placebo (WMD −2.30 kg, 95% CI: −2.73 to −1.88 kg, P < 0.001, in random‐effects); empagliflozin treatment resulted in a significantly greater weight reduction when compared with a placebo (WMD −1.95 kg, 95% CI: −2.07 to −1.83 kg, P < 0.001, in random‐effects); and ipragliflozin treatment also led to a significantly greater reduction in bodyweight when compared with a placebo (WMD −1.72 kg, 95% CI: −1.90 to −1.54 kg, P < 0.001, in random‐effects). Details are shown in Table 3.
Table 3.
Comparisons of the weight changes and glycated hemoglobin changes from baseline in different sodium‐glucose cotransporter 2 inhibitors treatment
| No. studies | No. participants (SGLT2i vs placebo) | WMD from baseline | 95% CI | P‐value | |
|---|---|---|---|---|---|
| Weight change from baseline (kg) | |||||
| Dapagliflozin | 20 | 2,954/2,971 | −1.92a | −2.11, −1.72 | <0.001 |
| Canagliflozin | 11 | 2,781/2,551 | −2.30a | −2.73, −1.88 | <0.001 |
| Empagliflozin | 13 | 2,495/2,288 | −1.95a | −2.07, −1.83 | <0.001 |
| Ipragliflozin | 4 | 370/237 | −1.72a | −1.90, −1.54 | <0.001 |
| Tofogliflozin | 2 | 122/122 | −2.15a | −2.82, −1.48 | <0.001 |
| Total | 51 | 8,710/8,151 | −2.01a | −2.18, −1.83 | <0.001 |
| HbA1c change from baseline (%) | |||||
| Dapagliflozin | 20 | 2,954/2,971 | −0.58a | −0.65, −0.52 | <0.001 |
| Canagliflozin | 11 | 2,781/2,551 | −0.75a | −0.82, −0.68 | <0.001 |
| Empagliflozin | 13 | 2,495/2,288 | −0.64a | −0.71, −0.56 | <0.001 |
| Ipragliflozin | 4 | 370/237 | −0.68a | −1.02, −0.35 | <0.001 |
| Tofogliflozin | 2 | 122/122 | −0.73a | −0.77, −0.69 | <0.001 |
| Total | 51 | 8,710/8,151 | −0.64a | −0.68, −0.60 | <0.001 |
P < 0.001. CI, confidence interval; HBA1c, glycated hemoglobin; SGLT2, sodium‐glucose cotransporter 2; WMD, weighted mean difference.
Weight changes in GLP‐1 analogs treatment
When GLP‐1 analogs treatment was compared with placebo treatment, the results suggested that GLP‐1 analogs led to a significantly greater decrease in bodyweight (WMD −1.59 kg, 95% CI: −1.86 to −1.32 kg, P < 0.001, in random‐effects). Compared with the placebo, GLP‐1 analogs as monotherapy led to a comparable decrease in bodyweight (WMD −1.22 kg, 95% CI: −1.61 to −0.83, P < 0.001, in random‐effects). As add‐on therapy, compared with the placebo, GLP‐1 analogs led to a significantly greater decrease in bodyweight (WMD −1.70 kg, 95% CI: −2.02 to −1.39 kg, P < 0.001, in random‐effects). Results from meta‐regression analysis suggested that the bodyweight changes in GLP‐1 analogs treatment was not associated with baseline BMI (β −0.058, 95% CI: −0.204 to 0.088, P > 0.05), or baseline HbA1c (β −0.524, 95% CI: −3.066 to 2.019, P > 0.05), or the HbA1c changes from baseline (β −1.716, 95% CI: −4.216 to 0.784, P > 0.05), but the bodyweight changes in GLP‐1 analogs treatment was significantly associated with baseline bodyweight (β 0.092, 95% CI: −0.154 to −0.03, P = 0.005). Details are shown in Figure S4.
Subgroup analysis was based on the efficacy of bodyweight in different kinds of GLP‐1 analogs treatment. The results suggested that exenatide treatment led to a significantly greater decrease in bodyweight when compared with the placebo (WMD −1.69 kg, 95% CI: −2.09 to −1.29 kg, P < 0.001, in random‐effects); liraglutide treatment resulted in a significantly greater reduction in bodyweight when compared with the placebo (WMD −2.51 kg, 95% CI: −3.33 to −1.69 kg, P < 0.001, in random‐effects); lixisenatide treatment was associated with a significantly greater weight reduction when compared with the placebo (WMD −0.90 kg, 95% CI: −1.24 to −0.56 kg, P < 0.001, in random‐effects); and taspoglutide treatment also led to a significantly greater weight reduction when compared with the placebo (WMD 1.40 kg, 95% CI: −1.45 to −1.35 kg, P < 0.001, in random‐effects). For treatment with GLP‐1 analogs daily dosage and weekly dosage one, the bodyweight decrease from baseline was also significant when compared with the placebo. Details are shown in Table 4.
Table 4.
Comparisons of the weight changes and glycated hemoglobin changes from baseline in different glucagon‐like peptide‐1analogs treatment
| No. studies | No. participants (GLP‐1 vs placebo) | WMD from baseline | 95% CI | P‐value | |
|---|---|---|---|---|---|
| Weight change from baseline (kg) | |||||
| Exenatide | 10 | 983/990 | −1.69a | −2.09, −1.29 | <0.001 |
| Liraglutide | 7 | 1,158/825 | −2.51a | −3.33, −1.69 | <0.001 |
| Lixisenatide | 12 | 2,350/1,915 | −0.90a | −1.24, −0.56 | <0.001 |
| Albiglutide | 3 | 450/316 | −0.21 | −0.50, 0.08 | 0.16 |
| Taspoglutide | 3 | 470/364 | −1.40a | −1.45, −1.35 | <0.001 |
| Dulaglutide | 2 | 99/98 | −1.07 | −3.74, 1.61 | 0.43 |
| Daily injections | 28 | 4,475/3,715 | −1.32a | −1.58, −1.06 | <0.001 |
| Weekly injections | 9 | 1,034/793 | −1.67a | −2.17, −1.17 | <0.001 |
| Total | 37 | 5,509/4,508 | −1.59a | −1.86, −1.32 | <0.001 |
| HbA1c change from baseline (%) | |||||
| Exenatide | 12 | 1,740/1,107 | −0.82a | −0.96, −0.68 | <0.001 |
| Liraglutide | 12 | 1,963/1,059 | −1.18a | −1.39, −0.97 | <0.001 |
| Lixisenatide | 12 | 2,350/1,915 | −0.47a | −0.57, −0.38 | <0.001 |
| Albiglutide | 3 | 450/316 | −0.78a | −0.94, −0.62 | <0.001 |
| Taspoglutide | 3 | 470/364 | −0.99a | −1.30, −0.69 | <0.001 |
| Dulaglutide | 5 | 436/243 | −1.15a | −1.45, −0.86 | <0.001 |
| Daily injections | 34 | 6,022/4,067 | −0.75a | −0.86, −0.65 | <0.001 |
| Weekly injections | 12 | 1,387/937 | −1.07a | −1.25, −0.89 | <0.001 |
| Total | 46 | 7,409/5,004 | −0.84a | −0.94, −0.74 | <0.001 |
P < 0.001. CI, confidence interval; GLP‐1, glucagon‐like peptide‐1; HBA1c, glycated hemoglobin; WMD, weighted mean difference.
Comparisons of weight changes from baseline between SGLT2 inhibitors and GLP‐1 analogs treatment
In total, comparisons of weight changes from baseline corrected by placebo between SGLT2 inhibitors and GLP‐1 analogs treatment showed that the difference was not significant (P > 0.05). For HbA1c changes from baseline corrected by placebo between SGLT2 inhibitors and GLP‐1 analogs treatment, neither showed a significant difference (P > 0.05).
Discussion
It is well known that treatment with GLP‐1 analogs both in monotherapy and add‐on therapy can lead to weight decrease from baseline in type 2 diabetes patients, which were reported by some randomized clinical trials8, 9, 10, 16, 17 and meta‐analyses18, 19, 20. Group analysis and subgroup analysis of the current meta‐analysis also showed the comparable effect of weight loss in GLP‐1 analogs treatment. Furthermore, from the results of the present meta‐analysis, SGLT2 inhibitors also resulted in significantly greater weight loss. Comparisons between the two kinds of treatment of the placebo‐corrected weight changes showed no significant difference. So far, few studies have made comparisons between these two kinds of hypoglycemic treatment in terms of weight change; therefore, the present meta‐analysis comprehensively evaluated the weight changes between these two groups of antidiabetes treatment.
Subgroup analysis showed that dapagliflozin, canagliflozin, empagliflozin and ipragliflozin all led to weight reductions, which is also consistent with previous results from randomized controlled trials in monotherapy and add‐on therapy in type 2 diabetes patients14, 21, 22, 23. Recently published longer‐term data of empagliflozin24 also showed that the placebo‐corrected reduction in bodyweight was 2.5 kg in average. Weight loss in SGLT2 inhibitors treatment might be explained as being due to caloric loss through glucose excretion in the urine, which could result in a shift toward negative net energy balance. Another explanation by Bolinder et al.14 suggested that with dapagliflozin treatment, ‘the bodyweight loss could be explained by reduced total body fat mass, visceral adipose tissue and subcutaneous adipose tissue volume.’ However, reasons for the weight loss in GLP‐1 analogs treatment were suggested as mediating through effects on appetite sensations and subsequent reduction of energy intake, rather than increasing energy expenditure25, which were different from those for SGLT2 inhibitors treatment.
So far, no head‐to‐head clinical comparative trial has reported on the weight changes between GLP‐1 analogs treatment and SGLT2 inhibitors treatment. However, these two antidiabetes agents have different mechanisms in glycemic control and weight control; one of which for SGLT2 inhibitors is through inhibition of renal glucose reabsorption by SGLT2, providing an insulin‐independent mechanism for lowering blood glucose26, another of which for GLP‐1 analogs is an analog of an incretin hormone that enhances glucose‐dependent insulin secretion, inhibiting glucagon secretion and slowing gastric emptying27, 28, 29. Therefore, the results of the present meta‐analysis should be cautiously explained. However, we might conclude that both of the two kinds of antidiabetes treatment could lead to significant weight reduction, which is an important issue for type 2 diabetes patients.
It was suggested that obesity was associated with diabetes and insulin resistance30, 31. Weight loss is an additional treatment goal for most patients with type 2 diabetes, and a degree of weight loss was associated with improvements in glycemic control and cardiovascular risk factors5, 6. Currently, treatments for type 2 diabetes associated with weight neutral are dipeptidyl peptidase‐4 inhibitors and alpha glucose inhibitor; treatments associated with weight increase are sulfonylureas, insulin and thiazolidinediones; and treatment associated with small weight reduction us metformin1, 3. The new mode therapies for type 2 diabetes, such as GLP‐1 analogs and SGLT2 inhibitors, has led to significant weight changes in type 2 diabetes treatments, as concluded from the present meta‐analysis. However, these two kinds of treatments also have some limitations. GLP‐1 analogs must be injected, and were reported to be associated with gastrointestinal side‐effects18, 19, whereas SGLT2 inhibitors were reported to be associated with ketoacidosis, osteoporosis and imbalance of electrolytes32, 33, 34, 35.
The present meta‐analysis compared the placebo‐corrected weight changes between GLP‐1 analogs treatment and SGLT2 inhibitors treatment in a large number of randomized controlled trials in type 2 diabetes patients. However, this meta‐analysis still had some limitations. First, although there were differences among separate studies in the inclusion criteria, baseline variables and so on, data should be combined together to evaluate the effects on bodyweight. Second, data on weight changes from baseline in each treatment group could only be collected from 53 studies, and 18 others that lacked this information were excluded from this analysis, which might indicate the presence of selection bias. Third, as the positive results might be published more easily than the negative results, there could be some publication bias in the meta‐analysis. We have carried out the visual inspection of the funnel plot to minimize this limitation. Additionally, the number of trials included in SGLT2 inhibitors treatment and GLP‐1 analogs treatment might not be comparable. Therefore, we should interpret the results from the present meta‐analysis with caution.
The results from the present meta‐analysis suggested that SGLT2 inhibitors, as well as GLP‐1 analogs, led to comparable placebo‐corrected bodyweight decrease in type 2 diabetes patients.
Disclosure
LJ has received fees for lecture presentations, and for consulting from Abbott, AstraZeneca, Bristol‐Myers Squibb, Merck, Metabasis, Novartis, Eli Lilly, Roche, Sanofi‐Aventis and Takeda. The other authors declare no conflict of interest.
Supporting information
Table S1 | Characteristics of randomized controlled trials in type 2 diabetes included in the meta‐analysis.
Figure S1 | Summary of risk of bias of included studies in glucagon‐like peptide‐1 analogs treatment.
Figure S2 | Summary of risk of bias of included studies in sodium‐glucose cotransporter 2 inhibitors treatment.
Figure S3 | Regression analysis of the associations between the weight changes from baseline and baseline body mass index (BMI), baseline glycated hemoglobin (HbA1c), HbA1c changes from baseline, and baseline weight in sodium‐glucose cotransporter 2 (SGLT2) inhibitors treatment. The size of the circles in this figure did represent the size of each study, which was represented as N.
Figure S4 | Regression analysis of the associations between the weight changes from baseline and baseline body mass index (BMI), baseline glycated hemoglobin (HbA1c), HbA1c changes from baseline, and baseline weight in glucagon‐like peptide‐1 (GLP‐1) analogs treatment. The size of the circles in this figure did represent the size of each study, which was represented as N.
Figure S5 | Funnel plot of studies included with glucagon‐like peptide‐1 (GLP‐1) analogs treatment.
Figure S6 | Funnel plot of studies included with sodium‐glucose cotransporter 2 inhibitors treatment.
Acknowledgments
We thank Dr Xueying Gao, Lijuan Sun, Fang Wang, Yumin Ma, and other doctors and nurses for their practical work during the study at Peking University People's Hospital Endocrinology and Metabolism Department. This meta‐analysis was supported by the National High‐Technology Research and Development Program of China (863 Program 2012AA02A509) and National Natural Science Foundation of China (NSFC) (81000334). The funding agencies had no role in the study design, data collection or analysis, decision to publish, or preparation of the manuscript.
J Diabetes Investig 2017; 8: 510–517
Clinical Trial Registry
PROSPERO International Prospective Register of Systematic Reviews
CRD42015027781
References
- 1. Inzucchi SE, Bergenstal RM, Buse JB. Management of hyperglycemia in type 2 diabetes: a patient‐centered approach. Position statement of the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD). Diabetes Care 2012; 35: 1364–1379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Klein S, Sheard NF, Pi‐Sunyer X. Weight management through lifestyle modification for the prevention and management of type 2 diabetes: rationale and strategies: a statement of the American Diabetes Association, the North American Association for the Study of Obesity, and the American Society for Clinical Nutrition. Diabetes Care 2004; 27: 2067–2073. [DOI] [PubMed] [Google Scholar]
- 3. American Diabetes Association . Diagnosis and classification of diabetes mellitus. Diabetes Care 2015; 38(Suppl 1): S8–S16. [DOI] [PubMed] [Google Scholar]
- 4. Pi‐Sunyer FX. The effects of pharmacologic agents for type 2 diabetes mellitus on body weight. Postgrad Med 2008; 120: 5–17. [DOI] [PubMed] [Google Scholar]
- 5. Maggio CA, Pi‐Sunyer FX. The prevention and treatment of obesity: application to type 2 diabetes. Diabetes Care 1997; 20: 1744–1766. [DOI] [PubMed] [Google Scholar]
- 6. Lavie CJ, Milani RV, Artham SM, et al The obesity paradox, weight loss, and coronary disease. Am J Med 2009; 122: 1106–1114. [DOI] [PubMed] [Google Scholar]
- 7. Bennett WL, Maruthur NM, Singh S. Comparative effectiveness and safety of medications for type 2 diabetes: an update including new drugs and 2‐drug combinations. Ann Intern Med 2011; 154: 602–613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Bergenstal RM. Efficacy and safety of Taspoglutide versus sitagliptin for type 2 diabetes mellitus (T‐Emerge 4 trial). Diabetes Ther 2012; 3: 13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Buse JB. Effects of Exenatide (Exendin‐4) on Glycemic Control Over 30 Weeks in Sulfonylurea‐Treated Patients With Type 2 Diabetes. Diabetes Care 2004; 27: 2628–2635. [DOI] [PubMed] [Google Scholar]
- 10. Nauck M. Efficacy and Safety Comparison of Liraglutide, Glimepiride, and Placebo, All in Combination With Metformin, in Type 2 Diabetes. Diabetes Care 2009; 32: 84–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Meng W, Ellsworth BA, Nirschl AA. Discovery of dapagliflozin: a potent, selective renal sodium‐dependent glucose cotransporter 2 (SGLT2) inhibitor for the treatment of type 2 diabetes. J Med Chem 2008; 51: 1145–1149. [DOI] [PubMed] [Google Scholar]
- 12. Rahmoune H, Thompson PW, Ward JM, et al Glucose transporters in human renal proximal tubular cells isolated from the urine of patients with non‐insulin‐dependent diabetes. Diabetes 2005; 54: 3427–3434. [DOI] [PubMed] [Google Scholar]
- 13. Hardman TC, Dubrey SW. Development and potential role of type‐2 sodium‐glucose transporter inhibitors for management of type 2 diabetes. Diabetes Therapy 2011; 2: 133–145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Bolinder J, Ljunggren O, Kullberg J. Effects of dapagliflozin on body weight, total fat mass, and regional adipose tissue distribution in patients with type 2 diabetes mellitus with inadequate glycemic control on metformin. J Clin Endocrinol Metab 2012; 97: 1020–1031. [DOI] [PubMed] [Google Scholar]
- 15. Cai X, Han X, Luo Y, et al Efficacy of dipeptidyl‐peptidase‐4 inhibitors and impact on β‐cell function in Asian and Caucasian type 2 diabetes mellitus patients: a meta‐analysis. J Diabetes 2015; 7: 347–359. [DOI] [PubMed] [Google Scholar]
- 16. Russell‐Jones D. Liraglutide vs insulin glargine and placebo in combination with metformin and sulfonylurea therapy in type 2 diabetes mellitus (LEAD‐5 met+SU): a randomised controlled trial. Diabetologia 2009; 52: 2046–2055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Seino Y. Dose‐dependent improvement in glycemia with once‐daily liraglutide without hypoglycemia or weight gain: a double‐blind, randomized, controlled trial in Japanese patients with type 2 diabetes. Diab Res Clin Prac 2008; 81: 161–168. [DOI] [PubMed] [Google Scholar]
- 18. Hermansen K, Mortensen LS. Bodyweight changes associated with antihyperglycaemic agents in type 2 diabetes mellitus. Drug Saf 2007; 30: 1127–1142. [DOI] [PubMed] [Google Scholar]
- 19. Wilding JP, Hardy K. Glucagon‐like peptide‐1 analogues for type 2 diabetes. BMJ 2011; 342: d410. [DOI] [PubMed] [Google Scholar]
- 20. Blonde L, Russell‐Jones D. The safety and efficacy of liraglutide with or without oral antidiabetic drug therapy in type 2 diabetes: an overview of the LEAD 1‐5 studies. Diabetes Obes Metab 2009; 11(Suppl 3): 26–34. [DOI] [PubMed] [Google Scholar]
- 21. Strojek K, Yoon KH, Hruba V, et al Effect of dapagliflozin in patients with type 2 diabetes who have inadequate glycaemic control with glimepiride: a randomised, 24‐week, double‐blind, placebo‐controlled trial. Diabetes Obes Metab 2011; 13: 928–938. [DOI] [PubMed] [Google Scholar]
- 22. Wilding JP, Norwood P, T'joen C, Bastien A, List JF, Fiedorek FT. A study of dapagliflozin in patients with type 2 diabetes receiving high doses of insulin plus insulin sensitizers: applicability of a novel insulin‐independent treatment. Diabetes Care 2009; 32: 1656–1662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Ferrannini E, Ramos SJ, Salsali A, et al Dapagliflozin monotherapy in type 2 diabetic patients with inadequate glycemic control by diet and exercise: a randomized, double‐blind, placebo‐controlled, phase 3 trial. Diabetes Care 2010; 33: 2217–2224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Zinman B, Wanner C, Lachin JM, et al EMPA‐REG OUTCOME Investigators. Empagliflozin, Cardiovascular Outcomes, and Mortality in Type 2 Diabetes. N Engl J Med 2015; 373: 2117–2128. [DOI] [PubMed] [Google Scholar]
- 25. van Can J, Sloth B, Jensen CB, et al Effects of the once‐daily GLP‐1 analog liraglutide on gastric emptying, glycemic parameters, appetite and energy metabolism in obese, non‐diabetic adults. Int J Obes (Lond) 2014; 38: 784–793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Neumiller JJ, White JR, Campbell RK. Sodium‐glucose co‐transport inhibitors: progress and therapeutic potential in type 2 diabetes mellitus. Drugs 2010; 70: 377–385. [DOI] [PubMed] [Google Scholar]
- 27. Meier JJ, Gethmann A, Gotze O, et al Glucagonlike peptide 1 abolishes the postprandial rise in triglyceride concentrations and lowers levels of non‐esterified fatty acids in humans. Diabetologia 2006; 49: 452–458. [DOI] [PubMed] [Google Scholar]
- 28. Wettergren A, Schjoldager B, Mortensen PE, et al Truncated GLP‐1 (proglucagon 78‐107‐amide) inhibits gastric and pancreatic functions in man. Dig Dis Sci 1993; 38: 665–673. [DOI] [PubMed] [Google Scholar]
- 29. Flint A, Raben A, Ersboll AK, et al The effect of physiological levels of glucagon‐like peptide‐1 on appetite, gastric emptying, energy and substrate metabolism in obesity. Int J Obes 2001; 25: 781–792. [DOI] [PubMed] [Google Scholar]
- 30. Freemantle N, Holmes J, Hockey A, et al How strong is the association between abdominal obesity and the incidence of type 2 diabetes? Int J Clin Pract 2008; 62: 1391–1396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Gastaldelli A, Cusi K, Pettiti M, et al Relationship between hepatic/visceral fat and hepatic insulin resistance in nondiabetic and type 2 diabetic subjects. Gastroenterology 2007; 133: 496–506. [DOI] [PubMed] [Google Scholar]
- 32. Taylor SI, Blau JE, Rother KI. SGLT2 Inhibitors May Predispose to Ketoacidosis. J Clin Endocrinol Metab 2015; 100: 2849–2852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Rosenstock J, Ferrannini E. Euglycemic diabetic Ketoacidosis: a predictable, detectable, and preventable safety concern with SGLT2Inhibitors. Diabetes Care 2015; 38: 1638–1642. [DOI] [PubMed] [Google Scholar]
- 34. Taylor SI, Blau JE, Rother KI. Possible adverse effects of SGLT2 inhibitors on bone. Lancet Diabetes Endocrinol 2015; 3: 8–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Kaur A, Winters SJ. Severe hypercalcemia and hypernatremia in a patient treated with canagliflozin. Endocrinol Diabetes Metab Case Rep 2015; 2015: 150042. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1 | Characteristics of randomized controlled trials in type 2 diabetes included in the meta‐analysis.
Figure S1 | Summary of risk of bias of included studies in glucagon‐like peptide‐1 analogs treatment.
Figure S2 | Summary of risk of bias of included studies in sodium‐glucose cotransporter 2 inhibitors treatment.
Figure S3 | Regression analysis of the associations between the weight changes from baseline and baseline body mass index (BMI), baseline glycated hemoglobin (HbA1c), HbA1c changes from baseline, and baseline weight in sodium‐glucose cotransporter 2 (SGLT2) inhibitors treatment. The size of the circles in this figure did represent the size of each study, which was represented as N.
Figure S4 | Regression analysis of the associations between the weight changes from baseline and baseline body mass index (BMI), baseline glycated hemoglobin (HbA1c), HbA1c changes from baseline, and baseline weight in glucagon‐like peptide‐1 (GLP‐1) analogs treatment. The size of the circles in this figure did represent the size of each study, which was represented as N.
Figure S5 | Funnel plot of studies included with glucagon‐like peptide‐1 (GLP‐1) analogs treatment.
Figure S6 | Funnel plot of studies included with sodium‐glucose cotransporter 2 inhibitors treatment.


