Abstract
Epilepsy is a common neurological disease in tropical countries, particularly in sub-Saharan Africa. Previous work on epilepsy in sub-Saharan Africa has shown that many cases are severe, partly a result of some specific causes, that it carries a stigma, and that it is not adequately treated in many cases. Many studies on the epidemiology, aetiology, and management of epilepsy in sub-Saharan Africa have been reported in the past 10 years. The prevalence estimated from door-to-door studies is almost double that in Asia, Europe, and North America. The most commonly implicated risk factors are birth trauma, CNS infections, and traumatic brain injury. About 60% of patients with epilepsy receive no antiepileptic treatment, largely for economic and social reasons. Further epidemiological studies should be a priority to improve understanding of possible risk factors and thereby the prevention of epilepsy in Africa, and action should be taken to improve access to treatment.
Introduction
Epilepsy is a major public health problem: it is common and can have serious physical and psychological consequences, including premature death, traumatic injury, and mental health disorders.1 Epilepsy was defined by the International League Against Epilepsy in 1993 as a condition characterised by recurrent seizures, at least two unprovoked, occurring in a period of more than 24 h.2 The prevalence of epilepsy is higher in less developed than in more developed countries.3,4
The stigma of epilepsy can be profound because it is widely thought to be contagious and associated with witchcraft or evil spirits.5,6 In Tanzanian and Kenyan studies,7,8 disturbed behaviour was significantly more common in children with active epilepsy than in those without the disorder (66 vs 19%; odds ratio [OR] 8·2, 95% CI 4·3–15·6; p<0·001), and children with active epilepsy had more behavioural problems than did those with inactive epilepsy (49% vs 26%; OR 7·86, 95% CI 1·23–50·06; p=0·029).7,8 The Global Burden of Diseases, Injuries, and Risk Factors Study 2010 found that the burden resulting from epilepsy is very high; only one other disorder (HIV infection) had greater disability weight than uncontrolled, severe epilepsy.9–11 Comorbidities greatly affect children with epilepsy in terms of quality of life, and life chances are reduced for adults with epilepsy in terms of employment and marriage.12 Left untreated, people with epilepsy face devastating social consequences, including stigma and discrimination, and premature mortality.13
Although effective antiepileptic drugs are available, a substantial treatment gap is evident in developing countries, because human and financial resources for diagnosis and treatment are limited and misconceptions and stigma surround the disorder.4
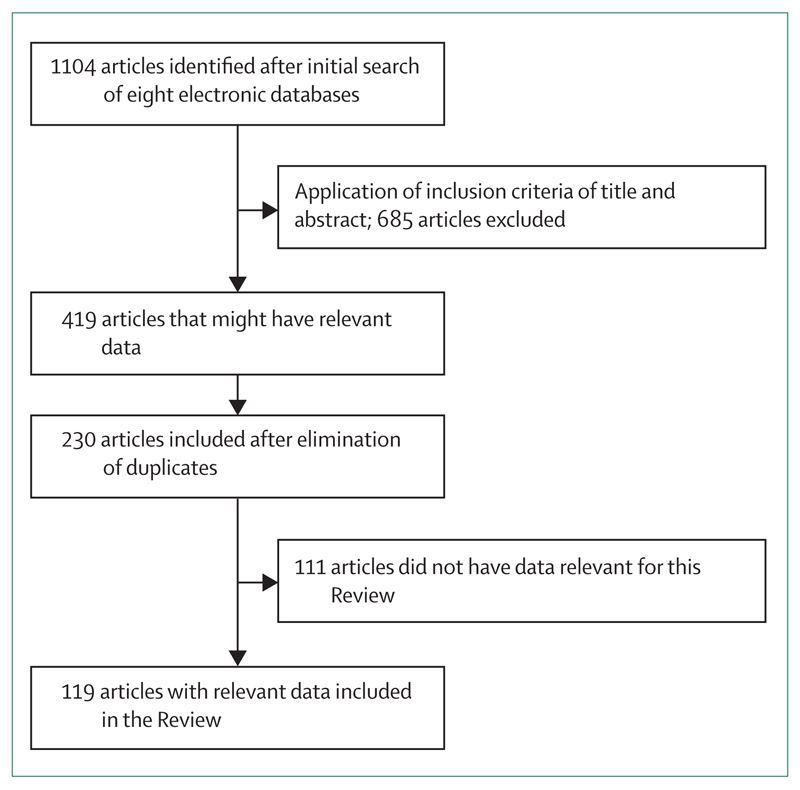
In 2005, Preux and Druet-Cabanac published a Review3 of studies on the epidemiology and aetiology of epilepsy in sub-Saharan Africa, which highlighted the paucity of studies in Africa. Since then, many studies have been published, particularly a major comparison of clinical features across continents14 and a large multisite epidemiological study (figure 1).15 New insights have been gained into the association between several parasitic diseases (malaria, onchocerciasis, cysticercosis, toxocariasis) and epilepsy,16–18 and epileptic syndrome (nodding disease) has re-emerged.19 Thus, an update of understanding of the epidemiology, aetiology, and management of epilepsy in sub-Saharan Africa is timely, to provide an overview of the situation now. We have not reviewed publications on stigma, comorbidities, consequences such as burns, or the role of traditional healers in the management of epilepsy in sub-Saharan Africa.
Figure 1. Flow chart showing selection procedure for articles in this Review.
Incidence
Few studies on the incidence of epilepsy in sub-Saharan Africa have been published, but the overall information suggests a high annual incidence of epilepsy in less developed countries of 81·7 per 100 000 (95% CI 28·0–239·5) compared with 45·0 per 100 000 (30·3–66·7) in more developed countries.20 We identified only eight studies estimating the incidence of epilepsy in sub-Saharan Africa, including four done after 2005 (table 1).21–28 These studies cannot be directly compared with one another because they used different methods.
Table 1. Studies of the incidence of epilepsy in sub-Saharan Africa.
| Year | N | Incidence (95% CI)* | Sex ratio (M/F) | Proportion aged <20 years | Type of study | |
|---|---|---|---|---|---|---|
| Ethiopia21 | 1997 | 61686 | 64·0 (44–84) | 1·2 | 79·0% | Prospective |
| Benin (Djidja)22 | 2013 | 11668 | 69·4 (30–137) | 0·9 | NA | Prospective |
| Tanzania23 | 1992 | 18183 | 73·3 (34–113) | 0·9 | 60·8% | Retrospective |
| Tanzania24 | 2009 | 7399 | 81·0 (65–101) | 1·0 | 59·1% | Prospective |
| Burkina Faso25 | 1993 | 16627 | 83·0 (40–126) | 1·7 | 76·2% | Retrospective |
| Uganda26 | 1998 | 4389 | 156·0 (145–166) | 1·2 | 97·5% | Prospective |
| Kenya27 | 2008 | 10218 | 187·0 (133–256) | 1·0 | NA | Prospective |
| Kenya28 | 2013 | 623004 | 77·0 (68–87) | 0·9 | 54·5% | Retrospective |
NA=not available.
Per 100 000 person-years of follow-up.
Prevalence
The prevalence of epilepsy in sub-Saharan Africa15,23–25,27,29–66 varied both between and within countries (table 2). The variation could result from the heterogeneity of the methods used, which could result from differences in the definitions of epilepsy used,1,2 the nature of the epilepsy studied (lifetime or active), the sample population (general or selected),14 and the incidence of the risk factors.67 Variations can also result from the use of differing screening methods and questionnaires. The most widely used questionnaires were the modified WHO questionnaire67,68 and that from the Institute of Epidemiology and Tropical Neurology, Limoges.69 A 2013 multicentre study, which used the same method in all centres, confirmed that the prevalence of active convulsive epilepsy in sub-Saharan Africa is high and within the range estimated for active epilepsy in countries of low and middle income.15 The prevalence in sub-Saharan Africa is related to the distribution and types of risk factors for epilepsy.
Table 2. Studies of the prevalence of epilepsy in sub-Saharan Africa.
| Year | N | Prevalence, per 1000 (95% CI) | Sex ratio (M/F) | Proportion aged <20 years | Method | Population | |
|---|---|---|---|---|---|---|---|
| West Africa | |||||||
| Benin38 | 2012 | 13046 | 8·0 (6·59–9·74) | 0·7 | 18·1% | DTD | Rural |
| Benin40 | 2007 | 1232 | 10·6 (5·9–18·5) | NA | NA | CS | Urban |
| Benin (Cotonou)37 | 2003 | 1400 | 7·9 (4·5–14·5) | 1·6 | 82·0% | CS | Urban |
| Benin (Dangbo)53 | 2007 | 737 | 31·0 (18·4–43·5) | 0·8 | 45·7% | CS | Rural |
| Benin (Zinvie)46 | 2000 | 3134 | 15·9 (22·3–44·3) | 0·8 | 52·4% | DTD | Rural |
| Burkina Faso25 | 1993 | 16627 | 10·6 (9·1–12·2) | 1·7 | 76·2% | CS | Rural |
| Burkina Faso56 | 2012 | 888 | 45·0 (33·0–60·0) | NA | NA | CS | Rural |
| The Gambia30 | 2002 | 16200 | 4·9 (4·5–5·3) | NA | NA | DTD | Rural |
| Ghana (Kintampo)15 | 2013 | 129812 | 4·9 (4·4–5·3) | 0·8 | NA | DTD | Rural |
| Côte d’lvoire36 | 1988 | 1176 | 7·6 (2·6–12·6) | 0·7 | 88·8% | CS | Rural |
| Côte d’lvoire58 | 1995 | 920 | 59·0 (43·7–74·2) | 1·4 | 36·4% | CS | Rural |
| Côte d’lvoire36 | 1990 | 309 | 74·4 (43·0–104·9) | 0·5 | 91·3% | CS | Rural |
| Liberia51 | 1983 | 4436 | 28·0 (23·1–32·8) | 1·1 | NA | CS | Rural |
| Mali43 | 2000 | 5243 | 13·3 (10·5–16·7) | 0·1 | NA | DTD | Rural |
| Nigeria32 | 1989 | 2925 | 6·2 (3·4–9·0) | 0·1 | 61·5% | CS | Rural |
| Nigeria (Aiyété)55 | 1982 | 903 | 37·0 (24·7–49·3) | 0·6 | NA | CS | Rural |
| Nigeria (Igbo-Ora)31* | 1987 | 18954 | 5·3 (4·2–6·3) | 0·9 | NA | CS | Urban |
| Senegal39 | 1986 | 7682 | 8·3 (6·2–10·4) | NA | 65·6% | CS | Rural |
| Senegal44 | 2005 | 4500 | 14·2 (10·7–17·7) | NA | 39·1% | CS | Urban |
| Togo (Kozah)47 | 1989 | 5264 | 16·7 (13·2–20·2) | 1·6 | NA | CS | Rural |
| Togo (Tone)49* | 2000 | 9155 | 18·6 (15·8–21·3) | 0·9 | NA | DTD | Rural |
| Togo (Batamariba)45* | 2007 | 6249 | 15·7 (12·7–19·2) | 1·4 | 29·6% | CS | Rural |
| East Africa | |||||||
| Ethiopia52* | 2006 | 1154 | 29·5 (19·7–39·3) | 1·1 | 57·0% | DTD | Rural |
| Kenya29 | 1994 | 7450 | 4·0 (2·6–5·4) | 0·8 | 50·6% | DTD | Rural |
| Kenya48 | 1988 | 2960 | 18·2 (13·3–23·0) | NA | NA | DTD | Rural |
| Kenya (Kilifi)27 | 2008 | 10218 | 41·0 (31·0–51·0) | 1·0 | NA | CS | Rural |
| Kenya (Kilifi)15 | 2013 | 233881 | 3·8 (3·5–4·0) | 1·0 | NA | DTD | Rural |
| Kenya62 | 2008 | 151408 | 2·9 (2·6–3·2) | 1·0 | 44·2% | DTD | Rural |
| Tanzania24 | 2009 | 7399 | 13·2 (11·9–14·5) | 1·0 | 59·1% | CS | Rural |
| Tanzania63 | 2012 | 38523 | 2·9 (2·4–3·5) | 1·0 | 23·9% | CS | Rural |
| Tanzania (Hai)64 | 2012 | 104889 | 2·9 (2·5–3·2) | 1·0 | NA | CS | Rural |
| Tanzania (Ifakara)15 | 2013 | 104889 | 7·2 (6·5–7·8) | 1·4 | NA | CS | Rural |
| Tanzania35* | 2005 | 4905 | 7·4 (5·0–9·8) | 0·8 | NA | DTD | Rural |
| Tanzania23* | 1992 | 18183 | 12·1 (10·5–13·7) | 0·9 | 60·8% | CS | Rural |
| Uganda42 | 1996 | 4743 | 13·0 (9·7–16·2) | NA | NA | DTD | Rural |
| Uganda65 | 2010 | 440 | 2·0 (1·94–2·20) | 1·0 | 100% | DTD | Rural |
| Uganda (Igangamayuge)15 | 2013 | 69186 | 5·0 (4·4–5·6) | 1·8 | NA | CS | Rural |
| Central Africa | |||||||
| Cameroon54 | 2007 | 1898 | 35·4 (27·4–43·4) | 1·2 | 89·2% | DTD | Rural |
| Cameroon (Kéleng)61 | 2008 | 181 | 134·5 (90·0–178·0) | 1·2 | NA | DTD | Rural |
| Cameroon (Bilomo)57 | 2000 | 1900 | 58·4 (47·8–69·0) | 0·9 | NA | CS | Rural |
| Cameroon59 | 1989 | 500 | 70·0 (47·6–92·3) | NA | NA | CS | Rural |
| Southern Africa | |||||||
| Madagascar50* | 2004 | 925 | 23·5 (11·6–30·0) | 0·5 | NA | DTD | Urban |
| Rwanda33 | 2008 | 6757 | 7·0 (5·0–9·0) | 0-8 | NA | CS | Rural/urban |
| South Africa34 | 2000 | 6692 | 7·3 (5·3–9·3) | NA | NA | CS | Rural |
| South Africa (Agincourt)15 | 2013 | 82818 | 3·4 (3·0–3·8) | 1·0 | NA | DTD | Rural |
| Zambia41 | 2004 | 55000 | 12·5 (11·6–13·4) | 1·3 | 70·9% | DTD | Rural |
DTD=door-to-door. CS=cross-sectional. NA=not available.
Active epilepsy.
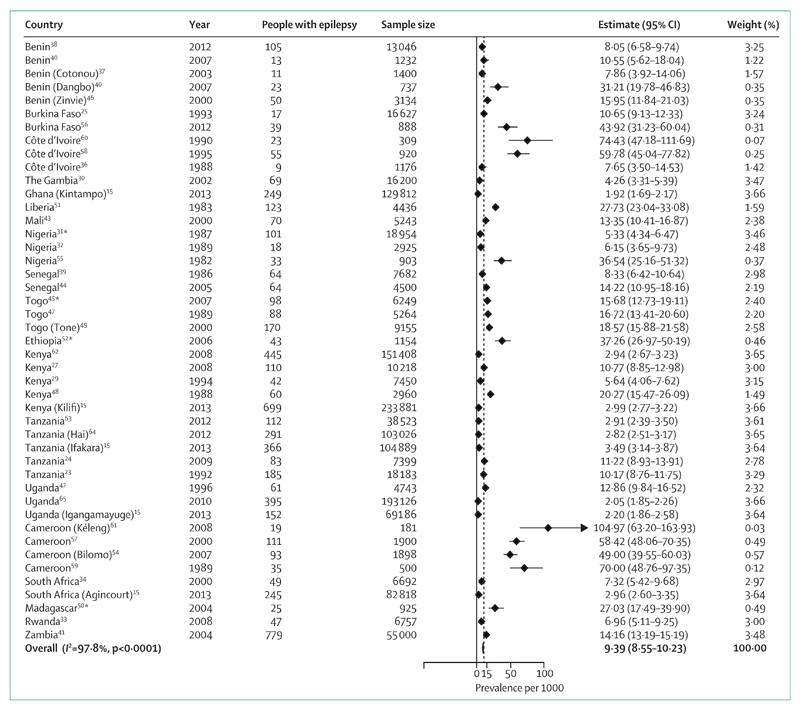
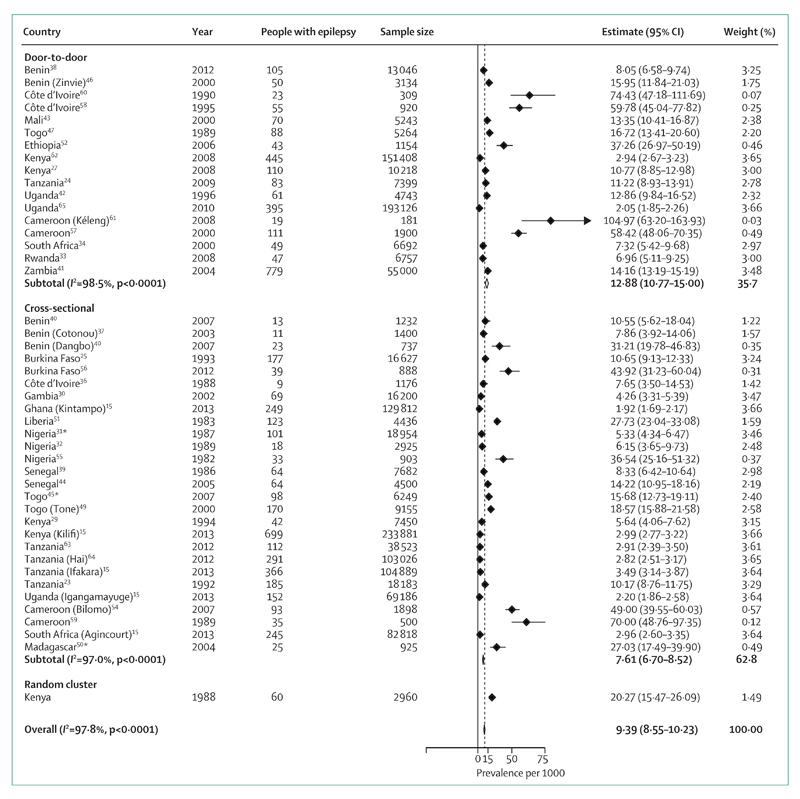
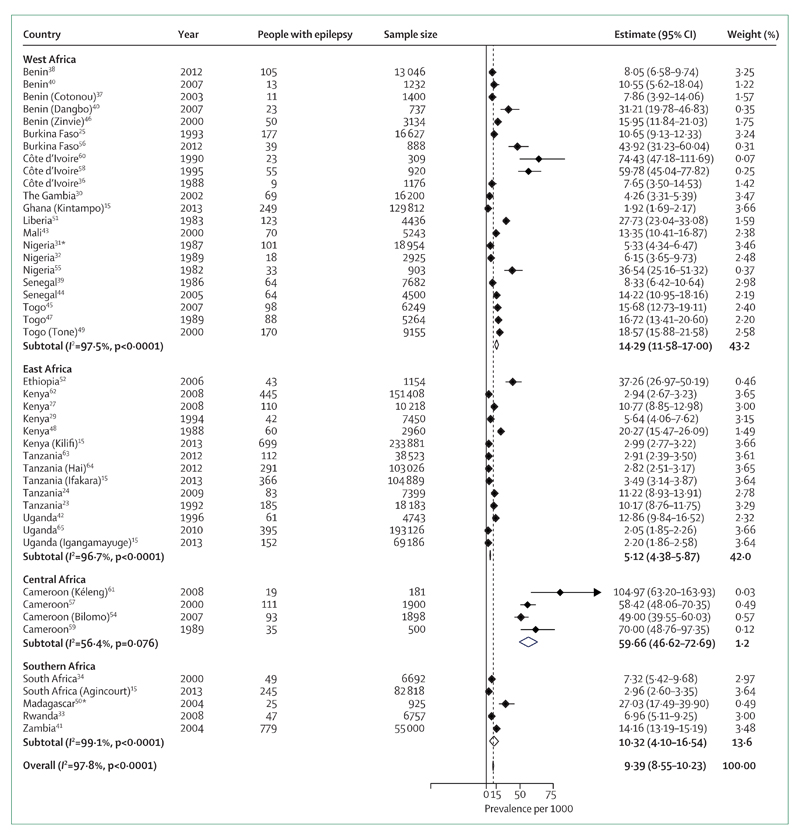
Our meta-analysis (figure 2) shows that the global prevalence of epilepsy in sub-Saharan Africa is 9·39 per 1000. By meta-analysis and meta-regression methods, we were able to calculate mean weighted estimates that took sample size into account. The stratified estimated prevalences according to the method used are shown in figure 3; the prevalence was higher in door-to-door than in cross-sectional studies. Estimates for each geographical African region are shown in figure 4. Although this estimate did not take account of the sample sizes of the included studies, we estimated the median prevalence so that we could compare our results with the 2005 Review.3 The median prevalence was 14·2 per 1000 (IQR 8·0–33·2), similar to that found in 2005 (15·4 per 1000; 5·0–74·0) and higher than that in more developed countries (5·8 per 1000; 2·7–12·4).4,71 In meta-regression, as in meta-analysis, door-to-door studies found higher prevalence than cross-sectional studies (p=0·078; table 3). Studies in west Africa reported significantly higher incidence than those in east Africa (p=0·027), as did those from central Africa (p<0·0001) though the number of studies in that region was small.
Figure 2. Global meta-analysis of epilepsy prevalence in sub-Saharan Africa.
For each item in figures 2–4, the following information was collected: authors, year of publication, journal, country, type of study, study population, method of data collection, and results on prevalence, incidence, seizure types, causes and risk factors, and treatment. Raw or adjusted prevalence expressed as the number of cases per 1000 population was presented in a summary table with 95% CI. Incidence is expressed as number of cases per 100 000 inhabitants per year. 95% CI were calculated by an exact method when not provided in the publication. Median prevalence was calculated from all studies that reported prevalence. Random-effects meta-analysis was done and forest plots obtained. Weighted prevalence rates were calculated.70 Weights were based on the precision of the estimates for each study (ie, SE of prevalence assuming a Poisson distribution for calculation of 95% CI). We also calculated I2, which reflects the percentage of total variation across studies that is due to heterogeneity rather than chance. Because heterogeneity was statistically significant, random-effects models were used. We calculated an overall pooled prevalence as well as one stratified by method (door-to-door vs cross-sectional) and geographical region (west, east, central, and southern Africa). Analyses used Stata v11.1. *Studies in which the estimates are based only on active epilepsy.
Figure 3. Meta-analysis of epilepsy prevalence in sub-Saharan Africa according to the type of study.
*Studies in which the estimates are based only on active epilepsy.
Figure 4. Meta-analysis of epilepsy prevalence in sub-Saharan Africa according to geographical region.
*Studies in which the estimates are based only on active epilepsy.
Table 3. Meta-regression of prevalence of epilepsy in sub-Saharan Africa.
| Prevalence estimate (95% CI) | |
|---|---|
| Door-to-door | |
| East Africa | 12·3 (4·8 to 19·8) |
| West Africa | 22·1 (14·2 to 30·0) |
| Central Africa | 64·8 (48·4 to 81·1) |
| Southern Africa | 13·7 (2·7 to 24·8) |
| Cross-sectional | |
| East Africa | 5·1 (−2·3 to 12·4) |
| West Africa | 14·9 (9·3 to 20·4) |
| Central Africa | 57·5 (41·4 to 73·6) |
| Southern Africa | 6·5 (0 to 18·2) |
Random-effects meta-regression as weighted variance of prevalence was done using Stata v11.1. We introduced explanatory variables as dummy variables in the model. Adjusted R2 of the model was 59%.
Case-fatality rate
Worldwide, mortality among people with epilepsy is reported to be two to three times higher than that in the general population.72 Few studies have estimated the mortality of epilepsy in sub-Saharan Africa; only six were identified for this Review.21,29,30,72–74 One study in Ethiopia in 199721 estimated the crude death rate in the general population as 16·4 per 1000 person-years; for people with epilepsy the estimated death rate was 31·6 per 1000 person-years. A 2 year community study in Kenya29 reported mortality of 3·5 per 100 person-years in people with epilepsy aged over 5 years, with 77% of the deaths occurring during status epilepticus. In a 10-year cohort study in Cameroon,72 the mortality rate was 28·9% in people with epilepsy compared with 4·7% in a control group (OR 5·6, 95% CI 2·0–12·6; p<0·0005).72 In The Gambia, mortality among people with epilepsy was 77 per 1000 person-years over a 2 year period, whereas that of the general population was eight per 1000 person-years (p<0·005).30 A 2007 study in Uganda reported a standardised mortality ratio in 61 patients with epilepsy of 7·2 (95% CI 4·4–11·6; p<0·0001) with a high proportion in patients aged between 10 and 20 years.73 In Kilifi, Kenya, the mortality from active convulsive epilepsy was 33·3 per 1000 person-years 25·9–42·8), with an overall standardised mortality ratio of 6·5 (5·0–8·3).74 Mortality was highest in the age-group 18–24 years. Risk factors for mortality among people with active convulsive epilepsy were non-adherence with antiepileptic drug treatment (adjusted rate ratio 3·4, 1·8–6·2), cognitive impairment (4·6, 2·5–8·3), and age over 50 years (relative risk 4·6, 1·3–15·9). Most deaths (56%) were directly related to epilepsy, with status epilepticus (38%) the most frequent cause of death.
Sociodemographic characteristics
According to a WHO report,75 the incidence of epilepsy in more developed countries is highest in the age-group 30–50 years. By contrast, in less developed countries, especially in sub-Saharan Africa, more than 90% of people with epilepsy are younger than 20 years (table 2).23,25,32,36,37,41,46,54,60 Of these patients, on average, the first seizure occurred before the age of 10 years in 35% and before the age of 20 years in 50% (table 4).
Table 4. Age at onset of seizures.
| Year | Proportion <10 years | Proportion 10–20 years | Proportion >20 years | |
|---|---|---|---|---|
| Benin37 | 2003 | 18% | 82% | NA |
| Benin (Dangbo)53 | 2007 | NA | 74% | 26% |
| Burkina Faso25 | 1993 | 58% | 19% | 23% |
| Burkina Faso56 | 2012 | 42% | 24% | 34% |
| Cameroon76 | 2003 | 25% | 68% | 7% |
| Cameroon61* | 2007 | 14% | 80% | 4% |
| Kenya77 | 2010 | 51% | NA | NA |
| Rwanda33† | 2008 | 55% | 23% | 21% |
NA=not available.
Age unknown in 2%.
Age unknown in 1%.
Most studies of epilepsy in more developed countries find that it is more common in men than in women, but the difference is rarely statistically significant. Outcomes in African countries are similar, although two studies in Benin37,53 found that the prevalence was higher in women than in the men (table 5). Some investigators suggested that the predominance in the female population can be explained by higher male mortality.36,39 Paul and colleagues66 showed that the prevalence of active epilepsy was very similar for the two sexes in age-groups 0–39 years, but the prevalence of active epilepsy was noticeably higher in women than in men in the age-group 40–59 years.66
Table 5. Prevalence of epilepsy in sub-Saharan Africa stratified by sex.
| Year | Male individuals | Female individuals | Prevalence (per 1000) in male population | Prevalence (per 1000) in female population | People with epilepsy | Method | |
|---|---|---|---|---|---|---|---|
| Benin37 | 2003 | 854 (61%) | 546 (39%) | 3·5 | 14·7 | 1400 | CS |
| Benin53 | 2007 | 12 (94%) | 1 (6%) | 10·4 | 13·5 | 13 | CS |
| Benin46 | 2000 | 30 (45%) | 36 (55%) | NA | NA | 66 | DTD |
| Benin38 | 2012 | 54 (51%) | 51 (49%) | 9·7 | 6·8 | 105 | DTD |
| Burkina Faso25 | 1993 | NA | NA | 13·8 | 8·1 | 177 | CS |
| Togo (Kozah)47 | 1989 | 48 (54%) | 30 (34%) | 26·2 | 10·7 | 88 | CS |
| Togo (Batamariba)45 | 2007 | 54 (61%) | 34 (39%) | 17·8 | 13·5 | 98 | CS |
NA=not available. DTD=door–to-door. CS-cross-sectional.
Type of seizure
Only a few study reports describe the distribution of types of seizure. Furthermore, comparison of results from different studies is difficult because of the heterogeneity of classifications used. We have summarised only studies based on the general population (table 6). Screening methods for epilepsy in sub-Saharan Africa, especially in rural areas, cannot generally detect all simple partial seizures or absence seizures. The lack of neurologists and electroencephalographic facilities means that patients having generalised tonic-clonic seizures (average 67%) are more likely to be reported because of their conspicuous presentation. The proportion of secondarily generalised partial seizures (average 8%) will be underestimated because the early stages are difficult to recognise clinically.85
Table 6. Types of seizure among patients with epilepsy in sub-Saharan Africa.
| Year | People with epilepsy | Proportion of patients with seizures of type |
EEG | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| GTC | Absence | PS | PC | PSG | Other | Not classified | ||||
| South Africa34 | 2000 | 49 | 96% | .. | 4% | .. | .. | .. | .. | No |
| Benin46 | 2000 | 66 | 68% | 6% | 6% | .. | 14% | 6% | .. | Yes |
| Benin37 | 2003 | 11 | 37% | 18% | 18% | 9% | .. | 18% | .. | No |
| Benin38 | 2012 | 105 | 80% | .. | 6% | .. | 14% | .. | .. | Yes |
| Burkina Faso25 | 1993 | 177 | 71% | .. | 13% | 16% | .. | .. | .. | No |
| Burundi78 | 2005 | 249 | 59% | .. | .. | .. | 31% | 8% | 2% | Yes |
| Burundi79 | 2007 | 191 | 39% | .. | 61% | .. | .. | .. | <1% | Yes |
| Cameroon80 | 2004 | 125 | 86% | .. | 4% | .. | .. | 9% | 1% | No |
| Cameroon61 | 2008 | 19 | 52% | .. | .. | 16% | 32% | .. | .. | Yes |
| Democratic Republic of the Congo (Mayama)81 | 1995 | 20 | 80% | 5% | .. | .. | 15% | .. | .. | No |
| Democratic Republic of the Congo (Kibouend)81 | 1995 | 24 | 92% | .. | 4% | .. | .. | 4% | .. | No |
| Ethiopia82 | 1990 | 316 | 75% | <1% | 14% | .. | 6% | .. | 5% | Yes |
| Ethiopia52 | 2006 | 82 | 82% | .. | .. | 3% | 6% | 9% | .. | No |
| The Gambia30 | 2002 | 69 | 48% | .. | 2% | 6% | 36% | 8% | .. | No |
| Kenya83 | 1991 | 302 | 59% | .. | .. | .. | 38% | 3% | .. | No |
| Kenya29 | 1994 | 30 | 53% | .. | 3% | 17% | 7% | .. | 20% | NA |
| Kenya27 | 2008 | 110 | 33% | 4% | 9% | 7% | 32% | 13% | 2% | Yes |
| Mali43 | 2000 | 70 | 67% | 7% | .. | .. | 18% | .. | 8% | No |
| Central African Republic84 | 1999 | 208 | 95% | .. | 1% | 4% | .. | .. | .. | NA |
| Senegal39 | 1986 | 64 | 60% | 8% | 16% | .. | 5% | 6% | 5% | No |
| Senegal44 | 2007 | 64 | 78% | .. | 3% | 5% | 14% | .. | .. | NA |
| Tanzania23 | 1992 | 207 | 57% | 1% | 1% | 9% | 22% | .. | 10% | No |
| Tanzania25 | 2009 | 83 | 54% | .. | 1% | .. | 22% | .. | 23% | No |
| Uganda85 | 2000 | 91 | 63% | .. | 24% | .. | .. | .. | 13% | Yes |
| Tanzania63 | 2012 | 112 | 17% | 1% | 2% | 10% | 64% | 3% | 3% | Yes |
| Tanzania 64 | 2012 | 291 | 2% | .. | 72% | .. | .. | .. | 26% | Yes |
| Uganda65 | 2010 | 395 | 61% | .. | 6% | 27% | .. | 6% | .. | Yes |
GTC=generalised tonic-clonic. PS=partial simple. PC=partial complex. PSG=partial secondarily generalised. NA=not available.
Causes and risk factors
The main risk factors for epilepsy in sub-Saharan Africa are family history of seizures, previous febrile seizures, perinatal trauma, head injury and CNS infections such as neurocysticercosis (table 7). However, there is little evidence from Africa on the association between risk factors and disease. The few case-control or cohort studies we identified are shown in table 8.43,79,80,84,86–91
Table 7. Risk factors for epilepsy in sub-Saharan Africa.
| Year | N | Cranial trauma | Perinatal cause | Infections | Tumour | Vascular | Febrile convulsions | Family history | Other or none | |
|---|---|---|---|---|---|---|---|---|---|---|
| Burkina Faso25 | 1993 | 177 | 1% | 10% | 6% | 2% | 2% | NA | 23% | 56% |
| Cameroon54 | 2007 | 66 | 5% | 19% | 10% | NA | NA | NA | 63% | 9% |
| Cameroon61 | 2008 | 181 | NA | NA | 11% | NA | NA | 22% | 100% | 33% |
| Côte d’lvoire58 | 1995 | 55 | 4% | 2% | 13% | NA | NA | 16% | 49% | 16% |
| Democratic Republic of the Congo (Kibouende)81 | 1995 | 24 | NA | NA | NA | NA | NA | NA | 40% | 60% |
| Democratic Republic of the Congo (Mayama)81 | 1995 | 20 | NA | NA | NA | NA | NA | NA | 60% | 40% |
| Ethiopia82 | 1990 | 316 | NA | NA | NA | NA | NA | NA | 32% | 68% |
| Ethiopia21 | 1997 | 139 | 6% | 6% | 1% | NA | 1% | NA | 22% | 64% |
| The Gambia30 | 2002 | 69 | NA | 67% | NA | NA | NA | 31% | 67% | NA |
| Kenya83 | 1991 | 302 | 4% | 6% | 8% | NA | NA | NA | 3% | 79% |
| Liberia51 | 1983 | 123 | 3% | 3% | NA | NA | NA | 38% | 53% | 3% |
| Mali43 | 2000 | 70 | 7% | 36% | 47% | NA | NA | NA | 30% | NA |
| Nigeria31 | 1987 | 100 | 6% | 2% | NA | 1% | 2% | 24% | 5% | 60% |
| Nigeria32 | 1989 | 580 | 3% | 9% | 7% | NA | NA | 6% | NA | 75% |
| Tanzania23 | 1992 | 207 | <1% | 1% | 3% | <1% | <1% | 13% | NA | 75% |
N=number of patients with epilepsy studied. NA=not available
Table 8. Case-control studies of risk factors for epilepsy in sub-Saharan Africa.
| Year | Cases | Controls | Multivariate analysis | Febrile convulsions | Family history | Cranial trauma | Perinatal cause | CNS infection | Onchocerciasis | Cysticercosis | Toxocariasis | Malaria | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Burundi79* | 2007 | 191 | 191 | Yes | NA | NA | NA | NA | NA | NA | NA | 2·1 (1·2–3·8) | NA |
| Burundi86† | 2003 | 324 | 648 | Yes | NA | 3·3 (2·3–4·7) | NA | 1·9 (1·1–3·7) | NA | NA | 4·1 (3·0–5·6) | NA | NA |
| Cameroon80 | 2004 | 93 | 81 | No | NA | NA | NA | NA | NA | NA | NS | NA | NA |
| Mali43 | 2000 | 70 | 140 | No | NA | NA | NA | NA | NA | 1·0 (0·5–2·3; NS) | NA | NA | NA |
| Uganda87 | 2011 | 38 | 38 | No | NA | NA | NA | NA | NA | 1·7 (0·6–4·6; NS) | NA | NA | NA |
| Central African Republic84 | 1999 | 187 | 374 | No | NA | NA | NA | NA | NA | 1·2 (0·8–1·8; NS) | NA | NA | NA |
| Tanzania88‡ | 2001 | 174 | 174 | Yes | 2·9 (1·6–6·2) | 3·3 (1·2–5·8) | NA | 4·5 (1·2–16·1) | 3·7 (0·72–19·6) | NA | NA | NA | NA |
| Tanzania63§ | 2012 | 112 | 113 | Yes | 2·4 (0·8–7·0) | 5·7 (1·0–27·5) | 7·6 (0·6–97·3) | 14·9 (1·4–151) | NA | NA | NA | NA | NA |
| Kenya89 | 2004 | 254 | 273 | No | NA | NA | NA | NA | NA | NA | NA | NA | 4·4 (1·4–13·7) |
| Mali90 | 2006 | 101 | 222 | No | NA | NA | NA | NA | NA | NA | NA | NA | 14·3 (1·6–132) |
| Gabon91 | 2006 | 296 | 296 | No | NA | NA | NA | NA | NA | NA | NA | NA | 3·9 (1·7–8·9) |
Data are odds ratio (95% CI), unless otherwise stated. NA=not available. NS=not significant.
Adjusted for seropositivity for Toxocara canis, seropositivity for cysticercosis, occupation, and religion.
Adjusted for seropositivity for cysticercosis, family history of epilepsy, severe disease during childhood, latrines near house, and exposure to pigs.
Adjusted for family history of epilepsy, febrile convulsions, cranial trauma, and neonatal or intrapartum complications.
Adjusted for perinatal event, head injury, history of febrile seizures, and poor educational attainment.
Genetic factors
A family history of epilepsy is commonly taken as equivalent to genetic risk; but, as environmental exposure common to a family would also produce such findings, this assumption has a major limitation. A family history was found in 6–60% of cases in the studies in sub-Saharan Africa compared with only 5% in the USA.92 To date, no evidence has been found for a dominant gene that could explain familial epilepsy, but individual polymorphisms seem to act synergistically with environmental factors.81 For cultural reasons, consanguineous marriages are common in certain African ethnic groups, which could increase the risk of genetic epilepsy, but this association is not well documented.23,93 Pedigree analysis of 23 patients with epilepsy in a study in Côte d’Ivoire showed that the incidence of the disease was 1·4 times higher in children of consanguineous marriages than in those of non-consanguinous marriages.60 In many cases, heredity has been shown to account for a large proportion of the expression of epilepsy. In 1951, Lennox94 found a high degree of concordance in the occurrence of seizures in monozygotic twins. By contrast, consanguinity in the families of patients with epilepsy was reported in 37% of cases in Mali.43 In a case-control study in Tanzania, a first-degree family history of epilepsy was recorded for 47% of patients compared with 18% of controls; 33% of patients were from consanguineous marriages.88
Perinatal causes
In sub-Saharan Africa, perinatal causes are implicated in between 2% and 65% of cases of epilepsy.30,54,83 Sequelae of birth injuries, often due to a difficult pregnancy or childbirth, can lead to epilepsy. Hypoxia and hypoglycaemia are frequently cited. In the absence of neuroimaging facilities, the association of epilepsy with a prenatal, perinatal, or early postnatal event is difficult. The link is commonly based on the history, which is not always recorded and is subject to recall bias.90 Traditional beliefs and large distances to maternity facilities lead frequently to deliveries at home without qualified assistance.62 A 2012 case-control study showed a strong association (OR 10·2, 95% CI 1·1–93·4) between epilepsy and adverse perinatal events, although this was based only on maternal recall.63 Ngugi and colleagues15 showed that risk factors during the antenatal and perinatal periods were most strongly associated with active convulsive epilepsy in children (population attributable fraction 0·33, 0·21–0·43).
Febrile seizures
Among children, the population most prone to epilepsy, febrile seizures are reported at all health facilities in Africa, and they are commonly associated with epileptic seizures, especially if prolonged.95 The studies in sub-Saharan Africa found that 6–38% of patients with epilepsy had a history of febrile seizures.32,51,61 In a case-control study in Nigeria, Ogunniyi and colleagues96 found an odds ratio of 11 for the association between febrile seizures and epilepsy, adjusted for other factors such as head trauma. In malaria-endemic areas, most acute seizures are caused by malaria, but whether they are febrile seizures or acute symptomatic seizures is unclear. Malaria-associated seizures were implicated in the occurrence of epilepsy in 71% of Tanzanian children in one study.97
Malnutrition
Few studies on a possible link between epilepsy and malnutrition in less developed countries have been published. Such a link has been suspected for many years.3 However, because of attitudes toward epilepsy and food taboos in sub-Saharan Africa, epilepsy could also contribute to malnutrition. In one study in Benin, a link between epilepsy and malnutrition was detected; the prevalence of malnutrition was higher in cases than in controls (22 vs 9%, p=0·0006).98
Neurological infections
Infectious causes that can lead to generalised seizures in equatorial Africa are mainly of parasitic origin. Among these conditions are malaria,88 cysticercosis, onchocerciasis, and toxocariasis.99
Neurocysticercosis is the most common neurological infection and a major cause of epilepsy in many countries in Africa, Asia, and Latin America.100–102 Neurocysticercosis is the main cause of partial epilepsy in adults in areas where Taenia solium is endemic.101,103,104 Cysticercosis is not common in Jewish and Muslim countries (there is little contact between people and pigs, and pork is not eaten) because of the low risk of infection with adult worms or environmental contamination by parasite eggs.105 An estimated 30% of epilepsy in endemic regions results from neurocysticercosis. Winkler17 calculated on the basis of this estimate that the number of people with neurocysticercosis in sub-Saharan Africa, who were therefore at risk of developing epilepsy, was between 1·9 and 6·16 million.17 In many resource-poor areas, with no access to neuroimaging, serological detection of anticysticercal antibodies or cysticercal antigen is the method of choice to assess the prevalence of human cysticercosis or neurocysticercosis.106 The prevalence of neurocysticercosis varies throughout sub-Saharan Africa. A meta-analysis in 2010 on eight African countries found a significant association between cysticercosis and epilepsy (overall OR 3·4, 95% CI 2·7–4·3).104
In Burundi, an OR of 3·8 (2·5–5·1) indicated a strong link between cysticercosis and epilepsy.86 The prevalence of cysticercosis in Togo is estimated at 38 per 1000 in the general population but 135 per 1000 in people with epilepsy.49 Neuroimaging is essential for diagnosis of neurocysticercosis. Access to CT is rare in sub-Saharan Africa. Winkler and colleagues24 found that in rural Tanzania more than half of people with epilepsy and abnormalities on CT had lesions related to neurocysticercosis. They also found that neurocysticercosis-related pathology on CT was significantly more common in people with epilepsy than in controls (38 of 212 [18%] vs ten of 198 [5%]; OR 4·1, 95% CI 2·0–8·5; p<0·0001).107
Another study in Tanzania found that among people with epilepsy, those who had neurocysticercosis were older and had their first seizure later in life than those without the infection.108 Neurocysticercosis should be considered as an underlying cause of epilepsy especially among patients with late-onset seizures. A 2011 study in the Democratic Republic of Congo found a prevalence of T solium antigen of 21·6%; the adjusted prevalence of active epilepsy in the community was 12·7 per 1000.109 Campaigns to increase awareness of the unknown burden of neurocysticercosis and improve prevention and care for patients in endemic areas are greatly needed.
Onchocerciasis (river blindness), caused by Onchocerca volvulus, has also been implicated in seizure disorders. Several studies have reported an association between the prevalence of onchocerciasis and of epilepsy in different areas of east, west, and central Africa,110–113 but the methods used have been subject to substantial criticism and other studies did not confirm the association.114 Studies from Mali,43 Burkina Faso,115 the Central African Republic,84 and Tanzania116 have shown no significant differences in microfilarial density or load between people with and without epilepsy. The Tanzanian study included analysis of CSF, which showed no trace of the parasite.116 A meta-analysis of these epidemiological studies in 2004 found no clear relation between onchocerciasis and epilepsy.117 However, other meta-analyses have shown an association (OR 2·82, 95% CI 1·43–5·56; p<0·005;16 and 2·49, 1·61–3·86; p<0·001118).
In Uganda, a prevalence of epilepsy of six to seven per 1000 was found in areas where onchocerciasis endemicity was below 50% compared with eight to 25 per 1000 where onchocerciasis endemicity was higher than 50%.42 In Nigeria, where the prevalence of onchocerciasis in 11 villages ranged from 8·3% to 36·0%, the highest frequency of the disease and the greatest densities of microfilariae were found in the villages with the highest prevalence of epilepsy.118 In case-control studies, the prevalence of onchocerciasis among patients with epilepsy was 40% versus 36% in controls in the Central African Republic (OR 1·21, 95% CI 0·81–1·80)84 and 24% versus 22% (1·02, 0·47–2·19) in Mali.43 In 2005, Pion and colleagues111 concluded that the average prevalence of epilepsy increased by 0·4% for each 10% increase in the prevalence of onchocerciasis. Overall, the evidence is conflicting and difficult to interpret.
Nodding syndrome is an epilepsy disorder that mainly affects children and has been confirmed only in three African regions so far: northern Uganda, South Sudan, and southern Tanzania.119,120 Nodding syndrome seems to be an epileptic encephalopathy found in children from the age of 5 years and in young adults, characterised by nodding of the head, often precipitated by food.121,122 The disease course in patients deteriorates, with multiple seizures, including myoclonic jerks, malnutrition, and cognitive impairment, and some die.123–125 The latest statistics from the Ugandan Ministry of Health reported about 3000 affected children and 170 deaths.126 Idro and colleagues127 thoroughly described clinical, electrophysiological, and brain imaging features and complications of nodding syndrome in 22 Ugandan children. The aetiology is unknown, but all studies that have assessed the association with onchocerciasis detected a trend toward more positive results for patients than for controls.128 In a study by Foltz and co-workers,129 positive onchocerciasis serology was associated with nodding syndrome (age-adjusted OR 14·4, 2·7–78·3).129 However, onchocerciasis has long been endemic in large parts of west and central Africa, as well as parts of Central and South America, but nodding syndrome has been reported only in small localised areas.119 Further investigations into nodding syndrome are needed to identify the cause, preventive measures, and treatments.
Malaria is one of the tropical parasitic diseases commonly thought to have a role in the development of epilepsy.130 The brain damage that occurs during falciparum malaria is acute encephalopathy, which can be fatal or have polymorphic sequelae. Seizures can occur in children with cerebral malaria who present with neurological sequelae.89,90,131 The first study to show an association between falciparum malaria and epilepsy found that epilepsy occurred in 9% (4·4, 1·4–13·7) of children exposed to cerebral malaria and 12% (6·1, 2·0–18·3) exposed to malaria and complex seizures.89
Two studies that used differing but complementary approaches to epidemiology found a relation between cerebral malaria and epilepsy with an adjusted relative risk of 14·3 (95% CI 1·6–132·0; p=0·01) in Mali90 and an adjusted odds ratio of 3·9 (1·7–8·9; p=0·001) in Gabon.132 In Malawi, 12 of 132 children with retinopathy-positive cerebral malaria developed epilepsy (p<0·0001).133 The other risk factors for epilepsy were a high maximum temperature (39·4°C [SD 1·2] vs 38·5°C [1·1]; p=0·01) and acute seizures (11 of 12 vs 76 of 120; OR 6·37, 95% CI 1·02–141·2).134
Toxocariasis is a zoonotic infection seen mainly in children and transmitted by Toxocara canis or T cati. The association between toxocariasis and epilepsy is known and a significant relationship has been observed in Burundi (OR 2·1, 1·2–3·8).79 A meta-analysis by Quattrocchi and colleagues18 showed a positive association between toxocariasis and epilepsy (OR 1·92, 1·50–2·44; p<0·001). Toxocara was associated with active convulsive epilepsy in five studies in Africa, with a population attributable fraction of 0·16 (0·08–0·24).15 However, two other studies found no significant relation between epilepsy and seropositivity.106,135 A positive association between seropositivity for Toxocara spp and epilepsy can be hypothesised, but even the association shown in the meta-analysis18 does not prove causality. Further studies are needed to estimate the effect of toxocariasis on the global burden of epilepsy.
Traumatic brain injury
In Africa, road accidents are the most common cause of brain injury owing to the lack of traffic regulation and failure to wear a seat belt or helmet (for motorcyclists). Brain damage can also result from accidents, assault, and injury in war, or violent sport.136 The risk of post-traumatic epilepsy depends on the degree and severity of the injury and the resulting complications. In a study in Mali, post-traumatic epilepsy was found in 7% of 70 patients with epilepsy.43
Brain tumours
The prevalence of brain tumours was low in the population in whom seizures occurred.137 A lack of diagnostic equipment, such as CT and MRI, in many developing countries means that brain tumours are not detected, and most are diagnosed in the terminal stages.
Cerebrovascular disease
Epilepsy can be an early or late complication of stroke, which is one of the most common causes of epilepsy in elderly people.138 Most studies in sub-Saharan Africa are biased and provide little reliable information about the incidence and prevalence of stroke. Indeed, because expertise and cerebral imaging are scarce, many cases of stroke are not diagnosed, especially transient ischaemic attacks.139,140 Most available studies of stroke in sub-Saharan Africa are hospital series, so do not reflect the true incidence of cerebral infarction in the population, owing to selection bias. Brain imaging is necessary for confirmation of the types of stroke but is not generally available.141
The reported frequency of epilepsy in cerebrovascular diseases in Africa varies widely (1% to 42%; mean 7% [SD 10]), whereas in more developed countries, 3–4% of patients with epilepsy have a history of cerebral stroke.137
Treatment
The management of epilepsy involves identification of the cause, administration of antiepileptic drugs to control seizures, and the prevention and treatment of the comorbidity. Epilepsy surgery is rarely available in sub-Saharan Africa. Early and appropriate care in more developed countries achieves seizure control in 70–80% of cases.142
The treatment gap is defined as the difference between the number of people with active epilepsy and the number whose seizures are treated appropriately in a given population at a given time, expressed as a percentage. It reflects the proportions seeking treatment for epilepsy and who adhere to the prescribed treatment.143 Support for people with epilepsy is very difficult in Africa, especially in rural areas, for several reasons: the scarcity of knowledgeable staff and investigative resources to ensure a diagnosis;69,144 non-acceptance and non-compliance with care by patients and their families because of their beliefs about the causes of epilepsy; the high cost of drugs and their relative unavailability; and the psychosocial effects of the disease.144–146 Several reasons for the treatment gap identified in a systematic review were lack of skilled manpower to make the diagnosis, cost of treatment, cultural beliefs, and unavailability of antiepileptic drugs.147 The distribution of health-care resources between rural and urban areas is probably crucial.148 A systematic review by WHO reported a treatment gap greater than 95% to exist in Ethiopia, The Gambia, Nigeria, Togo, Uganda, Tanzania, and Zambia.149
Procedures (identification, treatment, and follow-up) that will improve the identification and management of people with epilepsy in rural and semi-rural areas in sub-Saharan Africa must be implemented within the existing system of primary health care and with the participation of the community. The WHO Mental Health Gap Action Programme aims to scale-up services for mental, neurological, and substance-misuse disorders, especially for countries with low and middle income. The programme asserts that with proper care, psychosocial assistance, and medication, tens of millions could be treated for epilepsy, prevented from suicide, and allowed to lead normal lives—even where resources are scarce.150
About 80–85% of people with epilepsy are not treated adequately,151,152 because of economic, cultural, social, and legislative barriers, compounded by the lack of incentive for pharmaceutical companies because drug distribution is not lucrative.153 In sub-Saharan Africa, an average of 59% (95% CI 32–85) of patients do not take any antiepileptic treatment,37,38,52,53 and on average only 33% of patients receiving antiepileptic treatment are properly managed (table 9).29,61,80
Table 9. Proportions of types of treatment and treatment deficit.
| Year | N | Medical | Traditional | Mixed | None | |
|---|---|---|---|---|---|---|
| Benin38 | 2012 | 105 | 13·3% | NA | 29·5% | 57·2% |
| Benin53 | 2007 | 13 | 46·2% | 7·6% | NA | 46·2% |
| Benin37 | 2003 | 11 | 9·1% | 18·2% | 9·1% | 63·6% |
| Cameroon80 | 2004 | 125 | 68·8% | 16·0% | 9·6% | 5·6% |
| Cameroon61 | 2008 | 19 | 68·0% | 26·0% | NA | 6·0% |
| Ethiopia82 | 1990 | 316 | 8·5% | 55·9% | 10·8% | 24·8% |
| Ethiopia52 | 2006 | 82 | 38·0% | 9·0% | 9·0% | 44·0% |
| Kenya29 | 1994 | 30 | 70·0% | 23·0% | NA | 7·0% |
| Mali43 | 2000 | 70 | NA | 61·0% | 35·0% | 4·0% |
| Rwanda33 | 2008 | 47 | 41·0% | 38·0% | NA | 21·0% |
| Senegal44 | 2005 | 64 | 42·2% | 12·5% | 34·4% | 10·9% |
| South | 2000 | 49 | 22·5% | 22·5% | 20·4% | 34·6% |
| Africa34 | ||||||
| Tanzania7 | 2005 | 42 | NA | 35·7% | 4·8% | 59·5% |
NA=not available.
The sensitivity and specificity of self-reported adherence is poor, but by detection of antiepileptic drugs in blood, almost two-thirds of patients with epilepsy were not taking treatment.62,143 The risk factors for not seeking biomedical treatment in Kenya were traditional animistic religious beliefs (adjusted OR 1·85, 1·11–2·71), living more than 30 km from health facilities (3·89, 1·77–8·51), paying for drugs (2·99, 1·82–4·92), having learning difficulties (2·30, 1·29–4·11), having had epilepsy for longer than 10 years (4·60, 2·07–10·2), and having focal seizures (2·28, 1·50–3·47).143 The risk factors for non-adherence, as measured by blood concentrations of antiepileptic drugs, were negative attitudes about epilepsy (1·10, 1·03–1·18) and treatment with antiepileptic drugs for longer than 5 years (3·78, 1·79–7·98).
Phenobarbital is effective in all forms of epilepsy except typical absences. It is especially effective in generalised seizures, either immediately or secondarily, and because of its low cost it is the most prescribed antiepileptic drug worldwide. In Cameroon, phenobarbital is used in 75% of cases, carbamazepine in 15%, and phenytoin in 3%; each is prescribed as monotherapy in 94% of cases.80 With treatment, remission of seizures was observed in 70% of patients, a decrease in frequency in 16%, and failure to improve in 14%. A study in Mali found that almost 60% of the people who took phenobarbital were free of seizures at the last follow-up.154 However, phenobarbital is not always available in most pharmacies. A survey of pharmacies in Zambia found that almost half did not stock any antiepileptic drugs at all and that only a fifth stocked phenobarbital.155 The low availability of antiepileptic drugs in the public sector suggests that poor people are especially disadvantaged in terms of access to the drugs. Oral antiepileptic drugs are more likely to be available in the private than in the public sector, but availability is still inadequate.156 The border regulation procedures for importation of phenobarbital are a constraint; it is deemed to be a narcotic substance.
In most people with epilepsy, antiepileptic treatment is interrupted, resulting in ineffective control and drug resistance. The reasons given for treatment interruption are inconsistent drug supplies to clinics, and the cost to the patients or their families of the antiepileptic drugs and the visit to pick them up from the clinics. For example, in Rwanda, 74% of patients stop their treatment owing to lack of financial resources.33 The morbidity and premature mortality associated with epilepsy and the large economic burden that it imposes on health-care systems can only be mitigated by greater availability of effective antiepileptic treatment.
For the few individuals whose condition does not respond to pharmacotherapy, surgery is the only option for cure. Epilepsy surgery is not available in most of sub-Saharan Africa because of cost, the absence of neurosurgeons, and the lack of infrastructure to maintain advanced technology.157,158
Conclusion
Since the 2005 Review by Preux and Druet-Cabanac,3 several valid epidemiological studies have been carried out in sub-Saharan Africa. However, even now insufficient evidence is available on the incidence and mortality of epilepsy in the region. The use of different methods and variations in sample size and populations studied make the comparison of studies and drawing of robust conclusions very difficult. We have applied random-effects models to take into account this heterogeneity in a meta-analyses, and our meta-regression indicated that methodology and geographical area might explain 60% of variability in prevalence. Epidemiologists therefore must establish standard methodological rules that are applicable in less developed countries. These rules could be adapted from guidelines published lately.159
Despite the variety of survey methods and definitions, these studies consistently show that the prevalence reported in African studies is underestimated and does not reflect reality because of the social stigma surrounding epilepsy. Many people in Africa believe that epilepsy is contagious and some people avoid touching patients, especially during seizures, when some simple forms of help can avoid dangerous situations.160–162 Given the social morbidity of the condition, the need for interventions to reduce stigma is also urgent; few such intervention studies have been done and the capacity to measure stigma meaningfully is limited.
Many risk factors for epilepsy in sub-Saharan Africa are infectious and preventable. Perinatal factors have an important role in the onset of epilepsy, but are still very little studied. Better understanding of the relation between the risk factors and epilepsy is a key issue in improving prevention of epilepsy in Africa.
Finally, despite the importance of epilepsy and the availability of medication that is effective in many cases, epilepsy is rarely a public-health priority in Africa. For economic and social reasons, three-quarters of people with epilepsy worldwide, most of whom live in less developed countries, are not properly treated. Many of the risk factors for the treatment gap can be addressed, but appropriate strategies to prevent epilepsy and reduce the stigma and treatment gap in Africa are urgently needed.
Search strategy and selection criteria.
We searched eight electronic databases: PubMed, African Index Medicus, Scopus, Science Direct, African Journal of Neurological Sciences, African Journal Online, the African Virtual Library of Neurology, and the Sudoc Catalog of PhD Theses. The search was done without date limitation, for all 48 countries that make up sub-Saharan Africa, with the keyword “epilepsy”, combined with each of the following: “epidemiology”, “prevalence”, “incidence”, “aetiology”, “cohort”, “case-control”, “seizure”, and “treatment” for each country. Other keywords also used were: “malaria”, “neurocysticercosis”, “cysticercosis”, “mortality”, “therapy”, “risk factor”, “onchocerciasis”, and “toxocariasis”. The search was restricted to papers published in English or French.
The bibliographies of all articles included were searched for further references. Articles were included if they had at least an abstract in English or French and presented an explicit and correct epidemiological definition of epilepsy according to the International League Against Epilepsy, 1993.2
To avoid methodological biases, we selected only studies that focused on the general population, including cohort, cross-sectional (including door-to-door), and case-control studies; those based only on medical registries were excluded. There was no limitation on the date of publication of articles up to April 30, 2013.
We identified 1104 publications, covering 32 countries in sub-Saharan Africa. No information was found about epilepsy in 16 countries (Angola, Botswana, Cape Verde, Comoros, Djibouti, Eritrea, Guinea, Equatorial Guinea, Guinea-Bissau, Lesotho, Mauritius, Namibia, São Tomé and Príncipe, Seychelles, Somalia, and Chad). 119 articles were selected for review (figure 1). We have aggregated data as much as possible, but the paucity of published work means we also have to discuss some specific results.
Acknowledgments
We thank the Wellcome Trust, UK (083744), which funds CRN.
Footnotes
Contributors
AB-D and P-MP were responsible for the conception of the article. AB-D, MD-C, CRN, and P-MP did the literature review and BM did the statistical analysis. AB-D and P-MP created the tables, and BM and P-MP created the figures. AB-D, BM, EBN, CRN, and P-MP wrote the article. All authors revised the article and were part of the decision to submit for publication.
Declaration of interests
We declare no competing interests.
For the WHO Mental Health Gap Action Programme see http://www.who.int/mental_health/mhgap/en/
For the African Virtual Library of Neurology see http://wwwient.unilim.fr
For the Sudoc Catalog of PhD Theses see http://www.sudoc.abes.fr/
Contributor Information
Awa Ba-Diop, INSERM UMR1094, Tropical Neuroepidemiology, and Institute of Neuroepidemiology and Tropical Neurology, School of Medicine, University of Limoges, Limoges, France.
Benoît Marin, INSERM UMR1094, Tropical Neuroepidemiology, and Institute of Neuroepidemiology and Tropical Neurology, School of Medicine, University of Limoges, Limoges, France; CEBIMER: Center of Epidemiology, Biostatitics, and Research Methodology, CHU Limoges, France.
Prof Michel Druet-Cabanac, INSERM UMR1094, Tropical Neuroepidemiology, and Institute of Neuroepidemiology and Tropical Neurology, School of Medicine, University of Limoges, Limoges, France.
Prof Edgard B Ngoungou, INSERM UMR1094, Tropical Neuroepidemiology, and Institute of Neuroepidemiology and Tropical Neurology, School of Medicine, University of Limoges, Limoges, France; Unit of Neuroepidemiology and Tropical Infectious Diseases, Department of Epidemiology, Biostatistics, University of Health Sciences, Libreville, Gabon.
Prof Charles R Newton, KEMRI/Wellcome Trust Collaborative Programme, Centre for Geographical Medicine, Kilifi, Kenya; Department of Psychiatry, University of Oxford, Oxford, UK.
Prof Pierre-Marie Preux, INSERM UMR1094, Tropical Neuroepidemiology, and Institute of Neuroepidemiology and Tropical Neurology, School of Medicine, University of Limoges, Limoges, France; CEBIMER: Center of Epidemiology, Biostatitics, and Research Methodology, CHU Limoges, France.
References
- 1.Fisher RS, van Emde Boas W, Blume W, et al. Epileptic seizures and epilepsy: definitions proposed by the International League Against Epilepsy (ILAE) and the International Bureau for Epilepsy (IBE) Epilepsia. 2005;46:470–72. doi: 10.1111/j.0013-9580.2005.66104.x. [DOI] [PubMed] [Google Scholar]
- 2.Commission on Epidemiology and Prognosis, International League Against Epilepsy. Guidelines for Epidemiologic Studies on Epilepsy. Epilepsia. 1993;34:592–96. doi: 10.1111/j.1528-1157.1993.tb00433.x. [DOI] [PubMed] [Google Scholar]
- 3.Preux P-M, Druet-Cabanac M. Epidemiology and aetiology of epilepsy in sub-Saharan Africa. Lancet Neurol. 2005;4:21–31. doi: 10.1016/S1474-4422(04)00963-9. [DOI] [PubMed] [Google Scholar]
- 4.Ngugi AK, Bottomley C, Kleinschmidt I, Sander JW, Newton CR. Estimation of the burden of active and life-time epilepsy: a meta-analytic approach. Epilepsia. 2010;51:883–90. doi: 10.1111/j.1528-1167.2009.02481.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mushi D, Hunter E, Mtuya C, Mshana G, Aris E, Walker R. Social-cultural aspects of epilepsy in Kilimanjaro Region, Tanzania: Knowledge and experience among patients and carers. Epilepsy Behav. 2011;20:338–3. doi: 10.1016/j.yebeh.2010.11.016. [DOI] [PubMed] [Google Scholar]
- 6.Viteva E. Impact of stigma on the quality of life of patients with refractory epilepsy. Seizure. 2013;22:64–69. doi: 10.1016/j.seizure.2012.10.010. [DOI] [PubMed] [Google Scholar]
- 7.Burton K, Rogathe J, Hunter E, et al. Behavioural comorbidity in Tanzanian children with epilepsy: a community-based case-control study. Dev Med Child Neurol. 2011;53:1135–42. doi: 10.1111/j.1469-8749.2011.04109.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kariuki SM, Ikumi M, Ojal J, et al. Acute seizures attributable to falciparum malaria in an endemic area on the Kenyan coast. Brain. 2011;134:1519–28. doi: 10.1093/brain/awr051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lozano R, Naghavi M, Foreman K, et al. Global and regional mortality from 235 causes of death for 20 age groups in 1990 and 2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet. 2012;380:2095–128. doi: 10.1016/S0140-6736(12)61728-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Murray CJL, Vos T, Lozano R, et al. Disability-adjusted life years (DALYs) for 291 diseases and injuries in 21 regions, 1990-2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet. 2012;380:2197–223. doi: 10.1016/S0140-6736(12)61689-4. [DOI] [PubMed] [Google Scholar]
- 11.Salomon JA, Vos T, Hogan DR, et al. Common values in assessing health outcomes from disease and injury: disability weights measurement study for the Global Burden of Disease Study 2010. Lancet. 2012;380:2129–43. doi: 10.1016/S0140-6736(12)61680-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Burton K, Rogathe J, Whittaker RG, et al. Co-morbidity of epilepsy in Tanzanian children: a community-based case-control study. Seizure J Br Epilepsy Assoc. 2012;21:169–74. doi: 10.1016/j.seizure.2011.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Vos T, Flaxman AD, Naghavi M, et al. Years lived with disability (YLDs) for 1160 sequelae of 289 diseases and injuries 1990-2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet. 2012;380:2163–96. doi: 10.1016/S0140-6736(12)61729-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yemadje L-P, Houinato D, Quet F, Druet-Cabanac M, Preux P-M. Understanding the differences in prevalence of epilepsy in tropical regions. Epilepsia. 2011;52:1376–81. doi: 10.1111/j.1528-1167.2011.03099.x. [DOI] [PubMed] [Google Scholar]
- 15.Ngugi AK, Bottomley C, Kleinschmidt I, et al. Prevalence of active convulsive epilepsy in sub-Saharan Africa and associated risk factors: cross-sectional and case-control studies. Lancet Neurol. 2013;12:253–63. doi: 10.1016/S1474-4422(13)70003-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kaiser C, Pion SDS, Boussinesq M. Case-control studies on the relationship between onchocerciasis and epilepsy: systematic review and meta-analysis. PLoS Negl Trop Dis. 2013;7:e2147. doi: 10.1371/journal.pntd.0002147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Winkler AS. Neurocysticercosis in sub-Saharan Africa: a review of prevalence, clinical characteristics, diagnosis, and management. Pathog Glob Health. 2012;106:261–74. doi: 10.1179/2047773212Y.0000000047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Quattrocchi G, Nicoletti A, Marin B, Bruno E, Druet-Cabanac M, Preux P-M. Toxocariasis and epilepsy: systematic review and meta-analysis. PLoS Negl Trop Dis. 2012;6:e1775. doi: 10.1371/journal.pntd.0001775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mitchell KB, Kornfeld J, Adiama J, et al. Nodding syndrome in northern Uganda: overview and community perspectives. Epilepsy Behav EB. 2013;26:22–24. doi: 10.1016/j.yebeh.2012.10.030. [DOI] [PubMed] [Google Scholar]
- 20.Ngugi AK, Kariuki SM, Bottomley C, Kleinschmidt I, Sander JW, Newton CR. Incidence of epilepsy: a systematic review and meta-analysis. Neurology. 2011;77:1005–12. doi: 10.1212/WNL.0b013e31822cfc90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tekle-Haimanot R, Forsgren L, Ekstedt J. Incidence of epilepsy in rural central Ethiopia. Epilepsia. 1997;38:541–46. doi: 10.1111/j.1528-1157.1997.tb01138.x. [DOI] [PubMed] [Google Scholar]
- 22.Houinato D, Yemadje L-P, Glitho G, et al. Epidemiology of epilepsy in rural Benin: prevalence, incidence, mortality, and follow-up. Epilepsia. 2013;54:757–63. doi: 10.1111/epi.12082. [DOI] [PubMed] [Google Scholar]
- 23.Rwiza HT, Kilonzo GP, Haule J, et al. Prevalence and incidence of epilepsy in Ulanga, a rural Tanzanian district: a community-based study. Epilepsia. 1992;33:1051–56. doi: 10.1111/j.1528-1157.1992.tb01758.x. [DOI] [PubMed] [Google Scholar]
- 24.Winkler AS, Kerschbaumsteiner K, Stelzhammer B, Meindl M, Kaaya J, Schmutzhard E. Prevalence, incidence, and clinical characteristics of epilepsy–a community-based door-to-door study in northern Tanzania. Epilepsia. 2009;50:2310–13. doi: 10.1111/j.1528-1167.2009.02184.x. [DOI] [PubMed] [Google Scholar]
- 25.Debouverie M, Kaboré J, Dumas M, Giordano C, Gentilini M, Chieze F. Epidemiology of epilepsy in Burkina Faso. Neurol Trop. 1993:57–61. [Google Scholar]
- 26.Kaiser C, Asaba G, Leichsenring M, Kabagambe G. High incidence of epilepsy related to onchocerciasis in West Uganda. Epilepsy Res. 1998;30:247–51. doi: 10.1016/s0920-1211(98)00007-2. [DOI] [PubMed] [Google Scholar]
- 27.Mung’ala-Odera V, White S, Meehan R, et al. Prevalence, incidence and risk factors of epilepsy in older children in rural Kenya. Seizure. 2008;17:396–404. doi: 10.1016/j.seizure.2007.11.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ngugi AK, Bottomley C, Scott JAG, et al. Incidence of convulsive epilepsy in a rural area in Kenya. Epilepsia. 2013;54:1352–59. doi: 10.1111/epi.12236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Snow RW, Williams REM, Rogers JE, Mung'ala VO, Peshu N. The prevalence of epilepsy among a rural Kenyan population: its association with premature mortality. Trop Geogr Med. 1994;46:175–79. [PubMed] [Google Scholar]
- 30.Coleman R, Loppy L, Walraven G. The treatment gap and primary health care for people with epilepsy in rural Gambia. Bull World Health Organ. 2002;80:378–83. [PMC free article] [PubMed] [Google Scholar]
- 31.Osuntokun BO, Adeuja AO, Nottidge VA, et al. Prevalence of the epilepsies in Nigerian Africans: a community-based study. Epilepsia. 1987;28:272–79. doi: 10.1111/j.1528-1157.1987.tb04218.x. [DOI] [PubMed] [Google Scholar]
- 32.Longe AC, Osuntokun BO. Prevalence of neurological disorders in Udo, a rural community in southern Nigeria. Trop Geogr Med. 1989;41:36–40. [PubMed] [Google Scholar]
- 33.Simms V, Atijosan O, Kuper H, Nuhu A, Rischewski D, Lavy C. Prevalence of epilepsy in Rwanda: A national cross-sectional survey. Trop Med Int Health. 2008;13:1047–53. doi: 10.1111/j.1365-3156.2008.02108.x. [DOI] [PubMed] [Google Scholar]
- 34.Christianson AL, Zwane ME, Manga P, Rosen E, Venter A, Kromberg JG. Epilepsy in rural South African children—prevalence, associated disability and management. South Afr Med J Suid-Afr Tydskr Vir Geneeskd. 2000;90:262–66. [PubMed] [Google Scholar]
- 35.Dent W, Helbok R, Matuja WBP, Scheunemann S, Schmutzhard E. Prevalence of active epilepsy in a rural area in south Tanzania: a door-to-door survey. Epilepsia. 2005;46:1963–69. doi: 10.1111/j.1528-1167.2005.00338.x. [DOI] [PubMed] [Google Scholar]
- 36.Kouassi B, Koffi J, Diarra J, et al. Prévalence de l’épilepsie en milieu rural ivoirien: étude pilote. Pub Méd Afr. 1988;89:25–30. [Google Scholar]
- 37.Avode Dossou G, Houinato D, Tevoedjre M, Adjien C, Adoukonou T, Guedou F. Epilepsy in schools in Cotonou (Benin). Epilepsie en Milieu Scolaire a Cotonou (Benin) (English) Afr J Neurol Sci. 2003;22 doi: 10.4314/ajns.v22i2.7539. [DOI] [Google Scholar]
- 38.Yemadje L-P, Houinato D, Boumédiène F, Ngoungou EB, Preux P-M, Druet-Cabanac M. Prevalence of epilepsy in the 15 years and older in Benin: a door-to-door nationwide survey. Epilepsy Res. 2012;99:318–26. doi: 10.1016/j.eplepsyres.2011.12.012. [DOI] [PubMed] [Google Scholar]
- 39.Ndiaye IP, Mauferon JB, Diagne M. Épidémiologie de l’épilepsie au Sénégal. Communication presented to the 7th Congress of the Pan-African Association of Neurological Sciences; Abidjan, Côte d’Ivoire: 1986. Apr 23–30, [Google Scholar]
- 40.Houinato D, Tibarbache H, Houeze F, et al. L’épilepsie en milieu professionnel urbain au Sud-Bénin. Arch Mal Prof Environ. 2007;68:244–50. [Google Scholar]
- 41.Birbeck GL, Kalichi EMN. Epilepsy prevalence in rural Zambia: a door-to-door survey. Trop Med Int Health. 2004;9:92–95. doi: 10.1046/j.1365-3156.2003.01149.x. [DOI] [PubMed] [Google Scholar]
- 42.Kaiser C, Kipp W, Asaba G, et al. The prevalence of epilepsy follows the distribution of onchocerciasis in a west Ugandan focus. Bull World Health Organ. 1996;74:361–67. [PMC free article] [PubMed] [Google Scholar]
- 43.Farnarier G, Diop S, Coulibaly B, et al. Onchocerciasis and epilepsy: epidemiological survey in Mali. Méd Trop Rev Corps Santé Colon. 2000;60:151–55. [PubMed] [Google Scholar]
- 44.Ndoye NF, Sow AD, Diop AG, et al. Prevalence of epilepsy its treatment gap and knowledge, attitude and practice of its population in sub-urban Senegal an ILAE/IBE/WHO study. Seizure J Br Epilepsy Assoc. 2005;14:106–11. doi: 10.1016/j.seizure.2004.11.003. [DOI] [PubMed] [Google Scholar]
- 45.Balogou AAK, Grunitzky EK, Belo M, et al. Management of epilepsy patients in Batamariba district, Togo. Acta Neurol Scand. 2007;116:211–16. doi: 10.1111/j.1600-0404.2007.00871.x. [DOI] [PubMed] [Google Scholar]
- 46.Debrock C, Preux PM, Houinato D, et al. Estimation of the prevalence of epilepsy in the Benin region of Zinvié using the capture-recapture method. Int J Epidemiol. 2000;29:330–35. doi: 10.1093/ije/29.2.330. [DOI] [PubMed] [Google Scholar]
- 47.Dumas M, Grunitzky E, Deniau M, et al. Epidemiological study of neuro-cysticercosis in northern Togo (West Africa) Acta Leiden. 1989;57:191–96. [PubMed] [Google Scholar]
- 48.Kaamugisha J, Feksi AT. Determining the prevalence of epilepsy in the semi-urban population of Nakuru, Kenya, comparing two independent methods not apparently used before in epilepsy studies. Neuroepidemiology. 1988;7:115–21. doi: 10.1159/000110144. [DOI] [PubMed] [Google Scholar]
- 49.Balogou AA, Grunitzky KE, Beketi KA, Bouteille B, Dumas M. Cysticercosis and epilepsy in the city of Tone, north of Togo. Rev Neurol (Paris) 2000;156:270–73. [PubMed] [Google Scholar]
- 50.Andriantseheno L, Ralaizandriny D. Prévalence communautaire de l’épilepsie chez les Malgaches. Epilepsies Montrouge. 2004;16:83–86. [Google Scholar]
- 51.Goudsmit J, van der Waals FW, Gajdusek C. Epilepsy in the Gbawein and Wroughbarh Clan of Grand Bassa County, Liberia: the endemic occurrence of “See-ee” in the native population. Neuroepidemiology. 1983;2:24–34. [Google Scholar]
- 52.Almu S, Tadesse Z, Cooper P, Hackett R. The prevalence of epilepsy in the Zay Society, Ethiopia—an area of high prevalence. Seizure. 2006;15:211–13. doi: 10.1016/j.seizure.2006.01.004. [DOI] [PubMed] [Google Scholar]
- 53.Houinato D, Adjien K, Gnonlonfon D, Adoukonou T, Dema F, Avode D. Etude de la prévalence de l’épilepsie á Dangbo dans le département de l’Oueme au Benin. Benin Méd. 2007;37:14–177. [Google Scholar]
- 54.Njamnshi A, Sini V, Djientcheu V, et al. Risk factors associated with epilepsy in a rural area in Cameroon: a preliminary study. Afr J Neurol Sci. 2007;26:18–26. [Google Scholar]
- 55.Osuntokun BO, Schoenberg BS, Nottidge VA, et al. research protocol for measuring the prevalence of neurologic disorders in developing countries. Neuroepidemiology. 1982;1:143–53. [Google Scholar]
- 56.Nitiéma P, Carabin H, Hounton S, et al. Prevalence case-control study of epilepsy in three Burkina Faso villages. Acta Neurol Scand. 2012;126:270–78. doi: 10.1111/j.1600-0404.2011.01639.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Dongmo L, Ndo D, Atchou G, Njamnshi A. Epilepsie au Sud-Cameroun: enquête préliminaire dans le village Bilomo. Bull Soc Pethol Exot. 2000;93:266–67. [Google Scholar]
- 58.Kaudjhis P. Les agrégats de l’épilepsie de M’brou: approche électroclinique et étiologique. Medical Thesis; Abidjan, Côte-d’Ivoire: 1995. [Google Scholar]
- 59.Nkwi PN, Ndonko FT. The epileptic among the Bamileke of Maham in the Nde Division, West Province of Cameroon. Cult Med Psychiatry. 1989;13:437–48. doi: 10.1007/BF00052050. [DOI] [PubMed] [Google Scholar]
- 60.Kouadjo Y. Génétique et épilepsie: à propos d’un foyer d’épilepsie observé dans un village ivoirien. Medical Thesis; Abidjan, Côte-d’Ivoire: 1990. [Google Scholar]
- 61.Prischich F, De Rinaldis M, Bruno F, et al. High prevalence of epilepsy in a village in the Littoral Province of Cameroon. Epilepsy Res. 2008;82:200–10. doi: 10.1016/j.eplepsyres.2008.09.004. [DOI] [PubMed] [Google Scholar]
- 62.Edwards T, Scott AG, Munyoki G, et al. Active convulsive epilepsy in a rural district of Kenya: a study of prevalence and possible risk factors. Lancet Neurol. 2008;7:50–56. doi: 10.1016/S1474-4422(07)70292-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Burton KJ, Rogathe J, Whittaker R, et al. Epilepsy in Tanzanian children: association with perinatal events and other risk factors. Epilepsia. 2012;53:752–60. doi: 10.1111/j.1528-1167.2011.03395.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Hunter E, Rogathi J, Chigudu S, et al. Prevalence of active epilepsy in rural Tanzania: a large community-based survey in an adult population. Seizure J Br Epilepsy Assoc. 2012;21:691–98. doi: 10.1016/j.seizure.2012.07.009. [DOI] [PubMed] [Google Scholar]
- 65.Duggan MB. Epilepsy in rural Ugandan children: seizure pattern, age of onset and associated findings. Afr Health Sci. 2010;10:218. [PMC free article] [PubMed] [Google Scholar]
- 66.Paul A, Adeloye D, George-Carey R, Kolčič I, Grant L, Chan KY. An estimate of the prevalence of epilepsy in Sub-Saharan Africa: a systematic analysis. J Glob Health. 2012;2:020405. doi: 10.7189/jogh.02.020405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Newton CR, Garcia HH. Epilepsy in poor regions of the world. Lancet. 2012;380:1193–201. doi: 10.1016/S0140-6736(12)61381-6. [DOI] [PubMed] [Google Scholar]
- 68.Placencia M, Sander JW, Shorvon SD, Ellison RH, Cascante SM. Validation of a screening questionnaire for the detection of epileptic seizures in epidemiological studies. Brain J Neurol. 1992;115:783–94. doi: 10.1093/brain/115.3.783. [DOI] [PubMed] [Google Scholar]
- 69.Preux P, Druet-Cabanac M, Debrock C, Tapie P, Dumas M, the Comité de Recherche sur l’Epilepsie de l’Institut d’Epidémiologie Neurologique et de Neurologie Tropicale de Limoges Questionnaire d’investigation de l’épilepsie dans les pays tropicaux. Bull Soc Pathol Exot. 2000;93:276–78. [PubMed] [Google Scholar]
- 70.Higgins JPT, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ. 2003;327:557–60. doi: 10.1136/bmj.327.7414.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Forsgren L. Epidemiology and prognosis of epilepsy and its treatment. In: Shorvon S, Perucca E, Fish D, Dodson E, editors. The treatment of epilepsy. Malden: Blackwell Science Oxford; 2004. pp. 21–42. [Google Scholar]
- 72.Diop AG, Hesdorffer DC, Logroscino G, Hauser WA. Epilepsy and mortality in Africa: a review of the literature. Epilepsia. 2005;46(suppl 11):33–35. doi: 10.1111/j.1528-1167.2005.00405.x. [DOI] [PubMed] [Google Scholar]
- 73.Kaiser C, Asaba G, Kasoro S, Rubaale T, Kabagambe G, Mbabazi M. Mortality from epilepsy in an onchocerciasis-endemic area in West Uganda. Trans R Soc Trop Med Hyg. 2007;101:48–55. doi: 10.1016/j.trstmh.2006.06.003. [DOI] [PubMed] [Google Scholar]
- 74.Ngugi AK. Prevalence, incidence and mortality of epilepsy in four health and demographic surveillance sites in sub-Saharan Africa. London School of Hygiene and Tropical Medicine; (University of London): 2012. PhD thesis. [Google Scholar]
- 75.WHO. La prévention primaire des troubles mentaux, neurologiques et psychosociaux. Geneva: WHO; 1999. [Google Scholar]
- 76.Kamgno J, Pion SDS, Boussinesq M. Demographic impact of epilepsy in Africa: results of a 10-year cohort study in a rural area of Cameroon. Epilepsia. 2003;44:956–63. doi: 10.1046/j.1528-1157.2003.59302.x. [DOI] [PubMed] [Google Scholar]
- 77.Munyoki G, Edwards T, White S, et al. Clinical and neurophysiologic features of active convulsive epilepsy in rural Kenya: A population-based study. Epilepsia. 2010;51:2370–76. doi: 10.1111/j.1528-1167.2010.02653.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Diagana M, Nsengiyumva G, Tuillas M, et al. Electroencephalograms (EEG) in 250 patients with epilepsy in a cysticercosis endemic area in Burundi. Neurophysiol Clin. 2005;35:1–10. doi: 10.1016/j.neucli.2004.12.002. [DOI] [PubMed] [Google Scholar]
- 79.Nicoletti A, Bartoloni A, Sofia V, et al. Epilepsy and toxocariasis: a case-control study in Burundi. Epilepsia. 2007;48:894–99. doi: 10.1111/j.1528-1167.2007.01104.x. [DOI] [PubMed] [Google Scholar]
- 80.Dongmo L, Druet-Cabanac M, Moyou SR, et al. Cysticercosis and epilepsy: a case-control study in Mbam Valley, Cameroon. Bull Société Pathol Exo. 2004;97:105–08. [PubMed] [Google Scholar]
- 81.Petitjeans F, Gandin C, Sturtz F, Fila A, Chazot G, Vandenberghe A. Epilepsie dans les pays en voie de développement: recensement et description de cas dans deux villages congolais. Epilepsies. 1995;7:167–78. [Google Scholar]
- 82.Tekle-Haimanot R, Forsgren L, Abebe M, et al. Clinical and electroencephalographic characteristics of epilepsy in rural Ethiopia: a community-based study. Epilepsy Res. 1990;7:230–39. doi: 10.1016/0920-1211(90)90020-v. [DOI] [PubMed] [Google Scholar]
- 83.Feksi AT, Kaamugisha J, Gatiti S, Sander JWAS, Shorvon SD. A comprehensive community epilepsy programme: The Nakuru project. Epilepsy Res. 1991;8:252–59. doi: 10.1016/0920-1211(91)90072-n. [DOI] [PubMed] [Google Scholar]
- 84.Druet-Cabanac M, Preux PM, Bouteille B, et al. Onchocerciasis and epilepsy: a matched case-control study in the Central African Republic. Am J Epidemiol. 1999;149:565–70. doi: 10.1093/oxfordjournals.aje.a009853. [DOI] [PubMed] [Google Scholar]
- 85.Kaiser C, Benninger C, Asaba G, et al. Clinical and electro-clinical classification of epileptic seizure in west Uganda. Bull Société Pathol Exot. 1990;93:255–59. [PubMed] [Google Scholar]
- 86.Nsengiyumva G, Druet-Cabanac M, Ramanankandrasana B, Bouteille B, Nsizabira L, Preux P-M. Cysticercosis as a major risk factor for epilepsy in Burundi, east Africa. Epilepsia. 2003;44:950–55. doi: 10.1046/j.1528-1157.2003.55302.x. [DOI] [PubMed] [Google Scholar]
- 87.Kaiser C, Rubaale T, Tukesiga E, et al. Association between onchocerciasis and epilepsy in the Itwara hyperendemic focus, West Uganda: Controlling for time and intensity of exposure. Am J Trop Med Hyg. 2011;85:225–28. doi: 10.4269/ajtmh.2011.10-0544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Matuja WB, Kilonzo G, Mbena P, et al. Risk factors for epilepsy in a rural area in Tanzania. A community-based case-control study. Neuroepidemiology. 2001;20:242–47. doi: 10.1159/000054797. [DOI] [PubMed] [Google Scholar]
- 89.Carter JA, Neville BGR, White S, et al. Increased prevalence of epilepsy associated with severe falciparum malaria in children. Epilepsia. 2004;45:978–81. doi: 10.1111/j.0013-9580.2004.65103.x. [DOI] [PubMed] [Google Scholar]
- 90.Ngoungou EB, Dulac O, Poudiougou B, et al. Epilepsy as a consequence of cerebral malaria in area in which malaria is endemic in Mali, West Africa. Epilepsia. 2006;47:873–79. doi: 10.1111/j.1528-1167.2006.00558.x. [DOI] [PubMed] [Google Scholar]
- 91.Ngoungou EB, Koko J, Druet-Cabanac M, et al. Cerebral malaria and sequelar epilepsy: first matched case-control study in Gabon. Epilepsia. 2006;47:2147–53. doi: 10.1111/j.1528-1167.2006.00890.x. [DOI] [PubMed] [Google Scholar]
- 92.Ottman R, Lee JH, Hauser WA, Risch N. Birth cohort and familial risk of epilepsy: the effect of diminished recall in studies of lifetime prevalence. Am J Epidemiol. 1995;141:235–41. doi: 10.1093/oxfordjournals.aje.a117425. [DOI] [PubMed] [Google Scholar]
- 93.WHO. Global campaign against epilepsy: out of the shadows. Geneva: [accessed July 19, 2014]. http://www.who.int/mental_health/management/globalepilepsycampaign/en/ [Google Scholar]
- 94.Lennox WG. The heredity of epilepsy as told by relatives and twins. JAMA. 1951;146:529–36. doi: 10.1001/jama.1951.03670060005002. [DOI] [PubMed] [Google Scholar]
- 95.Diop AG, de Boer HM, Mandlhate C, Prilipko L, Meinardi H. The global campaign against epilepsy in Africa. Acta Trop. 2003;87:149–59. doi: 10.1016/s0001-706x(03)00038-x. [DOI] [PubMed] [Google Scholar]
- 96.Ogunniyi A, Osuntokun BO, Bademosi O, Adeuja AO, Schoenberg BS. Risk factors for epilepsy: case-control study in Nigerians. Epilepsia. 1987;28:280–85. doi: 10.1111/j.1528-1157.1987.tb04219.x. [DOI] [PubMed] [Google Scholar]
- 97.Matuja WB. Aetiological factors in Tanzanian epileptics. East Afr Med J. 1989;66:343–8. [PubMed] [Google Scholar]
- 98.Crepin S, Houinato D, Nawana B, Avode GD, Preux P-M, Desport J-C. Link between epilepsy and malnutrition in a rural area of Benin. Epilepsia. 2007;48:1926–33. doi: 10.1111/j.1528-1167.2007.01159.x. [DOI] [PubMed] [Google Scholar]
- 99.Rothenberg G, Schubert S. Assessment of late complications of malaria in travelers to the tropics. Z Für Gesamte Inn Med Ihre Grenzgeb. 1983;38:46–7. [PubMed] [Google Scholar]
- 100.Dada TO. Parasites and epilepsy in Nigeria. Trop Geogr Med. 1970;22:313–22. [PubMed] [Google Scholar]
- 101.Cruz M, Schantz P, Cruz I, et al. Epilepsy and neurocysticercosis in an Andean community. Int J Epidemiol. 1999;28:799–803. doi: 10.1093/ije/28.4.799. [DOI] [PubMed] [Google Scholar]
- 102.Medina MT, Dubon-Murcia SA, Aguilar-Estrada RL, Chaves-Sell F, Bu J. Neurocysticercosis and epilepsy. Epilepsies. 2010;22:126–33. [Google Scholar]
- 103.Nicoletti A, Bartoloni A, Reggio A, et al. Epilepsy, cysticercosis, and toxocariasis: a population-based case-control study in rural Bolivia. Neurology. 2002;58:1256–61. doi: 10.1212/wnl.58.8.1256. [DOI] [PubMed] [Google Scholar]
- 104.Quet F, Guerchet M, Pion SDS, Ngoungou EB, Nicoletti A, Preux P-M. Meta-analysis of the association between cysticercosis and epilepsy in Africa. Epilepsia. 2010;51:830–37. doi: 10.1111/j.1528-1167.2009.02401.x. [DOI] [PubMed] [Google Scholar]
- 105.Ibrahim N, Azman R, Basri H, Phadke P. Neurocysticercosis in a Malaysian muslim. Neurol J Southeast Asia. 2003;8:45–48. [Google Scholar]
- 106.Winkler AS, Blocher J, Auer H, Gotwald T, Matuja W, Schmutzhard E. Anticysticercal and antitoxocaral antibodies in people with epilepsy in rural Tanzania. Trans R Soc Trop Med Hyg. 2008;102:1032–38. doi: 10.1016/j.trstmh.2008.05.004. [DOI] [PubMed] [Google Scholar]
- 107.Winkler AS, Blocher J, Auer H, Gotwald T, Matuja W, Schmutzhard E. Epilepsy and neurocysticercosis in rural Tanzania—An imaging study. Epilepsia. 2009;50:987–93. doi: 10.1111/j.1528-1167.2008.01867.x. [DOI] [PubMed] [Google Scholar]
- 108.Blocher J, Schmutzhard E, Wilkins PP, et al. A cross-sectional study of people with epilepsy and neurocysticercosis in Tanzania: clinical characteristics and diagnostic approaches. PLoS Negl Trop Dis. 2011;5:e1185. doi: 10.1371/journal.pntd.0001185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Kanobana K, Praet N, Kabwe C, et al. High prevalence of Taenia solium cysticerosis in a village community of Bas-Congo, Democratic Republic of Congo. Int J Parasitol. 2011;41:1015–18. doi: 10.1016/j.ijpara.2011.06.004. [DOI] [PubMed] [Google Scholar]
- 110.Kipp W, Kasoro S, Burnham G. Onchocerciasis and epilepsy in Uganda. Lancet. 1994;343:183–84. doi: 10.1016/s0140-6736(94)90980-6. [DOI] [PubMed] [Google Scholar]
- 111.Pion SDS, Kalser C, Boutros-Toni F, et al. Epilepsy in onchocerciasis endemic areas: Systematic review and meta-analysis of population-based surveys. PLoS Negl Trop Dis. 2009;3:e461. doi: 10.1371/journal.pntd.0000461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Boussinesq M, Pion SDS, Demanga-Ngangue Kamgno J. Relationship between onchocerciasis and epilepsy: a matched case-control study in the Mbam Valley, Republic of Cameroon. Trans R Soc Trop Med Hyg. 2002;96:537–41. doi: 10.1016/s0035-9203(02)90433-5. [DOI] [PubMed] [Google Scholar]
- 113.Pion SDS, Boussinesq M. Significant association between epilepsy and presence of onchocercal nodules: case-control study in Cameroon. Am J Trop Med Hyg. 2012;86:5577. doi: 10.4269/ajtmh.2012.11-0603a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Preux P-M, Marin B, Druet-Cabanac M, Farnarier G. Onchocercose et épilepsie. Epilepsies. 2010;22:116–19. [Google Scholar]
- 115.Kabore JK, Cabore JW, Melaku Z, Druet-Cabanac M, Preux PM. Epilepsy in a focus of onchocerciasis in Burkina Faso. Lancet. 1996;347:836. doi: 10.1016/s0140-6736(96)90917-4. [DOI] [PubMed] [Google Scholar]
- 116.König R, Nassri A, Meindl M, et al. The role of Onchocerca volvulus in the development of epilepsy in a rural area of Tanzania. Parasitology. 2010;137:1559–68. doi: 10.1017/S0031182010000338. [DOI] [PubMed] [Google Scholar]
- 117.Druet-Cabanac M, Boussinesq M, Dongmo L, Farnarier G, Bouteille B, Preux PM. Review of epidemiological studies searching for a relationship between onchocerciasis and epilepsy. Neuroepidemiology. 2004;23:144–49. doi: 10.1159/000075958. [DOI] [PubMed] [Google Scholar]
- 118.Dozie INS, Onwuliri COE, Nwoke BEB, et al. Onchocerciasis and epilepsy in parts of the Imo river basin, Nigeria: a preliminary report. Public Health. 2006;120:448–50. doi: 10.1016/j.puhe.2005.10.010. [DOI] [PubMed] [Google Scholar]
- 119.Reik L, Abubakar A, Opoka M, et al. Nodding Syndrome—South Sudan, 2011. Cent Dis Control Prev CDC. 2012;61:52–54. [PubMed] [Google Scholar]
- 120.Donnelly J. CDC planning trial for mysterious nodding syndrome. Lancet. 2012;379:299. doi: 10.1016/s0140-6736(12)60126-3. [DOI] [PubMed] [Google Scholar]
- 121.Winkler AS, Friedrich K, Meindl M, et al. Clinical characteristics of people with head nodding in southern Tanzania. Trop Doct. 2010;40:173–75. doi: 10.1258/td.2010.090373. [DOI] [PubMed] [Google Scholar]
- 122.Sejvar JJ, Kakooza AM, Foltz JL, et al. Clinical, neurological, and electrophysiological features of nodding syndrome in Kitgum, Uganda: an observational case series. Lancet Neurol. 2013;12:166–74. doi: 10.1016/S1474-4422(12)70321-6. [DOI] [PubMed] [Google Scholar]
- 123.Dowell SF, Sejvar JJ, Riek L, et al. Nodding Syndrome. Emerg Infect Dis. 2013;19:1374–73. doi: 10.3201/eid1909.130401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Winkler AS, Friedrich K, König R, et al. The head nodding syndrome—clinical classification and possible causes. Epilepsia. 2008;49:2008–15. doi: 10.1111/j.1528-1167.2008.01671.x. [DOI] [PubMed] [Google Scholar]
- 125.Spencer PS, Vandemaele K, Richer M, et al. Nodding syndrome in Mundri county, South Sudan: Environmental, nutritional and infectious factors. Afr Health Sci. 2013;13:183–204. doi: 10.4314/ahs.v13i2.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Mitchell KB, Kornfeld J, Adiama J, et al. Nodding syndrome in northern Uganda: overview and community perspectives. Epilepsy Behav. 2013;26:22–24. doi: 10.1016/j.yebeh.2012.10.030. [DOI] [PubMed] [Google Scholar]
- 127.Idro R, Opoka RO, Aanyu HT, et al. Nodding syndrome in Ugandan children—clinical features, brain imaging and complications: a case series. BMJ Open. 2013;3:e002540. doi: 10.1136/bmjopen-2012-002540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Tumwine J, Vandemaele K, Chungong S, et al. Clinical and epidemiologic characteristics of nodding syndrome in Mundri County, southern Sudan. Afr Health Sci. 2012;12:242–48. doi: 10.4314/ahs.v12i3.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Foltz JL, Makumbi I, Sejvar JJ, et al. An epidemiologic investigation of potential risk factors for nodding syndrome in Kitgum District, Uganda. PloS One. 2013;8:e66419. doi: 10.1371/journal.pone.0066419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Ngoungou EB, Niare-Doumbo S, Preux P-M, Doumbo O. Paludisme et épilepsie en zone tropicale. Epilepsies. 2010;22:111–15. [Google Scholar]
- 131.Ngoungou EB, Preux P-M. Cerebral malaria and epilepsy. Epilepsia. 2008;49(suppl 6):19–24. doi: 10.1111/j.1528-1167.2008.01752.x. [DOI] [PubMed] [Google Scholar]
- 132.Postels DG, Taylor TE, Molyneux M, et al. Neurologic outcomes in retinopathy-negative cerebral malaria survivors. Neurology. 2012;79:1268–72. doi: 10.1212/WNL.0b013e31826aacd4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Birbeck GL, Molyneux ME, Kaplan PW, et al. Blantyre Malaria Project Epilepsy Study (BMPES) of neurological outcomes in retinopathy-positive paediatric cerebral malaria survivors: a prospective cohort study. Lancet Neurol. 2010;9:1173–81. doi: 10.1016/S1474-4422(10)70270-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Birbeck GL, Beare N, Lewallen S, et al. Identification of malaria retinopathy improves the specificity of the clinical diagnosis of cerebral malaria: findings from a prospective cohort study. Am J Trop Med Hyg. 2010;82:231–34. doi: 10.4269/ajtmh.2010.09-0532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Akyol A, Bicerol B, Ertug S, Ertabaklar H, Kiylioglu N. Epilepsy and seropositivity rates of Toxocara canis and Toxoplasma gondii. Seizure. 2007;16:233–37. doi: 10.1016/j.seizure.2006.12.008. [DOI] [PubMed] [Google Scholar]
- 136.Silberberg D, Katabira E. Neurological Disorders. In: Jamison DT, Feachem RG, Makgoba MW, et al., editors. Disease and Mortality in Sub-Saharan Africa. 2nd edn. Washington DC: World Bank; 2006. [accessed Nov 9, 2013]. http://www.ncbi.nlm.nih.gov/books/NBK2295/ [Google Scholar]
- 137.Sander JW, Shorvon SD. Epidemiology of the epilepsies. J Neurol Neurosurg Psychiatry. 1996;61:433–3. doi: 10.1136/jnnp.61.5.433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Silverman IE, R L. Poststroke seizures. Arch Neurol. 2002;59:195–201. doi: 10.1001/archneur.59.2.195. [DOI] [PubMed] [Google Scholar]
- 139.Howitt SC, Jones MP, Jusabani A, et al. A cross-sectional study of quality of life in incident stroke survivors in rural northern Tanzania. J Neurol. 2011;258:1422–30. doi: 10.1007/s00415-011-5948-6. [DOI] [PubMed] [Google Scholar]
- 140.Walker R, Whiting D, Unwin N, et al. Stroke incidence in rural and urban Tanzania: a prospective, community-based study. Lancet Neurol. 2010;9:786–92. doi: 10.1016/S1474-4422(10)70144-7. [DOI] [PubMed] [Google Scholar]
- 141.Sagui E. Les accidents vasculaires cerebraux en Afrique subsaharienne. Médecine Trop. 2007;67:596–600. [PubMed] [Google Scholar]
- 142.De Toffol B. Epilepsies: étiologie, diagnostic, évolution, pronostic, traitement. Rev Prat. 1995;45:885–91. [PubMed] [Google Scholar]
- 143.Mbuba CK, Ngugi AK, Fegan G, et al. Risk factors associated with the epilepsy treatment gap in Kilifi, Kenya: a cross-sectional study. Lancet Neurol. 2012;11:688–96. doi: 10.1016/S1474-4422(12)70155-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Diop A, Ndiaye M, Diagne M, et al. Filières des soins anti-épileptiques en Afrique. Epilepsies. 1998;10:115–21. [Google Scholar]
- 145.Adamolekun B, Meinardi H. Problems of drug therapy of epilepsy in developing countries. Trop Geogr Med. 1990;42:178–81. [PubMed] [Google Scholar]
- 146.Kaiser C, Asaba G, Mugisa C, et al. Antiepileptic drug treatment in rural Africa: involving the community. Trop Doct. 1998;28:73–77. doi: 10.1177/004947559802800206. [DOI] [PubMed] [Google Scholar]
- 147.Mbuba CK, Ngugi AK, Newton CR, Carter JA. The epilepsy treatment gap in developing countries: a systematic review of the magnitude, causes, and intervention strategies. Epilepsia. 2008;49:1491–503. doi: 10.1111/j.1528-1167.2008.01693.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Meyer A-CL, Dua T, Boscardin WJ, Escarce JJ, Saxena S, Birbeck GL. Critical determinants of the epilepsy treatment gap: a cross-national analysis in resource-limited settings. Epilepsia. 2012;53:2178–85. doi: 10.1111/epi.12002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Meyer A-C, Dua T, Ma J, Saxena S, Birbeck G. Global disparities in the epilepsy treatment gap: a systematic review. Bull World Health Organ. 2010;88:260–66. doi: 10.2471/BLT.09.064147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.WHO. [accessed Nov 8, 2013];WHO Mental Health Gap Action Programme (mhGAP) http://www.who.int/mental_health/mhgap/en/
- 151.Meinardi H, Scott RA, Reis R, Sander JW. The treatment gap in epilepsy: the current situation and ways forward. Epilepsia. 2001;42:136–9. doi: 10.1046/j.1528-1157.2001.32800.x. [DOI] [PubMed] [Google Scholar]
- 152.Ratsimbazafy V, Andrianabelina R, Randrianarisona S, Preux P-M, Odermatt P. Treatment gap for people living with epilepsy in Madagascar. Trop Doct. 2011;41:38–39. doi: 10.1258/td.2010.100254. [DOI] [PubMed] [Google Scholar]
- 153.Ilangaratne NB, Mannakkara NN, Bell GS, Sander JW. Phenobarbital: missing in action. Bull World Health Organ. 2012;90:871–71A. doi: 10.2471/BLT.12.113183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Bruno E, Nimaga K, Foba I, et al. Results of an Action-Research on Epilepsy in Rural Mali. PLoS One. 2012;7:e44469. doi: 10.1371/journal.pone.0044469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Chomba EN, Haworth A, Mbewe E, et al. The Current Availability of Antiepileptic Drugs in Zambia: Implications for the ILAE/WHO “Out of the Shadows” Campaign. Am J Trop Med Hyg. 2010;83:571–74. doi: 10.4269/ajtmh.2010.10-0100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Cameron A, Bansal A, Dua T, et al. Mapping the availability, price, and affordability of antiepileptic drugs in 46 countries. Epilepsia. 2012;53:962–69. doi: 10.1111/j.1528-1167.2012.03446.x. [DOI] [PubMed] [Google Scholar]
- 157.Boling W, Palade A, Wabulya A, et al. Surgery for pharmacoresistant epilepsy in the developing world: A pilot study. Epilepsia. 2009;50:1256–61. doi: 10.1111/j.1528-1167.2008.01984.x. [DOI] [PubMed] [Google Scholar]
- 158.Tran DS, Ngoungou EB, Quet F, Preux PM. Prise en charge de l’épilepsie dans les pays en développement. Med Trop (Mars) 2007;67:635–3. [PubMed] [Google Scholar]
- 159.Thurman DJ, Beghi E, Begley CE, et al. Standards for epidemiologic studies and surveillance of epilepsy. Epilepsia. 2011;52(suppl 7):2–26. doi: 10.1111/j.1528-1167.2011.03121.x. [DOI] [PubMed] [Google Scholar]
- 160.Osungbade KO, Siyanbade SL. Myths, misconceptions, and misunderstandings about epilepsy in a Nigerian rural community: implications for community health interventions. Epilepsy Behav. 2011;21:425–29. doi: 10.1016/j.yebeh.2011.05.014. [DOI] [PubMed] [Google Scholar]
- 161.Rafael F, Houinato D, Nubukpo P, et al. Sociocultural and psychological features of perceived stigma reported by people with epilepsy in Benin. Epilepsia. 2010;51:1061–68. doi: 10.1111/j.1528-1167.2009.02511.x. [DOI] [PubMed] [Google Scholar]
- 162.Rwiza HT, Matuja WB, Kilonzo GP, et al. Knowledge, attitude, and practice toward epilepsy among rural Tanzanian residents. Epilepsia. 1993;34:1017–23. doi: 10.1111/j.1528-1157.1993.tb02127.x. [DOI] [PubMed] [Google Scholar]