Diabetes is a highly prevalent condition worldwide, currently representing the seventh cause of death in the United States. The American Diabetes Association (ADA) estimates that up to 29.1 million Americans (i.e., 9.3% of the general population) suffer from diabetes, and as many as 8.1 million of these remain undiagnosed (1). A similar prevalence of disease has been reported in China, with an estimated 114 million Chinese adults affected by diabetes, so accounting for approximately 12% of the general population (2). These figures are especially concerning if one considers that diabetes has many unfavorable consequences on patient health, healthcare sustainability and society. This is mainly due to the many well-known complications of untreated or poorly managed diabetes, which include both microvascular (i.e., retinopathy, nephropathy, and neuropathy) and macrovascular (e.g., coronary heart disease and cerebral ischemic disease) disorders.
According to the ADA guidelines, which are actually the most followed recommendations worldwide, four criteria can be used for diagnosing diabetes, as summarized in Table 1. These essentially include the measurement of hemoglobin A1c (HbA1c), the assessment of fasting plasma glucose (FPG), the oral glucose tolerance test (OGTT) or the occasional finding of a random plasma glucose value >11.1 mmol/L (i.e., >200 mg/dL) (3).
Table 1. American Diabetes Association (ADA) criteria for diagnosing diabetes.
| Hemoglobin A1c (HbA1c) ≥6.5% (48 mmol/mol) |
| Fasting plasma glucose (FPG) ≥7.0 mmol/L (126 mg/dL) |
| 2-h plasma glucose ≥11.1 mmol/L (200 mg/dL) during an oral glucose tolerance test (OGTT) |
| Symptoms of hyperglycemia and random plasma glucose concentration ≥11.1 mmol/L (200 mg/dL) |
The main preanalytical issue emerging from the use of FPG for diagnosing diabetes is the progressive decrease of glucose concentration in whole (uncentrifuged) blood, which is attributable to blood cells (especially erythrocytes) metabolism. Briefly, glucose concentration may decrease by approximately 4–5% per hour in serum or lithium-heparin blood tubes kept at room temperature before centrifugation (4), thus making glucose measurement highly unreliable in this sample matrix when blood tubes cannot be immediately centrifuged and the plasma timely separated from blood cells, as increasingly occurring due to the worldwide consolidation process of small laboratories in larger facilities (5). To overcome this issue, it has been formerly recommended to measure FPG in blood tubes containing lithium-heparin and sodium fluoride (NaF) and/or potassium oxalate (KOx), which both work as rapid glycolysis inhibitors (6). Nevertheless, the efficiency of these compounds to stably inhibit glycolysis has recently been questioned. Bonetti et al. showed that glucose concentration measured in lithium-heparin blood tubes containing NaF exhibited a 6.8% and 8.0% decrease after 1 and 2 hours of storage at room temperature, respectively (4). Similar results were published in an elegant study by Juricic et al. who also showed that glucose concentration decreased by approximately 4% per hour in uncentrifuged serum tubes, but that also displayed a sharp decrease in NaF/KOx blood tubes stored for 1 hour at room temperature before centrifugation (7). In another study, Roccaforte et al. also showed that the concentration of glucose was decreased by over 6% in NaF/KOx tubes stored for 2.5 hours at room temperature before centrifugation (8). The general trend of blood glucose decay, reflecting data published in these and other studies, is shown in Figure 1.
Figure 1.
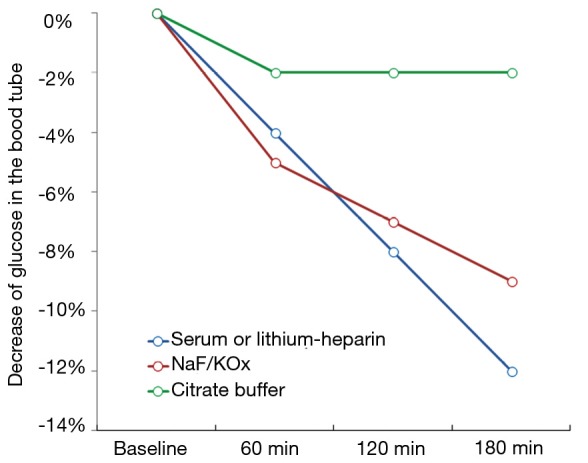
General trend of blood glucose concentration in uncentrifuged blood tubes containing different additives. NaF, sodium fluoride; KOx, potassium oxalate.
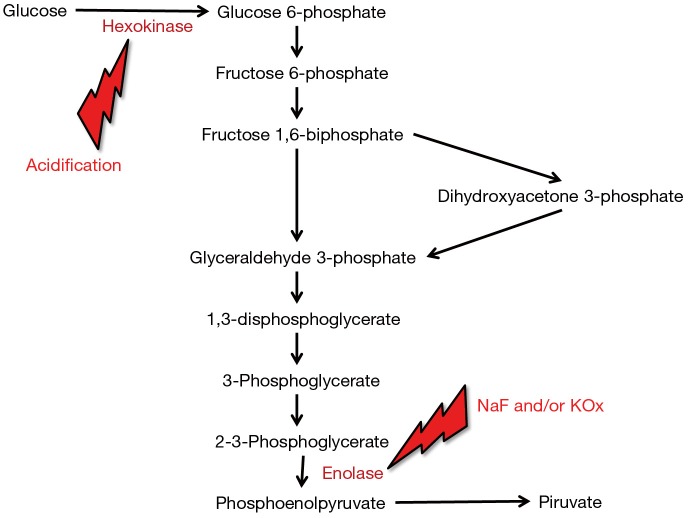
The unquestionable evidence that the total inhibition of glycolysis by NaF and/or KOx can take up to 4 h, during which glucose concentration may decrease between 9–12% in samples stored at room temperature, led the way to the search for more efficient glycolysis inhibitors. The main problem here is that NaF and/or KOx are efficient inhibitors of enolase, which only acts at a late stage of the glycolytic pathway. Unlike enolase, the enzyme hexokinase catalyzes the conversion of glucose to glucose-6-phosphate at a much earlier phase of the glycolytic pathway, but is only active with blood pH higher than 5.9 (9) (Figure 2). On this assumption, a new generation of blood tubes has been developed, containing three additives (citrate, NaF and/or EDTA) that combine different patterns of glycolysis inhibition (i.e., acidification, both hexokinase and enolase inhibition and blood anticoagulation). The better efficiency in stabilizing blood glucose of this mixture over traditional NaF and/or KOx has been demonstrated in some studies (4,7,8), whose results are also summarized in Figure 1. More recently, van der Hagen showed that collecting blood in citrated NaF-EDTA blood tubes was effective to directly and completely inhibit glycolysis, and also demonstrated that blood glucose concentration may be stable for up to 48 h at room temperature and for not less than 24 h at 37 °C in uncentrifuged blood tubes (10).
Figure 2.
Effect of hexokinase and enolase inhibition in the glycolytic pathway. NaF, sodium fluoride; KOx, potassium oxalate.
Although additional evidence may be needed before the use of citrated NaF-EDTA tubes can be widely recommended for diagnosing diabetes, recent data suggests that the measurement of FPG may be more accurate with these tubes than using those containing traditional glycolysis inhibitors. Some critical issues of citrated NaF-EDTA tubes should also be clearly acknowledged, such as the critical issue of minimum blood tube filling (i.e., with not less than 0.5 mL of blood) and the need of immediate mixing the tube after blood collection to enable appropriate contact of blood and additives (11). To overcome some of these drawbacks, HbA1c may also be seen as a valuable alternative, provided that test results are interpreted with caution in patients carrying hemoglobin disorders (12).
Acknowledgements
None.
Footnotes
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- 1.American Diabetes Association. Statistics About Diabetes. Available online: http://www.diabetes.org/diabetes-basics/statistics/. Last access, 23 March, 2017.
- 2.Xu Y, Wang L, He J, et al. Prevalence and control of diabetes in Chinese adults. JAMA 2013;310:948-59. 10.1001/jama.2013.168118 [DOI] [PubMed] [Google Scholar]
- 3.American Diabetes Association Standards of Medical Care in Diabetes—2017. Diabetes Care 2017;40:1-135. [Google Scholar]
- 4.Bonetti G, Carta M, Montagnana M, et al. Effectiveness of citrate buffer-fluoride mixture in Terumo tubes as an inhibitor of in vitro glycolysis. Biochem Med (Zagreb) 2016;26:68-76. 10.11613/BM.2016.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lippi G, Simundic AM. Laboratory networking and sample quality: a still relevant issue for patient safety. Clin Chem Lab Med 2012;50:1703-5. 10.1515/cclm-2012-0245 [DOI] [PubMed] [Google Scholar]
- 6.Sacks DB, Arnold M, Bakris GI, et al. Guidelines and recommendation for laboratory analysis in the diagnosis and management of diabetes mellitus. Diabetes Care 2011;34:e61-99. 10.2337/dc11-9998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Juricic G, Bakliza A, Saracevic A, et al. Glucose is stable during prolonged storage in un-centrifuged Greiner tubes with liquid citrate buffer, but not in serum and NaF/KOx tubes. Clin Chem Lab Med 2016;54:411-8. 10.1515/cclm-2015-0746 [DOI] [PubMed] [Google Scholar]
- 8.Roccaforte V, Daves M, Platzgummer S, et al. The impact of different sample matrices in delayed measurement of glucose. Clin Biochem 2016;49:1412-5. 10.1016/j.clinbiochem.2016.08.015 [DOI] [PubMed] [Google Scholar]
- 9.Peake MJ, Bruns DE, Sacks DB, et al. It's time for a better blood collection tube to improve the reliability of glucose results. Diabetes Care 2013;36:e2. 10.2337/dc12-1312 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.van der Hagen EA, Fokkert MJ, Kleefman AM, et al. Technical and clinical validation of the Greiner FC-Mix glycaemia tube. Clin Chem Lab Med 2017. [Epub ahead of print]. 10.1515/cclm-2016-0944 [DOI] [PubMed] [Google Scholar]
- 11.Dimeski G, Yow KS, Brown NN. What is the most suitable blood collection tube for glucose estimation? Ann Clin Biochem 2015;52:270-5. 10.1177/0004563214544708 [DOI] [PubMed] [Google Scholar]
- 12.Danese E, Montagnana M, Salvagno GL, et al. Can we still trust hemoglobin A1c? Clin Chem Lab Med 2017. doi: . [Epub ahead of print]. 10.1515/cclm-2017-0114 [DOI] [PubMed] [Google Scholar]