Abstract
In this article, we review ongoing novel clinical trials currently investigating immunotherapeutic approaches for patients with malignant pleural mesothelioma (MPM). There is a dearth of effective therapeutic options for patients diagnosed with MPM and metastatic cancers of the pleura; these diseases have an estimated annual incidence of 150,000. Modulating the immune microenvironment to promote antitumor immune responses by systemically and regionally delivered therapeutic agents is an active area of investigation. We have conducted a review of the clinical trials database for clinical trials actively recruiting MPM patients. We focused on novel therapeutic agents administered either systemically or intrapleurally to modulate the tumor immune microenvironment. Herein, we have summarized the published results of early phase clinical trials. A total of 43 clinical trials met our inclusion criteria. These trials are investigating immunologic agents (n=20) and antibody directed therapies (n=23). The regional intrapleural delivery technique (6 trials) is used to administer chemotherapy agents (3 of 6 trials) and immunotherapy agents (3 of 6 trials), including chimeric antigen receptor T cells (1 of 6 trials). Current clinical trials modulating the MPM immune microenvironment and the combination of these novel agents with standard of care therapy provide a promising area of investigation for MPM therapy.
Keywords: Chimeric antigen receptors, regional immunotherapy, mesothelin, adoptive T-cell therapy, checkpoint blockade
Introduction
Malignant pleural mesothelioma (MPM) is a regionally aggressive, treatment-resistant cancer characterized by a prolonged incubation period following asbestos exposure (1). The majority of MPM patients present with unexplained pleural effusion, chest wall discomfort, or no symptoms. A recent International Association for the Study of Lung Cancer (IASLC) mesothelioma project involved the analysis of outcomes of an international database of mesothelioma patients and resulted in proposed changes to the TNM staging of the disease (2-4). Although MPM is a regional disease confined to the hemithorax, distant metastasis is possible during later stages of the disease (5). Malignant pleural mesothelioma is classified into 3 histologic subtypes—epithelioid, biphasic, and sarcomatoid—with epithelioid being the most common and associated with improved survival following treatment compared with the other histologic subtypes (5-7). However, we propose that, within epithelioid MPM, patients with the pleomorphic subtype have a poor prognosis equivalent to sarcomatoid MPM (8). Current treatment options for patients with MPM include pleurectomy and decortication, extrapleural pneumonectomy for resectable disease with or without induction chemotherapy, and/or intensity-modulated radiation therapy (5). In the largest series published to date, we reported that median overall survival (OS) for patients with mesothelioma was 12.5 months (n=939) (5). The dismal prognosis for those diagnosed at an early stage despite multimodality therapy has led investigators to explore novel therapies to combat this disease. One aspect of the emerging novel therapy is immunotherapy and it already has shown promising results in other thoracic solid tumors such as non-small cell lung cancer (NSCLC) (9,10).
MPM tumor immune microenvironment
It was hypothesized that MPM was the result of chronic inflammation following trapped asbestos fibers in the pleura. To characterize the immune microenvironment in MPM tumors, we first performed a semi-quantitative assessment of inflammatory response in the tumor and stroma on routine hematoxylin and eosin stained (H&E) slides of epithelioid tumors obtained from MPM patients (n=175) (11). Each tumor was assessed histologically for acute and chronic inflammatory response within the tumor and the stromal components. Inflammatory response was graded as low or high. Patients with high chronic inflammatory response in the stroma (n=59) had improved survival compared with patients with low response (n=116; median OS 19.4 vs. 15.0 months, P=0.01). On multivariate analysis, chronic inflammation in the stroma was an independent predictor of survival (HR =0.659; 95% CI, 0.464–0.937, P=0.02) (11). As a next step, we conducted a comprehensive investigation of immune responses in tumor and tumor-associated stroma in epithelioid MPM with the goal of identifying prognostic immune markers. We investigated 8 types of tumor-infiltrating immune cells within the tumor nest and tumor-associated stroma, as well as tumor expression of 5 cytokine/chemokine receptors in 230 patients (12). On multivariate analysis, stage and presence of tumoral CD20 (B lymphocytes) were independently associated with survival. Analysis of immunologically relevant cell combinations showed that high CD163+ tumor-associated macrophages and low CD8+ lymphocyte infiltration had worse prognosis than other groups and low CD163+ tumor-associated macrophages and high CD20+ lymphocyte infiltration had better prognosis than other groups (12). Multiple studies have demonstrated the prognostic role of T and B lymphocytes and macrophages (12-15). Other investigators have published data showing the presence of immunosuppression in MPM through analysis of T-cell inhibitory receptors (16) and chemokines, such as C-C motif chemokine ligand 2 (CCL2), which is a factor in the protumor M2 macrophage recruitment (17). Recently available multi-color immunofluorescence techniques have allowed investigators to study the distribution and co-localization of immunostimulatory, as well as immunosuppressive cells within the tumor microenvironment on a single slide. Figure 1 is an example of a human MPM tumor characterized by our laboratory. To tilt the immune microenvironment balance towards an antitumor immune response, several immunomodulatory agents are currently being investigated.
Figure 1.
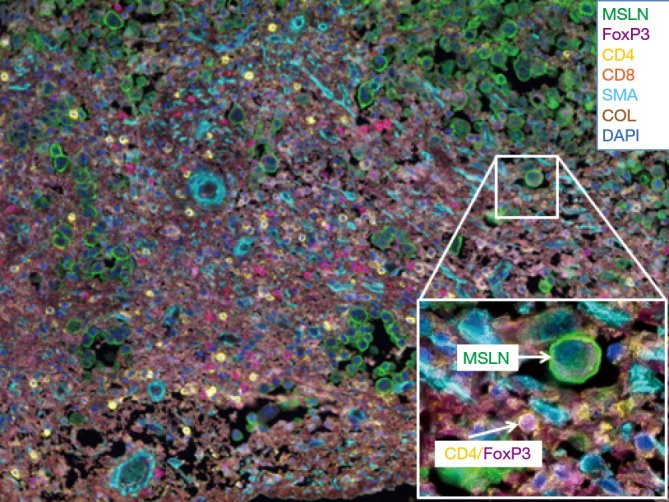
Multiplex immunofluorescence image of human mesothelioma. MSLN, mesothelin; FoxP3, forkhead box P3; CD4, CD4+ T-cell; CD8, CD8+ T-cell; SMA, smooth muscle actin; COL, collagen
Novel therapies for MPM
For the purpose of this review, we searched the term “pleural mesothelioma” on the publicly available clinical trials database (https://www.clinicaltrials.gov/); this yielded 189 results at the time our search was conducted (October 2016). We narrowed our focus to trials that are ongoing or actively recruiting patients and trials that are using either immunomodulatory agents or novel delivery methods. We excluded studies that were non-therapeutic, energy therapy focused (including radiation therapy), systemic chemotherapy focused, kinase inhibitor or other non-antibody inhibitor focused, and those designated as suspended, terminated, withdrawn, complete, or with an unknown status. Ultimately, 44 trials met our criteria; 2 additional relevant trials that were retrieved during a review of the published literature were also included. These trials have been categorized into different groups (Figure 2)—regional (intrapleural) therapies, immunotherapies, and antibody directed therapies.
Figure 2.
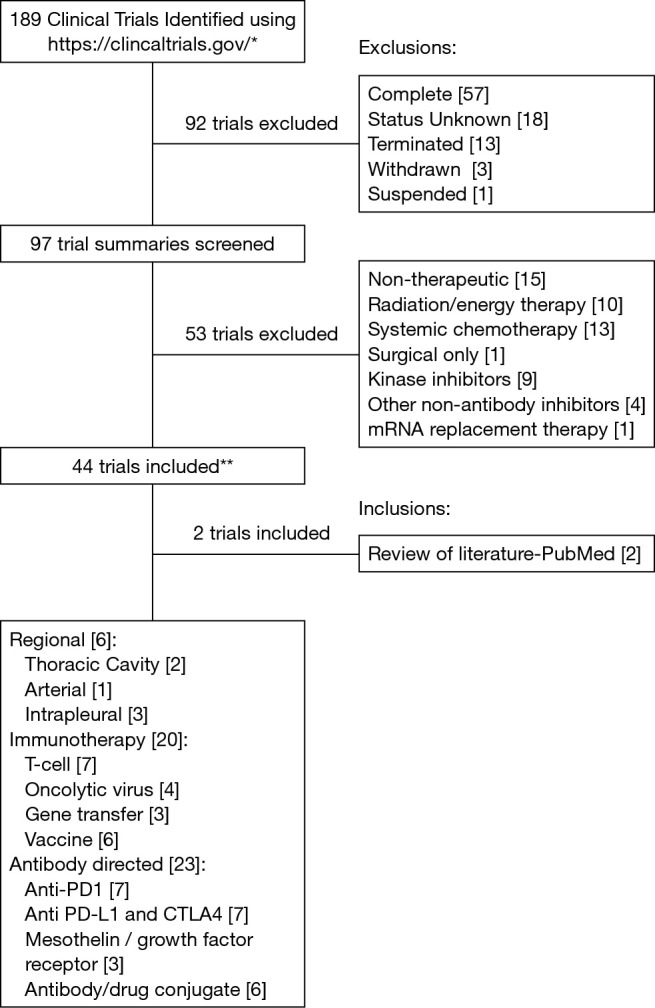
Flowchart of study methodology. *, search term consisted of “pleural mesothelioma”; **, 3 studies listed in multiple categories.
Regional therapies
Local recurrence after multimodality therapy for MPM is common and affects more than half of patients (18). Furthermore, systemic therapies lack sufficient infiltration and persistence within MPM tumors to be effective. As MPM is a regionally aggressive disease with rare metastases, regional therapies are being investigated in an effort to improve local control. In the hyperthermic intraoperative cisplatin (HIOC) chemotherapy approach, a cohort of MPM patients at low-risk for recurrence who had undergone macroscopic complete resection and received standard of care plus HIOC had a longer interval to recurrence (27.1 vs. 12.8 months, P=0.0084, n=103) and greater OS (35.3 vs. 22.8 months, P=0.026, n=103) than patients who received standard of care treatment alone (19). In the transarterial chemo-perfusion approach, mitomycin C, cisplatin, and gemcitabine were delivered by cannulation through the femoral artery to arteries that fed the tumor (20). This treatment resulted in 36% of the treated tumors (14 of 39) achieving a partial response and a decrease in tumor volume from 839.6±590 mL (range, 3.9–1,972.2 mL) to 137±399.8 mL (range, 0.88–1,131.4 mL; P=0.00012), as well as 49% of the treated tumors (19 of 39) experiencing stable disease (20).
Novel delivery methods are not only limited to chemotherapeutic agents. Regional delivery of novel agents into the pleural space is being conducted with oncolytic viruses and chimeric antigen receptor (CAR) T cells, as discussed in the following section.
Immunotherapies
T-cell therapy
During CAR T-cell therapy, a patient’s own T cells are harvested by apheresis and genetically engineered to express the CAR that specifically targets a cancer cell surface antigen. A CAR consists of a recombinant receptor that targets a defined antigen and activates the T-cell through one or more intracellular signaling domains (21). For instance, our construct, a fully human mesothelin (MSLN)-targeted CAR, incorporates an extracellular MSLN-specific scFv linked to transmembrane and intracellular signaling domains (CD28 and CD3-ζ) (22). Antigen-targeted CAR T cells are expanded ex vivo and infused into the patient following preconditioning, typically using cyclophosphamide. Upon encountering the target antigen in vivo, CAR T cells activate, proliferate, and persist. This is why they are called a “living drug” in contrast with other therapeutic agents that are typically metabolized.
Remarkable successes reported with CAR T-cell therapy in patients with acute lymphoblastic leukemia are attributed partly to the ability to target a single antigen, CD19, that is expressed on all neoplastic cells but not in any normal tissue other than the B-cell lineage (23). To select a cancer-associated antigen that is overexpressed in the majority of MPM cells with limited expression in normal tissue we and other investigators have selected MSLN. In a large cohort of epithelioid MPM tumors (n=139) we have shown that MSLN is uniformly and strongly expressed on MPM cancer cells with limited expression in the pleura and pericardium (24-26). In both preclinical models and patient tumors, MSLN expression is associated with invasion and MMP-9 secretion (24). Several clinical trials utilizing an immunotoxin or Listeria-based vaccine approach have already demonstrated that MSLN-expressing cancer cells can be targeted safely (27-29).
In our clinically relevant orthotopic MPM mouse model (30) a single, low dose of MSLN-targeted CAR T cells delivered into the pleural cavity eradicated the tumor at a 30-fold lower dose than systemically delivered CAR T cells and outperformed the systemically delivered T cells in terms of T-cell activation, proliferation, persistence, tumor eradication, and survival (22). More importantly, we have demonstrated that the beneficial effect of regionally administered CAR T cells is CD4 T-cell dependant. Intrapleurally administered CAR T cells were able to circulate and distribute to systemic sites. These promising results have led to the development of our phase I study of MSLN-targeted CAR T cells administered intrapleurally as a single infusion, with or without prior cyclophosphamide therapy, which is currently recruiting patients diagnosed with MPM or other secondary pleural malignancies from lung and breast cancers (NCT02414269). As an additional safety measure our CAR contains the iCaspase-9 suicide gene that acts as a safety switch. When AP1903, an otherwise inert molecule, is administered it dimerizes with iCaspase-9 to activate intrinsic T-cell apoptotic pathways (31-33).
Viral therapy
Advances in genetic analysis and recombinant engineering facilitated viruses to be mutated and repurposed for either oncolysis or gene transfer. An oncolytic virus is a genetically engineered virus that can selectively replicate within dividing cells, such as cancer cells, and kill cancer cells without damaging normal tissue (34). With advances in understanding tumor immunology, investigators have shown that treatment with viruses can break the immune tolerance within the tumor microenvironment (35,36). ONCOS-102 is a granulocyte-macrophage colony-stimulating factor (GM-CSF)-expressing oncolytic virus. GM-CSF expression within the tumor recruits antigen presenting cells (APC) and natural killer (NK) cells, as well as activates and matures APCs, which may induce antitumor immunity (36). A case study in a phase I clinical trial of treatment with ONCOS-102 demonstrated infiltration of CD8+ lymphocytes into the tumor and induction of Th1-type polarization in the tumor-infiltrating immune cells (36). Vaccinia is another oncolytic virus that is an attractive candidate for viral therapy due to its large genome that permits foreign gene insertion, its ability for tumor tropism, and its history of safety as a smallpox vaccine (37). Vaccinia has been shown to have an oncolytic effect on MPM cells in vitro and to be successful in treating MPM when delivered intrapleurally in a preclinical mouse model (38). These preclinical results led to a clinical trial (NCT01766739) that is currently recruiting patients.
In another approach to viral therapy, viruses are engineered to incorporate genes that may translate into therapeutic proteins in the virus-infected cells. For instance, the enzyme thymidine kinase (TK) produced by the genetically modified herpes simplex virus (AdvHSV-tk) is harmless to human cells as they naturally lack a substrate to TK (39). However, ganciclovir, a substrate administered intravenously, is metabolized into a cytotoxic nucleotide by the virus-infected cells. The treatment of malignant glioma patients with this approach resulted in increased median survival from 37.7 to 62.4 weeks (n=36) (39). A phase I study (NCT01997190) investigating the safety of this approach with valacyclovir as a prodrug in MPM patients. Similarly, Sterman et al. focused on intrapleural injection of replication-deficient adenoviruses, which encode genes for interferon (IFN) β or IFNα, that target mesothelial cells (40,41). The infected mesothelial cells deliver IFN into the pleural space to stimulate the immune system (40). In this study, patients (n=40) received first-line (n=18) or second-line (n=22) neoadjuvant chemotherapy prior to intrapleural infusion of the viruses; the overall response rate (ORR) was 25%, the disease control rate was 88%, and the median OS was either 12.5 months (first-line) or 21.5 months (second-line) depending on the type of neoadjuvant chemotherapy administered (40).
Vaccine
Dendritic cells are potent APCs that are capable of inducing immune responses by presenting neoantigens to naïve T cells in the secondary lymphoid organs; this characteristic has led to dendritic cells being increasingly used as vaccine adjuvants (42). In one approach, dendritic cells have been exogenously matured with cytokines, pulsed with tumor lysate, and re-infused as therapy in preclinical and clinical settings (42). In a completed clinical trial (NCT00280982) vaccination with antigen-pulsed dendritic cells induced cytotoxic activity against autologous tumor cells in a subgroup of patients (43). In another recent trial of MPM patients (n=10) (NCT01241682) dendritic cell vaccination composed of autologous tumor lysate-pulsed dendritic cells combined with cyclophosphamide resulted in 7 patients who survived ≥24 months and 2 patients who survived 50 and 66 months after treatment (44). Tumor cell lysates and a multipeptide vaccine are also being investigated as vaccines in two other clinical trials (NCT02395679 and NCT02661659, respectively). Similarly, synthetic peptide analogues of WT-1, a protein abundantly expressed in mesothelioma (45), have been shown to stimulate human T cells to kill mesothelioma cell lines (46). The safety and immunogenicity of the synthetic analogue WT-1 peptide vaccine has been shown in a pilot trial where 6 of 9 patients demonstrated CD4+ T-cell proliferation and 5 out of 6 HLA-A0201 patients mounted a CD8+ T-cell response against WT-1 specific peptides. The stimulated T cells were capable of cytotoxic response against the WT-1 positive cells (47,48). Currently, three clinical trials are utilizing the WT-1 peptide based approach (NCT02649829, NCT01890980, and NCT01265433). CRS-207 is a live, attenuated strain of Listeria monocytogenes that activates the innate and adaptive immune system, and is engineered to express MSLN. In an ongoing clinical trial (NCT01675765) 38 patients have been enrolled at the time of this report and, of those evaluable, 59% had partial response and 35% had stable disease (94% overall disease control rate). Median progression free survival (PFS) was 8.5 months and median OS had not been reached (49).
Antibody directed therapies
Immunotherapy treatments with antibodies in clinical trials exemplified by amatuximab have directly targeted MSLN, which has resulted in 40% of patients achieving partial responses and 51% achieving stable disease. Other findings included 6-month PFS of 51% (95% CI, 39.1–62.3), median PFS of 6.1 (95% CI, 5.8–6.4) months, and median OS of 14.8 (95% CI, 12.4–18.5) months (n=89) (28). Antibody treatments in clinical trial investigations also encompass inhibitory receptor blockade that produced objective responses in diseases such as NSCLC and melanoma (10), and applications that allow antibodies to induce cytotoxicity by conjugation to an immunotoxin (27).
Checkpoint blockade
The activated T-cell, as part of the cell mediated immune response, is regulated by checkpoint molecules, such as programmed cell death protein 1 (PD-1), its ligand programmed death receptor ligand-1 (PD-L1), and cytotoxic T lymphocyte-associated antigen-4 (CTLA-4), all of which prevent immune overstimulation. Checkpoint interactions lead to immune tolerance of tumors and several checkpoint inhibitors that modulate this function are currently being studied (50). High levels of PD-1 on CD4+ and CD8+ T cells have been shown in MPM (16). Also, PD-L1+ MPM tumors were found to have increased expression of PD-1 on their associated CD4+ and CD8+ T cells in comparison with PD-L1-tumors (51). Additionally, PD-L1+ tumors were associated with lower median survival (4.79 vs. 16.3 months, P=0.012, n=77) (52) and non-epithelioid histology (51,52).
Pembrolizumab is a monoclonal antibody against PD-1 used to treat patients with PD-L1+ tumors in the KEYNOTE-028 trial (NCT02399371). This trial yielded an ORR of 24% (confirmed and unconfirmed) and 52% of patients achieved stable disease, which is a disease control rate of 76% (n=25) (53,54). Multiple clinical trials utilizing pembrolizumab for patients with MPM, as well as trials targeting PD-L1, are ongoing and recruiting patients (Table 1). Avelumab is a human anti-PD-L1 IgG1 antibody being used to treat 53 patients with advanced unresectable mesothelioma in the JAVELIN trial (NCT01772004) (54,55). Results indicate that the unconfirmed ORR was 9.4%, stable disease was achieved in 47.2% of patients, disease control rate was 56.6%, and median PFS was 17.1 weeks (95% CI, 6.1–30.1) (55). In patients where PD-L1 status was determined, patients who were PD-L1+ (n=14) had an ORR of 14.3% and median PFS of 17.1 weeks (95% CI, 5.4–not evaluable) as opposed to patients who were PD-L1− (n=25) had an ORR of 8.0% and median PFS of 7.4 weeks (95% CI, 6.0–30.1) (55). Tremelimumab is a monoclonal antibody against CTLA-4 and several clinical trials have been completed that have studied tremelimumab as a treatment for MPM (56). In one clinical trial (NCT01655888) 29 patients with unresectable MPM received tremelimumab and disease control was achieved in 52% of patients with a median duration of 10.9 months (95% CI, 8.2–13.6), 1 patient achieved a partial response, and 38% achieved disease control rate (57).
Table 1. Current and upcoming trials (regional therapy and immunotherapy) for pleural mesothelioma.
| Description | Primary therapeutic agent | Trial status | Trial identifier |
|---|---|---|---|
| Regional therapy [6] | |||
| Thoracic cavity delivery | Cisplatin-fibrin | Recruiting | NCT01644994 |
| Cisplatin, pemetrexed | Recruiting | NCT02838745 | |
| Arterial delivery | Cisplatin, gemcitabine | Recruiting | NCT02611037 |
| Intrapleural delivery | Intrapleural GL-ONC1 (oncolytic virus) | Recruiting | NCT01766739 |
| Intrapleural herpes simplex (HSV1716; oncolytic virus) | Ongoing | NCT01721018 | |
| Intrapleural mesothelin targeted (iCasp9M28z) CAR T cells | Recruiting | NCT02414269 | |
| Immunotherapy [20] | |||
| T-cell | Autologous tumor infiltrating lymphocytes with IL-2 | Recruiting | NCT02414945 |
| Intrapleural mesothelin targeted (iCasp9M28z) CAR T cells | Recruiting | NCT02414269 | |
| Mesothelin targeted CIR T cells | Ongoing | NCT01355965 | |
| Mesothelin targeted (CART-meso) CAR T cells | Ongoing | NCT02159716 | |
| Mesothelin targeted CAR T cells | Recruiting | NCT01583686 | |
| FAP (fibroblast activation protein) targeted CAR T cells | Recruiting | NCT01722149 | |
| WT1 (Wilm’s tumor-1) targeted CAR T cells | Recruiting | NCT02408016 | |
| Oncolytic virus | Adenovirus (ONCOS 102) | Recruiting | NCT02879669 |
| Intrapleural herpes simplex (HSV1716) | Ongoing | NCT01721018 | |
| Intrapleural vaccinia (GL-ONC1) | Recruiting | NCT01766739 | |
| Measles (MV-NIS) | Recruiting | NCT01503177 | |
| Gene Transfer | AdV-tk (sensitizes tumor to anti-viral medication) | Ongoing | NCT01997190 |
| SCH 721015 (Interferon-alpha gene expressed) | Ongoing | NCT01119664 | |
| SCH 721015 (Interferon-alpha gene expressed) | Ongoing | NCT00299962 | |
| Vaccine | Multipeptide (S-588210) | Recruiting | NCT02661659 |
| Modified listeria (CRS-207) | Ongoing | NCT01675765 | |
| Tumor cell lysate (PheraLys) on dendritic cells (MesoCancerVac) | Recruiting | NCT02395679 | |
| WT-1 (Wilm’s tumor-1) | Ongoing | NCT01265433 | |
| WT-1 (Wilm’s tumor-1) | Ongoing | NCT01890980 | |
| WT-1 (Wilm’s tumor-1) on dendritic cells | Recruiting | NCT02649829 | |
| Antibody directed [23] | |||
| Anti-PD-1 | Nivolumab | Not yet recruiting | NCT02497508 |
| Nivolumab, ipilimumab | Ongoing | NCT02716272 | |
| Nivolumab, ipilimumab | Not yet recruiting | NCT02899299 | |
| Pembrolizumab | Recruiting | NCT02399371 | |
| Pembrolizumab | Recruiting | NCT02707666 | |
| Pembrolizumab | Not yet recruiting | NCT02784171 | |
| Pembrolizumab | Not yet recruiting | NCT02959463 | |
| Anti-PD-L1 and anti-CTLA4 | Atezolizumab | Recruiting | NCT02458638 |
| Durvalumab | Not yet recruiting | NCT02899195 | |
| Durvalumab, tremelimumab | Recruiting | NCT02592551 | |
| Avelumab | Recruiting | NCT01772004 | |
| Tremelimumab | Ongoing | NCT01843374 | |
| Tremelimumab, durvalumab | Recruiting | NCT02588131 | |
| Tremelimumab, durvalumab | Ongoing | NCT02141347 | |
| Mesothelin and growth factor receptor | Amatuximab (mesothelin targeting antibody) | Recruiting | NCT02357147 |
| Cetuximab (epidermal growth factor receptor targeting antibody) | Recruiting | NCT00996567 | |
| Cixutumumab (insulin-like growth factor-1 receptor targeting antibody) | Ongoing | NCT01160458 | |
| Antibody-drug conjugates | Anetumab (mesothelin targeted with tubulin inhibitor) | Recruiting | NCT02610140 |
| GSK3052230 (fibroblast growth factor receptor targeted with Fc) | Recruiting | NCT01868022 | |
| LMB-100 (mesothelin targeted with immunotoxin) | Recruiting | NCT02798536 | |
| NGR-hTNF (tumor vessel targeted with TNFα) | Recruiting | NCT01358084 | |
| NGR-hTNF (tumor vessel targeted with TNFα) | Ongoing | NCT01098266 | |
| SS1(dsFv)PE38 (mesothelin targeted with immunotoxin) | Ongoing | NCT01362790 |
Antibody drug conjugates
Antibody drug conjugates combine the specificity of an antibody with the killing ability of a toxophore (27) or other molecules such as a tubulin inhibitor (58). Investigators have combined a MSLN antibody with an immunotoxin SS1P (27) and Anetumab ravtansine, a tubulin inhibitor (58). Anetumab showed antiproliferative activity specific for tumor cell lines expressing MSLN, inhibited MSLN-expressing xenografts in vivo, and was reported to be superior to standard of care regimens in preclinical studies (58). There are currently 6 clinical trials, with 4 trials in active recruitment, utilizing antibody drug conjugates.
Combination immunotherapies
In a recent study, we have demonstrated that a combination of CAR T-cell therapy and followed by checkpoint blockade resulted in remarkable tumor regression and improved OS in mice with MPM (59). The “living drug” CAR T cells were reactivated multiple times by repeated administration of checkpoint blockade agents. Alternatively, we have demonstrated that cell-intrinsic checkpoint blockade can be achieved by genetic engineering methods that provided sustained T-cell activation without the need for repeated antibody administration (60). This approach is currently being translated to a clinical trial.
Anti-CTLA-4 antibodies are also being evaluated in combination with other immunotherapy antibodies. Three clinical trials, NCT02588131 (NIBIT-MESO-1 trial), NCT02592551, and NCT02141347, are evaluating the combination of tremelimumab and durvalumab (anti-PD-L1). One clinical trial (NCT0271672) is evaluating the combination of ipilimumab (anti-CTLA-4) and nivolumab (anti-PD-1) (54). Another clinical trial (NCT02588131) is utilizing tremelimumab and durvalumab, and currently recruiting patients with MPM.
The potential to combine existing standard of care with upcoming novel immunotherapeutic agents is still developing. Although we excluded the review of radiation therapy clinical trials, de Perrot et al. from the University of Toronto reported intriguing results following a new approach of Surgery for Mesothelioma After Radiation Therapy (SMART), which involves an accelerated hemithoracic intensity-modulated radiation therapy followed by extrapleural pneumonectomy (61). Preclinical investigations have demonstrated potential immune responses following radiation therapy and augmentation of these responses by combining with immunotherapeutic agents (62). We have reported that a single dose of thoracic radiation prior to administration of CAR T cells has improved survival outcomes in a preclinical MPM model (63). More mechanistic insights into these combination therapy approaches will help translate benefits to mesothelioma patients.
Conclusions
Immunotherapeutic approaches and novel delivery methods are a promising area of investigation for potential treatments of MPM. As data evolves in these early phase trials, it will create a foundation for further investigations of combination therapy approaches such as a rational combination of immunotherapeutic agents (i.e., combination of immunotherapeutic agents with traditional agents such as chemotherapy and/or radiation therapy). Intrapleural administration, either using a pleural catheter or via interventional radiologic measures, provides an opportunity to deliver these novel agents directly to the tumor bed. However, following delivery, strategies for effective tumor infiltration and functional persistence remain future areas of investigation in the preclinical models.
Acknowledgements
We thank Alex Torres of the MSK Thoracic Surgery Service for his editorial assistance.
Funding: Authors laboratory research is supported by funding from the National Institutes of Health (P30 CA008748), the Mesothelioma Applied Research Foundation, the Baker Street Foundation, the Mr. William H. Goodwin and Alice Goodwin, the Commonwealth Foundation for Cancer Research, and the Experimental Therapeutics Center, and Stand Up to Cancer—Cancer Research Institute Cancer Immunology Translational Cancer Research Grant (SU2C-AACR-DT1012). Stand Up To Cancer is a program of the Entertainment Industry Foundation administered by the American Association for Cancer Research.
Footnotes
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- 1.Robinson BW, Musk AW, Lake RA. Malignant mesothelioma. Lancet 2005;366:397-408. 10.1016/S0140-6736(05)67025-0 [DOI] [PubMed] [Google Scholar]
- 2.Nowak AK, Chansky K, Rice DC, et al. The IASLC Mesothelioma Staging Project: Proposals for Revisions of the T Descriptors in the Forthcoming Eighth Edition of the TNM Classification for Pleural Mesothelioma. J Thorac Oncol 2016;11:2089-99. [DOI] [PubMed] [Google Scholar]
- 3.Rice D, Chansky K, Nowak A, et al. The IASLC Mesothelioma Staging Project: Proposals for Revisions of the N Descriptors in the Forthcoming Eighth Edition of the TNM Classification for Pleural Mesothelioma. J Thorac Oncol 2016;11:2100-11. [DOI] [PubMed] [Google Scholar]
- 4.Rusch VW, Chansky K, Kindler HL, et al. The IASLC Mesothelioma Staging Project: Proposals for the M Descriptors and for Revision of the TNM Stage Groupings in the Forthcoming (Eighth) Edition of the TNM Classification for Mesothelioma. J Thorac Oncol 2016;11:2112-9. 10.1016/j.jtho.2016.09.124 [DOI] [PubMed] [Google Scholar]
- 5.Flores RM, Zakowski M, Venkatraman E, et al. Prognostic factors in the treatment of malignant pleural mesothelioma at a large tertiary referral center. J Thorac Oncol 2007;2:957-65. 10.1097/JTO.0b013e31815608d9 [DOI] [PubMed] [Google Scholar]
- 6.Travis WD, Brambilla E, Muller-Hermelink HK, et al. editors. Pathology and Genetics of Tumours of the Lung, Pleura, Thymus and Heart. 1 edition. Lyon: IARC Press, 2004:132-5. [Google Scholar]
- 7.Arrossi AV, Lin E, Rice D, Moran CA. Histologic assessment and prognostic factors of malignant pleural mesothelioma treated with extrapleural pneumonectomy. Am J Clin Pathol 2008;130:754-64. 10.1309/AJCPHV33LJTVDGJJ [DOI] [PubMed] [Google Scholar]
- 8.Kadota K, Suzuki K, Sima CS, et al. Pleomorphic epithelioid diffuse malignant pleural mesothelioma: a clinicopathological review and conceptual proposal to reclassify as biphasic or sarcomatoid mesothelioma. J Thorac Oncol 2011;6:896-904. 10.1097/JTO.0b013e318211127a [DOI] [PubMed] [Google Scholar]
- 9.Garon EB, Rizvi NA, Hui R, et al. Pembrolizumab for the treatment of non-small-cell lung cancer. N Engl J Med 2015;372:2018-28. 10.1056/NEJMoa1501824 [DOI] [PubMed] [Google Scholar]
- 10.Topalian SL, Hodi FS, Brahmer JR, et al. Safety, activity, and immune correlates of anti-PD-1 antibody in cancer. N Engl J Med 2012;366:2443-54. 10.1056/NEJMoa1200690 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Suzuki K, Kadota K, Sima CS, et al. Chronic inflammation in tumor stroma is an independent predictor of prolonged survival in epithelioid malignant pleural mesothelioma patients. Cancer Immunol Immunother 2011;60:1721-8. 10.1007/s00262-011-1073-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ujiie H, Kadota K, Nitadori JI, et al. The tumoral and stromal immune microenvironment in malignant pleural mesothelioma: A comprehensive analysis reveals prognostic immune markers. Oncoimmunology 2015;4:e1009285. 10.1080/2162402X.2015.1009285 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Anraku M, Cunningham KS, Yun Z, et al. Impact of tumor-infiltrating T cells on survival in patients with malignant pleural mesothelioma. J Thorac Cardiovasc Surg 2008;135:823-9. 10.1016/j.jtcvs.2007.10.026 [DOI] [PubMed] [Google Scholar]
- 14.Cornelissen R, Lievense LA, Maat AP, et al. Ratio of intratumoral macrophage phenotypes is a prognostic factor in epithelioid malignant pleural mesothelioma. PLoS One 2014;9:e106742. 10.1371/journal.pone.0106742 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yamada N, Oizumi S, Kikuchi E, et al. CD8+ tumor-infiltrating lymphocytes predict favorable prognosis in malignant pleural mesothelioma after resection. Cancer Immunol Immunother 2010;59:1543-9. 10.1007/s00262-010-0881-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lizotte PH, Jones RE, Keogh L, et al. Fine needle aspirate flow cytometric phenotyping characterizes immunosuppressive nature of the mesothelioma microenvironment. Sci Rep 2016;6:31745. 10.1038/srep31745 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chéné AL, d'Almeida S, Blondy T, et al. Pleural Effusions from Patients with Mesothelioma Induce Recruitment of Monocytes and Their Differentiation into M2 Macrophages. J Thorac Oncol 2016;11:1765-73. 10.1016/j.jtho.2016.06.022 [DOI] [PubMed] [Google Scholar]
- 18.Baldini EH, Recht A, Strauss GM, et al. Patterns of failure after trimodality therapy for malignant pleural mesothelioma. Ann Thorac Surg 1997;63:334-8. 10.1016/S0003-4975(96)01228-3 [DOI] [PubMed] [Google Scholar]
- 19.Sugarbaker DJ, Gill RR, Yeap BY, et al. Hyperthermic intraoperative pleural cisplatin chemotherapy extends interval to recurrence and survival among low-risk patients with malignant pleural mesothelioma undergoing surgical macroscopic complete resection. J Thorac Cardiovasc Surg 2013;145:955-63. 10.1016/j.jtcvs.2012.12.037 [DOI] [PubMed] [Google Scholar]
- 20.Vogl TJ, Lindemayr S, Naguib NN, et al. Nonselective transarterial chemoperfusion: a palliative treatment for malignant pleural mesothelioma. Radiology 2013;266:649-56. 10.1148/radiol.12111858 [DOI] [PubMed] [Google Scholar]
- 21.Sadelain M, Brentjens R, Rivière I. The basic principles of chimeric antigen receptor design. Cancer Discov 2013;3:388-98. 10.1158/2159-8290.CD-12-0548 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Adusumilli PS, Cherkassky L, Villena-Vargas J, et al. Regional delivery of mesothelin-targeted CAR T cell therapy generates potent and long-lasting CD4-dependent tumor immunity. Sci Transl Med 2014;6:261ra151. 10.1126/scitranslmed.3010162 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Davila ML, Riviere I, Wang X, et al. Efficacy and toxicity management of 19-28z CAR T cell therapy in B cell acute lymphoblastic leukemia. Sci Transl Med 2014;6:224ra25. 10.1126/scitranslmed.3008226 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Servais EL, Colovos C, Rodriguez L, et al. Mesothelin overexpression promotes mesothelioma cell invasion and MMP-9 secretion in an orthotopic mouse model and in epithelioid pleural mesothelioma patients. Clin Cancer Res 2012;18:2478-89. 10.1158/1078-0432.CCR-11-2614 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pastan I, Hassan R. Discovery of mesothelin and exploiting it as a target for immunotherapy. Cancer Res 2014;74:2907-12. 10.1158/0008-5472.CAN-14-0337 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chang K, Pastan I, Willingham MC. Isolation and characterization of a monoclonal antibody, K1, reactive with ovarian cancers and normal mesothelium. Int J Cancer 1992;50:373-81. 10.1002/ijc.2910500308 [DOI] [PubMed] [Google Scholar]
- 27.Hassan R, Sharon E, Thomas A, et al. Phase 1 study of the antimesothelin immunotoxin SS1P in combination with pemetrexed and cisplatin for front-line therapy of pleural mesothelioma and correlation of tumor response with serum mesothelin, megakaryocyte potentiating factor, and cancer antigen 125. Cancer 2014;120:3311-9. 10.1002/cncr.28875 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hassan R, Kindler HL, Jahan T, et al. Phase II clinical trial of amatuximab, a chimeric antimesothelin antibody with pemetrexed and cisplatin in advanced unresectable pleural mesothelioma. Clin Cancer Res 2014;20:5927-36. 10.1158/1078-0432.CCR-14-0804 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Le DT, Brockstedt DG, Nir-Paz R, et al. A live-attenuated Listeria vaccine (ANZ-100) and a live-attenuated Listeria vaccine expressing mesothelin (CRS-207) for advanced cancers: phase I studies of safety and immune induction. Clin Cancer Res 2012;18:858-68. 10.1158/1078-0432.CCR-11-2121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Servais EL, Colovos C, Kachala SS, et al. Pre-clinical mouse models of primary and metastatic pleural cancers of the lung and breast and the use of bioluminescent imaging to monitor pleural tumor burden. Curr Protoc Pharmacol 2011;Chapter 14:Unit14.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Clackson T, Yang W, Rozamus LW, et al. Redesigning an FKBP-ligand interface to generate chemical dimerizers with novel specificity. Proc Natl Acad Sci U S A 1998;95:10437-42. 10.1073/pnas.95.18.10437 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Iuliucci JD, Oliver SD, Morley S, et al. Intravenous safety and pharmacokinetics of a novel dimerizer drug, AP1903, in healthy volunteers. J Clin Pharmacol 2001;41:870-9. 10.1177/00912700122010771 [DOI] [PubMed] [Google Scholar]
- 33.Di Stasi A, Tey SK, Dotti G, et al. Inducible apoptosis as a safety switch for adoptive cell therapy. N Engl J Med 2011;365:1673-83. 10.1056/NEJMoa1106152 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Adusumilli PS, Stiles BM, Chan MK, et al. Imaging and therapy of malignant pleural mesothelioma using replication-competent herpes simplex viruses. J Gene Med 2006;8:603-15. 10.1002/jgm.877 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Workenhe ST, Pol JG, Lichty BD, et al. Combining oncolytic HSV-1 with immunogenic cell death-inducing drug mitoxantrone breaks cancer immune tolerance and improves therapeutic efficacy. Cancer Immunol Res 2013;1:309-19. 10.1158/2326-6066.CIR-13-0059-T [DOI] [PubMed] [Google Scholar]
- 36.Ranki T, Joensuu T, Jäger E, et al. Local treatment of a pleural mesothelioma tumor with ONCOS-102 induces a systemic antitumor CD8+ T-cell response, prominent infiltration of CD8+ lymphocytes and Th1 type polarization. Oncoimmunology 2014;3:e958937. 10.4161/21624011.2014.958937 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kelly KJ, Woo Y, Brader P, et al. Novel oncolytic agent GLV-1h68 is effective against malignant pleural mesothelioma. Hum Gene Ther 2008;19:774-82. 10.1089/hum.2008.036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Belin LJ, Ady JW, Lewis C, et al. An oncolytic vaccinia virus expressing the human sodium iodine symporter prolongs survival and facilitates SPECT/CT imaging in an orthotopic model of malignant pleural mesothelioma. Surgery 2013;154:486-95. 10.1016/j.surg.2013.06.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Immonen A, Vapalahti M, Tyynelä K, et al. AdvHSV-tk gene therapy with intravenous ganciclovir improves survival in human malignant glioma: a randomised, controlled study. Mol Ther 2004;10:967-72. 10.1016/j.ymthe.2004.08.002 [DOI] [PubMed] [Google Scholar]
- 40.Sterman DH, Alley E, Stevenson JP, et al. Pilot and Feasibility Trial Evaluating Immuno-Gene Therapy of Malignant Mesothelioma Using Intrapleural Delivery of Adenovirus-IFNα Combined with Chemotherapy. Clin Cancer Res 2016;22:3791-800. 10.1158/1078-0432.CCR-15-2133 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sterman DH, Recio A, Haas AR, et al. A phase I trial of repeated intrapleural adenoviral-mediated interferon-beta gene transfer for mesothelioma and metastatic pleural effusions. Mol Ther 2010;18:852-60. 10.1038/mt.2009.309 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Thomas A, Hassan R. Immunotherapies for non-small-cell lung cancer and mesothelioma. Lancet Oncol 2012;13:e301-10. 10.1016/S1470-2045(12)70126-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hegmans JP, Veltman JD, Lambers ME, et al. Consolidative dendritic cell-based immunotherapy elicits cytotoxicity against malignant mesothelioma. Am J Respir Crit Care Med 2010;181:1383-90. 10.1164/rccm.200909-1465OC [DOI] [PubMed] [Google Scholar]
- 44.Cornelissen R, Hegmans JP, Maat AP, et al. Extended Tumor Control after Dendritic Cell Vaccination with Low-Dose Cyclophosphamide as Adjuvant Treatment in Patients with Malignant Pleural Mesothelioma. Am J Respir Crit Care Med 2016;193:1023-31. 10.1164/rccm.201508-1573OC [DOI] [PubMed] [Google Scholar]
- 45.Ordóñez NG. The immunohistochemical diagnosis of mesothelioma: a comparative study of epithelioid mesothelioma and lung adenocarcinoma. Am J Surg Pathol 2003;27:1031-51. 10.1097/00000478-200308000-00001 [DOI] [PubMed] [Google Scholar]
- 46.May RJ, Dao T, Pinilla-Ibarz J, et al. Peptide epitopes from the Wilms' tumor 1 oncoprotein stimulate CD4+ and CD8+ T cells that recognize and kill human malignant mesothelioma tumor cells. Clin Cancer Res 2007;13:4547-55. 10.1158/1078-0432.CCR-07-0708 [DOI] [PubMed] [Google Scholar]
- 47.Zauderer MG, Krug LM. Novel therapies in phase II and III trials for malignant pleural mesothelioma. J Natl Compr Canc Netw 2012;10:42-7. 10.6004/jnccn.2012.0007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Krug LM, Dao T, Brown AB, et al. WT1 peptide vaccinations induce CD4 and CD8 T cell immune responses in patients with mesothelioma and non-small cell lung cancer. Cancer Immunol Immunother 2010;59:1467-79. 10.1007/s00262-010-0871-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Jahan T, Hassan R, Alley E, et al. 208O_PR: CRS-207 with chemotherapy (chemo) in malignant pleural mesothelioma (MPM): Results from a phase 1b trial. J Thorac Oncol 2016;11:S156. 10.1016/S1556-0864(16)30330-627198358 [DOI] [Google Scholar]
- 50.Karim S, Leighl N. Pembrolizumab for the treatment of thoracic malignancies: current landscape and future directions. Future Oncol 2016;12:9-23. 10.2217/fon.15.294 [DOI] [PubMed] [Google Scholar]
- 51.Awad MM, Jones RE, Liu H, et al. Cytotoxic T Cells in PD-L1-Positive Malignant Pleural Mesotheliomas Are Counterbalanced by Distinct Immunosuppressive Factors. Cancer Immunol Res 2016;4:1038-48. 10.1158/2326-6066.CIR-16-0171 [DOI] [PubMed] [Google Scholar]
- 52.Cedrés S, Ponce-Aix S, Zugazagoitia J, et al. Analysis of expression of programmed cell death 1 ligand 1 (PD-L1) in malignant pleural mesothelioma (MPM). PLoS One 2015;10:e0121071. 10.1371/journal.pone.0121071 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Alley EW, Lopez J, Santoro A, et al. Clinical safety and activity of pembrolizumab in patients with malignant pleural mesothelioma (KEYNOTE-028): preliminary results from a non-randomised, open-label, phase 1b trial. Lancet Oncol 2017;18:623-30. 10.1016/S1470-2045(17)30169-9 [DOI] [PubMed] [Google Scholar]
- 54.Thapa B, Watkins DN, John T. Immunotherapy for malignant mesothelioma: reality check. Expert Rev Anticancer Ther 2016. [Epub ahead of print]. 10.1080/14737140.2016.1241149 [DOI] [PubMed] [Google Scholar]
- 55.Hassan R, Thomas A, Patel MR, et al. Avelumab (MSB0010718C; anti-PD-L1) in patients with advanced unresectable mesothelioma from the JAVELIN solid tumor phase Ib trial: Safety, clinical activity, and PD-L1 expression. J Clin Oncol 2016; abstr 8503).
- 56.Guazzelli A, Hussain M, Krstic-Demonacos M, et al. Tremelimumab for the treatment of malignant mesothelioma. Expert Opin Biol Ther 2015;15:1819-29. 10.1517/14712598.2015.1116515 [DOI] [PubMed] [Google Scholar]
- 57.Calabrò L, Morra A, Fonsatti E, et al. Efficacy and safety of an intensified schedule of tremelimumab for chemotherapy-resistant malignant mesothelioma: an open-label, single-arm, phase 2 study. Lancet Respir Med 2015;3:301-9. 10.1016/S2213-2600(15)00092-2 [DOI] [PubMed] [Google Scholar]
- 58.Golfier S, Kopitz C, Kahnert A, et al. Anetumab ravtansine: a novel mesothelin-targeting antibody-drug conjugate cures tumors with heterogeneous target expression favored by bystander effect. Mol Cancer Ther 2014;13:1537-48. 10.1158/1535-7163.MCT-13-0926 [DOI] [PubMed] [Google Scholar]
- 59.Cherkassky L, Morello A, Villena-Vargas J, et al. Human CAR T cells with cell-intrinsic PD-1 checkpoint blockade resist tumor-mediated inhibition. J Clin Invest 2016;126:3130-44. 10.1172/JCI83092 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Chen N, Morello A, Tano Z, et al. CAR T-cell intrinsic PD-1 checkpoint blockade: A two-in-one approach for solid tumor immunotherapy. Oncoimmunology 2016;6:e1273302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.de Perrot M, Feld R, Leighl NB, et al. Accelerated hemithoracic radiation followed by extrapleural pneumonectomy for malignant pleural mesothelioma. J Thorac Cardiovasc Surg 2016;151:468-73. 10.1016/j.jtcvs.2015.09.129 [DOI] [PubMed] [Google Scholar]
- 62.Wu L, Wu MO, De la Maza L, et al. Targeting the inhibitory receptor CTLA-4 on T cells increased abscopal effects in murine mesothelioma model. Oncotarget 2015;6:12468-80. 10.18632/oncotarget.3487 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Zeltsman M, Villena-Vargas J, Rimner A, et al. MA13.07 Tumor-Targeted Radiation Promotes Abscopal Efficacy of Regionally Administered CAR T Cells: A Rationale for Clinical Trial. J Thorac Oncol 2017;12:s419 10.1016/j.jtho.2016.11.483 [DOI] [Google Scholar]


